Abstract
The copper-catalyzed azide–alkyne cycloaddition (CuAAC) is a highly effective method for the selective incorporation of deuterium atom into the C-5 position of the 1,2,3-triazole structure. Reactions of alkynes and azides can be conveniently carried out in a biphasic medium of CH2Cl2/D2O, using the CuSO4/Na ascorbate system. The mildness of the method renders it applicable to substrates of relatively high complexity, such as nucleosides. Good yields and high levels of deuterium incorporation were observed. A reaction conducted in equimolar H2O and D2O showed 2.7 times greater incorporation of hydrogen atom as compared to deuterium. This is consistent with the H+ and D+ ion concentrations in H2O and D2O, respectively. With appropriately deuterated precursors, partially to fully deuterated triazoles were assembled where the final deuterium atom was incorporated in the triazole-forming step.
INTRODUCTION
The Huisgen ligation of azides and alkynes is an exceptionally atom-economical reaction leading to the formation of 1,2,3-triazoles.1 However, the thermal process is not regioselective, resulting in the formation of 1,4- and 1,5-disubstituted 1,2,3-triazoles. The copper-catalyzed azide–alkyne cycloaddition (CuAAC) has emerged as an outstanding procedure for access to 1,4-disubstituted 1,2,3-triazoles, in a regioselective manner.2–4
The mechanism of the copper-catalyzed reaction of azides and terminal alkynes has been actively investigated5–8 and several notable factors have emerged. Under catalytic conditions, the reaction is second order in CuI, either due to the involvement of one Cu each with the azide and the alkyne,6,7 or due to the involvement of two Cu centers with the alkyne.5 There has also been a question of the involvement of alkyne with π-coordinated copper versus a copper acetylide.6–8 In this regard, the experimental evidence suggests intermediacy of Cu-acetylides from terminal alkynes. This is because the CuAAC reaction of PhC≡C–D with PhCH2N3 in t-BuOH/H2O gave only the C-5 protio triazole,8 consistent with a prior observation.6 Therefore, CuAAC reactions of alkynes with a terminal deuterium atom are unlikely to lead to C-5 deuterated triazoles under aqueous conditions. However, aqueous conditions produce faster reactions.7 To our knowledge, use of alkynes with a terminal deuterium atom in azide–alkyne cycloadditions is rare, and their use would be limited to processes where the deuterium will not be lost. In one example, an alkyne with a terminal deuterium atom has been employed in a thermal intramolecular reaction so as to control the cycloaddition regiochemistry and to ensure retention of the heavier isotope.9
A review of the literature indicated that although deuteration of 1,2,3-triazoles has been accomplished, procedures for C-5 deuteration are not particularly simple as compared to the CuAAC approach. For instance, C-5 deuterated triazoles can be obtained from alkynyl aluminum10 or copper11 intermediates or via lithiation of the triazole (Scheme 1).12 Notably, the isolated alkynyl copper intermediate in Scheme 1 did not undergo ligation with azide in the absence of MeCO2H(D).11 In a complementary reaction, a magnesiated alkyne yielded the C-4 deuterated triazole.13 Dideuterotriazoles can be made from acetylene gas generated in situ from CaC2, in the presence of Et3N/D2O.14
Scheme 1.
Known syntheses of deuterated triazoles
Despite the widespread application of the CuAAC reaction and its possible use for the deuteration of triazoles, we were surprised to find the absence of a general method for this purpose.15 In the broader context, the importance of isotopic labeling is well-recognized, for example, in mechanistic and biosynthetic considerations, mass spectrometry applications, and in metabolism studies, to name a few. The 1,2,3-triazolyl moiety is also prominent in medicinal chemistry, where it has been likened to an amide linkage, with the N-3 atom serving as a hydrogen-bond acceptor and the C-4 atom the hydrogen-bond donor.16–18 However, in contrast to amides, the triazole ring is chemically and biologically robust. These considerations have led to the design of bioactive entities containing a 1,2,3-triazolyl ring.16,17 Additionally, “heavy drugs,” namely pharmaceuticals that contain one or more deuterium atom(s) in place of hydrogen(s), have received increased attention due to the positive attributes gained upon deuteration.19 On the basis of these overall considerations, a method to deuterated triazoles is likely to be of broad relevance. Herein we report a simple, mild, and general route to deuterated 1,2,3-triazoles wherein introduction of the deuterium atom at the C-4 position occurs in the triazole-forming step.
RESULTS AND DISCUSSION
Of importance to the success of the approach would be the formation of Cu-acetylides as intermediates in CuAAC reactions. It has been proposed that π-coordination of CuI increases the acidity of the alkynyl proton, rendering the formation of Cu-acetylides feasible in aqueous media.7 This implies that terminal alkynes can be directly utilized since the alkynyl proton would be lost early in the process. Azide coordination to the Cu center occurs after formation of the Cu-acetylide.7 These mechanistic considerations supported our one-pot triazole-forming, deuteration strategy.
In recent work20 with the CuAAC reactions of 6-azido purine nucleosides we had encountered a significant problem that is not typical in the reactions of simpler azides. That is, the azido group underwent competing reduction in the presence of aqueous CuSO4/Na ascorbate.20 To remedy this, we resorted to a biphasic reaction medium, which suppressed the undesired azide reduction and gave good to excellent product recoveries. This led us to reason that use of biphasic reaction conditions with cheap, commercially available D2O as one phase, should allow for capture of a deuterium atom by the C-4 cuprated intermediates formed in the CuAAC while suppressing other undesired processes.
With these considerations we conducted a series of initial optimization reactions (Table 1). We opted to desiccate CuSO4•5H2O in order to increase the extent of deuterium incorporation by eliminating as much water as possible. Entry 1 in Table 1 indicates proof of principle. Here, simply drying the CuSO4•5H2O with a heat gun, use of D2O with 98% D, and use of low catalyst load, gave a respectable product yield and %D incorporation, but the reaction was incomplete. Use of better conditions (more rigorously dried CuSO4 and D2O with 99.8% D), gave excellent D incorporation, but again the reaction remained incomplete (entry 2). Complete reaction, with 96% D incorporation was achieved by increasing the amounts of CuSO4 and Na ascorbate (entry 3). In this case %D incorporation was evaluated in two ways. Use of CH2Cl2 as an internal NMR standard indicated 96% D whereas assessment by comparison of the residual H-5 to other product resonances indicated 94% D. In an attempt to further improve the extent of D incorporation, a reaction was carried out under conditions of entry 3, except that Na ascorbate was evaporated from D2O under vacuum prior to its use in the reaction. Under these conditions (entry 4), practically no further improvement in %D incorporation was observed and product yield remained essentially unchanged. Next, the amount of alkyne was lowered so as to reduce the amount of a proton source, but here incomplete reaction was observed (entry 5).
Table 1.
Conditions tested for the one-pot triazole formation and deuteration.a
 | ||
|---|---|---|
| entry | solvent, catalytic system, reaction time | product: resultb, c |
| 1d,e | 1:1 CH2Cl2/D2O, 2 equiv alkyne, 5 mol % CuSO4, 10 mol % Na ascorbate, 12 h | 1-D: 60% yield, 80% D incorporation, reaction was incompletef |
| 2g,h | 1:1 CH2Cl2/D2O, 2 equiv alkyne, 7 mol % CuSO4, 15 mol % Na ascorbate, 12 h | 1-D: 65% yield, 95% D incorporation, reaction was incompletei |
| 3g,h | 1:1 CH2Cl2/D2O, 2 equiv alkyne, 10 mol % CuSO4, 20 mol % Na ascorbate, 12 h | 1-D: 75% yield, 96% D incorporation |
| 4g,h | 1:1 CH2Cl2/D2O, 2 equiv alkyne, 10 mol % CuSO4, 20 mol % Na ascorbate (dried from D2O), 12 h | 1-D: 74% yield, 96% D incorporation |
| 5g,h | 1:1 CH2Cl2/D2O, 1 equiv alkyne, 10 mol % CuSO4, 20 mol % Na ascorbate (dried from D2O), 12 h | 1-D: reaction was incomplete (ca 80% azide consumed)j |
| 6g,h | 1:1 CH3CN/D2O, 2 equiv alkyne, 10 mol % CuSO4, 20 mol % Na ascorbate (dried from D2O), 24 h | 1-D: reaction was incomplete (<5% conversion)j |
| 7 | 1:1 CH3CN/H2O, 2 equiv alkyne, 10 mol % CuSO4, 20 mol % Na ascorbate, 5 d | 1-H: reaction was incomplete (<5% conversion)j |
| 8 | 1:1 CH2Cl2/H2O, 2 equiv alkyne, 20 mol % CuCl, 48 h | 1-H: reaction was incompletek |
Reactions were conducted at room temperature with 0.2 M azide in anhydrous CH2Cl2 or in CH3CN.
Where reported, yield is of isolated and purified product.
%D incorporation was assessed from the residual H-5 resonance in the 1H NMR spectra. Deuterium resonances are referenced to the 2H resonance of CDCl3 (δ = 7.24 ppm).
D2O contained 98% D (not a freshly opened sample).
CuSO4•5H2O was heated with a heat gun until the color changed from blue to gray.
Phenyl azide (10%) was recovered.
D2O contained 99.8% D.
CuSO4•5H2O was heated at 120 °C, under vacuum, until the color changed from blue to gray.
Phenyl azide (8%) was recovered.
Assessed by 1H NMR.
Assessed by TLC.
Attempts were then made to evaluate whether a water-miscible solvent offered any advantage. Use of CH3CN led to less than 5% product formation in 24 h (entry 6). Surprised by this result, we conducted a reaction with H2O rather than with D2O, over an extended period. Again, less than 5% product formation was observed in 5 days (entry 7, a repeat of this experiment gave a comparable result). A prior report documents the failure of azide–alkyne ligation reactions with CuSO4•5H2O/Na ascorbate in CH3CN/H2O, DMSO/H2O, EtOH/H2O, t-BuOH/H2O, and other solvent combinations.21 In that study, the superiority of the CH2Cl2/H2O system led the authors to propose a rate-enhancing influence of this solvent combination on the CuAAC reactions.21 Finally, in comparison to the CuSO4/Na ascorbate system (entries 3 and 4), CuCl was inferior at catalyzing this reaction (entry 8).
Having completed these initial studies, we next focused on evaluating the generality of this regioselective deuteration. Product structures, yields, and %D incorporation are shown in Table 2.
Table 2.
Reactions were conducted at room temperature with 0.2 M azide and 2 mol equiv of alkyne.
Yields are of isolated and purified products.
%D incorporation was assessed from the residual H-5 resonance in the 1H NMR spectra. Deuterium resonances are referenced to the 2H resonance of CDCl3 (δ = 7.24 ppm).
Syntheses of compounds 2-D to10-D were performed using 10 mol % CuSO4 (0.048 M in 99.8% D2O) and 20 mol % Na ascorbate (0.096 M in 99.8% D2O).
Synthesis of 11-D was performed using 5 mol % CuSO4 (0.048 M in 99.8% D2O) and 10 mol % Na ascorbate (0.096 M in 99.8% D2O).
As can be seen from Table 2, generally good-excellent product yields were obtained, with excellent D incorporation (92–98%). From a medicinal chemistry perspective, compounds 1-D to 7-D are structurally very similar to resveratrol analogues that have shown more potent cytotoxic and antiproliferative activity than resveratrol.22 Compound 9-D is a deuterated version of a highly active nicotinic acetylcholine receptor modulator.23 Although a 54% yield of the protio analogue 9-H is reported in the literature,23 under our conditions both 9-H and 9-D were obtained in 70% yield. Even the more complex nucleoside substrates gave high yields and showed excellent D incorporation. The 96% yield of 10-D compares very favorably to the microwave-mediated CuAAC reactions of AZT.24 Protio triazolyl nucleoside analogues such as 10-D, derived from AZT, have proven to be excellent substrates for thymidine kinase of the pathogen Ureaplasma parvum.24 We have previously shown that the C-6 azido purine nucleoside precursor to 11-D exhibits significant azide-tetrazole equilibrium and is prone to facile reduction under CuAAC conditions.20 However, as seen here, this substrate reacted smoothly. These results clearly indicate that the reaction conditions are generally very effective for a broad range of substrates.
In comparing protonation to deuteration, the former should be a more facile reaction due to the difference in the O–H and O–D bond energies.25 To assess the relative difference in protonation versus deuteration when both processes compete in the triazole-forming-deuteration reactions, two reactions of phenyl azide with 4-ethynyltoluene were conducted in parallel. One reaction was with D2O (99.8%) only and the other was with equimolar H2O/D2O (99.8%). By 1H NMR analysis, using CH2Cl2 as an internal standard, the amount of 1-H formed in the reaction with only D2O was estimated to be 4%, whereas in the H2O/D2O reaction 1-H was formed to an extent of 73% (77% observed – 4% formed in the control experiment). The isolated product yields from the two reactions were comparable, 75% for the D2O reaction and 76% in the H2O/D2O reaction. The ratio of 1-H/1-D formed in the latter experiment was 2.7. It is therefore plausible that the greater proportion of 1-H as compared to 1-D results from a more rapid protonation of the cuprated triazole. This could be a contributing factor toward the amounts of the protio products formed in these reactions, which exceed what would be predicted based solely upon the deuterium content of the D2O used.
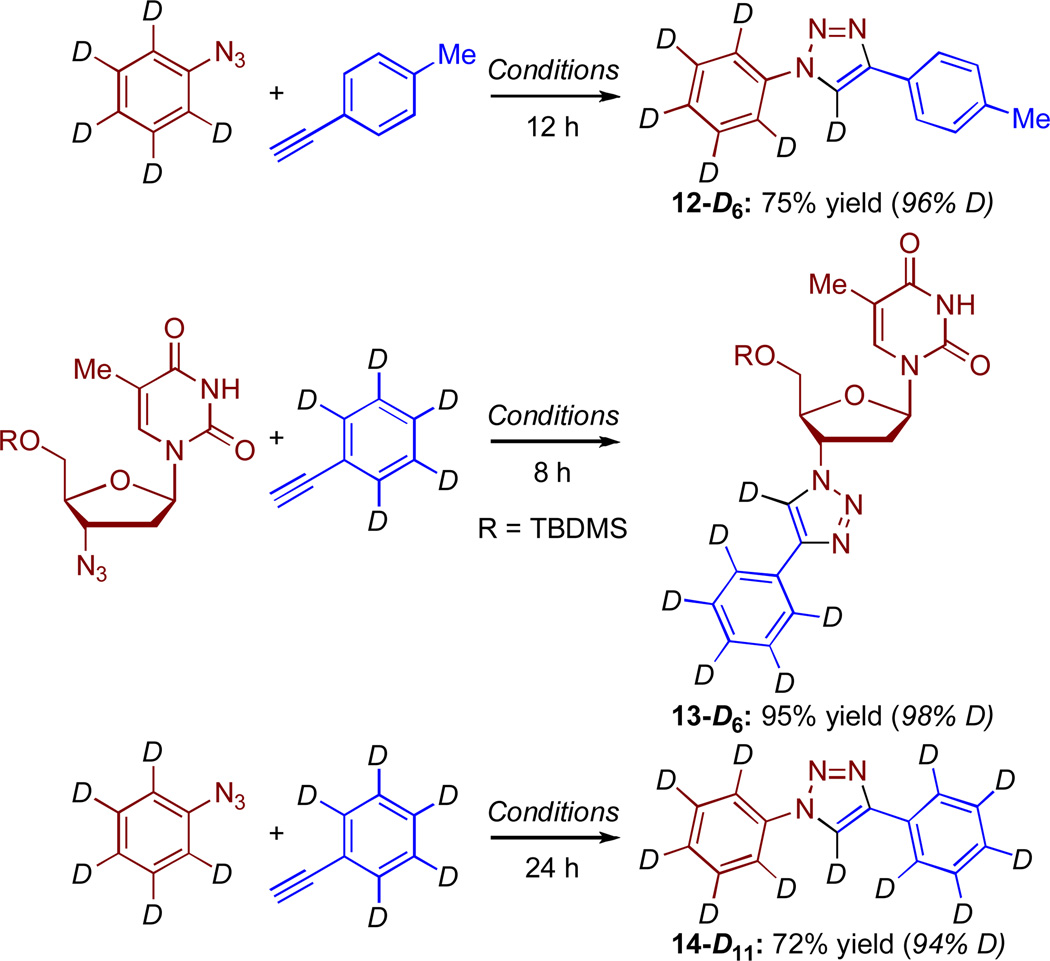
Although monodeuteration reactions proceeded efficiently, we were curious to assess the ability to build poly- and fully-deuterated molecules by this general methodology, where final deuteration of the triazole occurred in the CuAAC step. Therefore, using the optimized conditions, the synthesis of three compounds was undertaken (Scheme 2). For this, phenyl azide-D526–28 and ethynylbenzene-D529–32 were synthesized from bromobenzene-D5 (99% D).
Scheme 2.
Synthesis of poly- and fully-deuterated 1,2,3-triazoles
Reaction of phenyl azide-D5 with 4-ethynyltoluene yielded 12-D6, whereas reaction of TBDMS-protected AZT with ethynylbenzene-D5 gave 13-D6, a more extensively deuterated analogue of 10-D. Finally, reaction of phenyl azide-D5 with ethynylbenzene-D5 gave 14-D11, which is a fully deuterated version of 2-D. %D incorporation in 12-D6 and 13-D6 was assessed from the residual H-5 triazolyl resonances in the 1H NMR spectra. In the case of 14-D11, this assessment was done with CH2Cl2 as an internal standard.
CONCLUSIONS
In summary, a straightforward and mild procedure for regioselective C-5 deuteration of 1,2,3-triazoles has been developed via the CuAAC protocol, utilizing desiccated CuSO4 and Na ascorbate. Cuprated triazoles formed in these reactions capture deuterium from cheap and readily available D2O. Use of biphasic CH2Cl2/D2O for these reactions eliminates certain problems, such as azide reduction encountered during the CuAAC reactions of azido nucleosides, and this combination is superior to CH3CN/D2O. Mono to fully deuterated compounds can be readily assembled via this methodology. It is foreseeable that this deuteration process can be coupled with the incorporation of other atomic isotopes, such as 13C and/or 15N, to yield multiply labeled compounds. All these factors contribute to the importance of the described chemistry. This simple deuteration method circumvents chemical manipulations of the triazole that may not be easily accomplished with molecules of increased complexity, and this is also likely to be of value in medicinal chemistry applications.
EXPERIMENTAL SECTION
General Experimental Considerations
Thin layer chromatography was performed on aluminum foil-backed TLC plates of 200 µm thickness and column chromatographic purifications were performed on 200–300 mesh silica gel. CH2Cl2 and MeCN were distilled over CaH2, Et2O was distilled over LiAlH4 and then over Na. All other reagents were obtained from commercial sources and were used without further purification. Glassware used for reactions was dried at 150 °C in an oven and cooled in a desiccator. Melting points reported are of chromatographically purified materials and are uncorrected. 1H NMR spectra were recorded at 500 MHz and are referenced to the residual solvent resonance. 13C NMR spectra were recorded at 125 MHz in CDCl3 and are referenced to the solvent resonance. 2H NMR spectra were recorded at 77 MHz using CDCl3 as an internal standard. For this, first the 1H NMR spectrum of CDCl3 was recorded at 500 MHz and the residual protonated solvent resonance was set to δ 7.26 ppm. Next, the 2H NMR spectrum of the same sample was recorded at 77 MHz, and this showed a resonance at δ 7.24 ppm. From this analysis, CDCl3 was referenced to δ 7.24 ppm for all 2H NMR experiments. The 19F NMR spectrum was recorded at 282 MHz using CFCl3 as an internal standard. Chemical shifts (δ) are reported in parts per million (ppm) and coupling constants (J) are in hertz (Hz). HRMS analyses were performed using a TOF analyzer, the ionization methods are provided in the compound characterization.
Synthesis of 3-Ethynylpyridine
Step 1. Synthesis of 3-((triisopropylsilyl)ethynyl)pyridine
Following a literature procedure,31 in a clean, dry, round-bottom flask equipped with a stirring bar, 3-bromopyridine (122 µL, 1.26 mmol) was dissolved in iPr2NH (5 mL). TIPS-acetylene (336 µL, 1.512 mmol), PdCl2(Ph3P)2 (44.0 mg, 0.063 mmol) and CuI (12.0 mg, 0.063 mmol) were added, and the reaction mixture was stirred and heated at 100 °C under a reflux condenser. The reaction mixture was diluted with EtOAc (20 mL) and filtered through Celite. The filtrate was washed with deionized H2O (3 × 20 mL) and brine (15 mL). The organic layer was separated, dried over anhydrous Na2SO4, and concentrated under reduced pressure. Purification of the crude material, by passing it through a short silica gel plug with initial elution using hexanes followed by 5% EtOAc in hexanes, gave 294 mg (90% yield) of TIPS-protected ethynylpyridine as a pale-yellow, viscous liquid. Rf (SiO2/10% EtOAc in hexanes) = 0.40. 1H NMR (500 MHz, CDCl3): δ 8.69 (s, 1H, H-2), 8.51 (d, 1H, H-6, J = 4.1 Hz), 7.73 (dt, 1H, H-4, J = 1.9, 7.8 Hz), 7.22 (dd, 1H, H-5, J = 4.9, 7.8 Hz), 1.13 (s, 21H, Si(iPr)3). 13C NMR (125 MHz, CDCl3): δ 153.0, 148.8, 139.0, 123.0, 120.9, 103.8, 95.1, 18.7, 11.6. HRMS (ESI) calculated for C16H26NSi [M + H]+ 260.1829, found 260.1835.
Step 2. Synthesis of 3-ethynylpyridine33
Following a literature procedure,34 in a clean, dry, round-bottom flask equipped with a stirring bar, TIPS-protected ethynylpyridine (286 mg, 1.102 mmol) was dissolved in dry MeOH (15 mL). Powdered KOH (433 mg, 7.72 mmol) was added and the reaction mixture was stirred at 45 °C for 24 h. To the reaction mixture was added a saturated aqueous NH4Cl solution and the mixture was extracted with Et2O (3 × 15 mL). The organic layer was separated, washed with brine (10 mL), dried over anhydrous Na2SO4, and carefully evaporated. Purification of the crude material on a short silica gel column using 10% Et2O in hexanes yielded 51.0 mg (45% yield) of 3-ethynylpyridine as a white solid. Rf (SiO2/20% Et2O in hexanes) = 0.39. 1H NMR (500 MHz, CDCl3): δ 8.73 (s, 1H, H-2), 8.57 (d, 1H, H-6, J = 4.6 Hz), 7.77 (d, 1H, H-4, J = 7.8 Hz), 7.37 (t, 1H, H-5, J = 6.4 Hz), 3.22 (s, 1H, ≡CH).
Synthesis of Ethynylbenzene-D5
Step 1. Synthesis of triisopropyl(phenylethynyl)silane-D5
Following a literature procedure,31 in a clean, dry, round-bottom flask equipped with a stirring bar, bromobenzene-D5 (500 mg, 3.085 mmol) was dissolved in iPr2NH (12.5 mL). TIPS-acetylene (823 µL, 3.702 mmol), PdCl2(Ph3P)2 (108.3 mg, 0.154 mmol) and CuI (29.4 mg, 0.154 mmol) were added, and the reaction mixture was stirred and heated under a reflux condenser at 100 °C for 1.5 h, in a nitrogen atmosphere. The reaction mixture was diluted with EtOAc (20 mL) and filtered through Celite. The filtrate was washed with deionized H2O (3 × 20 mL) and brine (15 mL). The organic layer was separated, dried over anhydrous Na2SO4, and concentrated under reduced pressure. Purification of the crude material, by passing it through a short silica gel plug using hexanes as the eluting solvent gave 500 mg (61% yield) of TIPS-protected ethynylbenzene as a colorless, viscous liquid. Rf (hexanes/SiO2) = 0.70. 1H NMR (500 MHz, CDCl3): δ 1.16 (s, 21H, Si(iPr)3). 13C NMR (125 MHz, CDCl3): δ 131.8 (t, J = 24.8 Hz), 128.0 (t, J = 24.0 Hz), 127.8 (t, J = 24.5 Hz), 123.6, 107.3, 90.6, 18.9, 11.6. 2H NMR (77 MHz, CHCl3): δ 7.51, 7.33, 7.20. HRMS (ESI) calculated for C17H21D5Si [M]+ 263.2112, found 263.2101.
Step 2. Synthesis of ethynylbenzene-D530
By modifying a literature procedure,32 in a clean, dry, 25 mL round-bottom flask equipped with a stirring bar, TIPS-protected ethynylbenzene-D5 (700 mg, 2.656 mmol) was dissolved in dry Et2O (11 mL) with stirring. A 1 M solution of n-Bu4N+F− in THF (3.2 mL, 3.2 mmol) was added and the mixture was stirred for 5 min. The mixture was concentrated under a stream of nitrogen gas and loaded onto a short silica gel column. Elution with hexanes, followed by careful rotary evaporation of the fractions containing the product using a water bath at ≤ 65 °C, gave 171 mg (61% yield) of ethynylbenzene-D5 as a pale-yellow liquid. Rf (SiO2/hexanes) = 0.42. 1H NMR (500 MHz, CDCl3): δ 3.08 (s, 1H, ≡CH). 2H NMR (77 MHz, CHCl3): δ 7.52, 7.38, 7.35.
Synthesis of azides
The C-6 azido purine ribonucleoside precursor20 and 3’-azido-3’-deoxy-5’-O-(t-butyldimethylsilyl)thymidine35 were synthesized as described in the literature. Unless mentioned otherwise, all other azides were synthesized from the corresponding boronic acids.36
4-(t-Butyl)phenyl azide37
In a clean 10 mL round-bottom flask equipped with a stirring bar, 4-(t-butyl)phenylboronic acid (356 mg, 2 mmol) was dissolved in MeOH (6 mL). NaN3 (156 mg, 2.4 mmol) and CuSO4•5H2O (50 mg, 0.2 mmol) were added to the reaction mixture, and the mixture was stirred in an open flask at room temperature for 16 h. The solvent was evaporated under reduced pressure and EtOAc (10 mL) was added. The mixture was extracted with H2O (10 mL) and the aqueous layer was back extracted with EtOAc (3 × 10 mL). The combined organic layers were dried over Na2SO4 and concentrated under reduced pressure. Chromatography of the crude material on a short silica gel plug using hexanes yielded 311.5 mg (89% yield) of the title compound as an orange-red liquid. Rf (SiO2/hexanes) = 0.60. 1H NMR (500 MHz, CDCl3): δ 7.38 (d, 2H, Ar-H, J = 8.6 Hz), 6.98 (d, 2H, Ar-H, J = 8.6 Hz), 1.32 (s, 9H, t-Bu). 4-(t-Butyl)phenyl azide has previously been synthesized by diazotization of 4-t-butylaniline.37
m-Azidobenzonitrile38
Following a literature procedure,39 in a clean, dry, round-bottom flask equipped with a stirring bar, 3-aminobenzonitrile (200 mg, 1.69 mmol) was dissolved in dry MeCN (4 mL) and cooled to 0 °C. To the stirring solution, t-butyl nitrite (300 µL, 2.54 mmol) was added dropwise, followed by dropwise addition of Me3SiN3 (270 µL, 2.03 mmol). The mixture was warmed to room temperature and stirred for 2 h. This reaction mixture was diluted with EtOAc (15 mL) and washed with deionized H2O (3 × 15 mL), and brine (10 mL). The organic layer was separated, dried over anhydrous Na2SO4, and concentrated under reduced pressure. Chromatography of the crude material on a silica gel column using 5% EtOAc in hexanes yielded 190 mg (78% yield) of the title compound as a brown solid. Rf (SiO2/10% EtOAc in hexanes) = 0.30. 1H NMR (500 MHz, CDCl3): δ 7.49-7.42 (m, 2H), 7.29-7.26 (m, 2H). m-Azidobenzonitrile has previously been synthesized by diazotization of m-cyanoaniline.38
Phenyl azide-D527
Following a literature procedure,28 in a clean, dry, round-bottom flask equipped with a stirring bar, bromobenzene-D5 (400 mg, 2.468 mmol) was dissolved in 7:3 EtOH/H2O (4.8 mL). N,N’-Dimethylethylenediamine (40 µL, 0.37 mmol), NaN3 (321 mg, 4.936 mmol), Na ascorbate (24.4 mg, 0.123 mmol), and CuI (47 mg, 0.247 mmol) were added, and the mixture was stirred and heated at 100 °C under a reflux condenser for 2 h, in an argon atmosphere. The mixture was cooled to room temperature, and diluted with Et2O (20 mL) and deionized H2O (10 mL). The aqueous layer was separated and extracted with Et2O (3 × 20 mL). The organic layers were combined and washed with brine (10 mL), dried over anhydrous Na2SO4 and concentrated under reduced pressure. Chromatography of the crude material on a short silica gel column using hexanes yielded 261 mg (85% yield) of the title compound as a yellow liquid. Rf (SiO2/hexanes) = 0.50. 13C NMR (125 MHz, CDCl3): δ 139.8, 129.2 (t, J = 24.2 Hz), 124.3 (t, J = 24.2 Hz), 118.6 (t, J = 24.2 Hz). 2H NMR (77 MHz, CHCl3): δ 7.29, 7.08, 6.98.Phenyl azide-D5 has previously been synthesized by diazotization of aniline-D5.27
General procedure for the synthesis of deuterated triazoles (1-D to 14-D11)
In a clean, oven-dried, 10 mL round-bottom flask equipped with a stirring bar, was placed the appropriate azide (0.335 mmol) in 1.6 mL of dry CH2Cl2. To this stirring mixture the appropriate alkyne (0.67 mmol) was added. In a separate vial CuSO4•5H2O (8.4 mg, 0.0335 mmol, 10 mol %) was heated at 120 °C under vacuum until the color changed from blue to gray. Using this desiccated CuSO4, a 0.048 M solution was prepared in a glovebag by the addition of D2O (99.8% D, 0.7 mL), and this solution was transferred to the reaction mixture. To the reaction mixture a 0.096 M solution of Na ascorbate (13.3 mg in 0.7 mL of 99.8% D2O, 20 mol %) was added. The reaction mixture was sealed and stirred in a nitrogen atmosphere at room temperature until TLC showed consumption of azide. The organic layer of the reaction mixture was separated and the aqueous layer was back extracted with EtOAc (3 × 5 mL). The combined organic layer was washed with brine (5 mL), dried over anhydrous Na2SO4, and concentrated under reduced pressure. The crude material was purified by chromatography on a silica gel column. See compound headings below for details.
5-Deutero-1-phenyl-4-(p-tolyl)-1H-1,2,3-triazole (1-D)12a
Chromatography using hexanes followed by 20% EtOAc in hexanes gave 59.5 mg (75% yield) of 1-D as an off-white solid. M.p. = 156–158 °C. Rf (SiO2/25% EtOAc in hexanes) = 0.34. 1H NMR (500 MHz, CDCl3): δ 7.82-7.79 (m, 4H, Ar-H), 7.55 (t, 2H, Ar-H, J = 7.4 Hz), 7.46 (t, 1H, Ar-H, J = 7.3 Hz), 7.28 (d, 2H, Ar-H, J = 7.4 Hz), 2.41 (s, 3H, CH3). 13C NMR (125 MHz, CDCl3): δ 148.7, 138.5, 137.5, 129.9, 129.8, 128.8, 127.8, 126.1, 120.7, 117.2 (t, J = 30.4 Hz), 21.4. 2H NMR (77 MHz, CHCl3): δ 8.19. HRMS (ESI) calculated for C15H13DN3 [M + H]+ 237.1245, found 237.1248.
Assessment of %D incorporation using an external standard
CH2Cl2 (16 µL, 0.25 mmol) was added to a solution of the product (11.8 mg, 0.05 mmol) in CDCl3 (0.5 mL). The 1H NMR spectrum of this solution was acquired and the resonances were integrated. The signal at δ 5.30 ppm (CH2Cl2) was set to 10 whereby the signal at δ 8.16 ppm (residual triazolyl C5-H) integrated to 0.04. Thus, the %D incorporation in the reaction was estimated to be 4%.
5-Deutero-1,4-diphenyl-1H-1,2,3-triazole (2-D)
Chromatography using hexanes followed by 20% EtOAc in hexanes gave 53.5 mg (72% yield) of 2-D as a white solid. M.p. = 183–185 °C. Rf (SiO2/25% EtOAc in hexanes) = 0.34. 1H NMR (500 MHz, CDCl3): δ 7.92 (d, 2H, Ar-H, J = 8.1 Hz), 7.80 (d, 2H, Ar-H, J = 7.8 Hz), 7.55 (t, 2H, Ar-H, J = 7.8 Hz), 7.48-7.45 (m, 3H, Ar-H), 7.37 (t, 1H, Ar-H, J = 7.4 Hz). 13C NMR (125 MHz, CDCl3): δ 148.6, 137.4, 130.6, 130.0, 129.1, 128.9, 128.6, 126.2, 120.8, 117.6 (s, J = 28.1 Hz). 2H NMR (77 MHz, CHCl3): δ 8.22. HRMS (ESI) calculated for C14H11DN3 [M + H]+ 223.1089, found 223.1086.
5-Deutero-1-(4-methoxyphenyl)-4-(p-tolyl)-1H-1,2,3-triazole (3-D)
Chromatography using hexanes followed by 20% EtOAc in hexanes gave 89.0 mg (87% yield) of 3-D as a white solid. M.p. = 174–176 °C. Rf (SiO2/25% EtOAc in hexanes) = 0.26. 1H NMR (500 MHz, CDCl3): δ 7.80 (d, 2H, Ar-H, J = 7.6 Hz), 7.67 (d, 2H, Ar-H, J = 8.6 Hz), 7.26 (d, 2H, Ar-H, J = 7.6 Hz), 7.02 (d, 2H, Ar-H, J = 8.6 Hz), 3.87 (s, 3H, OCH3), 2.40 (s, 3H, CH3). 13C NMR (125 MHz, CDCl3): δ 159.9, 148.3, 138.4, 130.7, 129.7, 127.7, 125.9, 122.3, 117.5 (t, J = 28.9 Hz), 114.9, 55.8, 21.5. 2H NMR (77 MHz, CHCl3): δ 8.10. HRMS (ESI) calculated for C16H15DN3O [M + H]+ 267.1351, found 267.1356.
5-Deutero-4-(4-fluorophenyl)-1-(4-methoxyphenyl)-1H-1,2,3-triazole (4-D)
Chromatography using hexanes followed by 20% EtOAc in hexanes gave 90.5 mg (70% yield) of 4-D as an off-white solid. M.p. = 184–186 ° Rf (SiO2/25% EtOAc in hexanes) = 0.22. 1H NMR (500 MHz, CDCl3): δ 7.89-7.86 (m, 2H, Ar-H), 7.67 (d, 2H, Ar-H, J = 8.9 Hz), 7.14 (t, 2H, Ar-H, J = 8.6 Hz), 7.04 (d, 2H, Ar-H, J = 8.9 Hz), 3.88 (s, 3H, OCH3). 13C NMR (125 MHz, CDCl3): δ 163.1 (d, J = 247.7 Hz), 160.3, 147.5, 130.8, 127.8 (d, J = 8.1 Hz), 127.0 (d, J = 3.2 Hz), 122.4, 117.6 (t, J = 28.9 Hz), 116.1 (d, J = 21.8 Hz), 115.1, 55.9. 2H NMR (77 MHz, CHCl3): δ 8.09. 19F NMR (282 MHz, CDCl3): δ –113.8 (relative to CFCl3). HRMS (ESI) calculated for C15H12DFN3O [M + H]+ 271.1100, found 271.1105.
5-Deutero-1-(4-methoxyphenyl)-4-phenyl-1H-1,2,3-triazole (5-D)
Chromatography using hexanes followed by 20% EtOAc in hexanes gave 77.0 mg (91% yield) of 5-D as a white solid. M.p. = 166–169 °C. Rf (SiO2/25% EtOAc in hexanes) = 0.23. 1H NMR (500 MHz, CDCl3): δ 7.90 (d, 2H, Ar-H, J = 7.8 Hz), 7.69 (d, 2H, Ar-H, J = 8.9 Hz), 7.46 (t, 2H, Ar-H, J = 7.6 Hz), 7.37 (t, 1H, Ar-H, J = 7.4 Hz), 7.04 (d, 2H, Ar-H, J = 8.9 Hz), 3.88 (s, 3H, OCH3). 13C NMR (125 MHz, CDCl3): δ 160.2, 148.4, 130.9, 130.8, 129.1, 128.5, 126.1, 122.4, 117.8 (t, J = 28.3 Hz), 115.1, 55.9. 2H NMR (77 MHz, CHCl3): δ 8.16. HRMS (ESI) calculated for C15H13DN3O [M + H]+ 253.1194, found 253.1199.
1-(4-(t-Butyl)phenyl)-5-deutero-4-phenyl-1H-1,2,3-triazole (6-D)
Chromatography using hexanes followed by 20% EtOAc in hexanes gave 93.3 mg (85% yield) of 6-D as an off-white solid. M.p. = 153–156 °C. Rf (SiO2/25% EtOAc in hexanes) = 0.46. 1H NMR (500 MHz, CDCl3): δ 7.92 (d, 2H, Ar-H, J = 8.1 Hz), 7.70 (d, 2H, Ar-H, J = 8.5 Hz), 7.53 (d, 2H, Ar-H, J = 8.5 Hz), 7.44 (t, 2H, Ar-H, J = 7.6 Hz), 7.35 (dt, 1H, Ar-H, J = 1.0, 7.4 Hz), 1.38 (s, 9H, t-Bu). 13C NMR (125 MHz, CDCl3): δ 152.3, 148.3, 134.8, 130.5, 129.1, 128.5, 126.8, 126.0, 120.4, 117.8 (t, J = 28.9 Hz), 35.0, 31.5. 2H NMR (77 MHz, CHCl3): δ 8.20. HRMS (ESI) calculated for C18H19DN3 [M + H]+ 279.1715, found 279.1709.
5-Deutero-1-(naphthalen-1-yl)-4-phenyl-1H-1,2,3-triazole (7-D)
Chromatography using hexanes followed by 20% EtOAc in hexanes gave 80.0 mg (72% yield) of 7-D as a brown solid. M.p. = 86–88 °C. Rf (SiO2/25% EtOAc in hexanes) = 0.34. 1H NMR (500 MHz, CDCl3): δ 8.00-7.92 (m, 4H, Ar-H), 7.69 (d, 1H, Ar-H, J = 8.3 Hz), 7.59-7.49 (m, 4H, Ar-H), 7.46 (t, 2H, Ar-H, J = 7.7 Hz), 7.04 (dt, 1H, Ar-H, J = 1.0, 7.4 Hz). 13C NMR (125 MHz, CDCl3): δ 147.9, 134.5, 134.0, 130.6, 130.5, 129.1, 128.9, 128.6, 128.5, 128.1, 127.3, 126.1, 125.2, 123.7, 122.6, 122.2 (t, J = 28.6 Hz). 2H NMR (77 MHz, CHCl3): δ 8.17. HRMS (ESI) calculated for C18H13DN3 [M + H]+ 273.1245, found 273.1247.
5-Deutero-1-(4-methoxyphenyl)-4-(N-phthalimidomethyl)-1H-1,2,3-triazole (8-D)
Chromatography using hexanes followed by 40% EtOAc in hexanes gave 80.0 mg (71% yield) of 8-D as an off-white solid. M.p. = 181–183 °C. Rf (SiO2/50% EtOAc in hexanes) = 0.40. 1H NMR (500 MHz, CDCl3): δ 7.85 (dd, 2H, Ar-H, J = 3.1, 5.3 Hz), 7.72 (dd, 2H, Ar-H, J = 3.1, 5.3 Hz), 7.57 (d, 2H, Ar-H, J = 8.9 Hz), 6.97 (d, 2H, Ar-H, J = 8.9 Hz), 5.06 (s, 2H, CH2), 3.83 (s, 3H, OCH3). 13C NMR (125 MHz, CDCl3): δ 167.8, 159.9, 143.3, 134.3, 132.2, 130.5, 123.6, 122.4, 121.1 (t, J = 28.8 Hz), 114.8, 55.8, 33.2. 2H NMR (77 MHz, CHCl3): δ 7.97. HRMS (ESI) calculated for C18H14DN4O3 [M + H]+ 336.1201, found 336.1204.
1-(3-Cyanophenyl)-4-(pyridin-3-yl)-1H-1,2,3-triazole (9-H)23
In a clean, oven-dried, 10 mL round-bottom flask equipped with a stirring bar, m-azidobenzonitrile (57 mg, 0.335 mmol) was dissolved in CH2Cl2 (1.6 mL). 3-Ethynylpyridine (69 mg, 0.67 mmol) was added to the flask, followed by CuSO4•5H2O (8.4 mg, 0.0335 mmol), Na ascorbate (13.3 mg, 0.067 mmol), and H2O (1.4 mL). The mixture was stirred at room temperature for 48 h and then diluted with CH2Cl2 (5 mL). The aqueous layer was separated and back extracted with CH2Cl2 (3 × 5 mL). The combined organic layers were washed with brine (5 mL), dried over anhydrous Na2SO4, and concentrated under reduced pressure. Chromatography of the crude material on a short silica gel plug by sequential elution with 10% EtOAc in hexanes followed by EtOAc, gave 58.0 mg (70% yield) of 9-H as an off-white solid. M.p. = 215–217 °C (lit. 214–216 °C23). Rf (SiO2/EtOAc) = 0.34. 1H NMR (500 MHz, CDCl3): δ 9.13 (br s, 1H, Ar-H), 8.68 (br s, 1H, Ar-H), 8.33 (s, 1H, Ar-H), 8.31 (d, 1H, Ar-H, J = 7.7 Hz), 8.15 (t, 1H, Ar-H, J = 1.5 Hz), 8.11 (ddd, 1H, Ar-H, J = 1.1, 2.1, 8.1 Hz), 7.79 (dt, 1H, Ar-H, J = 1.2, 7.8 Hz), 7.73 (t, 1H, Ar-H, J = 7.9 Hz), 7.47 (br s, 1H, Ar-H). 13C NMR (125 MHz, CD3OD, 55 °C): δ 150.2, 147.8, 146.8, 139.1, 135.2, 133.7, 132.5, 128.4, 126.1, 125.7, 125.1, 121.4, 118.7, 115.4.
1-(3-Cyanophenyl)-5-deutero-4-(pyridin-3-yl)-1H-1,2,3-triazole (9-D)
Chromatography using 10% EtOAc in hexanes followed by EtOAc gave 52.8 mg (70% yield) of 9-D as an off-white solid. M.p. = 218–220 °C. Rf (SiO2/EtOAc) = 0.34. 1H NMR (500 MHz, CDCl3): δ 9.17 (br s, 1H, Ar-H), 8.73 (br s, 1H, Ar-H), 8.31 (d, 1H, Ar-H, J = 7.9 Hz), 8.15 (t, 1H, Ar-H, J = 1.6 Hz), 8.11 (ddd, 1H, Ar-H, J = 1.1, 2.2, 8.1 Hz), 7.79 (dt, 1H, Ar-H, J = 1.3, 7.7 Hz), 7.74 (t, 1H, Ar-H, J = 7.9 Hz), 7.47 (br s, 1H, Ar-H). 13C NMR (125 MHz, CD3OD, 55 °C): δ 150.2, 147.8, 146.8, 139.1, 135.2, 133.7, 132.5, 128.4, 126.1, 125.7, 125.1, 121.2 (t, J = 29.6 Hz), 118.7, 115.4. 2H NMR (77 MHz, CHCl3): δ 8.38. HRMS (ESI) calculated for C14H9DN5 [M + H]+ 249.0993, found 249.0997.
5’-O-(t-Butyldimethylsilyl)-3’-deoxy-3’-(5-deutero-4-(p-tolyl)-1H-1,2,3-triazol-1-yl)thymidine (10-D)
For this reaction 0.131 mmol of azide, 0.262 mmol of alkyne, 10 mol % of anhydrous CuSO4 in 273 µL of D2O, 20 mol % of Na ascorbate in 273 µl of D2O, and 0.65 mL of dry CH2Cl2 were used. Chromatography using hexanes followed by 45% EtOAc in hexanes gave 63.0 mg (96% yield) of 10-D as a white solid. M.p. = 176–178 °C. Rf (SiO2/75% EtOAc in hexanes) = 0.48. 1H NMR (500 MHz, CDCl3): δ 9.41 (br s, 1H, NH), 7.70 (d, 2H, Ar-H, J = 7.9 Hz), 7.49 (s, 1H, Ar-H), 7.22 (d, 2H, Ar-H, J = 7.9 Hz), 6.44 (t, 1H, H-1’, J = 6.5 Hz), 5.33 (app dt, 1H, H-3’, Japp = 4.6, 9.1 Hz), 4.49-4.47 (m, 1H, H-4’), 4.02 (dd, 1H, H-5’, J = 2.4, 11.6 Hz), 3.88 (dd, 1H, H-5’, J = 2.4, 11.6 Hz), 3.00 (app dt, 1H, H-2’, Japp = 5.9, 13.1 Hz), 2.63 (ddd, 1H, H-2’, J = 6.8, 8.5, 14.6 Hz), 2.37 (s, 3H, CH3), 1.94, (s, 3H, CH3), 0.93 (s, 9H, t-Bu), 0.13 and 0.12 (2s, 6H, SiCH3). 13C NMR (125 MHz, CDCl3): δ 163.8, 150.5, 148.6, 138.5, 135.5, 129.8, 127.7, 125.9, 118.5 (t, J = 28.7 Hz), 111.3, 85.8, 85.0, 62.9, 59.8, 38.8, 26.1, 21.4, 18.6, 12.7, –5.1, –5.2. 2H NMR (77 MHz, CHCl3): δ 7.85. HRMS (ESI) calculated for C25H35DN5O4Si [M + H]+ 499.2594, found 499.2600.
6-[5-Deutero-4-(p-tolyl)-1H-1,2,3-triazol-1-yl]-9-[2,3,5-tri-O-(t-butyldimethylsilyl)-β-D-ribofuranosyl]purine (11-D)
For this reaction 0.198 mmol of azide, 0.395 mmol of alkyne, 5 mol % of anhydrous CuSO4 in 0.2 mL of D2O, 10 mol % of Na ascorbate in 0.2 ml of D2O, and 0.95 mL of CH2Cl2 were used. Chromatography using hexanes followed by 15% EtOAc in hexanes gave 131.0 mg (88% yield) of 11-D as a pale-yellow, solid. M.p. = 89–91 °C. Rf (SiO2/20% EtOAc in hexanes) = 0.28. 1H NMR (500 MHz, CDCl3): δ 8.97 (s, 1H, Ar-H), 8.65 (s, 1H, Ar-H), 7.91 (d, 1H, Ar-H, J = 8.0 Hz), 7.29 (d, 2H, Ar-H, J = 8.0 Hz), 6.24 (d, 1H, H-1’, J = 5.1 Hz), 4.68 (t, 1H, H-2’, J = 4.7 Hz), 4.35 (t, 1H, H-3’, J = 3.9 Hz), 4.20 (app q, 1H, H-4’, Japp = 3.0 Hz), 4.06 (dd, 1H, H-5’, J = 3.5, 11.4 Hz), 3.85 (dd, 1H, H-5’, J = 2.4, 11.4 Hz), 2.41 (s, 3H, CH3), 0.99, 0.96, and 0.81 (3s, 27H, t-Bu), 0.19, 0.18, 0.13, 0.12, −0.02, and −0.21 (6s, 18H, Si-CH3). 13C NMR (125 MHz, CDCl3): δ 154.5, 152.5, 148.5, 145.2, 145.0, 138.8, 129.8, 127.5, 126.4, 123.5, 119.5 (t, J = 26 Hz), 88.9, 86.1, 76.5, 72.3, 62.8, 26.4, 26.1, 25.9, 21.5, 18.8, 18.3, 18.1, −4.1, −4.3, −4.4, −4.6, −4.7, −5.1. 2H NMR (77 MHz, CHCl3): δ 9.35. HRMS (ESI) calculated for C37H60DN7O4Si3Na [M + Na]+ 775.4048, found 775.4046.
5-Deutero-1-phenyl-D5-4-(p-tolyl)-1H-1,2,3-triazole (12-D6)
Chromatography using hexanes followed by 20% EtOAc in hexanes gave 60.5 mg (75% yield) of 12-D6 as an off-white solid. M.p. = 153–155 °C. Rf (SiO2/50% EtOAc in hexanes) = 0.60. 1H NMR (500 MHz, CDCl3): δ 7.83 (d, 2H, Ar-H, J = 7.8 Hz), 7.29 (d, 2H, Ar-H, J = 7.8 Hz), 2.42 (s, 3H, -CH3). 13C NMR (125 MHz, CDCl3): δ 148.6, 138.5, 137.2, 129.8, 129.5 (t, J = 25.0 Hz), 128.4 (t, J = 25.0 Hz), 127.6, 126, 120.3 (t, J = 24.9 Hz), 117.2 (t, J = 29.4 Hz), 21.5. 2H NMR (77 MHz, CHCl3): δ 8.19, 7.82, 7.58, 7.49. HRMS (ESI) calculated for C15H8D6N3 [M + H]+ 242.1559, found 242.1553.
5’-O-(t-butyldimethylsilyl)-3’-deoxy-3’-(5-deutero-4-(phenyl-D5)-1H-1,2,3-triazol-1-yl)thymidine (13-D6)
For this reaction 0.131 mmol of azide, 0.262 mmol of alkyne, 10 mol % of anhydrous CuSO4 in 0.273 mL of D2O, 20 mol % of Na ascorbate in 0.273 ml of D2O, and 0.65 mL of dry CH2Cl2 were used. Chromatography using hexanes followed by 50% EtOAc in hexanes gave 61.0 mg (95% yield) of 13-D6 as a white solid. M.p. = 96–98 °C. Rf (SiO2/50% EtOAc in hexanes) = 0.21. 1H NMR (500 MHz, CDCl3): δ 9.20 (br s, 1H, NH), 7.50 (s, 1H, Ar-H), 6.45 (t, 1H, H-1’, J = 6.5 Hz), 5.34 (app dt, 1H, H-3’, Japp = 4.6, 8.8 Hz), 4.49-4.51 (m, 1H, H-4’), 4.03 (dd, 1H, H-5’, J = 2.1, 11.5 Hz), 3.88 (dd, 1H, H-5’, J = 1.8, 11.5 Hz), 3.01 (ddd, 1H, H-2’, J = 5.2, 6.3, 14.0 Hz), 2.64 (ddd, 1H, H-2’, J = 6.5, 8.1, 14.3 Hz), 1.95 (s, 3H, CH3), 0.94 (s, 9H, t-Bu), 0.14 and 0.13 (2s, 6H, SiCH3). 13C NMR (125 MHz, CDCl3): δ 163.8, 150.5, 148.4, 135.5, 130.1, 128.6 (t, J = 23.8 Hz), 128.1 (t, J = 23.0 Hz), 125.6 (t, J = 24.1 Hz), 118.7 (t, J = 28.8 Hz), 111.4, 85.6, 85.0, 62.8, 59.8, 38.9, 26.1, 18.6, 12.8, −5.1, −5.2. 2H NMR (77 MHz, CHCl3): δ 7.87, 7.45. HRMS (ESI) calculated for C24H28D6N5O4Si [M + H]+ 490.2751, found 490.2766.
5-Deutero-1,4-diphenyl-D10-1H-1,2,3-triazole (14-D11)
Chromatography using hexanes followed by 20% EtOAc in hexanes gave 58.4 mg (72% yield) of 14-D11 as an off-white solid. M.p. = 183–186 °C. Rf (SiO2/50% EtOAc in hexanes) = 0.60. 13C NMR (125 MHz, CDCl3): δ 148.5, 137.2, 130.3, 129.5 (t, J = 24.3 Hz), 128.6 (t, J = 23.3 Hz), 128.5(t, J = 23.1 Hz), 128.1 (t, J = 21.8 Hz), 125.7 (t, J = 24.2 Hz), 120.3 (t, J = 25.2 Hz), 117.6 (t, J = 26.9 Hz). 2H NMR (77 MHz, CHCl3): δ 8.23, 7.94, 7.83, 7.58, 7.49. HRMS (ESI) calculated for C14HD11N3 [M + H]+ 233.1716, found 233.1717.
Assessment of %D in 14-D11
CH2Cl2 (16 µL, 0.25 mmol) was added to the solution of 14-D11 (11.7 mg, 0.05 mmol) in CDCl3 (0.5 mL). The 1H NMR spectrum of this solution was acquired and the resonances were integrated. The signal at δ 5.30 ppm (CH2Cl2) was set to 10 whereby the signal at δ 8.19 ppm (residual triazolyl C5-H) integrated to 0.06. Thus, the %D incorporation in the reaction was estimated to be 94%.
Competitive Experiment using H2O and D2O
In a clean, oven-dried, 10 mL round-bottom flask equipped with a stirring bar, was placed the phenyl azide (40.0 mg, 0.335 mmol) in dry CH2Cl2 (1.6 mL). To this stirring solution 4-ethynyltoluene (85 µL, 0.67 mmol) was added. In a separate vial CuSO4•5H2O (8.4 mg, 0.0335 mmol, 10 mol %) was heated at 120 °C under vacuum until the color changed from blue to gray. Using this dehydrated CuSO4, a 0.048 M solution was prepared in a glovebag by the addition of D2O (99.8% D, 0.7 mL) and this solution was transferred to the reaction mixture. To the reaction mixture a 0.096 M solution of Na ascorbate (13.3 mg in 0.7 mL of H2O, 20 mol %) was then added. The reaction mixture was sealed and stirred under nitrogen gas at room temperature until TLC showed consumption of azide. The organic layer of the reaction mixture was separated and the aqueous layer was back extracted with EtOAc (3 × 5 mL). The organic layers were combined and washed with brine (5 mL), dried over anhydrous Na2SO4, and concentrated under reduced pressure. The crude material was purified by chromatography on a silica gel column to give 60 mg of an off-white solid. On the basis of the 1H NMR analysis, this mixture contained 15.6 mg (19.7 %) of 1-D and 44.4 mg (56.3 %) of 1-H. Rf (25% EtOAc in hexanes/SiO2) = 0.34.
Assessment of the amounts of 1-H and 1-D in the product mixture
CH2Cl2 (16 µL, 0.25 mmol) was added to a solution of the product mixture (11.8 mg, 0.05 mmol) in CDCl3 (0.5 mL). The 1H NMR spectrum of this solution was acquired and the resonances were integrated. The signal at δ 5.30 ppm (CH2Cl2) was set to 10 whereby the signal at δ 8.16 ppm (residual triazolyl C5-H) integrated to 0.77. On the basis of these values the amount of 1-H was estimated at 9.058 mg (77%) and that of 1-D was estimated as 2.741 mg (23%).
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by NIH (NIAID) Grant AI094545-01. Infrastructural support at CCNY was provided by National Institutes of Health Grants 2G12RR03060-26A1 from the National Center for Research Resources and by 8G12MD007603-27 from the National Institute on Minority Health and Health Disparities. We thank Mr. Gerry Guilfoy of Cambridge Isotope Laboratories, Inc. for a sample of 99.8% D2O, Ms. Nonka Sevova and Dr. Bill Boggess (University of Notre Dame Mass Spectrometry and Proteomics Facility) for some of the HRMS analyses, which were supported by NSF Grant CHE-0741793.
Footnotes
SUPPORTING INFORMATION AVAILABLE
Spectral data for 1-D to 14-D11, 9-H, as well as for other relevant intermediates. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.(a) Huisgen R, Szeimies G, Möbius L. Chem. Ber. 1967;100:2492–2507. [Google Scholar]; (b) Huisgen R, Knorr R, Möbius L, Szeimies G. Chem. Ber. 1965;98:4041–4021. [Google Scholar]; (c) Huisgen R. Angew. Chem. Int. Ed. 1963;2:633–645. [Google Scholar]; (d) Huisgen R. Angew. Chem. Int. Ed. 1963;2:565–598. [Google Scholar]
- 2.Tornøe CW, Christensen C, Meldal M. J. Org. Chem. 2002;67:3057–3064. doi: 10.1021/jo011148j. [DOI] [PubMed] [Google Scholar]
- 3.Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. Angew. Chem. Int. Ed. 2002;41:2596–2599. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 4.For reviews see: ; (a) Kolb HC, Finn MG, Sharpless KB. Angew. Chem. Int. Ed. 2001;40:2004–2021. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]; (b) Gil MV, Arévalo MJ, López O. Synthesis. 2007:1589–1620. [Google Scholar]
- 5.Ahlquist M, Fokin VV. Organometallics. 2007;26:4389–4391. [Google Scholar]
- 6.Rodionov VO, Fokin VV, Finn MG. Angew. Chem. Int. Ed. 2005;44:2210–2215. doi: 10.1002/anie.200461496. [DOI] [PubMed] [Google Scholar]
- 7.Himo F, Lovell T, Hilgraf R, Rostovtsev VV, Noodleman L, Sharpless KB, Fokin VV. J. Am. Chem. Soc. 2005;127:210–216. doi: 10.1021/ja0471525. [DOI] [PubMed] [Google Scholar]
- 8.Cantillo D, Àvalos M, Babiano R, Cintas P, Jiménez JL, Palacios JC. Org. Biomol. Chem. 2011;9:2952–2958. doi: 10.1039/c0ob01001d. [DOI] [PubMed] [Google Scholar]
- 9.Bew SP, Hiatt-Gipson GD, Lovell JA, Poullain C. Org. Lett. 2012;14:456–459. doi: 10.1021/ol2029178. [DOI] [PubMed] [Google Scholar]
- 10.Zhou Y, Lecourt T, Micouin L. Angew Chem. Int. Ed. 2010;49:2607–2610. doi: 10.1002/anie.200907016. [DOI] [PubMed] [Google Scholar]
- 11.Shao C, Wang X, Xu J, Zhao J, Zhang Q, Hu Y. J. Org. Chem. 2010;75:7002–7005. doi: 10.1021/jo101495k. [DOI] [PubMed] [Google Scholar]
- 12.(a) Chuprakov S, Chernyak N, Dudnik AS, Gevorgyan V. Org. Lett. 2007;9:2333–2336. doi: 10.1021/ol070697u. (see the Supporting Information). [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Couty F, Durrat F, Prim D. Tetrahedron Lett. 2004;45:3725–3728. [Google Scholar]; (c) Ohta S, Kawasaki I, Uemura T, Yamashita M, Yoshioka T, Yamaguchi S. Chem. Pharm. Bull. 1997;45:1140–1145. [Google Scholar]
- 13.Krasiński A, Fokin VV, Sharpless KB. Org. Lett. 2004;6:1237–1240. doi: 10.1021/ol0499203. [DOI] [PubMed] [Google Scholar]
- 14.Gonda Z, Lőrincz K, Novák Z. Tetrahedron. 2010;51:6275–6277. [Google Scholar]
- 15.In reference 7, the authors indicate that conducting CuAAC in D2O leads to quantitative deuteration at the C-5 position (reference 18 of that paper). However, no other details are available.
- 16.Kolb HC, Sharpless KB. Drug Discovery Today. 2003;24:1128–1137. doi: 10.1016/s1359-6446(03)02933-7. [DOI] [PubMed] [Google Scholar]
- 17.Appendino G, Bacchiega S, Minassi A, Cascio MG, De Petrocellis L, Di Marzo V. Angew. Chem. Int. Ed. 2007;46:9312–9315. doi: 10.1002/anie.200703590. [DOI] [PubMed] [Google Scholar]
- 18.Agnell YL, Burgess K. Chem. Soc. Rev. 2007;36:1674–1689. doi: 10.1039/b701444a. [DOI] [PubMed] [Google Scholar]
- 19.(a) Tung R. Innovations Pharm. Tech. 2010;32:24–26,28. [Google Scholar]; (b) Sanderson K. Nature. 2009;458:269. doi: 10.1038/458269a. [DOI] [PubMed] [Google Scholar]; (c) Yarnell AT. Chem. Eng. News. 2009;87:36–39. [Google Scholar]; (d) O’Driscoll C. Chem. Ind. 2009:24–26. [Google Scholar]; (e) Agres T. Drug Discovery Dev. 2009;12:6–8. [Google Scholar]
- 20.Lakshman MK, Singh MK, Parrish D, Balachandran R, Day BW. J. Org. Chem. 2010;75:2461–2473. doi: 10.1021/jo902342z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee B-Y, Park SR, Jeon HB, Kim KS. Tetrahedron Lett. 2006;47:5105–5109. [Google Scholar]
- 22.Pagliai F, Pirali T, Del Grosso E, Di Brisco R, Tron GC, Sorba G, Genazzani AA. J. Med. Chem. 2006;49:467–470. doi: 10.1021/jm051118z. [DOI] [PubMed] [Google Scholar]
- 23.Dahl BH, Olsen GM, Christensen JK, Peters D. PCT Int. Appl. 2010 2010026134. [Google Scholar]
- 24.Lin J, Roy V, Wang L, You L, Agrofoglio LA, Deville-Bonne D, McBrayer TR, Coats SJ, Schinazi RF, Eriksson S. Bioorg. Med. Chem. 2010;18:3261–3269. doi: 10.1016/j.bmc.2010.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olgun A, Öztürk K, Bayr S, Akman S, Erbil MK. Ann. N.Y. Acad. Sci. 2007;1100:400–403. doi: 10.1196/annals.1395.044. (ionization constant of H2O = 1.008 × 10−14 and that of D2O = 1.95 × 10−15; pH of H2O = 6.998 and pD D2O = 7.355). Thus, the ratio of H+ to D+ ion concentration of H2O and D2O = 2.28. [DOI] [PubMed] [Google Scholar]
- 26.Phenyl azide-D527 was synthesized from bromobenzene-D5 as described for the unlabeled compound in reference 28.
- 27.Bencivenni G, Cesari R, Nanni D, El Mkami H, Walton JC. Org. Biomol. Chem. 2010;8:5097–5104. doi: 10.1039/c0ob00084a. [DOI] [PubMed] [Google Scholar]
- 28.Andersen J, Madsen U, Björkling F, Liang X. Synlett. 2005:2209–2213. [Google Scholar]
- 29.Ethynylbenzene-D530 was synthesized in two steps from bromobenzene-D5 via literature procedures. First, triisopropyl(phenylethynyl)silane-D5 was synthesized via a Sonogashira cross-coupling as reported in reference 31. Next, triisopropyl(phenylethynyl)silane-D5 was desilylated as reported in reference 32.
- 30.Dougherty TK, Lau KSY, Hedberg FL. J. Org. Chem. 1983;48:5273–5280. [Google Scholar]
- 31.Sakai N, Komatsu R, Uchida N, Ikeda R, Konakahara T. Org. Lett. 2010;12:1300–1303. doi: 10.1021/ol100180j. [DOI] [PubMed] [Google Scholar]
- 32.Keller JM, Schanze KS. Organometallics. 2009;28:4210–4216. [Google Scholar]
- 33.Alagille D, Baldwin RM, Roth BL, Wroblewski JT, Grajkowska E, Tamagnan GD. Bioorg. Med. Chem. 2005;13:197–209. doi: 10.1016/j.bmc.2004.09.042. [DOI] [PubMed] [Google Scholar]
- 34.Pahadi NK, Camacho DH, Nakamura I, Yamamoto Y. J. Org. Chem. 2005;71:1152–1155. doi: 10.1021/jo052262v. [DOI] [PubMed] [Google Scholar]
- 35.Varizhuk A, Kochetkova S, Kolganova N, Timofeev E, Florent’ev V. Russ. J. Bioorg. Chem. 2010;36:199–206. doi: 10.1134/s1068162010040138. [DOI] [PubMed] [Google Scholar]
- 36.Tao C-Z, Cui X, Li J, Liu A-X, Liu L, Guo Q-X. Tetrahedron Lett. 2007;48:3525–3529. [Google Scholar]
- 37.Lee S, Hua Y, Park H, Flood AH. Org. Lett. 2010;12:2100–2102. doi: 10.1021/ol1005856. [DOI] [PubMed] [Google Scholar]
- 38.Leffler JE, Temple RD. J. Am. Chem. Soc. 1967;89:5235–5246. [Google Scholar]
- 39.Barral K, Moorhouse AD, Moses JE. Org. Lett. 2007;9:1809–1811. doi: 10.1021/ol070527h. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.