Abstract
The “basal ganglia” refers to a group of subcortical nuclei responsible primarily for motor control, as well as other roles such as motor learning, executive functions and behaviors, and emotions. Proposed more than two decades ago, the classical basal ganglia model shows how information flows through the basal ganglia back to the cortex through two pathways with opposing effects for the proper execution of movement. Although much of the model has remained, the model has been modified and amplified with the emergence of new data. Furthermore, parallel circuits subserve the other functions of the basal ganglia engaging associative and limbic territories. Disruption of the basal ganglia network forms the basis for several movement disorders. This article provides a comprehensive account of basal ganglia functional anatomy and chemistry and the major pathophysiological changes underlying disorders of movement. We try to answer three key questions related to the basal ganglia, as follows: What are the basal ganglia? What are they made of? How do they work? Some insight on the canonical basal ganglia model is provided, together with a selection of paradoxes and some views over the horizon in the field.
The basal ganglia are responsible for motor control, and their proper functioning requires dopamine to be released at the input nuclei. Dopamine dysfunction is associated with several movement disorders (e.g., Parkinson’s).
The basal ganglia and related nuclei consist of a variety of subcortical cell groups engaged primarily in motor control, together with a wider variety of roles such as motor learning, executive functions and behavior, and emotions. The term basal ganglia in the strictest sense refers to nuclei embedded deep in the brain hemispheres (striatum or caudate-putamen and globus pallidus), whereas related nuclei consist of structures located in the diencephalon (subthalamic nucleus), mesencephalon (substantia nigra), and pons (pedunculopontine nucleus). Ideas and concepts regarding the functions of the basal ganglia were strongly influenced by clinical observations during the 20th century, which showed that lesions of the lenticular nucleus (putamen and globus pallidus) and the subthalamic nucleus (STN) were associated with parkinsonian signs, dystonia, and hemiballismus (Wilson 1925; Purdon-Martin 1927). Thus, the terms extra-pyramidal system and extra-pyramidal syndrome were frequently used in the past to refer to the pathological basis of movement disorders in an attempt to make a clear distinction from the pyramidal system (corticofugal neurons that give rise to corticospinal projections). At present, these terms and distinctions are considered obsolete and misleading and, therefore, will not be used in this article.
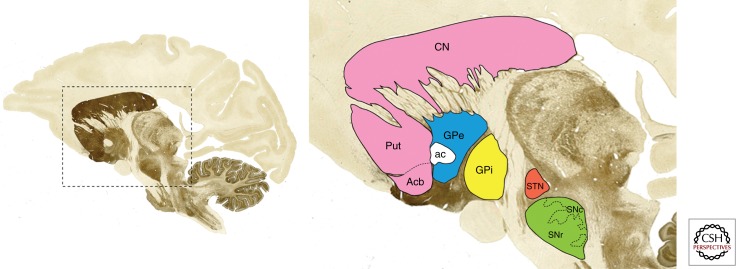
The basal ganglia and related nuclei can be broadly categorized as (1) input nuclei, (2) output nuclei, and (3) intrinsic nuclei. Input nuclei are those structures receiving incoming information from different sources, mainly cortical, thalamic, and nigral in origin. The caudate nucleus (CN), the putamen (Put), and the accumbens nucleus (Acb) are all considered input nuclei. The output nuclei are those structures that send basal ganglia information to the thalamus and consist of the internal segment of the globus pallidus (GPi) and the substantia nigra pars reticulata (SNr). Finally, intrinsic nuclei such as the external segment of the globus pallidus (GPe), the STN and the substantia nigra pars compacta (SNc) are located between the input and output nuclei in the relay of information. Cortical and thalamic efferent information enters the striatum (CN, Put, and Acb) to be processed further within the basal ganglia system. The output nuclei (GPi and SNr) project mainly to the thalamus (ventral nuclei), which, in turn, project back to the cerebral cortex (mainly frontal lobe). The appropriate functioning of the basal ganglia system requires dopamine to be released at the input nuclei. Dopamine dysfunction is associated with several basal ganglia movement disorders such as the parkinsonian syndrome (i.e., Parkinson’s disease), dystonia, chorea, and tics. All major basal ganglia components are illustrated in Figure 1.
Figure 1.
Basal ganglia nuclei. Parasagittal section through the monkey brain (stained with the acetylcholinesterase method) showing the localization and boundaries of all major components of the basal ganglia system.
BASAL GANGLIA NUCLEI
Input Nuclei: The Striatum
The striatum is by far the largest subcortical brain structure in the mammalian brain, with an estimated volume of 10 cm3 in the human brain (Schröeder et al. 1975). It is a heterogeneous structure that receives afferents from several cortical and subcortical structures and projects to various basal ganglia nuclei.
Striatal Neurons: Projection Neurons and Interneurons
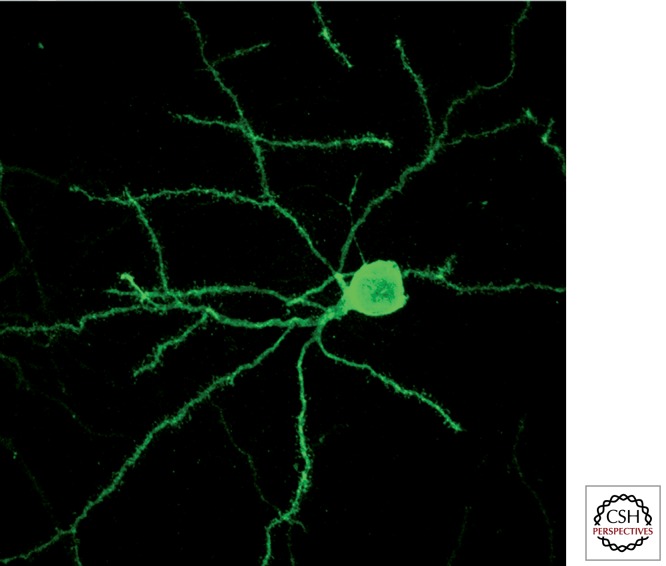
The striatum contains two different types of neurons: projection neurons and interneurons (90% and 10% of striatal neurons, respectively). Projection or striatofugal neurons are also called medium-sized spiny neurons (MSNs) because these multipolar neurons have small to medium cellular somata (20 µm in diameter), and their dendritic processes are covered by postsynaptic specializations called dendritic spines (Fig. 2). All striatal MSNs are inhibitory neurons that use GABA as the neurotransmitter. Striatofugal MSNs could be divided further, according to their projection targets, into those innervating the GPe nucleus and those projecting to the output nuclei GPi and SNr. Striatal MSNs innervating the GPe nucleus express the dopamine receptor subtype 2 (D2R), which inhibits intracellular adenyl-cyclase trough G-protein signaling, and give rise to the indirect pathway (striato-GPe-STN-GPi/SNr). On the other hand, striatal MSNs projecting directly to GPi and SNr contain dopamine receptor subtype 1 receptors (D1R), which activate adenyl-cyclase signaling (D1-containing neurons), and give rise to the direct striatopallidal pathway. Furthermore, striatal MSNs projecting through the indirect pathway are known to contain the neuropeptide enkephalin, whereas the neuropeptides substance P and dynorphin are expressed in those MSNs projecting directly to the GPi and SNr.
Figure 2.
Striatal medium-sized spiny neurons (MSNs). Striatal MSNs are the most abundant neuronal phenotype in the striatum (representing up to 95% of the total number of striatal neurons). These medium-sized neurons (20 µm in diameter) are multipolar stellate cells with radially oriented dendrites. These dendrites are covered by small postsynaptic specializations called dendritic spines. Photomicrograph taken from a striatonigral-projecting MSN, retrogradely labeled following the delivery of rabies virus into the substantia nigra pars reticulata.
In addition to these spiny projection neurons, the striatum also contains several different classes of local-circuit neurons (interneurons), all of which show smooth dendrites. Interneurons in the striatum are often classified into four groups depending on their neurochemical profiles and morphological characteristics (for review, see Kawaguchi et al. 1995). The most abundant group of interneurons consists of large aspiny cholinergic neurons that use acetylcholine as neurotransmitter. Electrophysiologically, these neurons show a rather continuous and constant firing pattern of activity and are therefore also known as tonically active neurons (TANs). Another type of striatal interneuron is GABAergic and also contains the calcium-binding protein parvalbumin. Based on their electrophysiological fingerprint, these neurons are also called fast-spiking interneurons (FSIs). Another group of GABAergic interneurons with a different phenotype is those containing the calcium-binding protein calretinin. In addition to parvalbumin and calretinin, a third type of GABAergic interneuron, known as nitrergic interneuron, uses nitric oxide as the neurotransmitter. Both TAN and FSI interneurons modulate the activity of striatofugal neurons and are, in turn, under dopaminergic control, forming a complex intrastriatal microcircuit, which is discussed below (see section “Classic Basal Ganglia Model”). Calretinin-positive and nitrergic interneurons innervate TAN and FSI interneurons.
Striatal Compartments: Striosomes and Matrix
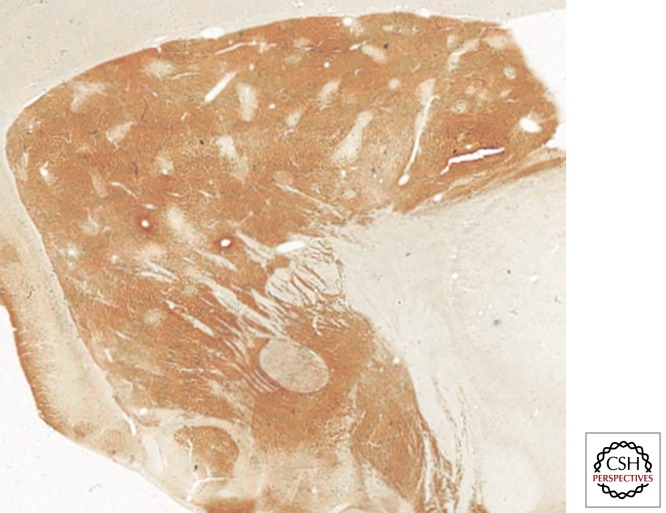
The striatum appears as a rather homogeneous structure when viewed with cytoarchitectural techniques; however, the use of some specific immunohistochemical markers highlights two subdivisions: striosomes and matrix compartments. The first evidence for these striatal compartments, shown using the histochemical detection of the enzyme acetylcholinesterase (AChE), was published in the late 1970s by Graybiel and Ragsdale (1978). The pattern of AChE staining revealed the presence of several patchy areas showing weak AChE activity (striosomes, sometimes also called patches) immersed within a background of more intense AChE staining (matrix). Moreover, the immunocytochemical detection of the μ opioid receptor revealed an enriched expression of this receptor within the striosomes, together with a weaker expression in the matrix (Perth et al. 1976; Desban et al. 1995). From a neurochemical perspective, striosomes are characterized by strong immunoreactivity against enkephalin, substance P, GABA, and neurotensin (Graybiel et al. 1981; Gerfen 1984), whereas the matrix compartment is enriched with calcium-binding proteins such as parvalbumin (Prensa et al. 1999) and calbindin (Fig. 3) (Gerfen et al. 1985).
Figure 3.
Striatal compartments. Although the striatum appears as a rather homogeneous structure, several histochemical and immunohistochemical stains evidence the presence of two compartments named striosomes and matrix. The photomicrograph is taken from a parasagittal section through the primate striatum. The immunohistochemical detection of the calcium-binding protein calbindin reveals the presence of a number of patchy areas with weak calbindin stain (striosomes) immersed within a background showing higher calbindin stain (matrix).
In terms of striatal afferent and efferent systems, striosomes and the matrix could be viewed as isolated compartments. Although striatal MSNs show profuse arborization throughout the striatum (Fig. 2), their dendritic domains and local axon collaterals remain restricted within the striatal compartment in which they are located. In other words, dendrites from MSNs located in a striosomal compartment never enter the matrix, and the same holds true for matrix MSNs. Such a distinct compartmental segregation of MSNs was first reported by Penny et al. (1988) and Kawaguchi et al. (1989) and more recently confirmed by Fujiyama et al. (2011). Furthermore, matrix MSNs innervate the GPe, GPi, and SNr, whereas MSNs located in striosomes preferentially project to the SNc, also giving rise to axon collaterals targeting the GPe, GPi, and SNr (Gerfen 1984; Bolam et al. 1988; Kawaguchi et al. 1989; Giménez-Amaya and Graybiel 1990; Fujiyama et al. 2011).
Striatal glutamatergic afferents originating from the cerebral cortex, thalamus, and amygdala, as well as dopaminergic afferents arising from SNc are heterogeneously distributed throughout the striosome/matrix compartments. Motor and sensory areas of the cerebral cortex, together with thalamostriatal projections and dopaminergic neurons from the SNc, mainly innervate the matrix compartment, whereas cortical limbic areas, the basolateral amygdala, and ventral parts of the SNc preferentially target striosomes (Graybiel 1984, 1990; Donoghue and Herkenham 1986; Ragsdale and Graybiel 1988; Gerfen 1992; Sadikot et al. 1992a,b; Kincaid and Wilson 1996). At present, an accurate explanation of the functional correlate underlying this striosome/matrix compartmental organization is still lacking.
Striatal Afferents: Cortex, Thalamus, Substantia Nigra, and Raphe
The entire cerebral cortex projects to the striatum. Both ipsilateral and contralateral cortices send glutamatergic projections that make asymmetric synaptic contacts mainly with dendritic spines of striatal MSNs. In addition to cortical limbic areas innervating the striosomal compartment (Gerfen 1984; Donoghue and Herkenham 1986), the bulk of corticostriatal projections spread through the matrix compartment, and these projections are topographically organized (Selemon and Goldman-Rakic 1985). Pyramidal neurons located in cortical layers III and Va innervate the matrix, whereas cortical input from prelimbic cortices originating from pyramidal neurons in deeper layer V reaches the striosomal compartment. Up to two different types of layer V pyramidal neurons innervating the striatum have been described: the so-called pyramidal tract neurons (PT-type), which consist of striatal collaterals that project through the cortico-pyramidal tract, and the intratelencephalic neurons (IT-type). PT-type neurons provide ipsilateral input to the striatum, whereas IT-type neurons innervate the striatum bilaterally (Reiner et al. 2003; for review, see Reiner et al. 2010). Furthermore, IT-type neurons mainly innervate striatal MSNs giving rise to the direct pathway, whereas PT-type neurons mainly contact striatal MSNs in the indirect circuit (Lei et al. 2004). More recently, such a selective pattern of striatal innervation from IT-type neurons has been challenged by Baillon et al. (2008), who show that IT-type neurons equally excite striatonigral and striatopallidal neurons (direct and indirect pathway neurons in rodents). However, this is not completely inconsistent with preferential IT-type input to MSNs of the direct pathway and PT-type innervation of striato-GPe neurons (Lei et al. 2004).
In addition to the corticostriatal system, thalamostriatal projections are a major source of glutamatergic afferents reaching the striatum. Initial descriptions of the thalamostriatal system were made by Vogt and Vogt (1941) as well as by Powell and Cowan (1956) in the mid-20th century. Although this pathway has been properly characterized anatomically, very little is known regarding the role it plays in basal ganglia function and dysfunction. Until recently, the thalamostriatal pathway had been largely neglected in most modern models summarizing basal ganglia circuits. Virtually all types of striatal neurons are contacted by thalamostriatal afferents, which include projection neurons (Dubé et al. 1988; Sidibé and Smith 1999; Gonzalo et al. 2002; Bacci et al. 2004; Lanciego et al. 2004; Smith et al. 2004; Castle et al. 2005) and striatal interneurons (Meredith and Wouterlood 1990; Lapper and Bolam 1992; Lapper et al. 1992; Bennet and Bolam 1993; Rudkin and Sadikot 1999; Sidibé and Smith 1999; Thomas et al. 2000). It has been generally considered that thalamostriatal axons preferentially contact the dendritic shafts of striatal MSNs (Sadikot et al. 1990; Smith and Bolam 1990; Sidibé and Smith 1996; Smith et al. 2004), whereas corticostriatal afferents mainly synapse with dendritic spines. This distinct segregation of postsynaptic structures has been challenged by recent studies showing that thalamostriatal afferents originating from the centromedian-parafascicular (CM-Pf) thalamic complex only target dendritic shafts, whereas thalamic inputs arising from nuclei other than the CM-Pf complex contact dendritic spines (Raju et al. 2006). Furthermore, although the midline and intralaminar thalamic nuclei are often considered the main source of the thalamostriatal system (Berendse and Groenewegen 1990; Groenewegen and Berendse 1994), it is worth noting that the ventral thalamic motor nuclei also contribute to thalamostriatal projections (McFarland and Haber 2001).
The corticostriatal and thalamostriatal projections can be differentiated based on the type of vesicular glutamate transporter expressed. The vesicular glutamate transporter isoform 1 (vGlut1) has been commonly used as a marker for corticostriatal projections, whereas the vesicular glutamate transporter isoform 2 (vGlut2) typically characterizes thalamostriatal terminals (Kaneko et al. 2002; Fujiyama et al. 2004, 2006; Raju and Smith 2005; Raju et al. 2006). Despite this apparent segregation of glutamate transporters, vGlut1 transcripts have been found in ventral relay and associative thalamic nuclei (Barroso-Chinea et al. 2007), which contribute substantially to the thalamostriatal system (Beckstead 1984a,b; Smith and Parent 1986; Berendse and Groenewegen 1990; Erro et al. 2001, 2002; McFarland and Haber 2001; Van der Werf et al. 2002). In this regard, recent data from our group have shown coexpression of vGlut1 and vGlut2 transcripts within single thalamostriatal-projection neurons located in thalamic territories other than the midline and intralaminar nuclei (Barroso-Chinea et al. 2008).
In summary, the thalamostriatal system is currently viewed as a dual system, consisting of a projection originating from midline and intralaminar thalamic nuclei (vGlut2-positive projections), together with another projection that arises from ventral and associative relay nuclei that uses both isoforms of the vesicular glutamate transporter.
The nigrostriatal system originates from the SNc (A9 group from Dahlström and Fuxe 1964) and provides the major source of dopaminergic innervation reaching the striatum. The retrorubral field (A8) and the ventral tegmental area (A10) also participate in the mesostriatal and mesolimbic dopaminergic projections. At least two different territories have been described within the SNc: dorsal and ventral tiers. Dopaminergic neurons in the ventral tier are organized in columns, enter the SNr, and give rise to a topographically organized nigrostriatal projection (Lynd-Balta and Haber 1994a,b; Haber et al. 2000). Dopaminergic terminals densely innervate the striatal matrix compartment, whereas striosomes display different densities of nigrostriatal innervation (Prensa and Parent 2001). Nigrostriatal axons synapse with both types of striatal MSNs. It has been generally accepted that dopaminergic input exerts a facilitatory effect on D1R-containing MSNs (direct pathway neurons) and an inhibitory effect on D2R-containing MSNs (indirect pathway neurons). Such a dichotomous effect on striatal projection neuron activity represents one of the foundations of the basal ganglia model, as explained below; however, it should not be taken for granted in physiological terms. An important morphological feature of the nigrostriatal projection is the very large number of axonal arborizations it expresses. In the rat, one dopaminergic axon is estimated to innervate some 75,000 MSNs, and one MSN is under the influence of 95–194 dopaminergic neurons on average (Matsuda et al. 2009). These figures are thought to be much higher in the primate, where one dopaminergic neuron may make up to 1 million synaptic contacts onto striatal neurons. This huge profusion of dopaminergic connectivity implies a wide modulatory effect of dopamine by the nigrostriatal projection.
Finally, other sources of glutamatergic inputs to the striatum arise from the amygdaloid complex reaching striosomes (Ragsdale and Graybiel 1988) and serotoninergic projections originating from the raphe nuclei, with a preponderant role for the dorsal raphe nuclei. The presence of serotoninergic projections has long been characterized (Andén et al. 1966; Bobillier et al. 1976; Szabo 1980), and its functional correlate remains unknown. More recently, this projection has gained increasing attention because serotoninergic axons have been suggested to underlie graft-induced dyskinesias and by extension levodopa-induced dyskinesias (López et al. 2001, López-Real et al. 2003; Carta et al. 2007, 2008a,b, 2010).
Striatal Efferents: Striatum-GPe and Striatum-GPi/SNr
Two main routes for striatofugal projections have been properly characterized: D2R-expressing striatal MSNs innervating the GPe and D1R-expressing striatal MSNs projecting downstream to the GPi and SNr. It has been formerly considered that striatal fibers reaching GPe and GPi were, in fact, collaterals of striatonigral projections (Fox and Rafols 1975), although the current view of the striatofugal system describes three different types of striatofugal MSNs, innervating the GPe, GPi, or SNr (Beckstead and Cruz 1986). However, it is well known that efferent striatal neurons interact with different target structures through axon collaterals (for review, see Parent et al. 2000), and, indeed, modern dual retrograde tract-tracing studies have shown that a substantial proportion of striatonigral-projecting MSNs send collaterals to GPe (Castle et al. 2005). Although much anatomical, neurochemical, and electrophysiological evidence has supported the complementary distribution of D1R and D2R within different types of striatofugal neurons (Beckstead et al. 1988; Gerfen et al. 1990; for review, see Bertrán-González et al. 2010), such a sharp degree of D1R/D2R complementary expression still remains controversial. In this regard, two fundamental questions remain unanswered: whether the segregation of striatofugal MSNs is matched by the differential expression of dopamine receptors and whether the two populations of striatofugal MSNs are fully segregated from each other. The recent implementation of BAC-transgenic mice has shown that only a small number of striatal efferent neurons (<6% in the dorsolateral striatum) coexpress D1R and D2R (Bertrán-González et al. 2008). These newly available data challenge earlier indications that roughly half of striatal MSNs coexpress both dopamine receptors (for review, see Bertrán-González et al. 2010). When considering anatomical data gathered from juxtacellular tract-tracing studies and single axon reconstruction, approximately half of striatal MSNs innervating the GPi and SNr also send axon collaterals to the GPe, whereas most striatal MSNs were found to innervate GPe directly (Kawaguchi et al. 1990; Parent et al. 2000; Wu et al. 2000). It is also worth noting that these types of anatomical data have their own limitations, including a limited number of axons being reconstructed and the lack of information on the relative importance of each axon collateral when compared with the main axonal terminal field.
Output Nuclei: Globus Pallidus, Internal Segment, and Substantia Nigra Pars Reticulata
The GPi, also known as the medial division of the globus pallidus, and the SNr are often grouped together as basal ganglia output nuclei because they share a number of cyto- and chemoarchitectural characteristics and to some extent similar types of afferent and efferent systems. Both nuclei consist of inhibitory GABAergic neurons with a high rate of discharge that fire tonically to inhibit their targets. In comparison with the striatum, the GPi shows a smaller cellular density. This feature, together with the presence of a large number of myelinated striatofugal fibers crossing the GPi nucleus, provides the GPi with a paler appearance when compared with the striatum.
As discussed above, the GPi and SNr receive two different types of inputs. D1R-containing striatal MSNs that give rise to the direct pathway are a major source of inhibition to the GPi and SNr. The STN provides an excitatory glutamatergic projection to the GPi and SNr, part of the indirect pathway. The subthalamopallidal and subthalamonigral projections are highly collateralized (simultaneously reaching GPi and SNr neurons) and can be easily characterized immunohistochemically using antibodies against vGlut2. Both types of afferent systems (GABAergic and glutamatergic) converge onto basal ganglia output neurons, which, in turn, innervate thalamic targets and brainstem (i.e., superior colliculus, PPN). Thalamic-recipient areas of basal ganglia output neurons differ slightly when considering pallidothalamic versus nigrothalamic projections, differences that paved the way for introducing an accurate parcellation of the ventral thalamic nuclei based on hodological criteria (Ilinsky and Kultas-Ilinsky 1987; Percheron et al. 1996). In this respect, pallidothalamic projections innervate the densicellular and parvicellular territories of the ventral anterior motor thalamus (VAdc and VApc, respectively), whereas nigrothalamic projections mainly target the magnocellular division of the ventral anterior motor thalamus (VAmc). Moreover, thalamic intralaminar nuclei (mainly the CM-Pf complex) are also known to be innervated by basal ganglia output. There are two routes by which pallidothalamic projections can gain access to their respective thalamic targets: (1) projections that arise from neurons located in lateral GPi territories form the ansa lenticularis that course around the internal capsule finally entering the so-called field H of Forel (also known as the prerubral field); and (2) axons from more medially located GPi neurons that course through the internal capsule to form the lenticular fasciculus (Forel’s field H2) located between the STN and the zona incerta. The lenticular fasciculus merges with the ansa lenticularis at the level of field H of Forel to enter the thalamic fasciculus (H1 of Forel), the latter finally reaching ventral and intralaminar thalamic nuclei.
INTRINSIC NUCLEI
Globus Pallidus, External Segment
The GPe (also known as the lateral division of the globus pallidus) is part of the pallidal complex together with GPi and the ventral pallidum. The GPe is surrounded laterally by the putamen, from which it is separated by the lateral medullarly lamina. The boundary between the GPe and GPi is made of another thin layer of myelinated fibers named the medial medullarly lamina. The GPe shares with the GPi several cyto- and chemoarchitectural features, because both nuclei are made of sparsely distributed GABAergic neurons with large somas. All GPi and GPe neurons are also characterized by an enriched expression of the calcium-binding protein parvalbumin.
Two principal afferent systems innervate GPe neurons. The GPe receives a GABAergic projection from D2R-containing striatal MSNs, an inhibitory projection that represents the first synaptic relay station of the indirect pathway as is discussed below. Moreover, striatal projections innervating GPe neurons also show an enriched expression of adenosine type 2A receptors (A2A-Rs), and thus antibodies against A2A-Rs have been used to define the boundaries of the GPe nucleus accurately (Rosin et al. 1988; Bogenpohl et al. 2012). GPe neurons are also reciprocally connected with STN neurons from which they receive strong glutamatergic excitatory projections (vGlut2-positive). The existence of such a close interrelationship between the GPe and STN has provided the foundation for no longer considering the GPe as simply a relay station between the striatum and the STN (Shink et al. 1996; Joel and Weiner 1997; Nambu 2004). Furthermore, it has long been known that both segments of the globus pallidus (GPe and GPi) are also reciprocally interconnected. Finally, another minor source of glutamatergic afferents is represented by inputs received from the caudal intralaminar nuclei (Kincaid et al. 1991; Marini et al. 1999; Yashukawa et al. 2004).
Subthalamic Nucleus
Extensive research has been performed in the last few decades focused on the subthalamic nucleus of Luys. This small subcortical nucleus, with densely packed neurons, is located immediately ventral to the zona incerta and rostral to the substantia nigra. It has become very relevant and well known over the last decade because it is the best surgical target for electrode placement during deep brain stimulation (STN-DBS). The canonical basal ganglia model (Albin et al. 1989; DeLong 1990) provided an oversimplified view of afferent and efferent STN connectivity. This simplistic view, which only considered GABAergic input from the GPe and STN efferents targeting basal ganglia output nuclei, has been superseded by much experimental evidence that emerged shortly after the model was first introduced (see section, “Pathophysiology: A Summary,” below). Besides the classical GABAergic projection from GPe neurons (second relay station of the indirect pathway), the STN also receives glutamatergic projections from the cerebral cortex (known as the hyperdirect pathway) (see Nambu et al. 2002; Nambu 2004, 2005) and the ipsilateral thalamic caudal intralaminar nuclei (Sugimoto and Hattori 1983; Sugimoto et al. 1983; Royce and Mourey 1985; Sadikot et al. 1992a,b; Sidibé and Smith 1996; Marini et al. 1999; Gonzalo et al. 2002; Lanciego et al. 2004, 2009; Castle et al. 2005), as well as a minor projection from the contralateral caudal intralaminar nuclei (Gerfen et al. 1982; Castle et al. 2005). Finally, it is also worth noting that the STN also receives a sparse dopaminergic projection from the SNc, as a part of the so-called nigro-extrastriatal projection system (for review, see Rommelfanger and Wichmann 2010).
The cerebral cortex sends direct projections to the STN, which, in turn, innervates the output nuclei, thus providing a way for motor-related cortical areas to access the output nuclei directly, bypassing the input nuclei. This pathway is characterized by shorter conduction times than those funneled through direct and indirect basal ganglia pathways and therefore exerts powerful excitation onto basal ganglia output. Furthermore, cortical inputs arising from the primary motor cortex, the supplementary motor area, premotor cortices, frontal eye field area, and supplementary eye field area are conveyed through the cortico-subthalamic hyperdirect pathway, somatotopically organized at the level of the STN (Nambu et al. 1996, 2002). The direct projection arising from the caudal intralaminar nuclei is another important source of glutamatergic activation, described in rodents, cats, and primates (Sugimoto and Hattori 1983; Sugimoto et al. 1983; Royce and Mourey 1985; Sadikot et al. 1992; Sidibé and Smith 1996; Marini et al. 1999; Gonzalo et al. 2002; Lanciego et al. 2004, 2009; Castle et al. 2005). Thalamo-subthalamic projections are topographically organized (Sadikot et al. 1992; Lanciego et al. 2004), reaching STN efferent neurons innervating the GPe and SNr/GPi (Castle et al. 2005). Furthermore, thalamo-subthalamic projections are bilateral, albeit with an ipsilateral predominance (Castle et al. 2005). Indeed, opposite changes in STN activity have been found following drug-induced stimulation or inhibition of the caudal intralaminar nuclei (Mouroux et al. 1995).
The bulk of STN efferent projections are directed toward the basal ganglia nuclei, a projection typically characterized by highly branched neuronal processes. It has been postulated that most STN efferent neurons send axons that simultaneously innervate the GPi, GPe, and SNr (Van der Kooy and Hattori 1980; Kita et al. 1983; Kita and Kitai 1987; Plenz and Kitai 1999; Castle et al. 2005). The pattern of axonal collateralization has served to identify up to five distinct types of STN efferent neurons (Parent et al. 2000; Sato et al. 2000). In addition to STN projections to the GPi, GPe, and SNr, efferent STN neurons also innervate thalamic targets: those projecting ipsilaterally to ventral thalamic motor nuclei (Nauta and Cole 1978; Rico et al. 2010) and a smaller contralateral projection linking the STN with the parafascicular nucleus (Gerfen et al. 1982; Castle et al. 2005). Furthermore, dual retrograde tract-tracing studies have shown that subthalamic projections reaching the GPi and ventral thalamic nuclei largely arise from different subpopulations of STN neurons (Rico et al. 2010).
Substantia Nigra Pars Compacta
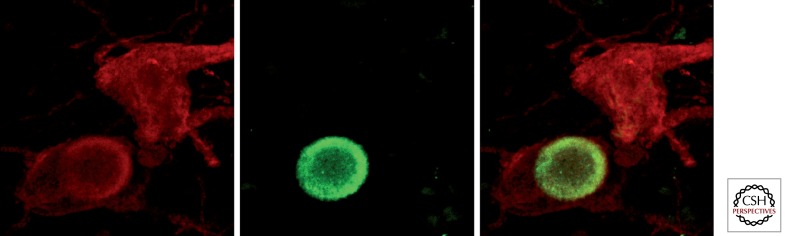
Deep in the ventral midbrain, several groups of tyrosine-positive neurons supplying the basal ganglia nuclei with dopamine can be found. According to Dahlström and Fuxe (1964), these dopaminergic neurons could be classified in up to three main groups: A10 neurons (ventral tegmental area), A9 neurons (SNc), and A8 neurons (retrorubral field). Neurons from the A10 field mainly innervate the accumbens nucleus as well as several limbic-related areas, whereas the nigrostriatal projection mainly arises from the A9 and A8 dopaminergic groups. In the past few years, efforts were made to subdivide the SNc into several clusters or cell groups. Because this issue is beyond the scope of this article, this will not be addressed here. However, more information can be found elsewhere (González-Hernández and Rodríguez 2000; Fu et al. 2012). In humans, dopaminergic neurons located in the SNc contain the dopamine precursor neuromelanin, a pigment that produces the black color inherent to SNc territories as seen in freshly extracted brains in human necropsies. Interestingly, this black pigment of the SNc is still maintained in human albinism, because the gene coding cutaneous melanin is different from the one coding neuromelanin. Dopaminergic neurons in the SNc degenerate progressively in Parkinson’s disease (PD), leading to severe dopamine deficiency and the appearance of the cardinal features of the disease. Neurons still alive often display intracellular aggregates of misfolded proteins, from which α-synuclein is the most prominent, giving rise to circular shape aggregates known as Lewy bodies (Fig. 4), which represent the histopathological hallmark of PD.
Figure 4.
Lewy bodies in dopaminergic neurons from the substantia nigra pars compacta. In humans suffering from Parkinson’s disease, dopaminergic neurons accumulate intracytoplasmatic aggregates of misfolded α-synuclein. These aggregates often adopt a circular shape and form the so-called Lewy bodies, which represent the pathological hallmark of this disease. Dual immunofluorescent detection of tyrosine hydroxylase (red channel) and α-synuclein (green channel), identifying two dopaminergic neurons from a PD patient, one of them showing a typical intracytoplasmic Lewy body.
FUNCTIONAL ORGANIZATION OF THE BASAL GANGLIA: THE CANONICAL MODEL
Classic Basal Ganglia Model
The functional organization of the basal ganglia formulated in the 1980s was based on the concept that neuronal signals from the cortex flow to the striatum, through the GPi and SNr, and project back to the cortex via the thalamus, forming parallel cortico–basal ganglia–thalamo–cortical loops. Although the model was primarily formulated to understand the pathophysiology of movement, the existence of parallel circuits subserving oculo-motor control, executive functions, and emotions was also recognized.
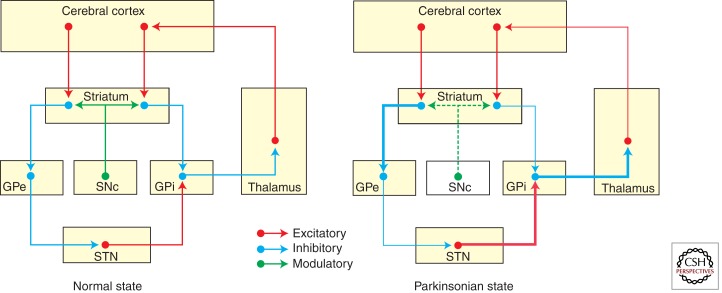
The basis of the model resides in the striatopallidal connections via the direct and the indirect projections (Fig. 5), which have an opposite functional effect on basal ganglia output (Albin et al 1989; DeLong 1990). Activation of GABAergic MSNs that give rise to the direct pathway inhibits GPi/SNr tonic activity, inducing a pause of neuronal firing. At the same time, activation of MSNs of the indirect pathway first inhibits GPe neurons, followed by disinhibition of the STN, which then excites GPi/SNr neurons. Because pauses of neuronal firing in the basal ganglia output are associated with the occurrence of an action (e.g., an eye movement saccade), and discharges are associated with stopping or halting movement, the direct and indirect pathways are viewed as opposite functional projection systems that facilitate and inhibit movements and behaviors, respectively. As previously mentioned, the nigrostriatal projection exerts a differential effect on D1R/D2R MSNs, facilitating and inhibiting the direct and indirect circuits, respectively (Gerfen et al. 1990). Accordingly, dopaminergic depletion reduces the facilitation of MSNs in the direct pathway and increases the activation of indirect circuit neurons, which leads to increased activity of the STN, which, in turn, overactivates inhibitory output neurons in the GPi/SNr, such that the reduction of GABAergic inhibition of direct MSNs combined with an increased glutamatergic driving force from the disinhibited STN increases activity in the GPi/SNr. This effectively reduces the likelihood of phasic inhibitory activity in these output neurons, thus impeding movement initiation and execution. Seminal studies in the monkey by Crossman (1987) led to a similar understanding of the dyskinetic state (i.e., hemichorea-ballism and levodopa-induced dyskinesias), essentially considered to result from decreased GPi output and, therefore, functionally opposed to the parkinsonian state (Obeso et al. 2000, 2008).
Figure 5.
Schematic summary of the original basal ganglia model. The motor circuit is composed of a corticostriatal (putaminal) projection, two major striatofugal projection systems giving rise to the direct and indirect pathways, and the efferent pallido–thalamo–cortical projections to close the motor loop. The thickness of arrows represents the functional state of a given circuit. Thicker arrows illustrate hyperactive pathways, whereas thinner arrows represent hypoactive circuits.
This dual concept of the classic basal ganglia model gained considerable support from many different experimental and clinical sources. Over the years, quite a few difficulties were encountered in trying to fit together new findings with the classical model, creating paradoxes that have been hotly discussed. Among these, the two best recognized and puzzling paradoxes are the following: (1) Lesion of the basal ganglia output should provoke a marked reduction in inhibition of the thalamocortical projection and therefore facilitate or disinhibit movements. However, in monkeys and patients, pallidotomy does not induce involuntary movements, but on the contrary, it abolishes dyskinesias (Carpenter et al. 1950; Lozano et al. 1995). (2) Lesion of the STN, GPi, or motor thalamus singly or in combination does not deteriorate motor function (Marsden and Obeso 1994), but it is actually associated with marked improvement in PD patients (Obeso et al. 2009).
Nevertheless, the model served to stimulate considerable advances in the understanding of basal ganglia organization and, importantly, paved the way for the resurgence of functional neurosurgery for Parkinson’s disease (see below). Interestingly, two recent studies in the rat using optogenetics have provided strong support for the classical model. Kravitz et al. (2010) showed that selective stimulation of MSNs expressing D2R (indirect circuit) provoked movement arrest, whereas activation of MSNs expressing D1R led to movement activation. This essentially confirmed the notion that the indirect and direct circuits are in functional equilibrium, inhibiting and facilitating movement, respectively. Bateup et al. (2010) found that the loss of DARPP-32 in the “direct” striatonigral projection abolished levodopa-induced dyskinesias, whereas in striatopallidal neurons produced a robust increase in locomotor activity and reduced cataleptic response to haloperidol. These two elegant studies selectively activating or inhibiting the direct and indirect striatopallidal circuits corroborated the functional balance of both projection systems.
Current Concepts
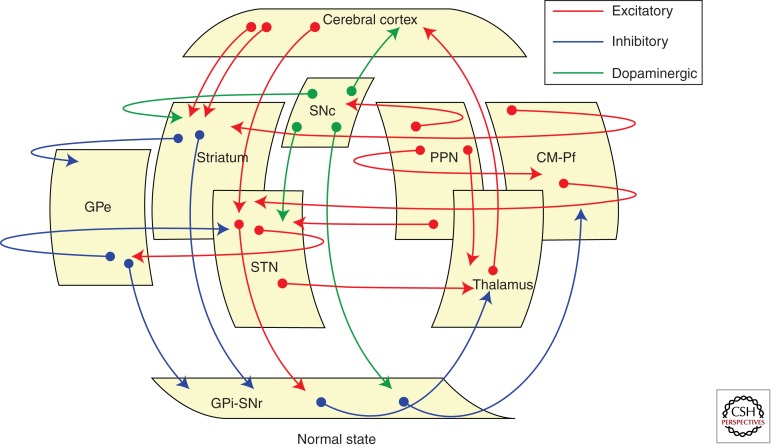
The original functional organization of the basal ganglia was conceived as a loop, in which cortical afferent activity is dispatched to and modulated by the basal ganglia, which subsequently sends back a signal to the cortex to facilitate (or inhibit) motor activity. Accordingly, the basal ganglia were featured as a “go through” station within the motor loop. Current thinking has modified this functional model on several fronts and in fundamental aspects. It is now known that the basal ganglia has several loops, where cortical and subcortical projections interact with internal reentry loops (Fig. 6) forming a complex network, ideally designed for selecting and inhibiting simultaneously occurring events and signals.
Figure 6.
Basal ganglia circuits. Cartoon showing the main circuits linking the basal ganglia nuclei. Besides traditional cortico-basal ganglia-thalamocortical circuits, several transverse loops have been described in the last few years, most of them with a putative modulatory role.
Functional Subdivisions: Basal Ganglia Domains
The basal ganglia are functionally subdivided into motor, associative, and limbic/emotional domains based on their relationship with relevant cortical projection areas and the engagement of these regions. Thus, in addition to the well-known role of the basal ganglia in motor control, there is now a better appreciation for other functions such as attention and time estimation, implicit learning and habit formation, and reward-related behavior and emotions, all of which are associated with the activation of cortical loops that connect with the CN, the anterior putamen, or the ventral putamen (Hikosaka et al. 2002; Buhusi and Meck 2005; Yin and Knowlton 2006). Important evidence for this concept has arisen from studies in non-human and human primates.
In monkeys, bicuculline injections in the motor putamen elicited focal, brief muscle jerks (tic-like) that were accompanied by increased neuronal activity in the GPe, marked reduction in the GPi, and increased firing in the primary motor cortex (M1) time-locked to the jerks (McCairn et al. 2009). This is a firm indication that movement release is punctually associated with a reduction of GPi output activity. Moreover, focal bicuculline injections, to block specific subregions of the striatum, GPe, and STN produced dyskinesias, stereotopies, and hyperactivity among other behavioral abnormalities when injected into the postero–lateral (motor) segment, associative and limbic regions (François et al. 2004; Karachi et al. 2009). Injections in the ventral striatum also elicited sexual behaviors (erection and ejaculation) and vomiting (Worbe et al. 2009).
Human activation studies using functional magnetic resonance imaging (fMRI) and positron emission tomography (15-0 H2O-PET) showed topographical segregation according to the requested task and underlying functions. Thus, activation in the posterior (i.e., sensorimotor) putamen was consistently encountered for movements and presented a somatotopical organization, with the leg lying dorsal, face ventral, and arm in between, as expected (Lehéricy et al. 2005). Preparatory activation as well as finger movement sequencing was located more rostrally in the anterior putamen, whereas activation of the associative territory was observed during tasks such as motor internal representation selection and planning of sequential actions (Monchi et al. 2006), whereas tasks evoking emotional responses typically activate the region of the ventral striatum, where the accumbens nucleus is recognized.
Functional Anatomy
Corticostriatal Connections
GABAergic MSNs receive profuse glutamatergic inputs from most cortical regions and the thalamus, which predominantly form asymmetric contacts with the heads of dendritic spines. Input to MSNs also comes from dopaminergic, cholinergic, and GABAergic neurons, which tend to form symmetric synaptic contacts. In the rat, approximately 5000 glutamatergic afferents project to each striatal MSN, and 100 MSNs project onto each pallidal neuron. This arrangement is probably the anatomical basis for fundamental striatal function, that is, selection and filtering of incoming/outgoing signals. The capacity of the striatum to filter massive incoming signals is facilitated by several mechanisms, among which the nigrostriatal dopaminergic projection plays a paramount role. Thus, dopamine exerts a dual effect on MSNs (Onn et al. 2000), inhibiting striatal D2R-containing neurons and exciting striatal neurons that express D1Rs through L-type calcium channels. Activation of selected nigrostriatal projections leads to dopamine elevation in innervated striatal regions in parallel with a marked dopamine reduction in adjacent zones. This is supported by experimental evidence indicating that dopamine enhances synchronous corticostriatal afferent volleys while simultaneously inhibiting other inputs (Bamford et al. 2004). The creation of a gradient or salient pattern of activity is thought to govern dopaminergic signaling, probably underlying the responses to reward (and addiction), habit formation, and motor learning. Another important component of striatal internal functioning is provided by striatal cholinergic TANs and GABAergic FISs interneurons. Phasic release of dopamine induces firing pauses in TANs that facilitate corticostriatal transmission (Bonsi et al. 2011). GABAergic interneurons, which are also modulated by dopamine, along with axon collaterals from MSNs, mediate a powerful intrastriatal inhibition (Tepper et al. 2004). Together, interneurons modulate striatal excitability providing a mechanism to facilitate a given pool of MSNs excited from the cortex, while competing stimuli are canceled.
Corticosubthalamic Connections
The STN is subdivided into a motor, associative, and limbic region. The dorsolateral STN corresponds with the motor region. STN neurons in the healthy monkey fire at 20–30 Hz and provide tonic excitatory input to both segments of the globus pallidus. Lesion or blockade of the STN is associated with a significant reduction in the mean firing rate of pallidal neurons.
Initial studies recording STN activity in awake monkeys performing a task indicated that neuronal firing occurred after or coinciding with movement initiation (DeLong et al. 1985). This suggested that the STN was not involved with signals associated with movement onset, in keeping with the prevailing idea that the STN was mainly related to movement inhibition. However, recent studies recording single cell activity and local field potentials in patients with Parkinson’s disease have shown clear evidence of activation up to 1 sec before initiation of movement (Rodriguez-Oroz et al. 2001; Alegre et al. 2005). Thus, when a voluntary movement is going to be performed, the same cortical volley that arrives to the putamen is dispatched to the STN. Indeed, studies in monkeys have shown that the cortico-STN-GPe/GPi projection is a fast-conducting system, well placed to modulate basal ganglia output (Nambu 2004).
Subcortical Connections. Thalamo-Basal Ganglia Loops and Brainstem Circuits
The role and importance of projections and feedback loops between the basal ganglia and several subcortical regions were not well considered in the initial basal ganglia model, but have been extensively documented in the last decade (McHaffie et al. 2005). Interestingly, it is now acknowledged that re-entrant loops involving the neocortex are neither novel nor the first evolutionary example of closed-loop architecture involving the basal ganglia. Thus, there are phylogenetically older subcortical loops between the basal ganglia and brainstem motor structures, including the superior and inferior colliculi, periaqueductal gray, pedunculopontine nucleus, cuneiform area and parabrachial complex, and various pontine and medullary reticular nuclei. The interested reader may consult recent excellent reviews on this subject (Redgrave et al. 2010, 2011; Smith et al. 2011).
Pathophysiology: A Summary
Movement disorders comprise a variety of motor problems, not all of which are associated with dysfunction of the basal ganglia. Those that have a clearly established pathological basis and are caused by pathophysiological mechanisms directly involving the basal ganglia are the following: (1) the parkinsonian syndrome composed of rigidity, akinesia/bradykinesia, and resting tremor; (2) dystonia characterized by prolonged muscle spasms and abnormal postures; and (3) chorea-ballism, in which fragments of movements flow irregularly from one body segment to another, to cause a dance-like appearance. When the amplitude of the movement is very large, the term ballism is applied. From a practical point of view, levodopa-induced dyskinesias in PD patients, which are by and large choreic in nature, represent the most frequent cause of choreic dyskinesias in the general population.
The Parkinsonian Syndrome
The best known cause and example of the parkinsonian syndrome is Parkinson’s disease, which is mainly characterized by poverty of spontaneous movements (hypokinesia) and slowness of voluntary movement (bradykinesia), as well as increased muscle tone (rigidity) and tremor at rest. Dopamine striatal depletion secondary to cell loss in the SNc is the cause of the major clinical features of Parkinson’s disease. A large bulk of evidence has been accumulated from both experimental models and patients with Parkinson’s disease to show that dopamine depletion shifts the balance of basal ganglia activity toward the indirect circuit, leading to excessive activity of the STN that overstimulates the GPi/SNr. Increased output from the GPi/SNr overinhibits the thalamocortical projection, reducing cortical neuronal activation associated with movement initiation (DeLong 1990; Obeso et al. 2000).
The way in which the basal ganglia modulate and respond to external input is also distorted in the parkinsonian state. The putamen, STN, and GPi show augmented neuronal responses to peripheral stimulation, which impairs physiological selection or filtering of incoming signals discussed above. Overall, the basal ganglia are shifted toward inhibiting cortically aided movements by an increased activation of the STN–GPi network and reduced excitability in the direct cortico-putaminal-GPi projection (Obeso et al. 2008).
Remarkably, our current understanding of basal ganglia pathophysiology does not provide an adequate explanation for the two other cardinal features of Parkinson’s disease, namely, rigidity and tremor. Regarding the latter, studies in PD patients during surgery and in the monkey MPTP model have started to unravel some interesting features (Heimer et al. 2002). Nevertheless, it is fascinating to realize that when nearly reaching 200 years after James Parkinson described the “Shaking Palsy,” and therefore, the parkinsonian tremor, its origin remains a mystery.
Pathophysiology of Dyskinesias
Chorea-ballism is the abnormal release of fragments of normal movement. In simple terms, the pathophysiology of dyskinetic movements may be seen as the opposite of parkinsonism. Chorea is mainly caused by drugs, such as levodopa in Parkinson’s disease, in neurodegenerative diseases such as Huntington’s disease and secondary to systemic conditions like hyperthyroidism or lupus erythemathosus.
According to the basal ganglia model, dyskinesias (i.e., chorea or ballism) result from reduced activity in the STN-GPi projection leading to decreased firing in GPi output (Crossman 1987; DeLong 1990). Choreatic or ballistic movements can occur secondary to focal lesion in the basal ganglia, such as the STN. In the 1980s, fundamental work performed in control monkeys showed that local administration of bicuculline to block the striato-GPe projection induced chorea-ballism in the contralateral hemibody. This was associated with increased 2-deoxiglucose uptake in the STN as seen with autoradiography (Mitchell et al. 1989), indicating excessive inhibition from the GPe onto the STN, consequently decreasing glutamategic excitatory activity directed to the GPi. Neuronal recording of extracellular activity in the GPi of control monkeys with chorea induced by an injection of bicuculline into the GPe showed a reduction in neuronal firing rate and an abnormal pattern of neuronal synchronization (Filion 2000). The initial evidence, therefore, supported a major role for the indirect pathway for the induction of dyskinesias. Subsequently, recording of neuronal activity in the GPe and GPi in MPTP-treated monkeys and PD patients during surgery, although showing levodopa-induced dyskinesias, further showed the increase and reduction of GPe and GPi neuronal activity, respectively, as a basic pathophysiological feature of choreic dyskinesias (Papa et al. 1999). More recently, evidence has accumulated for increased and extra-synaptic shifting of NMDA glutamatertic receptors (Gardoni et al. 2006) and abnormal D1R receptor activation in the striatum of dyskinetic animals (Berthet et al. 2009), suggesting a paramount role of the direct circuit as a mediator of levodopa-induced dyskinesias (Bateup et al. 2010).
CONCLUSIONS
The basal ganglia network may be viewed as multiple parallel loops and re-entering circuits whereby motor, associative, and limbic territories are engaged mainly in the control of movement, behavior, and emotions. This is likely to be sustained by the same basic architectural and functional organization, differentially applied to (1) selection and facilitation of pre-frontal-striatopallidal activity during the performance and acquisition of new activities and task (goal-directed system); (2) reinforcement learning to create habitual responses automatically performed by the motor circuit (habit system); and (3) stopping an ongoing activity and switching to a new one if necessary, which is mainly mediated by the inferior frontal cortex/STN-cortical circuit. Abnormalities in these domains and functions lead to movement disorders such as parkinsonism and dyskinesias, obsessive compulsive disorders, and alterations of mood (i.e., apathy, euphoria).
Footnotes
Editor: Serge Przedborski
Additional Perspectives on Parkinson’s Disease available at www.perspectivesinmedicine.org
REFERENCES
- Albin RL, Young AB, Penney JB 1989. The functional anatomy of basal ganglia disorders. Trends Neurosci 12: 366–375 [DOI] [PubMed] [Google Scholar]
- Alegre M, Alonso-Frech F, Rodriguez-Oroz MC, Guridi J, Zamarbide I, Valencia M, Marique M, Obeso JA, Artieda J 2005. Movement-related changes in oscillatory activity in the human subthalamic nucleus: Ipsilateral vs. contralateral movements. Eur J Neurosci 22: 2315–2324 [DOI] [PubMed] [Google Scholar]
- Andén NE, Dahlström A, Fuxe K, Olson L, Ungerstedt U 1966. Ascending noradrenaline neurons from the pons and the medulla oblongata. Experientia 22: 44–45 [DOI] [PubMed] [Google Scholar]
- Bacci JJ, Kachidian P, Kerkerian-Le Goff L, Salin P 2004. Intralaminar thalamic nuclei lesions: Widespread impact on dopamine denervation-mediated cellular defects in the rat basal ganglia. J Neuropathol Exp Neurol 63: 20–31 [DOI] [PubMed] [Google Scholar]
- Baillon B, Mallet N, Bézard E, Lanciego JL, Gonon F 2008. Intratelencephalic corticostriatal neurons equally excite striatonigral and striatopallidal neurons and their discharge activity is selectively reduced in experimental parkinsonism. Eur J Neurosci 27: 2313–2321 [DOI] [PubMed] [Google Scholar]
- Bamford NS, Zhang H, Schmitz Y, Wu NP, Cepeda C, Levine MS, Schmauss C, Zakharenko SS, Zablow L, Sulzer D 2004. Heterosynaptic dopamine neurotransmission selects sets of corticostriatal terminals. Neuron 42: 653–663 [DOI] [PubMed] [Google Scholar]
- Barroso-Chinea P, Castle M, Aymerich MS, Pérez-Manso M, Erro E, Tuñón T, Lanciego JL 2007. Expression of the mRNAs encoding for the vesicular glutamate transporters 1 and 2 in the rat thalamus. J Comp Neurol 501: 703–715 [DOI] [PubMed] [Google Scholar]
- Barroso-Chinea PJ, Castle M, Aymerich MS, Lanciego JL 2008. Expression of vesicular glutamate transporters 1 and 2 in the cells of origin of the rat thalamostriatal pathway. J Chem Neuroanat 35: 101–107 [DOI] [PubMed] [Google Scholar]
- Bateup HS, Santini E, Shen W, Birnbaum S, Valjent E, Surmeier DJ, Fisone G, Nestler EJ, Greengard P 2010. Distinct subclasses of medium spiny neurons differentially regulate striatal motor behaviors. Proc Natl Acad Sci 107: 14845–14850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckstead RM 1984a. The thalamostriatal projection in the cat. J Comp Neurol 223: 313–346 [DOI] [PubMed] [Google Scholar]
- Beckstead RM 1984b. A direct projection to the striatum from the medial subdivision of the posterior group of the thalamus in the cat. Brain Res 300: 351–356 [DOI] [PubMed] [Google Scholar]
- Beckstead RM 1988. Association of dopamine D1 and D2 receptors with specific cellular elements in the basal ganglia of the cat: The uneven topography of dopamine receptors in the striatum is determined by intrinsic striatal cells, not nigrostriatal axons. Neuroscience 27: 851–863 [DOI] [PubMed] [Google Scholar]
- Beckstead RM, Cruz CJ 1986. Striatal axons to the globus pallidus, entopeduncular nucleus and substantia nigra come mainly from separate cell populations in the cat. Neuroscience 19: 147–158 [DOI] [PubMed] [Google Scholar]
- Bennet BD, Bolam JP 1993. Characterization of calretinin-immunoreactive structures in the striatum of the rat. Brain Res 609: 137–148 [DOI] [PubMed] [Google Scholar]
- Berendse HW, Groenewegen HJ 1990. Organization of the thalamostriatal projections in the rat, with special emphasis on the ventral striatum. J Comp Neurol 299: 187–228 [DOI] [PubMed] [Google Scholar]
- Berthet A, Porras G, Doudnikoff E, Stark H, Cador M, Bezard E, Bloch B 2009. Pharmacological analysis demonstrates dramatic alteration of D1 dopamine receptor neuronal distribution in the rat analog of l-DOPA-induced dyskinesia. J Neurosci 29: 4829–4835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrán-González J, Bosch C, Maroteaux M, Matamales M, Hervé D, Valjent E, Girault JA 2008. Opposing patterns of signaling activation in dopamine D1 and D2 receptor-expressing striatal neurons in response to cocaine and haloperidol. J Neurosci 28: 5671–5685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrán-González J, Hervé D, Girault JA, Valjent E 2010. What is the degree of segregation between striatonigral and striatopallidal projections? Front Neuroanat 4: 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobillier R, Seguin S, Petitjean F, Salvert D, Turet M, Jouvet M 1976. The raphe nuclei of the cat brain stem: A topographical atlas of their efferent projections as revealed by autoradiography. Brain Res 113: 449–486 [DOI] [PubMed] [Google Scholar]
- Bogenpohl JW, Ritter SL, Hall RA, Smith Y 2012. Adenosine A2A receptor in the monkey basal ganglia: Ultrastructural localization and colocalization with the metabotropic glutamate receptor 5 in the striatum. J Comp Neurol 520: 570–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolam JP, Izzo PN, Graybiel AM 1988. Cellular substrates of the histochemically defined striosome/matrix system of the caudate nucleus: A combined Golgi and immunocytochemical study in cat and ferret. Neuroscience 24: 853–875 [DOI] [PubMed] [Google Scholar]
- Bonsi P, Cuomo D, Martella G, Madeo G, Schirinzi T, Puglisi F, Ponterio G, Pisani A 2011. Centrality of striatal cholinergic transmission in basal ganglia function. Front Neuroanat 5: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhusi CV, Meck WH 2005. What makes us tick? Functional and neural mechanisms of interval timing. Nat Rev Neurosci 6: 755–765 [DOI] [PubMed] [Google Scholar]
- Carpenter MB, Whittier JR, Mettler FA 1950. Analysis of choreoid hyperkinesia in the Rhesus monkey; surgical and pharmacological analysis of hyperkinesia resulting from lesions in the subthalamic nucleus of Luys. J Comp Neurol 92: 293–331 [DOI] [PubMed] [Google Scholar]
- Carta M, Carlsson T, Kirik D, Björklund A 2007. Dopamine released from 5-HT terminals is the cuase of l-DOPA-induced dyskinesia in parkinsonian rats. Brain 130: 1819–1833 [DOI] [PubMed] [Google Scholar]
- Carta M, Carlsson T, Muñoz A, Kirik D, Björklund A 2008a. Involvement of the serotonin system in l-dopa-induced dyskinesias. Parkinsonism Relat Disord 14: S154–S158 [DOI] [PubMed] [Google Scholar]
- Carta M, Carlsson T, Muñoz A, Kirik D, Björklund A 2008b. Serotonin–dopamine interaction in the induction and maintenance of l-DOPA-induced dyskinesias. Progr Brain Res 172: 465–478 [DOI] [PubMed] [Google Scholar]
- Carta M, Carlsson T, Muñoz A, Kirik D, Björklund A 2010. Role of serotonin neurons in the induction of levodopa- and graft-induced dyskinesias in Parkinson’s disease. Mov Disord 25: S174–S179 [DOI] [PubMed] [Google Scholar]
- Castle M, Aymerich MS, Sánchez-Escobar C, Gonzalo N, Obeso JA, Lanciego JL 2005. Thalamic innervation of the direct and indirect basal ganglia pathways in the rat: Ipsi- and contralateral projections. J Comp Neurol 483: 143–153 [DOI] [PubMed] [Google Scholar]
- Crossman AR 1987. Primate models of dyskinesia: The experimental approach to the study of basal ganglia-related involuntary movement disorders. Neuroscience 21: 1–40 [DOI] [PubMed] [Google Scholar]
- Dahlström A, Fuxe K 1964. Localization of monoamines in the lower brain stem. Experientia 20: 398–399 [DOI] [PubMed] [Google Scholar]
- DeLong MR 1990. Primate models of movement disorders of basal ganglia origin. Trends Neurosci 13: 281–285 [DOI] [PubMed] [Google Scholar]
- DeLong MR, Crutcher MD, Georgopoulos AP 1985. Primate globus pallidus and subthalamic nucleus functional organization. J Neurophysiol 53: 530–543 [DOI] [PubMed] [Google Scholar]
- Desban M, Gauchy C, Glowinski J, Kemel ML 1995. Heterogeneous topographical distribution of the striatonigral and striatopallidal neurons in the matrix compartment of the cat caudate nucleus. J Comp Neurol 352: 117–133 [DOI] [PubMed] [Google Scholar]
- Donoghue JP, Herkenham M 1986. Neostriatal projections from individual cortical fields conform to histochemically distinct striatal compartments in the rat. Brain Res 365: 397–403 [DOI] [PubMed] [Google Scholar]
- Dubé L, Smith AD, Bolam JL 1988. Identification of synaptic terminals of thalamic or cortical origin in contact with distinct medium-sized spiny neurons in the rat neostriatum. J Comp Neurol 267: 455–471 [DOI] [PubMed] [Google Scholar]
- Erro E, Lanciego JL, Arribas J, Giménez-Amaya JM 2001. Striatal input from the ventrobasal complex of the rat thalamus. Histochem Cell Biol 115: 447–454 [DOI] [PubMed] [Google Scholar]
- Erro ME, Lanciego JL, Giménez-Amaya JM 2002. Re-examination of the thalamostriatal projections in the rat with retrograde tracers. Neurosci Res 42: 45–55 [DOI] [PubMed] [Google Scholar]
- Filion M 2000. Physiologic basis of dyskinesia. Ann Neurol 47: S35–S40 [PubMed] [Google Scholar]
- Fox CA, Rafols JA 1975. The radial fibers in the globus pallidus. J Comp Neurol 159: 177–200 [DOI] [PubMed] [Google Scholar]
- François C, Grabli D, McCairn K, Jan C, Karachi C, Hisrch EC, Féger J, Tremblay L 2004. Behavioural disorders induced by external globus pallidus dysfunction in primates II. Anatomical study. Brain 127: 2055–2070 [DOI] [PubMed] [Google Scholar]
- Fu Y, Yuan Y, Halliday G, Rusznák Z, Watson C, Paxinos G 2012. A cytoarchitectonic and chemoarchitectonic analysis of the dopamine cell groups in the substantia nigra, ventral tegmental area, and retrorubral field in the mouse. Brain Struct Funct 217: 591–612 [DOI] [PubMed] [Google Scholar]
- Fujiyama F, Kuramoto E, Okamoto K, Hioki H, Furuta T, Zhou L, Nomura S, Kaneko T 2004. Presynaptic localization of an AMPA-type glutamate receptor in corticostriatal and thalamostriatal axon terminals. Eur J Neurosci 20: 3322–3330 [DOI] [PubMed] [Google Scholar]
- Fujiyama F, Unzai T, Nakamura K, Nomura S, Kaneko T 2006. Difference in organization of corticostriatal and thalamostriatal synapses between patch and matrix compartments of rat neostriatum. Eur J Neurosci 24: 2813–2824 [DOI] [PubMed] [Google Scholar]
- Fujiyama F, Sohn J, Nakano T, Furuta T, Nakamura KC, Matsuda W, Kaneko T 2011. Exclusive and common targets of neostriatofugal projections of rat striosome neurons: A single neuron-tracing study using a viral vector. Eur J Neurosci 33: 668–677 [DOI] [PubMed] [Google Scholar]
- Gardoni F, Picconi B, Ghiglieri V, Pilli F, Bagetta V, Bernardi G, Cattabeni F, Di Luca M, Calabresi P 2006. A critical interaction between NR2B and MAGUK in l-DOPA induced dyskinesia. J Neurosci 26: 2914–2922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR 1984. The neostriatal mosaic: Compartmentalization of corticostriatal input and striatonigral output systems. Nature 311: 461–464 [DOI] [PubMed] [Google Scholar]
- Gerfen CR 1992. The neostriatal mosaic: Multiple levels of compartmental organization. Trends Neurosci 15: 133–139 [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Stained WA, Arbuthnott GW, Fibiger HC 1982. Crossed connections of the substantia nigra in the rat. J Comp Neurol 207: 283–303 [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Baimbridge KG, Miller JJ 1985. The neostriatal mosaic: Compartmental distribution of calcium-binding protein and parvalbumin in the basal ganglia of the rat and monkey. Proc Natl Acad Sci 82: 8780–8784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR, Engber T, Mahan L, Susel Z, Chase T, Monsma F, Sibley D 1990. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science 250: 1429–1432 [DOI] [PubMed] [Google Scholar]
- Giménez-Amaya JM, Graybiel AM 1990. Compartmental origins of the striatopallidal projections in the primate. Neuroscience 34: 111–126 [DOI] [PubMed] [Google Scholar]
- González-Hernández T, Rodríguez M 2000. Compartmental organization and chemical profile of dopaminergic and GABAergic neurons in the substantia nigra of the rat. J Comp Neurol 421: 107–135 [DOI] [PubMed] [Google Scholar]
- Gonzalo N, Lanciego JL, Castle M, Vázquez A, Erro E, Obeso JA 2002. The parafascicular thalamic complex and basal ganglia circuitry: Further complexity to the basal ganglia model. Thal Rel Sys 1: 341–348 [Google Scholar]
- Graybiel AM 1984. Correspondance between the dopamine islands and striosomes of the mammalian striatum. Neuroscience 13: 1157–1187 [DOI] [PubMed] [Google Scholar]
- Graybiel AM 1990. Neurotransmitters and neuromodulators in the basal ganglia. Trends Neurosci 13: 244–254 [DOI] [PubMed] [Google Scholar]
- Graybiel AM, Ragsdale CW 1978. Histochemically distinct compartments in the striatum of human, monkey and cat demonstrated by acetylcholinesterase staining. Proc Natl Acad Sci 75: 5723–5726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel AM, Ragsdale CW, Yoneoka ES, Elde RH 1981. An immunohistochemical study of enkephalins and other neuropeptides in the striatum of the cat with evidence that the opiate peptides are arranged to form mosaic patterns in register with the striosomal compartments visible by acetylcholinesterase staining. Neuroscience 6: 377–397 [DOI] [PubMed] [Google Scholar]
- Goenewegen HJ, Berendse HW 1994. The specificity of the “nonspecific” midline and intralaminar thalamic nuclei. Trends Neurosci 17: 52–57 [DOI] [PubMed] [Google Scholar]
- Haber SN, Fudge JL, McFarland NR 2000. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci 20: 2369–2382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimer G, Bar-Gad I, Goldberg JA, Bergman H 2002. Dopamine replacement therapy reverses abnormal synchronization of pallidal neurons in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine primate model of Parkinsonism. J Neurosci 22: 7850–7855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka O, Nakamura K, Sakai K, Nakahara H 2002. Central mechanisms of motor learning. Curr Opin Neurobiol 12: 217–222 [DOI] [PubMed] [Google Scholar]
- Ilinsky IA, Kultas-Ilinsky K 1987. Sagittal cytoarchitectonic maps of the Macaca mulatta thalamus with a revised nomenclature of the motor-related nuclei validated by observations of their connectivity. J Comp Neurol 262: 331–364 [DOI] [PubMed] [Google Scholar]
- Joel D, Weiner I 1997. The organization of the basal ganglia-thalamocortical circuits: Open interconnected rather than closed segregated. Brain Res Rev 23: 62–78 [DOI] [PubMed] [Google Scholar]
- Kaneko T, Fujiyama F, Hioki H 2002. Immunohistochemical localization of candidates for vesicular glutamate transporters in the rat brain. J Comp Neurol 444: 39–62 [DOI] [PubMed] [Google Scholar]
- Karachi C, Grabli D, Baup N 2009. Dysfunction of the subthalamic nucleus induces behavioral and movement disorders in monkeys. Mov Disord 24: 1183–1192 [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Wilson CJ, Emson PC 1989. Intracellular recording of identified neostriatal patch and matrix spiny cells in a slice preparation preserving cortical inputs. J Neurophysiol 62: 1052–1068 [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Wilson CJ, Emson PC 1990. Projection subtypes of rat neostriatal matrix cells revealed by intracellular injection of biocytin. J Neurosci 10: 3421–3438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Wilson CJ, Augood SJ, Emson PC 1995. Striatal interneurons: Chemical, physiological and morphological characterization. Trends Neurosci 18: 527–535 [DOI] [PubMed] [Google Scholar]
- Kincaid AE, Wilson CJ 1996. Corticostriatal innervation of the patch and matrix in the neostriatum. J Comp Neurol 374: 578–592 [DOI] [PubMed] [Google Scholar]
- Kincaid Y, Wilson CJ, Emson PC 1991. Projection subtypes of rat neostriatal matrix cells revealed by intracellular injection of biocytin. J Neurosci 10: 3421–3438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita H, Kitai ST 1987. Efferent projections of the subthalamic nucleus in the rat: Light and electron microscopic analysis with the PHA-L method. J Comp Neurol 260: 435–452 [DOI] [PubMed] [Google Scholar]
- Kita H, Chang HT, Kitai ST 1983. The morphology of intracellularly labelled rat subthalamic nucleus: A light microscopic analysis. J Comp Neurol 215: 245–257 [DOI] [PubMed] [Google Scholar]
- Kravitz AV, Freeze BS, Parker PR, Kay K, Thwin MT, Deisseroth K, Kreitzer AC 2010. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature 466: 622–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanciego JL, Gonzalo N, Castle M, Sánchez-Escobar C, Aymerich MS, Obeso JA 2004. Thalamic innervation of striatal and subthalamic neurons projecting to the rat entopeduncular nucleus. Eur J Neurosci 19: 1267–1277 [DOI] [PubMed] [Google Scholar]
- Lanciego JL, Rodríguez-Oroz MC, Blesa FJ, Alvarez-Erviti L, Guridi J, Barroso-Chinea P, Smith Y, Obeso JA 2008. Lesion of the centromedian thalamic nucleus in MPTP-treated primates. Mov Disord 23: 708–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanciego JL, López IP, Rico AJ, Aymerich MS, Pérez-Manso M, Conte L, Combarro C, Roda E, Molina C, Gonzalo N, et al. 2009. The search for a role of the caudal intralaminar nuclei in the pathophysiology of Parkinson’s disease. Brain Res Bull 78: 55–59 [DOI] [PubMed] [Google Scholar]
- Lapper SR, Bolam JP 1992. Input from the frontal cortex and the parafascicular nucleus to cholinergic interneurons in the dorsal striatum of the rat. Neuroscience 51: 533–545 [DOI] [PubMed] [Google Scholar]
- Lapper SR, Smith Y, Sadikot AF, Parent A, Bolam JP 1992. Cortical input to parvalbumin-immunoreactive neurones in the putamen of the squirrel monkey. Brain Res 580: 215–224 [DOI] [PubMed] [Google Scholar]
- Lehéricy S, Benali H, Van de Moortele PF, Pélégrini-Issac M, Waechter T, Ugurbil K, Doyon J 2005. Distinct basal ganglia territories are engaged in early and advanced motor sequence learning. Proc Nat Acad Sci 102: 12566–12571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei W, Jiao Y, Del Mar N, Reiner A 2004. Evidence for differential cortical input to direct pathway versus indirect pathway striatal projection neurons in rats. J Neurosci 24: 8289–8299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López A, Muñoz A, Guerra MJ, Labandeira-Garcia JL 2001. Mechanisms of the effects of exogenous levodopa on the dopamine-denervated striatum. Neuroscience 103: 639–651 [DOI] [PubMed] [Google Scholar]
- López-Real A, Rodriguez-Pallares J, Guerra MJ, Labandeira-Garcia JL 2003. Localization and functional significance of striatal neurons immunoreactive to aromatic l-amino acid decarboxylase or tyrosine hydroxylase in rat parkinsonian models. Brain Res 969: 135–146 [DOI] [PubMed] [Google Scholar]
- Lozano AM, Lang AE, Galvez-Jimenez N, Miyasaki J, Duff J, Hutchinson WD, Dostrovsky JO 1995. The effect of GPi pallidotomy on motor function in Parkinson’s disease. Lancet 346: 1383–1387 [DOI] [PubMed] [Google Scholar]
- Lynd-Balta E, Haber SN 1994a. The organization of midbrain projections to the striatum in the primate: Sensorimotor-related striatum versus ventral striatum. Neuroscience 59: 625–640 [DOI] [PubMed] [Google Scholar]
- Lynd-Balta E, Haber SN 1994b. The organization of midbrain projections to the ventral striatum in the primate. Neuroscience 59: 609–623 [DOI] [PubMed] [Google Scholar]
- Marini G, Pianca L, Tredici G 1999. Descending projections arising from the parafascicular nucleus in rats. Trajectory of fibers, projection pattern and mapping of terminations. Somatosens Motor Res 16: 207–222 [DOI] [PubMed] [Google Scholar]
- Marsden CD, Obeso JA 1994. The functions of the basal ganglia and the paradox of stereotaxic surgery in Parkinson’s disease. Brain 117: 877–897 [DOI] [PubMed] [Google Scholar]
- Matsuda W, Furuta T, Nakamura KC, Hioki H, Fujiyama F, Arai R, Kaneko T 2009. Single nigrostriatal dopaminergic neurons form widely spread and highly dense axonal arborizations in the neostriatum. J Neurosci 29: 444–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCairn KW, Bronfeld M, Belelovsky K, Bar-Gad I 2009. The neurophysiological correlates of motor tics following focal striatal disinhibition. Brain 132: 2125–2138 [DOI] [PubMed] [Google Scholar]
- McFarland NR, Haber SN 2001. Organization of thalamostriatal terminals from the ventral motor nuclei in the macaque. J Comp Neurol 429: 321–336 [DOI] [PubMed] [Google Scholar]
- McHaffie JG, Stanford TR, Stein BE, Coizet V, Redgrave P 2005. Subcortical loops through the basal ganglia. Trends Neurosci 28: 401–417 [DOI] [PubMed] [Google Scholar]
- Meredith GE, Wouterlood FG 1990. Hippocampal and midline thalamic fibers and terminals in relation to the choline acetyltransferase-immunoreactive neurons in nucleus accumbens of the rat: A light and electron microscopic study. J Comp Neurol 296: 204–221 [DOI] [PubMed] [Google Scholar]
- Mitchell IJ, Clarke CE, Boyce S, Robertson RG, Peggs D, Sambrook MA, Crossman AR 1989. Neural mechanisms underlying parkinsonian symptoms based upon regional uptake of 2-deoxyglucose in monkeys exposed to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Neuroscience 32: 213–226 [DOI] [PubMed] [Google Scholar]
- Monchi O, Petrides M, Strafella AP, Worsley KJ, Doyon J 2006. Functional role of the basal ganglia in the planning and execution of actions. Ann Neurol 59: 257–264 [DOI] [PubMed] [Google Scholar]
- Mouroux M, Hassani OK, Féger J 1995. Electrophysiological study of the excitatory parafascicular projection to the subthalamic nucleus and evidence for ipsi- and contralateral controls. Neuroscience 67: 399–407 [DOI] [PubMed] [Google Scholar]
- Nambu A 2004. A new dynamic model of the cortico-basal ganglia loop. Prog Brain Res 143: 461–466 [DOI] [PubMed] [Google Scholar]
- Nambu A 2005. A new approach to understand the pathophysiology of Parkinson’s disease. J Neurol 252: 1–4 [DOI] [PubMed] [Google Scholar]
- Nambu A, Takada M, Inase M, Tokuno H 1996. Dual somatotopical representations in the primate subthalamic nucleus: Evidence for ordered but reverse body-map transformations from the primary motor cortex and the supplementary motor area. J Neurosci 16: 2671–2683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambu A, Tokuno H, Takada M 2002. Functional significance of the cortico-subthalamo-palidal “hyperdirect” pathway. Neurosci Res 43: 111–117 [DOI] [PubMed] [Google Scholar]
- Nauta HJW, Cole M 1978. Efferent projections of the subthalamic nucleus: An autoradiographic study in monkey and cat. J Comp Neurol 180: 1–16 [DOI] [PubMed] [Google Scholar]
- Obeso JA, Rodriguez-Oroz MC, Rodriguez M, Lanciego JL, Artieda J, Gonzalo N, Olanow CW 2000. Pathophysiology of the basal ganglia in Parkinson’s disease. Trends Neurosci 23: S8–S19 [DOI] [PubMed] [Google Scholar]
- Obeso JA, Rodriguez-Oroz MC, Benitez-Termino B, Blesa FJ, Guridi J, Marin C, Rodriguez M 2008. Functional organization of the basal ganglia. Therapeutic implications for Parkinson’s disease. Mov Disord 23: S548–S559 [DOI] [PubMed] [Google Scholar]
- Obeso JA, Jahanshahi M, Alvarez L, Macias R, Pedroso I, Wilkinson L, Pavon N, Day B, Pinto S, Rodriguez-Oroz MC, et al. 2009. What can man do without basal ganglia motor output? The effect of combined unilateral subthalamotomy and pallidotomy in a patient with Parkinson’s disease. Exp Neurol 220: 283–292 [DOI] [PubMed] [Google Scholar]
- Onn SP, West AR, Grace AA 2000. Dopamine-mediated regulation of striatal neuronal and network interactions. Trends Neurosci 23: 48–56 [DOI] [PubMed] [Google Scholar]
- Papa SM, Desimone R, Fiorani M, Oldfield EH 1999. Internal globus pallidus discharge is nearly suppressed during levodopa-induced dyskinesias. Ann Neurol 46: 732–738 [DOI] [PubMed] [Google Scholar]
- Parent A, Sato F, Wu Y, Gauthier J, Lévesque M, Parent M 2000. Organization of the basal ganglia: The importance of axonal collateralization. Trends Neurosci 23: S20–S27 [DOI] [PubMed] [Google Scholar]
- Penny GR, Wilson CJ, Kitai ST 1988. Relationship of the axonal and dendritic geometry of spiny projection neurons to the compartmental organization of the neostriatum. J Comp Neurol 269: 275–289 [DOI] [PubMed] [Google Scholar]
- Percheron G, François C, Talbi B, Yelnik J, Fénelon G 1996. The primate motor thalamus. Brain Res Rev 22: 93–181 [PubMed] [Google Scholar]
- Perth CB, Kuhar MI, Snyder SH 1976. Opiate receptor: Autoradiographic localization in rat brain. Proc Natl Acad Sci 73: 3729–3733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plenz D, Kitai ST 1999. A basal ganglia pacemaker formed by the subthalamic nucleus and external globus pallidus. Nature 400: 677–682 [DOI] [PubMed] [Google Scholar]
- Powell TPS, Cowan WM 1956. A study of thalamo-striate relations in the monkey. Brain 79: 364–390 [DOI] [PubMed] [Google Scholar]
- Prensa L, Parent A 2001. The nigrostriatal pathway in the rat: A single-axon study of the relationship between dorsal and ventral tier nigral neurons and the striosome/matrix striatal compartments. J Neurosci 21: 7247–7260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prensa L, Giménez-Amaya JM, Parent A 1999. Chemical heterogeneity of the striosomal compartment in the human striatum. J Comp Neurol 413: 603–618 [PubMed] [Google Scholar]
- Purdon Martin J 1927. Hemichorea resulting from a location lesion of the brain (the syndrome of the body of Luys). Brain 50: 637–651 [Google Scholar]
- Ragsdale CW, Graybiel AM 1988. Fibers from the basolateral nucleus of the amygdala selectively innervate striosomes in the caudate nucleus of the cat. J Comp Neurol 269: 506–522 [DOI] [PubMed] [Google Scholar]
- Raju DV, Smith Y 2005. Differential localization of vesicular glutamate transporters 1 and 2 in the rat striatum. In The basal ganglia VIII (ed. JP Bolam, et al. ), pp. 601–610 Springer, New York [Google Scholar]
- Raju DV, Shah DJ, Wright TM, Hall RA, Smith Y 2006. Differential synaptology of vGluT2-containing thalamostriatal afferents between the patch and matrix compartments in rats. J Comp Neurol 499: 231–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redgrave P, Coizet V, Comoli E, McHaffie JG, Leriche M, Vautrelle N, Haves LM, Overton P 2010. Interactions between the midbrain superior colliculus and the basal ganglia. Front Neuroanat 4: 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redgrave P, Vautrelle N, Reynolds JN 2011. Functional properties of the basal ganglia’s re-entrant loop architecture: Selection and reinforcement. Neuroscience 198: 138–151 [DOI] [PubMed] [Google Scholar]
- Reiner A, Jiao Y, Del Mar N, Laverghetta AV, Lei WL 2003. Differential morphology of piramidal-tract type and intratelencephalically-projecting type corticostriatal neurons and their intrastriatal terminals in rats. J Comp Neurol 457: 420–440 [DOI] [PubMed] [Google Scholar]
- Reiner A, Hart NM, Lei W, Deng Y 2010. Corticostriatal projection neurons: Dichotomous types and dichotomous functions. Front Neuroanat 4: 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rico AJ, Barroso-Chinea P, Conte-Perales L, Roda E, Gómez-Bautista V, Gendive M, Obeso JA, Lanciego JL 2010. A direct projection from the subthalamic nucleus to the ventral thalamus in monkeys. Neurobiol Dis 39: 381–392 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Oroz MC, Rodriguez M, Guridi J, Mewes K, Chockkman V, Vitek J, DeLong MR, Obeso JA 2001. The subthalamic nucleus in Parkinson’s disease: Somatotopic organization and physiological characteristics. Brain 124: 1777–1790 [DOI] [PubMed] [Google Scholar]
- Rommelfanger KS, Wichmann T 2010. Extrastriatal dopaminergic circuits of the basal ganglia. Front Neuroanat 4: 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosin DL, Robeya A, Woodard RL, Guyenet PG, Linden J 1998. Immunohistochemical localization of adenosine A2A receptors in the rat central nervous system. J Comp Neurol 401: 163–186 [PubMed] [Google Scholar]
- Royce GJ, Mourey RJ 1985. Efferent connections of the centromedian and parafascicular thalamic nuclei: An autoradiography investigation in the cat. J Comp Neurol 235: 277–300 [DOI] [PubMed] [Google Scholar]
- Rudkin TM, Sadikot AF 1999. Thalamic input to parvalbumin-immunoreactive GABAergic interneurons: Organization in normal striatum and effect on neonatal decortication. Neuroscience 88: 1165–1175 [DOI] [PubMed] [Google Scholar]
- Sadikot AF, Parent A, François C 1990. The centre median and parafascicular thalamic nuclei project respectively to the sensorimotor and associative-limbic striatal territories in the squirrel monkey. Brain Res 510: 161–165 [DOI] [PubMed] [Google Scholar]
- Sadikot AF, Parent A, François C 1992a. Efferent connections of the centromedian and parafascicular thalamic nuclei in the squirrel monkey: A PHA-L study of subcortical connections. J Comp Neurol 315: 137–159 [DOI] [PubMed] [Google Scholar]
- Sadikot AF, Parent A, Smith Y, Bolam JP 1992b. Efferent connections of the centromedian and parafascicular thalamic nuclei in the squirrel monkey: A light and electron microscopic study of the thalamostriatal projection in relation to striatal heterogeneity. J Comp Neurol 320: 228–242 [DOI] [PubMed] [Google Scholar]
- Sato F, Parent M, Lévesque M, Parent A 2000. Axonal branching pattern of neurons of the subthalamic nucleus in primates. J Comp Neurol 424: 142–152 [DOI] [PubMed] [Google Scholar]
- Schröeder KF, Hopf A, Lange H, Thörner G 1975. Strukturanalysen des Striatum, Pallidum und Nucleus subthalamicus beim Menschen. I. Striatum. J Hirnforschung 16: 333–350 [PubMed] [Google Scholar]
- Selemon LD, Goldman-Rakic PS 1985. Longitudinal topography and interdigitation of corticostriatal projections in the rhesus monkey. J Neurosci 5: 776–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shink E, Bevan MD, Bolam JP, Smith Y 1996. The subthalamic nucleus and the external globus pallidum: Two tightly interconnected structures that control the ourput of the basal ganglia in the monkey. Neuroscience 73: 335–357 [DOI] [PubMed] [Google Scholar]
- Sidibé M, Smith Y 1996. Differential synaptic innervation of striatofugal neurons projecting to the internal and external segments of the globus pallidus by thalamic afferents in the squirrel monkey. J Comp Neurol 365: 445–465 [DOI] [PubMed] [Google Scholar]
- Sidibé M, Smith Y 1999. Thalamic input to striatal interneurons in monkeys: Synaptic organization and co-localization of calcium-binding proteins. Neuroscience 89: 1189–1208 [DOI] [PubMed] [Google Scholar]
- Smith Y, Bolam JP 1990. The neural network of the basal ganglia as revealed by the study of synaptic connections of identified neurons. Trends Neurosci 13: 259–265 [DOI] [PubMed] [Google Scholar]
- Smith Y, Parent A 1986. Differential connections of caudate nucleus and putamen in the squirrel monkey (Saimiri scireus). Neuroscience 18: 347–371 [DOI] [PubMed] [Google Scholar]
- Smith Y, Raju DV, Pare JF, Sidibé M 2004. The thalamostriatal system: A highly specific network of the basal ganglia circuitry. Trends Neurosci 27: 520–527 [DOI] [PubMed] [Google Scholar]
- Smith Y, Surmeier DJ, Redgrave P, Kimura M 2011. Thalamic contributions to basal ganglia-related behavioral switching and reinforcement. J Neurosci 31: 16102–16106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto T, Hattori T 1983. Confirmation of thalamosubthalamic projection by electron microscope autoradiography. Brain Res 267: 335–339 [DOI] [PubMed] [Google Scholar]
- Sugimoto T, Hattori T, Mizuno N, Itoh K, Sato M 1983. Direct projections from the centromedian–parafascicular complex to the subthalamic nucleus in the cat and rat. J Comp Neurol 214: 209–216 [DOI] [PubMed] [Google Scholar]
- Szabo J 1980. Organization of the ascending striatal afferents in monkeys. J Comp Neurol 189: 307–321 [DOI] [PubMed] [Google Scholar]
- Tepper MJ, Koós T, Wilson CJ 2004. GABAergic microcircuits in the neostriatum. Trends Neurosci 27: 662–669 [DOI] [PubMed] [Google Scholar]
- Thomas TM, Smith Y, Levey AI, Hersch SM 2000. Cortical inputs to m2-immunoreactive striatal interneurons in rat and monkey. Synapse 37: 252–261 [DOI] [PubMed] [Google Scholar]
- Van der Kooy D, Hattori T 1980. Single subthalamic nucleus neurons project to both the globus pallidus and substantia nigra in rat. J Comp Neurol 192: 751–768 [DOI] [PubMed] [Google Scholar]
- Van der Werf YD, Witter MP, Groenewegen HJ 2002. The intralaminar and midline nuclei of the thalamus. Anatomical and functional evidence for participation in processes of arousal and awareness. Brain Res Rev 39: 107–140 [DOI] [PubMed] [Google Scholar]
- Vogt C, Vogt O 1941. Thalamusstudien I-III. Sur Einfürung. II. Homogenität und Grenzgestaldung der Grisea des Thalamus. III. Das Griseum centrale (centrum medianum Luys). I. Psychol Neurol (Leipzig) 50: 31–154 [Google Scholar]
- Wilson SAK 1925. Disorders of motility and tone. Lancet Neurol 1: 1–103 [Google Scholar]
- Worbe Y, Baup N, Grabli D, Chaigneau M, Mounayar S, McCairn K, Féger J, Tremblay L 2009. Behavioral and movement disorders induced by local inhibitory dysfunction in primate striatum. Cereb Cortex 19: 1844–1856 [DOI] [PubMed] [Google Scholar]
- Wu Y, Richard S, Parent A 2000. The organization of the striatal output system: A single-cell juxtacellular labeling study in the rat. Neurosci Res 38: 49–62 [DOI] [PubMed] [Google Scholar]
- Yashukawa T, Kita T, Xue Y, Kita H 2004. Rat intralaminar thalamic nuclei projections to the globus pallidus: A biotinylated dextran amine anterograde tracing study. J Comp Neurol 471: 153–167 [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton B 2006. The role of the basal ganglia in habit formation. Nat Neurosci Rev 7: 464–476 [DOI] [PubMed] [Google Scholar]