Abstract
Objectives
The non-nucleoside reverse transcriptase inhibitor MC1220 has potent in vitro activity against HIV type 1 (HIV-1). A liposome gel formulation of MC1220 has previously been reported to partially protect rhesus macaques against vaginal challenge with a simian HIV (SHIV). Here, we describe the pre-clinical development of an MC1220-releasing silicone elastomer vaginal ring (SEVR), including pharmacokinetic (PK) and efficacy studies in macaques.
Methods
In vitro release studies were conducted on SEVRs loaded with 400 mg of MC1220, using simulated vaginal fluid (SVF, n = 4) and 1 : 1 isopropanol/water (IPA/H2O, n = 4) as release media. For PK evaluation, SEVRs were inserted into adult female macaques (n = 6) for 30 days. Following a 1week washout period, fresh rings were placed in the same animals, which were then challenged vaginally with RT-SHIV162P3 once weekly for 4 weeks.
Results
SEVRs released 1.66 and 101 mg of MC1220 into SVF and IPA/H2O, respectively, over 30 days, the differential reflecting the low aqueous solubility of the drug. In macaque PK studies, MC1220 was consistently detected in vaginal fluid (peak 845 ng/mL) and plasma (peak 0.91 ng/mL). Kaplan–Meier analysis over 9weeks showed significantly lower infection rates for animals given MC1220-containing SEVRs than placebo rings (hazard ratio 0.20, P = 0.0037).
Conclusions
An MC1220-releasing SEVR partially protected macaques from vaginal challenge. Such ring devices are a practical method for providing sustained, coitally independent protection against vaginal exposure to HIV-1.
Keywords: HIV-1 microbicide, silicone elastomer vaginal ring, rhesus macaque, pharmacokinetics, RT-SHIV challenge
Introduction
Microbicide-releasing vaginal rings are currently among the leading strategies being evaluated for impeding the heterosexual transmission of HIV type 1 (HIV-1).1–9 One attractive feature of this method is coital independence; unlike short-lasting gels that must be applied soon before sexual intercourse, a single vaginal ring might provide continuous and sustained protection for ≥1month. Furthermore, non-adherence to protocols has a serious adverse effect on the outcome of clinical trials of antiretroviral drugs (ARVs) that must be applied, or taken orally, on a regular basis. This problem might be lessened if the drug could be delivered continuously from a vaginal ring. A matrix-type, silicone elastomer vaginal ring (SEVR) containing 25 mg of dapivirine, a potent non-nucleoside reverse transcriptase inhibitor (NNRTI), has successfully completed early clinical testing and is scheduled to enter Phase III efficacy and long-term safety trials in 2012.3,4 Pharmacokinetic (PK) studies with the 25 mg dapivirine ring during 28 days of continuous use in women have shown that the drug is maintained in vaginal fluid at concentrations >20 μg/mL. Plasma levels were very much lower (<1 ng/mL), which is important from the perspective of minimizing the emergence of resistant strains in HIV-1-infected women who may use the ring without knowing their infection status.3 However, a critical uncertainty is whether the concentrations of active drug in the vagina are sufficient for protection against HIV-1 transmission. That kind of information should become available in 2014–15, when the results from the planned Phase III trial are reported. Until then, the only way to gauge the protective potential of vaginal rings is to conduct experiments in a macaque vaginal challenge model.7,8,10–12
Here, we describe the results of a rhesus macaque PK and vaginal challenge study using vaginal rings of a similar design to those used in women, but of an appropriately reduced size.12 The microbicide candidate we tested was MC1220, an NNRTI with potent anti-HIV-1 activity in vitro and similar physicochemical properties to dapivirine. In earlier studies, MC1220 partially protected Chinese rhesus macaques (2/5 infected) when administered vaginally in a liposomal gel formulation (0.5% MC1220) followed by a vaginal challenge with RT-SHIV, a hybrid virus containing the reverse transcriptase of HIV-1 in the backbone of SIVmac239 that was engineered to be appropriately susceptible to this class of ARV.13–15 In the present study, we formulated MC1220 in an SEVR, obtained in vitro and in vivo PK data, and then conducted a vaginal challenge experiment using RT-SHIV162P3.
Materials and methods
Materials
A platinum-catalysed, medical grade, silicone elastomer two-part kit (LSR9-9509-30/DDU-4320) was supplied by NuSil Silicone Technology Inc. (Carpinteria, CA, USA). MC1220 (99%) was provided by Cittadella Universitaria (Monserrato, Italy). RT-SHIV162P3 was obtained from the NIH AIDS Reagent and Reference Program, contributed by James Smith of the Centers for Disease Control and expanded by Ranajit Pal of Advanced BioSciences Laboratories. Isopropanol (IPA), HPLC-grade acetonitrile, dichloromethane, HPLC-grade methanol and potassium chloride were obtained from VWR International Ltd (Dublin, Ireland). Simulated vaginal fluid (SVF, pH 4.2) was prepared using analytical-grade reagents according to the recipe described previously.16 HPLC-grade water was obtained using a Millipore Direct-Q 3 UV Ultrapure Water System (Watford, UK). Trifluoroacetic acid (TFA), 19-norethindrone (N4128), hydrochloric acid (0.5 M), potassium hydroxide (concentrate) and potassium hydrogen phthalate were all obtained from Sigma–Aldrich (Gillingham, UK). Thermo Scientific (Loughborough, UK) supplied phosphate buffer (pH 7) for use with the Sirius instrument.
Determination of MC1220 physicochemical characteristics
Ionization constants (pKa) for MC1220 were measured with a Sirius T3 instrument (Sirius Analytical Instruments, UK). Titrations were performed in 0.15 M KCl under a nitrogen atmosphere. Apparent ionization constants (psKa) were determined in methanol/water mixtures with extrapolation to 0% methanol to derive aqueous pKa values. The solubilities of MC1220 in water and SVF were determined in quadruplicate using a shake flask method. Excess compound (25 mg) was added to 10 mL of HPLC-grade water or SVF, vortexed for 30 s and placed in a rotating orbital incubator (37°C, 60 rpm; Infors HT Unitron, Switzerland) for 72 h. The samples were equilibrated at room temperature before filtering, using a 0.22 μm mixed cellulose nitrate and acetate ester membrane syringe filter (Millex®; Millipore, Ireland), and analysis by HPLC.
Thermal gravimetric analysis (TGA) of the supplied MC1220 was conducted using a TA Instruments Q500™ Thermogravimetric Analyser and standard aluminium pans (part number 900779.901) (both TA Instruments, New Castle, DE, USA). Samples (5 mg) were heated in open pans at a rate of 10°C/min from room temperature to 300°C under a nitrogen atmosphere.
Differential scanning calorimetry (DSC) analysis was performed on MC1220 (as supplied) and on a sample of MC1220-loaded, cured silicone elastomer (obtained during SEVR manufacture), using a calibrated DSC 2920™ machine (TA Instruments). Each sample (10 mg) was accurately weighed into an aluminium pan and heated from room temperature to 220°C at a rate of 10°C/min under a nitrogen atmosphere, alongside an open empty reference pan.
Ring manufacture and in vitro testing
Macaque-sized [25 × 6.0 mm (overall and cross-sectional diameter, respectively)] matrix-type SEVRs containing 400 mg of MC1220 were manufactured by reaction injection moulding of a 23% w/w mixture of MC1220 in LSR9-9509-30 platinum-catalysed silicone elastomer at 80°C, according to a method described previously.8 The rings weighed 1.831 g (±0.003 g).
Mechanical compression testing of rings containing MC1220 was performed using a TA.XT2 Texture Analyser (Stable Micro Systems, Godalming, UK), both before and after a 30 day in vitro release study. Each ring was placed vertically in a specially designed ring holder on the base of the Texture Analyser and compressed five times through a distance of 2.50 mm at a rate of 2 mm/s. Eight placebo rings were also tested, both before and after storage for 30 days in a 1 : 1 mixture of IPA/H2O (n = 4) or in SVF (n = 4).
The in vitro release of MC1220 from eight rings was assessed over a 30 day period. Each ring was placed in a glass flask containing 200 mL of IPA/H2O (n = 4) or 50 mL of SVF (n = 4). Mixtures of IPA/H2O have been widely used in the release testing of SEVRs, since they are much more effective than aqueous media, such as SVF, at dissolving poorly water-soluble, non-polar drug compounds.1,2,8 The flasks were sealed and placed in a rotating orbital incubator (37°C, 60 rpm, throw 25 mm). After 24 h (±15 min), each flask was removed from the incubator and a sample of the release medium was retained for HPLC analysis. The remaining release medium was discarded and replaced with a fresh aliquot (100 mL of IPA/H2O or 25 mL of SVF). This procedure was carried out on a daily basis, except at weekends; higher volumes of release medium were added to the flasks on Fridays so as to maintain sink conditions until the following Monday (200 mL of IPA/H2O or 50 mL of SVF).
MC1220 release from the rings was quantified by reverse-phase HPLC using a Waters system (1525 Binary HPLC pump, 717 Plus Autosampler, In-line Degasser AF Unit; Waters Corporation, Milford, UK) with UV detection at 210 nm (2487 Dual λ Absorbance Detector). A 20 μL aliquot of each sample was injected onto a Phenomenex® Luna 5 μm C18(2) 100 Å column (150 × 4.6 mm; Phenomenex, Cheshire, UK) held at 30°C. HPLC was conducted in isocratic mode with a mobile phase of 0.1% TFA in HPLC-grade water (40%) and HPLC-grade acetonitrile (60%) at a flow rate of 1 mL/min. MC1220 had a retention time of 3 min. Standard solutions of MC1220 in IPA/H2O (0.5–50 μg/mL) were used to construct a linear calibration plot for each set of samples analysed (R2 = 0.998).
In vitro inhibition of RT-SHIV162P3 replication
Inhibition of RT-SHIV162P3 replication was measured in the TZM-bl cell assay, as described previously.17 MC1220 was compared with another NNRTI, the licensed drug efavirenz. Five viruses were used: RT-SHIV162P3 (the challenge virus in the macaque study), SHIV162P3, and the HIV-1 isolates SF162, DJ258 and NL4-3. The viruses all have the R5 phenotype, except for NL4-3 (X4), their env genes are all derived from Clade B, except for DJ258 (Clade A), and their reverse transcriptase enzymes are all from HIV-1, except for SHIV162P3 (SIV). The effects of MC1220 and efavirenz on the viability of TZM-bl cells were assessed by the MTT assay.18
Macaque PK study
A PK study in adult female cycling rhesus macaques (age 4–14 years) was performed at the Tulane National Primate Research Center in accordance with recommendations in the Guide for the Care and Use of Laboratory Animals of the NIH, and following approval from the Tulane University Institutional Animal Care and Use Committee. Ring application and vaginal fluid and plasma sampling methods have been described previously.8 The animals were not treated with Depo-Provera at any time during these studies.
Quantification of MC1220 in biological samples
Plasma and vaginal fluid MC1220 concentrations were quantified by gradient reverse-phase HPLC (Prominence, Shimadzu) coupled to a triple-quadrupole mass spectrometer (API3200; Applied Biosystems). The method was similar to that described previously for maraviroc, with the following changes.8,19 An internal standard (13C,2H3-MC1220) in 200 μL of acetonitrile was added to 25 μL of either plasma or vaginal fluid. Supernatant (100 μL) was diluted 1 : 1 with the aqueous mobile phase before analysis. The linear range in plasma was 0.25–10 ng/mL and in vaginal fluid was 5–5000 ng/mL. Chromatography was performed on a 50 × 2 mm, 3 μm Polaris C18 Ether column (Varian). The initial mobile-phase composition, a 60 : 40 mixture of 0.1% formic acid in water and acetonitrile, was held for 0.5 min and then increased to 70% organic at 1.0 min, with a 2 min hold at 70% organic before equilibration to the initial conditions. Analytes were detected by LC/MS as described previously, except that the temperature was 550°C and the ion-spray voltage was 4500 V (MC1220 m/z 294 → 260; 13C,2H3-MC1220 m/z 298 → 260).
Pre- and post-use content of MC1220 rings
The MC1220 content of four unused active rings was measured to determine the initial drug loading. The residual drug contents of all rings used in in vitro and in vivo experiments were also quantified. Each ring was cut into small sections and placed in a round-bottomed glass flask containing 95 mL of dichloromethane and 5 mL of a 5 mg/mL solution of norethindrone in methanol (internal standard). A condensing column was fitted to each flask and MC1220 was extracted from the ring sections by refluxing for 2 h. After cooling, a 2 mL aliquot was removed from each flask, evaporated to dryness, reconstituted in 10 mL of methanol and then diluted (1 : 5) to obtain a sample suitable for HPLC analysis. Samples were analysed by reverse-phase HPLC using a similar method to that outlined above (mobile-phase composition: 1 : 1 mix of water with 0.1% TFA and acetonitrile). MC1220 and norethindrone retention times were 4.2 and 5.8 min, respectively. A standard solution of MC1220 and norethindrone in methanol was used to quantify the mass of MC1220 in each ring.
Macaque challenge study
Female rhesus macaques aged from 13 to 21 years were challenged vaginally with RT-SHIV162P3 (250 TCID50 in 1 mL of culture medium, where TCID50 is the median tissue culture infective dose) once a week for 4 weeks.20 The animals were not pre-treated with Depo-Provera. The infection status of the animals was determined by measuring plasma viraemia, using a quantitative RT–PCR assay performed by the University of Wisconsin AIDS Vaccine Research Laboratory.
Statistical analyses
Statistical results and AUC were derived using GraphPad Prism software. Residual content data were analysed using one-way ANOVA with Tukey's post hoc test. Significance was noted when P < 0.05. The forces required to compress rings (active and placebo) before and after 30 days of storage in IPA/H2O or SVF were compared for each set of rings using a two-tailed paired t-test. The hazard ratio was derived from the Kaplan–Meier plot and the significance of the difference in the infection rate over time was analysed by the log-rank test. Peak viral loads were compared by a two-tailed Mann–Whitney U-test.
Results
Solubility and pKa values for MC1220
The experimental solubility values for MC1220 in water and SVF at ambient temperature (∼20°C) were 0.31 ± 0.08 and 0.77 ± 0.08 μg/mL, respectively. The ionization profile for MC1220 as a function of pH shows two pKa values (Figure 1a). The first, pKa = 2.69 ± 0.14, is attributable to the loss of a proton from the protonated dimethylamine moiety that predominates at low pH (Figure 1a and b); the second, pKa = 10.74 ± 0.15, is due to deprotonation of the pyrimidinone moiety in the neutral form of the molecule that predominates at intermediate pH values (Figure 1b and c).
Figure 1.
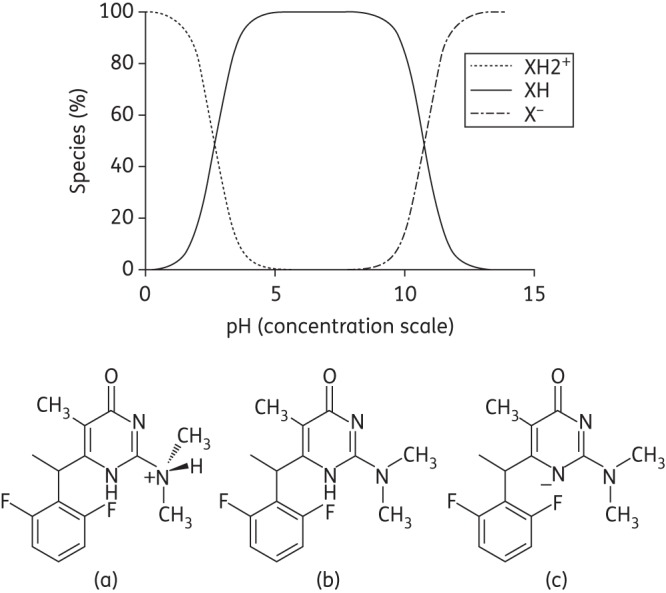
MC1220 ionisation profile as a function of pH. The molecule primarily exists in the single positively charged state (a), the neutral state (b) and the negatively charged state (c) at low, medium and high pH values, respectively.
Thermal analysis of MC1220
The thermal stability of MC1220 under the cure-temperature conditions used for SEVR manufacture (i.e. 80°C) was determined by TGA. No weight change was observed below 200°C (Figure 2a), confirming that no volatile MC1220 degradation products were likely formed during ring manufacture.
Figure 2.
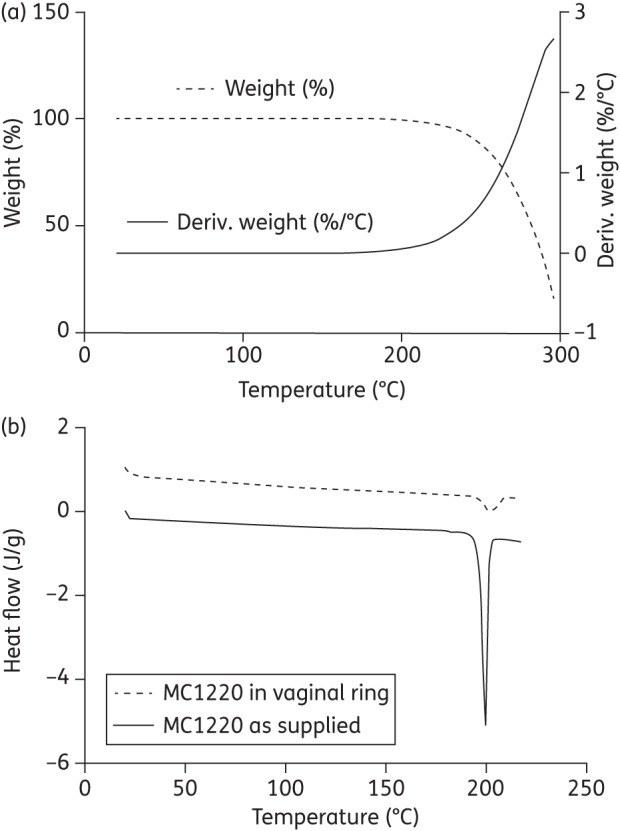
(a) TGA trace illustrating how the weight of MC1220 (dashed line, expressed as % of original weight) and the rate of change of weight (solid line) vary with temperature. Deriv. weight is the first derivative of the weight loss curve. (b) DSC traces of MC1220 both as supplied and in a sample of cured silicone elastomer at 23% w/w.
DSC profiles for MC1220, both as supplied and after incorporation (at 23% w/w) into silicone elastomer, showed MC1220 had crystalline melting endotherms at 199.4°C (onset 196.8°C, enthalpy 93.62 J/g) and 201.7°C (onset 196.8°C, enthalpy 14.58 J/g), respectively (Figure 2b). The lower melting enthalpy value for the silicone elastomer sample reflects both the lower MC1220 concentration present compared with the pure sample and an increase in the amount dissolved in the elastomer at the elevated temperature.21
In vitro release of MC1220 from SEVRs
Daily and cumulative MC1220 in vitro release profiles from matrix-type SEVRs into IPA/H2O and SVF over 30 days are presented in Figure 3. The amount of MC1220 released daily into IPA/H2O was between one and two orders of magnitude greater than into SVF at each timepoint, reflecting the differences in MC1220 solubility in the two release media (Figure 3a). Thus, the day 1 values were 12.3 and 0.12 mg for IPA/H2O and SVF, respectively, while the corresponding day 30 values were 1.85 and 0.06 mg. For comparison, the 25 mg dapivirine human rings release 2 and 0.2 mg on day 1 and day 28, respectively, using the same IPA/H2O system in vitro.22,23 After 30 days, the total amounts of MC1220 released from the rings into IPA/H2O and SVF were 101 and 1.66 mg, respectively (Figure 3b); under comparable conditions, the 25 mg dapivirine human ring releases 12 mg into IPA/H2O medium over 28 days.22,23
Figure 3.
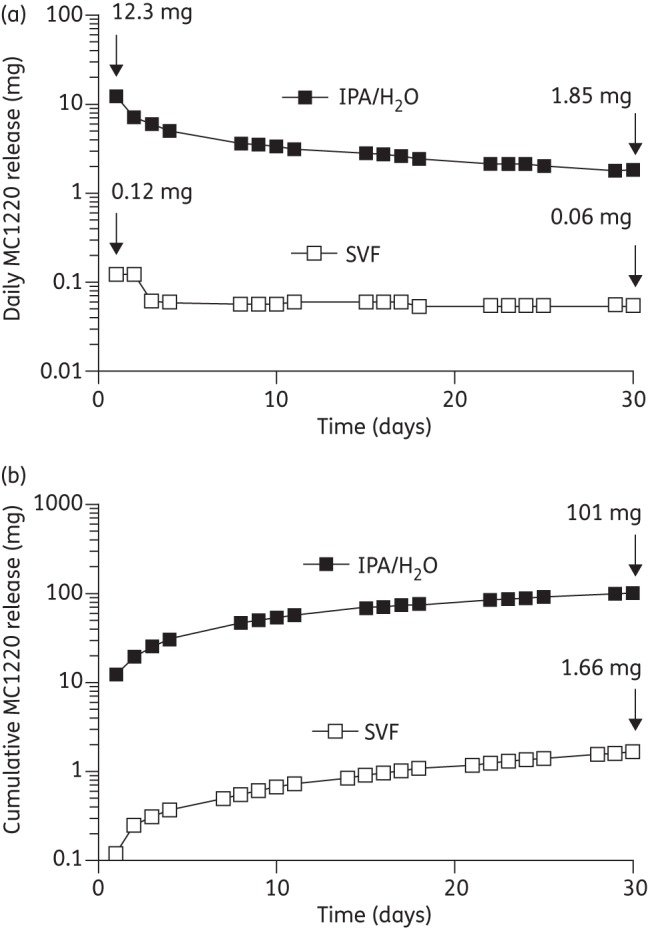
In vitro release into SVF or IPA/H2O for SEVRs containing ∼400 mg of MC1220. (a) Daily release and (b) cumulative release. The data are mean values (±SD, n = 4). The SD error bars were smaller than the plot symbol height for all data points.
The release of MC1220 into the IPA/H2O medium obeyed root time (t½) kinetics, confirmed by a linear cumulative release versus square-root time profile (R2 = 1.000, gradient 20.2 mg/day0.5; data not shown). In contrast, the release of MC1220 into the SVF medium did not obey t½ kinetics (R2 = 0.974). Instead, the release data (days 3–30) were effectively modelled by zero-order kinetics, confirmed by a linear cumulative release versus time profile (R2 = 0.999, gradient 50.0 μg/day; data not shown).
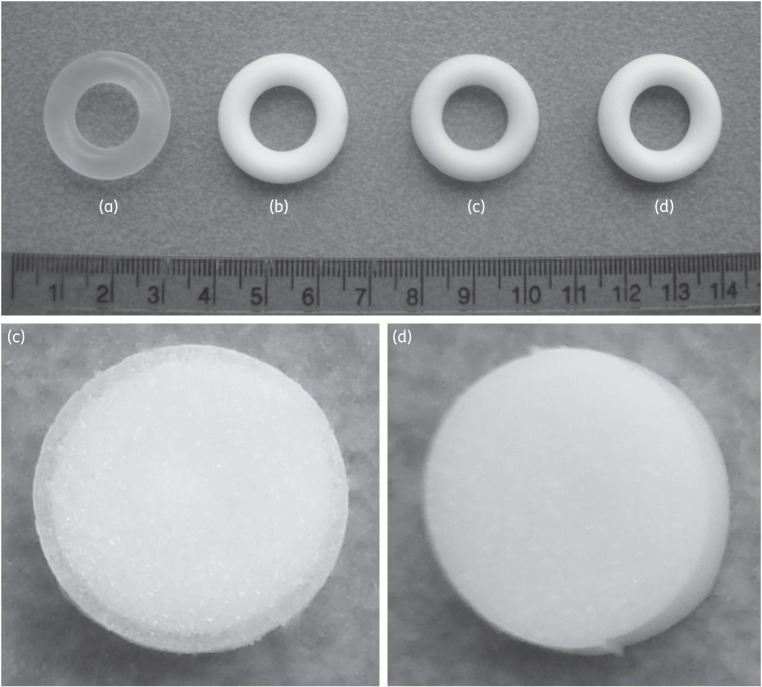
Photographs of placebo and MC1220-containing macaque-sized SEVRs were taken before and after in vitro use (Figure 4). A drug-depletion zone was visible adjacent to the surface of ring c (post-release into IPA/H2O), but not ring d (post-release into SVF). The initial MC1220 content in the rings was determined to be 432.2 ± 0.6 mg (23.65 ± 0.34% w/w) (n = 4). In mechanical testing studies, the mean compression forces of 10 N were similar to those measured previously for silicone elastomer rings of comparable size that have a good safety profile in macaques.12 There were no significant differences between the forces required to compress the SEVRs before and after in vitro release (Figure 5) (P > 0.05).
Figure 4.
The top photograph shows macaque-sized, matrix-type SEVRs: (a) placebo ring; (b) unused MC1220-loaded ring; and (c, d) MC1220-loaded rings after release into (c) IPA/H2O or (d) SVF for 30 days in vitro. The bottom photographs show cross sections from rings c and d. Note the appearance of a drug-depletion zone adjacent to the ring surface in cross section c, which is not apparent in cross section d.
Figure 5.
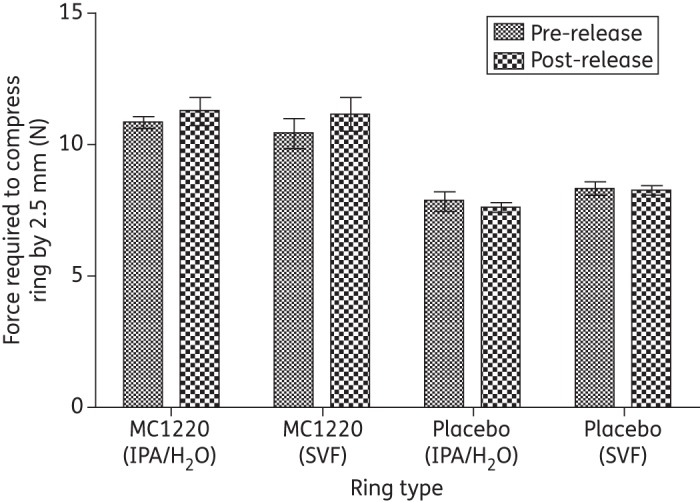
Mean force required to compress macaque vaginal rings by 2.50 mm, before and after storage in IPA/H2O or SVF for 30 days (±SD, n = 4). There were no significant differences for any of the pre- and post-release comparisons (P > 0.05).
Antiviral activity of MC1220 in vitro
The RT-SHIV162P3 challenge virus was engineered to contain the HIV-1 reverse transcriptase, which replaces the simian immunodeficiency virus (SIV) version present in the parental SIVmac251 and SHIV162P3 viruses.13 MC1220 is active only against viruses that carry the HIV-1 reverse transcriptase, including RT-SHIV162P3, as like most NNRTIs it does not bind to the SIV version of the enzyme. Its half-maximal inhibitory concentration (IC50) in the TZM-bl infectivity assay against RT-SHIV162P3 was 0.15 ± 0.023 ng/mL, i.e. 0.52 ± 0.080 nM (mean ± SEM, n = 2), but it was, as expected, inactive against SHIV162P3 (Table 1). MC1220 was slightly less active (3-fold) against the HIV-1 isolates, but 4- to 8-fold more potent against the same viruses than another NNRTI, the licensed drug efavirenz (Table 1). Neither inhibitor was toxic to the TZM-bl cells at effective antiviral concentrations: no cell death was detected at the highest concentrations used (11 μM for MC1220 and 70 μM for efavirenz) in three repeats of the MTT assay.
Table 1.
Inhibition of simian HIV (SHIV) and HIV-1 replication by MC1220 in vitro
| Virus | IC50 (ng/mL)a |
|||
|---|---|---|---|---|
| MC1220 | n | efavirenz | n | |
| RT-SHIV162P3 | 0.15 ± 0.023 | 4 | 1.3 ± 0.57 | 2 |
| SF162 | 0.41 ± 0.065 | 6 | 2.1 ± 0.44 | 4 |
| SHIV162P3 | >3000 | 5 | >3000 | 3 |
| DJ258 | 0.50 ± 0.12 | 4 | 3.5 ± 0.76 | 4 |
| NL4-3 | 0.59 ± 0.10 | 4 | 2.7 ± 0.69 | 4 |
aThe 50% inhibitory concentration (IC50) values are means (±SEM) from n replicate TZM-bl cell assays. For MC1220 and efavirenz, 1 ng/mL corresponds to 3.4 and 3.2 nM, respectively.
PK studies in rhesus macaques
After insertion of the rings into macaques, the mean MC1220 concentrations in vaginal fluid rose rapidly and then declined slowly but steadily, with values ranging from 844 (8 h) to 207 ng/mL (day 28) (Figure 6a). The unusually low mean value on day 10 (120 ng/mL) was because concentrations in two of the six macaques were below the quantifiable limit, for unknown reasons. The mean plasma concentrations of MC1220 were much lower, averaging 0.72 ng/mL over the 28 day ring insertion period and not differing significantly over time (Figure 6b). The statistical PK parameters of maximum concentration (Cmax), time to maximum concentration (Tmax) and AUC are presented in Table 2.
Figure 6.
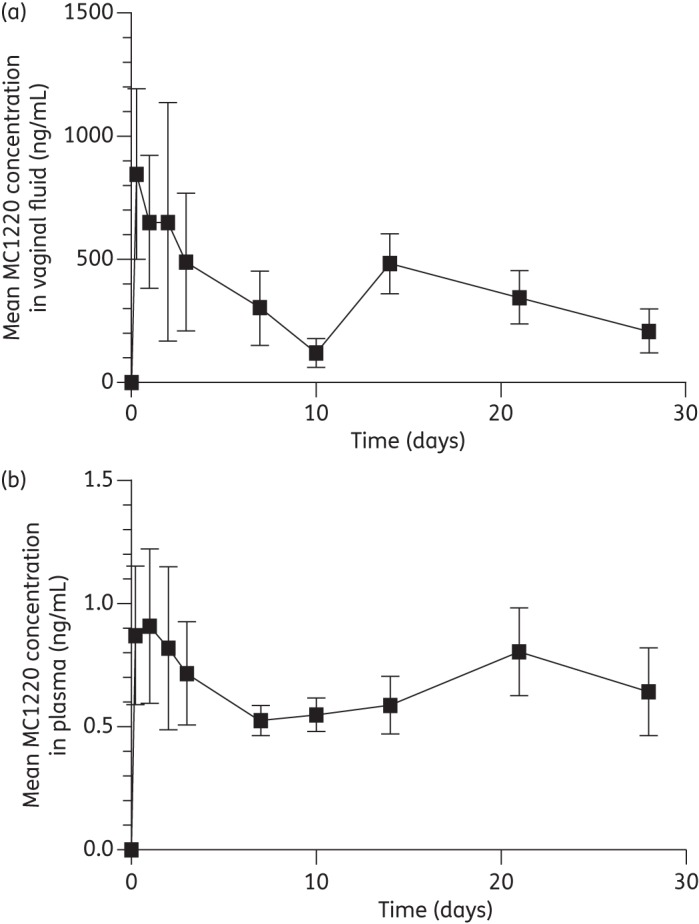
Mean concentrations (±SEM; n = 6) of MC1220 measured in (a) the vaginal fluid and (b) the plasma of rhesus macaques during a 28 day period of continuous ring placement.
Table 2.
Statistical PK parameters for MC1220 in macaque plasma and vaginal fluid after release from SEVRs in vivo
| Cmax (μg/mL) | Tmax (h) | AUC (μg · h/mL) | |
|---|---|---|---|
| Plasma | 0.91 | 1.0 | 18.75 |
| Vaginal fluid | 844.6 | 0.3 | 10100 |
Efficacy of MC1220 SEVRs against vaginal challenge of macaques
We assessed whether SEVRs containing MC1220 could protect macaques against vaginal infection with RT-SHIV162P3, a virus that is susceptible to this and other NNRTIs (Table 1).20 When considering the design of the challenge study, we elected not to use Depo-Provera to thin the vaginal epithelium and increase the susceptibility of the animals to a single challenge. We have previously observed that Depo-Provera use reduces the vaginal fluid concentration of the CCR5 inhibitors maraviroc and CMPD167 after release from SEVRs, while increasing the penetration of the drug into the plasma compartment.8 However, for a ring-challenge experiment to be conducted successfully, it is necessary for the control animals to become infected consistently and rapidly, i.e. within the 4 week period when the rings are in situ. Our pilot studies indicated that the RT-SHIV162P3 virus, unlike the parental SHIV162P3, was appropriately infectious after vaginal inoculation even when Depo-Provera was not used.11
MC1220 SEVRs were inserted into six animals, while four received placebo rings. Seven days later, all the animals were given the first of four weekly challenges with RT-SHIV162P3. Plasma viral loads were measured at weekly intervals to assess infection status. The macaques given the MC1220 SEVRs remained uninfected for significantly longer, during and after the challenge period, compared with the placebo recipients (P = 0.0037, log-rank test, hazard ratio 0.20 with 95% CI 0.0049–0.36) (Figure 7). Moreover, two animals containing MC1220 SEVRs remained uninfected after the four challenges, compared with none of the control animals. Hence, MC1220 was released from the rings in sufficient quantity to exert a protective effect against vaginal challenge, albeit not a complete one. The placebo-treated animals had peak viral loads of 3.7 × 104–3.3 × 106 RNA copies/mL on days 21–35; the infected MC1220 recipients had peak viral loads of 3.4 × 105–3.5 × 107 on days 28–63 (last day of analysis). These peak viral loads did not differ significantly between the groups.
Figure 7.
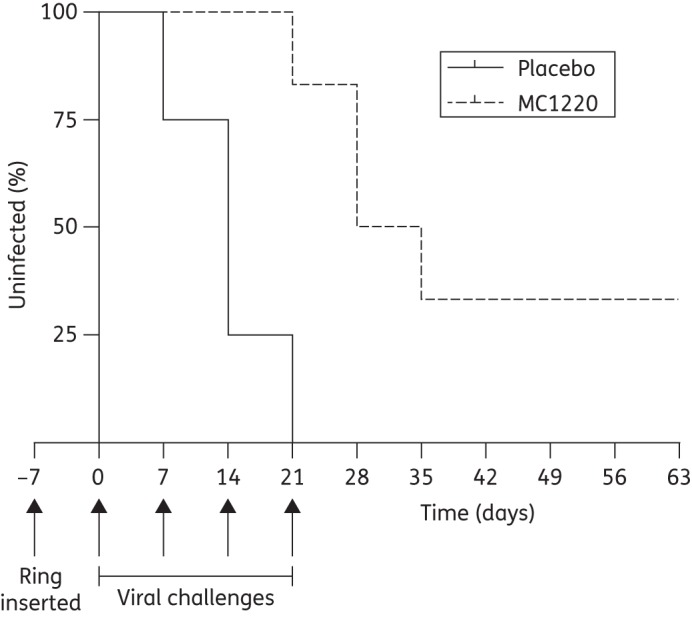
Kaplan–Meier plot showing the percentage of uninfected animals as a function of time. The arrows indicate when the rings were inserted and when the four vaginal challenges with RT-SHIV162P3 took place.
Residual MC1220 content of SEVRs post-use
The amounts of MC1220 remaining in SEVRs after in vitro and in vivo release studies were quantified (Figure 8). Before use, the mean MC1220 content of the rings was 432 mg. Of this amount, ∼100 mg was released into IPA/H2O over 30 days in vitro, but only a negligible amount was released into SVF. The total amounts released into the macaques during the 28 day insertion period, calculated from the initial and residual contents, were 20 and 5 mg in the PK and challenge studies, respectively.
Figure 8.
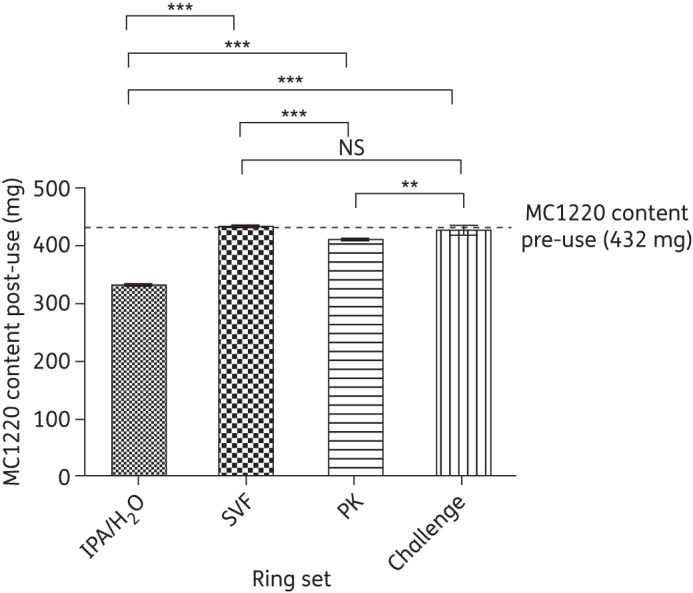
Mean residual content (±SD) of MC1220 in macaque vaginal rings after in vitro release into IPA/H2O and SVF (n = 4 in both cases), and after macaque PK and challenge studies (n = 6 in both cases). The dashed line indicates the content of MC1220 in unused rings immediately after manufacture (n = 4). One-way ANOVA indicated significant differences between the data: P < 0.0001. Post hoc statistical analysis is indicated by horizontal bars: NS, not significant; *, significant (0.01 < P < 0.05); **, very significant (0.001 < P < 0.01); ***, extremely significant (P < 0.001).
Discussion
MC1220 is a potent NNRTI with similar antiviral and physicochemical properties to the microbicide candidate dapivirine that is currently being evaluated clinically as a matrix-type SEVR.3,4,6 Although dapivirine-releasing SEVRs have been assessed for safety and PK in women,3,4 challenge studies have never been conducted in non-human primate models to gauge their efficacy.10 We therefore elected to assess the protective potential of MC1220 SEVRs in a rhesus macaque vaginal challenge model, to obtain proof-of-concept data for NNRTI-containing vaginal rings.
How the active compounds react to elevated temperatures is an important consideration in the manufacture of vaginal rings (both silicone elastomer and thermoplastic), since these devices are generally made by injection moulding at temperatures >150°C. TGA analysis showed that a dry powder sample of MC1220 did not change in weight when heated to temperatures as high as 200°C (Figure 2), indicating compound stability under injection moulding conditions. The similar melting points for MC1220 in powder form and as a 23% w/w dispersion in the heat-cured silicone elastomer provide further evidence for its stability in this system. Accordingly, we found that we could manufacture MC1220 SEVRs successfully.
To be effective against HIV-1 transmission, the active compound must be released efficiently from the SEVR into the aqueous milieu of the vagina and then penetrate into the surrounding tissues. The efficiencies of these processes are governed by the physicochemical properties of the compound and how it interacts with the local environment. Based on the principles of pH-partition theory, neutral molecules are better absorbed (i.e. into tissue and the systemic compartment) than their ionized counterparts.24 The ionization profile of MC1220 indicates that its neutral form (solid line, Figure 1) predominates at pH values representative of the normal human and macaque vaginas (pH 4–5 and 6–7, respectively).16,25,26 However, since high water solubility is a prerequisite for a compound to be efficiently absorbed into tissues via conventional passive diffusion-controlled mechanisms, the very low solubility of MC1220 in aqueous media (0.31 μg/mL in water and 0.77 μg/mL in SVF) may be a substantial limitation to its intravaginal efficacy. The same concern may also apply to dapivirine, whose water solubility is even lower (0.02 μg/mL; R. K. Malcolm, unpublished data, Table 3).
Table 3.
Physicochemical properties for dapivirine and MC1220
| Property | Dapivirine | MC1220 |
|---|---|---|
| Molecular weight (g/mol) | 329.40 | 293.31 |
| Water solubility (μg/mL) | 0.02 ± 0.003a | 0.31 ± 0.08b |
| SVF solubility (μg/mL) | N/A | 0.77 ± 0.08b |
| pKa at 25°C | ||
| measured | 5.54 ± 0.02c | 2.69 ± 0.14, 10.74 ± 0.15d |
| predicted | 4.74 ± 0.10e | 3.86 ± 0.10, 9.62 ± 0.50e |
| Log P at 25°Cf | ||
| measured | 2.27g | NA |
| predicted | 5.03 ± 0.55e | 2.55 ± 0.57e |
NA, not available.
aModified shake flask method (R. K. Malcolm, unpublished data).
bShake flask method (this article).
cTitrimetry in a water/methanol mixture (R. K. Malcolm, unpublished data).
dTitrimetry in a water/methanol mixture (this article).
eCalculated using Advanced Chemistry Development (ACD/Labs) Software V11.02 (© 1994–2012 ACD/Labs) as accessed using the SciFinder database.
fLogarithm of octanol/water partition coefficient.
gMaterial Safety Data Sheet (Ajinomoto Omnichem nv).
The highly hydrophobic nature of MC1220 is further illustrated by comparing the in vitro release data (Figure 3). Thus, the quantities released daily into the less polar IPA/H2O medium are between 30- (day 30) and 100-fold (day 1) greater than those released into SVF. The different solubilities of MC1220 in the two release media also influenced the release kinetics. The t½ kinetics observed in IPA/H2O is indicative of diffusion-controlled release under sink conditions. In other words, molecular diffusion of the drug through the silicone elastomer matrix is the factor that controls the release rate. In contrast, the pseudo-zero-order kinetics observed using the SVF medium are consistent with solubility-controlled release. The inference is that the release of MC1220 from the rings into SVF is controlled by the limited aqueous solubility of the drug, rather than its rate of diffusion through the silicone elastomer. Of course, the in vivo environment is significantly more complex than a flask containing a simple release medium, in that fluid, tissue and systemic compartments are all involved. Nonetheless, it is possible that similar solubility control mechanisms would still apply in vivo, influencing the release of poorly water-soluble compounds such as MC1220 and, by analogy, dapivirine. Such a scenario could have important implications for the future design of ARV-releasing rings, since the normal strategies for increasing drug release rates (e.g. using a higher drug loading in matrix-type devices) may not be effective. A PK study testing different ring loadings of a poorly water-soluble compound (e.g. MC1220 or dapivirine) would shed light on this critical issue.
Newly manufactured placebo SEVRs are clear and transparent (Figure 4a). However, owing to dispersion of solid drug particles throughout the elastomeric matrix, MC1220 rings appear white and opaque (Figure 4b). After in vitro incubation in SVF or IPA/H2O, the appearances of the MC1220 rings correlated with the quantities of the compound released. Thus, the MC1220 ring was visually unchanged after the SVF-release experiment (Figure 4d), while the corresponding exposure to the IPA/H2O medium led to a dewhitening that was associated with a substantial loss of MC1220 from the surface layers (Figure 4c). The difference was particularly apparent when cross sections of the rings were compared: a clearly defined drug-depletion zone was observed in the ring exposed to IPA/H2O, but not in the one immersed in SVF (Figure 4c and d, respectively). The rings used in the macaque studies, although typically discoloured, also did not show a drug-depletion zone, which is consistent with their exposure to an aqueous environment (photographs not shown).
MC1220 was released from SEVRs in vivo, leading to readily quantifiable, albeit highly variable, concentrations in macaque vaginal fluid that were generally sustained in the 120–650 ng/mL range over the 28 day study period. These levels are 800- to 4300-fold greater than the IC50 values for MC1220 activity against RT-SHIV162P3 replication in vitro (Table 1). Much lower MC1220 concentrations (<1 ng/mL, comparable to in vitro IC50 concentrations) were measured in plasma. Whether these plasma concentrations would be sufficient to drive the emergence of resistance in unknowingly infected women is not known, but the possibility cannot be excluded. The ratio between the plasma and vaginal fluid MC1220 concentrations is of the order of 1 × 10−3. For comparison, the corresponding concentration ratios for dapivirine, maraviroc, CMPD167 and tenofovir, calculated from data obtained in other PK studies of various gel and ring formulations, are substantially and consistently smaller at ∼1 × 10–6, independently of the formulation type.3,8,19,27–30 Unlike MC1220, these four compounds are all predominantly in the ionized state at normal vaginal pH. We conclude that the small quantity of MC1220 released from a ring penetrates into the vaginal tissues and thereafter the plasma very efficiently, a consequence of both its hydrophobicity and its charge neutrality at vaginal pH.
Although the MC1220 rings did yield sustained vaginal fluid concentrations that approached the μM (μg/mL) range, the only way to determine whether such levels are sufficient to exert a protective effect against virus transmission was to conduct a vaginal challenge experiment. The outcome was that significantly more weekly challenges with the RT-SHIV162P3 virus were required to infect the animals given MC1220 SEVRs, compared with placebo ring recipients. Although there are study design differences, the degree of partial protection seen here might be usefully compared with those found in recent macaque studies of leading vaccine candidates.31–35
When we estimated the amounts of MC1220 released from the SEVRs in the two in vivo studies, there was a small apparent difference (20 mg versus 5 mg in the PK and challenge experiments, respectively). The discrepancy is most likely an artefact that arises from variation in the initial MC1220 loadings when different SEVR batches are manufactured. Since only a very small proportion of the incorporated MC1220 is released, any variation in the initial amount present has a substantial influence on estimates of how much is released from the SEVR. At present, it is not possible to non-destructively and accurately measure the drug loading of each individual vaginal ring prior to in vitro release or in vivo testing, although we are in the process of developing such a quantitative method using Raman spectroscopy, based on previous work.36 For comparison, a 25 mg dapivirine matrix ring releases ∼5 mg of drug in women during a 28 day insertion (R. K. Malcolm, unpublished data).
Macaque studies are valuable for generating PK data and for assessing the protective potential of a microbicide candidate. The extent to which protection data, in particular, can be extrapolated to what might happen in humans remains to be fully understood, and there are additional uncertainties when cross-comparing different ARVs. Nonetheless, it is worth considering how our studies of MC1220 rings in macaques might predict the outcome of the current Phase III clinical study of the 25 mg dapivirine ring in human females. Dapivirine and MC1220 are both potent NNRTIs that are active against HIV-1 at ng/mL (nM) concentrations and they have similar physicochemical characteristics, including very poor aqueous solubility (Table 3). Here, we observed that an SEVR loaded with 430 mg of MC1220 provided partial protection over a 28 day period. The incomplete nature of the protection is, we believe, attributable to the limited release (only low mg quantities) of MC1220 from the rings in vivo, which is in turn caused by the compound's poor solubility in aqueous fluids. Given the similarity in physicochemical and antiviral properties between the two NNRTIs, it is plausible that a dapivirine version of the MC1220 ring would behave similarly in macaques in respect of both release and protection. If so, there might be a concern that the 25 mg dapivirine ring (0.31% w/w drug loading, compared with 23.6% w/w for the MC1220 macaque ring) currently under evaluation in humans might not be strongly protective. However, despite the much lower drug loading in the ring, dapivirine concentrations in vaginal fluid and plasma during 28 days of use of the 25 mg dapivirine ring were two to three orders of magnitude greater than those we measured in the present study of MC1220 rings in macaques.3 Certainly, one factor that influences the diffusion-controlled release of compounds from matrix-type SEVRs is the ring's surface area. However, the 3.2-fold difference between the surface areas of macaque- and human-sized rings is not sufficient to account for the substantially different local and systemic drug concentrations (albeit for different NNRTIs) seen in the two species. It is possible that variations in vaginal physiology might influence drug distribution and absorption between humans and macaques. A comparative study in the two species using SEVRs containing the same microbicide candidate would resolve this issue.
Overall, we have shown that MC1220 SEVRs provide partial protection against vaginal challenge of rhesus macaques. By extrapolation, these rings may be a useful approach to developing a coitally independent vaginal microbicide for women. It would, therefore, seem worthwhile to evaluate the safety and PK of MC1220 rings in a Phase I clinical study. To improve the chances of protection, MC1220 could be coformulated with another ARV with a complementary mechanism of action, including but not limited to the CCR5 inhibitor, maraviroc. Another approach worth considering is to use MC1220 SEVRs (or those based on another ARV) in combination with a vaccine. The beneficial effect of combining gel-based microbicides and systemically administered vaccines that induce cellular immunity has now been established in the macaque model.32 As SEVRs and vaccines both have the potential to provide long-lasting protection, they may be particularly suitable for use as a combination in humans. Furthermore, a new type of ring device that permits the simultaneous vaginal administration of multiple microbicides and a protein antigen has recently been developed.37,38 Additional macaque studies intended to determine whether these various combination concepts provide reinforced protection seem justified.
Funding
This work was supported by NIH Cooperative Agreement U19 AI76982. Previous studies supporting the development of MC1220 were funded through the following projects: AIDS (ISS), SHIVA & EUROPRIZE (EU Commission FP6).
Transparency declarations
P. L. C. is a named inventor on a granted patent for MC1220 (US 6,635,636), which is entirely owned by UNICA. No commercial agreements are presently in place. All other authors: none to declare.
Acknowledgements
We thank James Smith (CDC) and Ranajit Pal (Advanced BioSciences Laboratories) for providing RT-SHIV162P3. We also thank Robin Shattock for his helpful comments and guidance.
References
- 1.Malcolm RK, Woolfson AD, Toner CF, et al. Long-term, controlled release of the HIV microbicide TMC120 from silicone elastomer vaginal rings. J Antimicrob Chemother. 2005;56:954–6. doi: 10.1093/jac/dki326. doi:10.1093/jac/dki326. [DOI] [PubMed] [Google Scholar]
- 2.Woolfson AD, Malcolm RK, Toner CF, et al. Potential use of vaginal rings for prevention of heterosexual transmission of HIV a controlled-release strategy for HIV microbicides. Am J Drug Deliv. 2006;4:7–20. doi:10.2165/00137696-200604010-00002. [Google Scholar]
- 3.Nel A, Smythe S, Young K, et al. Safety and pharmacokinetics of dapivirine delivery from matrix and reservoir intravaginal rings to HIV-negative women. J Acquir Immune Defic Syndr. 2009;51:416–23. doi: 10.1097/qai.0b013e3181acb536. doi:10.1097/QAI.0b013e3181acb536. [DOI] [PubMed] [Google Scholar]
- 4.Romano R, Variano B, Coplan P, et al. Safety and availability of dapivirine (TMC120) delivered from an intravaginal ring. AIDS Res Human Retrovir. 2009;25:483–8. doi: 10.1089/aid.2008.0184. doi:10.1089/aid.2008.0184. [DOI] [PubMed] [Google Scholar]
- 5.Johnson TJ, Gupta KM, Fabian J, et al. Segmented polyurethane intravaginal rings for the sustained combined delivery of antiretroviral agents dapivirine and tenofovir. Eur J Pharm Sci. 2010;39:203–12. doi: 10.1016/j.ejps.2009.11.007. doi:10.1016/j.ejps.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 6.Malcolm RK, Edwards K-L, Kiser P, et al. Advances in microbicide vaginal rings. Antivir Res. 2010;88:S30–9. doi: 10.1016/j.antiviral.2010.09.003. doi:10.1016/j.antiviral.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 7.Johnson TF, Srinivasan P, Albright TH, et al. Safe and sustained vaginal delivery of pyrimidinedione HIV-1 inhibitors from polyurethane intravaginal rings. Antimicrob Agents Chemother. 2012;56:1291–9. doi: 10.1128/AAC.05721-11. doi:10.1128/AAC.05721-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malcolm RK, Veazey RS, Geer L, et al. Sustained release of the CCR5 inhibitors CMPD167 and maraviroc from vaginal rings in rhesus macaques. Antimicrob Agents Chemother. 2012;56:2251–8. doi: 10.1128/AAC.05810-11. doi:10.1128/AAC.05810-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mesquita PMM, Rastogi R, Segarra TJ, et al. Intravaginal ring delivery of tenofovir disoproxil fumarate for prevention of HIV and herpes simplex virus infection. J Antimicrob Chemother. 2012;67:1730–8. doi: 10.1093/jac/dks097. doi:10.1093/jac/dks097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Veazey RS. Microbicide safety/efficacy studies in animals—macaques and small animal models. Curr Opin HIV AIDS. 2008;3:567–73. doi: 10.1097/COH.0b013e32830891bb. doi:10.1097/COH.0b013e32830891bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Veazey RS, Shattock RJ, Klasse PJ, et al. Animal models for microbicide studies. Curr HIV Res. 2012;10:79–87. doi: 10.2174/157016212799304715. doi:10.2174/157016212799304715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Promadej-Lanier N, Smith JM, Srinivasan P, et al. Development and evaluation of a vaginal ring device for sustained delivery of HIV microbicides to non-human primates. J Med Primatol. 2009;38:263–71. doi: 10.1111/j.1600-0684.2009.00354.x. doi:10.1111/j.1600-0684.2009.00354.x. [DOI] [PubMed] [Google Scholar]
- 13.Pal R, Galmin L, Pereira LE, et al. Virological and molecular characterization of a simian human immunodeficiency virus (SHIV) encoding the envelope and reverse transcriptase genes from HIV-1. Virology. 2012;432:173–83. doi: 10.1016/j.virol.2012.05.034. doi:10.1016/j.virol.2012.05.034. [DOI] [PubMed] [Google Scholar]
- 14.Caron M, Besson G, Etenna SLD, et al. Protective properties of non-nucleoside reverse transcriptase inhibitor (MC1220) incorporated into liposome against intravaginal challenge of rhesus macaques with RT-SHIV. Virology. 2010;405:225–33. doi: 10.1016/j.virol.2010.06.008. doi:10.1016/j.virol.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 15.Stolte-Leeb N, Loddo R, Antimisiaris S, et al. Topical nonnucleoside reverse transcriptase inhibitor MC1220 partially prevents vaginal RT-SHIV infection of macaques. AIDS Res Human Retrovir. 2011;27:933–43. doi: 10.1089/AID.2010.0339. doi:10.1089/aid.2010.0339. [DOI] [PubMed] [Google Scholar]
- 16.Owen DH, Katz DF. A vaginal fluid simulant. Contraception. 1999;59:91–5. doi: 10.1016/s0010-7824(99)00010-4. doi:10.1016/S0010-7824(99)00010-4. [DOI] [PubMed] [Google Scholar]
- 17.Ketas TJ, Holuigue S, Matthews K, et al. Env-glycoprotein heterogeneity as a source of apparent synergy and enhanced cooperativity in inhibition of HIV-1 infection by neutralizing antibodies and entry inhibitors. Virology. 2012;422:22–36. doi: 10.1016/j.virol.2011.09.019. doi:10.1016/j.virol.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mosman T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:1–2. doi: 10.1016/0022-1759(83)90303-4. doi:10.1016/0022-1759(83)90300-9. [DOI] [PubMed] [Google Scholar]
- 19.Forbes CJ, Lowry D, Geer L, et al. Non-aqueous silicone elastomer gels as a vaginal microbicide delivery system for the HIV-1 entry inhibitor maraviroc. J Control Rel. 2011;156:161–9. doi: 10.1016/j.jconrel.2011.08.006. doi:10.1016/j.jconrel.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith JM, Dauner A, Li B, et al. Generation of a dual RT Env SHIV that is infectious in rhesus macaques. J Med Primatol. 2010;39:213–23. doi: 10.1111/j.1600-0684.2010.00434.x. doi:10.1111/j.1600-0684.2010.00434.x. [DOI] [PubMed] [Google Scholar]
- 21.Gramaglia D, Conway BR, Kett VL, et al. High speed DSC (hyper-DSC) as a tool to measure the solubility of a drug within a solid or semi-solid matrix. Int J Pharm. 2005;301:1–5. doi: 10.1016/j.ijpharm.2005.04.038. doi:10.1016/j.ijpharm.2005.04.038. [DOI] [PubMed] [Google Scholar]
- 22.McBride M, Boyd P, Fetherston S, et al. Preclinical stability evaluation of vaginal rings containing dapivirine and maraviroc. Abstracts of the Microbicides 2010 Conference, Pittsburgh, PA, 2010; Abstract 250, p. 173. [Google Scholar]
- 23.Fetherston S, Boyd P, McBride M, et al. Preclinical stability evaluation of dapivirine and maraviroc release in vitro from combination silicone elastomer vaginal rings. Abstracts of the 37th Annual Meeting & Exposition of the Controlled Release Society, Portland, OR, 2010; Abstract 838, p. 58. Controlled Release Society, St Paul, MN, USA. [Google Scholar]
- 24.Shore PA, Brodie BB, Hogben CAM. The gastric secretion of drugs: a pH partition hypothesis. J Pharmacol Exp Therapeut. 1957;119:361–9. [PubMed] [Google Scholar]
- 25.Spear GT, Gilbert D, Sikaroodi M, et al. Identification of rhesus macaque genital microbiota by 16S pyrosequencing shows similarities to human bacterial vaginosis: implications for use as an animal model for HIV vaginal infection. AIDS Res Hum Retrovir. 2010;26:193–200. doi: 10.1089/aid.2009.0166. doi:10.1089/aid.2009.0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith KPB. Estrogens and the urogenital tract. Studies on steroid hormone receptors and a clinical study on a new estradiol releasing vaginal ring. Acta Obstet Gynecol Scand. 1993;72(Suppl 157):S1–26. [PubMed] [Google Scholar]
- 27.Moss JA, Malone AM, Smith TJ, et al. Simultaneous delivery of tenofovir and acyclovir via an intravaginal ring. Antimicrob Agents Chemother. 2012;56:875–82. doi: 10.1128/AAC.05662-11. doi:10.1128/AAC.05662-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwartz JL, Rountree W, Kashuba ADM, et al. A multi-compartment, single and multiple dose pharmacokinetic study of the vaginal candidate microbicide 1% tenofovir gel. PLoS ONE. 2011;6:e25974. doi: 10.1371/journal.pone.0025974. doi:10.1371/journal.pone.0025974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nuttall J, Kashuba A, Wang R, et al. Pharmacokinetics of tenofovir following intravaginal and intrarectal administration of tenofovir gel to rhesus macaques. Antimicrob Agents Chemother. 2012;56:103–9. doi: 10.1128/AAC.00597-11. doi:10.1128/AAC.00597-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abdool Karim SS, Kashuba ADM, Werner L, et al. Drug concentrations after topical and oral antiretroviral pre-exposure prophylaxis: implications for HIV prevention in women. Lancet. 2011;378:279–81. doi: 10.1016/S0140-6736(11)60878-7. doi:10.1016/S0140-6736(11)60878-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barouch DH, Klasse PJ, Dufour J, et al. Macaque studies of vaccine and microbicide combinations for preventing HIV-1 sexual transmission. Proc Natl Acad Sci USA. 2012;109:8694–8. doi: 10.1073/pnas.1203183109. doi:10.1073/pnas.1203183109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Letvin NL, Rao SS, Montefiori DC, et al. Immune and genetic correlates of vaccine protection against mucosal infection by SIV in monkeys. Sci Transl Med. 2011;3:81ra36. doi: 10.1126/scitranslmed.3002351. doi:10.1126/scitranslmed.3002351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barouch DH, Liu J, Li H, et al. Vaccine protection against acquisition of neutralization-resistant SIV challenges in rhesus monkeys. Nature. 2012;482:89–93. doi: 10.1038/nature10766. doi:10.1038/nature10766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lai L, Kwa SF, Kozlowski PA, et al. SIVmac239 MVA vaccine with and without a DNA prime, similar prevention of infection by a repeated dose SIVsmE660 challenge despite different immune responses. Vaccine. 2012;30:1737–45. doi: 10.1016/j.vaccine.2011.12.026. doi:10.1016/j.vaccine.2011.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xiao P, Patterson LJ, Kuate S, et al. Replicating adenovirus-SIV recombinant priming and envelope protein boosting elicits localized, mucosal IgA immunity in rhesus macaques correlated with delayed acquisition following a repeated low dose rectal SIVmac251 challenge. J Virol. 2012;86:4644–57. doi: 10.1128/JVI.06812-11. doi:10.1128/JVI.06812-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bell SE, Dennis AC, Fido LA, et al. Characterization of silicone elastomer vaginal rings containing HIV microbicide TMC120 by Raman spectroscopy. J Pharm Pharmacol. 2007;59:203–7. doi: 10.1211/jpp.59.2.0007. doi:10.1211/jpp.59.2.0007. [DOI] [PubMed] [Google Scholar]
- 37.Morrow RJ, Woolfson AD, Donnelly L, et al. Sustained release of proteins from a modified vaginal ring device. Eur J Pharm Biopharm. 2011;77:3–10. doi: 10.1016/j.ejpb.2010.10.010. doi:10.1016/j.ejpb.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pattani A, Lowry D, Curran RM, et al. Characterisation of protein stability in rod-insert vaginal rings. Int J Pharm. 2012;430:89–97. doi: 10.1016/j.ijpharm.2012.03.036. doi:10.1016/j.ijpharm.2012.03.036. [DOI] [PubMed] [Google Scholar]