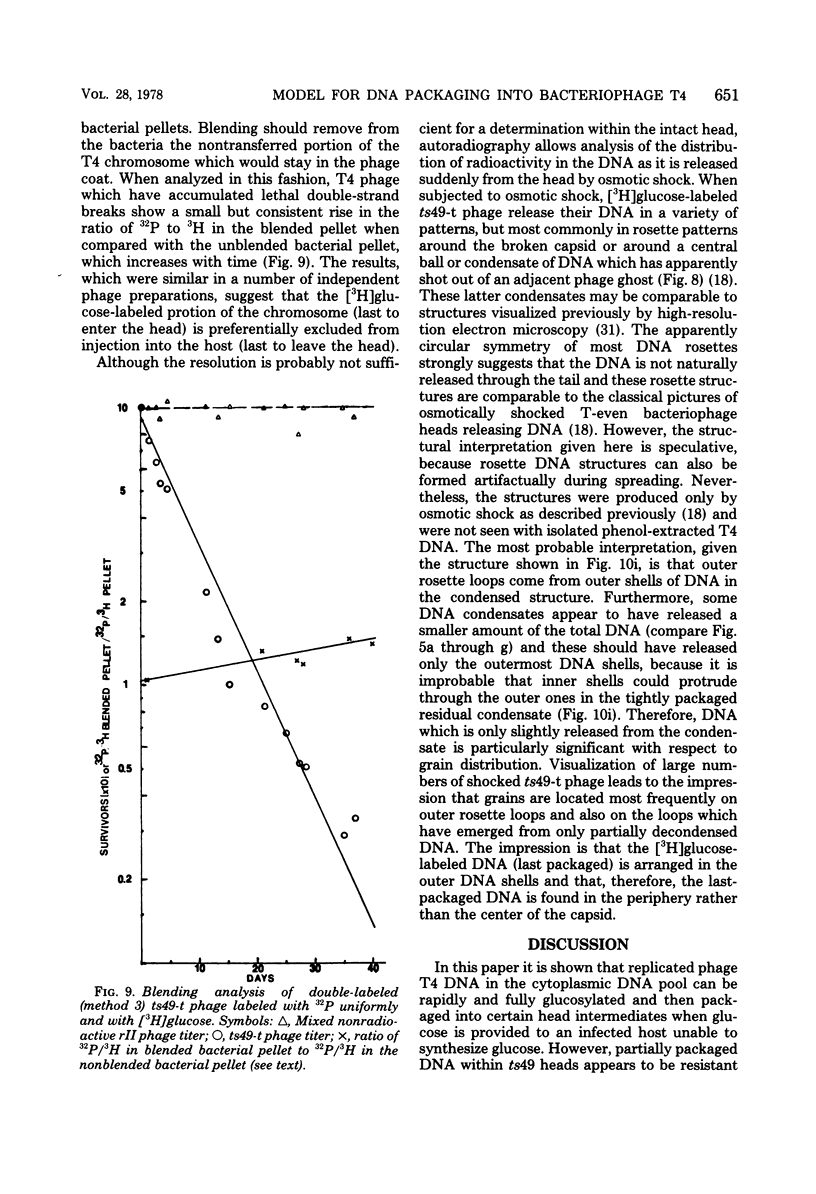
Abstract
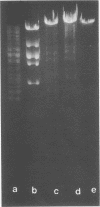
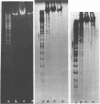
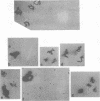
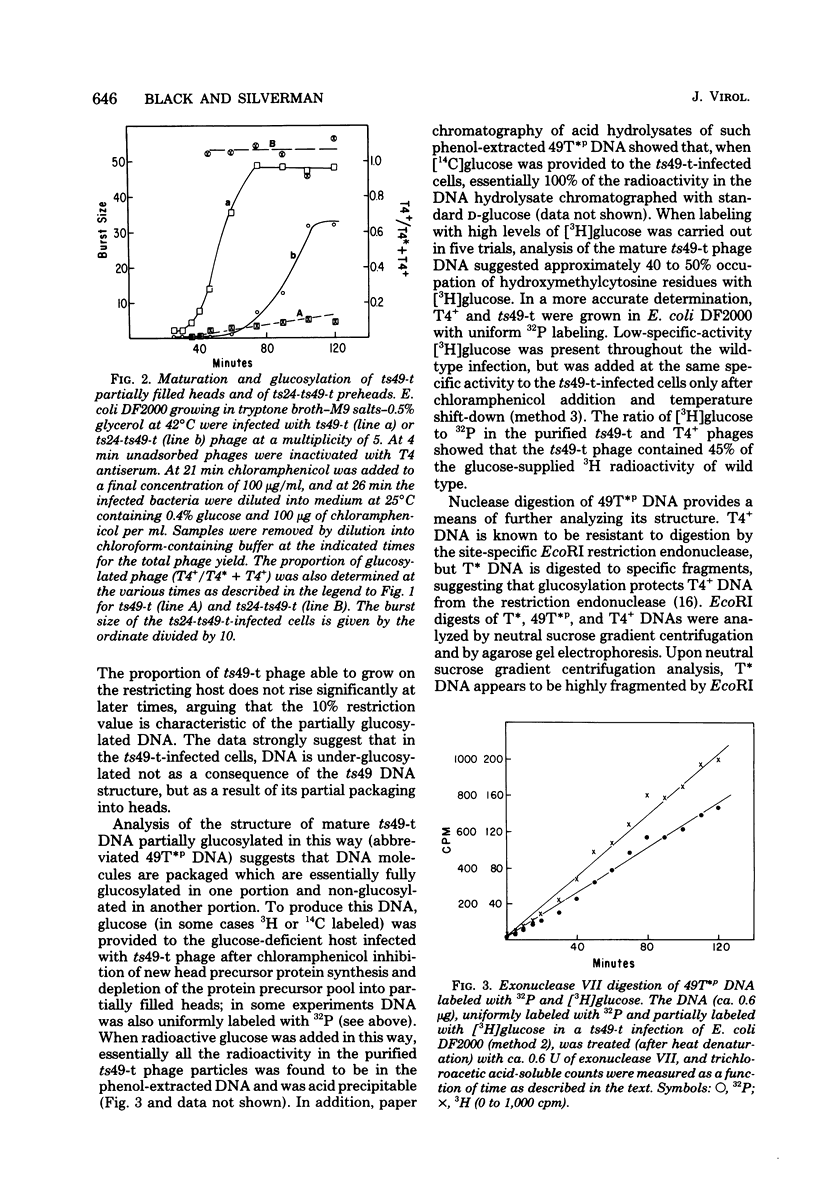
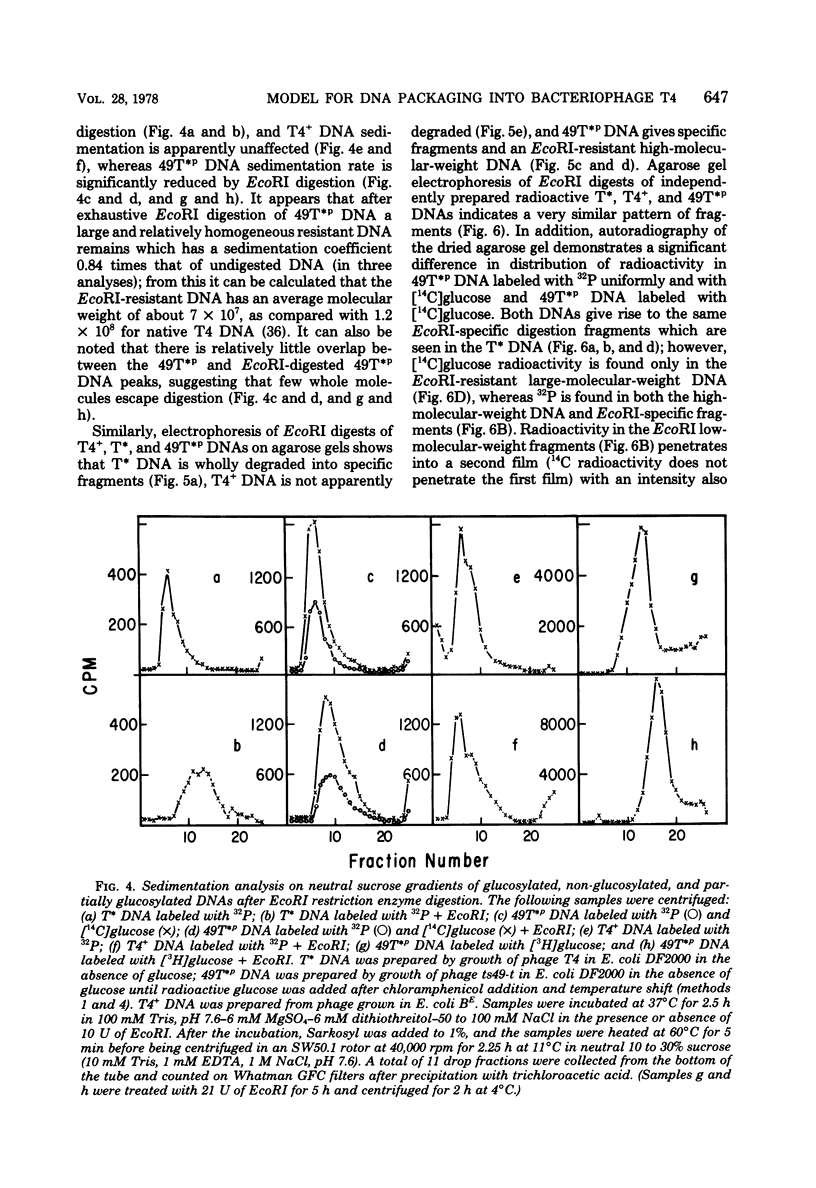
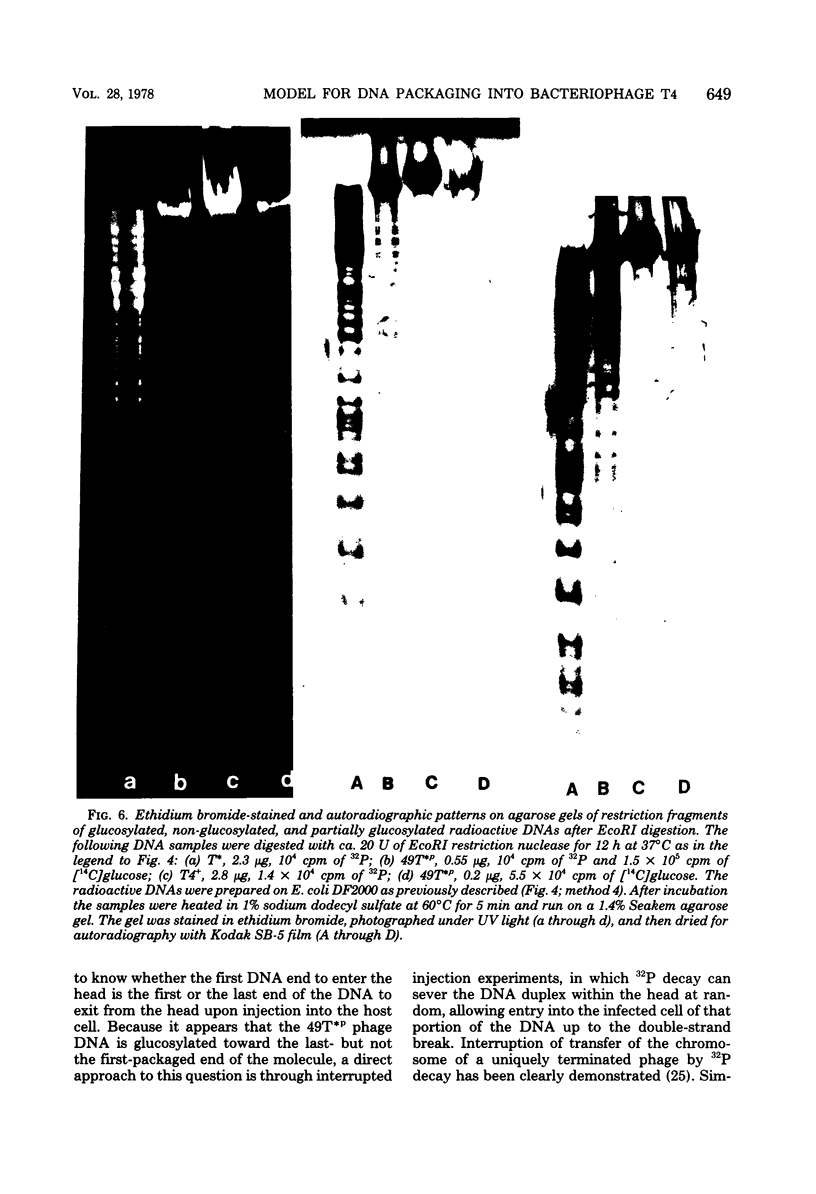
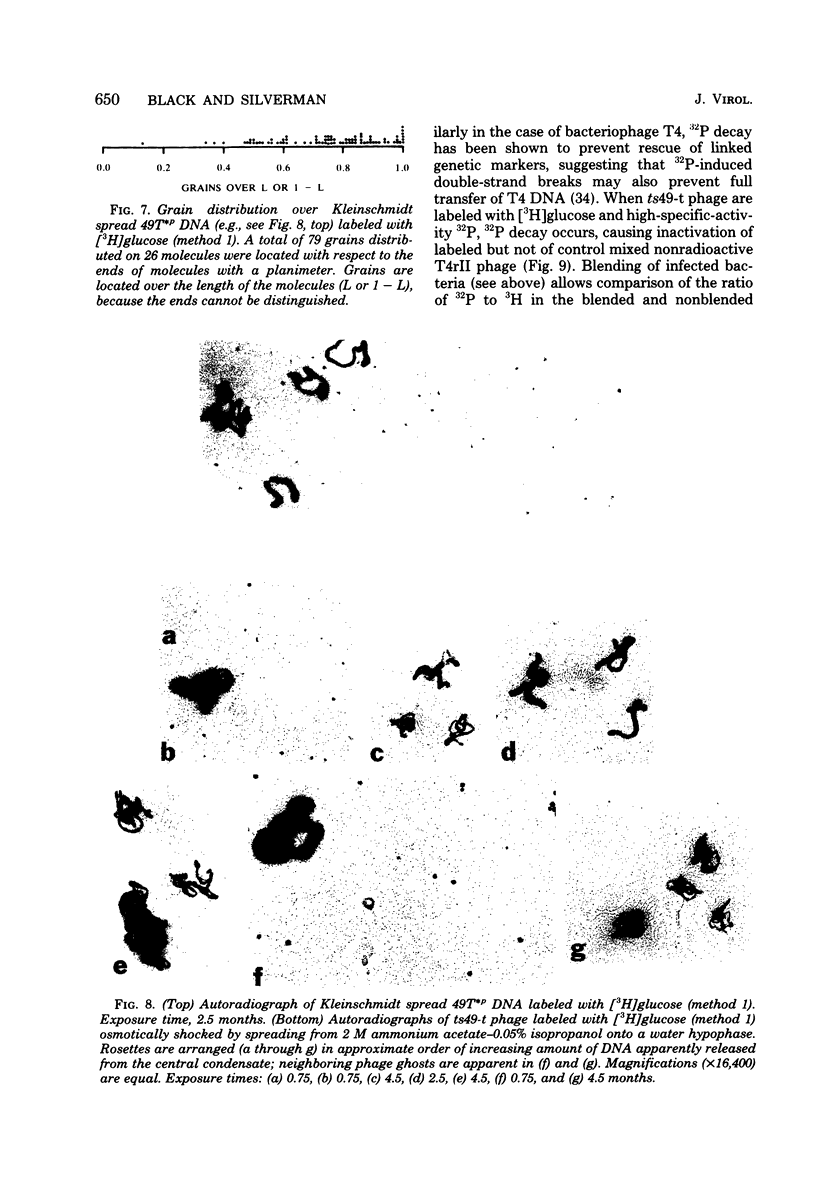
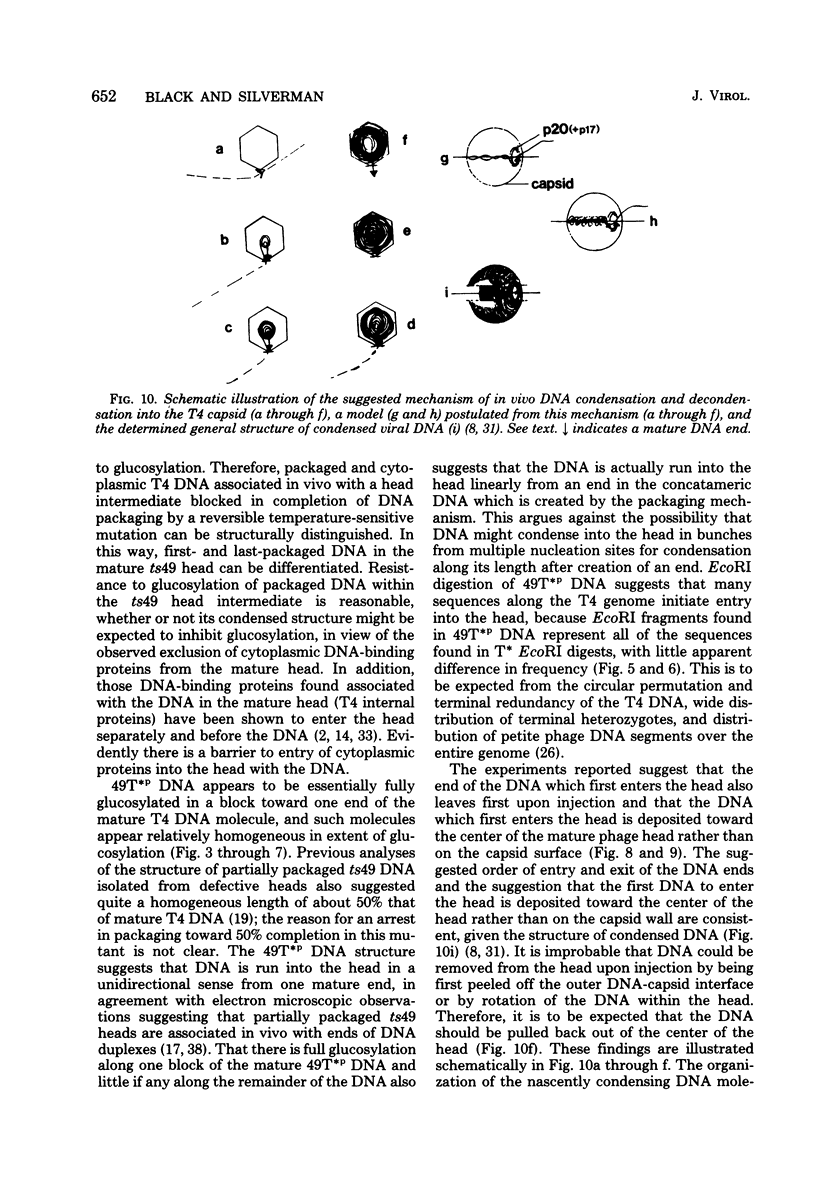
The mechanism of DNA packaging into bacteriophage T4 heads in vivo was investigated by glucosylation of hydroxymethylcytosine residues in a conditionally glucose-deficient host. Cytoplasmic DNA associated with partially packaged ts49 heads can be fully glucosylated, whereas DNA already packaged into these heads is shown to be resistant to glucosylation. After temperature shift and completion of arrested packaging into the reversible temperature-sensitive ts49 head, the structure of the DNA in the mature ts49 phage was investigated by restriction enzyme digestion, autoradiography, and other techniques. Such mature DNA appears to be fully glucosylated along part of its length and nonglucosylated on the remainder. Its structure suggests that the DNA is run into the head linearly and unidirectionally from one mature end and that there is little sequence specificity in that portion of the T4 DNA which first enters the capsid. This technique should be useful in investigation of the three-dimensional structure of first- and last-packaged DNA within the head; preliminary studies including autoradiography of osmotically shocked phage suggest that the DNA which first enters the head is deposited toward the center of the capsid and that the end of the DNA which first enters the head exits first upon injection. In conjunction with studies of the structure of condensed DNA, the positions and functions of T4 capsid proteins in DNA packaging, and the order of T4 packaging functions [Earnshaw and Harrison, Nature (London) 268:598-602, 1977; Hsiao and Black, Proc. Natl. Acad. Sci. U.S.A. 74:3652-3656, 1977; Müller-Salamin et al., J. Virol. 24:121-134, 1977; Richards et al., J. Mol. Biol. 78:255-259, 1973], the features described above suggest the following model: the first DNA end is fixed to the proximal apex of the head at p20 and the DNA is then pumped into the head enzymatically by proteins (p20 + p17) which induce torsion in the DNA molecule.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bijlenga R. K., Scraba D., Kellenberger E. Studies on the morphopoiesis of the head of T-even phage. IX. Gamma-particles: their morphology, kinetics of appearance and possible precursor function. Virology. 1973 Nov;56(1):250–267. doi: 10.1016/0042-6822(73)90304-8. [DOI] [PubMed] [Google Scholar]
- Black L. W. Bacteriophage T4 internal protein mutants: isolation and properties. Virology. 1974 Jul;60(1):166–179. doi: 10.1016/0042-6822(74)90374-2. [DOI] [PubMed] [Google Scholar]
- Casjens S., King J. Virus assembly. Annu Rev Biochem. 1975;44:555–611. doi: 10.1146/annurev.bi.44.070175.003011. [DOI] [PubMed] [Google Scholar]
- Castillo C. J., Hsiao C. L., Coon P., Black L. W. Identification and perperties of bacteriophage T4 capsid-formation gene products. J Mol Biol. 1977 Mar 5;110(3):585–601. doi: 10.1016/s0022-2836(77)80113-7. [DOI] [PubMed] [Google Scholar]
- Chase J. W., Richardson C. C. Exonuclease VII of Escherichia coli. Purification and properties. J Biol Chem. 1974 Jul 25;249(14):4545–4552. [PubMed] [Google Scholar]
- Chattoraj D. K., Inman R. B. Location of DNA ends in P2, 186, P4 and lambda bacteriophage heads. J Mol Biol. 1974 Jul 25;87(1):11–22. doi: 10.1016/0022-2836(74)90556-7. [DOI] [PubMed] [Google Scholar]
- Earnshaw W. C., Harrison S. C. DNA arrangement in isometric phage heads. Nature. 1977 Aug 18;268(5621):598–602. doi: 10.1038/268598a0. [DOI] [PubMed] [Google Scholar]
- Emmons S. W. Bacteriophage lambda derivatives carrying two copies of the cohesive end site. J Mol Biol. 1974 Mar 15;83(4):511–525. doi: 10.1016/0022-2836(74)90511-7. [DOI] [PubMed] [Google Scholar]
- Fraenkel D. G. The accumulation of glucose 6-phosphate from glucose and its effect in an Escherichia coli mutant lacking phosphoglucose isomerase and glucose 6-phosphate dehydrogenase. J Biol Chem. 1968 Dec 25;243(24):6451–6457. [PubMed] [Google Scholar]
- Gellert M., Mizuuchi K., O'Dea M. H., Nash H. A. DNA gyrase: an enzyme that introduces superhelical turns into DNA. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3872–3876. doi: 10.1073/pnas.73.11.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glinert S. J., Luftig R. B. Bacteriophage T4D head morphogenesis. VIII. DNA-protein associations in intermediate head structures that accumulate in gene 49--mutant-infected cells. J Virol. 1977 Jun;22(3):758–777. doi: 10.1128/jvi.22.3.758-777.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohn B., Wurtz M., Klein B., Lustig A., Hohn T. Phage lambda DNA packaging, in vitro. J Supramol Struct. 1974;2(2-4):302–317. doi: 10.1002/jss.400020220. [DOI] [PubMed] [Google Scholar]
- Hsiao C. L., Black L. W. DNA packaging and the pathway of bacteriophage T4 head assembly. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3652–3656. doi: 10.1073/pnas.74.9.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josslin R. The lysis mechanism of phage T4: mutants affecting lysis. Virology. 1970 Mar;40(3):719–726. doi: 10.1016/0042-6822(70)90216-3. [DOI] [PubMed] [Google Scholar]
- KLEINSCHMIDT A. K., LANG D., JACHERTS D., ZAHN R. K. [Preparation and length measurements of the total desoxyribonucleic acid content of T2 bacteriophages]. Biochim Biophys Acta. 1962 Dec 31;61:857–864. [PubMed] [Google Scholar]
- Kaplan D. A., Nierlich D. P. Cleavage of Nonglucosylated Bacteriophage T4 deoxyribonucleic acid by Restriction Endonuclease Eco RI. J Biol Chem. 1975 Mar 25;250(6):2395–2397. [PubMed] [Google Scholar]
- Kemper B., Brown D. T. Function of gene 49 of bacteriophage T4. II. Analysis of intracellular development and the structure of very fast-sedimenting DNA. J Virol. 1976 Jun;18(3):1000–1015. doi: 10.1128/jvi.18.3.1000-1015.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Paulson J. R., Hitchins V. Maturation of the head of bacteriophage T4. V. A possible DNA packaging mechanism: in vitro cleavage of the head proteins and the structure of the core of the polyhead. J Supramol Struct. 1974;2(2-4):276–301. doi: 10.1002/jss.400020219. [DOI] [PubMed] [Google Scholar]
- Lenk E., Casjens S., Weeks J., King J. Intracellular visualization of precursor capsids in phage P22 mutant infected cells. Virology. 1975 Nov;68(1):182–199. doi: 10.1016/0042-6822(75)90160-9. [DOI] [PubMed] [Google Scholar]
- Lickfeld K. G., Menge B. Morphogenesis of bacteriophage lambda: electron microscopy of thin sections. J Mol Biol. 1976 May 15;103(2):299–318. doi: 10.1016/0022-2836(76)90314-4. [DOI] [PubMed] [Google Scholar]
- Luftig R. B., Wood W. B., Okinaka R. Bacteriophage T4 head morphogenesis. On the nature of gene 49-defective heads and their role as intermediates. J Mol Biol. 1971 May 14;57(3):555–573. doi: 10.1016/0022-2836(71)90109-4. [DOI] [PubMed] [Google Scholar]
- McAllister W. T., Green D. M. Effects of the decay of incorporated radioactive phosphorus on the transfer of the bacteriophage SP82G genome. J Virol. 1973 Aug;12(2):300–309. doi: 10.1128/jvi.12.2.300-309.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minagawa T. Endonuclease of T4 ghosts. Virology. 1977 Jan;76(1):234–245. doi: 10.1016/0042-6822(77)90299-9. [DOI] [PubMed] [Google Scholar]
- Müller-Salamin L., Onorato L., Showe M. K. Localization of minor protein components of the head of bacteriophage T4. J Virol. 1977 Oct;24(1):121–134. doi: 10.1128/jvi.24.1.121-134.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piechowski M. M., Susman M. Acridine-resistance in phage T4D. Genetics. 1967 May;56(1):133–148. doi: 10.1093/genetics/56.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revel H. R., Luria S. E. DNA-glucosylation in T-even phage: genetic determination and role in phagehost interaction. Annu Rev Genet. 1970;4(0):177–192. doi: 10.1146/annurev.ge.04.120170.001141. [DOI] [PubMed] [Google Scholar]
- Richards K. E., Williams R. C., Calendar R. Mode of DNA packing within bacteriophage heads. J Mol Biol. 1973 Aug 5;78(2):255–259. doi: 10.1016/0022-2836(73)90114-9. [DOI] [PubMed] [Google Scholar]
- STAHL F. W. The effects of the decay of incorporated radioactive phosphorus on the genome of bacteriophage T4. Virology. 1956 Apr;2(2):206–234. doi: 10.1016/0042-6822(56)90018-6. [DOI] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Serwer P. Buoyant density sedimentation of macromolecules in sodium iothalamate density gradients. J Mol Biol. 1975 Mar 5;92(3):433–448. doi: 10.1016/0022-2836(75)90290-9. [DOI] [PubMed] [Google Scholar]
- Showe M. K., Black L. W. Assembly core of bacteriophage T4: an intermediate in head formation. Nat New Biol. 1973 Mar 21;242(116):70–75. doi: 10.1038/newbio242070a0. [DOI] [PubMed] [Google Scholar]
- Sternberg N., Weisberg R. Packaging of prophage and host DNA by coliphage lambda. Nature. 1975 Jul 10;256(5513):97–103. doi: 10.1038/256097a0. [DOI] [PubMed] [Google Scholar]
- Thomas J. O. Chemical linkage of the tail to the right-hand end of bacteriophage lambda DNA. J Mol Biol. 1974 Jul 25;87(1):1–9. doi: 10.1016/0022-2836(74)90555-5. [DOI] [PubMed] [Google Scholar]
- Wagner J., Laemmli U. K. Studies on the maturation of the head of bacteriophage T4. Philos Trans R Soc Lond B Biol Sci. 1976 Nov 30;276(943):15–28. doi: 10.1098/rstb.1976.0093. [DOI] [PubMed] [Google Scholar]
- Wunderli H., vd Broek J., Kellenberger E. Studies related to the head-maturation pathway of bacteriophages T4 and T2:I. morphology and kinetics of intracellular particles produced by mutants in the maturation genes. J Supramol Struct. 1977;7(2):135–161. doi: 10.1002/jss.400070202. [DOI] [PubMed] [Google Scholar]