Abstract
Aims/Hypothesis
We examined the role of the protein kinase C-τ (PKC-ι) in mediating alterations in expression of enzymes in hepatocytes of type 2 diabetic humans that contribute importantly to development of lipid and carbohydrate abnormalities in type 2 diabetes.
Methods
We examined insulin signalling in isolated hepatocytes of non-diabetic and type 2 diabetic humans, and effects of two newly developed small molecule PKC-ι inhibitors on aberrant signalling and downstream processes.
Results
Opposite to PKC-ι deficiency in diabetic muscle, which diminishes glucose transport, "PKC-ι in diabetic hepatocytes was overexpressed and overactive, basally and following insulin treatment, and, moreover, was accompanied by increased expression of "PKC-ι-dependent lipogenic, proinflammatory and gluconeogenic enzymes. Heightened "PKC-ι activity most likely reflected heightened activity of insulin receptor substrate(IRS)-2-dependent phosphatidylinositol-3-kinase (PI3K), as IRS-1 levels and IRS-1/PI3K activity were markedly diminished.. Importantly, insulin stimulated "PKC-ι expression and its overexpression in diabetic hepatocytes was reversed in vitro by both insulin deprivation and "PKC-ι inhibitors; this suggested operation of an insulin-driven, feed-forward/positive-feedback mechanism. In contrast to "PKC-ι, Akt2 activity and activation by insulin was diminished, apparently reflecting IRS-1 deficiency. Treatment of diabetic hepatocytes with "PKC-ι/λ inhibitors diminished expression of lipogenic, proinflammatory and gluconeogenic enzymes.
Conclusions/Interpretations
Our findings suggest that a vicious cycle of "PKC-ι overactivity and overexpression exists in hepatocytes of type 2 diabetic humans and contributes importantly to maintaining overactivity of lipogenic, proinflammatory and gluconeogenic pathways that underlie lipid and carbohydrate abnormalities in type 2 diabetes.
Keywords: Protein Kinase C-iota, Type 2 Diabetes, Hepatocytes
Introduction
Alterations in hepatic metabolism in type 2 diabetes mellitus (T2DM) lead to overproduction of glucose and lipids, which in turn abet development of glucose intolerance and dyslipidaemias. Hepatic glucose overproduction is understandable, as insulin resistance in type 2 diabetes is expected to diminish the ability of insulin to repress expression of gluconeogenic enzymes. On the other hand, lipid overproduction in type 2 diabetes is not readily explained by insulin resistance, as insulin increases expression of lipogenic enzymes, and resistance to this effect of insulin should diminish lipogenesis. Elucidation of the signalling aberrations underlying these paradoxical alterations in gluconeogenesis and lipogenesis is essential for developing new therapeutic approaches for type 2 diabetes.
Insulin controls metabolic processes largely by activating Akt and atypical protein kinase C (aPKC), which function distal to insulin receptor substrate(IRS)-1- and/or IRS-2-dependent phosphatidylinositol 3-kinase (PI3K). Germane to the above-stated paradox, in rodent forms obesity and diabetes, hepatic aPKC activation by insulin is fully conserved or heightened, even when hepatic Akt activation is markedly diminished in diabetic rodents [1–3]. Divergence of Akt and aPKC pathways in diabetic liver [1, 3] most likely reflects downregulation of IRS-1/PI3K, which is of major importance for hepatic Akt activation [4–7], as opposed to conserved activation of IRS-2/PI3K, which, without IRS-1, controls hepatic aPKC [4, 6, 7]. In contrast to liver, in muscle, IRS-1/PI3K controls aPKC as well as Akt [5, 6], which together control glucose transport, and all three signalling factors are generally downregulated in obesity and diabetes [8].
Conserved hepatic aPKC activation in obesity and type 2 diabetes is problematic, as, hyperinsulinaemia inordinately activates hepatic aPKC and aPKC-dependent processes: (a) expression/activation of sterol receptor element binding protein-1c (SREBP-1c), which transactivates multiple lipogenic genes, including, fatty acid synthase (FAS) and acetyl-CoA carboxylase (ACC) [2, 3, 9, 10]; and (b) activation of IKKβ which phosphorylates IκBβ, the inhibitor of nuclear factor κ-B (NFκB), thus releasing NFκB for nuclear uptake and transactivation of proinflammatory cytokine genes, including, tissue necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) [2, 3, 10].
Activation of hepatic aPKC, SREBP-1c and NFκB in hyperinsulinaemic states apparently contributes importantly to the development of hepatosteatosis, hypertriglyceridaemia, hypercholesterolaemia, impaired insulin signalling in muscle, and systemic insulin resistance. Indeed, tissue-selective inhibition of hepatic aPKC by adenoviral-mediated expression of kinase-inactive aPKC or knockdown of IRS-2 diminishes aPKC activity and activation of SREBP-1c-dependent lipogenic and NFκB-dependent proinflammatory pathways, and, moreover, reverses or improves clinical abnormalities in several rodent obesity and type 2 diabetes models [2, 3]. Additionally, adenoviral-mediated inhibition of hepatic aPKC diminishes expression of gluconeogenic enzymes, phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase (G6Pase) [2].
Presently, we have only limited knowledge about insulin signalling mechanisms in human hepatocytes, particularly in hepatocytes obtained from diabetic subjects, and particularly in which insulin effects on both aPKC and Akt were examined. Here, we examined insulin signalling to aPKC, Akt, IRS-1 and IRS-2, and alterations in expression of downstream factors that regulate lipogenic, proinflammatory and gluconeogenic pathways in hepatocytes isolated from non-diabetic and type 2 diabetic humans. We tested the role of "PKC-ι in provoking aberrations in these pathways in hepatocytes of type 2 diabetic humans by examining effects of two newly developed, highly specific, small molecule inhibitors of "PKC-ι. We found that both inhibitors diminished these aberrations in hepatocytes of type 2 diabetic humans.
Methods
Human Hepatocytes
Cryo-preserved hepatocytes (70–90% viability; purchased from Zen-Bio Corp, Research Triangle, North Carolina, USA) were prepared from collagenase-perfused livers of non-diabetic [2 females and 6 males; ages, 43–60 years, 51 ± 3 (mean ± SEM); BMI, 30 ± 2] and type 2 diabetic [2 females and 4 males, ages, 46-68 years, 60 ± 4; BMI, 27 ± 2] patients who were being maintained on life support as transplant donors, following irreversible brain damage caused by traumatic injuries, anoxia or cerebral vascular accidents. The diagnosis of type 2 diabetes in these patients was made by the Attending Physicians, apparently (as per supplier’s information) historically, on the basis of an adult onset, lack of history of ketoacidosis, and, in some cases, a history of maintenance on oral agents. All diabetic patients were undergoing active insulin treatment for hyperglycemia until their demise, but other clinical and laboratory data are limited in transplant donors.
Hepatocyte Incubations
As per supplier’s instructions, hepatocytes were incubated (106 cells/100mm plate) overnight (16–20 hours) in Dulbecco’s minimal essential medium containing 5% fetal calf serum, 100 units/ml sodium-penicillin, 100 µg/ml streptomycin-sulfate, 1µmol/l insulin and 2µmol/l dexamethasone, then for 2 hours in William’s E medium (Sigma, St.Louis, Missouri, USA) containing Glutamax (Invitrogen, Carlsbad, California, USA), 100 units/ml sodium-penicillin, 100 µg/ml streptomycin-sulfate, 10µmol/l insulin, 100nmol/l dexamethasone, 25mg/ml transferrin and 0.25µg/ml sodium selenite, then for 3 hours in similar but insulin-free medium, and finally for 30 min with or without aPKC inhibitor before incubation for either 15 min with or without 100nmol/l insulin in acute signalling studies, or for 4–6 hours with or without 1µmol/l insulin in expression studies. These concentrations of insulin in the final incubations were used to ensure that (a) insulin actions were maximal in both acute signalling and longer expression studies, and (b) insulin-dependent diabetic abnormalities were maintained throughout all incubations. In this regard, note that diabetic abnormalities involving heightened activity of aPKC and aPKC-dependent processes (i.e., heightened expression of lipogenic, proinflammatory and gluconeogenic enzymes) were comparable in hepatocytes analyzed directly without incubation, and in hepatocytes in which the insulin concentration in the final 6-hour incubation was either 1µmol/l, as presently used, or 100nmol/l, as used in a subsequent unpublished study. Also note that effects of "PKC-ι inhibition on diabetic abnormalities were comparable regardless of whether 100nmol/l or 1µmol/l insulin was present in the 6-hour incubation.
In some cases, hepatocytes were analyzed directly, i.e., without overnight pre-incubation, or incubated overnight in insulin-free medium.
As shown below, the presence of insulin in the overnight pre-incubation medium was essential for maintaining the type 2 diabetic cellular phenotype.
After incubation, cells were sonicated in homogenizing buffer.
Human Muscle Samples
Vastus lateralis muscles biopsies were obtained from non-diabetic and type 2 diabetic humans as described [11]. All experimental procedures involving humans, including muscle-biopsy studies, were approved by the Institutional Review Board of the University of South Florida College of Medicine, and the James A. Haley Veterans Administration Medical Center Research and Development Committee, Tampa, Fl, and conducted in accordance with the Declaration of Helsinki and Good Clinical Practice.
"PKC-ι Inhibitors
We used two "PKC-ι inhibitors: aurothiomalate (ATM; Monochrysine, Taylor Pharmaceuticals; Decatur, Illinois, USA; see [12–15]); and 1H-imidazole-4-carboxamide, 5-amino-1-[2, 3–dihydroxy-4-[(phosphono-oxy)methyl]cyclopentyl-[1R-(1a, 2b, 3b, 4a)] (ICAPP; Southern Research, Birmingham, Alabama, USA; see [16].
Tissue and Immunoprecipitate Preparations
As described [1, 2, 3, 10, 11], hepatocytes and tissues were homogenized in ice-cold buffer containing 0.25 mol/l sucrose, 20 mmol/l Tris/HCl (pH 7.5), 2 mmol/l EGTA, 2 mmol/l EDTA, 1 mmol/l phenlysulfonlyfluoride (PMSF), 20 µg/ml leupeptin, 10 µg/ml aprotinin, 2 mmol/l Na4P2O7, 2 mmol/l Na3VO4, 2 mmol/l NaF, and 1µmol/l microcystin, and then supplemented with 1% TritonX-100, 0.6% Nonidet and 150 mmol/l NaCl, and cleared by low-speed centrifugation.
PKC, Akt2, IRS/PI3K and AMPK Assays
As described [1, 2, 3, 10, 11], aPKCs were immunoprecipitated from lysates with rabbit polyclonal antiserum (Santa Cruz Biotechnologies, Santa Cruz, California, USA) which recognizes C-termini of PKC-ζ and PKC-λ/τ ["PKC-ι is the human homolog of mouse PKC-λ (98% homology); note that whereas human muscle contains primarily "PKC-ι and small amounts of PKC-ζ, human liver contains substantial amounts of both PKC-ζ and PKC-λ/τ (unpublished)]. Immunoprecipitates were collected on Sepharose-AG beads (Santa Cruz Biotechnologies) and incubated for 8 min at 30°C in 100µl buffer containing 50mmol/l Tris/HCl (pH, 7.5), 100µmol/l Na3VO4, 100µmol/l Na4 P2O7, 1mmol/l NaF, 100µmol/l PMSF, 4µg phosphatidylserine (Sigma), 50µmol/l [γ-32P]ATP (NEN Life Science Products, Beverly, Massachussetts, USA), 5mmol/l MgCl2, and, as substrate, 40µmol/l serine analogue of the PKC-ε pseudosubstrate (Millipore, Bedford, Massachussetts, USA). After incubation, 32P-labeled substrate was trapped on P-81 filter paper and counted.
aPKC activation was also assessed by immunoblotting for phosphorylation of the auto(trans)phosphorylation site, threonine-555/560 in "PKC-ι/ζ, required for, and reflective of, activation [8].
For assays of recombinant aPKCs, "PKC-ι and PKC-ζ 10ng (Biovision, Mountain, California, USA) aPKC was incubated in the standard aPKC assay with 10fM phosphatidylinositol-3, 4, 5-(PO4)3 (PIP3; Matreya, Pleasant Gap, Pennsylvania, USA) to maximally activate and define aPKC activity.
To examine effects of "PKC-ι inhibitors on conventional and novel PKCs, we used baculovirus/Sf9 insect cell-derived highly-purified conventional and novel PKCs (gift of Dr. Larry Ballas, Sphinx Division, Eli Lilly Co) as described [17]. Conventional PKCs (α, β2) were assayed in the presence of 1 mmol/l CaCl2 and 100nmol/l phorbol-myristic acid (PMA); novel PKCs (δ,ε) were assayed with 100nmol/l PMA, as described (17).
AMP-activated protein kinase (AMPK) was immunoprecipitated from human hepatocytes and assayed ± "PKC-ι inhibitor as described [18].
Immunoprecipitable Akt2, IRS-1/PI3K and IRS-2/PI3K activities were measured (kit for Akt2 and anti-IRS-1 antiserum from Millipore; anti-IRS-2 antiserum from Santa Cruz Biotechnologies) as described [1, 2, 3, 10]. For IRS-1/PI3K and IRS-2/PI3K, immunoprecipitates were incubated with PI and [γ-32P]ATP, and the end product PI-3’-[32PO4] was purified by thin layer chromatography.
Western Analyses
Western analyses were conducted as described [1, 2, 3, 10, 11] using: anti-IRS-2, anti-PKC-ζ/λ, anti-p65/RelA-NFκB subunit, and anti-Akt1/2 antisera (Millipore); anti-phospho-serine-473-Akt1/2, anti-phospho-threonine-410-PKC-ζ/-403-"PKC-ι/λ and glyceraldehyde-phosphate dehydrogenase (GAPDH) antisera (Santa Cruz Biotechnologies); anti-phospho-threonine-560-PKC-ζ/-555-"PKC-ι/λ antiserum (Invitrogen); anti-PKC-ζ antiserum (gift, Dr. Todd Sacktor, New York, NY, USA); and mouse monoclonal anti-"PKC-ι/λ antibodies (Transduction Labs, Bedford, Massachussets, USA). Samples from experimental groups were compared on the same blots, and corrected for recovery as needed by measurement of GAPDH immunoreactivity.
mRNA Measurements
As described [2, 3, 10], tissues were added to Trizol reagent (Invitrogen) and RNA extracted and purified with RNA-Easy Mini-Kit and RNAase-free DNAase set (Qiagen, Valencia, California, USA), quantified (A260/A280), checked for purity by electrophoresis on 1.2% agarose gels, and mRNA quantified by quantitative real-time reverse transcriptase-polymerase chain reaction (RT-PCR), using TaqMan reverse transcription reagent (Applied Biosystems, Carlsbad, California, USA). mRNAs were measured with a SYBR Green kit (Applied Biosystems) and human nucleotide primers as provided in electronic supplementary materials.
Statistical Evaluations
Data are expressed as mean ± SE, and P values were determined by a one-way ANOVA and the least-significant multiple comparison method.
Results
Overexpression of "PKC-ι and Increased Activity of "PKC-ι and "PKC-ι-dependent Functions in Hepatocytes of Type 2 Diabetic Humans
Prior to culturing, resting aPKC activity, "PKC-ι protein and mRNA levels, and expression of hepatic lipogenic, proinflammatory, and gluconeogenic hepatic enzymes were increased in hepatocytes of type 2 diabetic, as compared to non-diabetic, humans (Table 1). Moreover, this pattern of "PKC-ι overexpression and hyperactivity, and activation of lipogenic, proinflammatory and gluconeogenic pathways in hepatocytes of type 2 diabetic humans was comparably maintained when hepatocytes were cultured overnight in insulin-containing pre-incubation medium, but lost if insulin was omitted from the pre-incubation medium (Table 2). On the other hand, note that a comparable diabetic cellular phenotype was not induced by comparable addition of insulin to the pre-incubation medium of non-diabetic hepatocytes. Dependency of the diabetic cellular phenotype on the continued presence of insulin is discussed further below.
Table 1.
Activity and Levels of aPKCs and aPKC-dependent Expression of Lipogenic, Proinflammatory and Gluconeogenic Enzymes in Uncultured Hepatocytes of Non-diabetic and Type 2 Diabetic Humans
| Parameter | Uncultured Non-Diabetic |
Uncultured T2DM |
P Values |
|---|---|---|---|
| aPKC Activity | 1 ± 0.14 | 2.53 ± 0.23 | <0.001 |
| "PKC-ι mRNA | 1 ± 0.23 | 3.54 ± 0.72 | <0.01 |
| "PKC-ι Protein | 1 ± 0.36 | 2.41 ± 0.67 | <0.05 |
| SREBP-1c mRNA | 1 ± 0.17 | 3.08 ± 0.70 | <0.05 |
| FAS mRNA | 1 ± 0.26 | 3.85 ± 1.15 | <0.05 |
| ACC mRNA | 1 ± 0.26 | 2.77 ± 0.61 | <0.05 |
| TNFα mRNA | 1 ± 0.26 | 2.55 ± 0.59 | <0.05 |
| PEPCK mRNA | 1 ± 0.23 | 2.87 ± 0.61 | <0.05 |
| G6Pase mRNA | 1 ± 0.22 | 3.32 ± 0.89 | <0.05 |
| PKC-ζ mRNA | 1 ± 0.20 | 0.80 ± 0.19 | NS |
Hepatocytes of 5 non-diabetic and 5 type 2 diabetic (T2DM) subjects were analyzed directly, i.e., without overnight culturing, and results for each patient were expressed relative to the mean value of all non-diabetic patients, set at unity. Values are mean ± SEM of 4–5 determinations. P values were determined ANOVA. NS, not significant
Table 2.
Effects of Overnight Culturing in Presence or Absence of Insulin on Activity, Levels and Expression of aPKC and aPKC-dependent Expression of Lipogenic, Proinflammatory and Gluconeogenic Enzymes in Hepatocytes of Type 2 Diabetic and Non-diabetic Humans
| Parameter | Cultured without Insulin T2DM/Non-diabetic |
Cultured with Insulin T2DM/Non-diabetic |
P Values |
|---|---|---|---|
| aPKC Activity | 0.98 ± 0.34 | 1.85 ± 0.24 | <0.05 |
| "PKC-ι mRNA | 1.14 ± 0.23 | 4.48 ± 0.72 | <0.05 |
| "PKC-ι Protein | 0.98 ± 0.19 | 2.01 ± 0.14 | <0.001 |
| SREBP-1c mRNA | 0.84 ± 0.20 | 2.90 ± 0.53 | <0.001 |
| FAS mRNA | 1.08 ± 0.52 | 2.76 ± 0.37 | <0.05 |
| IL1β mRNA | 1.99 ± 0.54 | 3.78 ± 0.97 | NS |
| TNFα mRNA | 3.02 ± 0.41 | 8.39 ± 1.87 | <0.05 |
| PEPCK mRNA | 1.26 ± 0.23 | 2.37 ± 0.97 | NS |
| G6Pase mRNA | 1.91 ± 0.88 | 3.03 ± 1.24 | NS |
Hepatocytes of 5 non-diabetic and 5 T2DM subjects were cultured overnight in insulin-containing or insulin-free, but otherwise identical, medium (see Methods), and T2DM values in each medium were compared as the ratio to the mean non-diabetic value in the same medium. Note that aPKC activity and expression of "PKC-ι and aPKC-dependent enzymes in non-diabetic hepatocytes were not significantly different following overnight (20–24 hr) culturing in insulin-containing or insulin-free medium, followed by 3 hr incubation in insulin-free medium (data not shown). Values are mean ± SEM of 4–5 determinations.
aPKC Inhibitors
To elucidate the role of hepatic aPKC in maintaining the phenotype of hepatocytes of type 2 diabetic humans, we used two highly specific aPKC inhibitors developed by high throughput screening methods. The first inhibitor, 1H-imidazole-4-carboxamide, 5-amino-1-[2, 3–dihydroxy-4-[(phosphonooxy)methyl]cyclopentyl-[1R-(1a, 2b, 3b, 4a)] (ICAPP) binds to the substrate-binding the site of "PKC-ι, but not PKC-ζ [16], and had no effect on activities of PKC-α, PKC-β2, PKC-δ, PKC-ε and AMP-activated protein kinase (AMPK) (Table 3). The second inhibitor, aurothiomalate (ATM), binds to cysteine-69 in "PKC-ι, thereby inhibiting PB1 domain-dependent binding (present in all aPKCs, τ/λ/ζ, but not other PKCs) to factors that regulate downstream processes [12–16]. Note that ATM does not directly inhibit "PKC-ι, but, as seen below, diminishes insulin-induced increases in aPKC enzyme activity in intact tissues. Thus, inhibitory effects of ATM on downstream processes are mediated by inhibition of both PB1-dependent scaffolding and enzymatic activation of aPKC.
Table 3.
Effects of ICAPP on activities of conventional PKCs (α and β2), novel PKCs (δ and ε), atypical "PKC-ι and AMPK
| ICAPP (nmol/l) |
PKC α | PKC β2 | PKC δ | PKC ε | PKC τ | ICAPP (nmol/l) |
AMPK |
|---|---|---|---|---|---|---|---|
| 0 | 5522 ± 227 | 6509 ± 239 | 4962 ± 393 | 7129 ± 269 | 5461 ± 226 | 0 | 2427 ± 83 |
| 1 | 5866 ± 707 | 7286 ± 102 | 5279 ± 265 | 7068 ± 280 | 3980 ± 45 | 0.1 | 2352 ± 342 |
| 10 | 5088 ± 128 | 6757 ±92 | 5293 ± 173 | 6694 ± 285 | 2239 ± 64 | 1 | 2803 ± 69 |
| 100 | 4965 ± 137 | 6545 ± 118 | 5504 ± 729 | 6732 ± 332 | 1882 ± 246 | 10 | 2815 ± 15 |
| 1000 | 6187 ± 1670 | 6422 ± 698 | 5634 ± 201 | 6156 ± 282 | 1762 ± 105 | 100 | 2830 ± 15 |
| 10,000 | 4737 ± 238 | 6225 ± 242 | 4443 ± 232 | 5837 ± 191 | 1300 ± 40 | 1000 | 3173 ± 5 |
Conventional PKCs were maximally stimulated with 1mmol/l CaCl2 and 100nmol/l phorbol ester. Novel PKCs were maximally stimulated with 100nmol/l phorbol esters. "PKC-ι was maximally stimulated with 10fmol/l PIP3. See Methods for other details. Kinase activity values (cpm) are mean ± range or SEM of duplicates or quadruplicates, respectively; comparable results were seen in a repeat experiment.
Insulin Signalling in Hepatocytes of Non-diabetic and Type 2 Diabetic Humans
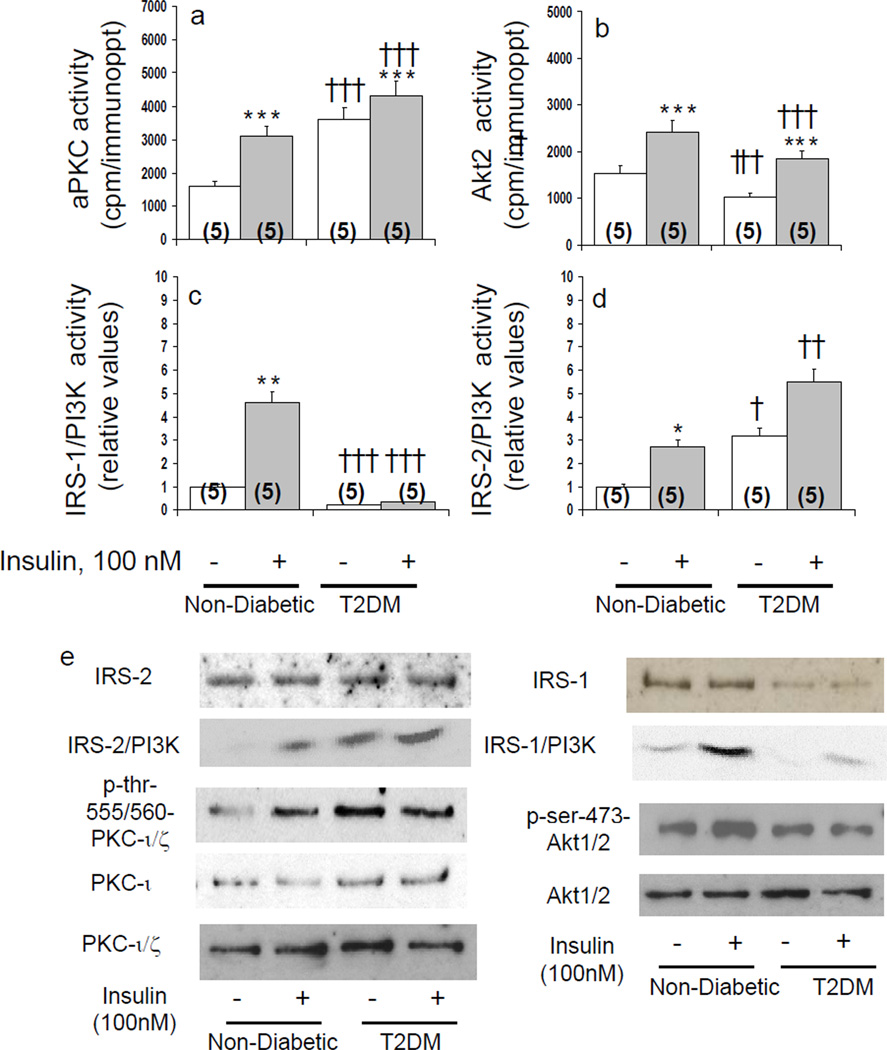
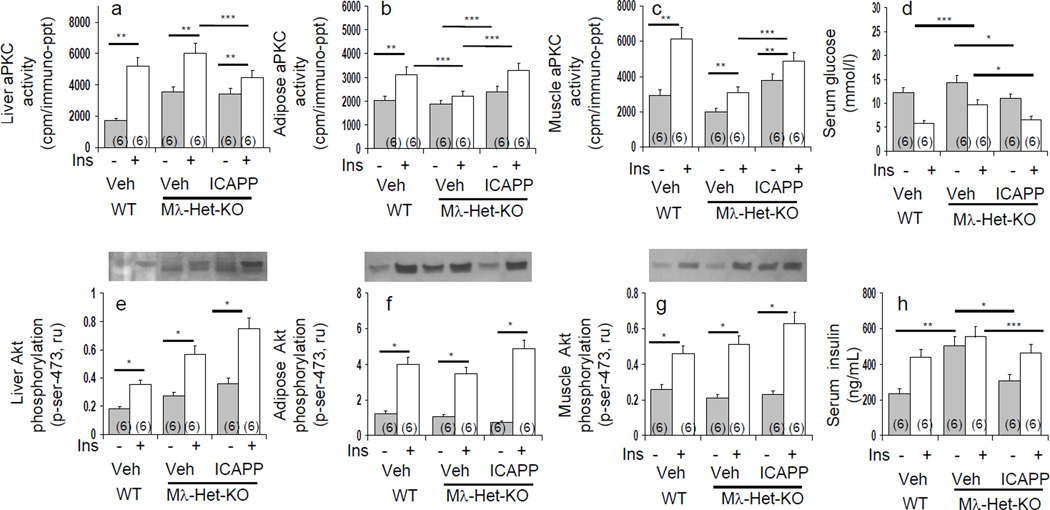
Basal and acute insulin-stimulated activities of aPKC and its major if not sole activator during insulin action in liver, IRS-2/PI3K, were increased in hepatocytes of type 2 diabetic, relative to non-diabetic, humans (Figs 1 and 2). In contrast, activities of Akt2 and its primary activator, IRS-1/PI3K, were diminished (Figs 1 and 2). Like enzyme activity, aPKC auto(trans)phosphorylation (p-threonine-555/560-"PKC-ι/ζ) was increased basally in diabetic hepatocytes and by insulin in non-diabetic hepatocytes (Figs 1 and 2). Moreover, auto(trans)phosphorylation was diminished by both aPKC inhibitors (Fig 2).
Figure 1.
Effects of insulin on basal and insulin-stimulated enzyme activities of total aPKC (panel a), Akt2 (panel b), IRS-1-dependent PI3K (panel c) and IRS-2-dependent PI3K (panel d) in hepatocytes of non-diabetic and type 2 diabetic humans. Hepatocytes of all subjects, 5 non-diabetic and 5 type 2 diabetic (T2DM), were compared simultaneously during a 15-minute incubation with or without 100nmol/l insulin. Shown in panel e are representative immunoblots of levels and autoradiograms portraying PI3K activity of IRS-1 and IRS-2, auto(trans)phosphorylation of "PKC-ι/ζ (threonine-555/560), PDK2-dependent phosphorylation of Akt1/2 (serine-473), and levels of "PKC-ι, "PKC-ι/ζ (total aPKC) and Akt1/2. Bargram values are mean ± SEM of 5 patients. Asterisks [*, P<0.05; **, P P<0.01; ***, P<0.001] reflect P values (ANOVA) of adjacent basal and insulin-stimulated groups. Crosses [†, P<0.05; ††, P<0.01; †††, P<0.001] reflect P values of basal versus basal groups, or insulin-stimulated versus insulin-stimulated groups.
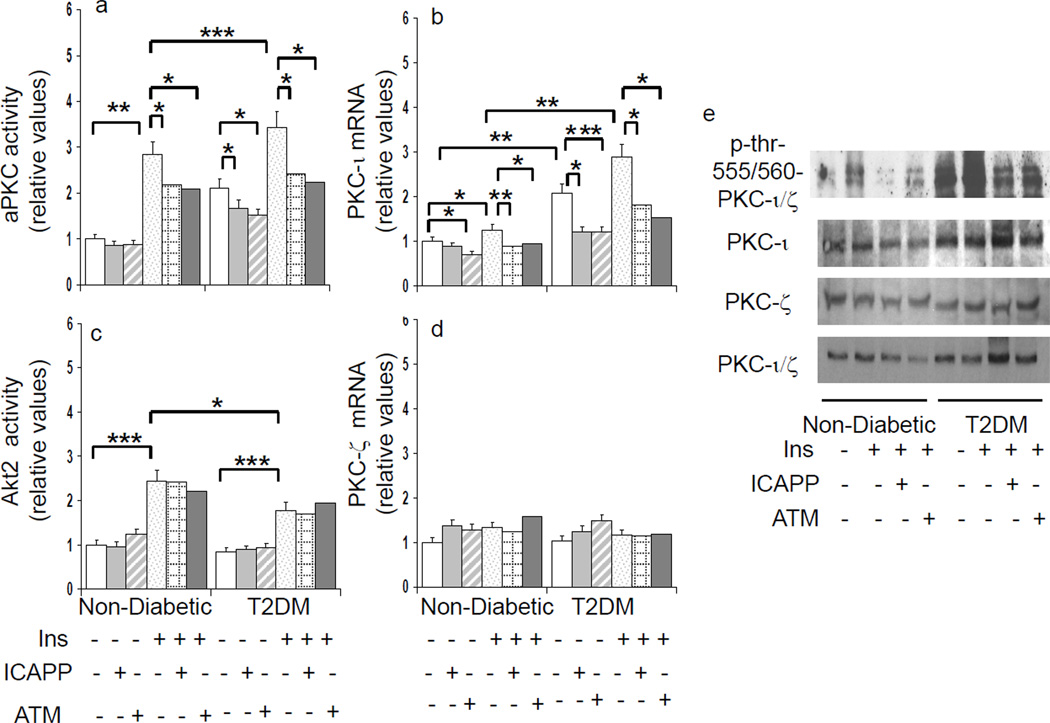
Figure 2.
Effects of aPKC inhibitors, ICAPP and ATM, on basal and insulin-stimulated total aPKC activity (panel a), "PKC-ι expression (panel b), Akt2 activity (panel c) and PKC-ζ expression (panel d) in hepatocytes of non-diabetic and type 2 diabetic (T2DM) humans. Incubation time, 4 or 6 hours with or without 1µmol/l insulin (values at both times were comparable and therefore pooled). Bargram values, normalized to unity, are mean ± SEM of findings in 5–8 patients. Asterisks reflect significant differences (*, P<0.05; **, P<0.01: ***, P<0.001; ANOVA) between bracketed groups. Representative immunoblots reflecting changes in basal, insulin stimulated, and inhibitor-treated levels of phospho-threonine-555/560-"PKC-ι/ζ, "PKC-ι, PKC-ζ and "PKC-ι/ζ ( total aPKC) are shown at right in panel e.
Like aPKC activity, protein levels of "PKC-ι, but not PKC-ζ, were increased 2-fold in hepatocytes of type 2 diabetic humans [1 ± 0.04 (mean ± SEM; N=4) non-diabetic versus 2.07 ± 0.05 (N=4) diabetic, relative units (P<0.001)] (blots, Figs 1 and 2). Total aPKC levels in diabetic hepatocytes were increased less than "PKC-ι, presumably reflecting the presence of sizeable but unchanged amounts of PKC-ζ (Figs 1 and 2). Unlike "PKC-ι Akt levels were comparable in diabetic and non-diabetic hepatocytes (Fig 1).
Like IRS-1/PI3K activity, IRS-1 levels were diminished by 61% in diabetic hepatocytes [1 ± 0.01 (N=5) non-diabetic versus 0.39 ± 0.10 (N=5) diabetic, relative units (P<0.001)] (blot, Fig 1). In contrast, IRS-2 levels were comparable in diabetic and non-diabetic hepatocytes (blot, Fig 1).
Expression of aPKCs in Hepatocytes of Non-diabetic and Type 2 Diabetic Humans
As in acute signalling studies, activities of aPKC and Akt2 continued to be excessive and diminished, respectively, in hepatocytes of type 2 diabetic humans during more prolonged 4–6-hour expression studies (Fig 2). Along with increases in activity and protein levels, "PKC-ι expression (mRNA) was increased in diabetic hepatocytes (Fig 2). Moreover, "PKC-ι expression in non-diabetic hepatocytes was increased modestly but significantly by 4–6-hour insulin treatment, and similar upward trends were seen in diabetic hepatocytes. Importantly, diabetes-induced and insulin-stimulated "PKC-ι expression was diminished by "PKC-ι inhibitors (Fig 2), suggesting operation of an insulin-driven, feed-forward/positive-feedback mechanism. That protein levels of "PKC-ι did not change with inhibitor treatment probably reflects a slower turnover rate relative to that of mRNA.
Unlike "PKC-ι, expression and protein levels of PKC-ζ were unaltered by insulin, diabetes, and aPKC inhibitors (Fig 2).
Expression of "PKC-ι in Muscles of Non-diabetic and T2DM Humans
Different from hepatocytes, but consonant with reports of diminished total aPKC levels in muscles of type 2 diabetic humans [11, 19], muscle "PKC-ι mRNA levels were diminished by 57% in diabetic subjects [1 ± 0.14 (N=5) non-diabetic versus 0.43 ± 0.07 (N=5) diabetic, relative units (P<0.05)]. Muscle "PKC-ι protein levels were similarly diminished by 52% [1 ± 0.12 (N=12) non-diabetic versus 0.48 ± 0.08 (N=12) diabetic, relative units (P<0.001)].
Effects of aPKC Inhibitors on aPKC Activity in Human Hepatocytes
ICAPP potently (IC50, 10–100nM) inhibited both recombinant "PKC-ι activated by PIP3 in vitro (Table 3) and insulin-stimulated aPKC activity in human hepatocytes (Figs 2 and 3). Note that residual insulin-dependent hepatic aPKC activity that is resistant to ICAPP probably reflects PKC-ζ, which is not inhibited by ICAPP. Also note that basal immunoprecipitable aPKC activity is partially resistant to ICAPP (Figs 2 and 3), and this probably reflects some non-aPKC kinase activity in the immunoprecipitates.
Figure 3.
Dose-related effects of "PKC-ι/λ inhibitors, ICAPP (panels a and d) and ATM (panels c and f) on basal (open bars) and insulin-stimulated (shaded bars) activities of total aPKC and Akt2 in hepatocytes of non-diabetic humans. Incubation time, 4 hours with or without 1µmol/l insulin. Also shown are dose-related effects of ICAPP on: (1) activity of 10ng recombinant "PKC-ι activated by maximally-effective 10fmol/l PIP3 (panel b), and (2) serine-256 phosphorylation of FoxO1 in hepatocytes (panel e). Values are mean ± SEM of 4 or more determinations.
Like ICAPP, ATM diminished insulin-stimulated aPKC activity in intact hepatocytes (Figs 2 and 3), presumably reflecting a requirement for a PB1-dependent scaffolding complex during aPKC activation.
Also note: neither ICAPP nor ATM inhibited insulin-stimulated Akt activation (Figs 2 and 3); ICAPP did not inhibit increases in PDK1-dependent phosphorylation of "PKC-ι/ζ (threonine-403/410) (not shown); and (c) both ICAPP and ATM diminished aPKC auto(trans)phosphorylation in intact hepatocytes (Fig 2).
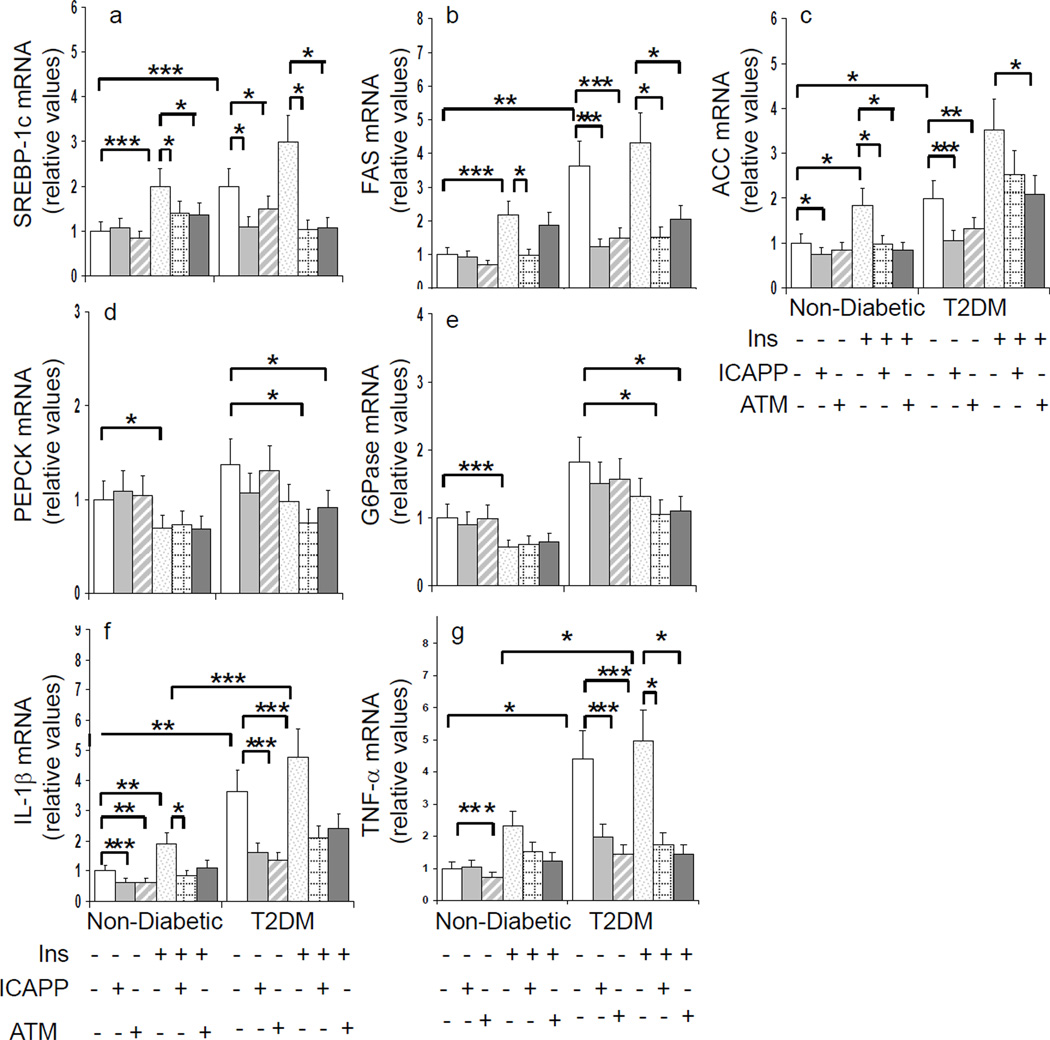
Effects of aPKC Inhibitors on aPKC-dependent Processes in Human Hepatocytes
Accompanying increases in aPKC activity seen basally in hepatocytes of type 2 diabetic humans and following insulin treatment of hepatocytes of non-diabetic and type 2 diabetic humans, there were increases in expression of SREBP-1c and SREBP-1c-dependent enzymes, FAS and ACC, and NFκB-dependent cytokines, TNF-α and IL-1β (Figs 4, 5 and 6). Accompanying decreases in aPKC activity induced by ICAPP and ATM, expression of SREBP-1c, FAS, ACC, TNF-α, and IL-1β diminished in hepatocytes of non-diabetic and type 2 diabetic humans (Figs 4, 5 and 6). Note that unaltered expression of PKC-ζ (see Fig 2) served as a negative control in expression studies.
Figure 4.
Dose-related effects of "PKC-ι/λ inhibitor, ICAPP, on expression of lipogenic (SREBP-1c, FAS, ACC), inflammatory (TNF-α, ILl-1β) and glucogenic (PEPCK, G6Pase) enzymes in hepatocytes of non-diabetic humans. Incubation time, 4 hours with or without 1µmol/l insulin. Values are mean ± SEM of 4 or more determinations. Data are from cells used in the Fig 3.
Figure 5.
Dose-related effects of "PKC-ι/λ inhibitor, ATM, on expression of lipogenic (SREBP-1c, FAS, ACC), inflammatory (TNF-α, ILl-1β) and gluconeogenic (PEPCK, G6Pase) enzymes in hepatocytes of non-diabetic humans. Incubation time, 4 hours with or without 1µmol/l insulin. Values are mean ± SEM of 4 or more determinations. Data are from cells used in Fig 3.
Figure 6.
Effects of aPKC inhibitors, ICAPP and ATM, on basal and insulin-stimulated expression of lipogenic (SREBP-1c, FAS, ACC), inflammatory (TNF-α, IL-1β) and gluconeogenic (PEPCK, G6Pase) enzymes in hepatocytes of non-diabetic and type 2 diabetic (T2DM) humans. Incubation time, 4 or 6 hours with or without 1µmol/l insulin (values at both times were comparable and therefore pooled). Bargram values (normalized to unity) are mean ± SEM of findings in 5–8 patients. Asterisks reflect significant differences (*, P<0.05; **, P<0.01: ***, P<0.001; ANOVA) between bracketed groups. See Figure 3 for effects of ICAPP and ATM on activities and/or expression aPKC and Akt in these hepatocytes.
Insulin maintains blood glucose levels largely by decreasing expression of gluconeogenic enzymes, PEPCK and G6Pase, thereby diminishing hepatic glucose output. Akt at least partly mediates this inhibitory effect of insulin by phosphorylating ser-256-FoxO1, thus causing nuclear exclusion and diminished ability of FoxO1 to promote gluconeogenic enzyme expression in response to A-kinase activators. Interestingly, along with decreased Akt activity in hepatocytes of type 2 diabetic humans, there were upward trends in basal expression of, and diminished effects of insulin on, PEPCK/G6Pase expression (Fig 6). Moreover, consonant with previous findings that adenoviral-mediated inhibition of hepatic aPKC elicits insulin-like decreases in fasting-dependent expression of PEPCK/G6Pase in rodent liver [2], ICAPP and ATM had insulin-like effects, or enhanced insulin effects, on FoxO1 phosphorylation (Fig 3 and other unpublished findings) and PEPCK/G6Pase expression in hepatocytes of non-diabetic and diabetic humans (Figs 4, 5 and 6).
Discussion
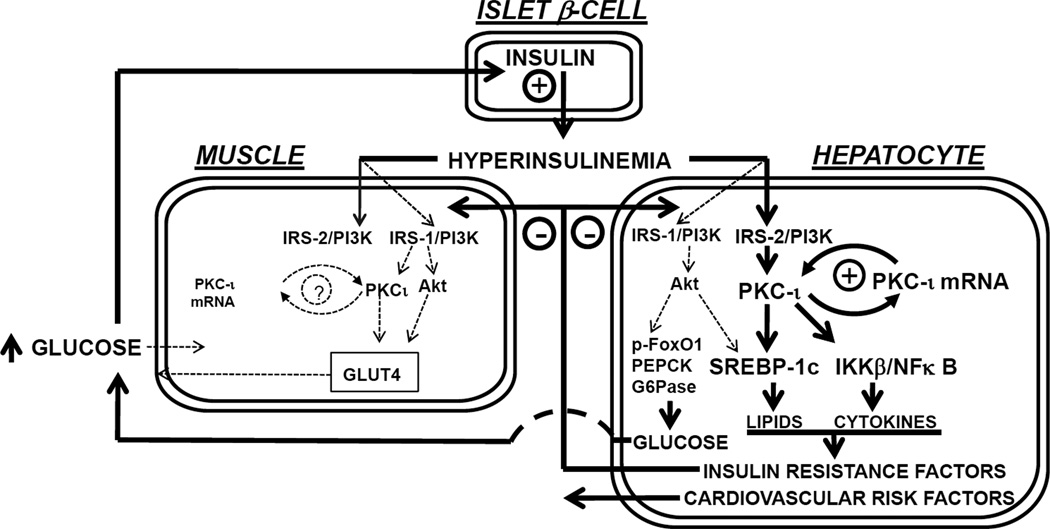
The major findings (depicted in Fig 7) are: (a) "PKC-ι is maintained in an overactive/overexpressed state by an insulin-driven, IRS-2-dependent mechanism in hepatocytes of type 2 diabetic humans; (b) overactive hepatic "PKC-ι contributes importantly to excessive activation of hepatic lipogenic, proinflammatory and gluconeogenic pathways that presumably underlie or contribute to lipid and carbohydrate abnormalities in type 2 diabetes; and (c) "PKC-ι inhibitors are capable of reversing or markedly improving aPKC-dependent excesses in expression of lipogenic, proinflammatory and gluconeogenic enzymes in hepatocytes of type 2 diabetic humans.
Figure 7.
Schematic showing a cycle of "PKC-ι hyperactivation and overexpression in hepatocytes of type 2 diabetic humans. This cycle is driven by insulin and dependent on "PKC-ι itself, as well as IRS-2/PI3K, and is therefore, vicious. This cycle in turn drives lipogenic and inflammatory pathways, thereby leading to insulin resistance, dyslipidaemia and other clinical abnormalities. In contrast to "PKC-ι, IRS-1/PI3K-dependent Akt activation is diminished in diabetic hepatocytes, thereby leading to increases in gluconeogenic factors and hyperglycemia. Opposite to hepatic "PKC-ι, muscle "PKC-ι is underexpressed and underactive, and, along with underactive IRS-1/PI3K and Akt, leads to further decreases in glucose tolerance. Note that the overactive hepatic "PKC-ι cycle in liver exacerbates the underactive "PKC-ι cycle in muscle and vice versa.
Ability to observe hyperactivity and overexpression of "PKC-ι in overnight cultured as well as uncultured hepatocytes of type 2 diabetic humans seems best explained by the fact that "PKC-ι expression was increased by an insulin- and "PKC-ι-dependent mechanism. Accordingly, we postulate that: (a) hyperinsulinaemia in vivo initiated a feed-forward, positive-feedback cycle of "PKC-ι activation/expression in hepatocytes of type 2 diabetic humans; and (b) this aberrant cycle was maintained ex vivo by continued exposure to high concentrations of insulin, but could be effectively downregulated by sufficiently long deprivation of insulin. On the other hand, a comparable cycle was not elicited by simple exposure of non-diabetic hepatocytes to overnight insulin treatment, suggesting that the aberrant "PKC-ι-cycle in type 2 diabetes develops more slowly than it dissipates, and/or co-requires other factors, such as IRS-2/PI3K hyperactivity, which, for presently uncertain reason, exists in diabetic hepatocytes. In any case, dissipation of the aberrant "PKC-ι activation/expression cycle by insulin deprivation demonstrates that it is an acquired, reversible abnormality.
That insulin-stimulated aPKC activation was excessive and Akt2 activation was simultaneously diminished probably reflects differential regulation of upstream activators in hepatocytes of type 2 diabetic humans. Thus: conserved levels and excessive activity of IRS-2 would account for heightened aPKC activation/expression; and diminished levels and diminished activity of IRS-1 would account for diminished Akt activation. These findings in hepatocytes of type 2 diabetic humans agree with: (a) an IRS-2 requirement for hepatic aPKC activation and an IRS-1 requirement for hepatic Akt activation in mouse knockout studies [4–7]; and (b) conserved or hyperactive IRS-2/PI3K/aPKC signalling versus diminished IRS-1/PI3K/Akt signalling in rodent diabetic liver [1, 3].
Although decreased IRS-1 levels and activity seem to account for diminished Akt activation in hepatocytes of type 2 diabetic humans, it is also possible that overactivity of "PKC-ι may have contributed to diminished hepatic Akt activity. In support of this idea, hepatic aPKC inhibition by ICAPP provoked increases in ser-256-phosphorylation of Akt substrate FoxO1. On the other hand, aPKC inhibition provoked increases in FoxO1 phosphorylation even in the absence of concurrent insulin stimulation of Akt, and the underlying mechanism is presently uncertain. In any case, increased FoxO1 phosphorylation may explain insulin-like decreases in PEPCK/G6Pase expression elicited by "PKC-ι inhibition elicited presently by ICAPP, and previously by hepatic expression of kinase-inactive aPKC (2).
That "PKC-ι expression was increased in hepatocytes but suppressed in muscles of type 2 diabetic humans may seem puzzling. However, as increased "PKC-ι expression in diabetic hepatocytes most likely reflects operation of an overactive, insulin-driven, /IRS-2/PI3K-dependent, feed-forward, positive-feedback cycle, the fact that expression of "PKC-ι in muscle is diminished could reasonably reflect diminished activation of IRS-1/PI3K and "PKC-ι in diabetic muscle [11, 19].
As partial "PKC-ι/λ deficiency in muscle induces obesity, metabolic syndrome features and glucose intolerance [10], the combination of diminished muscle "PKC-ι and excessive hepatic "PKC-ι activity in type 2 diabetic humans is particularly problematic. Thus: deficient "PKC-ι expression/activation and glucose transport in muscle would exacerbate glucose intolerance; resultant hyperinsulinaemia would increase hepatic "PKC-ι expression/activation and expression of lipogenic, proinflammatory and gluconeogenic pathways; and these hepatic abnormalities would further impair "PKC-ι activation in muscle. This imbalance between underactive muscle and overactive liver "PKC-ι is itself a multi-organ vicious cycle that is superimposed on the intra-hepatic "PKC-ι vicious cycle (see Fig 8). Fortunately, both vicious cycle are likely to be ameliorated by inhibition of hepatic "PKC-ι.
With aPKC, Akt is co-required for insulin effects on expression of SREBP-1c and lipogenic enzymes in rodent hepatocytes [20, 21]. Indeed, we found that the Akt chemical inhibitor used by Li et al [21] markedly (>90%) inhibited SREBP-1c activation by insulin in human hepatocytes, while inhibiting Akt2 enzyme activity by 40% (not shown). This lack of correlation suggests that there are multiple Akt pools with notable differences in responsiveness to inhibitors. By analogy, similar heterogeneity in aPKC pools, as well as different biological effects of "PKC-ι and PKC-ζ and unquantifiable scaffolding effects of ATM may explain the lack of strict parallelism between alterations in hepatic aPKC activity and hepatic enzyme expression following ATM and ICAPP treatment.
It should be emphasized that, because of limited availability of donor livers, in particular, diabetic donors, we were able to study only a relatively small number of human subjects, and further studies in various human populations are needed to see if the present findings have widespread application.
To summarize, opposite to underexpression and underactivation of "PKC-ι in muscle, "PKC-ι is overexpressed and overactive in hepatocytes of type 2 diabetic humans, most likely largely owing to an insulin-driven, IRS-2- and "PKC-ι-dependent, feed-forward/positive-feedback cycle. Moreover, this vicious cycle of "PKC-ι overexpression/overactivity in liver is accompanied by overexpression of hepatic lipogenic, proinflammatory and glucogenic factors that are presumed to be key players in the pathogenesis of clinical abnormalities in obesity, the metabolic syndrome and type 2 diabetes. Importantly, two newly-developed "PKC-ι inhibitors specifically inhibit activity and expression of "PKC-ι, and expression of "PKC-ι-dependent pathogenetic factors in isolated human hepatocytes. Further studies are needed to see if these or other "PKC-ι inhibitors are useful for treating obesity and type 2 diabetes.
Supplementary Material
Acknowledgements
Supported by funds from the Department of Veterans Affairs Merit Review Program and the National Institutes of Health Grants [DK 065969 to R.V.F.}.
Abbreviations
- ACC
acetyl-CoA carboxykinase
- AMPK
AMP-activated protein kinase
- Akt
protein kinase B
- ATM
aurothiomalate
- BMI
basal metabolic index
- ESM
electronic supplementary materials
- FAS
fatty acyl synthase
- G6Pase
glucose-6-phosphatase
- IRS-1
insulin receptor substrate-1
- IRS-2
insulin receptor substrate-2
- ICAPP
1H-imidazole-4-carboxamide, 5-amino-1-[2, 3-dihydroxy-4-[(phosphonooxy)methyl]cyclopentyl-[1R-(1a,2b,3b,4a)]
- IKKβ
inhibitor of kappa-B kinase-beta
- IκBβ
inhibitor of nuclear factor kappa-B-beta
- IL-1β
interleukin-1beta
- NFκB
nuclear factor kappa-B
- NEFA
Non-esterified fatty acid
- PDK1
phosphoinositide-dependent kinase-1
- PDK2
phosphoinositide-dependent kinase-2
- PEPCK
phosphoenolpyruvate carboxykinase
- PIP3
phosphatidylinositol-3,4,5-trisphosphate
- PI3K
phosphatidylinositol 3-kinase
- PKC
protein kinase C
- aPKC
atypical protein kinase C
- SREBP-1c
sterol receptor element binding protein-1c
- T2DM
type 2 diabetes mellitus
- TNF-α
tissue necrosis factor-alpha
Footnotes
Contribution Statement
RV Farese conceived, designed and directed the studies, analyzed data, and wrote the paper. MP Sajan conducted studies and assays, and assembled and assisted in interpretation of data.
Duality of Interest
There are no conflicts of interest amongst the authors.
References
- 1.Standaert ML, Sajan MP, Miura A, et al. Insulin-induced activation of atypical protein kinase C, but not protein kinase B, is maintained in diabeticob/oband Goto-Kakizaki liver. Contrasting insulin signaling patterns in liver versus muscle define phenotypes of type 2 diabetic and high fat-induced insulin-resistant states. J Biol Chem. 2004;279:24929–24934. doi: 10.1074/jbc.M402440200. [DOI] [PubMed] [Google Scholar]
- 2.Sajan MP, Standaert ML, Nimal S, et al. Critical role of atypical protein kinase C in activating hepatic SREBP-1c and NFκB in obesity. J Lipid Res. 2009;50:1133–1145. doi: 10.1194/jlr.M800520-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sajan MP, Standaert ML, Rivas J, et al. Role of atypical protein kinase C in activation of sterol regulatory element binding protein-1c and nuclear factor kappa B (NFkappaB) in liver of rodents used as model of diabetes, and relationships to hyperlipidaemia and insulin resistance. Diabetologia. 2009;52:1197–1207. doi: 10.1007/s00125-009-1336-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valverde AM, Burks DJ, Fabregat I, et al. Molecular mechanisms of insulin resistance in IRS-2-deficient hepatocytes. Diabetes. 2003;52:2239–2248. doi: 10.2337/diabetes.52.9.2239. [DOI] [PubMed] [Google Scholar]
- 5.Ueki K, Yamauchi T, Tamemoto H, et al. Restored insulin-sensitivity in IRS-1-deficient mice treated by adenovirus-mediated gene therapy. J Clin Invest. 2000;105:1437–1445. doi: 10.1172/JCI7656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sajan MP, Standaert ML, Miura A, Farese RV. Tissue-specific differences in activation of atypical protein kinase C and protein kinase B in muscle, liver and adipocytes of insulin receptor substrate-1 knockout mice. Mol Endocrinol. 2004;18:2513–2521. doi: 10.1210/me.2004-0045. [DOI] [PubMed] [Google Scholar]
- 7.Guo S, Copps KD, Park S, et al. The Irs1 branch of the insulin signaling cascade plays a dominant role in hepatic nutrient homeostasis. Mol Cell Biol. 2009;29:5070–5083. doi: 10.1128/MCB.00138-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farese RV, Sajan MP. Metabolic Functions of Atypical Protein Kinase C: “Good and Bad” as Defined by Nutritional Status. (Invited Review) Am J Physiol Endocrinol Metab. 2010;298:E385–E394. doi: 10.1152/ajpendo.00608.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsumoto M, Ogawa W, Akimoto K, et al. PKCλ in liver mediates insulin-induced SREBP-1c expression and determines both hepatic lipid content and overall insulin sensitivity. J Clin Invest. 2003;112:935–944. doi: 10.1172/JCI18816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farese RV, Sajan MP, Yang H, et al. Muscle-specific Knockout of Protein Kinase C-λ Impairs Glucose Transport and Induces Metabolic and Diabetic Syndromes. J Clin Invest. 2007;117:2289–2301. doi: 10.1172/JCI31408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beeson M, Sajan MP, Dizon M, et al. Activation of protein kinase C-ζ by insulin and phosphatidylinositol-3,4,5-(PO4)3 is defective in muscle in type 2 diabetes and impaired glucose intolerance. Amelioration by rosiglitazone and exercise. Diabetes. 2003;52:1926–1934. doi: 10.2337/diabetes.52.8.1926. [DOI] [PubMed] [Google Scholar]
- 12.Stallings-Mann M, Jamieson L, Regala RP, Weems C, Murray NR, Fields AP. A novel small molecule inhibitor of protein kinase Cτ blocks transformed growth of non-small cell lung cancer. Cancer Res. 2006;66:1767–1774. doi: 10.1158/0008-5472.CAN-05-3405. [DOI] [PubMed] [Google Scholar]
- 13.Erdogan E, Lamark T, Stallings-Mann M, et al. Aurothiomalate inhibits transformed growth by targeting the PB1 domain of atypical protein kinase Ciota. J Biol Chem. 2006;281:28450–28459. doi: 10.1074/jbc.M606054200. [DOI] [PubMed] [Google Scholar]
- 14.Fields AP, Frederick LA, Regala RP. Targeting the oncogenic protein kinase Ciota for the treatment of cancer. Biochem Soc Trans. 2007;23:1996–2000. doi: 10.1042/BST0350996. [DOI] [PubMed] [Google Scholar]
- 15.Regala RP, Thompson EA, Fields AP. Atypical protein kinase Cτ expression and aurothiomalate sensitivity in human lung cancer cells. Cancer Res. 2008;68:5888–5895. doi: 10.1158/0008-5472.CAN-08-0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pillai P, Desai S, Patel R, Sajan MP, Farese RV, Acevedo-Duncan M. ICA-1: A novel "PKC-ι inhibitor that abrogates cell proliferation and induces apoptosis in neuroblastoma. Internat J Biochem and Cell Biol. 2011;43:784–794. doi: 10.1016/j.biocel.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 17.Standaert ML, Galloway L, Bandyopadhyay G, Moscat J, Farese RV. PKC-ζ as a Downstream Effector of PI 3-kinase during Insulin Stimulation in Rat Adipocytes. Potential Role in Glucose Transport. J. Biol. Chem. 1997;272:30075–30082. doi: 10.1074/jbc.272.48.30075. [DOI] [PubMed] [Google Scholar]
- 18.Sajan MP, Bandyopadhyay G, Miura A. AICAR and metformin, but not exercise, increase muscle glucose transport through AMPK-, ERK- and PDK1-dependent activation of atypical PKC. Am J Physiol Endocrinol Metab. 2010;298:E179–E192. doi: 10.1152/ajpendo.00392.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim Y-B, Kotani K, Ciaraldi TP, Henry RR, Kahn BB. Insulin-stimulated protein kinase C-λ/ζ activity is reduced in skeletal muscle of humans with obesity and type 2 diabetes; reversal with weight reduction. Diabetes. 2003;52:1935–1942. doi: 10.2337/diabetes.52.8.1935. [DOI] [PubMed] [Google Scholar]
- 20.Fleischmann M, Iynedjian PB. Regulation of sterol regulatory element binding protein 1 gene expression in liver: Role of insulin and protein kinase B/cAkt. Biochem J. 2000;349:13–17. doi: 10.1042/0264-6021:3490013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li S, Brown MS, Goldstein JL. Bifurcation of insulin signaling pathway in rat liver: mTORC1 required for stimulation of lipogenesis, but not inhibition of gluceoneogenesis. Proc Natl Acad Sci USA. 2010;107:3441–3446. doi: 10.1073/pnas.0914798107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.