Abstract
HIV is a disease in which the original clinical observations of severe opportunistic infections gave the first clues regarding the underlying pathology, namely that HIV is essentially an infection of the immune system. HIV infects and deletes CD4+ T cells that normally coordinate the adaptive T- and B-cell response to defend against intracellular pathogens. The immune defect is immediate and profound: At the time of acute infection with an AIDS virus, typically more than half of the gut-associated CD4+ T cells are depleted, leaving a damaged immune system to contend with a life-long infection.
At the time of acute HIV infection, more than half of the gut-associated CD4+ T cells are depleted, leaving a damaged immune system to contend with a life-long infection.
The earliest studies of T-cell function among infected persons revealed aspects of HIV immunopathogenesis that are still not fully understood. Despite initial and persistent damage to CD4+ T cells, and a lack of detectable HIV-specific CD4+ T helper cells (Murray et al. 1984; Lane et al. 1985), the magnitude and breadth of CD8+ T-cell responses to HIV in infected humans were found to be robust, with direct effector function of such a magnitude that it could be readily detected in freshly isolated lymphocytes from peripheral blood and brochoalveolar lavage in persons with AIDS (Plata et al. 1987; Walker et al. 1987; Nixon et al. 1988). HIV was already known to be an immunosuppressive disease, yet these cells were present in such robust quantity that they could be detected by assays measuring the ability of freshly isolated peripheral blood cells to lyse autologous B cells infected with recombinant vaccinia-HIV vectors or peptide pulsed targets (Walker et al. 1987; Nixon et al. 1988). Moreover, CD8+ T cells from infected persons were able to inhibit HIV replication (Walker et al. 1986), clearly showing that these cells were functional at least in vitro, but despite this, persons were progressing to AIDS.
Subsequent studies using other approaches such as interferon γ Elispot (Dalod et al. 1999), intracellular cytokine (Maecker et al. 2001), and peptide MHC tetramer assays (Altman et al. 1996) confirmed and quantified these robust CD8+ T-cell responses. Most chronically infected persons target more than a dozen CD8+ epitopes simultaneously (Addo et al. 2003), and in some instances up to 19% of CD8+ T cells are specific for HIV (Richardson 2000; Betts et al. 2001; Papagno et al. 2002), yet control of viremia is not achieved. At the same time, HIV-specific CD4+ T-cell responses were found to be severely impaired, particularly as measured by the ability of these cells to proliferate to viral antigens (Wahren et al. 1987). Thus, from the early studies there was a clear disconnect between the lack of HIV-specific CD4+ T cells and the abundance of HIV-specific CD8+ T cells.
Despite these conundrums, there were early signs that CD8+ T cells play a role in controlling HIV disease progression. Sequencing of autologous virus from infected persons revealed evidence of immune selection pressure mediated by these responses (Phillips et al. 1991) and an association with the initial decline in peak viremia after acute infection (Borrow et al. 1994; Koup et al. 1994). The development of HLA-class I-peptide tetramers confirmed the presence of robust induction of responses to multiple epitopes (Altman et al. 1996), and as larger numbers of patients were studied, it also became clear that among the strongest associations with disease outcome was the expression of certain HLA class I alleles (Kaslow et al. 1996; Migueles et al. 2000; Gao et al. 2001), implicating class I restricted cytotoxic T lymphocytes (CTLs) as a major modulator of disease progression. The relationship between CD8+ T-cell immune function and viral control was shown by experimental depletion of CD8+ T cells in animal models of AIDS virus infection (Jin et al. 1999; Schmitz et al. 1999). As sensitive viral load assays became available, it also became clear that some infected persons were able to control viremia to levels below detection by the most sensitive RNA assays, and that these persons were characterized by robust HIV-specific CD4+ T-cell responses (Rosenberg et al. 1997). However, now a quarter century into the epidemic the precise role of T cells in HIV control, and the precise phenotype, specificity and function of T cells that should be induced with a vaccine, remain unclear. And from the standpoint of vaccine development, it remains controversial as to how much insight is to be gained from the study of persons who have become infected, because by definition these are not protective responses.
CTL RESPONSES IN ACUTE INFECTION
Acute HIV infection is often associated with a transient febrile illness, much like infectious mononucleosis (Ho et al. 1985), which has facilitated the identification of persons in the earliest stages of infection, who are HIV RNA positive but not yet HIV antibody positive. Important justification for the intense study of acute infection is the demonstration that early events predict subsequent disease progression (Mellors et al. 1996; Lyles et al. 2000). Acute infection is associated with a decline in peak viremia from ∼1–5 million viral copies/mL to a steady state of ∼30,000 copies (Lyles et al. 2000), but peak levels of viremia may be much lower in asymptomatic persons (Fiebig et al. 2003). Very few studies have actually examined CD8+ T-cell responses in the earliest stages of infection (Fiebig stage I and II), but when those studies have been performed it is clear that initial immune responses are very narrowly directed (Goonetilleke et al. 2009) and remain narrowly directed as viral set point is achieved (Dalod et al. 1999; Altfeld et al. 2001). Even when responses to autologous virus are measured, the initial detectable response has usually been to only one to three epitopes (Goonetilleke et al. 2009), even though the autologous viruses may contain wild-type epitopes to which CD8+ T cells are subsequently generated (Radebe et al. 2011). An important caveat is that there may be much more happening in the tissues than is sampled in the blood in these early stages. Studies comparing lymph node CD8+ T-cell responses to those in peripheral blood have shown the former to be detectable well in advance of detectable responses in the periphery (Altfeld et al. 2002). Even so, there is a clear hierarchy in initial responses and immunodominance that correlates with set point viremia, which is lost during the transition to chronic infection (Streeck et al. 2009).
Despite the narrowness of the acute phase CD8+ T-cell response, it is associated with a dramatic decline in initial viremia, suggesting that these early, presumably narrowly directed responses are at least partially effective. Although functional studies of early CTL responses are few (Ferrari et al. 2011), evidence of CD8+ T-cell efficacy is suggested by detailed analysis of viral genomes during these early phases of infection, and by modeling studies. 80% of acute infections are established by a single founder virus, which first begins to diversify at around the time of peak infection (Salazar-Gonzalez et al. 2009; Shaw and Hunter 2011). By single genome amplification one can detect viral evolution at sites of CTL pressure as early as peak viremia (Goonetilleke et al. 2009), clear indication that there is effective pressure being applied because it can lead to a wholesale turnover in the replicating virus population. This has been modeled, suggesting that 15%–35% of infected cells can be killed by CTLs of a single specificity per day in vivo during acute infection (Goonetilleke et al. 2009). This CD8+ T-cell-mediated immune pressure is also apparent in population studies, which have shown clear HLA-associated signature mutations following acute infection (Brumme et al. 2008), persisting in the chronic phase of infection (Moore et al. 2002), and transmission and reversion of CTL escape variants (Goulder et al. 2001; Goepfert et al. 2008).
The characteristics and antiviral efficacy of the acute phase CD8+ T-cell responses are being progressively defined. These clearly occur in the setting of acute phase proteins and proinflammatory cytokines (Stacey et al. 2009). The initial response is narrowly directed, predominantly at epitopes in Env and Nef, regions that are among the most variable in the virus (Lichterfeld et al. 2004; Goonetilleke et al. 2009; Turnbull et al. 2009). Indeed, early responses appear to be targeted to epitopes of higher entropy for reasons that are not clear (Bansal et al. 2005). The breadth of responses increases over time, as do the number of HLA alleles that are involved in recognition of infected cells (Altfeld et al. 2006; Streeck et al. 2009). Some acute phase epitopes are not those that are targeted in chronic infection and may be novel (Goonetilleke et al. 2009), as revealed by studies using peptides representing autologous virus. Often these earliest responses fade away as soon as the epitope escape has occurred. Although these detailed findings have been made in just four patients, ongoing studies in a further 12 show very similar patterns (N Goonetilleke, M Liu, and AJ McMichael, unpubl. data).
The influence of host genetics on acute phase CTL responses is readily apparent. HLA alleles associated with protection from disease progression are preferentially targeted in acute infection, whereas coexpression of risk alleles does not induce responses, a process called immunodominantion (Altfeld et al. 2006). Clinical symptoms of acute infection have been shown to be reduced in persons expressing HLA B57, and this allele is associated with improved outcome and also dominates the early response in persons who express it (Altfeld et al. 2003a). At least one study indicates the selective loss of high-avidity CTLs in acute infection, and when these are maintained they are associated with a lower viral load (Lichterfeld et al. 2007). However, the ability of CD8+ T cells to produce interferon γ in primary infection is a poor predictor of viral set point and disease progression (Gray et al. 2009), suggesting that if CTLs do influence viral set point, this assay may not discern their function. In contrast to the wealth of information regarding CD8+ T-cell responses in the peripheral blood, almost nothing is known about responses in tissues in acute infection, which is the major site of HIV replication.
There has been considerable interest and some controversy over whether highly exposed but HIV seronegative people, such as sex workers in high-HIV-1-prevalence communities, make CTL responses to HIV-1 and whether these are helping them avoid infection (Rowland-Jones et al. 1995). The controversy has been partly resolved by using very sensitive highly controlled and blinded assays that show anti-HIV-specific CD4+ T cells in both HIV-1-exposed and -unexposed uninfected people, with stronger and more sustained T-cell responses in the former (Ritchie et al. 2011). No CD8+ T-cell responses were found in that study but the level of exposure in the exposed cohort in the study was much less than in African sex workers during the early 1990s, so the earlier findings cannot be disregarded. More likely, as shown by (Ritchie et al. 2011), there are relatively frequent CD4+ T-cell responses, primed by crossreacting antigens, in unexposed people and that these are amplified by HIV-1 exposure without infection, for example, in persons homozygous for the CCR5 Δ32 mutation. With very high exposure these T-cell responses could include CD8+ T cells. However, there is no evidence that either of these T-cell responses is protective.
CTL EVOLUTION FOLLOWING ACUTE INFECTION
Although a narrowly directed immune response is found at the time of maximal decline in peak viremia, responses subsequently broaden, such that during chronic infection the average person targets a median of 14 epitopes simultaneously (Addo et al. 2003), and given that these studies were performed with a reference set of peptides rather than autologous peptides, the actual number may be 20%–30% higher (Altfeld et al. 2003b; Goonetilleke et al. 2009). Immunization studies in animal models indicate that the CD8+ T-cell compartment has enormous expansion capacity, without affecting the size of the naïve CD4+, CD8+, or B-cell populations, and while preserving memory CD8+ T-cell populations to other pathogens (Vezys et al. 2009). HIV-specific CD8+ T-cell responses remain detectable throughout the course of disease, and are actually broader and higher in persons with progressive infection than in those with controlled infection (Pereyra et al. 2008).
The relationship between the earliest CTL responses, viral set point, and disease progression remains controversial. Kinetics of immune responses in acute infection are associated with serial waves of responses (Turnbull et al. 2009), but detectable responses tend to be narrowly directed and of low magnitude (Dalod et al. 1999; Altfeld et al. 2001; Radebe 2011). This may be caused by localization of responses at inductive and effector sites within lymphoid and gut mucosa, respectively, and responses detected in lymph nodes precede those detectable in peripheral blood and are of higher magnitude (Altfeld et al. 2002). There is a high predictability of initial and subsequent epitopes targeted, based on the HLA type of the individual (Goulder et al. 2001; Moore et al. 2002; Yu et al. 2002; Draenert et al. 2006; Brumme et al. 2009). Remarkably, most responses detectable at the time a quasi-set point is achieved persist even in very late stage infection (Koibuchi et al. 2005), though function may be lost, as evidenced by up-regulation of negative immunoregulatory molecules such as PD-1 (Day et al. 2006; Petrovas et al. 2006; Trautmann et al. 2006), and up-regulation of transcription factors that inhibit cell proliferation and cytokine secretion, such as BATF-1 (Quigley et al. 2010).
Specificity of responses during the chronic phase of infection repeatedly suggests that Gag targeting is associated with lower viral load (Edwards et al. 2002; Zuniga et al. 2006; Kiepiela et al. 2007). In a large study of persons with clade C virus infection, the broader the Gag-specific response the lower the viral load, and somewhat paradoxically, the broader the Env-specific response the higher the viral load (Kiepiela et al. 2007; Ngumbela et al. 2008). A similar association between targeting of Gag and viral load is also seen in children (Huang et al. 2008). It is noteworthy that the most protective HLA molecules B57, B58, B81, B14, and B27 target epitopes in Gag, often in relatively conserved regions. It may also be relevant that Gag p24 variation may often carry a fitness cost to the virus, possibly because mutants affect the complex assembly of the viral capsid (Schneidewind et al. 2008). This may make T-cell responses to these epitopes more favorable to the host because the virus is either controlled or escapes with a loss of virulence (Martinez-Picado et al. 2006). Eventually, however, the virus may restore its virulence with compensatory mutations (Schneidewind et al. 2007, 2008, 2009; Goepfert et al. 2008; Crawford et al. 2009).
In addition to targeting epitopes within the nine expressed HIV proteins, recent studies show that antisense peptides and alternative reading frame products are also targeted by CD8+ T cells, as shown first in the SIV model (Maness et al. 2007, 2009) and more recently in humans infected with HIV (Bansal et al. 2010; Berger et al. 2010). Moreover, almost all studies have used peptide-pulsed target cells to define responses, and emerging evidence suggests that processing and presentation are likely critical (Allen et al. 2004; Draenert et al. 2004; Lazaro et al. 2009). Mutations surrounding epitopes are associated with altered processing and lack of presentation (Le Gall et al. 2007), and this is only detectable when the epitopes are processed and presented in infected cells. Even the infected cell type appears to influence this processing, based on digestion of longer peptides using cytosolic extracts—and perhaps importantly CD4+ T cells have much lower proteolytic activity than monocytes, suggesting that antigen processing may be least effective in the cells that are the most critical to target (Lazaro et al. 2009).
CD4+ T-CELL RESPONSES IN ACUTE AND CHRONIC HIV-1 INFECTION
Generally in those infected with HIV-1, the T-cell responses are dominated by CD8+ T cells. These are much stronger than CD4+ T-cell responses (Ramduth et al. 2005), which are damaged by the virus. Given the important role that CD4+ T cells have in maintaining CD8+ T-cell responses, it is remarkable that the latter are so strong (Kalams et al. 1999). In murine models in which CD4+ T cells are depleted either with antibody infusion or genetically, CD8+ T-cell responses are greatly impaired (Janssen et al. 2003; Shedlock and Shen 2003; Sun and Bevan 2003). On antigen stimulation, they expand rapidly to exhaustion and their IL-2-dependent progression to long term memory populations is abrogated (Kamimura and Bevan 2007). In HIV-1 infection CD4+ T cells, though greatly depleted, are not entirely absent, but abnormalities in the development of CD8+ T-cell responses could be consistent with partial loss of CD4+ T-cell help, or impaired function of what cells remain (Pitcher et al. 1999).
In acute HIV-1 infection, memory CD4+ T cells are massively depleted from the lymphoid system, particularly in the gut involving both direct targeting by the virus and bystander activation-induced cell death (Mattapallil et al. 2005; Douek et al. 2009). This applies to all memory CD4+ T-cell populations but those specific for HIV may be preferentially infected and destroyed (Douek et al. 2002). However, the percentage of HIV-specific CD4+ T cells that are infected, even in the presence of high level viremia, is typically only a few percent or less, suggesting that the majority of these cells somehow escape infection despite being activated at a time of very high viremia.
Early studies showed a lack of CD4+ T-cell responses, measured by antigen stimulated proliferation assays both in early and late infection (Lane et al. 1985). However, Pitcher et al. (1999) found using antigen stimulated cytokine production, that HIV-specific T cells were present at all stages of infection, though in relatively low numbers. A lack of IL-2 production by antigen-specific CD4+ T cells and CD8+ T cells may account for the apparent discrepancy between results with the two assays (Zimmerli et al. 2005). In very early infection however, HIV-specific CD4+ T cells may be present in larger numbers. Rosenberg et al. (2000) showed that when patients were treated very early with antiretroviral drugs, that strong CD4+ T-cell responses to HIV antigens could be rescued, as measured by lymphocyte proliferation assays. Recently, Gray et al. (C Riou, VV Ganusov, C Gray, et al., submitted) have supported this by showing strong CD4+ T-cell responses around the time of peak viremia, but these T cells are rapidly lost as the infection progresses. This loss may reflect the damage to the whole CD4+ T-cell compartment, but it should be noted that in other acute and chronic virus infections (including responses to live vaccines) CD4+ T-cell responses may peak early and then decline to a relatively low level in the blood, in the absence of damage to CD4+ T cells (e.g., Amyes et al. 2003; Goonetilleke et al. 2009). At this point almost nothing is known about HIV-specific CD4+ T-cell responses in lymphoid tissues and the gut, a clearly needed focus for future studies.
Persons who progress very slowly to AIDS, in the absence of treatment, make stronger CD4+ T-cell responses and there is a correlation between the strength of the response and slow progression (Rosenberg et al. 1997), which is also striking in the slow progression of HIV-2 infection (Duvall et al. 2006). In a subset of persons who control viremia spontaneously, the gene expression profile of CD4+ cells is similar to that of uninfected persons (Vigneault et al. 2011). Cross-sectional data in chronically infected persons indicate a link between strong CD4+ T-cell responses and effective CD8+ T-cell responses (Kalams et al. 1999). Recent data implicate CD4+ T cells that make IL21 as particularly important in maintaining CD8+ responses (Chevalier et al. 2011; Williams et al. 2011).
The epitopes recognized by CD4+ T cells occur in all viral proteins but there is a preponderance of T cells specific for Gag and Nef and several epitopes have been defined, though far fewer than the number defined for CD8+ T-cell responses (Kaufmann et al. 2004) (LANL HIV Immunology database: http://www.hiv.lanl.gov/content/immunology/compendium.html). As is often the case for HLA class II restricted T-cell responses, the peptides can be presented by more than one, often several, different HLA class II molecules, in contrast to the generally tighter restriction by HLA class I molecules. In marked contrast to HLA class I presented peptides, mutational escape seems rare, or possibly absent, in HLA class II restricted T-cell responses (Rychert et al. 2007). This may reflect a lack of direct cytotoxic action on virus-infected cells as well as the relative lack of HLA class II on infected cells and also their propensity to interact primarily with dendritic cells, but additional studies are clearly warranted. And genetic studies in a number of cohorts suggest a relationship between expression of certain HLA class II alleles and HIV control/disease progression, again suggesting a possible role for these responses in active immune containment (Lacap et al. 2008; Julg et al. 2011).
Weak HIV-specific CD4+ T-cell responses have been reported in HIV exposed but seronegative donors in a number of studies (Goh et al. 1999; Schenal et al. 2005). Remarkably around 25% of HIV unexposed persons make similar, though weaker and less sustained than in those exposed, CD4+ T-cell responses to previously described HIV epitopes (Ritchie et al. 2011). These responses are probably cross-reactive with other pathogens and other antigens to which the person has been exposed. At least some of these epitopes are the same as those found in HIV infection (Kaufmann et al. 2004), so these preinfection cross-reactive responses could help explain why these particular epitopes are immunodominant after infection.
Several of the vaccine candidates that have been tested in humans were more effective at stimulating CD4+ T-cell responses than CD8+ T cells, particularly when plasmid DNA and pox virus vectors were used (Goonetilleke et al. 2006; Graham et al. 2006; Harari et al. 2008). Similar results have been found in macaques vaccinated with DNA and pox virus vectors (Sun et al. 2010). In the latter study, the vaccine-induced CD4+ T-cell response did not protect against challenge with the SHIV 89.6P virus, although an earlier study had shown that longer survival in vaccinated monkeys challenged with SIV mac251 was associated with vaccine-induced SIV-specific CD4+ T-cell responses (Sun et al. 2006). Although concern has been expressed that vaccine induction of HIV-specific CD4+ T-cell responses may increase the risk of HIV-1 acquisition by providing more activated CD4+ T cells to the site of infection (Douek et al. 2002; Staprans et al. 2004), this has only been observed in the absence of concomitant CD8+ T-cell responses (Staprans et al. 2004).
IMMUNE SELECTION PRESSURE AND VIRAL ESCAPE
The impact of CTL selection pressure is readily observed by sequencing autologous virus, which undergoes rapid immune-driven evolution in vivo following acute infection (Fig. 1). New approaches involving single genome amplification techniques have shown clear evidence of CTL escape within 25–32 days of infection, around the time of peak viremia following acute infection, and before full seroconversion (Goonetilleke et al. 2009). Indeed, deep sequencing has revealed that the virus explores multiple pathways to escape before going to fixation, indicating that there are constraints on HIV evolution that appear to be caused by imposed fitness alterations (Fischer et al. 2010). The pathways to immune escape appear limited, as shown by studies of genetically identical twins infected by the same virus as adults through shared injection drug use, in whom the earliest targeted epitopes, kinetics of escape and earliest escape variants that arose were strikingly similar (Draenert et al. 2006), despite differences in T-cell receptor (TCR) usage (Yu et al. 2007).
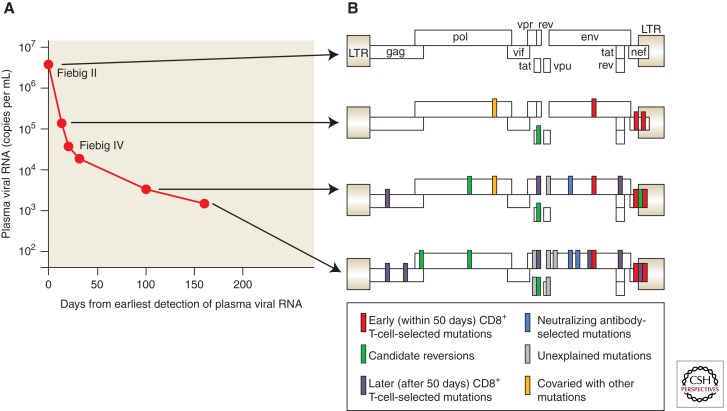
Figure 1.
Evolution of the transmitted/founder virus in acute HIV-1 infection is largely driven by CD8+ T-cell responses. (A) The graph shows the falling viral load from the time of peak viremia, about 3 weeks after infection, to the establishment of the viral set point 160 days later. Virus taken at the first sampling (the time of screening or “day 0”) was characteristic of a single founder virus when sequenced; this is represented on the genetic map of the virus at the top right (B). At subsequent time points mutations appeared in all the viruses sequenced, shown by the bars on the genetic map to indicate the sites of the mutations. The bars are color coded to indicate rapid escape selected by CD8+ T cells, slower or late escape selected by CD8+ T cells, selection by neutralizing antibodies targeting the virus envelope, “reversions” from unusual sequences in the founder virus sequence to the sequence consensus in the whole database (the transmitted sequences may have been selected by CD8+ T cells in the transmitting sexual partner of the patient). At a few sites it was not clear what was selecting the amino acid change and these are shown in gray. (Adapted from McMichael et al. 2010; reprinted, with express permission, from the author.)
Escape from CTL responses can occur through multiple mechanisms. Mutations in regions flanking CTL epitopes can clearly impact processing and subsequent recognition (Draenert et al. 2004; Le Gall et al. 2007; Tenzer et al. 2009), as can mutations within epitopes. Mutations in TCR contact residues have a variable impact on CTL recognition, and the ability to cross-recognize variants appears to be influenced by thymic selection (Kosmrlj et al. 2010). Those HLA alleles associated with better outcome following infection are associated with presentation of fewer self-peptides in the thymus, resulting in a repertoire in which more clones survive negative selection, and are thus are likely to be more cross-reactive to variants that arise. This may also explain the association between protective HLA alleles in HIV infection and an association between these same alleles and autoimmunity/hypersensitivity (Kosmrlj et al. 2010).
The strongest temporal relationship between CTL escape and viral load is associated with HLA B*27 restricted recognition of an epitope in the p24 Gag protein, which requires an initial mutation within the epitope followed by a second distant mutation in order for the anchor residue to mutate and completely abrogate recognition (Schneidewind et al. 2007). However, for other mutations the effects on viral load are not as clear, likely because of competing effects of immune escape and alterations in viral fitness. Studies clearly indicate that some mutations come at the cost of a significant decrease in viral replicative capacity, suggesting that escape can be beneficial to the host (Martinez-Picado et al. 2006). In persons who control viremia without therapy, their viruses have impaired replication capacity, and this has also been shown for acute infection (Miura et al. 2010); moreover, there is a clear link between certain HLA class I alleles and viral fitness (Miura et al. 2009a), suggesting that this is immune mediated by class I restricted CD8+ T cells. The evolving view is thus that CTL efficacy is not only caused by inhibiting viral replication, but is also impacted by the mutations induced by these responses. Such a view helps to reconcile the finding that the breadth of responses to Gag, a necessarily conserved protein, are associated with lower viral load, whereas breadth of responses to Env, which readily tolerates mutations without affecting viral fitness, is associated with higher viral loads (Fig. 2) (Kiepiela et al. 2007).
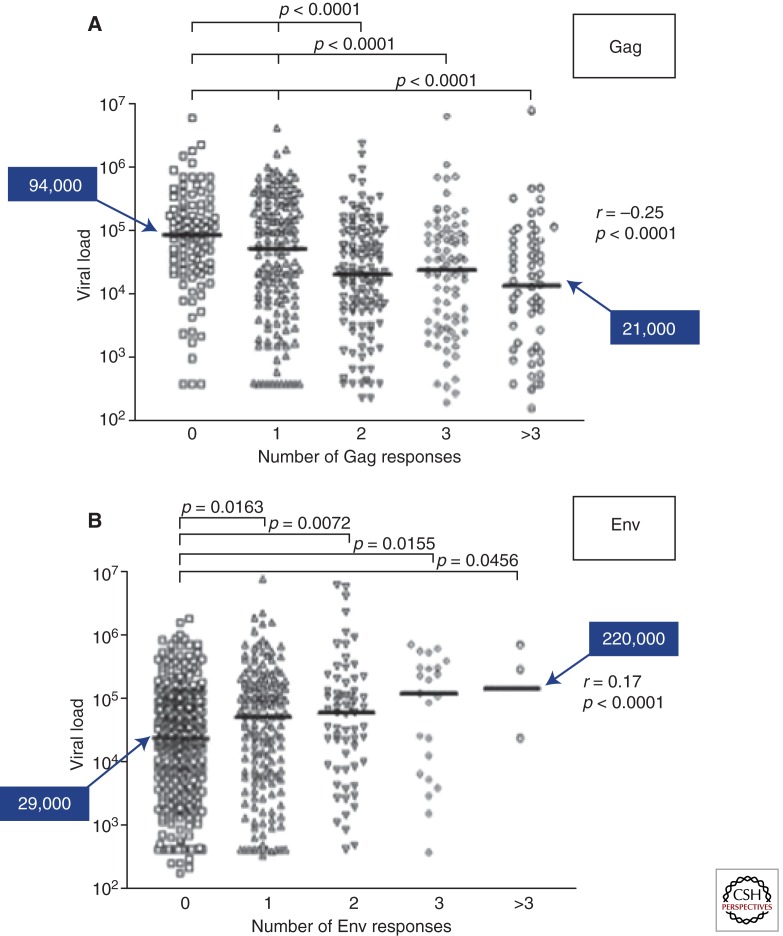
Figure 2.
Breadth of protein-specific CD8+ T-cell responses in relation to viral load. 578 persons infected with clade C virus were recruited, and comprehensive analysis of epitope targeting by PBMC was determined using a panel of overlapping peptides spanning all expressed viral proteins. Viral load of individuals in terms of the number of Gag (A)- and Env (B)-specific responses detected is shown, revealing that the broader the Gag-specific response, the lower the viral load, and the higher the Env-specific response, the higher the viral load, indicating that specificity impacts control. (Adapted from Kiepiela et al. 2007; reprinted, with permission, from Nature Publishers © 2007.)
Some additional complexities of CTL escape have been revealed by studies of an immunodominant epitope restricted by the protective allele HLA B*57. Population studies have shown this to be one of the earliest escape mutations to arise for HLA B57 is mutation with the dominant B57-restricted epitope TW10 in Gag (Brumme et al. 2008) that causes a clear decrease in replication capacity (Martinez-Picado et al. 2006). In elite controllers, HLA B57 can be associated with additional rare mutations at other sites within and surrounding the epitope that are even more fitness impairing (Miura et al. 2009b). In acute infection a large population study showed that viruses from individuals expressing protective HLA alleles are less fit than in persons lacking these alleles, and replication capacity in the acute stage of infection correlated with the HLA B-associated Gag polymorphisms (Goepfert et al. 2008; Brockman et al. 2010; Wright et al. 2011). However, these relationships were lost in chronic infection, but compensatory mutations that restore fitness were significantly increased. Together these data suggest that protective alleles may function in part through induction of fitness-impairing mutations. Indeed, in elite controllers, who maintain undetectable viral loads in the absence of therapy (Deeks and Walker 2007), viruses are significantly less fit (Miura et al. 2009a), and this is associated with specific HLA alleles, and more pronounced for Gag than for Env (Troyer et al. 2009). Studies of HIV controllers from the time of acute infection indicate that fitness impairing mutations are selected early or are transmitted from the donor (Miura et al. 2010), and early impairment of viral replication, including those caused by antiviral drug resistance in the donor virus, may impact long term control (Miura et al. 2010; Wright et al. 2011).
The impact of escape at a population level is becoming recognized. Transmitted escape mutations are associated with lower viral loads (Chopera et al. 2008; Goepfert et al. 2008; Crawford et al. 2009), and reversion of these mutations can be slow. Population studies show that the frequency of specific mutations increases with the expression of the selecting HLA allele in the population, and that in some geographic areas escape mutants have become the dominant circulating viruses (Kawashima et al. 2009). Because compensatory mutations can also be transmitted, the end result can be the deletion of an epitope with no associated impairment in viral fitness (Schneidewind et al. 2009). Thus, the host CD8+ T-cell response is helping to drive global HIV evolution, and the geographic differences in HIV clades relates at least in part to selection of mutations by locally prevalent HLA alleles.
IMMUNOGENETICS OF HIV-SPECIFIC T-CELL RESPONSES
Population studies have repeatedly shown dramatically different outcomes following HIV infection, with some persons succumbing to AIDS within less than a year of infection, and others surviving for more than three decades without the need for antiviral therapy, and with no apparent adverse effects (Deeks and Walker 2007). Early on, it was noted that there was a strong association between expression of certain class I alleles and disease outcome, but the mechanistic basis for this was unclear (Migueles et al. 2000). Indeed, whether this was a simple association or actually causally related could not be determined. Remarkably, both the positive and negative associations between viral load and HLA class I alleles were observed to be limited to HLA B alleles (Kiepiela et al. 2004), suggesting an active immune control mechanism. Although some associations with HLA C alleles were noted, these may be entirely explained by linkage disequilibrium (Kiepiela et al. 2004).
The first large scale attempt to define a genetic association with viral control was performed on a cohort of persons with acute HIV infection, in whom set point viral load data were available (Fellay et al. 2007). This study, and others that followed, revealed a SNP that is a proxy for HLA B57, as well as another that is associated with HLA C expression, to be the only significant associations with the level of viremia in the year following infection. This unbiased approach confirmed something that had already been recognized in cohort studies, namely that HLA B57 is in some way associated with better control, and also shed new light on HLA C, unique among class I alleles in that it is not down-regulated by the effects of Nef (Le Gall et al. 2000).
Recent population genetic studies comparing persons who progress rapidly to disease with controllers who maintain low viral loads in the absence of antiviral therapy have now shed additional light on these associations (Pereyra et al. 2010). At a viral load below 2000 copies the chances of disease progression drop dramatically, as does the likelihood of transmission (Quinn et al. 2000; Wawer et al. 2005). Through an international collaboration (the International HIV Controllers Study 2010) involving nearly 300 collaborators, a genome wide association study was performed on a cohort of nearly 974 HIV controllers, who maintain viral loads of less than 2000 copies/mL in the absence of therapy, and 2648 HIV progressors with viral loads in excess of 50,000 copies. Analysis of more than one million SNPs in each person revealed over 300 that were statistically associated with influencing viral load, and remarkably all of these lay within the HLA region of chromosome 6. However, after correction for multiple comparisons, only four SNPs remained significant, two of which were the same as those identified to be associated with lower set point viral load after acute infection (Fellay et al. 2007), as well as a SNP associated with psoriasis and with a noncoding SNP in the MICA gene.
The above findings were still unable to reconcile whether these were simply associations, and not causal, or whether this reflected an HLA-mediated impact on HIV control. Subsequent sequence analysis within the HLA region, and stepwise regression analysis, revealed that 5 amino acids within HLA B, all of which are involved in binding of viral peptide in the HLA binding groove (Fig. 3), along with a minor effect of a SNP associated with HLA C expression, explained the entire genetic signal being detected, and revealed the major genetic influence on HIV control (Pereyra et al. 2010). The specific amino acids at these positions reconcile both the protective and risk HLA alleles, and thus provide a parsimonious explanation for the long-recognized HLA associations with HIV control, namely that it is the nature of viral peptide presentation that is the major genetic determinant of viral control, likely caused by differences in the relative efficacy of CD8+ T-cell responses induced in the context of slightly altered T-cell-infected cell interactions. Indeed, the site with the strongest association with control is position 97 in HLA B, which sits at the base of the C pocket and depending on its allelic form, has the most impact on the nature of the binding groove and the way in which peptides are bound. Extension of these studies has now shown that the HLA C-associated SNP actually marks a polymorphism that affects microRNA binding, and thereby expression of HLA C (Kulkarni et al. 2011).
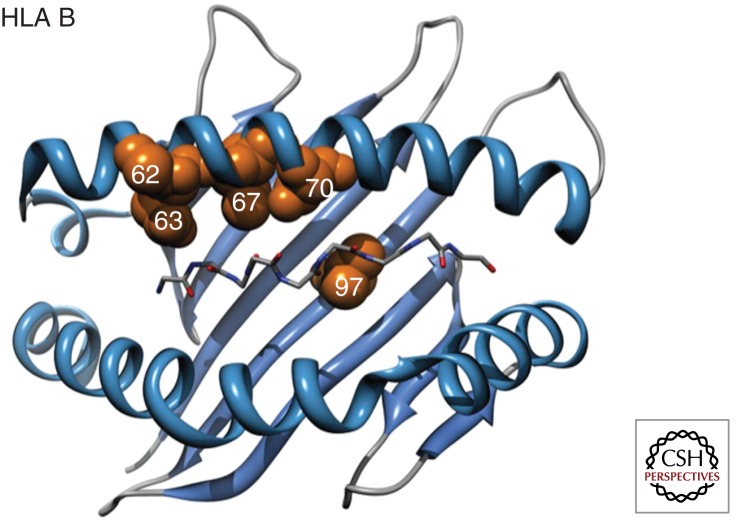
Figure 3.
Three-dimensional ribbon representation of the HLA B protein, highlighting amino acid positions 62, 63, 67, 70, and 97 lining the peptide binding pocket that are significantly associated with HIV control. The peptide backbone of the epitope is also displayed. (Adapted from the International HIV Controllers Study 2010; reprinted, with permission, from Science © 2010.)
The overall impact of CD8+ T-cell-mediated immune pressure on the virus is readily apparent from population analysis of expressed HLA types and sequencing of autologous viruses. Such selection pressure, first shown associated with mother to child transmission (Goulder et al. 2001) and revealed through examination of RT sequences and HLA alleles (Moore et al. 2002), has not only shown clear adaptation of HIV to host HLA genes, but also that some immunodominant epitopes are being eliminated over time (Kawashima et al. 2009). The extent to which CD4+ T-cell responses are driving HIV evolution appears to be negligible, although mutations within CD4+ T-cell epitopes have been documented in early infection (Rychert et al. 2007). And the impact of transmitted CD8+ T-cell-selected mutations on viral set point and disease progression, likely caused by fitness impairing mutations, is clear (Goepfert et al. 2008; Matthews et al. 2008), and consistent with defects in viral fitness as described above.
FUNCTION OF CD8+ T CELLS IN HIV INFECTION
Given the strong evidence that HIV-1-specific CD8+ T cells contribute to the control of HIV-1 in the acute and chronic stages of infection, it is important to know what T-cell functions are responsible for this. Antiviral CD8+ T cells were first identified as T cells that mediate lysis of virus-infected cells and are often referred to as cytotoxic T lymphocytes (Plata et al. 1975). Although most antigen-specific CD8+ T cells have this activity, they can use other effector mechanisms in addition. These include production of interferon-γ, IL-2, TNF-α, MIP-1α (renamed CCL3), MIP-1β (CCL4), and RANTES (CCL5) (Betts et al. 2006; Streeck et al. 2008). This list is probably not complete as revealed by transcriptional profiling, which also reveals subtle interplay between multiple pathways linking function, proliferation, and survival (Quigley et al. 2010). It is also clear that all CD8+ T cells do not display all functions at all times (Ferrari et al. 2011). Naive T cells are quiescent and require days of antigen stimulation to show these functions (Veiga-Fernandes et al. 2000; Peixoto et al. 2007), in contrast memory CD8+ T cells respond rapidly producing interferon-γ within a few hours (Lalvani et al. 1997). Production of lytic granules requires a bit longer but once activated, effector memory CD8+ T cells can release perforin and granzymes within minutes (Barber et al. 2003). The delay in activating lytic functions in memory T cells probably protects the body from autoimmune attack when the TCR encounters weakly binding self antigens, but has the disadvantage that it may take many hours or days before these T cells can be recruited into lytic antipathogen activity. This may be a disadvantage for vaccines that are unlikely to maintain effector CD8+ T cells in a fully primed state and therefore only kill infected cells after some hours.
The relative importance of lytic mechanisms versus cytokine or chemokine production in anti-HIV activity is much discussed. In very early HIV-1 infection CD8+ T cells rapidly select virus escape mutants, often selecting a new mutant to replace all of the previous virus population in a few days (Goonetilleke et al. 2009). It is easiest to explain this by effector CD8+ T cells killing HIV-infected target cells they recognize and thereby reducing their life span and capacity to generate new virus particles. However, new virus production could also be lowered by, for instance, β-chemokines (CCL3, 4, and 5) secreted by the CD8+ T cells reducing infection of new target cells by direct competition for binding to CCR5 or by reducing its expression on the target cells. However, this would not shorten the life of the infected cells, which is what the mathematical model, described by Goonetilleke et al. (2009), implied. These studies suggest that lysis of infected cells is the most important antiviral function of the CD8+ T cells in acute infection. This is consistent with the finding that the T cells that select early virus escape mutants are high perforin expressors (Hersperger et al. 2010) whereas these early T cells are rather limited in their expression of cytokines and chemokines (Ferrari et al. 2011). This importance of cytolysis in HIV-1 infection contrasts with some other virus infections such as hepatitis B virus infection where, in animal models, perforin-deficient T cells are highly effective because the main antiviral activity is mediated nonlytically by interferon-γ (Yang et al. 2010). And it is important to note that there are data from in vivo analysis of SIV-infected macaques indicating that depletion of CD8+ T cells 2 and 6 months after infection does not result in a measureable change in the lifespan of infected cells, suggesting that direct killing may not be not be the main mechanism of control, at least in this model.
During chronic infection, once viral set point is established, the other functions of CD8+ T cells may become more important, although lytic potential may still be essential (Betts and Harari 2008). In patients who control virus well, the T cells are more quiescent than in acute infection. Many studies have shown that T cells in those who control HIV-1 well are polyfunctional, showing not only cytolytic potential but also have the capacity to produce cytokines and chemokines (Betts and Harari 2008), although it is not clear whether this is cause or effect. Prolonged antigen stimulation in the absence of excessive activation and exhaustion, as occurs in slow progressors, could favor expression of multiple functions. Production of IL-2 may be important in the long term persistence of CD8+ T cells and can be provided by the CD8+ T cell itself or by CD4+ T cells, which survive much better in those whose disease progresses slowly (Rosenberg et al. 1997; Zimmerli et al. 2005). Similar observations have been made in HIV-2 infection in which elite controllers are relatively common (Duvall et al. 2008). These findings are entirely consistent with data in CD4+ T-cell-depleted mice that show the importance of IL-2 in the maintenance of long-term CD8+ T-cell memory (Williams et al. 2006).
THE INTERSECTION OF VIRAL FITNESS AND IMMUNE CONTROL
Although most of the focus on immune control and lack of control has been on CD8+ T-cell function and differential induction of negative immunoregulatory molecules, an increasing body of data suggests that immune-mediated mutations within CD8+ T-cell epitopes lead to reduced viral fitness. These data include assays in which replication of virus containing a B57-selected mutation is out-competed by wild-type virus (Martinez-Picado et al. 2006), evidence of reduced viral fitness in Gag-PR in persons who control virus spontaneously (Miura et al. 2009a), and evidence of compensatory mutations leading to restoration of fitness (Schneidewind et al. 2007, 2009). Importantly, mutations in Env do not lead to reduced fitness, suggesting that structural constraints are likely key to this effect (Troyer et al. 2009). More recent studies have shed further light on this, by demonstrating that there are multidimensional constraints on HIV evolution because certain combinations of mutations must occur in a coordinated manner to maintain virus viability, and thus constrain immune escape pathways (Dahirel et al. 2011). Persons who spontaneously control HIV without medications preferentially target sites that are most constrained, providing further evidence that the specific sites targeted by the immune system may have a major impact on overall control (Dahirel et al. 2011).
FUTURE DIRECTIONS: TOWARD A UNIFIED EXPLANATION FOR HIV PATHOGENESIS
A unified view of HIV pathogenesis is emerging, but there remain many unknowns, and a better understanding will undoubtedly require an integrated analysis of innate and adaptive immune responses, host genetics, and viral genetics. Although both viral load and CD4+ cell count predict disease progression, we remain convinced that the adaptive immunologic response is also predictive of the subsequent course. Clear signals are there: CD8+ T cells are associated with initial control, depletion of these cells in animal models of AIDS leads to an increase in viremia, virus is evolving to escape detection by CTLs, specific functions mediated by CD8+ T cells have demonstrable antiviral effects in vivo, the effect of HLA far outweighs any other genetic factors, CD8+ and CD4+ T-cell dysfunction is associated with lack of viral control, and immune-induced mutations reduce viral fitness and likely contribute to the antiviral efficacy of the CD8+ T-cell response. But important questions remain, and in particular questions regarding the actual functional profile of CD8+ T cells that might lead to long-term control of HIV, or prevention of disseminated infection. And the extent to which such data will be important to vaccine design remains unclear—although animal models suggest that CD8+ T cells may be able to prevent progressive systemic dissemination of infection (Hansen et al. 2009) and even reduce the level of virus to barely detectable levels (Hansen et al. 2011).
Footnotes
Editors: Frederic D. Bushman, Gary J. Nabel, and Ronald Swanstrom
Additional Perspectives on HIV available at www.perspectivesinmedicine.org
REFERENCES
*Reference is also in this collection.
- Addo MM, Yu XG, Rathod A, Cohen D, Eldridge RL, Strick D, Johnston MN, Corcoran C, Wurcel AG, Fitzpatrick CA, et al. 2003. Comprehensive epitope analysis of human immunodeficiency virus type 1 (HIV-1)-specific T-cell responses directed against the entire expressed HIV-1 genome demonstrate broadly directed responses, but no correlation to viral load. J Virol 77: 2081–2092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen TM, Altfeld M, Yu XG, O’Sullivan KM, Lichterfeld M, Le Gall S, John M, Mothe BR, Lee PK, Kalife ET, et al. 2004. Selection, transmission, and reversion of an antigen-processing cytotoxic T-lymphocyte escape mutation in human immunodeficiency virus type 1 infection. J Virol 78: 7069–7078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altfeld M, Rosenberg ES, Shankarappa R, Mukherjee JS, Hecht FM, Eldridge RL, Addo MM, Poon SH, Phillips MN, Robbins GK, et al. 2001. Cellular immune responses and viral diversity in individuals treated during acute and early HIV-1 infection. J Exp Med 193: 169–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altfeld M, van Lunzen J, Frahm N, Yu XG, Schneider C, Eldridge RL, Feeney ME, Meyer-Olson D, Stellbrink HJ, Walker BD 2002. Expansion of pre-existing, lymph node-localized CD8+ T cells during supervised treatment interruptions in chronic HIV-1 infection. J Clin Invest 109: 837–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altfeld M, Addo MM, Rosenberg ES, Hecht FM, Lee PK, Vogel M, Yu XG, Draenert R, Johnston MN, Strick D, et al. 2003a. Influence of HLA-B57 on clinical presentation and viral control during acute HIV-1 infection. AIDS 17: 2581–2591 [DOI] [PubMed] [Google Scholar]
- Altfeld M, Addo MM, Shankarappa R, Lee PK, Allen TM, Yu XG, Rathod A, Harlow J, O’Sullivan K, Johnston MN, et al. 2003b. Enhanced detection of human immunodeficiency virus type 1-specific T-cell responses to highly variable regions by using peptides based on autologous virus sequences. J Virol 77: 7330–7340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altfeld M, Kalife ET, Qi Y, Streeck H, Lichterfeld M, Johnston MN, Burgett N, Swartz ME, Yang A, Alter G, et al. 2006. HLA alleles associated with delayed progression to AIDS contribute strongly to the initial CD8+ T cell response against HIV-1. PLoS Med 3: e403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman JD, Moss PA, Goulder PJ, Barouch DH, McHeyzer-Williams MG, Bell JI, McMichael AJ, Davis MM 1996. Phenotypic analysis of antigen-specific T lymphocytes. Science 274: 94–96 [DOI] [PubMed] [Google Scholar]
- Amyes E, Hatton C, Montamat-Sicotte D, Gudgeon N, Rickinson AB, McMichael AJ, Callan MF 2003. Characterization of the CD4+ T cell response to Epstein-Barr virus during primary and persistent infection. J Exp Med 198: 903–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal A, Gough E, Sabbaj S, Ritter D, Yusim K, Sfakianos G, Aldrovandi G, Kaslow RA, Wilson CM, et al. 2005. CD8 T-cell responses in early HIV-1 infection are skewed towards high entropy peptides. AIDS 19: 241–250 [PubMed] [Google Scholar]
- Bansal A, Carlson J, Yan J, Akinsiku OT, Schaefer M, Sabbaj S, Bet A, Levy DN, Heath S, Tang J, et al. 2010. CD8 T cell response and evolutionary pressure to HIV-1 cryptic epitopes derived from antisense transcription. J Exp Med 207: 51–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber DL, Wherry EJ, Ahmed R 2003. Cutting edge: Rapid in vivo killing by memory CD8 T cells. J Immunol 171: 27–31 [DOI] [PubMed] [Google Scholar]
- Berger CT, Carlson JM, Brumme CJ, Hartman KL, Brumme ZL, Henry LM, Rosato PC, Piechocka-Trocha A, Brockman MA, Harrigan PR, et al. 2010. Viral adaptation to immune selection pressure by HLA class I-restricted CTL responses targeting epitopes in HIV frameshift sequences. J Exp Med 207: 61–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betts MR, Ambrozak DR, Douek DC, Bonhoeffer S, Brenchley JM, Casazza JP, Koup RA, Picker LJ 2001. Analysis of total human immunodeficiency virus (HIV)-specific CD4+ and CD8+ T-cell responses: Relationship to viral load in untreated HIV infection. J Virol 75: 11983–11991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, Lederman MM, Benito JM, Goepfert PA, Connors M, et al. 2006. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood 107: 4781–4789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betts MR, Harari A 2008. Phenotype and function of protective T cell immune responses in HIV. Curr Opin HIV AIDS 3: 349–355 [DOI] [PubMed] [Google Scholar]
- Borrow P, Lewicki H, Hahn BH, Shaw GM, Oldstone MB 1994. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J Virol 68: 6103–6110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockman MA, Brumme ZL, Brumme CJ, Miura T, Sela J, Rosato PC, Kadie CM, Carlson JM, Markle TJ, Streeck H, et al. 2010. Early selection in Gag by protective HLA alleles contributes to reduced HIV-1 replication capacity that may be largely compensated in chronic infection. J Virol 84: 11937–11949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumme ZL, Brumme CJ, Carlson J, Streeck H, John M, Eichbaum Q, Block BL, Baker B, Kadie C, Markowitz M, et al. 2008. Marked epitope- and allele-specific differences in rates of mutation in human immunodeficiency type 1 (HIV-1) Gag, Pol, and Nef cytotoxic T-lymphocyte epitopes in acute/early HIV-1 infection. J Virol 82: 9216–9227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumme ZL, John M, Carlson JM, Brumme CJ, Chan D, Brockman MA, Swenson LC, Tao I, Szeto S, Rosato P, et al. 2009. HLA-associated immune escape pathways in HIV-1 subtype B Gag, Pol and Nef proteins. PLoS One 4: e6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier MF, Jülg B, Pyo A, Flanders M, Ranasinghe S, Soghoian DZ, Kwon DS, Rychert J, Lian J, Muller MI, et al. 2011. HIV-1-specific interleukin-21+ CD4+ T cell responses contribute to durable viral control through the modulation of HIV-specific CD8+ T cell function. J Virol 85: 733–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopera DR, Woodman Z, Mlisana K, Mlotshwa M, Martin DP, Seoighe C, Treurnicht F, de Rosa DA, Hide W, Karim SA, et al. 2008. Transmission of HIV-1 CTL escape variants provides HLA-mismatched recipients with a survival advantage. PLoS Pathog 4: e1000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford H, Lumm W, Leslie A, Schaefer M, Boeras D, Prado JG, Tang J, Farmer P, Ndung’u T, Lakhi S, et al. 2009. Evolution of HLA-B*5703 HIV-1 escape mutations in HLA-B*5703-positive individuals and their transmission recipients. J Exp Med 206: 909–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahirel V, Shekhar K, Pereyra F, Miura T, Artyomov M, Talsania S, Allen TM, Altfeld M, Carrington M, Irvine DJ, et al. 2011. Coordinate linkage of HIV evolution reveals regions of immunologic vulnerability. Proc Natl Acad Sci 108: 11530–11535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalod M, Dupuis M, Deschemin JC, Goujard C, Deveau C, Meyer L, Ngo N, Rouzioux C, Guillet JG, Delfraissy JF, et al. 1999. Weak anti-HIV CD8+ T-cell effector activity in HIV primary infection. J Clin Invest 104: 1431–1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, et al. 2006. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 443: 350–354 [DOI] [PubMed] [Google Scholar]
- Deeks SG, Walker BD 2007. Human immunodeficiency virus controllers: Mechanisms of durable virus control in the absence of antiretroviral therapy. Immunity 27: 406–416 [DOI] [PubMed] [Google Scholar]
- Douek DC, Brenchley JM, Betts MR, Ambrozak DR, Hill BJ, Okamoto Y, Casazza JP, Kuruppu J, Kunstman K, Wolinsky S, et al. 2002. HIV preferentially infects HIV-specific CD4+ T cells. Nature 417: 95–98 [DOI] [PubMed] [Google Scholar]
- Douek DC, Roederer M, Koup RA 2009. Emerging concepts in the immunopathogenesis of AIDS. Annu Rev Med 60: 471–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draenert R, Le Gall S, Pfafferott KJ, Leslie AJ, Chetty P, Brander C, Holmes EC, Chang SC, Feeney ME, Addo MM, et al. 2004. Immune selection for altered antigen processing leads to cytotoxic T lymphocyte escape in chronic HIV-1 infection. J Exp Med 199: 905–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draenert R, Allen TM, Liu Y, Wrin T, Chappey C, Verrill CL, Sirera G, Eldridge RL, Lahaie MP, Ruiz L, et al. 2006. Constraints on HIV-1 evolution and immunodominance revealed in monozygotic adult twins infected with the same virus. J Exp Med 203: 529–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvall MG, Jaye A, Dong T, Brenchley JM, Alabi AS, Jeffries DJ, van der Sande M, Togun TO, McConkey SJ, Douek DC, et al. 2006. Maintenance of HIV-specific CD4+ T cell help distinguishes HIV-2 from HIV-1 infection. J Immunol 176: 6973–6981 [DOI] [PubMed] [Google Scholar]
- Duvall MG, Precopio ML, Ambrozak DA, Jaye A, McMichael AJ, Whittle HC, Roederer M, Rowland-Jones SL, Koup RA 2008. Polyfunctional T cell responses are a hallmark of HIV-2 infection. Eur J Immunol 38: 350–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards BH, Bansal A, Sabbaj S, Bakari J, Mulligan MJ, Goepfert PA 2002. Magnitude of functional CD8+ T-cell responses to the gag protein of human immunodeficiency virus type 1 correlates inversely with viral load in plasma. J Virol 76: 2298–2305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellay J, Frahm N, Shianna KV, Cirulli ET, Casimiro DR, Robertson MN, Haynes BF, Geraghty DE, McElrath MJ, Goldstein DB, et al. 2007. A whole-genome association study of major determinants for host control of HIV-1. Science 317: 944–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari G, Korber B, Goonetilleke N, Liu MK, Turnbull EL, Salazar-Gonzalez JF, Hawkins N, Self S, Watson S, Betts MR, et al. 2011. Relationship between functional profile of HIV-1 specific CD8 T cells and epitope variability with the selection of escape mutants in acute HIV-1 infection. PLoS Pathog 7: e1001273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiebig EW, Wright DJ, Rawal BD, Garrett PE, Schumacher RT, Peddada L, Heldebrant C, Smith R, Conrad A, Kleinman SH, et al. 2003. Dynamics of HIV viremia and antibody seroconversion in plasma donors: Implications for diagnosis and staging of primary HIV infection. AIDS 17: 1871–1879 [DOI] [PubMed] [Google Scholar]
- Fischer W, Ganusov VV, Giorgi EE, Hraber PT, Keele BF, Leitner T, Han CS, Gleasner CD, Green L, Lo CC, et al. 2010. Transmission of single HIV-1 genomes and dynamics of early immune escape revealed by ultra-deep sequencing. PLoS One 5: e12303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Nelson GW, Karacki P, Martin MP, Phair J, Kaslow R, Goedert JJ, Buchbinder S, Hoots K, Vlahov D, et al. 2001. Effect of a single amino acid change in MHC class I molecules on the rate of progression to AIDS. N Engl J Med 344: 1668–1675 [DOI] [PubMed] [Google Scholar]
- Goepfert PA, Lumm W, Farmer P, Matthews P, Prendergast A, Carlson JM, Derdeyn CA, Tang J, Kaslow RA, Bansal A, et al. 2008. Transmission of HIV-1 Gag immune escape mutations is associated with reduced viral load in linked recipients. J Exp Med 205: 1009–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh WC, Markee J, Akridge RE, Meldorf M, Musey L, Karchmer T, Krone M, Collier A, Corey L, Emerman M, et al. 1999. Protection against human immunodeficiency virus type 1 infection in persons with repeated exposure: Evidence for T cell immunity in the absence of inherited CCR5 coreceptor defects. J Infect Dis 179: 548–557 [DOI] [PubMed] [Google Scholar]
- Goonetilleke N, Moore S, Dally L, Winstone N, Cebere I, Mahmoud A, Pinheiro S, Gillespie G, Brown D, Loach V, et al. 2006. Induction of multifunctional human immunodeficiency virus type 1 (HIV-1)-specific T cells capable of proliferation in healthy subjects by using a prime-boost regimen of DNA- and modified vaccinia virus Ankara-vectored vaccines expressing HIV-1 Gag coupled to CD8+ T-cell epitopes. J Virol 80: 4717–4728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goonetilleke N, Liu MK, Salazar-Gonzalez JF, Ferrari G, Giorgi E, Ganusov VV, Keele BF, Learn GH, Turnbull EL, Salazar MG, et al. 2009. The first T cell response to transmitted/founder virus contributes to the control of acute viremia in HIV-1 infection. J Exp Med 206: 1253–1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulder PJ, Brander C, Tang Y, Tremblay C, Colbert RA, Addo MM, Rosenberg ES, Nguyen T, Allen R, Trocha A, et al. 2001. Evolution and transmission of stable CTL escape mutations in HIV infection. Nature 412: 334–338 [DOI] [PubMed] [Google Scholar]
- Graham BS, Koup RA, Roederer M, Bailer RT, Enama ME, Moodie Z, Martin JE, McCluskey MM, Chakrabarti BK, Lamoreaux L, et al. 2006. Phase 1 safety and immunogenicity evaluation of a multiclade HIV-1 DNA candidate vaccine. J Infect Dis 194: 1650–1660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray CM, Mlotshwa M, Riou C, Mathebula T, de Assis Rosa D, Mashishi T, Seoighe C, Ngandu N, van Loggerenberg F, Morris L, Mlisana K, et al. 2009. Human immunodeficiency virus-specific γ interferon enzyme-linked immunospot assay responses targeting specific regions of the proteome during primary subtype C infection are poor predictors of the course of viremia and set point. J Virol. 83: 470–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen SG, Vieville C, Whizin N, Coyne-Johnson L, Siess DC, Drummond DD, Legasse AW, Axthelm MK, Oswald K, Trubey CM, et al. 2009. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nat Med 15: 293–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen SG, Ford JC, Lewis MS, Ventura AB, Hughes CM, Coyne-Johnson L, Whizin N, Oswald K, Shoemaker R, Swanson T, et al. 2011. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature 473: 523–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harari A, Bart PA, Stohr W, Tapia G, Garcia M, Medjitna-Rais E, Burnet S, Cellerai C, Erlwein O, Barber T, et al. 2008. An HIV-1 clade C DNA prime, NYVAC boost vaccine regimen induces reliable, polyfunctional, and long-lasting T cell responses. J Exp Med 205: 63–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersperger AR, Pereyra F, Nason M, Demers K, Sheth P, Shin LY, Kovacs CM, Rodriguez B, Sieg SF, Teixeira-Johnson L, et al. 2010. Perforin expression directly ex vivo by HIV-specific CD8+ T-cells is a correlate of HIV elite control. PLoS Pathog 6: e1000917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho DD, Sarngadharan MG, Resnick L, Dimarzoveronese F, Rota TR, Hirsch MS 1985. Primary human T-lymphotropic virus type III infection. Ann Intern Med 103: 880–883 [DOI] [PubMed] [Google Scholar]
- Huang S, Dunkley-Thompson J, Tang Y, Macklin EA, Steel-Duncan J, Singh-Minott I, Ryland EG, Smikle M, Walker BD, Christie CD, et al. 2008. Deficiency of HIV-Gag-specific T cells in early childhood correlates with poor viral containment. J Immunol 181: 8103–8111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- International HIV Controllers Study, Pereyra F, Jia X, McLaren PJ, Telenti A, de Bakker PI, Walker BD, Ripke S, Brumme CJ, Pulit SL, Carrington M, et al. 2010. The major genetic determinants of HIV-1 control affect HLA class I peptide presentation. Science 330: 1551–1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen EM, Lemmens EE, Wolfe T, Christen U, von Herrath MG, Schoenberger SP 2003. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature 421: 852–856 [DOI] [PubMed] [Google Scholar]
- Jin X, Bauer DE, Tuttleton SE, Lewin S, Gettie A, Blanchard J, Irwin CE, Safrit JT, Mittler J, Weinberger L, et al. 1999. Dramatic rise in plasma viremia after CD8+ T cell depletion in simian immunodeficiency virus-infected macaques. J Exp Med 189: 991–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julg B, Moodley ES, Qi Y, Ramduth D, Reddy S, Mncube Z, Gao X, Goulder PJ, Detels R, Ndung'u T, et al. 2011. Possession of HLA Class II DRB1*1303 associates with reduced viral loads in chronic HIV-1 clade C and B infection. J Infect Dis 203: 803–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalams SA, Buchbinder SP, Rosenberg ES, Billingsley JM, Colbert DS, Jones NG, Shea AK, Trocha AK, Walker BD 1999. Association between virus-specific cytotoxic T-lymphocyte and helper responses in human immunodeficiency virus type 1 infection. J Virol 73: 6715–6720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamimura D Bevan MJ 2007. Naive CD8+ T cells differentiate into protective memory-like cells after IL-2 anti IL-2 complex treatment in vivo. J Exp Med 204: 1803–1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaslow RA, Carrington M, Apple R, Park L, Munoz A, Saah AJ, Goedert JJ, Winkler C, O'Brien SJ, Rinaldo C, et al. 1996. Influence of combinations of human major histocompatibility complex genes on the course of HIV-1 infection. Nat Med 2: 405–411 [DOI] [PubMed] [Google Scholar]
- Kaufmann DE, Bailey PM, Sidney J, Wagner B, Norris PJ, Johnston MN, Cosimi LA, Addo MM, Lichterfeld M, Altfeld M, et al. 2004. Comprehensive analysis of human immunodeficiency virus type 1-specific CD4 responses reveals marked immunodominance of gag and nef and the presence of broadly recognized peptides. J Virol 78: 4463–4477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima Y, Pfafferott K, Frater J, Matthews P, Payne R, Addo M, Gatanaga H, Fujiwara M, Hachiya A, Koizumi H, et al. 2009. Adaptation of HIV-1 to human leukocyte antigen class I. Nature 458: 641–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiepiela P, Leslie AJ, Honeyborne I, Ramduth D, Thobakgale C, Chetty S, Rathnavalu P, Moore C, Pfafferott KJ, Hilton L, et al. 2004. Dominant influence of HLA-B in mediating the potential co-evolution of HIV and HLA. Nature 432: 769–775 [DOI] [PubMed] [Google Scholar]
- Kiepiela P, Ngumbela K, Thobakgale C, Ramduth D, Honeyborne I, Moodley E, Reddy S, de Pierres C, Mncube Z, Mkhwanazi N, et al. 2007. CD8+ T-cell responses to different HIV proteins have discordant associations with viral load. Nat Med 13: 46–53 [DOI] [PubMed] [Google Scholar]
- Klatt NR, Shudo E, Ortiz AM, Engram JC, Paiardini M, Lawson B, Miller MD, Else J, Pandrea I, Estes JD, et al. 2010. CD8+ Lymphocytes control viral replication in SIVmac239-infected rhesus macaques without decreasing the lifespan of productively infected cells. PLoS Pathog 6: e1000747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koibuchi T, Allen TM, Lichterfeld M, Mui SK, O'Sullivan KM, Trocha A, Kalams SA, Johnson RP, Walker BD 2005. Limited sequence evolution within persistently targeted CD8 epitopes in chronic human immunodeficiency virus type 1 infection. J Virol 79: 8171–8181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosmrlj A, Read EL, Qi Y, Allen TM, Altfeld M, Deeks SG, Pereyra F, Carrington M, Walker BD, Chakraborty AK 2010. Effects of thymic selection of the T-cell repertoire on HLA class I-associated control of HIV infection. Nature 465: 350–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koup RA, Safrit JT, Cao Y, Andrews CA, McLeod G, Borkowsky W, Farthing C, Ho DD 1994. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol 68: 4650–4655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni S, Savan R, Qi Y, Gao X, Yuki Y, Bass SE, Martin MP, Hunt P, Deeks SG, Telenti A, et al. 2011. Differential microRNA regulation of HLA-C expression and its association with HIV control. Nature 472: 495–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacap PA, Huntington JD, Luo M, Nagelkerke NJ, Bielawny T, Kimani J, Wachihi C, Ngugi EN, Plummer FA 2008. Associations of human leukocyte antigen DRB with resistance or susceptibility to HIV-1 infection in the Pumwani Sex Worker Cohort. AIDS 22: 1029–1038 [DOI] [PubMed] [Google Scholar]
- Lalvani A, Brookes R, Hambleton S, Britton WJ, Hill AV, McMichael AJ 1997. Rapid effector function in CD8+ memory T cells. J Exp Med 186: 859–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane HC, Depper JM, Greene WC, Whalen G, Waldmann TA, Fauci AS 1985. Qualitative analysis of immune function in patients with the acquired immunodeficiency syndrome. Evidence for a selective defect in soluble antigen recognition. N Engl J Med 313: 79–84 [DOI] [PubMed] [Google Scholar]
- Lazaro E, Godfrey SB, Stamegna P, Ogbechie T, Kerrigan C, Zhang M, Walker BD, Le Gall S 2009. Differential HIV epitope processing in monocytes and CD4 T cells affects cytotoxic T lymphocyte recognition. J Infect Dis 200: 236–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Gall S, Buseyne F, Trocha A, Walker BD, Heard JM, Schwartz O 2000. Distinct trafficking pathways mediate Nef-induced and clathrin-dependent major histocompatibility complex class I down-regulation. J Virol 74: 9256–9266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Gall S, Stamegna P, Walker BD 2007. Portable flanking sequences modulate CTL epitope processing. J Clin Invest 117: 3563–3575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichterfeld M, Yu XG, Cohen D, Addo MM, Malenfant J, Perkins B, Pae E, Johnston MN, Strick D, Allen TM, et al. 2004. HIV-1 Nef is preferentially recognized by CD8 T cells in primary HIV-1 infection despite a relatively high degree of genetic diversity. AIDS 18: 1383–1392 [DOI] [PubMed] [Google Scholar]
- Lichterfeld M, Yu XG, Mui SK, Williams KL, Trocha A, Brockman MA, Allgaier RL, Waring MT, Koibuchi T, Johnston MN, et al. 2007. Selective depletion of high-avidity human immunodeficiency virus type 1 (HIV-1)-specific CD8+ T cells after early HIV-1 infection. J Virol 81: 4199–4214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyles RH, Munoz A, Yamashita TE, Bazmi H, Detels R, Rinaldo CR, Margolick JB, Phair JP, Mellors JW 2000. Natural history of human immunodeficiency virus type 1 viremia after seroconversion and proximal to AIDS in a large cohort of homosexual men. Multicenter AIDS Cohort Study. J Infect Dis 181: 872–880 [DOI] [PubMed] [Google Scholar]
- Maecker HT, Dunn HS, Suni MA, Khatamzas E, Pitcher CJ, Bunde T, Persaud N, Trigona W, Fu TM, Sinclair E, et al. 2001. Use of overlapping peptide mixtures as antigens for cytokine flow cytometry. J Immunol Methods 255: 27–40 [DOI] [PubMed] [Google Scholar]
- Maness NJ, Valentine LE, et al. 2007. AIDS virus specific CD8+ T lymphocytes against an immunodominant cryptic epitope select for viral escape. J Exp Med 204: 2505–2512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maness NJ, Sacha JB, Piaskowski SM, Weisgrau KL, Rakasz EG, May GE, Buechler MB, Walsh AD, Wilson NA, Watkins DI 2009. Novel translation products from the SIVmac239 Env-encoding mRNA contain both Rev and cryptic T cell epitopes. J Virol 83: 10280–10285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Picado J, Prado JG, Fry EE, Pfafferott K, Leslie A, Chetty S, Thobakgale C, Honeyborne I, Crawford H, Matthews P, et al. 2006. Fitness cost of escape mutations in p24 Gag in association with control of human immunodeficiency virus type 1. J Virol 80: 3617–3623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattapallil JJ, Douek DC, Hill B, Nishimura Y, Martin M, Roederer M 2005. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature 434: 1093–1097 [DOI] [PubMed] [Google Scholar]
- Matthews PC, Prendergast A, Leslie A, Crawford H, Payne R, Rousseau C, Rolland M, Honeyborne I, Carlson J, Kadie C, et al. 2008. Central role of reverting mutations in HLA associations with human immunodeficiency virus set point. J Virol 82: 8548–8559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMichael AJ, Borrow P, Tomaras GD, Goonetilleke N, Haynes BF 2010. The immune response during acute HIV-1 infection: Clues for vaccine development. Nat Rev Immunol 10: 11–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellors JW, Rinaldo CR Jr, Gupta P, White RM, Todd JA, Kingsley LA 1996. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science 272: 1167–1170 [DOI] [PubMed] [Google Scholar]
- Migueles SA, Sabbaghian MS, Shupert WL, Bettinotti MP, Marincola FM, Martino L, Hallahan CW, Selig SM, Schwartz D, Sullivan J, et al. 2000. HLA B*5701 is highly associated with restriction of virus replication in a subgroup of HIV-infected long term nonprogressors. Proc Natl Acad Sci 97: 2709–2714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura T, Brockman MA, Brumme ZL, Brumme CJ, Pereyra F, Trocha A, Block BL, Schneidewind A, Allen TM, Heckerman D, et al. 2009a. HLA-associated alterations in replication capacity of chimeric NL4-3 viruses carrying gag-protease from elite controllers of human immunodeficiency virus type 1. J Virol 83: 140–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura T, Brockman MA, Schneidewind A, Lobritz M, Pereyra F, Rathod A, Block BL, Brumme ZL, Brumme CJ, Baker B, et al. 2009b. HLA-B57/B*5801 human immunodeficiency virus type 1 elite controllers select for rare gag variants associated with reduced viral replication capacity and strong cytotoxic T-lymphocyte [corrected] recognition. J Virol 83: 2743–2755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura T, Brumme ZL, Brockman MA, Rosato P, Sela J, Brumme CJ, Pereyra F, Kaufmann DE, Trocha A, Block BL, Daar ES, et al. 2010. Impaired replication capacity of acute/early viruses in persons who become HIV controllers. J Virol 84: 7581–7591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CB, John M, James IR, Christiansen FT, Witt CS, Mallal SA 2002. Evidence of HIV-1 adaptation to HLA-restricted immune responses at a population level. Science 296: 1439–1443 [DOI] [PubMed] [Google Scholar]
- Murray HW, Rubin BY, Masur H, Roberts RB 1984. Impaired production of lymphokines and immune (γ) interferon in the acquired immunodeficiency syndrome. N Engl J Med 310: 883–889 [DOI] [PubMed] [Google Scholar]
- Ngumbela KC, Day CL, Mncube Z, Nair K, Ramduth D, Thobakgale C, Moodley E, Reddy S, de Pierres C, Mkhwanazi N, et al. 2008. Targeting of a CD8 T cell env epitope presented by HLA-B*5802 is associated with markers of HIV disease progression and lack of selection pressure. AIDS Res Hum Retroviruses 24: 72–82 [DOI] [PubMed] [Google Scholar]
- Nixon DF, Townsend AR, Elvin JG, Rizza CR, Gallwey J, McMichael AJ 1988. HIV-1 gag-specific cytotoxic T lymphocytes defined with recombinant vaccinia virus and synthetic peptides. Nature 336: 484–487 [DOI] [PubMed] [Google Scholar]
- Papagno L, Appay V, Sutton J, Rostron T, Gillespie GM, Ogg GS, King A, Makadzanhge AT, Waters A, Balotta C et al. 2002. Comparison between HIV- and CMV-specific T cell responses in long-term HIV infected donors. Clin Exp Immunol 130: 509–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peixoto A, Evaristo C, Munitic I, Monteiro M, Charbit A, Rocha B, Veiga-Fernandes H 2007. CD8 single-cell gene coexpression reveals three different effector types present at distinct phases of the immune response. J Exp Med 204: 1193–1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereyra F, Addo MM, Kaufmann DE, Liu Y, Miura T, Rathod A, Baker B, Trocha A, Rosenberg R, Mackey E, Ueda P, Lu Z, et al. 2008. Genetic and immunologic heterogeneity among persons who control HIV infection in the absence of therapy. J Infect Dis 197: 563–571 [DOI] [PubMed] [Google Scholar]
- Petrovas C, Casazza JP, Brenchley JM, Price DA, Gostick E, Adams WC, Precopio ML, Schacker T, Roederer M, Douek DC, et al. 2006. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J Exp Med 203: 2281–2292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips RE, Rowland-Jones S, Nixon DF, Gotch FM, Edwards JP, Ogunlesi AO, Elvin JG, Rothbard JA, Bangham CR, Rizza CR 1991. Human immunodeficiency virus genetic variation that can escape cytotoxic T cell recognition. Nature 354: 453–459 [DOI] [PubMed] [Google Scholar]
- Pitcher CJ, Quittner C, Peterson DM, Connors M, Koup RA, Maino VC, Picker LJ 1999. HIV-1-specific CD4+ T cells are detectable in most individuals with active HIV-1 infection, but decline with prolonged viral suppression. Nat Med 5: 518–525 [DOI] [PubMed] [Google Scholar]
- Plata F, Cerottini JC, et al. 1975. Primary and secondary in vitro generation of cytolytic T lymphocytes in the murine sarcoma virus system. Eur J Immunol 5: 227–233 [DOI] [PubMed] [Google Scholar]
- Plata F, Autran B, Martins LP, Wain-Hobson S, Raphaël M, Mayaud C, Denis M, Guillon JM, Debré P 1987. AIDS virus-specific cytotoxic T lymphocytes in lung disorders. Nature 328: 348–351 [DOI] [PubMed] [Google Scholar]
- Quigley M, Pereyra F, Nilsson B, Porichis F, Fonseca C, Eichbaum Q, Julg B, Jesneck JL, Brosnahan K, Imam S, et al. 2010. Transcriptional analysis of HIV-specific CD8+ T cells shows that PD-1 inhibits T cell function by upregulating BATF. Nat Med 16: 1147–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn TC, Wawer MJ, et al. 2000. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med 342: 921–929 [DOI] [PubMed] [Google Scholar]
- Radebe M, Nair K, Chonco F, Bishop K, Wright JK, van der Stok M, Bassett IV, Mncube Z, Altfeld M, Walker BD, Ndung'u T 2011. Limited immunogenicity of HIV CD8+ T-cell epitopes in acute clade C virus Infection. J Infect Dis 204: 768–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramduth D, Chetty P, et al. 2005. Differential immunogenicity of HIV-1 clade C proteins in eliciting CD8+ and CD4+ cell responses. J Infect Dis 192: 1588–1596 [DOI] [PubMed] [Google Scholar]
- Richardson JS 2000. Early ribbon drawings of proteins. Nat Struct Biol 7: 624–625 [DOI] [PubMed] [Google Scholar]
- Ritchie AJ, Campion SL, Kopycinski J, Moodie Z, Wang ZM, Pandya K, Moore S, Liu MK, Brackenridge S, Kuldanek K, et al. 2011. Differences in HIV-specific T cell responses between HIV-exposed and -unexposed HIV-seronegative individuals. J Virol 85: 3507–3516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg ES, Billingsley JM, Caliendo AM, Boswell SL, Sax PE, Kalams SA, Walker BD 1997. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science 278: 1447–1450 [DOI] [PubMed] [Google Scholar]
- Rosenberg ES, Altfeld M, Poon SH, Phillips MN, Wilkes BM, Eldridge RL, Robbins GK, D’Aquila RT, Goulder PJ, Walker BD 2000. Immune control of HIV-1 after early treatment of acute infection. Nature 407: 523–526 [DOI] [PubMed] [Google Scholar]
- Rowland-Jones S, Sutton J, Ariyoshi K, Dong T, Gotch F, McAdam S, Whitby D, Sabally S, Gallimore A, Corrah T 1995. HIV-specific cytotoxic T-cells in HIV-exposed but uninfected Gambian women. Nat Med 1: 59–64 [DOI] [PubMed] [Google Scholar]
- Rychert J, Saindon S, Placek S, Daskalakis D, Rosenberg E 2007. Sequence variation occurs in CD4 epitopes during early HIV infection. J Acquir Immune Defic Syndr 46: 261–267 [DOI] [PubMed] [Google Scholar]
- Salazar-Gonzalez JF, Salazar MG, Keele BF, Learn GH, Giorgi EE, Li H, Decker JM, Wang S, Baalwa J, Kraus MH, et al. 2009. Genetic identity, biological phenotype, and evolutionary pathways of transmitted/founder viruses in acute and early HIV-1 infection. J Exp Med 206: 1273–1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenal M, Lo Caputo S, Fasano F, Vichi F, Saresella M, Pierotti P, Villa ML, Mazzotta F, Trabattoni D, Clerici M 2005. Distinct patterns of HIV-specific memory T lymphocytes in HIV-exposed uninfected individuals and in HIV-infected patients. AIDS 19: 653–661 [DOI] [PubMed] [Google Scholar]
- Schmitz JE, Kuroda MJ, Santra S, Sasseville VG, Simon MA, Lifton MA, Racz P, Tenner-Racz K, Dalesandro M, Scallon BJ, et al. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283: 857–860 [DOI] [PubMed] [Google Scholar]
- Schneidewind A, Brockman MA, Yang R, Adam RI, Li B, Le Gall S, Rinaldo CR, Craggs SL, Allgaier RL, Power KA, et al. 2007. Escape from the dominant HLA-B27-restricted cytotoxic T-lymphocyte response in Gag is associated with a dramatic reduction in human immunodeficiency virus type 1 replication. J Virol 81: 12382–12393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneidewind A, Brockman MA, Sidney J, Wang YE, Chen H, Suscovich TJ, Li B, Adam PI, Allgaier RL, Mothé BR, et al. 2008. Structural and functional constraints limit options for cytotoxic T-lymphocyte escape in the immunodominant HLA-B27-restricted epitope in human immunodeficiency virus type 1 capsid. J Virol 82: 5594–5605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneidewind A, Brumme ZL, Brumme CJ, Power KA, Reyor LL, O'Sullivan K, Gladden A, Hempel U, Kuntzen T, Wang YE, et al. 2009. Transmission and long-term stability of compensated CD8 escape mutations. J Virol. 83: 3993–3997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Shaw GM, Hunter E 2011. HIV transmission. Cold Spring Harb Perspect Med 10.1101/cshperspect.a006965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shedlock DJ, Shen H 2003. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science 300: 337–339 [DOI] [PubMed] [Google Scholar]
- Stacey AR, Norris PJ, Qin L, Haygreen EA, Taylor E, Heitman J, Lebedeva M, DeCamp A, Li D, Grove D, et al. 2009. Induction of a striking systemic cytokine cascade prior to peak viremia in acute human immunodeficiency virus type 1 infection, in contrast to more modest and delayed responses in acute hepatitis B and C virus infections. J Virol 83: 3719–3733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staprans SI, Barry AP, Silvestri G, Safrit JT, Kozyr N, Sumpter B, Nguyen H, McClure H, Montefiori D, Cohen JI, et al. 2004. Enhanced SIV replication and accelerated progression to AIDS in macaques primed to mount a CD4 T cell response to the SIV envelope protein. Proc Natl Acad Sci 101: 13026–13031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streeck H, Brumme ZL, Anastario M, Cohen KW, Jolin JS, Meier A, Brumme CJ, Rosenberg ES, Alter G, Allen TM, et al. 2008. Antigen load and viral sequence diversification determine the functional profile of HIV-1-specific CD8+ T cells. PLoS Med 5: e100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streeck H, Jolin JS, Qi Y, Yassine-Diab B, Johnson RC, Kwon DS, Addo MM, Brumme C, Routy JP, Little S, et al. 2009. Human immunodeficiency virus type 1-specific CD8+ T-cell responses during primary infection are major determinants of the viral set point and loss of CD4+ T cells. J Virol 83: 7641–7648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JC, Bevan MJ 2003. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science 300: 339–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Schmitz JE, Buzby AP, Barker BR, Rao SS, Xu L, Yang ZY, Mascola JR, Nabel GJ, Letvin NL 2006. Virus-specific cellular immune correlates of survival in vaccinated monkeys after simian immunodeficiency virus challenge. J Virol 80: 10950–10956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Santra S, Buzby AP, Mascola JR, Nabel GJ, Letvin NL 2010. Recombinant vector-induced HIV/SIV-specific CD4+ T lymphocyte responses in rhesus monkeys. Virology 406: 48–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenzer S, Wee E, Burgevin A, Stewart-Jones G, Friis L, Lamberth K, Chang CH, Harndahl M, Weimershaus M, Gerstoft J, et al. 2009. Antigen processing influences HIV-specific cytotoxic T lymphocyte immunodominance. Nat Immunol 10: 636–646 [DOI] [PubMed] [Google Scholar]
- Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, Boulassel MR, Delwart E, Sepulveda H, Balderas RS, Routy JP, Haddad EK, Sekaly RP 2006. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med 12: 1198–1202 [DOI] [PubMed] [Google Scholar]
- Troyer RM, McNevin J, Liu Y, Zhang SC, Krizan RW, Abraha A, Tebit DM, Zhao H, Avila S, Lobritz MA, et al. 2009. Variable fitness impact of HIV-1 escape mutations to cytotoxic T lymphocyte (CTL) response. PLoS Pathog 5: e1000365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbull EL, Wong M, Wang S, Wei X, Jones NA, Conrod KE, Aldam D, Turner J, Pellegrino P, Keele BF, et al. 2009. Kinetics of expansion of epitope-specific T cell responses during primary HIV-1 infection. J Immunol 182: 7131–7145 [DOI] [PubMed] [Google Scholar]
- Veiga-Fernandes H, Walter U, Bourgeois C, McLean A, Rocha B 2000. Response of naive and memory CD8+ T cells to antigen stimulation in vivo. Nat Immunol 1: 47–53 [DOI] [PubMed] [Google Scholar]
- Vezys V, Yates A, Casey KA, Lanier G, Ahmed R, Antia R, Masopust D 2009. Memory CD8 T-cell compartment grows in size with immunological experience. Nature 457: 196–199 [DOI] [PubMed] [Google Scholar]
- Vigneault F, Woods M, Buzon MJ, Li C, Pereyra F, Crosby SD, Rychert J, Church G, Martinez-Picado J, Rosenberg ES, et al. 2011. Transcriptional profiling of CD4 T cells identifies distinct subgroups of HIV-1 elite controllers. J Virol 85: 3015–3019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahren B, Morfeldt-Månsson L, Biberfeld G, Moberg L, Sönnerborg A, Ljungman P, Werner A, Kurth R, Gallo R, Bolognesi D 1987. Characteristics of the specific cell-mediated immune response in human immunodeficiency virus infection. J Virol 61: 2017–2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker CM, Moody DJ, Stites DP, Levy JA 1986. CD8+ lymphocytes can control HIV infection in vitro by suppressing virus replication. Science 234: 1563–1566 [DOI] [PubMed] [Google Scholar]
- Walker BD, Chakrabarti S, Moss B, Paradis TJ, Flynn T, Durno AG, Blumberg RS, Kaplan JC, Hirsch MS, et al. 1987. HIV-specific cytotoxic T lymphocytes in seropositive individuals. Nature 328: 345–348 [DOI] [PubMed] [Google Scholar]
- Wawer MJ, Gray RH, Sewankambo NK, Serwadda D, Li X, Laeyendecker O, Kiwanuka N, Kigozi G, Kiddugavu M, Lutalo T, et al. 2005. Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J Infect Dis 191: 1403–1409 [DOI] [PubMed] [Google Scholar]
- Williams MA, Tyznik AJ, Bevan MJ 2006. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature 441: 890–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LD, Bansal A, Sabbaj S, Heath SL, Song W, Tang J, Zajac AJ, Goepfert PA 2011. Interleukin-21-producing HIV-1-specific CD8 T cells are preferentially seen in elite controllers. J Virol 85: 2316–2324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright JK, Novitsky V, Brockman MA, Brumme ZL, Brumme CJ, Carlson JM, Heckerman D, Wang B, Losina E, Leshwedi M, et al. 2011. Influence of gag-protease-mediated replication capacity on disease progression in individuals recently infected with HIV-1 subtype C. J Virol 85: 3996–4006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang PL, Althage A, Chung J, Maier H, Wieland S, Isogawa M, Chisari FV 2010. Immune effectors required for hepatitis B virus clearance. Proc Natl Acad Sci 107: 798–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu XG, Addo MM, Rosenberg ES, Rodriguez WR, Lee PK, Fitzpatrick CA, Johnston MN, Strick D, Goulder PJ, Walker BD, et al. 2002. Consistent patterns in the development and immunodominance of human immunodeficiency virus type 1 (HIV-1)-specific CD8+ T-cell responses following acute HIV-1 infection. J Virol 76: 8690–8701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu XG, Lichterfeld M, Williams KL, Martinez-Picado J, Walker BD 2007. Random T-cell receptor recruitment in human immunodeficiency virus type 1 (HIV-1)-specific CD8+ T cells from genetically identical twins infected with the same HIV-1 strain. J Virol 81: 12666–12669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerli SC, Harari A, Cellerai C, Vallelian F, Bart PA, Pantaleo G 2005. HIV-1-specific IFN-γ/IL-2-secreting CD8 T cells support CD4-independent proliferation of HIV-1-specific CD8 T cells. Proc Natl Acad Sci 102: 7239–7244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuñiga R, Lucchetti A, Galvan P, Sanchez S, Sanchez C, Hernandez A, Sanchez H, Frahm N, Linde CH, Hewitt HS, et al. 2006. Relative dominance of Gag p24–specific cytotoxic T lymphocytes is associated with human immunodeficiency virus control. J Virol 80: 3122–3125 [DOI] [PMC free article] [PubMed] [Google Scholar]