Abstract
Objective
To identify factors that predict treatment success or failure in patients treated with intravitreal ranibizumab for diabetic macular edema (DME).
Methods
Thirty-seven baseline demographic, systemic, ocular, optical coherence tomography (OCT), and fundus photographic variables were assessed for association with change in visual acuity (VA) or OCT between baseline and 1 year in 361 eyes thatwere assigned randomly to intravitreal ranibizumab with prompt or deferred laser within a trial of ranibizumab, triamcinolone, and laser for center involved DME. A categorical variable describing follow-up anatomic responses to therapy was added to the vision outcome model.
Results
After adjusting for baseline VA, a larger VA treatment benefit was associated with younger age (P < 0.001), less severe diabetic retinopathy on clinical exam (P = 0.003), and absence of surface wrinkling retinopathy (P<0.001). Central subfield (CSF) thickness evolution during the first treatment year also predicted better vision outcomes (P<0.001). After adjusting for baseline CSF thickness, lipid was associated with more favorable OCT improvement (P = 0.004). As only11 eyes experienced vision loss and 6 eyes experienced CSF worsening, factors for poor outcomes could not be evaluated.
Conclusion
A review of baseline factors and anatomic responses during the first year of ranibizumab therapy for association with vision outcome did not identify any features that would preclude ranibizumab treatment. However, baseline thickness is the strongest predictor of anatomic outcome and reduction in thickness during the first treatment year is associated with better vision outcomes.
Diabetic macular edema (DME) has been the leading cause of moderate vision loss in people with diabetes.1 The prevalence of diabetes is expected to rise as the prevalence of obesity continues to increase markedly, therefore improving treatment of DME will become increasingly important.2 Based on findings from the Early Treatment Diabetic Retinopathy Study, focal/grid laser photocoagulation has been the mainstay of treatment for DME since 1985.3 However, recent studies suggesting a role of vascular endothelial growth factors (VEGF) in the pathogenesis of DME have prompted evaluation of anti-VEGF drugs, such as ranibizumab (Lucentis, Genentech, South San Francisco, CA), bevacizumab (Avastin, Genentech, South San Francisco, CA), and VEGF Trap-Eye (Regeneron Pharmaceuticals, Inc., Tarrytown, NY and Bayer Healthcare Pharmaceuticals, Berlin, Germany) for the treatment of DME.3-6
A multicenter-randomized Diabetic Retinopathy Clinical Research network (DRCR.net) trial found that intravitreal ranibizumab, either with prompt or deferred focal/grid laser, resulted in superior visual acuity and central retinal thickness outcomes compared with focal/grid laser treatment alone or triamcinolone with laser at both 1 and 2 years of follow-up. 7, 8 The two ranibizumab groups, with a median baseline best-corrected visual acuity of 20/50 (∼Snellen equivalent), had nearly identical outcomes at one year. Both groups averaged nearly 2 lines of visual acuity improvement, almost 30% improved 3 or more lines of visual acuity, and fewer than 5% lost 2 or more visual acuity lines. Findings from this clinical trial support the use of ranibizumab for the management of center-involved DME with vision impairment. Identifying risk factors that predict treatment success or failure could help investigators to make informed decisions as to which patients should be treated with intravitreal ranibizumab. Therefore, we are presenting additional analyses on 361 eyes that were assigned randomly to either 0.5-mg ranibizumab + prompt laser (n=180) or 0.5-mg ranibizumab + deferred laser (≥24 weeks) (n=181).
Methods
All data utilized for this analysis were collected from baseline to 1-year by clinical sites. 7 The protocol for this trial is available at the DRCR.net website (www.drcr.net).
Synopsis of Study Design
Major eligibility criteria for participation in this trial included: 1) best-corrected Electronic-Early Treatment Diabetic Retinopathy Study visual acuity (E-ETDRS Visual Acuity Test) letter score 78 to 24 (∼Snellen equivalent 20/32 to 20/320), 2) definite retinal thickening from DME involving the foveal center on clinical examination as the cause of vision loss, and 3) time domain optical coherence tomography (OCT) confirmation of foveal edema with a central subfield thickness (CSF) of ≥250 μm.
At baseline, and at each follow-up visit, best-corrected visual acuity letter score was measured at 3 meters by a certified masked examiner using an E-ETDRS Visual Acuity Test and OCT images were obtained by a certified operator using the Zeiss Stratus OCT machine (Carl Zeiss Meditec, Inc., Dublin, CA). Additional testing at baseline included slit-lamp examination, fundus examination, and standard ETDRS 7-field color stereoscopic fundus photographs. Fundus photographs were graded at the Fundus Photograph Reading Center (University of Wisconsin, Madison, WI).
During the first year, follow-up visits occurred every 4 weeks (± 1 week) with the potential to receive ranibizumab at each visit. Eyes assigned to either ranibizumab arm were required to have four consecutive monthly ranibizumab injections. Treatment was at investigator discretion at any visit that fulfilled “success” criteria (defined as a vision equivalent of 20/20 or OCT <250 μm) beginning with the 16-week study visit. Two additional ranibizumab injections were required through week 20 if success had not been met. Beginning at week 24 treatment was at investigator discretion if the participant met the criteria for “failure”; however, treatment could be deferred for “futility” criteria beginning at week 52. Unless failure or futility criteria were met, investigators were encouraged to continue intravitreal injections when edema persisted or recurred.
Eyes assigned to the ranibizumab + prompt laser group received focal/grid laser treatment within 3 to 10 days of their baseline ranibizumab injection. Repeat laser was administered as often as every 13 weeks if persistent DME involved or threatened the fovea and if complete laser treatment had not been previously administered. Eyes in the ranibizumab + deferred laser group first became eligible for laser anytime at week 24 or beyond if DME persisted or threatened the fovea and the “futility” criteria were met.
Additional details of the treatment protocol can be found in the article that summarizes the primary outcome of the trial.7 In particular, Appendices I-3 depict flowcharts of patient management and Table 1 provides important definitions used to guide management (available at http://aaojournal.org).
Table 1. Baseline Factors Evaluated – N (%) or Mean (SD) (online).
| Participant Characteristics | |
|---|---|
| Race/Ethnicity | |
| Black/African American | 55 (15) |
| Hispanic or Latino | 32 (9) |
| Other | 9 (2) |
| White | 265 (73) |
| Gender | |
| Women | 155 (43) |
| Men | 206 (57) |
| Age | |
| <60 years | 124 (34) |
| ≥60 years | 237 (66) |
| Mean (SD) | 63 (10) |
| Diabetes Type | |
| Type 1 | 26 (7) |
| Type 2 | 329 (91) |
| Uncertain | 6 (2) |
| Diabetes Duration | |
| <15 years | 137 (38) |
| ≥15 years | 224 (62) |
| Mean (SD) | 18 (9) |
| Mean Arterial Blood Pressure* | |
| ≥100 mmHg | 151 (42) |
| <100 mmHg | 210 (58) |
| Mean (SD) | 98 (12) |
| HbA1c | |
| < 7.5 % | 182 (52) |
| ≥ 7.5 % | 170 (48) |
| Mean (SD) | 7.6 (1.5) |
| Hypertension† | |
| No | 72 (20) |
| Yes | 289 (80) |
| Elevated Cholesterol† | |
| No | 130 (36) |
| Yes | 231 (64) |
| Cardiovascular Disease† | |
| No | 260 (72) |
| Yes | 101 (28) |
| Renal Disease† | |
| No | 337 (93) |
| Yes | 24 (7) |
| Neurologic Disease† | |
| No | 286 (79) |
| Yes | 75 (21) |
| Insulin Use | |
| No | 138 (38) |
| Yes | 223 (62) |
| Prescribed Glitazones† | |
| No | 290 (80) |
| Yes | 71 (20) |
| Prescribed Statins† | |
| No | 156 (43) |
| Yes | 205 (57) |
|
| |
| Historical Ocular Characteristics Present | |
| Prior DME Treatment | |
| No | 134 (37) |
| Yes | 227 (63) |
| Timing of Most Recent Prior DME Treatment‡ | |
| ≤ 16-weeks | 26 (11) |
| > 16-weeks – 32-weeks | 95 (42) |
| > 32-weeks – 1 year | 20 (9) |
| > 1 year | 86 (38) |
| Prior Laser for DME | |
| No | 159 (44) |
| Yes | 202 (56) |
| Timing of Prior Laser for DME‡ | |
| ≤ 1 year | 107 (53) |
| > 1 year | 95 (47) |
| Prior PRP§ | |
| No | 264 (73) |
| Yes | 97 (27) |
| Timing of Prior PRP‡,□ | |
| ≤ 1 year | 19 (28) |
| > 1 year | 48 (72) |
| Timing of Prior Cataract Extraction‡ | |
| ≤ 1 year | 22 (25) |
| > 1 year | 67 (75) |
| History of YAG Capsulotomy | |
| No | 344 (95) |
| Yes | 17 (5) |
|
| |
| Baseline Ocular Characteristics | |
| Visual Acuity | |
| ≤ 65 (20/50) letters | 185 (51) |
| ≥ 66 (20/50) letters | 176 (49) |
| Mean (SD) | 62 (12) |
| ∼Snellen equivalent | 20/63 |
| Visual Acuity in Non-study Eye | |
| ≤ 65 (20/50) letters | 110 (30) |
| ≥ 66 (20/50) letters | 251 (70) |
| Mean (SD) | 68 (19) |
| ∼Snellen equivalent | 20/50 |
| Lens Status on Exam | |
| Pseudophakic | 110 (30) |
| Phakic | 251 (70) |
| Diabetic Retinopathy Severity on Clinical Exam as Judged by Investigator | |
| No DR/MA/Mild/Moderate NPDR | 202 (56) |
| Severe NPDR | 71 (20) |
| PDR or prior PRP** | 88 (24) |
| Type of DME on Clinical Exam as Judged by Investigator | |
| Predominantly focal | 115 (32) |
| Neither predominantly focal or diffuse | 86 (24) |
| Predominantly diffuse | 160 (44) |
|
| |
| Optical Coherence Tomography Characteristics Provided by Reading Center | |
| OCT Central Subfield Thickness | |
| < 400 μm | 202 (56) |
| ≥ 400 μm | 158 (44) |
| Mean (SD) | 406 (130) |
| OCT Retinal Volume | |
| <9.0 mm3 | 180 (64) |
| ≥9.0 mm3 | 102 (36) |
| Mean (SD) | 8.9 (1.9) |
| OCT Cystoid Abnormalities | |
| No evidence | 14 (4) |
| Questionable/Definite | 342 (96) |
| OCT Subretinal Fluid | |
| No evidence | 276 (77) |
| Questionable/Definite | 81 (23) |
| OCT Vitreoretinal Abnormalities | |
| No evidence | 181 (52) |
| Questionable/Definite | 170 (48) |
|
| |
| Ocular Characteristics Provided by Fundus Photograph Reading Center | |
| ETDRS Diabetic Retinopathy Severity | |
| No DR/MA/Mild/Moderate NPDR (Levels 10, 12, 14, 15, 20, 35, 43) | 89 (26) |
| Moderately Severe/Severe NPDR (Levels 47, 53) | 147 (42) |
| PDR or prior PRP (Levels 60, 61, 65, 71, 75) | 110 (32) |
| Hemorrhages/MA in Macular Grid | |
| None/Questionable/<std1 | 74 (21) |
| <std2a/<std2b/>std2b | 273 (79) |
| Hard Exudates in Macular Grid | |
| None | 91 (26) |
| Questionable/Definite | 256 (74) |
| Surface Wrinkling Retinopathy | |
| None | 286 (83) |
| Questionable/Definite | 57 (17) |
SD = standard deviation; HbA1c = hemoglobin A1c; DME = diabetic macular edema; PRP = panretinal photocoagulation; ETDRS = Early Treatment Diabetic Retinopathy Study; OCT = optical coherence tomography; NPDR = non-proliferative diabetic retinopathy; PDR = proliferative diabetic retinopathy, std = standard; DR= diabetic retinopathy; MA=microaneurisms
Mean Arterial Pressure = Diastolic + 1/3(Systolic-diastolic)
By history or review of medication lists
Includes only eyes with previous corresponding treatment/procedure
Categorized as “Yes” if any of the following characteristics were met: medical history of PRP, scars of PRP present or questionably present on clinical exam, or reading center confirmed presence of PRP based on fundus photographs.
Timing of prior PRP was collected from the medical history form,this variable is missing for 30 eyes without prior PRP recorded on the medical history form where PRP was determined to be present based on alternate criteria mentioned in footnote “§”
All but one had prior PRP
Missing or ungradable data: HbA1c (9), OCT central subfield thickness (1), OCT volume (79), OCT cystoid abnormalities (5), OCT subretinal fluid (4), OCT vitreoretinal abnormalities (10), fundus photographs retinopathy severity (15), photographic grade within the ETDRS 6 mm grid of hemorrhages/microaneurysms (14), or hard exudates (14), or surface wrinkling retinopathy presence (18).
Statistical Methods
Three hundred and sixty one eyes randomly assigned at baseline to either of the two ranibizumab groups within this trial were eligible for these analyses. Thirty-seven baseline demographic, systemic, ocular, OCT, and fundus photographic factors were assessed for association with change in visual acuity or OCT between baseline and 1 year (Table 1, available online).Change in visual acuity (using “letter score” as a continuous variable) was assessed as the primary dependent variable. The association between baseline visual acuity and the proportion of participants reaching 74 (∼ snellen equivalent 20/32) or better visual acuity and the percent reduction in visual acuity deficit at 1 year were also explored. The baseline visual acuity deficit was defined as 84 minus the baseline visual acuity letter score; a letter score of 84 (∼Snellen equivalent 20/20) was considered ‘normal’ visual acuity. The percent reduction in visual acuity deficit at 1 year was defined as the change in visual acuity letter score from baseline to one year divided by the baseline visual acuity deficit and multiplied by 100%. We were unable to evaluate factors associated with poor visual acuity outcomes because the numbers of individuals in the ranibizumab treatment groups with vision loss or an increase in CSF thickness were too small to analyze. Only 5 eyes (1%) lost 10 to 14 letters of visual acuity and 6 eyes (2%) lost at least 15 letters in the ranibizumab assigned treatment arms 1 year following treatment initiation.
Associations with anatomic change were also investigated using change in OCT CSF thickness measured in microns as the dependent variable. Other dependent variables included CSF thickness < 250 μm and the percent reduction in excessive retinal thickness. The percent reduction in excess retinal thickness was calculated in a similar fashion to the percent reduction in visual acuity deficit. Baseline excess retinal thickness was defined as the baseline CSF thickness measurement minus 201 μm (the mean value of a cohort of persons with diabetes with no or mild retinopathy and no clinical evidence of DME9).The percent reduction in excess retinal thickness was defined as the change in CSF thickness from baseline to one year divided by the baseline excessive retinal thickness and multiplied by 100%. We were unable to evaluate factors associated with poor OCT CSF thickness outcomes because the number of individuals in the ranibizumab treatment groups with an increase in CSF thickness was too small to analyze. OCT CSF thickness increased 20% (1-step worsening of logOCT) in only 6 eyes receiving ranibizumab between study entry and the 1 year exam.
A composite outcome of both favorable functional and anatomic endpoints was also evaluated because the simultaneous presence of reduction in retinal thickness at the time of vision improvement may provide a greater level of confidence that the observed vision changes are attributed to the biologic effects of the drug. The composite outcome was defined as at least a 10 letter improvement and at least a 20% reduction in CSF thickness (the equivalent of a 1-step reduction of logOCT10).
Linear regression (visual acuity and OCT CSF thickness outcomes) and Poisson regression (composite outcome) models with robust variance estimation were used to assess potential risk factors for each of the three outcome variables specified above.11 Vision analyses were adjusted for baseline visual acuity, anatomic analyses were adjusted for baseline CSF thickness, and the composite outcome was adjusted for both. Potential risk factors with P value <0.10 in the univariate models were included in multivariate models. Final multivariate models consist of factors with P value <0.01 following a backwards selection process, and each eliminated covariate was tested by adding to the final model, one at a time, to confirm P value ≥ 0.01.When continuous or ordinal values were available for baseline features, these were used in the variable selection process; categories are shown for these variables for ease of interpretation, to report estimates and relative risks, and 95% confidence intervals. In the multivariate models, missing values of covariates were handled by adding a separate missing category for discrete covariates and adding a missing indicator variable for continuous covariates.
A second vision outcome model was constructed that included a categorical variable created to separate groups by their OCT behavior at follow-up visits, or thickness evolution, in response to treatment during the first treatment year. All study eyes were differentiated into one of four separate categories based on whether they had at least a 20% reduction from baseline CSF thickness at the 16-week, 32 week, and 1-year study visits. The ‘early and consistent’ group of anatomic responders experienced at least a 20% reduction in CSF thickness by the 16-week study visit and sustained at least this much improvement at32-week and 1 year study visits. The ‘early but inconsistent’ group of anatomic responders experienced this threshold level of CSF thickness reduction by the 16-week study visit but failed to sustain this anatomic improvement consistently at the 32-week or 1-year study visits or both. The ‘slow and variable’ group of anatomic responders did not manifest at least a 20% reduction in CSF thickness at the 16-week study visit but did so at either the 32-week or 1-year study visit or both. Finally, the ‘non-responder’ group did not manifest this threshold level of CSF thickness improvement at the 16-week, 32-week, or 1-year study visits. Pairwise comparisons among macular thickness evolution subgroups were performed using linear contrasts. The number of injections received during year 1 was included as an additional adjustment covariate (continuous and categorical in separate models) to confirm that the relationship seen was not due to differences in number of injections among the macular thickness evolution subgroups.
Univariate analyses were performed initially on each of the individual ranibizumab groups (prompt or deferred laser). Results were consistent between groups, therefore we have only reported the combined analyses. All factors, with one exception (prior DME treatment), showing evidence of strong association (P <0.001) in one of the treatment groups also showed evidence of association (P <0.10) in the other treatment group. The observation that one factor was associated with vision outcome in the deferred laser group, but not associated with vision outcome in the prompt laser group may be due to chance in view of the large number of factors evaluated.
Data to assess baseline predictors for the visual acuity and OCT outcomes were available for 338 eyes (94%) and 334 eyes (93%) respectively. A complete set of CSF thickness measurements at baseline, 16-weeks, 32-weeks, and 1-year were available on 288 (80%) study eyes to explore the impact of anatomic change during treatment on the 1 year visual acuity outcome.
Results
Results for analyses limited to participant demographics (3 variables), systemic conditions (9 variables) and medication use (3 variables) are shown in Table 2; prior ocular history (8 variables) in Table 3; ocular examination characteristics at study entry (5 variables) in Table 4, and ocular characteristics obtained from the initial visit OCT (5 variables) and fundus photographs (4 variables) in Table 5.
Table 2. Change in Visual Acuity and Optical Coherence Tomography Central Subfield Thickness from Baseline to 1 Year by Participant Demographic and Medical Disease Characteristics.
| Change in Visual Acuity | Change in OCT CSF Thickness | Visual Acuity Improvement of >10 letters and ≥20% reduction in OCT CSF Thickness | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| N | Median (25th, 75th Quartile) | Univariate/Multivariate P-values† | N | Median (25th, 75thQuartile) | Univariate/MultivariateP-values† | N | % | Univariate/MultivariateP-values† | |
| Total | 338 | +9 (+4, +15) | 334 | −116 (−209, −44) | 334 | 37% | |||
|
| |||||||||
| Race | 0.87/-- | 0.008/-- | 0.08/-- | ||||||
| Non-White | 91 | +9 (+5, +15) | 88 | −151 (−227, −78) | 88 | 44% | |||
| White | 247 | +9 (+3, +15) | 246 | −104 (−192, −34) | 246 | 35% | |||
| Gender | 0.94/-- | 0.81/-- | 0.36/-- | ||||||
| Women | 145 | +9 (+5, +15) | 145 | −119 (−210, −34) | 145 | 39% | |||
| Men | 193 | +9 (+3, +15) | 189 | −115 (−207, −46) | 189 | 36% | |||
| Age‡ | <0.001/<0.001 | 0.26/-- | 0.002/0.003 | ||||||
| <60 years | 114 | +11 (+5, +16) | 114 | −133 (−258, −63) | 114 | 48% | |||
| ≥60 years | 224 | +8 (+3, +14) | 220 | −109 (−192, −37) | 220 | 32% | |||
| Diabetes Type | 0.65/-- | 0.03/-- | 0.31/-- | ||||||
| Type 1 | 23 | +7 (+3, +12) | 23 | −95 (−127, −44) | 23 | 26% | |||
| Type 2 | 309 | +9 (+4, +15) | 305 | −122 (−212, −44) | 305 | 38% | |||
| Diabetes Duration‡ | <0.001/-- | 0.16/-- | 0.04/-- | ||||||
| <15 years | 128 | +10 (+5, +16) | 128 | −151 (−226, −57) | 128 | 40% | |||
| ≥15 years | 210 | +8 (+3, +15) | 206 | −103 (−181, −35) | 206 | 36% | |||
| Mean Arterial Blood Pressure‡ | 0.03/-- | 0.57/-- | 0.19/-- | ||||||
| ≥100 mmHg | 140 | +11 (+5, +16) | 138 | −142 (−242, −47) | 138 | 44% | |||
| <100 mmHg | 198 | +8 (+2, +15) | 196 | −109 (−179, −37) | 196 | 33% | |||
| HbA1c‡ | 0.18/-- | 0.94/-- | 0.67/-- | ||||||
| < 7.5 % | 170 | +8 (+3, +14) | 167 | −133 (−221, −49) | 167 | 35% | |||
| ≥ 7.5 % | 161 | +9 (+4, +16) | 160 | −101 (−195, −34) | 160 | 39% | |||
| Hypertension | 0.43/-- | 0.96/-- | 0.71/-- | ||||||
| No | 69 | +9 (+4, +15) | 68 | −105 (−238, −43) | 68 | 38% | |||
| Yes | 269 | +9 (+4, +15) | 266 | −120 (−203, −44) | 266 | 37% | |||
| Elevated Cholesterol | 0.94/-- | 0.64/-- | 0.83/-- | ||||||
| No | 117 | +9 (+3, +16) | 115 | −130 (−241, −62) | 115 | 40% | |||
| Yes | 221 | +9 (+4, +15) | 219 | −110 (−189, −35) | 219 | 36% | |||
| Cardiovascular Disease | 0.55/-- | 0.57/-- | 0.74/-- | ||||||
| No | 246 | +9 (+3, +15) | 243 | −122 (−206, −44) | 243 | 38% | |||
| Yes | 92 | +10 (+4, +15) | 91 | −99 (−212, −43) | 91 | 35% | |||
| Renal Disease | 0.87/-- | 0.26/-- | 0.71/-- | ||||||
| No | 314 | +9 (+4, +15) | −113 (−209, −42) | 310 | 37% | ||||
| Yes | 24 | +10 (+6, +15) | 24 | −136 (−215, −73) | 24 | 46% | |||
| Neurologic Disease | 0.21/-- | 0.48/-- | 0.87/-- | ||||||
| No | 266 | +9 (+3, +15) | 262 | −113 (−210, −44) | 262 | 37% | |||
| Yes | 72 | +11 (+6, +18) | 72 | −130 (−194, −44) | 72 | 39% | |||
| Insulin Use | 0.56/-- | 0.30/-- | 0.56/-- | ||||||
| No | 132 | +9 (+5, +15) | 131 | −141 (−235, −62) | 131 | 37% | |||
| Yes | 206 | +9 (+3, +15) | 203 | −100 (−189, −34) | 203 | 38% | |||
| Prescribed Glitazones | 0.71/-- | 0.07/-- | 0.57/-- | ||||||
| No | 269 | +9 (+4, +15) | 266 | −110 (−192, −44) | 266 | 36% | |||
| Yes | 69 | +8 (+4, +16) | 68 | −160 (−263, −45) | 68 | 41% | |||
| Prescribed Statins | 0.87/-- | 0.88/-- | 0.93/-- | ||||||
| No | 143 | +9 (+4, +16) | 141 | −120 (−210, −62) | 141 | 38% | |||
| Yes | 195 | +9 (+4, +15) | 193 | −112 (−206, −34) | 193 | 37% | |||
DME = diabetic macular edema; OCT = optical coherence tomography; CSF= central subfield thickness, HbA1c = hemoglobin A1c
‘Univariate’ P-values are adjusted for baseline level of the outcome (visual acuity, OCT CSF thickness, or both). Characteristics with P<0.10 were included in a multivariate model and those with P<0.01 were retained; P-values < 0.01 in the final multivariate models appear in bold.
Continuous version of variable used in model to obtain P-value, categories are shown for ease of interpretation only
Table 3. Change in Visual Acuity and Optical Coherence Tomography Central Subfield Thickness from Baseline to 1 Year by Ocular History Prior to Study Entry.
| Change in Visual Acuity | Change in OCT CSF Thickness | Visual Acuity Improvement of >10 Letters and ≥20% Reduction in OCT CSF Thickness | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| N | Median(25th, 75thQuartile) | Univariate/MultivariateP-values† | N | Median(25th, 75th Quartile) | Univariate/MultivariateP-values† | N | % | Univariate/MultivariateP-values† | |
| Total | 338 | +9 (+4, +15) | 334 | −116 (−209, −44) | 334 | 37% | |||
|
| |||||||||
| Prior DME Treatment | 0.004/-- | 0.08/-- | 0.10/-- | ||||||
| No | 126 | +11 (+6, +16) | 126 | −140 (−242, −63) | 126 | 43% | |||
| Yes | 212 | +8 (+2, +15) | 208 | −101 (−183, −35) | 208 | 34% | |||
| Timing of Most Recent Prior DME Treatment | 0.92/-- | 0.23/-- | 0.77/-- | ||||||
| ≤ 16-weeks | 24 | +8 (−1, +16) | 24 | −123 (−179, −56) | 24 | ||||
| > 16-weeks – 32-weeks | 88 | +8 (+2, +15) | 87 | −135 (−191, −48) | 87 | 36% | |||
| > 32-weeks – 1 year | 19 | +7 (+2, +14) | 18 | −76 (−105, −5) | 18 | 22% | |||
| > 1 year | 81 | +9 (+4, +14) | 79 | −81 (−192, −26) | 79 | 33% | |||
| Prior Laser for DME | 0.15/-- | 0.20/-- | 0.18/-- | ||||||
| No | 148 | +10 (+5, +15) | 146 | −129 (−230, −53) | 146 | 41% | |||
| Yes | 190 | +8 (+3, +15) | 188 | −101 (−186, −35) | 188 | 35% | |||
| Timing of Prior Laser for DME | 0.73/-- | 0.33/-- | 0.51/-- | ||||||
| ≤ 1 year | 100 | +8 (+3, +15) | 99 | −113 (−180, −47) | 99 | 36% | |||
| > 1 year | 90 | +8 (+3, +14) | 89 | −92 (−215, −26) | 89 | 33% | |||
| Prior PRP | 0.003/-- | 0.24/-- | 0.57/-- | ||||||
| No | 245 | +9 (+5, +16) | 244 | −136 (−218, −61) | 244 | 39% | |||
| Yes | 93 | +8 (+2, +13) | 90 | −81 (−161, −15) | 90 | 34% | |||
| Timing of Prior PRP | 0.74/-- | 0.52/-- | 0.14/-- | ||||||
| ≤ 1 year | 18 | +9 (0, +15) | 18 | −106 (−140, −5) | 18 | 44% | |||
| > 1 year | 48 | +9 (+1, +13) | 46 | −64 (−167, −11) | 46 | 28% | |||
| Timing of Prior Cataract Extraction | 0.18/-- | 0.79/-- | 0.33/-- | ||||||
| ≤ 1 year | 22 | +9 (+7, +15) | 22 | −169 (−238, −29) | 22 | 27% | |||
| > 1 year | 61 | +6 (+1, +12) | 60 | −121 (−166, −47) | 60 | 28% | |||
| History of YAG Capsulotomy | 0.60/-- | 0.89/-- | 0.05/-- | ||||||
| No | 323 | +9 (+4, +15) | 320 | −120 (−210, −44) | 320 | 38% | |||
| Yes | 15 | +6 (+1, +13) | 14 | −77 (−137, −42) | 14 | 14% | |||
OCT= optical coherence tomography; CSF= central subfield thickness; DME= diabetic macular edema; PRP= panretinal photocoagulation; YAG= yttrium aluminum garnet
‘Univariate’ P-values are adjusted for baseline level of the outcome (visual acuity, OCT CSF thickness, or both). Characteristics with P<0.10 were included in a multivariate model and those with P<0.01 were retained.
Table 4. Change in Visual Acuity and Optical Coherence Tomography Central Subfield Thickness from Baseline to 1 Year by Ocular Examination Characteristics at Study Entry.
| Change in Visual Acuity | Change in OCT CSF Thickness | Visual Acuity improvement of >10 Letters and ≥20% Reduction in OCT CSF Thickness | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| N | Median (25th, 75thQuartiles) | Univariate/MultivariateP-values† | N | Median (25th, 75thQuartiles) | Univariate/multivariateP-values† | N | % | Univariate/MultivariateP-values† | |
| Total | 338 | +9 (+4, +15) | 334 | −116 (−209, −44) | 334 | 37% | |||
|
| |||||||||
| Visual Acuity‡ | <0.001/<0.001 | 0.24/-- | 0.09/0.003 | ||||||
| ≤ 65 (20/50) letters | 170 | +13 (+7, +20) | 168 | −151 (−238, −50) | 168 | 49% | |||
| ≥ 66 (20/50) letters | 168 | +6 (+1, +11) | 166 | −97 (−178, −42) | 166 | 25% | |||
| Visual Acuity in Non-study Eye‡ | 0.05/-- | 0.79/-- | 0.17/-- | ||||||
| ≤ 65 (20/50) letters | 103 | +9 (+4, +15) | 103 | −137 (−238, −49) | 103 | 37% | |||
| ≥ 66 (20/50) letters | 235 | +9 (+4, +15) | 231 | −110 (−189, −36) | 231 | 38% | |||
| Lens Status | 0.005/-- | 0.44/-- | 0.01/-- | ||||||
| Pseudophakic | 103 | +7 (+2, +13) | 102 | −123 (−185, −36) | 102 | 27% | |||
| Phakic | 235 | +10 (+5, +15) | 232 | −113 (−216, −47) | 232 | 42% | |||
| DR Severity on Clinical Exam as Judged by Investigators§ | 0.007/0.003 | 0.72/-- | 0.80/-- | ||||||
| No DR/MA/Mild/Moderate NPDR | 188 | +9 (+5, +15) | 187 | −115 (−199, −52) | 187 | 35% | |||
| Severe NPDR | 66 | +12 (+4, +18) | 66 | −183 (−276, −92) | 66 | 53% | |||
| PDR or prior PRP | 84 | +8 (+1, +12) | 81 | −70 (−140, −11) | 81 | 31% | |||
| Type of DME on Clinical Exam as Judged by Investigator | 0.66/-- | 0.39/-- | 0.26/-- | ||||||
| Predominantly focal | 105 | +9 (+4, +13) | 104 | −94 (−158, −30) | 104 | 34% | |||
| Neither predominantly focal or diffuse | 83 | +10 (+2, +16) | 82 | −114 (−212, −43) | 82 | 40% | |||
| Predominantly diffuse | 150 | +9 (+3, +16) | 148 | −161 (−254, −63) | 148 | 39% | |||
OCT= optical coherence tomography; CSF= central subfield; DR= diabetic Retinopathy; MA= microaneurysms; NPDR= non proliferative diabetic retinopathy; PDR= proliferative diabetic retinopathy; PRP= panretinal photocoagulation.
‘Univariate’ P-values for factors other than baseline level of outcome are adjusted for baseline level of the outcome (visual acuity, OCT CSF thickness, or both). Characteristics with P<0.10 were included in a multivariate model and those with P<0.01 were retained; P-values < 0.01 in the final multivariate models appear in bold.
Continuous version of variable used in model to obtain P-value, categories are shown for ease of interpretation only
Ordinal version of variable used in model to obtain P-value
Table 5. Change in Visual Acuity and Optical Coherence Tomography Central Subfield Thickness from Baseline to 1 Year by Optical Coherence Tomography and Fundus Photograph Features at Study Entry.
| Change in Visual Acuity | Change in OCT CSF Thickness | Visual Acuity Improvement of >10 Letters and ≥20% Reduction in OCT CSF Thickness | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| N | Median (25th, 75thQuartiles) | Univariate/MultivariateP-values† | N | Median (25th, 75th Quartiles) | Univariate/MultivariateP-values† | N | % | Univariate/MultivariateP-values† | |
| Total | 338 | +9 (+4, +15) | 334 | −116 (−209, −44) | 334 | 37% | |||
|
| |||||||||
| OCT CSF Thickness‡ | 0.03/-- | <0.001/<0.001 | <0.001/0.005 | ||||||
| < 400 μm | 187 | +7 (+2, +13) | 185 | −71 (−119, −15) | 185 | 26% | |||
| ≥ 400 μm | 150 | +11 (+6, +17) | 149 | −219 (−304, −154) | 149 | 51% | |||
| OCT Retinal Volume‡ | 0.10/-- | 0.03/-- | 0.55/-- | ||||||
| <9.0 mm3 | 167 | +7 (+2, +12) | 167 | −84 (−139, −29) | 167 | 27% | |||
| ≥9.0 mm3 | 96 | +13 (+7, +18) | 96 | −227 (−300, −149) | 96 | 55% | |||
| OCT Cystoid Abnormalities§ | 0.81/-- | 0.95/-- | 0.88/-- | ||||||
| No evidence | 14 | +7 (+4, +20) | 14 | −19 (−46, +11) | 14 | 7% | |||
| Questionable/Definite | 318 | +9 (+4, +15) | 315 | −124 (−212, −49) | 315 | 39% | |||
| OCT Subretinal Fluid | 0.04/-- | 0.34/-- | 0.64/-- | ||||||
| No evidence | 258 | +8 (+3, +14) | 256 | −98 (−178, −34) | 256 | 32% | |||
| Questionable/Definite | 76 | +13 (+7, +21) | 75 | −215 (−306, −110) | 75 | 53% | |||
| OCT Vitreoretinal Abnormality§ | 0.01/-- | 0.01/-- | 0.01/-- | ||||||
| No evidence | 171 | +9 (+4, +15) | 170 | −127 (−206, −54) | 170 | 41% | |||
| Questionable/Definite | 157 | +8 (+3, +14) | 154 | −104 (−207, −34) | 154 | 32% | |||
| Ocular Characteristics Provided by Fundus Photograph Reading Center | |||||||||
| Photograph DR Severity§ | 0.03/-- | 0.62/-- | 0.66/-- | ||||||
| No DR/MA/Mild/Moderate NPDR | 86 | +8 (+4, +15) | 85 | −111 (−186, −46) | 85 | 29% | |||
| Severe NPDR | 135 | +11 (+6, +17) | 135 | −151 (−249, −63) | 135 | 44% | |||
| PDR or prior PRP | 103 | +8 (+2, +13) | 100 | −94 (−165, −18) | 100 | 34% | |||
| Hemorrhages/MA in Grid (Photographic)§ | 0.02/-- | 0.03/-- | 0.18/-- | ||||||
| None/Questionable/<std1 | 70 | +6 (0, +13) | 69 | −76 (−137, −20) | 69 | 28% | |||
| <std2a/<std2b/>std2b | 255 | +10 (+5, +16) | 252 | −133 (−234, −58) | 252 | 40% | |||
| Hard Exudates in Grid (Photographic)§ | <0.001/-- | 0.004/0.004 | <0.001/0.002 | ||||||
| Questionable/Ddefinite | 240 | +10 (+5, +15) | 238 | −140 (−230, −64) | 238 | 43% | |||
| None | 85 | +7 (+1, +12) | 83 | −70 (−140, −21) | 83 | 20% | |||
| Surface Wrinkling Retinopathy (Photographic)§ | <0.001/<0.001 | 0.03/-- | <0.001/-- | ||||||
| None | 268 | +10 (+4, +15) | 268 | −129 (−216, −61) | 268 | 41% | |||
| Questionable/Definite | 54 | +7 (+2, +12) | 50 | −45 (−157, −18) | 50 | 20% | |||
‘Univariate’ P-values for factors other than baseline level of outcome are adjusted for baseline level of the outcome (visual acuity, OCT CSF thickness, or both). Characteristics with P<0.10 were included in a multivariate model and those with P<0.01 were retained; P-values < 0.01 in the final multivariate models appear in bold.
Continuous version of variable used in model to obtain P-value, categories are shown for ease of interpretation only.
Ordinal version of variable used in model to obtain P-value.
Relationship between Baseline Characteristics and Visual Acuity Outcome
The average visual acuity at baseline was 62 letters (∼Snellen equivalent 20/63). Eyes that started with lower visual acuity scores (poorer levels of acuity) were more likely to realize larger gains in visual acuity over time (Table 4), but less likely to reach near normal visual acuity (≥74 letters, ∼Snellen equivalent 20/32 or better, Table 6, available online). The overall median percent reduction in visual acuity deficit between baseline and 1 year was 52% and did not significantly differ across the range of baseline visual acuity (P = 0.92). For example, the median percent reduction in the deficit was 55% among those with baseline visual acuity 20/80 or better compared with 36% for those with baseline visual acuity worse than 20/80 (P = 0.64).
Table 6. Relationship between Baseline Visual Acuity and Visual Acuity Outcomes (online).
| N | Change in Visual Acuity from Baseline | 20/32 or Better at 1-year | Reduction in Visual Acuity Deficit | |
|---|---|---|---|---|
|
| ||||
| Median (letter score) (25th, 75thQuartiles) | % | Median (%) (25th, 75thQuartiles) | ||
|
|
||||
| Visual Acuity at Baseline | ||||
| 24-53 (20/320-20/100) | 69 | +13 (+8, +24) | 17 | +36 (+19, +64) |
| 54-63 (20/80–20/63) | 79 | +12 (+6, +18) | 47 | +54 (+25, +73) |
| 64-68 (20/50) | 63 | +10 (+5, +15) | 68 | +56 (+29, +82) |
| 69-73 (20/40) | 65 | +7 (+2, +11) | 75 | +55 (+17, +85) |
| 74-78 (20/32) | 62 | +5 (0, +8) | --* | +55 (0, +100) |
Excluded from analysis as baseline visual acuity was already ≥74 (∼ snellen equivalent 20/32 or better)
After adjusting for baseline visual acuity, three factors were associated with a larger magnitude of treatment benefit on visual acuity at 1 year: younger age (P < 0.001, Table 2), less severe diabetic retinopathy on clinical exam (P = 0.003, Table 4) and absence of surface wrinkling retinopathy on fundus photographs (P<0.001, Table 5). There was an average of 2.2 letter increase in visual acuity gains at 1 year for every 10 years of younger age (95% confidence interval [CI] 1.1 – 3.3) across participants. Hence, a person aged 50 years old would be predicted to have an additional 4 letters of vision improvement compared with an individual aged 70 years old. Compared with eyes having proliferative diabetic retinopathy (PDR) or prior panretinal photocoagulation (PRP), eyes with severe non-proliferative diabetic retinopathy (NPDR) or moderate NPDR or less were estimated to average 4 more letters improvement (95% CI 1-7 and 2-7, respectively). Eyes that had no evidence of surface wrinkling retinopathy were estimated to average an additional 4 (95% CI 1-7) letters improvement compared with eyes that had either questionable or definite wrinkling on fundus photographs.
Relationship between Baseline Characteristics and OCT Defined Anatomic Outcomes
At baseline, the mean CSF thickness of eyes assigned to ranibizumab was 406 μm. Eyes that started with greater CSF thickness were more likely to realize greater reductions in thickness over time (Table 5), but less likely to reach normal or near normal thickness (CSF thickness < 250 μm, data not shown).
This relationship of greater reduction in CSF thickness with greater baseline thickness was confirmed when median percent change in excess retinal thickness was explored among baseline thickness groups. For example, the median percent decrease in excessive retinal thickness was 46% for eyes with baseline thickness of < 300 μm versus 82% for eyes with ≥ 500 μm thickness at study entry (P = 0.003).
After adjusting for baseline CSF thickness, hard exudates within the 6 mm ETDRS grid on fundus photographs were found to be associated with change in OCT CSF thickness (P = 0.004, Table 5). Eyes with questionable or definite lipid in the macular region at baseline were estimated to have 31 more microns reduction (95% CI 8-53) than eyes without any lipid.
Relationship between Baseline Characteristics and Composite Visual Acuity and OCT Outcome
The composite 1 year outcome of 10 or more letter improvement in visual acuity and 20% or more reduction in CSF thickness was observed in 37% of the overall cohort. After adjusting for baseline visual acuity and CSF thickness, age and hard exudates within the 6 mm ETDRS grid on fundus photographs were found to be associated with this composite outcome. For every 10 years of younger age, there was a 27% increase in the relative probability of this composite outcome (Relative Risk [RR, 95% CI]:1.27 [1.09, 1.48]; P = 0.003). Eyes with questionable or definite lipid in the macular region at baseline were twice as likely (43% versus 20%; RR 2.01 [95% CI 1.29, 3.14]; P = 0.002) to have this favorable combined functional and anatomic outcome than eyes without any lipid.
Relationship between Baseline Characteristics and Evolution of Central Macular Thickness during Treatment with Visual Acuity Outcome
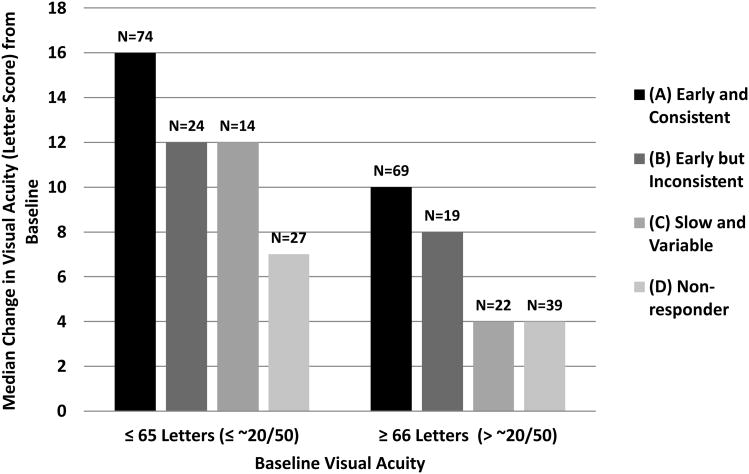
The 4 patterns of CSF thickness evolution during the first year of ranibizumab treatment showed differences in visual acuity outcome at 1 year (Table 7, available online). ‘Early and consistent’ anatomic responders had mean improvement of 13 ± 9 letters (column A), ‘early but inconsistent’ responders, 9 ± 9 letters (column B), ‘slow and variable’ responders, 7 ± 11 letters (column C), and ‘non-responders”, 4 ± 9 letters (column D) (P<0.001). These differences were not explained by differences in number of intravitreal injections administered to these subgroups (P remains <0.001 after adjustment for number of injections). The number of injections (mean ± SD) varied from 8±3 to 10±3 among these subgroups; the group with the most robust OCT evolution pattern had the least number of injections.
Table 7. Visual Acuity at One Year based on Categorization of Optical Coherence Tomography Central Subfield Thickness During Year 1 of Treatment (n=288) (online).
| Categorization of OCT CSF Thickness Improvement of at least 20% (1-step reduction of logOCT║) from Baseline | ||||
|---|---|---|---|---|
|
|
||||
| Change in Visual Acuity From Baseline to 1 Year* | (A) Early and Consistent N=143 | (B) Early but Inconsistent N=43 | (C) Slow and Variable N=36 | (D) Non-responder N=66 |
|
| ||||
| Improved ≥15 Letters | 60 (42%) | 11 (26%) | 5 (14%) | 5 (8%) |
| Improved 14-10 Letters | 32 (22%) | 12 (28%) | 7 (19%) | 8 (12%) |
| Improved 9-5 Letters | 33 (23%) | 10 (23%) | 10 (28%) | 18 (27%) |
| Within ±4 Letters | 14 (10%) | 5 (12%) | 12 (33%) | 24 (36%) |
| Worsened 5-9 Letters | 2 (1%) | 4 (9%) | 0 | 8 (12%) |
| Worsened 10-14 Letters | 1 (1%) | 1 (2%) | 1 (3%) | 2 (3%) |
| Worsened ≥15 Letters | 1 (1%) | 0 | 1 (3%) | 1 (2%) |
| Median(25th, 75th Quartiles) | +12 (+7, +17) | +11 (+6, +15) | +8 (+3, +11) | +4 (−1, +8) |
| Mean ± SD | +13 ± 9 | +9 ± 9 | +7 ± 11 | +4 ± 9 |
|
| ||||
| Number of Injections in Year 1 | ||||
|
| ||||
| Median(25th, 75th Quartiles) | 8 (6, 10) | 8 (7, 10) | 10 (8, 12) | 10 (7, 12) |
| Mean ± SD | 8 ± 3 | 9 ± 2 | 10 ± 3 | 9 ± 3 |
| Minimum-Maximum | 2 - 13 | 4 - 13 | 4 – 13 | 2 - 13 |
OCT= optical coherence tomography; CSF= central subfield, ‘Early and consistent’= Improved at the 16-week study visit and was sustained at the 32-week and 1-year study visits. ‘Early but inconsistent’= Improved at the 16-week study visit but not at both the 32-week and 1-year study visits. ‘Slow and variable’= Did not improve at the 16-week study visit but did improve at the 32-week and/or 1-year study visits. ‘Non-responder’= Did not improve at the 16-week, 32-week, or 1-year study visit.
The following pairwise comparisons were statistically significant (P< 0.01): (A) versus (C), (A) versus (D).
Logarithmic transformation of OCT central subfield thickness (logOCT) is calculated by taking the log base 10 of the ratio of the central subfield thickness divided by 200 and rounding to the nearest hundredth.The change is the change in the log values.10
When OCT evolution was added to the change in visual acuity outcome regression model, eyes that demonstrated an ‘early and consistent’ anatomic response had vision improvement of approximately 6 letters on average (95% CI: 4 - 9 letters) greater than eyes that did not manifest anatomic improvement at any visit and 4 letters (approximately 1 line, 95% CI: 0.4 - 7 letters) greater than eyes that have a late (at 16-week and/or 1-year study visits) anatomic response (P < 0.001, Table 8).Age and surface wrinkling retinopathy also remained associated with change in visual acuity, similar to the model containing only baseline factors.
Table 8. Visual Acuity Outcome Regression Model Incorporating Baseline Characteristics and Central Macular Thickness Evolution During Year 1*.
| N | Change in Visual Acuity Median (25TH, 75TH quartiles) | Multivariate Full Model*P Value | Final Multivariate Model† | ||
|---|---|---|---|---|---|
|
| |||||
| Estimate (Increase in Letter Score; 95% CI) | P Value | ||||
|
| |||||
| OCT CSF Thickness Evolution Pattern | <0.001 | <0.001 | |||
| Early and Consistent | 143 | +12 (+7, +17) | +6 (+4, +9) | ||
| Early but Inconsistent | 43 | +11 (+6, +15) | +3 (−1, +6) | ||
| Slow and Variable | 36 | +8 (+3, +11) | +3 (−1, +6) | ||
| Non-responder | 66 | +4 (-1, +8) | -- | ||
CI= confidence interval; OCT= optical coherence tomography; CSF= central subfield, ‘Early and consistent’= Improved at the 16-week study visit and was sustained at the 32-week and 1-year study visits. ‘Early but inconsistent’= Improved at the 16-week study visit but not at both the 32-week and 1-year study visits. ‘Slow and variable’= Did not improve at the 16-week study visit but did improve at the 32-week and/or 1-year study visits. ‘Non-responder’= Did not improve at the 16-week, 32-week, or 1-year study visit.
All factors with P<0.10 in univariate models were included in multivariate full models, however, only factors that were in the final multivariate models were shown in the table.
Includes factors that remained in the multivariate model after a backward selection process using P<0.01 to stay in the model, P-values < 0.01 in the final multivariate models appear in bold.
Figure 1 shows the effect of OCT CSF thickness evolution on median change in visual acuity between baseline and 1 year within subgroups with higher and lower baseline visual acuity.
Figure 1.
Median Visual Acuity Change (Letter Score) from Baseline to 1 Year by Baseline Visual Acuity and Categorization of Optical Coherence Tomography Central Subfield Thickness Evolution During Year 1.
‘Early and consistent’ = Improved at the 16-week study visit and was sustained at the 32-week and 1-year study visits. ‘Early but inconsistent’= Improved at the 16-week study visit but not at both the 32-week and 1-year study visits. ‘Slow and variable’= Did not improve at the 16-week study visit but did improve at the 32-week and/or 1-year study visits. ‘Non-responder’= Did not improve at the 16-week, 32-week, or 1-year study visit.
Discussion
Several clinical trials have recently demonstrated that intravitreal ranibizumab therapy, with either prompt or various degrees of deferred laser, results in improved visual acuity outcomes at 1 and 2 years compared with focal/grid laser alone.5-8, 12-14 Identification of factors associated with relatively good or poor outcomes can help inform treating ophthalmologists and patients as to what they can expect on average when choosing intravitreal ranibizumab as a treatment for DME. We were unable to evaluate factors associated with poor visual acuity outcomes or poor OCT CSF thickness outcomes because the numbers of individuals in the ranibizumab treatment groups with vision loss or an increase in CSF thickness were too small to analyze. Thus, our attention was focused on predictors of improvement in visual acuity, retinal thickness, or the simultaneous presence of both outcomes when treated with ranibizumab for central involving DME.
A large number of baseline features, 37, were evaluated for their relationship with vision and anatomic improvement at 1 year (Table 1, available online). The selected variables covered a wide range of demographic, medical history, medication use, past ocular history with particular emphasis on past interventions for diabetic retinopathy, present ocular manifestations of diabetic eye disease, and morphologic features identified on retinal images as interpreted by a reading center. No features were identified that would predict groups for whom treatment appeared to be harmful at one year. Some features were identified that were associated with a relatively better outcome. These analyses suggest that intravitreal ranibizumab could be considered for all patients with center involved DME. This additional information on relative risks and benefits could help patients and their physicians in their choice of treatment and their expectations.
Four factors were identified that were associated with a larger magnitude of visual acuity increase at 1 year: visual acuity at the time of treatment initiation, younger age, less severe retinopathy level as assessed by the treating ophthalmologist, and absence of surface wrinkling retinopathy. The observed association between worse visual acuity at the time of treatment and an increased degree of visual acuity improvement may be at least partially affected by “ceiling effects” on the degree of improvement possible for those with better visual acuity at the time of treatment. Our secondary analysis which substituted percent reduction in visual acuity deficit at 1 year for change in visual acuity represented an attempt to eliminate the ceiling effect of baseline visual acuity. In this analysis, the association between baseline visual acuity and visual acuity outcomes was not confirmed. The association with age and surface wrinkling retinopathy remained, suggesting that the ceiling effect may be largely responsible for the observed association of 1 year vision outcome with baseline visual acuity.
It is unknown why younger age is associated with superior vision outcomes. Younger participant age has also been found to be associated with superior vision outcomes when treating patients with neovascular age-related macular degeneration with ranibizumab.15 The present analysis found a median difference of 3 letters for those under age 60 versus age 60 or older. However, at the extremes of the age distribution of our participants, the median difference was as large as 7 letters (i.e., participants age 47 and younger [5th percentile] versus participants age 78 and older [95th percentile]).
Eyes graded by the participating investigators as NPDR, rather than PDR or post-PRP, had a greater magnitude of visual acuity improvement, but this was not confirmed in the analysis in which retinopathy level at baseline was assessed by the independent reading center. This association may have been a chance observation, although, it is plausible that eyes with the most advanced grades of retinopathy may have more ischemia or permanent damage/scarring that would limit their potential for vision improvement.
With respect to the association of superior vision outcomes in the absence of surface wrinkling retinopathy, persons with DME and evidence of surface wrinkling retinopathy might be anticipated to do less well than those with DME and without surface wrinkling since the former could have an effect on the macular anatomy which could retard functional and anatomic improvement. As part of the study inclusion criteria, investigators were asked to include only those eyes that had definite retinal thickening due to DME on clinical examination involving the center of the macula believed to be the main cause of visual loss. Therefore, enrollment of eyes with some degree of abnormal vitreo-retinal interface anatomy was permitted as long as the investigator did not interpret that finding to have a major impact on visual function. Thus, the 17% of individuals in this trial with surface wrinkling retinopathy, identified as questionable or definite on color fundus photographs at a reading center, likely represent eyes in a mild range of the spectrum of abnormal vitreo-retinal relationships. As noted in Table 5, there was a positive gain in median vision and a reduction in thickness when these eyes were treated with ranibiumab. However, the difference in outcome was roughly an average of 4 letters increase in visual acuity for the group without surface wrinkling retinopathy compared with the group with surface wrinkling. In this cohort this corresponds to approximately 15% fewer patients experiencing at least 10 or 15 letter gains in visual acuity in the presence of surface wrinkling retinopathy when receiving treatment for DME with ranibizumab.
An additional variable associated with 1 year visual acuity outcomes was the evolution of OCT thickness response during the first year of treatment. The finding that eyes that rapidly and consistently demonstrate a favorable anatomic response are more likely to have superior vision outcomes would be anticipated. However, the treatment protocol instructed investigators to continue monthly injections until criteria for “success” were met or until stabilization (successive treatments not yielding incremental improvements in vision or CSF thickness). When investigators applied this protocol, a larger number of injections were administered to the groups of individuals with the less favorable OCT evolution patterns. This suggests that some eyes are simply less responsive (at the anatomic level) than others to the intervention. However, we were unable to identify, in advance, predictors of who would or would not have a rapid and consistent response to treatment and even those subgroups that responded late or appeared to be refractory still had positive changes in both mean and median visual acuity relative to baseline. In other words, a less favorable OCT response pattern is not a reason to discontinue treatment and the early non-responders may convert to later responders.
One baseline factor, other than the initial thickness value, associated with more favorable OCT improvement at 1 year, was the presence of lipid within 6 mm of the foveal center. Lipid may be a marker for hyperpermeability and fluid turnover, as well as a marker of areas of retina with intact “blood retinal barrier.” These are the pathophysiologic characteristics that ranibizumab would be anticipated to most favorably affect. In addition, eyes without lipid might have additional underlying mechanisms for central retinal thickening including ischemia, traction, or cystoid degeneration; these mechanisms would not be anticipated to be altered by the administration of ranibizumab. The relationship of initial thickness value with a more favorable OCT outcome may be influenced by statistical issues of floor effects and regression to the mean, rather than representing a true biologic relationship, however, our analysis of change in percent excessive retinal thickness suggests it may be a true relationship.
When a composite outcome was constructed that consisted of both a favorable OCT change and visual acuity change at 1 year, predictors of this outcome were baseline visual acuity, age and the presence of lipid in the macula at the time of treatment initiation. This result is consistent with the results discussed above as each of these factors had been identified with either a more favorable vision or OCT outcome.
In summary, multiple studies have demonstrated that, for eyes with center involved DME, intravitreal ranibizumab improves both functional and anatomic outcomes compared with laser alone. An extensive post-hoc search of baseline factors to differentiate these outcomes among these individuals being treated with ranibizumab, with a median number of 8 injections in the first year, did not identify any factors that could be considered to be contraindications to treatment.It did identify some factors that may be associated with a greater improvement and this can be helpful in discussions with patients when balancing the risks and benefits of treatment for center involved DME.
Acknowledgments
Financial Support: Supported through a cooperative agreement from the National Eye Institute and the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Department of Health and Human Services EY14231, EY14229, EY018817
Footnotes
An address for reprints will not be provided
Financial Disclosure: The funding organization (National Institutes of Health) participated in oversight of the conduct of the study and review of the manuscript but not directly in the design or conduct of the study, nor in the collection, management, analysis, or interpretation of the data, or in the preparation of the manuscript. Genentech provided the ranibizumab for the study and Allergan, Inc. provided the triamcinolone for the study.In addition, Genentech and Allergan, Inc. provided funds to DRCR.net to defray the study's clinical site costs. As described in the Diabetic Retinopathy Clinical Research Network (DRCR.net) Industry Collaboration Guidelines (available at www.drcr.net), the DRCR.net had complete control over the design of the protocol, ownership of the data, and all editorial content of presentations and publications related to the protocol. A complete list of all DRCR.net investigator financial disclosures can be found at www.drcr.net.
References
- 1.Moss S, Klein R, Klein B. The 14-year incidence of visual loss in a diabetic population. Ophthalmology. 1998;105(6):998–1003. doi: 10.1016/S0161-6420(98)96025-0. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. [Accessed December 14,2011];Obesity and overweight for professionals, data and statistics: Adult obesity. http://www.cdc.gov/obesity/data/adult.html.
- 3.Early Treatment Diabetic Retinopathy Study Research Group. Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Arch Ophthalmol. 1985;103(12):1796–806. [PubMed] [Google Scholar]
- 4.Antonetti DA, Barber AJ, Hollinger LA, Wolpert EB, Gardner TW. Vascular endothelial growth factor induces rapid phosphorylation of tight junction proteins occludin and zonula occluden 1. A potential mechanism for vascular permeability in diabetic retinopaty and tumors. J Bio Chem. 1999;274(33):23463–7. doi: 10.1074/jbc.274.33.23463. [DOI] [PubMed] [Google Scholar]
- 5.Michaelides M, Kaines A, Hamilton RD, et al. A prospective randomized trial of intravitreal bevacizumab or laser therapy in the management of diabetic macular edema (BOLT study) 12-month data: report 2. Ophthalmology. 2010;117(6):1078–86 e2. doi: 10.1016/j.ophtha.2010.03.045. [DOI] [PubMed] [Google Scholar]
- 6.Do DV, Schmidt-Erfurth U, Gonzalez VH, et al. The DA VINCI Study: phase 2 primary results of VEGF Trap-Eye in patients with diabetic macular edema. Ophthalmology. 2011;118(9):1819–26. doi: 10.1016/j.ophtha.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 7.Diabetic Retinopathy Clinical Research Network. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010;117(6):1064–77 e35. doi: 10.1016/j.ophtha.2010.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elman MJ, Bressler NM, Qin H, et al. Expanded 2-year follow-up of ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2011;118(4):609–14. doi: 10.1016/j.ophtha.2010.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bressler NM, Edwards AR, Antoszyk AN, et al. Retinal thickness on Stratus optical coherence tomography in people with diabetes and minimal or no diabetic retinopathy. Am J Ophthalmol. 2008;145(5):894–901. doi: 10.1016/j.ajo.2007.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferris FL, 3rd, Miller KM, Glassman AR, Beck RW. A proposed method of logarithmic transformation of optical coherence tomography data for use in clinical research. Ophthalmology. 2010(117):1512–16. doi: 10.1016/j.ophtha.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–6. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen QD, Shah SM, Khwaja AA, et al. Two-year outcomes of the ranibizumab for edema of the macula in diabetes (READ-2) study. Ophthalmology. 2010;117(11):2146–51. doi: 10.1016/j.ophtha.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 13.Mitchell P, Bandello F, Schmidt-Erfurth U, et al. The RESTORE study ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology. 2011;118(4):615–25. doi: 10.1016/j.ophtha.2011.01.031. [DOI] [PubMed] [Google Scholar]
- 14.Cunningham ET, Jr, Adamis AP, Altaweel M, et al. A phase II randomized double-masked trial of pegaptanib, an anti-vascular endothelial growth factor aptamer, for diabetic macular edema. Ophthalmology. 2005;112(10):1747–57. doi: 10.1016/j.ophtha.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 15.Boyer DS, Antoszyk AN, Awh CC, Bhisitkul RB, Shapiro H, Acharya NR. Subgroup analysis of the MARINA study of ranibizumab in neovascular age-related macular degeneration. Ophthalmology. 2007;114(2):246–52. doi: 10.1016/j.ophtha.2006.10.045. [DOI] [PubMed] [Google Scholar]