Abstract
In zebrafish, the organizer is thought to consist of two regions, the yolk syncytial layer (YSL) and the shield. The dorsal YSL appears to send signals that affect formation of the shield in the overlying mesendoderm. We show here that a domain of dorsal deep cells located between the YSL and the shield is marked by expression of the iro3 gene. As gastrulation proceeds, the iro3 positive domain involutes and migrates to the animal pole. Iro3 expression is regulated by Nodal and bone morphogenic protein antagonists. Overexpression of iro3 induced ectopic expression of shield-specific genes. This effect was mimicked by an Iro3-Engrailed transcriptional repressor domain fusion, whereas an Iro3-VP16 activator domain fusion behaved as a dominant negative or antimorphic form. These results suggest that Iro3 acts as a transcriptional repressor and further implicate the iro3 gene in regulating organizer formation. We propose that the iro3-expressing dorsal deep cells represent a distinct organizer domain that receives signals from the YSL and in turn sends signals to the forming shield, thereby influencing its expansion and differentiation.
In the early vertebrate embryo, specification of the dorso-anterior axis is a critical step in body patterning that is promoted by a small dorsal region called the Spemann organizer. In Xenopus laevis, multiple regions are involved in organizer formation and function (1). The Nieuwkoop center, residing in the dorsal endodermal layer at mid to late blastula, has a role in organizer induction, and its formation is initiated by β-catenin signaling (2–4). In the zebrafish embryo, the dorsal yolk syncytial layer (YSL) may be analogous to the Nieuwkoop center (5–7), and its position is marked by the homeobox gene bozozok (dharma, nieuwcoid) (8–10). The shield, a region of dorsal mesendoderm that mainly gives rise to the prechordal plate and notochord, is the zebrafish equivalent of the Spemann organizer (11, 12).
In this article, we propose an additional organizer subdomain in zebrafish residing at the vegetal side of the shield in dorsal mesendoderm as identified by expression of the homeobox gene iroquois3 (iro3). Iroquois genes were discovered in Drosophila as neural prepattern genes regulating proneural genes in the achaete–scute complex (as–c) (13, 14). Loss of iroquois genes alters neural differentiation, wing formation, and dorsoventral polarity in the head region (15–19). Iroquois genes have recently been isolated from several vertebrates. Xenopus iro1, iro2, and iro3 induce proneural markers including the as–c homolog Xash3 (20, 21). In chicken, irx4 is expressed in heart ventricles and regulates ventricle/atrium cell fate determination (22). Zebrafish iro3 was reported to be expressed in the notochord in the late gastrula and in the neural tube during somitogenesis (23). Here, we report isolation of an additional iro3 cDNA, which we demonstrate to be the major splicing variant. We show that iro3 is also expressed in the dorsal deep layer during blastula and gastrula and that early iro3 expression is regulated by Nodal and anti-bone morphogenic protein (BMP) signals. We further show that ectopic expression of iro3 can induce several organizer genes. These results suggest that the iro3-expressing region delineates a distinct organizer subdomain and that iro3 has a role in organizer formation and function.
Materials and Methods
Radiation Hybrid Mapping.
Iro3 was mapped on the LN54 radiation hybrid panel (24), using the primers cattgtaagcatgtcctgtg and ttcggatcacaagtatatac.
Reverse Transcriptase (RT)-PCR of iro3 Transcripts.
Total RNA (2 μg) was reverse-transcribed by Superscript II reverse transcriptase in 20-μl reactions, and 1 μl of this reaction was amplified by PCR using Taq polymerase (Roche Molecular Biochemicals) in a 50-μl reaction under the following conditions: 94°C, 1 min; 60°C, 1 min; and 72°C, 2 min for 35 cycles, using primers 1062F/ggcgaaccggtcaaaatcaa, 1459R/gcttccaaggcactagatc, 1439F/gatctagtgccttggaagc, and 1743R/acatcaaatcctcacagagc.
Iro3, En-iro3, and VP-iro3 Expression Constructs.
The Iro3 coding region was amplified by PCR and subcloned into the pCS2 + expression vector. For the construction of En-iro3, the iro3 sequence encoding the homeodomain and flanking region (82–204 aa) was amplified by PCR, using primers ggcgaattcgccgccatgggcgtccagcatcctggatttg and ggcgaattccgtaaacatttccttcctcgtc. The product was inserted into the EcoRI site upstream of the engrailed repressor domain in pCS2 + En (25). To generate VP-iro3, the same region was amplified by using primers gcctcgagggcgtccagcatcctggatttgc and gctctagagtaaacatttccttcctcg and inserted into the XbaI/XhoI sites upstream of the VP16 activator domain in pCS2 + VP16 (25).
mRNA Synthesis and Embryo Injections.
mRNA was synthesized by MMESSAGE MMACHINE (Ambion, Austin, TX). The following genes were injected: iro3, En-iro3, VP-iro3, squint (sqt) and cyclops (cyc) (26), TaramA* (27), β-catenin (4), and mouse noggin (gift of M. Hibi, Osaka University, Osaka).
Whole-Mount in Situ Hybridization.
Embryos were fixed in 4% paraformaldehyde in PBS at 4°C and manually dechorionated. Embryos were stored in methanol, permeabilized in acetone for 8 min, and rehydrated in 0.1% Tween-20 in PBS. The in situ hybridization was performed essentially as described (28). The following probes were used: iro3, ntl, gsc, sqt, cyc, axl, lim1, BMP2b, BMP4, chd, nog1, follistatin, dkk1, sox17, and mixer. β-galactosidase (β-gal) was stained by Salmon-β-D-galactoside (Biosynth, Naperville, IL).
Mutant Analysis.
The following mutants were used for in situ hybridization: one eye pinheadm134 (oep), squintcz35 (sqt), cyclopsb16 (cyc), no tail (ntl), floating headn1 (flh), swirltc300 (swr), and chordinott250 (din).
Results
Cloning of iro3 cDNA.
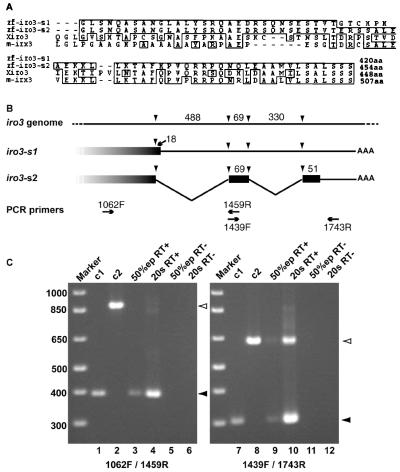
We isolated a cDNA for the iro3 homeobox gene during an in situ-based screen (T. K. M. Tsang, N. A. Hukriede, J. Chen, M. Dedekian, C. J. Clarke, A. Kiang, S. Schultz, J. A. Epstein, R. Toyama & I.B.D., unpublished observation). Recently, Tan et al. (23) reported the cloning of an iro3 cDNA. Our iro3 isolate encodes a different sequence in the C-terminal 40 aa; we refer to the Tan et al. clone as iro3-s1 and to our clone as iro3-s2. Iro3-s2 is similar to Xiro3 and mouse Irx3 throughout the C terminus, whereas similarity of Iro3-s1 and the frog and mouse proteins ends abruptly upstream of the C terminus (Fig. 1A). Iro3 genomic DNA was sequenced, proving to be very similar to iro3-s1 in this region, with only a few differences that may be polymorphisms (not shown), suggesting that iro3-s1 represents incompletely spliced mRNA that terminates prematurely (Fig. 1B).
Figure 1.
(A) Alignment of C-terminal sequences between zebrafish Iro3 splice variants, Xenopus iro3 (Xiro3), and mouse Irx3 (m-irx3) proteins. Iro3-s1 is from Tan et al. (23); Iro3-s2 is reported in the present work. Iro3-s2 contains 40 aa at the C terminus that are different from Iro3-s1; this region has high homology to Xiro3 and m-irx3. (B) Structure of iro3-s1 and iro3-s2 mRNA at the 3′ region compared with iro3 genomic sequence. In this genomic region, four splice sites (arrowheads) could be identified, defining two introns of 488 and 330 nt in length. Whereas iro3-s2 is fully spliced, iro3-s1 is essentially identical to iro3 genomic DNA in this region. Translation of the penultimate intron in iro3-s1 leads to the addition of six nonhomologous amino acids and premature termination. Two pairs of RT-PCR primers, designed to detect these two putative introns, are indicated below the maps. (C) Ratio of spliced and unspliced mRNA of iro3. C1 and C2 represent iro3-s2 cDNA and iro3 genomic DNA, respectively, used as control templates. Total RNA was prepared from 50% epibody (50%ep) and 20 somite stages (20s), reverse-transcribed (RT+), and amplified by PCR using the primers indicated. Open arrowhead, unspliced product; closed arrowhead, spliced product.
The ratio of splicing variants of iro3 was examined by RT-PCR with RNA from 50% epibody and 20 somite embryos. The spliced variant was the major product at both stages, although the unspliced variant, including the last intron, was easily detectable (Fig. 1C).
The iro3 gene was mapped by the radiation hybrid method with the LN54 panel (24). The gene resides on LG7, with the closest marker being Z6819 (see http://mgchd1.nichd.nih.gov:8000/zfrh/current.html for details).
Iro3 Expression in the Blastula and Gastrula Embryo.
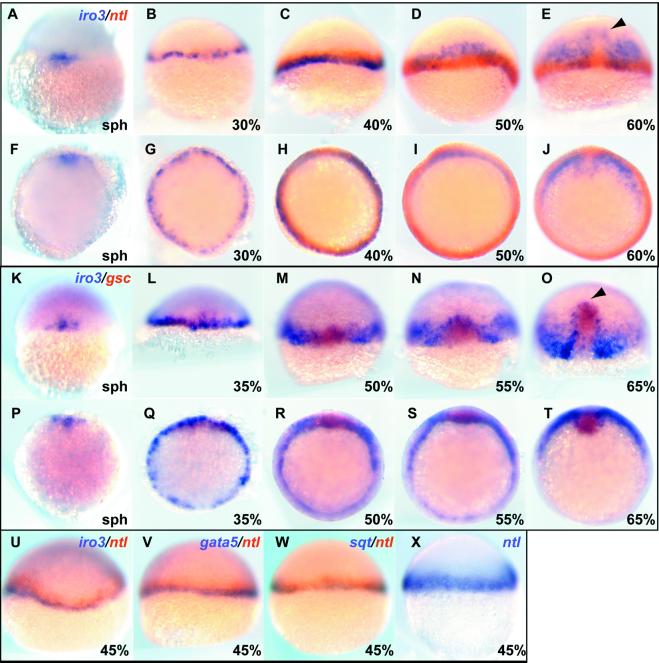
We found expression of the iro3 gene in a unique pattern in the mesendodermal layer of the early embryo, as illustrated by double-label in situ hybridization with the mesodermal marker no tail (ntl) and the organizer marker goosecoid (gsc) (Fig. 2). Iro3 was expressed initially at the sphere stage on one side of the blastoderm margin, expanding throughout the margin by 30% epibody, when iro3 and ntl domains largely coincide. At 40% epibody, ntl expands slightly toward the animal pole, whereas iro3 remains restricted to the vegetal side of the margin (Fig. 2C), similar to the pattern of the endodermal marker gata5 (29) (Fig. 2V), and squint (sqt) (26) (Fig. 2W). The similarity to gata5 implies that iro3 expression marks a presumptive endodermal domain. At the 50% epibody stage, when the marginal layer starts to involute, iro3 expression becomes restricted to the dorsal side. The iro3 domain involutes toward the animal pole, and at midgastrula a few iro3-positive cells surround the anterior edge of the prechordal plate that is marked by gsc (Fig. 2 E and O, arrowhead). Iro3 RNA disappears from the deep layer around the bud stage (not shown). As previously described, iro3 is also expressed in the notochord at the late gastrula stage and in the neural tube during somitogenesis (23).
Figure 2.
Iro3 expression in the early mesendodermal layer. Iro3 (purple) and the mesodermal marker ntl (red) (A–J), or the anterior axial marker gsc (red) (K–T), were visualized by double-staining in situ hybridization. At the sphere stage, iro3 starts to be expressed at one side of the blastoderm margin (A), spreading across the margin subsequently (B). At 40% epibody, ntl expression extends slightly toward the animal pole, whereas iro3 stays at the lower margin (C) but subsequently involutes as a deep layer (D, I, and N). At 60–65% epibody, axial mesendoderm differentially expresses gsc (anterior) and ntl (posterior). The iro3 positive region extends to the most anterior end at the edges of the gsc expression domain but is excluded from the axial domain (E and O, arrowhead). Lower marginal expression of iro3 is similar to the expression of gata5 (V) and sqt (W), whereas ntl extends slightly further in the animal direction (U and X). A–E, K–O, and U–X, dorsal-lateral view; F–J and P–T, animal view.
Iro3 Expression Is Regulated by a Nodal Signal.
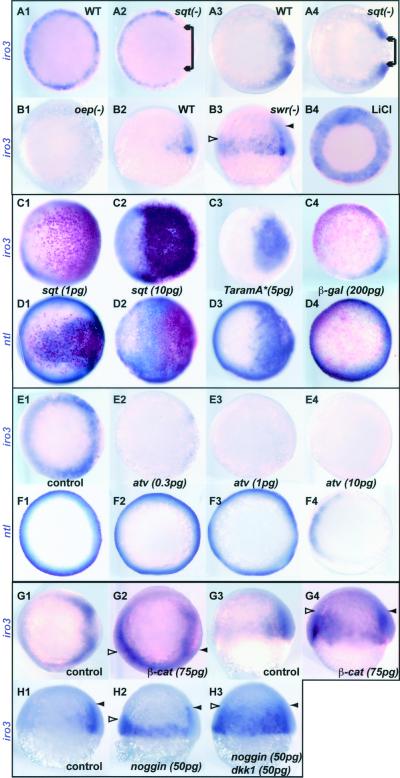
In sqt−/− embryos, where a nodal family gene is defective (30), iro3 expression was specifically lost on one side of the margin at 40% epibody (Fig. 3A2, arrow). This is most likely the dorsal side, as sqt embryos at 65% epibody miss iro3 expression dorsally, whereas some staining remains at the edges of the defective dorsal domain (Fig. 3A4, arrow). In one eye pinhead (oep) mutants where the activity of all Nodal factors is impaired (31), iro3 expression was lost entirely (Fig. 3B1). Other mesendodermal mutants including cyclops (cyc), no tail (ntl), and floating head (flh) did not show obvious defects in iro3 expression during gastrulation.
Figure 3.
Iro3 expression is regulated by Nodal, β-catenin, and BMP antagonists. (A and B) Embryos mutant for sqt (A2 and A4), oep (B1), and swr (B3), and LiCl-treated embryos (B4), were hybridized with iro3. In sqt embryos, there is a gap in iro3 expression at the dorsal side (A2 and A4, arrows). Zygotic oep mutant embryos do not express iro3 during blastula and gastrula stages (B1). Iro3 expression in the dorsal deep layer at early gastrula was expanded ventrally in swr embryos (B3) and LiCl-treated embryos (B4). (C and D) Sqt and TaramA* mRNAs were injected into two- to four-cell stage embryos together with β-gal mRNA. Embryos were fixed at 45–55% epibody and stained for β-gal (red) and iro3 (C) or ntl (D) (purple). Sqt induces iro3 only at a high dose (C2), but ntl at all doses (D1 and D2). (E and F) Antivin (atv) mRNA, encoding an antagonist of Nodal, was injected at different doses, and embryos at 45% epibody were stained for iro3 (E) and ntl (F). Even a low dose of atv mRNA suppressed iro3 (E2), whereas only a high dose suppressed ntl (F4). (G and H) Embryos were injected with RNAs encoding β-catenin and the BMP antagonist Noggin with or without the Wnt antagonist Dkk1, and were stained with iro3 at 60% epibody. Note that in swr mutant and noggin-injected embryos, the iro3 domain expanded ventrally but not toward the animal pole (B3 and H2, open arrowhead), whereas in β-catenin or noggin+dkk1 RNA-injected embryos, the ectopic iro3 domain did expand toward the animal pole (G4 and H3, open arrowhead). (A, B1, B4, C–F, G1, and G2) Animal view. (B2, B3, G3, G4, and H) Lateral view.
We compared the responses of iro3 and ntl to Nodal signaling. Whereas ntl was induced by injection of low doses of sqt mRNA, iro3 was only induced at high doses (Fig. 3 C1, C2, D1, and D2). Further, ntl was induced at a distance from the sqt injection domain, which was marked by β-gal, but iro3 was restricted to the marked region (Fig. 3 C2 and D2). Similar results were obtained by overexpression of the Nodal factor Cyclops, by Activin, and by the activated form of the putative Nodal receptor TaramA* (27) (Fig. 3 C3 and D3; data not shown). When mRNA encoding the Nodal inhibitor Antivin was injected, iro3 expression was inhibited at a low dose, whereas ntl was suppressed only at a higher dose (Fig. 3 E1–F4). Thus, ntl responds to a low level of Nodal signaling, whereas iro3 requires a high level for its activation. The difference in levels of Nodal signaling required for induction of ntl and iro3 is consistent with previous reports in Xenopus and zebrafish, showing that endoderm induction requires higher levels of Nodal/Activin signaling than mesoderm induction (29, 32–34).
Iro3 Expression Is Regulated by β-Catenin and BMP Signals.
In swirl (swr) mutants, which are defective in bmp2b (35), the normal dorsally restricted iro3 expression was expanded ventrally (Fig. 3B3). Consistently, injection of RNA encoding the BMP antagonist Noggin induced ventral expansion of iro3 (Fig. 3H2), and hyperdorsalized embryos generated by LiCl treatment expressed iro3 strongly in the entire blastoderm margin (Fig. 3B4). Likewise, β-catenin mRNA injection induced ectopic iro3 expression (Fig. 3 G2 and G4). These results suggest that iro3 expression during gastrulation is maintained dorsally by β-catenin-induced BMP antagonists. In swr mutants and in noggin-injected embryos, ventral deep cells expressed iro3, but this expression domain did not migrate toward the animal pole, unlike the natural, dorsal expression domain of iro3 (Fig. 3 B3 and H2, open and filled arrowheads, respectively). In contrast, the iro3-positive ventral region expanded toward the animal pole in β-catenin-injected embryos (Fig. 3G4). The same expansion was observed in embryos in which Noggin and the Wnt inhibitor Dkk1 were coexpressed (Fig. 3H3, open arrowhead); it is known that inhibition of BMP and Wnt signaling is required for anterior development in the gastrula (36).
Iro3 Induces Organizer Markers in a Noncell Autonomous Manner.
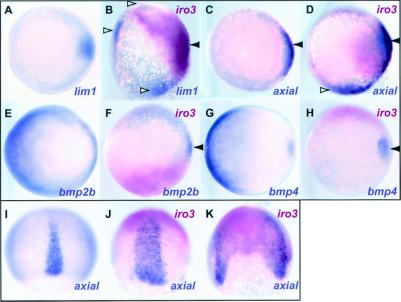
To test the possible role of iro3 in organizer formation, we injected iro3 mRNA into two-to four-cell stage embryos and looked for the induction of organizer markers. Iro3 was capable of inducing the organizer genes lim1, axial (axl), chd, flh, and cyc (Fig. 4 and data not shown). These genes were mostly induced just outside of the injected domain as visualized by β-gal staining, suggesting that iro3 induces organizer gene expression in a noncell autonomous manner. In contrast to the genes mentioned above, gsc, a marker for the anterior-most axial mesoderm, was induced only weakly and inconsistently by iro3.
Figure 4.
Iro3 induces dorsal and represses ventral markers in a noncell autonomous manner. Iro3 mRNA (200 pg) was injected as indicated at the two- to four-cell stage together with β-gal mRNA, and embryos were fixed at the shield stage (A–H; animal view) or 90% epibody stage (I–K; dorsal view). Organizer markers, lim1 (A and B) and axial (C and D), and ventral markers, bmp2b (E and F) and bmp4 (G and H), were stained purple by in situ hybridization, and red staining identifies the injected region. Lim1 and axial were activated in iro3-injected embryos outside the β-gal-positive region. The intrinsic expression domain (shield) and ectopic domain are indicated by filled and open arrowheads, respectively (A–D). Ventral expression of bmp2b and bmp4 was suppressed in both the injected and uninjected side (F and H), but the dorsal expression domain of the bmp2b and bmp4 was retained (arrowhead). At 90% epibody, the axial-stained notochord was expanded (J) or duplicated (K) in injected embryos.
We sought to identify putative secreted molecules that might mediate iro3 induction of organizer genes. However, iro3 injection did not activate sqt, cyc, dkk1, chd, follistatin, or noggin1 in the injected domain (not shown). We also examined the effect of iro3 injection on the ventral markers bmp2b and bmp4. Embryos injected at the two-cell stage with iro3 RNA showed decreased ventral expression of the bmp genes not only on the injected (red) but also the uninjected side (Fig. 4 F and H); the dorsal bmp domain was unaffected or occasionally expanded (arrowhead). As iro3 seems to be expressed in the endoderm, we examined the endodermal genes sox17, mixer, and gata5 in iro3-injected embryos but found none to be induced.
When iro3 mRNA was injected into eight- to 16-cell stage embryos, a low incidence (less than 10%) of secondary axis formation was observed. At least part of the reason for this low incidence may be that the injections could not be targeted to the future ventral domain. In addition, many embryos showed gastrulation defects and therefore could not be analyzed. Those secondary axes that did arise were incomplete, missing anterior structures. We also injected iro3 mRNA into Xenopus embryos and observed efficient induction of incomplete secondary axes (65%, n = 86). Likewise, Xenopus iro3 mRNA injected into Xenopus embryos induced secondary axes at similarly high efficiency. Thus, iro3 is capable of inducing organizer activity in the ventral domain of different vertebrate embryos.
VP-iro3 Inhibits Organizer Formation.
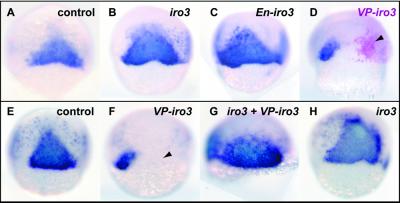
Artificial repressor (En-iro3) and activator (VP-iro3) forms of Iro3 were constructed, and the corresponding mRNAs were injected into one blastomere of two-cell zebrafish embryos (Fig. 5). En-iro3 mRNA induced axl like wild-type iro3 mRNA (Fig. 5 B and C). In contrast, VP-iro3 mRNA injection decreased axl expression; repression of axl expression was seen in the injected side of the embryo, although both cells that were and that were not injected with tracer were affected (Fig. 5D). Coinjection of iro3 and VP-iro3 mRNAs induced axl like the wild type alone, showing that the interfering effect of VP-iro3 could be rescued by coexpression of the wild-type form (Fig. 5G). These results indicate that iro3 functions as a transcriptional repressor in organizer formation and that VP-iro3 acts as a dominant negative or antimorphic form. The fact that Iro3, a repressor, activates expression of several genes suggests that this effect is indirect. This agrees with the noncell autonomous nature of the induction, which also implies involvement of a mediator.
Figure 5.
Opposite effects of iro3/en-iro3 and VP-iro3 on axial induction. Iro3 (B and H), en-iro3 (C), orVP-iro3 (D and F) mRNAs (200 pg) were injected at the two-cell stage into one blastomere. Axial was stained at 60% epibody. Whereas iro3 and en-iro3 injection induced axial strongly, VP-iro3 injection suppressed axial around the injected area visualized by β-gal staining (D, arrowhead). When iro3 and VP-iro3 mRNAs (200 pg each) were coinjected, axial expanded (G), as by iro3 single injection (H). All panels shown are dorsal views.
Discussion
Iro3 Marks a Specific Domain in the Early Mesendodermal Layer.
Iro3 is expressed in the lower blastoderm margin at late blastula in a pattern similar to that of gata5 (29) and different from ntl, which is expressed more widely, suggesting that most of the iro3 expression domain gives rise to endodermal derivatives. During gastrulation, ventral iro3 expression is lost while the dorsal iro3 domain involutes, continuing to occupy a deep layer that migrates toward the animal pole. This behavior is consistent with lineage analysis, indicating that vegetal cells in the dorsal germ ring give rise to pharyngeal endoderm (37, 38).
Iro3 Induction Requires a Nodal Signal.
Iro3 was suppressed in the presumptive organizer region but not in ventro-lateral marginal cells in sqt mutants in a similar way as several other genes such as lim1, ntl, gata5, and mixer (29, 39) (data not shown). It appears that all mesendoderm specification is defective in the dorsal-most quadrant of sqt embryos. In contrast, iro3 expression was unaffected in cyc embryos. The fact that the oep mutation affects iro3 expression more strongly than sqt implies that cyc does play a role in iro3 expression that is not apparent in cyc mutants because of some redundancy between the two Nodal factors (30, 31, 40, 41). In zygotic oep mutants where Nodal signaling is depressed (31, 42, 43), iro3 expression was lost. Such mutants, however, retain some Nodal signal as a result of maternal oep mRNA. The total loss of iro3 expression in zygotic oep embryos can be explained by the dependence of iro3 on a strong Nodal signal (Fig. 3).
The BMP Pathway Regulates Dorsal Restriction of iro3 Expression.
Dorsal iro3 expression was expanded ventrally by LiCl treatment, β-catenin, or noggin injection, and in swr mutants. These results suggest that ventral loss of iro3 expression at the onset of gastrulation is caused by a BMP signal. Iro3 expression was not affected in the din mutant where chordin activity is missing, presumably because of redundancy between BMP antagonists. In the mouse, noggin and chordin double knockout mutants show strong dorso-anterior defects, but single mutants do not (44, 45).
In noggin-injected and swr mutant embryos, iro3 expression expanded ventrally but did not expand toward the animal pole, as the dorsal iro3-expressing domain does (Fig. 3 B3 and H2). This suggests that suppression of the BMP signal is sufficient to activate iro3 in the deep marginal cell layer but not to recruit these cells to anterior migration. In contrast, anterior expansion of the ectopic iro3 domain was seen in β-catenin-injected embryos (Fig. 3G4), in agreement with the ability of β-catenin to induce complete secondary axes (2–4). A combination of BMP and Wnt antagonists likewise can induce complete axes (46), and coinjection of noggin and dkk1 mRNAs led to ventral expression of iro3 and expansion of the positive domain toward the animal pole (Fig. 3H3).
Noncell Autonomous Induction of Organizer Genes by iro3.
Iro3 mRNA injection induced ectopic expression of several organizer markers and suppressed ventral genes, both in a noncell autonomous manner. As dorsally restricted iro3 expression is found adjacent to axial mesoderm forming the shield, an indirect effect of Iro3 on organizer formation may be expected. Such an indirect role also is suggested by the results indicating that Iro3 acts as a repressor, requiring at least one intermediate step in a pathway in which Iro3 elicits gene activation in the organizer. In the simplest model, Iro3 would repress the expression of a signaling factor that inhibits organizer formation. More complex models involving additional intermediate steps can be envisioned and remain to be explored.
Role of the iro3-Expressing Domain in Patterning the Mesendoderm.
We suggest that iro3, activated by Nodal factors and restricted to the dorsal side by signals ultimately dependent on β-catenin, has an important role in patterning the mesendoderm in the early gastrula. The iro3-expressing domain may define a region with a distinct role in organizer formation in the early embryo. A multiple-organizer model, in which distinct regions act in temporal and spatial succession, has been proposed in Xenopus (1). The Nieuwkoop center is thought to induce the late blastula organizer in the vegetal half of the Spemann organizer; the blastula organizer region gives rise to head mesoderm, anterior notochord, anterior somites, and pharyngeal endoderm. The late blastula organizer, in turn, induces immediately above it the gastrula organizer, which develops into the notochord.
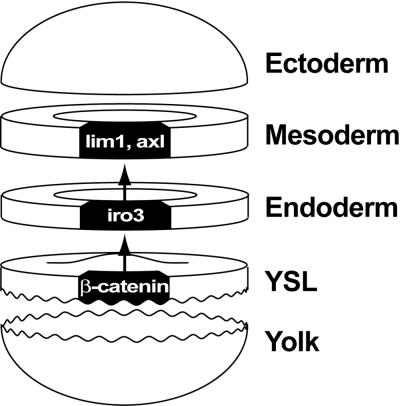
This model in Xenopus has some similarities in temporal and spatial aspects to a model we suggest for zebrafish on the basis of our studies on iro3 (Fig. 6). Here, the iro3-expressing domain corresponds to the Xenopus late blastula organizer and, like this structure, includes pharyngeal endoderm precursor cells. We suggest that the iro3 domain is spatially and functionally interspersed between the YSL, which represents the Nieuwkoop center equivalent, and the gastrula organizer. In sum, we propose that the early expression domain of iro3 marks a distinct region of the evolving organizer, and that Iro3 activity in this domain has an important role in organizer expansion and differentiation.
Figure 6.
A model for the position of iro3 and the iro3-expressing dorsal endodermal domain in organizer patterning. In the dorsal YSL, the β-catenin signal is activated and sqt is expressed. The activation of iro3 in the overlying endoderm depends on the function of the YSL. In turn, iro3 has a role in inducing organizer genes in the overlying mesoderm, presumably through the mediation of an unknown secreted molecule. Each of the arrows implies multiple steps in molecular pathways. This model is similar to a model in Xenopus that distinguishes the Nieuwkoop center, late blastula organizer, and gastrula organizer. See text for additional details.
Acknowledgments
We thank Elizabeth Laver for assistance with zebrafish; N. Hukriede, A. Kawahara, and M. Tsang for discussion; M. Halpern and D. Stainier for comments on the manuscript; M. Mullins, A. Schier, and M. Halpern for mutant zebrafish lines; B. Thisse for antivin and noggin1; R. Patient for gata5; F. Rosa for TaramA*; N. Ueno for bmp cDNAs; and M. Hibi for mouse noggin and dkk1.
Abbreviations
- BMP
bone morphogenic protein
- YSL
yolk syncytial layer
- RT
reverse transcriptase
- β-gal
β-galactosidase
Footnotes
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AF340184).
References
- 1.Gerhart J C, Stewart R, Doniach T. In: Gastrulation: Movement, Patterns, and Molecules. Keller R, Clark W Jr, Griffin F, editors. New York: Plenum; 1991. pp. 57–77. [Google Scholar]
- 2.Funayama N, Fagotto F, McCrea P, Gumbiner B M. J Cell Biol. 1995;128:959–968. doi: 10.1083/jcb.128.5.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heasman J, Crawford A, Goldstone K, Garner-Hamrick P, Gumbiner B, McCrea P, Kintner C, Noro C Y, Wylie C. Cell. 1994;79:791–803. doi: 10.1016/0092-8674(94)90069-8. [DOI] [PubMed] [Google Scholar]
- 4.Kelly G M, Erezyilmaz D F, Moon R T. Mech Dev. 1995;53:261–273. doi: 10.1016/0925-4773(95)00442-4. [DOI] [PubMed] [Google Scholar]
- 5.Long W L. J Exp Zool. 1983;228:91–97. [Google Scholar]
- 6.Mizuno T, Yamanaka M, Wakahara A, Kuroiwa A, Takeda H. Nature (London) 1996;383:131–132. [Google Scholar]
- 7.Schneider S, Steinbeisser H, Warga R M, Hausen P. Mech Dev. 1996;57:191–198. doi: 10.1016/0925-4773(96)00546-1. [DOI] [PubMed] [Google Scholar]
- 8.Fekany K, Yamanaka Y, Leung T, Sirotkin H I, Topczewski J, Gates M A, Hibi M, Renucci A, Stemple D, Radbill A, et al. Development (Cambridge, UK) 1999;126:1427–1438. doi: 10.1242/dev.126.7.1427. [DOI] [PubMed] [Google Scholar]
- 9.Koos D S, Ho R K. Curr Biol. 1998;8:1199–1206. doi: 10.1016/s0960-9822(07)00509-x. [DOI] [PubMed] [Google Scholar]
- 10.Yamanaka Y, Mizuno T, Sasai Y, Kishi M, Takeda H, Kim C H, Hibi M, Hirano T. Genes Dev. 1998;12:2345–2353. doi: 10.1101/gad.12.15.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kodjabachian L, Dawid I B, Toyama R. Dev Biol. 1999;213:231–245. doi: 10.1006/dbio.1999.9392. [DOI] [PubMed] [Google Scholar]
- 12.Bouwmeester T, Leyns L. BioEssays. 1997;19:855–863. doi: 10.1002/bies.950191005. [DOI] [PubMed] [Google Scholar]
- 13.Gomez-Skarmeta J L, del Corral R D, de la Calle-Mustienes E, Ferre-Marco D, Modolell J. Cell. 1996;85:95–105. doi: 10.1016/s0092-8674(00)81085-5. [DOI] [PubMed] [Google Scholar]
- 14.McNeill H, Yang C H, Brodsky M, Ungos J, Simon M A. Genes Dev. 1997;11:1073–1082. doi: 10.1101/gad.11.8.1073. [DOI] [PubMed] [Google Scholar]
- 15.Cavodeassi F, Diez Del Corral R, Campuzano S, Dominguez M. Development (Cambridge, UK) 1999;126:4933–4942. doi: 10.1242/dev.126.22.4933. [DOI] [PubMed] [Google Scholar]
- 16.Cavodeassi F, Modolell J, Campuzano S. Development (Cambridge, UK) 2000;127:1921–1929. doi: 10.1242/dev.127.9.1921. [DOI] [PubMed] [Google Scholar]
- 17.Diez del Corral R, Aroca P, Gomez-Skarmeta J L, Cavodeassi F, Modolell J. Genes Dev. 1999;13:1754–1761. doi: 10.1101/gad.13.13.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grillenzoni N, van Helden J, Dambly-Chaudiere C, Ghysen A. Development (Cambridge, UK) 1998;125:3563–3569. doi: 10.1242/dev.125.18.3563. [DOI] [PubMed] [Google Scholar]
- 19.Leyns L, Gomez-Skarmeta J L, Dambly-Chaudiere C. Mech Dev. 1996;59:63–72. doi: 10.1016/0925-4773(96)00577-1. [DOI] [PubMed] [Google Scholar]
- 20.Bellefroid E J, Kobbe A, Gruss P, Pieler T, Gurdon J B, Papalopulu N. EMBO J. 1998;17:191–203. doi: 10.1093/emboj/17.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gomez-Skarmeta J L, Glavic A, de la Calle-Mustienes E, Modolell J, Mayor R. EMBO J. 1998;17:181–190. doi: 10.1093/emboj/17.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bao Z Z, Bruneau B G, Seidman J G, Seidman C E, Cepko C L. Science. 1999;283:1161–1164. doi: 10.1126/science.283.5405.1161. [DOI] [PubMed] [Google Scholar]
- 23.Tan J T, Korzh V, Gong Z. Mech Dev. 1999;87:165–168. doi: 10.1016/s0925-4773(99)00121-5. [DOI] [PubMed] [Google Scholar]
- 24.Hukriede N A, Joly L, Tsang M, Miles J, Tellis P, Epstein J A, Barbazuk W B, Li F N, Paw B, Postlethwait J H, et al. Proc Natl Acad Sci USA. 1999;96:9745–9750. doi: 10.1073/pnas.96.17.9745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kessler D S. Proc Natl Acad Sci USA. 1997;94:13017–13022. doi: 10.1073/pnas.94.24.13017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rebagliati M R, Toyama R, Fricke C, Haffter P, Dawid I B. Dev Biol. 1998;199:261–272. doi: 10.1006/dbio.1998.8935. [DOI] [PubMed] [Google Scholar]
- 27.Renucci A, Lemarchandel V, Rosa F. Development (Cambridge, UK) 1996;122:3735–3743. doi: 10.1242/dev.122.12.3735. [DOI] [PubMed] [Google Scholar]
- 28.Toyama R, Curtiss P E, Otani H, Kimura M, Dawid I B, Taira M. Dev Biol. 1995;170:583–593. doi: 10.1006/dbio.1995.1238. [DOI] [PubMed] [Google Scholar]
- 29.Rodaway A, Takeda H, Koshida S, Broadbent J, Price B, Smith J C, Patient R, Holder N. Development (Cambridge, UK) 1999;126:3067–3078. doi: 10.1242/dev.126.14.3067. [DOI] [PubMed] [Google Scholar]
- 30.Feldman B, Gates M A, Egan E S, Dougan S T, Rennebeck G, Sirotkin H I, Schier A F, Talbot W S. Nature (London) 1998;395:181–185. doi: 10.1038/26013. [DOI] [PubMed] [Google Scholar]
- 31.Gritsman K, Zhang J, Cheng S, Heckscher E, Talbot W S, Schier A F. Cell. 1999;97:121–132. doi: 10.1016/s0092-8674(00)80720-5. [DOI] [PubMed] [Google Scholar]
- 32.Born J, Geithe H-P, Tiedemann H, Tiedemann H, Kocher-Becker U. Z Phys Chem (Leipzig) 1972;353:1075–1084. doi: 10.1515/bchm2.1972.353.2.1075. [DOI] [PubMed] [Google Scholar]
- 33.Weber H, Symes C E, Walmsley M E, Rodaway A R, Patient R K. Development (Cambridge, UK) 2000;127:4345–4360. doi: 10.1242/dev.127.20.4345. [DOI] [PubMed] [Google Scholar]
- 34.Yasuo H, Lemaire P. Curr Biol. 1999;9:869–879. doi: 10.1016/s0960-9822(99)80391-1. [DOI] [PubMed] [Google Scholar]
- 35.Kishimoto Y, Lee K H, Zon L, Hammerschmidt M, Schulte-Merker S. Development (Cambridge, UK) 1997;124:4457–4466. doi: 10.1242/dev.124.22.4457. [DOI] [PubMed] [Google Scholar]
- 36.Kimelman D, Griffin K J P. Curr Opin Genet Dev. 2000;10:350–356. doi: 10.1016/s0959-437x(00)00095-2. [DOI] [PubMed] [Google Scholar]
- 37.Kimmel C B, Warga R M, Schilling T F. Development (Cambridge, UK) 1990;108:581–594. doi: 10.1242/dev.108.4.581. [DOI] [PubMed] [Google Scholar]
- 38.Warga R M, Nusslein-Volhard C. Development (Cambridge, UK) 1999;126:827–838. doi: 10.1242/dev.126.4.827. [DOI] [PubMed] [Google Scholar]
- 39.Alexander J, Stainier D Y. Curr Biol. 1999;9:1147–1157. doi: 10.1016/S0960-9822(00)80016-0. [DOI] [PubMed] [Google Scholar]
- 40.Sampath K, Rubinstein A L, Cheng A M, Liang J O, Fekany K, Solnica-Krezel L, Korzh V, Halpern M E, Wright C V. Nature (London) 1998;395:185–189. doi: 10.1038/26020. [DOI] [PubMed] [Google Scholar]
- 41.Rebagliati M R, Toyama R, Haffter P, Dawid I B. Proc Natl Acad Sci USA. 1998;95:9932–9937. doi: 10.1073/pnas.95.17.9932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schier A F, Neuhauss S C, Helde K A, Talbot W S, Driever W. Development (Cambridge, UK) 1997;124:327–342. doi: 10.1242/dev.124.2.327. [DOI] [PubMed] [Google Scholar]
- 43.Hammerschmidt M, Pelegri F, Mullins M C, Kane D A, Brand M, van Eeden F J, Furutani-Seiki M, Granato M, Haffter P, Heisenberg C P, et al. Development (Cambridge, UK) 1996;123:143–151. doi: 10.1242/dev.123.1.143. [DOI] [PubMed] [Google Scholar]
- 44.McMahon J A, Takada S, Zimmerman L B, Fan C M, Harland R M, McMahon A P. Genes Dev. 1998;12:1438–1452. doi: 10.1101/gad.12.10.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bachiller D, Klingensmith J, Kemp C, Belo J A, Anderson R M, May S R, McMahon J A, McMahon A P, Harland R M, Rossant J, De Robertis E M. Nature (London) 2000;403:658–661. doi: 10.1038/35001072. [DOI] [PubMed] [Google Scholar]
- 46.Glinka A, Wu W, Onichtchouk D, Blumenstock C, Niehrs C. Nature (London) 1997;389:517–519. doi: 10.1038/39092. [DOI] [PubMed] [Google Scholar]