Abstract
Di(2-ethylhexyl) phthalate (DEHP) is a ubiquitous environmental contaminant. Epidemiological studies suggest that DEHP decreases masculinization of male fetuses. Numerous rat studies report DEHP reduces fetal testosterone production at doses greatly exceeding human exposure. We fed pregnant CD-1 mice 0.5 - 500,000 μg/kg/day DEHP from gestation day (GD) 9 - 18 and examined mothers and male fetuses on GD 18. We assessed non-monotonic dose-response by adding a quadratic term to a simple linear regression model. Except at the 500,000-μg/kg/day dose, DEHP stimulated an increase in maternal and fetal serum testosterone and increased anogenital distance (AGD). Non-monotonic dose-response curves were noted for AGD and maternal, and testis testosterone (p values 0.013 to 0.021). Because data from our highest dose (500,000-μg/kg/day) did not differ significantly from controls, this dose could have been incorrectly assumed to be the NOAEL had we only tested very high doses, as is typical in studies for regulatory agencies.
Keywords: Phthalates; DEHP; MEHP; Testosterone; Anogenital distance, non-monotonic dose-response
1. Introduction
The phthalate ester di (2-ethylhexyl) phthalate (DEHP) is a plasticizer added to polyvinyl chloride (PVC) plastic. DEHP's distinct elastic and transparent properties impart flexibility in otherwise brittle PVC products, such baby toys, upholstery, electrical cables, shower curtains, vinyl flooring, syringes, blood storage bags, catheters, and plastic food containers [1, 2]. Since the introduction of phthalates as plasticizers in the 1920s, their annual worldwide production has steadily increased. Current use of phthalates is estimated at 9 million tons per year, of which approximately 2 million tons is DEHP, making it the most abundantly produced phthalate ester and the focus of our study [3, 4].
Since phthalates are not covalently bound to the PVC polymer in the products in which they are incorporated, phthalates leach out of these product into the environment and ultimately into humans and other animals [5]. Humans are exposed to phthalates via skin contact, consumption and inhalation, and fetuses and newborns are exposed indirectly during gestation and lactation as a consequence of maternal exposure [1, 5]. Upon entering the body, phthalates are rapidly metabolized via hydrolysis and glucuronide conjugation. In the hydrolysis phase, phthalates are converted to their respective primary monoesters by lipases and esterases. In phase II metabolism, uridine 5’-diphosphoglucuronyl transferase (UDP) catalyzes the conversion of monoesters to hydrophilic glucuronide conjugates that are readily excreted by the kidneys [3, 6].
Humans are exposed to numerous types of phthalates, including approximately 10 μg/kg bodyweight/day DEHP [7], although the amount and type of phthalates to which humans are exposed varies with age. Fetuses are at risk for in utero exposure to phthalate esters, as metabolites of DEHP have been detected in the cord blood of newborns as well as maternal urine and amniotic fluid [8, 9]. Infants in neonatal intensive care units are exposed to high levels of DEHP metabolites due to the use of DEHP in medical products [10].
During male sexual differentiation, androgens released by the testes are necessary for normal formation of the reproductive tract. In utero exposure to phthalates during the critical perinatal period of sexual differentiation in rats has been shown to permanently alter the reproductive tract [11-13]. Rat studies involving in utero exposure to phthalates, including DEHP, the subject of our investigation, produce changes that are referred to as the “phthalate syndrome”, which include cryptorchidism (undescended testicles), hypospadias (abnormal placement of male external urethral orifice), shortened anogenital distance (AGD), malformations of internal male genitalia, reduced spermatogenesis and reduced testicular testosterone production [14-17]. There is much interest in the phthalate syndrome due to its similarity to human testicular dysgenesis syndrome (TDS), which also includes cryptorchidism, hypospadias, decreased spermatogenesis, and testicular cancer [12, 13].
Most toxicological rat studies with DEHP have examined exposure levels at doses above 100 mg/kg/day, although a few studies have examined lower doses [12, 18-22]. At very high doses, male rats exposed during sexual differentiation showed modulation of testis structure as well as phenotypic effects typically associated with developmental exposure to anti-androgenic chemicals. For example, oral administration of DEHP to pregnant Sprague-Dawley rats at 750 mg/kg/day from gestation day (GD) 14 to postnatal day 3 (PND 3) reduced pup weight at birth, shortened male pup AGD, and reduced testis weights [23]. Pregnant Wistar rats dosed by gavage at 100 and 300 mg/kg/day from GD 7- 21 produced male offspring that displayed reduced testicular testosterone levels as well as a reduction in the transcription of genes for steroidogenic acute regulatory protein (StAR) and P450ssc mRNA as well as the levels of these proteins. StAR and P450ssc are factors involved in steroidogenesis, and their reduced expression is likely to be involved in reduced testosterone production by the testis [24].
A study by Blystone and colleagues on reproductive development in rats [25] estimated that the no observed adverse effect level (NOAEL) for in utero exposure to DEHP was approximately 4.8 mg/kg/day. Human exposure estimates are far lower, at approximately 0.5-25 μg/kg/day of DEHP [7, 26]. However, in a cohort study of pregnant women, there was a significant inverse relationship between urine levels of maternal DEHP metabolites and certain external genital measurements in male offspring. Specifically, increased urine DEHP metabolites during pregnancy were associated with significantly shorter weight-adjusted AGD and an increased incidence of incomplete testicular descent [27]. Of considerable interest is that these potentially adverse reproductive effects were associated with phthalate exposures that were many fold below the predicted NOAEL based on rat studies [27, 28]. There is thus a large discrepancy between this suggestive evidence from epidemiological studies that DEHP has adverse anti-androgenic effects at these low exposure levels and studies with rats that have primarily focused on examining doses of DEHP many orders of magnitude greater than human exposure levels. This kind of discrepancy has led scientists to ponder the differences in methodology used to study endocrine-active compounds in endocrinology and regulatory toxicology as well as the current assumptions upon which chemical risk assessments of DEHP and other endocrine disrupting chemicals are based [29-32].
In order to understand the discrepancies between the doses of DEHP used in rat studies and human epidemiology findings, we conducted a study of the effects of DEHP over a 106 range of maternal exposures. We used CD-1 mice and examined male mouse fetuses for testicular testosterone production and serum levels of testosterone as well as on AGD, which has been shown to be a sensitive biomarker that differs as a result of very small changes in androgen exposure in male and female fetuses in rodents and also in human males [27, 33-35]. Maternal doses of DEHP ranged from the very low dose of 0.5 μg/kg/day to the typical dose in a toxicological risk assessment study of 500,000 μg/kg/day. Our consistent finding is that for no outcome was the effect at 500,000 μg/kg/day predictive of the effects of lower doses. Comparison of linear and non-linear statistical models revealed that all outcomes of interest showed non-monotonic dose-response curves.
2. Materials and Methods
2.1 Animals and housing
Virgin female CD-1 mice (Mus musculus domesticus) were purchased from Charles River Laboratory (Raleigh, NC). Animals were housed in standard polypropylene mouse cages on corncob bedding with ad libitum access to water purified by reverse osmosis and carbon filtration and Purina 5001 soy-based chow. A soy-based diet was selected because we [36] and others [37] have shown that diets that do not contain phytoestrogens elevate fetal serum estradiol and lead to metabolic syndrome and reproductive abnormalities in CD-1 mice. Thus, the use of soy-free diets is not appropriate in this strain of mouse.
Rooms were kept at 23°C, with 12h light and 12h dark. Adult virgin female mice were time-mated. Up to three females were placed with a stud male for 3-4 hours, after which females were checked for copulatory plugs. The date a plug was observed was designated as Gestation Day (GD) 0. Females positive for plugs were weighed, marked for individual identification, and housed up to 3 per cage. Beginning on GD 0, females were fed Purina 5008 soy-based chow ad libitum. All animal studies were conducted in full compliance with the rules governing the humane treatment of animals based on the NIH Guide for the Care and Use of Laboratory Animals. The study was approved by the University of Missouri Animal Care and Use Committee.
2.2 Animal treatment
On GD 9 females were weighed again to check for pregnancy and were randomly assigned to a treatment group. Females were dosed orally one time per day from GD 9 through GD 18 with either tocopherol-stripped corn oil (vehicle control) or DEHP in oil at 0.5, 1.0, 5, 500, 50,000, and 500,000 μg/kg/day. The volume administered, targeted at 30 μl/50 g, was adjusted daily, based on body weight at the time of dosing. The solutions were administered using a micropipetter placed into the corner of an animal's mouth, since the mice readily drink the solutions, and this is less stressful than gavage. The experiment was conducted in three blocks; the first block involved examination of the 0, 5, 500, 50,000 and 500,000 μg/kg/day doses. In the second block we added two more low-dose treatment groups and repeated the negative control and lowest dose from the first block (0, 0.5, 1.0, 5 μg/kg/day). The third block included all of the treatment groups to obtain a total of 9-21 litters per treatment (Table 1).
Table 1.
Litter size, litter sex ratio, fetal male body weight, AGD, testis weight and testicular testosterone concentration for each treatment group on GD 18. There was no statistically significant effect of treatment on litter size, male:female ratio or body weight of male fetuses at birth. AGD at the highest dose was not significantly different from controls, although there was a tendency for reduced AGD at the highest dose group compared to the lower doses of DEHP based on the PROC GLM ANOVA. However, the regression analysis on the AGD data for 1MF males that included the quadratic term was statistically significant (P = 0.014). The overall analysis of log transformed testis weight of 1MF males showed a trend for some doses of DEHP to decrease testis weight (PROC GLM; ANOVA, P = 0.06). Testis weight was significantly reduced in the 50,000 and 500,000 μg/kg/day dose groups relative to controls (a vs. bc P < 0.05, a vs. bc P<0.01), and male fetuses exposed to the 500,000 μg/kg/day dose also had significantly reduced testicular weight relative to males in the 5 and 500 μg/kg/day dose groups (ab vs. c, P < 0.05). PROC GLM ANOVA on log-transformed data indicated no significant effects of DEHP treatment. However, when a regression analysis was conducted with the quadratic term added to the model, the effect of dose on testicular testosterone was statistically significant (P = 0.034). There was a tendency toward elevated testosterone in animals treated with 500 μg/kg/day dose relative to controls (P = 0.08), and the 500 μg/kg/day dose was significantly higher than the 0.5 μg/kg/day dose (P < 0.05). Treatments with the same letter are not significantly different from each other but are statistically different from groups with other letters.
| Treatment (μg/kg/day) |
|||||||
|---|---|---|---|---|---|---|---|
| 0 | 0.5 | 1 | 5 | 500 | 50,000 | 500,000 | |
| Litter size | 12.38 | 12.83 | 12.79 | 12.64 | 12.08 | 12.44 | 12.67 |
| SEM | ±0.66 | ± 0.59 | ±0.58 | ±0.56 | ±0.58 | ±0.25 | ±0.74 |
| n | 21 | 12 | 14 | 14 | 13 | 18 | 18 |
| ANOVA P=0.98 | |||||||
| Male:Female ratio | 1.35 | 1.24 | 1.64 | 1.38 | 1.18 | 1.16 | 0.87 |
| SEM | 0.19 | 0.23 | 0.47 | 0.32 | 0.27 | 0.28 | 0.31 |
| n | 16 | 12 | 13 | 14 | 13 | 13 | 12 |
| ANOVA P=0.68 | |||||||
| Body Weight (g) 1MF males | 1.36 | 1.38 | 1.33 | 1.30 | 1.36 | 1.37 | 1.34 |
| SEM | ±0.03 | ±0.03 | ±0.03 | ±0.03 | ±0.02 | ±0.03 | ±0.03 |
| n | 21 | 11 | 13 | 13 | 13 | 17 | 17 |
| ANOVA P=0.47 | |||||||
| AGD (mm) 1MF males | 1.78 | 1.96 | 1.88 | 1.93 | 1.92 | 1.86 | 1.76 |
| SEM | ±0.03 | ±0.05 | ±0.07 | ±0.07 | ±0.07 | ±0.06 | ±0.06 |
| n | 13 | 5 | 7 | 9 | 9 | 13 | 14 |
| ANOVA P=0.28; linear model P = 0.52; quadratic model P = 0.01 | |||||||
| Testis weight (mg) | 1.49a | 1.25ab | 1.26ab | 1.50ac | 1.42ac | 1.27bc | 1.19c |
| SEM | ±0.08 | ±0.07 | ±0.06 | ±0.16 | ±0.08 | ±0.10 | ±0.11 |
| n | 21 | 12 | 14 | 14 | 13 | 18 | 17 |
| ANOVA P=0.06; linear model P = 0.12; quadratic model P = 0.10 | |||||||
| Testicular testosterone (pg/testis) | 122.08ab | 89.03a | 141.70ab | 138.54ab | 161.88b | 135.23ab | 104.88ab |
| SEM | ±18.74 | ±13.06 | ±29.09 | ±23.55 | ±28.01 | ±23.96 | ±17.63 |
| n | 18 | 12 | 11 | 14 | 13 | 18 | 15 |
| ANOVA P=0.30; linear model P = 0.42; quadratic model P = 0.03 | |||||||
2.3 Serum collection and morphological measurements
Two to four hours after dosing on GD 18, pregnant females were killed by CO2 followed by cervical dislocation. Maternal blood was collected into a 12×75 mm glass tube, which was immediately placed on ice. The fetuses were quickly delivered via caesarian section. The sex and intrauterine position were recorded and weight for 1MF male fetuses was measured as previously described [38]. Because it is known that in rodents intrauterine position influences serum concentrations of estradiol and testosterone due to steroid hormone transport between adjacent fetuses [39], we chose to work with 1MF males (males that were located between one male and one female fetus within a uterine horn) rather than males located between either two males or two females. A 1MF male was randomly selected from each litter for measurements and testes collection. Female fetuses were euthanized and were not examined in this study.
Blood was collected from all male fetuses in each litter, including the 1MF males. The fetal blood for the litter was collected using a heparinized microcapillary tube and pooled in a 0.5 ml polypropylene microcentrifuge tube, and placed on ice. The data for fetal serum testosterone thus represent the average concentration for all males within a litter. Maternal and fetal blood were kept at 4°C overnight and centrifuged for 30 minutes at 3000 RPM the following day. The serum was transferred to clean polypropylene tubes and stored at -20°C for later analysis.
After blood collection, the anogenital distance (AGD, the length from the caudal base of the genital tubercle to the anterior aspect of the anus) of the selected 1MF males was measured using a micrometer lens on an Olympus dissecting microscope. Two researchers blind to the treatment group of the male fetus (RPD and JAT) measured AGD, and a comparison of control group measurements by the two investigators was not significantly different (t-test; P > 0.1). The testes of the selected 1MF males were collected and weighed (body weights had been measured prior to blood collection). The testes were placed on ice and then stored at -20°C for subsequent extraction of testosterone.
2.4 Testosterone radioimmunoassay
Testosterone in maternal serum, fetal plasma and fetal testes was measured by radioimmunoassay as previously described [34]. All samples were assayed in duplicate. Approximately 100 μl of maternal and 10 μl of fetal sera were extracted twice with 1 ml methyl tert-butyl ether for assay. Fetal testes were first incubated in 100 μl phosphate buffered saline at 37°C in a water bath overnight, and then extracted twice with 2 ml ethyl acetate:chloroform (80:20). Samples were then assayed in the same manner as fetal and maternal serum. Iodinated testosterone, the primary anti-testosterone antibody and second antibody (goat anti-rabbit) were obtained from MP Biomedicals (Santa Ana, CA). Intra-assay and inter-assay coefficients of variation were 2.5% and 18.4% respectively, and the sensitivity of the assay was 0.5 pg/ml. Testicular testosterone extraction recovery was estimated using 3H-testosterone (PerkinElmer, Waltham MA) and ranged from 85%-93%.
2.5 Measurement of MEHP in mouse serum by LC/MSMS
Solvents were HPLC grade and were obtained from Fisher Scientific. Mono-2-ethylhexyl phthalate (MEHP) was obtained from Cambridge Isotope Laboratories Inc. (catalog # ULM-4583-1.2). Samples predicted to have low MEHP concentrations (dosing range 0.5-500 μg/kg) were extracted using the method of Takatori et al. [40] with minor modifications. Briefly, pooled serum samples (80 - 185 μl, n = 1 pooled sample per low-dose treatment) were vortexed with 2 ml acetone and then centrifuged at 3,000 rpm for 10 minutes. The pellet was washed with a further 1 ml of acetone. The acetone extracts were combined and dried under nitrogen. The samples were reconstituted in 0.5 ml H2O containing 4-μl acetic acid and then washed four times with 1 ml hexane. The hexane washes were pooled and dried under nitrogen. The dried samples were reconstituted in 0.1 ml acetonitrile for HPLC. Samples predicted to have a high concentration of MEHP (dosing range 50,000-500,000 μg/kg) were extracted directly into acetonitrile (n = 5-6/dose). Sample aliquots (10 μl) were vortexed well in 2.5 – 2.7 ml acetonitrile, and then centrifuged at 3,000 rpm for 10 min. A 1 ml aliquot of the supernatant was taken for HPLC. Recovery was calculated from spiked serum samples run alongside the test samples, and averaged 99-100% for both procedures. Procedural blanks were also run alongside the samples to monitor for reagent contamination or interference, which, as expected for this in vivo metabolite of DEHP, was not detected.
MEHP was quantified by liquid chromatography with mass spectrometry (LC-MSMS) using a Thermo Surveyor TSQ plus connected to an integrated Thermo-Accela LC system; analytes were detected using electrospray ionization with negative polarity, and conditions (tube lens setting, collision energy) were optimized for MEHP using the instrument software. Separations were performed isocratically on a 3 micron Hyperclone HPLC column (100×4.6 mm), at a flow rate of 350 μl/min. The mobile phase consisted of 75% methanol, 25% 50:50 methanol:H2O+0.1% formic acid. Thermo LCQuan software was used to autotune, acquire, and process the LC/MS data. MEHP was detected using selected reaction monitoring for m/z 277>134, and quantitation was made against a standard curve of the MEHP at concentrations ranging from 2-200 ng/ml. In this assay the limit of quantification (LOQ) for MEHP in serum was ~2 ng/ml on-column; for a 200 μl serum sample this is a serum LOQ of 1 ng/ml. Values below these levels were estimated by extrapolation of the standard curve to zero. Low-dose measurements were of single pooled serum samples from each treatment, and values represent the mean (±SEM) of duplicate measurements. High-dose values represent the mean (±SEM) concentration of 5-6 individual serum samples at each data point.
2.6 Statistical analysis
As described above, our outcome variables were AGD (with and without weight-adjustment) and testosterone measured in fetal testis from a randomly selected 1MF male fetuses per litter. We also measured testosterone in maternal serum and in pooled serum from all male fetuses in each litter. We initially analyzed all outcomes by ANOVA using PROC GLM in SAS (6.0) followed by Fisher's LSD test. All data are presented as mean ± SEM but, except for AGD and AGD/body weight, were log-transformed prior to analysis.
Since visual examination of the data suggested non-monotonic dose-response patterns in several outcome variables, we then explored the statistical significance of these patterns with two linear regression models: 1) a simple linear regression model and 2) a regression model that included a quadratic term to test for non-monotonicity. The response variables investigated using this approach were maternal serum testosterone, fetal serum testosterone, testicular testosterone, testis weight, fetal AGD, fetal body weight, and fetal AGD / body weight ratio, since many prior studies have reported AGD data corrected for body weight in rodents and humans [27, 35, 41]. We used Box-Cox transformations to normalize these response variables. Dose was log-transformed (after adding 0.05 to all doses to enable log transform of dose = 0). The statistical measure of a quadratic fit was the p value of the squared dose term, which is also the p value of the improvement in model fit over the simple linear model. These analyses were performed within the R statistical system (R v2.14.2; RStudio v0.95.263). The Companion to Applied Regression (“car”) R package was used for Box-Cox transformations (v2.0-12). Statistical significance was set at P < 0.05.
3. Results
For all outcomes the controls from the different blocks did not differ significantly, and the data from the different blocks were thus combined together for the overall analyses to examine dose effects.
3.1 Litter size
There was no maternal mortality due to treatment. Early gestation pup reabsorption was seen in a few litters from each treatment, but these were not treatment-dependent (data not shown). As shown in Table 1, litter size was approximately 12 fetuses per litter, and DEHP treatment did not affect implantation number, litter size (live fetuses on GD 18) or sex ratio.
3.2 Fetal male and maternal serum MEHP Concentrations
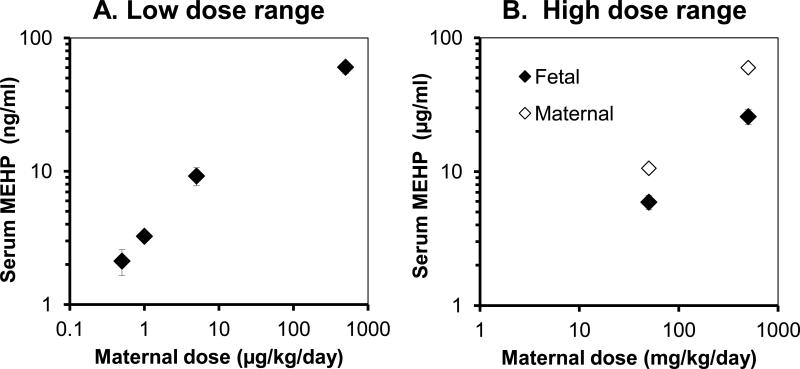
The data for serum MEHP concentrations are presented separately for the low dose range (0, 0.5, 1, 5 and 500 μg/kg/day; Figure 1-A) and the high dose range (50,000 and 500,000 μg/kg/day; Figure 1-B). The fetal serum concentrations of MEHP for the DEHP doses: 0.5, 1, 5, 500, 50,000, and 500,000 μg/kg/day were 2.1±0.5, 3.3±0.1, 9.2±1.4, 60.4±6.1, 5,918±718, 25,767±3,304 ng/ml, respectively. Thus, as the maternal administered oral dose increased from 0.5 to 500,000 μg/kg/day, the concentration of MEHP in male fetal serum at 2- 4 hr after maternal ingestion of DEHP on GD 18 (one day prior to parturition) increased from 2 to 23,000 ng/ml. We also measured MEHP for mothers in the two highest dose groups. Maternal MEHP levels were 10.6±2.7 and 59.8±6.3 μg/ml, approximately 1.8-fold and 2.3-fold, respectively, higher than male fetal serum concentrations for the 50,000 and 500,000-μg/kg/day dose groups.
Figure 1.
Serum MEHP concentrations as a function of maternal oral administration of doses of DEHP in A) the low dose range (0, 0.5, 1, 5 and 500 μg/kg/day) or B) the high dose range (50 or 500 mg/kg/day). Fetal male serum concentrations are given for both low- and high-dose ranges and maternal serum MEHP concentrations are given for the high dose range only. Low-dose measurements were of single pooled serum samples from each treatment, and values represent the mean (±SEM) of duplicate measurements; in B) values represent the mean (±SEM) concentration of 5-6 individual serum samples at each data point.
3.3 Maternal serum testosterone
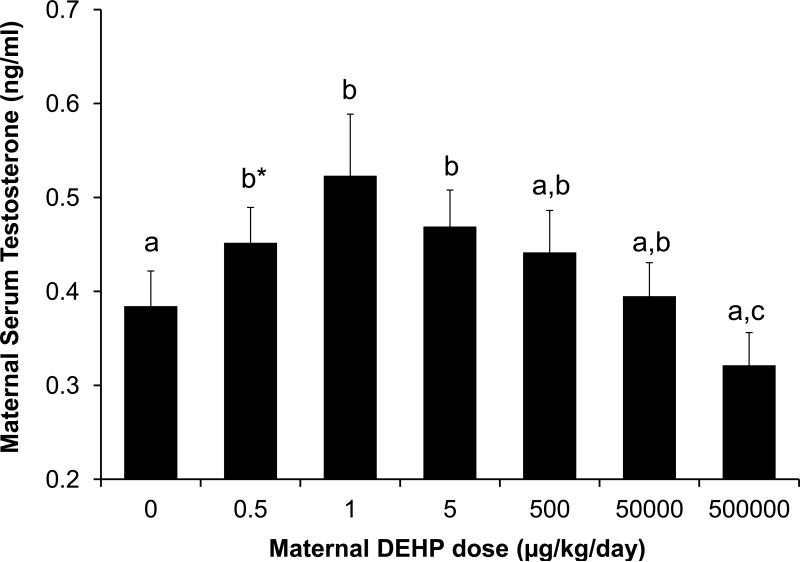
Exposure to different doses of DEHP from GD 9 - GD 18 significantly affected maternal serum testosterone levels (ANOVA of log-transformed values in SAS; P < 0.05). As DEHP treatment increased from 0.5 - 1 μg/kg/day, maternal testosterone significantly increased relative to controls (Figure 2). The lowest dose tested (0.5 μg/kg/day) tended (P = 0.07) to produce higher serum testosterone relative to controls. Maternal testosterone was highest at 1 μg/kg/day. However, as the dose continued to increase (5-500,000 μg/kg/day), maternal testosterone levels followed a decreasing trend ending with the highest dose (500,000 μg/kg/day) being significantly lower than all other DEHP groups (0.5-50000 μg/kg/day, P < 0.05 – 0.001) but not different from controls. There was thus an inverted-U dose-response relationship between DEHP dose and maternal serum testosterone. We also analyzed these data using the two regression models identified in the methods section. Regression analysis of log-transformed maternal serum testosterone data using the simple linear model resulted in P = 0.065. However, when the quadratic term was added to the model the effect of dose was statistically significant (P = 0.0013). Thus, for the effect of DEHP dose on maternal serum testosterone, the non-linear model fit the data significantly better than the simple linear model.
Figure 2.
Effect of different doses of DEHP on maternal serum testosterone concentrations on GD 18 (ANOVA on log-transformed data, P<0.05). Values represent the mean ±SEM. Treatments with the same letter are not significantly different from each other but are statistically different from groups with other letters. b*, P=0.07 relative to controls; b**, P = 0.09 relative to 500,000 group. Sample sizes were: Oil n=20, 0.5 μg n=9, 1 μg n=11, 5 μg n=12, 500 μg n=13, 50 mg n=16, 500 mg n=17.
3.4 Fetal male serum testosterone
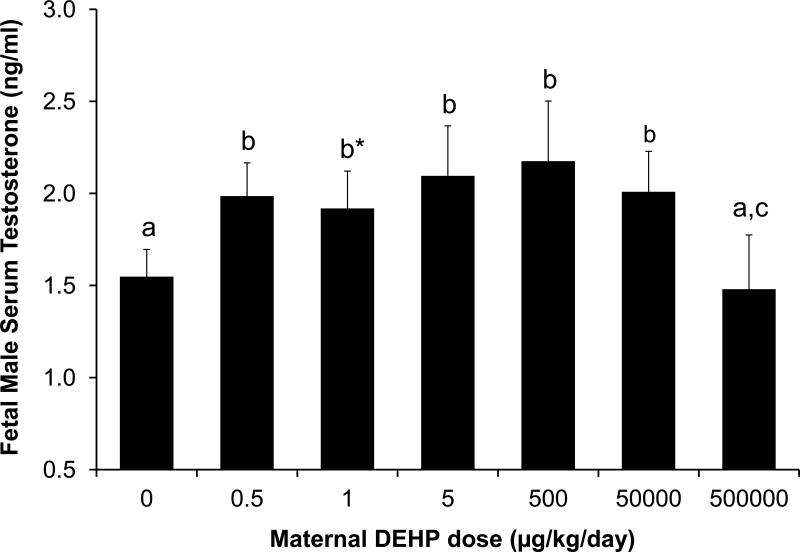
In utero exposure to increasing DEHP doses between 0.5 and 50,000 μg/kg/day relative to controls resulted in a tendency (P = 0.07) for an increase in fetal male serum testosterone (using pooled serum from all males within each litter) based on analysis using ANOVA of log-transformed values in SAS. As shown in Figure 3, below the dose of 50,000 μg/kg/day, DEHP exposure tended to increase testosterone levels in serum (P < 0.1). The 0.5, 5, and 500 μg/kg/day doses tended to increase serum testosterone (P < 0.1). However, in response to the highest DEHP dose (500,000 μg/kg/day), male serum testosterone concentrations were significantly decreased back to control levels. Relative to other DEHP treatment groups, excluding the oil controls, male serum testosterone levels in the 500,000-μg/kg/day group were significantly reduced by approximately 0.5 ng/ml (P<0.05 for all except for the 1 μg/kg dose, where P = 0.08). We also analyzed these data using the two regression models. Regression analysis of log-transformed male fetus serum testosterone data using the simple linear model resulted in P = 0.54. However, when the quadratic term was added to the model the effect of dose was statistically significant (P = 0.0015). Thus, for the effect of DEHP dose on male fetal serum testosterone, the non-linear model fit the data significantly better than the simple linear model.
Figure 3.
Effect of different doses of DEHP on serum testosterone concentrations in fetal males on GD 18 (ANOVA on log-transformed data, P <0.05). Values represent the mean ±SEM. Treatments with the same letter are not significantly different from each other but are statistically different from groups with other letters. a vs. b, P<0.1; b vs. c, P<0.05, b*vs. c, P=0.08. Sample sizes were: Oil n=21, 0.5 μg n=12, 1 μg n=11, 5 μg n=11, 500 μg n=13, 50 mg n=16, 500 mg n=18.
3.5 Fetal 1MF male testicular testosterone
Testosterone extracted from testes collected on GD 18 from 1MF male fetuses did not differ statistically as a function of dose based on using ANOVA of log-transformed values in SAS. However, as shown in Table, as the levels of DEHP increased from 0.5 – 500 μg/kg/day, testicular testosterone levels exhibited an increasing trend, with the highest serum testosterone level produced by the 500 μg/kg/day dose. DEHP-exposed males in groups higher than the 500-μg/kg/day group tended to secrete lower levels of testosterone as the dose increased, resulting in an inverted-U dose-response relationship. There was a tendency toward elevated testosterone in animals treated with the 500 μg/kg/day dose relative to controls (P = 0.08), and the 500 μg/kg/day dose was significantly higher than the 0.5 μg/kg/day dose (P < 0.05). We also analyzed these data using the two regression models. Regression analysis of log-transformed fetal male testicular testosterone data using the simple linear model resulted in P = 0.42. However, when the quadratic term was added to the model the effect of dose was statistically significant (P = 0.034). Thus, for the effect of DEHP dose on testicular testosterone in male fetuses, the non-linear model fit the data significantly better than the simple linear model.
3.6 Fetal 1MF male testis weight
The overall analysis using ANOVA in SAS of log-transformed testis weight (collected from 1MF males) showed a trend for some doses of DEHP to decrease testis weight (ANOVA, P = 0.06). Specifically, testis weight was significantly reduced in the 50,000 and 500,000 μg/kg/day dose groups relative to controls (P < 0.05), and male fetuses exposed to the 500,000 μg/kg/day dose also had significantly reduced testis weight relative to males in the 5 and 500 μg/kg/day dose groups (P < 0.05). We also analyzed these data using the two regression models. Regression analysis of log-transformed fetal male testicular testosterone data using the simple linear model resulted in P = 0.12. When the quadratic term was added to the model the effect of dose was again not statistically significant (P = 0.10). Thus, the non-linear model did not fit the data for 1MF male testis weight significantly better than the simple linear model (Table 1).
3.7 Fetal 1MF male AGD
Differences due to DEHP dose in 1MF male fetal AGD on GD 18 were overall not statistically significant using ANOVA in SAS (P > 0.1). However, there was a tendency for increased AGD at all doses except for the highest dose of DEHP, suggesting an inverted-U shaped dose-response curve (Table 1). When we analyzed these data using the two regression models, the regression analysis of fetal male AGD data using the simple linear model resulted in P = 0.52. However, when the quadratic term was added to the model the effect of dose was statistically significant (P = 0.014). Thus, for the effect of DEHP dose on male fetal AGD, the non-linear model fit the data significantly better than the simple linear model.
3.8 Fetal 1MF male body weight
In utero exposure to DEHP treatment did not affect fetal 1MF male body weight on GD 18 based on using ANOVA in SAS (P > 0.1; Table 1). The additional regression analyses also did not yield significant differences, with the simple linear model resulting in P = 0.54 and the addition of the quadratic term in model 2 resulting in P = 0.80. There was thus no significant effect of DEHP dose on fetal 1MF male body weight on GD 18.
3.9 Ratio of fetal male AGD / body weight (BW)
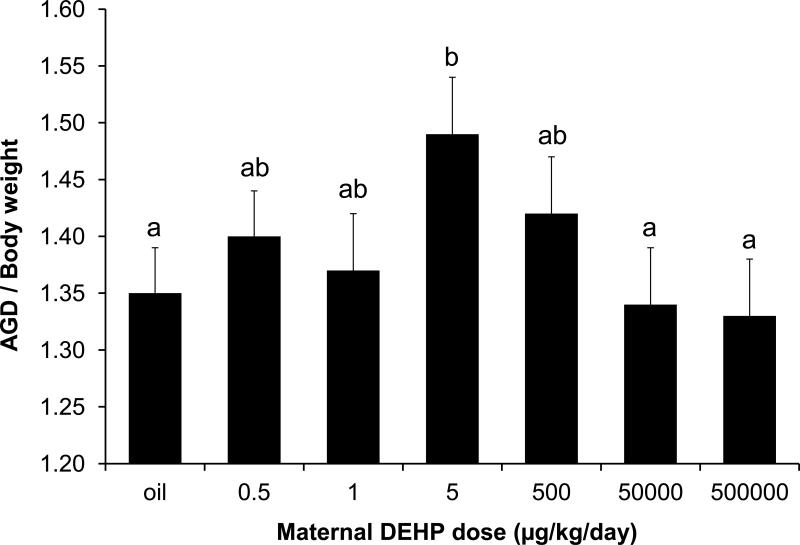
Since body weight can influence morphological measures such as AGD, we examined the effect of DEHP dose on AGD/BW of 1MF male fetuses by first using ANOVA in SAS. The overall ANOVA was not statistically significant (P > 0.1), although the LSD analysis showed that the 5-μg/kg/day group differed from controls (P = 0.05) as well as the 50,000 and 500,000 μg/kg/day groups (P < 0.05). The analysis using the two regression models revealed that the simple linear regression model was not statistically significant (P = 0.36). However, addition of the quadratic term to the model resulted in a statistically significant effect of dose on AGD/BW (P = 0.044). In addition, we used the method of Gallavan and colleagues [41] and analyzed the ratio of AGD and the cube root of BW, and the effect of dose was again statistically significant (p = 0.021). Thus, there was a statistically significant non-linear effect of DEHP dose on AGD with different approaches for correction for body weight (Figure 4). However, correction for body weight did not result in a better non-linear fit in comparison to the data for AGD without correction for body weight.
Figure 4.
Effect of DEHP on the ratio of 1MF male anogenital distance (AGD) to body weight (BW). Values represent the mean ±SEM. There was a significant effect of dose based on regression analysis that included a quadratic term (P < 0.05). Treatments with the same letter are not significantly different from each other but are statistically different from groups with other letters. Sample sizes were: Oil n=13, 0.5 μg n=5, 1 μg n=7, 5 μg n=9, 500 μg n=9, 50 mg n=13, 500 mg n=13.
4. Discussion
DEHP results in reproductive tract malformations in male rat offspring when mothers are exposed at 100 mg/kg/day and higher [20, 24, 42]. While the mechanism of actions of DEHP is currently not completely understood, numerous studies have confirmed that DEHP does not compete for androgen receptor binding sites. Therefore, it is likely that very high doses of DEHP disrupt male reproductive tract development in rodents by interfering with enzymes involved in androgen production such as StAR and Cyp11a [11, 16, 24, 43].
The “low dose” issue has generated controversy in the chemical risk assessment community. The approach that was used here involved studying an endocrine disrupting chemical, DEHP, for effects beginning at a low dose and moving up the dose-response curve rather than using the approach in guideline compliant studies of starting at the maximum tolerated dose and typically testing about 2 more doses over about a 50-fold lower range [29]. Our approach is based on the prediction than when the dose-response relationship is non-monotonic, the high-dose testing paradigm will not identify potentially unique low-dose effects of endocrine disrupting chemicals such as DEHP [31]. For example, results of a prior study on brain aromatase activity in male rats suggested that opposite effects occurred due to fetal exposure to low and high doses of DEHP [19]. Of particular relevance to our findings, a prior study reported that testicular testosterone production was significantly increased in male rat fetuses exposed to DEHP via the dam at 10 mg/kg/day, while testicular testosterone was significantly decreased by exposure at 750 mg/kg/day [21].
In our present study conducted with CD-1 mice, the doses of DEHP fed to pregnant females below 500 μg/kg/day produced serum MEHP concentrations of 2-60 ng/ml in male mouse fetuses (Figure 1), which are below those reported in human umbilical cord blood at term (~0.5 μg/ml; [8]) whereas the 50,000 and 500,000 μg/kg/day doses of DEHP produced serum MEHP concentrations of ~6μg/ml and 26 μg/ml respectively, far greater than levels detected in human cord blood. When pregnant CD-1 mice were exposed to the low levels of DEHP that produced internal concentrations of MEHP relevant to human exposures, maternal serum testosterone was significantly increased relative to controls. Specifically, at the 1-μg/kg/day dose maternal serum testosterone was significantly increased. However, as the dose of DEHP increased beyond the 1-μg/kg/day dose, maternal serum testosterone gradually decreased, with the highest dose of 500,000 μg/kg/day resulting in significantly lower serum testosterone relative to the 0.5, 1.0 and 5 μg/kg/day doses. DEHP thus acted as an endocrine disruptor at an oral dose of 1 μg/kg/day in pregnant mice; it is well recognized that elevating testosterone in pregnant females, both animals and humans, causes adverse effects in offspring [44]. However, the 500,000-μg/kg/day dose did not cause a change in maternal serum testosterone relative to controls and so would have been considered the NOAEL if the dosing paradigm had started at higher doses and this was the lowest dose examined.
Male fetuses carried by these DEHP-exposed mothers had elevated serum testosterone levels at low doses of DEHP. In addition, a subset of the male fetuses (those positioned in utero between a male and a female fetus; 1MF males) had elevated testicular testosterone production and increased AGD at doses below 500,000 μg/kg/day, while 1MF male fetuses exposed to the 500,000-μg/kg/day dose showed no difference relative to controls in these measures. For male fetal serum testosterone, the lowest adverse effect level (LOAEL) that significantly altered serum testosterone was 0.5 μg/kg/day. In contrast to some studies in rats, the 500,000-μg/kg/day dose did not result in serum testosterone levels in male fetuses that were significantly lower than controls. This finding is consistent with results from other studies in mice [45].
Importantly, the conclusion that the dose-response relationship was non-monotonic for maternal serum testosterone, fetal male serum testosterone, testicular testosterone and AGD is based on the significant improvement to our regression models resulting from the addition of a quadratic term to the simple linear model. Specifically, to statistically assess the apparent non-monotonicity in several of our results, we added a quadratic term to a simple regression model. A closely related method is ANOVA with polynomial contrasts, which tests for linear, quadratic, cubic, and greater trends across categories. This method produced very similar results in our data, such that linear (and cubic) fits were non-significant, whereas quadratic fits were significant. For example, ANOVA with quadratic contrast applied to (normalized) maternal and fetal testosterone vs. dose gave p values of 0.0002 and 0.002, respectively, as compared to 0.0013 and 0.0015 using regression with the quadratic term. In this study, we applied both methods post-hoc, but one could plan such comparisons a priori, which seems appropriate when studying non-linear systems such as endocrine and other cell signaling endpoints.
These findings are important because standard risk assessments have as a core assumption that high dose effects can be used to predict effects at low doses [29, 31]. Our multiple non-monotonic dose-response findings reveal that high dose effects cannot be used to predict a “safe” dose using linear extrapolation procedures such as the application of 10-fold safety factors from an apparent NOAEL based only on testing very high doses [30]; the high-dose testing apparent NOAEL for our data would been 500,000 μg/kg/day for all outcomes, when in fact unpredicted significant effects occurred at a one-million fold lower dose of 0.5 μg/kg/day. While some have challenged whether non-monotonic dose-response curves should be expected for endocrine disrupting chemicals [46], our findings here clearly demonstrate that for the outcomes we examined in relation to maternal DEHP dose (maternal and fetal serum testosterone, testicular testosterone and AGD of fetal males), the dose-response relationship was non-monotonic.
Of considerable interest is that we observed effects of DEHP in fetal mice at exposure levels consistent within the back-calculated exposure range of human male fetuses in which DEHP appears to interfere with masculinization [27, 28, 47]. This is likely due to the fact that the mouse (and the rat) placenta primarily secretes androgens (androstenedione and testosterone) rather than progesterone [48]. There is not enough progesterone secreted by the mouse or rat placenta to maintain pregnancy without the maternal ovaries. Also, in marked contrast to the human placenta, aromatase is not present in the mouse or rat placenta [49].
The regulation of steroid secretion by the rodent placenta is not well understood, but this important difference between the steroidogenic potential of the human and rodent placenta suggests that rodents might not be a sensitive model for predicting low-dose demasculinizing effects of DEHP in human fetuses. We thus do not interpret the present findings as suggesting that epidemiological studies in which phthalates, including DEHP, are related to demasculinization in males should be discounted [27, 28]. The increased prevalence of testicular dysgenesis syndrome (TDS) symptoms has drawn attention to environmental causes, since rapid changes in genotype cannot explain the dramatic changes that have occurred in some countries in diseases such as testicular cancer [12, 50]. The symptoms of TDS are similar to the effects of high doses of phthalates in rats. Thus, one possible explanation for the similarity of high-dose effects of phthalates in rats and low-dose effects of phthalates in humans is that rats are less sensitive to the androgen disrupting action of phthalates associated with species differences in maternal-placental-fetal physiology [11, 27, 28, 48, 49].
Research Highlights.
DEHP is reported to alter male fetus masculinization and reduce anogenital distance.
Most studies are of high-dose effects, beyond human exposure levels
We dosed pregnant mice with DEHP at 0.5 - 500,000 μg/kg/day.
DEHP increased maternal and fetal serum testosterone and AGD at lower doses
No effects of DEHP were seen at the highest dose.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Heudorf U, Mersch-Sundermann V, Angerer J. Phthalates: toxicology and exposure. Int J Hyg Environ Health. 2007;210:623–34. doi: 10.1016/j.ijheh.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 2.Thomas JA, Thomas MJ. Biological effects of di-(2-ethylhexyl) phthalate and other phthalic acid esters. Crit Rev Toxicol. 1984;13:283–317. doi: 10.3109/10408448409023761. [DOI] [PubMed] [Google Scholar]
- 3.Latini G. Monitoring phthalate exposure in humans. Clinica Chimica Acta. 2005;361:20–9. doi: 10.1016/j.cccn.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 4.Schmitzer JL, Scheunert I, Korte F. Fate of bis(2-ethylhexyl) [14C]phthalate in laboratory and outdoor soil-plant systems. Journal of Agricultural and Food Chemistry. 1988;36:210–5. [Google Scholar]
- 5.Latini G, Verrotti A, De Felice C. DI-2-Ethylhexyl Phthalate and Endocrine Disruption: A Review. Current Drug Targets - Immune, Endocrine & Metabolic Disorders. 2004;4:37–40. doi: 10.2174/1568008043340017. [DOI] [PubMed] [Google Scholar]
- 6.Frederiksen H, Skakkebaek NE, Andersson A-M. Metabolism of phthalates in humans. Molecular Nutrition & Food Research. 2007;51:899–911. doi: 10.1002/mnfr.200600243. [DOI] [PubMed] [Google Scholar]
- 7.CDC, Prevention CfDCa Fourth National Report on Human Exposure to Environmental Chemicals. 2012. Updated Tables, February 2012.
- 8.Latini G, De Felice C, Presta G, Del Vecchio A, Paris I, Ruggieri F, et al. In Utero Exposure to Di-(2-ethylhexyl)phthalate and Duration of Human Pregnancy. Environ Health Perspect. 2003:111. doi: 10.1289/ehp.6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silva MJ, Reidy JA, Herbert AR, Preau JL, Needham LL, Calafat AM. Detection of Phthalate Metabolites in Human Amniotic Fluid. Bulletin of Environmental Contamination and Toxicology. 2004;72:1226–31. doi: 10.1007/s00128-004-0374-4. [DOI] [PubMed] [Google Scholar]
- 10.Green R, Hauser R, Calafat AM, Weuve J, Schettler T, Ringer S, et al. Use of di(2-ethylhexyl) phthalate-containing medical products and urinary levels of mono(2-ethylhexyl) phthalate in neonatal intensive care unit infants. Environ Health Perspect. 2005;113:1222–5. doi: 10.1289/ehp.7932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Howdeshell KL, Rider CV, Wilson VS, Gray LE., Jr Mechanisms of action of phthalate esters, individually and in combination, to induce abnormal reproductive development in male laboratory rats. Environmental Research. 2008;108:168–76. doi: 10.1016/j.envres.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 12.Bay K, Asklund C, Skakkebaek NE, Andersson AM. Testicular dysgenesis syndrome: possible role of endocrine disrupters. Best Pract Res Clin Endocrinol Metab. 2006;20:77–90. doi: 10.1016/j.beem.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 13.Sonne SB, Kristensen DM, Novotny GW, Olesen IA, Nielsen JE, Skakkebæk NE, et al. Testicular dysgenesis syndrome and the origin of carcinoma in situ testis. International Journal of Andrology. 2008;31:275–87. doi: 10.1111/j.1365-2605.2007.00855.x. [DOI] [PubMed] [Google Scholar]
- 14.Fisher JS. Environmental anti-androgens and male reproductive health: focus on phthalates and testicular dysgenesis syndrome. Reproduction. 2004;127:305–15. doi: 10.1530/rep.1.00025. [DOI] [PubMed] [Google Scholar]
- 15.Foster PMD. Disruption of reproductive development in male rat offspring following in utero exposure to phthalate esters. International Journal of Andrology. 2006;29:140–7. doi: 10.1111/j.1365-2605.2005.00563.x. [DOI] [PubMed] [Google Scholar]
- 16.Hannas BR, Lambright CS, Furr J, Howdeshell KL, Wilson VS, Gray LE. Dose-Response Assessment of Fetal Testosterone Production and Gene Expression Levels in Rat Testes Following In Utero Exposure to Diethylhexyl Phthalate, Diisobutyl Phthalate, Diisoheptyl Phthalate, and Diisononyl Phthalate. Toxicological Sciences. 2011;123:206–16. doi: 10.1093/toxsci/kfr146. [DOI] [PubMed] [Google Scholar]
- 17.Wilson VS, Howdeshell KL, Lambright CS, Furr J, Earl Gray L., Jr Differential expression of the phthalate syndrome in male Sprague–Dawley and Wistar rats after in utero DEHP exposure. Toxicology Letters. 2007;170:177–84. doi: 10.1016/j.toxlet.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 18.Akingbemi BT, Youker RT, Sottas CM, Ge R, Katz E, Klinefelter GR, et al. Modulation of Rat Leydig Cell Steroidogenic Function by Di(2-Ethylhexyl)Phthalate. Biology of Reproduction. 2001;65:1252–9. doi: 10.1095/biolreprod65.4.1252. [DOI] [PubMed] [Google Scholar]
- 19.Andrade AJM, Grande SW, Talsness CE, Grote K, Chahoud I. A dose–response study following in utero and lactational exposure to di-(2-ethylhexyl)-phthalate (DEHP): Non-monotonic dose–response and low dose effects on rat brain aromatase activity. Toxicology. 2006;227:185–92. doi: 10.1016/j.tox.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 20.Andrade AJM, Grande SW, Talsness CE, Grote K, Golombiewski A, Sterner-Kock A, et al. A dose–response study following in utero and lactational exposure to di-(2-ethylhexyl) phthalate (DEHP): Effects on androgenic status, developmental landmarks and testicular histology in male offspring rats. Toxicology. 2006;225:64–74. doi: 10.1016/j.tox.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 21.Lin H, Ge RS, Chen GR, Hu GX, Dong L, Lian QQ, et al. Involvement of testicular growth factors in fetal Leydig cell aggregation after exposure to phthalate in utero. Proc Natl Acad Sci U S A. 2008;105:7218–22. doi: 10.1073/pnas.0709260105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayashi Y, Ito Y, Yanagiba Y, Kamijima M, Naito H, Nakajima T. Differences in metabolite burden of di(2-ethylhexyl)phthalate in pregnant and postpartum dams and their offspring in relation to drug-metabolizing enzymes in mice. Archives of Toxicology. 2012;86:563–9. doi: 10.1007/s00204-011-0790-2. [DOI] [PubMed] [Google Scholar]
- 23.Gray LE, Ostby J, Furr J, Price M, Veeramachaneni DNR, Parks L. Perinatal Exposure to the Phthalates DEHP, BBP, and DINP, but Not DEP, DMP, or DOTP, Alters Sexual Differentiation of the Male Rat. Toxicological Sciences. 2000;58:350–65. doi: 10.1093/toxsci/58.2.350. [DOI] [PubMed] [Google Scholar]
- 24.Borch J, Metzdorff SB, Vinggaard AM, Brokken L, Dalgaard M. Mechanisms underlying the anti-androgenic effects of diethylhexyl phthalate in fetal rat testis. Toxicology. 2006;223:144–55. doi: 10.1016/j.tox.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 25.Blystone CR, Kissling GE, Bishop JB, Chapin RE, Wolfe GW, Foster PMD. Determination of the di-(2-ethylhexyl) phthalate (DEHP) NOAEL for reproductive development in the rat: Importance of the retention of extra animals to adulthood. Toxicological Sciences. 2010 doi: 10.1093/toxsci/kfq147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wittassek M, Koch HM, Angerer J, Brüning T. Assessing exposure to phthalates – The human biomonitoring approach. Molecular Nutrition & Food Research. 2011;55:7–31. doi: 10.1002/mnfr.201000121. [DOI] [PubMed] [Google Scholar]
- 27.Swan SH, Main Katharina M., Liu Fan, Stewart Sara L., Kruse Robin L., Calafat Antonia M., Mao Catherine S., Redmon J. Bruce, Ternand Christine L., Sullivan Shannon, Teague J. Lynn. Decrease in Anogenital Distance among Male Infants with Prenatal Phthalate Exposure. Environ Health Perspect. 2005;118:1056–61. doi: 10.1289/ehp.8100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Swan SH. Environmental phthalate exposure in relation to reproductive outcomes and other health endpoints in humans. Environmental Research. 2008;108:177–84. doi: 10.1016/j.envres.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.vom Saal FS, Sheehan DM. Challenging risk assessment. Forum for Applied Research and Public Policy. 1998:11–8. [Google Scholar]
- 30.Welshons WV, Thayer KA, Judy BM, Taylor JA, Curran EM, vom Saal FS. Large Effects from Small Exposures. I. Mechanisms for Endocrine-Disrupting Chemicals with Estrogenic Activity. Environ Health Perspect. 2003:111. doi: 10.1289/ehp.5494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR, Lee D- H, et al. Hormones and Endocrine-Disrupting Chemicals: Low-Dose Effects and Nonmonotonic Dose Responses. Endocrine Reviews. 2012 doi: 10.1210/er.2011-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zoeller RT, Brown TR, Doan LL, Gore AC, Skakkebaek NE, Soto AM, et al. Endocrine-Disrupting Chemicals and Public Health Protection: A Statement of Principles from The Endocrine Society. Endocrinology. 2012 doi: 10.1210/en.2012-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.vom Saal FS, Bronson FH. In utero proximity of female mouse fetuses to males: effect on reproductive performance during later life. Biol Reprod. 1978;19:842–53. doi: 10.1095/biolreprod19.4.842. [DOI] [PubMed] [Google Scholar]
- 34.vom Saal FS, Quadagno DM, Even MD, Keisler LW, Keisler DH, Khan S. Paradoxical effects of maternal stress on fetal steroids and postnatal reproductive traits in female mice from different intrauterine positions. Biology of Reproduction. 1990;43:751–61. doi: 10.1095/biolreprod43.5.751. [DOI] [PubMed] [Google Scholar]
- 35.Vandenbergh JG, Huggett CL. The anogenital distance index, a predictor of the intrauterine position effects on reproduction in female house mice. Laboratory animal science. 1995;45:567–73. [PubMed] [Google Scholar]
- 36.Ruhlen RL, Howdeshell KL, Mao J, Taylor JA, Bronson FH, Newbold RR, et al. Low phytoestrogen levels in feed increase fetal serum estradiol resulting in the “fetal estrogenization syndrome” and obesity in CD-1 mice. Environ Health Perspect. 2008;116:322–8. doi: 10.1289/ehp.10448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cederroth CR, Vinciguerra M, Kuhne F, Madani R, Doerge DR, Visser TJ, et al. A phytoestrogen-rich diet increases energy expenditure and decreases adiposity in mice. Environ Health Perspect. 2007;115:1467–73. doi: 10.1289/ehp.10413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.vom Saal FS. Sexual differentiation in litter-bearing mammals: influence of sex of adjacent fetuses in utero. Journal of animal science. 1989;67:1824–40. doi: 10.2527/jas1989.6771824x. [DOI] [PubMed] [Google Scholar]
- 39.Even MD, Dhar MG, vom Saal FS. Transport of steroids between fetuses via amniotic fluid in relation to the intrauterine position phenomenon in rats. Journal of Reproduction and Fertility. 1992;96:709–16. doi: 10.1530/jrf.0.0960709. [DOI] [PubMed] [Google Scholar]
- 40.Takatori S, Kitagawa Y, Kitagawa M, Nakazawa H, Hori S. Determination of di(2-ethylhexyl)phthalate and mono(2-ethylhexyl)phthalate in human serum using liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;804:397–401. doi: 10.1016/j.jchromb.2004.01.056. [DOI] [PubMed] [Google Scholar]
- 41.Gallavan RH, Jr., Holson JF, Stump DG, Knapp JF, Reynolds VL. Interpreting the toxicologic significance of alterations in anogenital distance: potential for confounding effects of progeny body weights. Reprod Toxicol. 1999;13:383–90. doi: 10.1016/s0890-6238(99)00036-2. [DOI] [PubMed] [Google Scholar]
- 42.Moore RW, Rudy TA, Lin TM, Ko K, Peterson RE. Abnormalities of sexual development in male rats with in utero and lactational exposure to the antiandrogenic plasticizer Di(2-ethylhexyl) phthalate. Environ Health Perspect. 2001;109:229–37. doi: 10.1289/ehp.01109229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parks LG, Ostby JS, Lambright CR, Abbott BD, Klinefelter GR, Barlow NJ, et al. The Plasticizer Diethylhexyl Phthalate Induces Malformations by Decreasing Fetal Testosterone Synthesis during Sexual Differentiation in the Male Rat. Toxicological Sciences. 2000;58:339–49. doi: 10.1093/toxsci/58.2.339. [DOI] [PubMed] [Google Scholar]
- 44.Diamond M. Clinical implications of the organizational and activational effects of hormones. Hormones and behavior. 2009;55:621–32. doi: 10.1016/j.yhbeh.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 45.Gaido KW, Hensley JB, Liu D, Wallace DG, Borghoff S, Johnson KJ, et al. Fetal mouse phthalate exposure shows that Gonocyte multinucleation is not associated with decreased testicular testosterone. Toxicol Sci. 2007;97:491–503. doi: 10.1093/toxsci/kfm049. [DOI] [PubMed] [Google Scholar]
- 46.Rhomberg LR, Goodman JE. Low-dose effects and nonmonotonic dose-responses of endocrine disrupting chemicals: Has the case been made? Regulatory toxicology and pharmacology : RTP. 2012;64:130–3. doi: 10.1016/j.yrtph.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 47.Marsee K, Woodruff TJ, Axelrad DA, Calafat AM, Swan SH. Estimated daily phthalate exposures in a population of mothers of male infants exhibiting reduced anogenital distance. Environ Health Perspect. 2006;114:805–9. doi: 10.1289/ehp.8663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Soares MJ, Talamantes F. Gestational effects on placental and serum androgen, progesterone and prolactin-like activity in the mouse. J Endocrinol. 1982;95:29–36. doi: 10.1677/joe.0.0950029. [DOI] [PubMed] [Google Scholar]
- 49.vom Saal FS, Dhar MG. Blood flow in the uterine loop artery and loop vein is bidirectional in the mouse: implications for transport of steroids between fetuses. Physiol Behav. 1992;52:163–71. doi: 10.1016/0031-9384(92)90447-a. [DOI] [PubMed] [Google Scholar]
- 50.Skakkebæk NE, Rajpert-De Meyts E, Main KM. Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects: Opinion. Human Reproduction. 2001;16:972–8. doi: 10.1093/humrep/16.5.972. [DOI] [PubMed] [Google Scholar]