Abstract
Introduction
Transforming growth factor-β1 (TGF-β1) has been implicated in the pathogenesis of Peyronie’s disease (PD) and also plays a role in collagen and elastin metabolism. Pentoxifylline (PTX) antagonizes the effects of TGF-β1 and has been utilized in our clinic for the management of PD.
Aim
We studied the effects of TGF-β1 and PTX on collagen metabolism and elastogenesis in tunica albuginea-derived fibroblasts (TADFs).
Methods
TADFs from men with and without PD were cultured and treated with TGF-β1 and PTX as monotherapy at differing concentrations and time points. Combination treatment (TGF-β1 followed by PTX and vice versa) was also investigated.
Main Outcome Measures
Cell proliferation assay, enzyme-linked immunosorbent assay, and immunohistochemistry were utilized to assess the impact of TGF-β1 and PTX on TADF with respect to elastin and collagen I metabolism.
Results
PTX inhibited fibroblast proliferation at doses of 100 μM. TGF-β1 stimulated elastogenesis and collagen I fiber deposition in TADF in a dose- and time-dependent fashion. Pretreatment with PTX dramatically attenuated TGF-β1-mediated elastogenesis and collagen fiber deposition in TADF from men with and without PD. Interestingly, production of collagen I was higher in untreated Peyronie’s tunica (PT) cells relative to normal tunica (NT) cells; furthermore, PTX attenuated collagen production to levels similar to untreated control TADF in PT cells but not in NT cells, suggesting important intrinsic differences between PT and NT cells.
Conclusion
Both elastin and collagen are upregulated by TGF-β1 in TADF. This likely contributes to the PD phenotype. Pretreatment with PTX attenuates both collagen fiber deposition and elastogenesis in TADF exposed to TGF-β1; these effects suggest a useful role for PTX in the management of PD.
Keywords: TGF-Beta, Pentoxifylline, Peyronie’s Disease, Elastin, Tunica Albuginea, Fibroblasts
Introduction
Peyronie’s disease (PD) is the most common cause of acquired deformity of the penis and is characterized by the development of fibrotic, collagen-containing plaques within the tunica albuginea [1,2]. The condition is thought to occur when genetically predisposed men suffer trauma to the tunica; this injury may lead to a prolonged inflammatory response with subsequent remodeling of connective tissue into a dense fibrotic plaque with abundant collagen deposition [2,3]. It has been established in prior studies using cultured tunica fibroblasts that a variety of factors involved in wound healing and fibrosis (including alpha-actin, beta-catenin, heat-shock proteins, fibronectin, transforming growth factor-β1 (TGF-β1) receptor, and several currently unknown proteins) are upregulated in PD-plaque-derived cells compared with normal-tunica-derived cells [4]. Investigation of the molecular underpinnings of PD has the potential to further improve our capacity to understand and treat this disorder.
TGF-β1 has been implicated in the pathogenesis of numerous human fibrotic disorders and has been linked to the PD phenotype [5]. Treatment with TGF-β1 has been shown to induce a PD-like condition in animal models [6,7]. Much of this effect has been attributed to TGF-β1 induced enhancement of collagen production in fibroblasts and corporal smooth muscle cells [8,9]. TGF-β1 has also been demonstrated to increase elastin mRNA levels in both dermal and lung fibroblasts [10,11]. A report by Stewart et al. suggested that PD is associated with an increase in elastin fiber production and degradation [12]. Histological studies have demonstrated that tunical plaque tissue from men with PD contains dense, abnormal bundles of elastin [13]. It is logical to speculate that elastin metabolism may influence the PD phenotype; however, study of elastin in PD has been for the most part neglected in favor of studies of collagen metabolism.
Pentoxifylline (PTX), a nonselective phosphodiesterase inhibitor with anti-inflammatory properties in vivo and in vitro, has been used in a variety of inflammatory and fibrotic conditions [14–17]. PTX has been shown in both in vitro and in vivo (rat) experiments to induce regression of collagen and TGF-β1 mediated plaque formation [18]. In clinical practice PTX has been utilized for the treatment of PD although published data on treatment outcomes are limited [19].
In the current study, we studied the impact of TGF-β1 on elastin and collagen metabolism in tunica albuginea-derived fibroblasts (TADF). TADF are localized to the tissue in which PD lesions typically develop and therefore represent a potentially more accurate and informative model system than foreskin-derived fibroblasts. We also investigated the impact of PTX on this in vitro model system. Our hypothesis was that TGF-β1 induces dysregulation of collagen and elastin metabolism in TADF and that PTX ameliorates these effects.
Materials and Methods
Tissue Harvesting and Cell Culture
Our Institutional Committee on Human Research approved all procedures regarding the collection and use of human tissues. Plaque containing specimens of tunica from men with PD (PT) were harvested from 12 patients with chronic (>12 months duration) PD who were undergoing surgery for correction of penile curvature. Normal tunica (NT) was harvested from six patients who were undergoing penile prosthesis placement. None of the patients had undergone radical prostatectomy and all were free of diabetes. All cavernosal tissue was stripped from the biopsy specimens so as to ensure a pure culture of tunica-derived tissues.
TADF were procured as previously described [20]. Briefly, the tunica tissues were washed three times in sterile phosphate-buffered saline (PBS) and cut into 2- to 3-mm3 segments. The segments were placed evenly onto a 100-mm cell culture dish (Falcon-Becton Dickinson Labware, Franklin Lakes, NJ, USA). Ten minutes later, 10 mL of Dulbecco’s modified Eagle medium (DMEM) containing penicillin (100 units/mL), streptomycin (100 ug/mL), and 10% fetal bovine serum (FBS) was pipetted into the dish. The dish was kept undisturbed in a humidified 37°C incubator with 5% CO2. Five days later, tissue segments that had detached from the dish were removed, and the culture medium was replaced. This process was repeated after another 5 days of culture. When small islands of cells were noticeable, wells were treated with trypsin and transferred to a fresh dish. Expansion of each cell line was continued with change of medium every 3 days and passages approximately every 10 days. It has been demonstrated that chromosomal and cellular biological characteristics may change after multiple passages in cultured fibroblasts [21,22]; therefore, all cells used in the following experiments were from passages 4 through 10. All experiments were repeated in triplicate on TADF from each subject (i.e., 12 PT and 6 NT) and all data are presented as the average of three independent experiments.
Cell Proliferation Assay
The CellTiter-96 kit (Promega, Inc., Madison, WI, USA) was used for cell proliferation assay in the presence of PTX. One flat bottom 96-well cell culture plate was used for each assay. The plate was divided into 12 rows and each row was used for a set concentration of PTX. Each well in the same row received 50 μL of serum-free DMEM with 1% bovine serum albumin (BSA) mixed with PTX 0 nM, 100 nM, 10 μM, or 100 μM. Thereafter, cells that had grown to 70% confluence were rinsed with phosphate buffered saline, trypsinized, and resuspended in serum-free DMEM supplemented with 1% BSA at 1 ×10 [5] cells per mL. A total 50 μL of the cell suspension was then transferred to each well so that each well contained ~5,000 cells in a final volume of 100 μL. The plate was incubated in a 37°C humidified incubator with 5% CO2 for 48 hours. After 48 hours, 20 μL of CellTiter 96 Aqueous were added to each well and the plate was incubated an additional 1.5 and 3 hours at 37°C in the humidified 5% CO2 incubator. Color development was recorded with a plate reader (Molecular Devices Corp., Sunnyvale, CA, USA) at 490 nm absorbance.
Elastogenesis Assay and Immunofluorescence Staining
Protoelastin proteins are secreted out of the cell and assembled into elastin fibers by the actions of the proteins fibulin5 (aka DANCE) and lysyl oxidase-like 1 (LOXL). This biological process is termed elastogenesis. To study elastogenesis in vitro, TADF were seeded into eight-well plated glass at 40–60% confluence in DMEM with 10% FBS. At the time of experimental intervention cells were starved of FBS for 12 hours and subsequently treated with either 1 ng/mL TGF-β1 or PTX at 0, 0.01, 10, and 100 uM followed 6 hours later by 1 ng/mL TGF-β1 treatment. After treatment cells were maintained in 5% FBS DMEM for 2 weeks, the TADF were fixed with ice-cold methanol for 8 minutes, permeabilized with 0.05% Triton X-100 for 5 minutes, and blocked with 5% normal horse serum in PBS for 1 hour at room temperature. The cells were then incubated with Goat anti-elastin antibody and rabbit anti-DANCE antibody for 1 hour at room temperature. After washing with PBS three times, the cells were incubated with FITC-conjugated donkey anti-goat antibody for 1 hour at room temperature. After three washes with PBS, the cells were incubated with DexRed-conjugated Goat anti-rabbit antibody for 1 hour at room temperature. After three washes with PBS, the cells were stained with 4′,6-diamidino-2-phenylindole (for nuclear staining) for 5 minutes, examined under a fluorescence microscope, and photographed.
Quantification of Collagen I
Cells were seeded at 5 × 10 [4] cells per well in 1 mL of DMEM with 10% FBS in 12-well culture plates. In 48–72 hours, the cells reached 80% confluence and were then treated with 1 ng/mL TGF-β1 only or followed PTX at 10 uM, or PTX at 10 uM for 6 hours followed by 1 ng/mL TGF-β1. The culture media were harvested 24 hours and assayed with the collagen I enzyme-linked immunosorbent assay kit (Chondrex, Redmond, WA, USA) according to the protocol from the manufacturer. All assays were performed in triplicate in each experiment and all data presented under results are the average of three independent experiments.
Statistics
Statistical analysis was performed according to the Primer of Biostatistics, 3rd edition (Glantz SA, McGraw-Hill, Inc, New York, NY, USA). Data are expressed as means ± standard deviation. Analysis of variance on data from all groups was used to determine the P value. If the P value was less than 0.05, multiple comparisons of data from paired groups were performed with Bonferroni correction. All calculations were performed using Prism GraphPad v 4.0 (GraphPad Software, La Jolla, CA, USA). Statistical significance was set at P ≤ 0.05.
Results
PTX Inhibits Cell Proliferation at Doses of 100 Micromolar
Proliferation of both PT and NT TADF was slightly but significantly inhibited by PTX at doses of 100 μM PTX; at lower doses of PTX this inhibition was not statistically significant.
TGF-β1 Promotes Elastogenesis in TADF
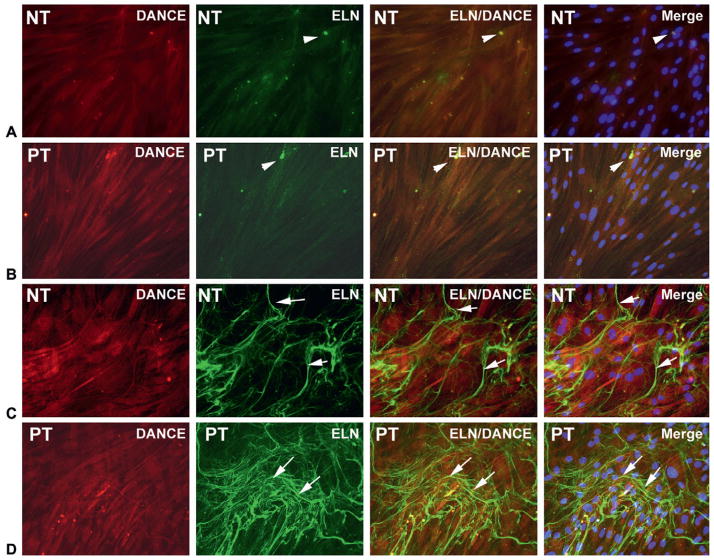
TADF did not form elastin fibers in vitro without exposure to TGF-β1, although small quantities of tropoelastin were identified (Figure 1A, B). After treatment with TGF-β1 at 1 ng/mL for 2 weeks in 5% FBS DMEM medium, TADF formed complex elastin fibers as determined by immunohistochemical staining for DANCE and elastin. A trend towards more robust TGF-β1 mediated elastogenesis was noted in PT relative to NT cells but this difference was not quantitated, and therefore cannot be definitively concluded (Figure 1C, D).
Figure 1.
Tunica albuginea-derived fibroblast cells were treated with vehicle or transforming growth factor-β1 (TGF-β1; 1 ng/mL) in Dulbecco’s modified Eagle medium with 5% fetal bovine serum for 14 days. In the native status (vehicle treated; A, B), there were no mature elastic fibers formed although small quantities of tropoelastin were identified (arrowheads). After treatment with TGF-β1, elastogenesis was activated. Many matured elastic fibers (arrows) were formed in the extracellular matrix (green) (C, D). Elastin fiber formation was more robust in PT cells.
Pretreatment with PTX Attenuates TGF-β1-Mediated Elastogenesis in TADF
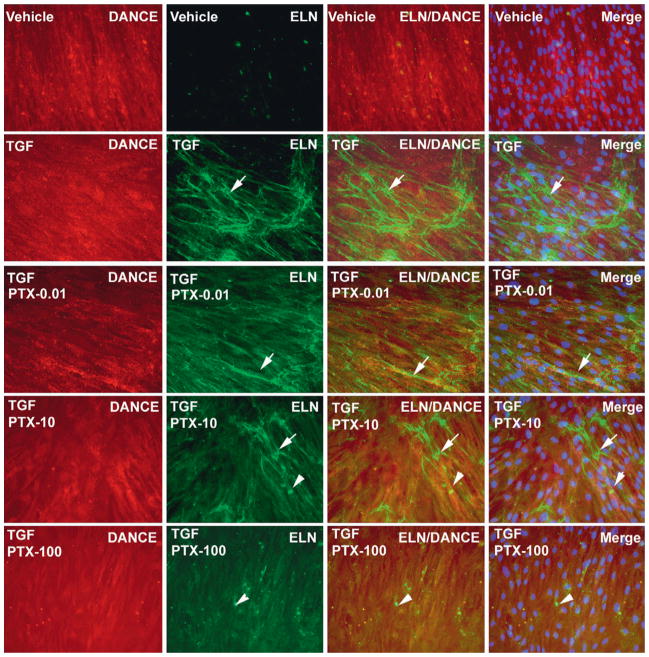
Pretreatment with PTX 6 hours prior to exposure to TGF-β1 inhibited elastogenesis in TADF in a dose-dependent fashion in PT cells (Figure 2). At 100 μM, PTX completely attenuated elastogenesis in both NT and PT cells (Figure 3). PTX alone had no impact on elastogenesis, and PTX given 6 hours after TGF-β1 did not inhibit elastogenesis to a significant extent (data not shown).
Figure 2.
Pentoxifylline (PTX) attenuates elastogenesis activated by transforming growth factor-β1 (TGF-β1) in tunica albuginea-derived fibroblast. PT TADF cells were pretreated with 0, 0.01, 10, and 100 uM PTX for 6 hours followed by 1 ng/mL TGF-β1 for 14 days in Dulbecco’s modified Eagle medium with 5% fetal bovine serum. Inhibition of elastogenesis showed a dose–response increase with progressively higher levels of PTX in PT cells; a PTX dose–response relationship was not clearly evident in normal tunica cells (arrows indicate elastic fibers, arrowhead indicates tropoelastin. ×200).
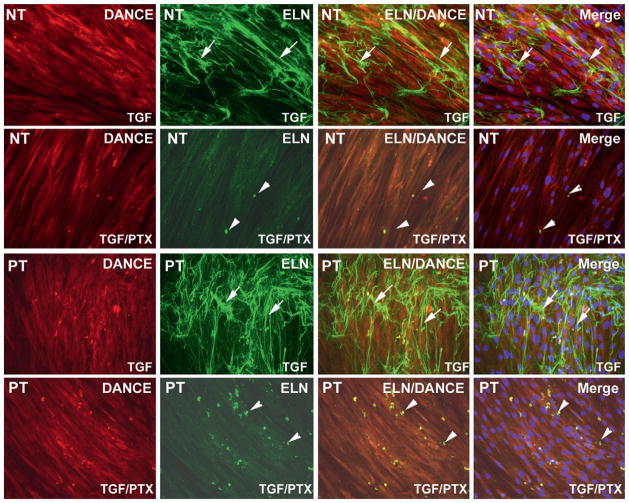
Figure 3.
Pentoxifylline (PTX) attenuates elastogenesis activated by transforming growth factor-β1 (TGF-β1) in normal tunica (NT) and PT cells. (A) PT tunica albuginea-derived fibroblast cells were treated with TGF-β1 (1 ng/mL) or combination TGF-β1 (1 ng/mL) and PTX (100 uM). PTX attenuated elastogenesis in both PT and NT cells.
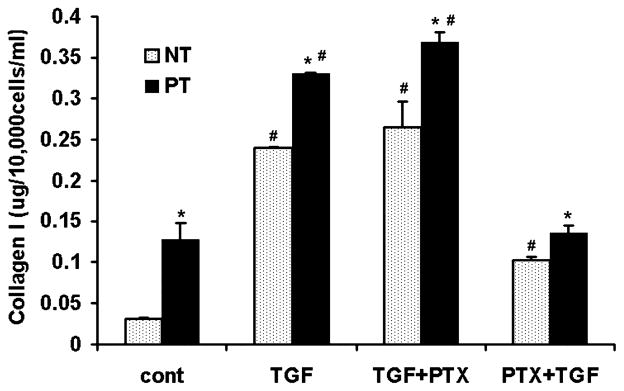
PTX Attenuates TGF-β1-Mediated Collagen Production in TADF
Expression of collagen I was significantly higher in untreated (control) PT vs. untreated NT cells. A significant decline in the production of Collagen I was detected in TGF-β1 treated TADF which received PTX pretreatment compared with cells that received TGF-β1 alone and TGF-β1 followed by PTX (Figure 4). Interestingly, NT cells that were treated with PTX followed by TGF-β1 expressed collagen in significantly higher amounts than untreated control NT cells but lower amounts than what was observed in the other two treatment arms; in contrast, PT cells that were pretreated with PTX followed by TGF-β1 expressed collagen at levels similar to completely untreated PT cells (Figure 4). Cells that received PTX after TGF-β1 had a significant difference in collagen expression relative to cells that were treated with TGF-β1 alone; this applied to both NT and PT cells.
Figure 4.
Pentoxifylline (PTX) decreases collagen I in tunica albuginea-derived fibroblast. Normal tunica (NT) and PT were treated with transforming growth factor-β1 (TGF-β1), TGF-β followed by PTX, and PTX followed by TGF-β1. Expression of collagen I was higher in PT relative to NT in the untreated control group (*P < 0.05). TGF-β1 significantly increased collagen I in NT and PT cells from 0.169 3 0.02 and 0.703 3 0.1 ug/mL to 1.342 3 0.01 and 1.8525 3 0.05 ug/mL, respectively (#P < 0.05 vs. controls). This effect was not observed in cells pretreated with PTX prior to TGF-β1 (0.569 3 0.02 and 0.75 3 0.05 ug/mL in NT and PT, respectively, P < 0.05 vs. TGF-β1 treated groups) although collagen I production in these NT cells remained significantly higher than what was observed in NT controls (#P < 0.05 vs. control cells). PTX following TGF-β1 treatment did not affect collagen I increase relative to TGF-β1 alone (P > 0.05; *P < 0.05 vs. NT cells, #P < 0.05 vs. control cells [PT and NT]).
Discussion
TGF-β1 is a regulator of normal biological functions including cell proliferation, apoptosis, differentiation, adhesion, and mobility [23]. TGF-β1 also contributes to the pathogenesis and progression of many human diseases, particularly those involving fibrosis [24,25]; PD has been intimately linked with TGF-β1 [6,26] and TGF-β1 injection has been demonstrated to produce fragmentation of penile elastic fibers and thickening of collagen in rat models [27].
Our study is novel relative to prior in vitro work with PD tissues in that we have investigated elastin metabolism in cultured TADF and the impact of PTX in this model system. This differs from other important work that has been done in PD cell cultures [28–30]. As would be expected, treatment with TGF-β1 stimulated an increase in collagen I production in all TADF. Interestingly, baseline production of collagen I was significantly higher in PT relative to NT cells, suggesting some intrinsic difference in the collagen-producing properties of these cells. The upregulation of collagen in our study is in line with prior reports in PD and is supportive of our model system as a proxy for studies of PD.
Although collagen findings are of some interest for validation of the model system, the principal novel finding of our study pertains to elastin metabolism. Upregulation of elastin production in inflammatory conditions has been previously reported [31,32]. Based on antibody studies, Stewart et al. suggested that elastin production and breakdown is upregulated in clinical PD [12]. However, other reports have suggested that elastin metabolism is down-regulated in PD plaques. Iacono et al. reported a significant decline in the number of elastin fibers found in tunica specimens from men with PD compared with controls; furthermore, men with erectile dysfunction (ED) and PD had significantly fewer elastin fibers than men with PD alone [1]. Costa et al. reported on stereological density of collagen and elastin in a series of plaque and corporal specimens in 7 men with PD compared with five men with no clinical evidence of PD. It was determined that the elastin content in both the plaque and the corporal tissue was significantly lower than what was observed in specimens from men without PD [33]. Similarly, in a study of 64 men Akkus et al. reported significantly lower elastin content in men with ED and/or PD relative to men without either condition; the elastin that was present tended to be densely packed and abnormal [13]. Loss of elasticity is consistent on a conceptual level with fibrotic conditions and therefore our determination of increases in elastin production with TGF-β1 is counterintuitive.
In our own examination of more than 20 plaques excised during surgery, the distribution and amount of elastic fibers has varied even within the same plaque. In the center of a mature plaque elastic fibers are scanty because of the dominance of the packed collagen fibers. In the peripheral part of the plaque, the elastic fibers content can be abundant but tend to be variable in length, width and arrangement (unpublished data). Therefore, based on our findings, we believe that the apparent total volume of elastic fibers can be increased or decreased in PD depending on the stage of the disease and the location from which histological samples are taken; this may explain the prior report by Akkus et al. in which dense buncles of elastin were observed but overall elastin content was thought to be diminished. It may be conjectured that many of these elastin fibers produced in clinical PD are phenotypically abnormal and not truly elastic, perhaps more similar to a “rubber ball” than a “rubber band.” In this situation elastin fibers may contribute to the PD phenotype rather than oppose it; ergo, upregulation of elastin production would tend to worsen clinical PD. It may also be the case that elastogenesis effects of the disease process(es) leading to the clinical condition of PD are overwhelmed by collagen effects, rendering elastin metabolism perturbations a moot point. These hypotheses are at this time entirely speculation; further histochemical, functional tissue, and in vivo studies using the variety of experimental models currently available will be required to ascertain whether they have any validity and to either refute or confirm prior published reports [34].
PTX, a non-specific phosphodiesterase inhibitor, has been used in a variety of clinical inflammatory and fibrotic conditions [14–17]. Oral bioavailablity of PTX is about 20–30% and the serum half-life of PTX is about 1 hour; to compensate for this a 400-mg extended release formulation taken three times daily is the standard regimen for use of this drug. At the conclusion of a 9 day study of routine dose PTX at 400 mg three times daily, peak serum concentration was 248 ng/mL with AUC 922 ± 512 ng/mL*hour; with a molecular weight of PTX of 278.3 g/Mole, this translates to approximate peak concentration of 0.89 uM and AUC of 3.3 uM/hour [35]. We are not aware of any published data concerning soft tissue concentration of PTX in human subjects after treatment. Assuming soft tissue levels of PTX are similar to serum levels in vivo, these concentrations are in the mid-range of doses utilized for this study. However, PTX is metabolized into at least seven active metabolites in vivo, and therefore serum concentrations of PTX alone may not be reflective of the level of biological activity of the drug in vivo [36].
In this study, PTX had a modest and likely clinically insignificant inhibitory effect on cell proliferation at high doses. PTX also had inhibitory effects on both elastogenesis and collagen fiber deposition. However, these effects occurred only when cells were incubated with PTX prior to exposure to TGF-β1. It is logical to speculate therefore that PTX will have a modest effect in ameliorating existing plaque tissue but may have an important role in PD by inhibiting the TGF-β1-mediated processes leading to further plaque formation. As PD is a progressive and chronic disease, interruption of the cascade of events leading to deposition of extracellular matrix would likely be of clinical utility when administered during the active phase of the disease. These findings suggest that PTX may be useful in the management of early phase PD or in cases where there is a concern about possible development of PD (i.e., penile fracture or other penile trauma).
It is particularly intriguing that PTX was able to return collagen production to what was seen in untreated control TADF in PT but not in NT. One possible explanation for this finding is that TGF-β1 induced a transformation in NT that made them similar to PT cells, with similar levels of collagen production that could not be modulated by PTX treatment; indeed, there was very little difference in collagen production between NT and PT treated with PTX followed by TGF-β1. In this scenario, PTX might play an inhibitory role in collagen production but cannot completely restore collagen metabolism to what is observed in healthy (i.e., NT), untreated TADF.
Limitations of this study include the in vitro study design; the behavior of cultured monolayers of fibroblasts in vitro may not be representative of what occurs in the more complex in vivo environment. Furthermore, we did not assess collagen III metabolism, which has been shown to play a role in PD in vivo. Without precise quantification of elastin fiber content it is difficult to definitively claim that elastin fiber content was higher in TGF-β1 treated cells. The mechanism by which PTX mediates elastin and collagen metabolism is the subject of another investigation that will be published separately; in this related study elastin mRNA and protein content are also quantified, lending support to our histological findings from this study. PTX treatment had to be applied prior to the application of TGF-β1 for maximal efficacy. Patients with PD typically present after disease is already manifest. This finding begs the question of whether or not application of PTX in a patient with existing PD could produce clinically meaningful results. However, as the inflammatory injury of PD is thought to be progressive and likely involves autocrine factors, we believe that application of PTX may have utility in disrupting progression of active phase PD lesions. PTX may also be useful when given before the development of palpable plaque in a patient at high risk of developing PD, i.e., after penile injury.
Conclusions
Pretreatment of TADF with PTX attenuates the TGF-β1 mediated increase in elastogenesis and collagen deposition, suggesting that PTX may have utility in the management of PD. The precise mechanisms by which PTX attenuates TGF-β1 mediated elastogenesis and collagen deposition remain unclear. Further studies are required to elucidate the molecular mechanisms underlying these observations; additionally, randomized, and controlled clinical studies in men with PD should be undertaken to accurately determine the clinical utility of PTX in the management of this troublesome and controversial condition.
Acknowledgments
This research was funded in part by the United States National Institute of Health (Grants DK64538, DK045370, DK069655).
Footnotes
Conflict of Interest: Alan W. Shindel is an informal consultant for Boeheringer-Ingelheim, Tom F. Lue has the following relationships: Consultant: Pfizer, Lilly, Bayer, Medtronic, Auxillium. Research Grant: American Medical Systems, Board member: Genix.
Statement of Authorship
Category 1
-
Conception and DesignGuiting Lin; Tom F. Lue; Ching-Shwun Lin
-
Acquisition of DataLia Banie; Hongxiu Ning; Yun-Ching Huang; Gang Liu; Guiting Lin
-
Analysis and Interpretation of DataGuiting Lin; Tom F. Lue; Ching-Shwun Lin; Alan W. Shindel
Category 2
-
Drafting the ArticleAlan W. Shindel
-
Revising It for Intellectual ContentGuiting Lin; Ching-Shwun Lin; Tom F. Lue; Alan W. Shindel
Category 3
-
Final Approval of the Completed ArticleGuiting Lin; Tom F. Lue; Ching-Shwun Lin; Alan W. Shindel
References
- 1.Iacono F, Barra S, De Rosa G, Boscaino A, Lotti T. Microstructural disorders of tunica albuginea in patients affected by Peyronie’s disease with or without erection dysfunction. J Urol. 1993;150:1806–9. doi: 10.1016/s0022-5347(17)35901-3. [DOI] [PubMed] [Google Scholar]
- 2.Bella AJ, Perelman MA, Brant WO, Lue TF. Peyronie’s disease (CME) J Sex Med. 2007;4:1527–38. doi: 10.1111/j.1743-6109.2007.00614.x. [DOI] [PubMed] [Google Scholar]
- 3.Gonzalez-Cadavid NF. Mechanisms of penile fibrosis. J Sex Med. 2009;6(suppl 3):353–62. doi: 10.1111/j.1743-6109.2008.01195.x. [DOI] [PubMed] [Google Scholar]
- 4.De Young LX, Bella AJ, O’Gorman DB, Gan BS, Lim KB, Brock GB. Protein biomarker analysis of primary Peyronie’s disease cells. J Sex Med. 2010;7:99–106. doi: 10.1111/j.1743-6109.2009.01556.x. [DOI] [PubMed] [Google Scholar]
- 5.Pohlers D, Brenmoehl J, Loffler I, Müller CK, Leipner C, Schultze-Mosgau S, Stallmach A, Kinne RW, Wolf G. TGF-beta and fibrosis in different organs—molecular pathway imprints. Biochim Biophys Acta. 2009;1792:746–56. doi: 10.1016/j.bbadis.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 6.El-Sakka AI, Hassoba HM, Chui RM, Bhatnagar RS, Dahiya R, Lue TF. An animal model of Peyronie’s-like condition associated with an increase of transforming growth factor beta mRNA and protein expression. J Urol. 1997;158:2284–90. doi: 10.1016/s0022-5347(01)68236-3. [DOI] [PubMed] [Google Scholar]
- 7.El-Sakka AI, Hassoba HM, Pillarisetty RJ, Dahiya R, Lue TF. Peyronie’s disease is associated with an increase in transforming growth factor-beta protein expression. J Urol. 1997;158:1391–4. [PubMed] [Google Scholar]
- 8.Moreland RB, Traish A, McMillin MA, Smith B, Goldstein I, Saenz de Tejada I. PGE1 suppresses the induction of collagen synthesis by transforming growth factor-beta 1 in human corpus cavernosum smooth muscle. J Urol. 1995;153:826–34. [PubMed] [Google Scholar]
- 9.Cutroneo KRTG. F-beta-induced fibrosis and SMAD signaling: Oligo decoys as natural therapeutics for inhibition of tissue fibrosis and scarring. Wound Repair Regen. 2007;15(suppl 1):S54–60. doi: 10.1111/j.1524-475X.2007.00226.x. [DOI] [PubMed] [Google Scholar]
- 10.Kuang PP, Zhang XH, Rich CB, Foster JA, Subramanian M, Goldstein RH. Activation of elastin transcription by transforming growth factor-beta in human lung fibroblasts. Am J Physiol Lung Cell Mol Physiol. 2007;292:L944–52. doi: 10.1152/ajplung.00184.2006. [DOI] [PubMed] [Google Scholar]
- 11.Wagenseil JE, Mecham RP. New insights into elastic fiber assembly. Birth Defects Res C Embryo Today. 2007;81:229–40. doi: 10.1002/bdrc.20111. [DOI] [PubMed] [Google Scholar]
- 12.Stewart S, Malto M, Sandberg L, Colburn KK. Increased serum levels of anti-elastin antibodies in patients with Peyronie’s disease. J Urol. 1994;152:105–6. doi: 10.1016/s0022-5347(17)32828-8. [DOI] [PubMed] [Google Scholar]
- 13.Akkus E, Carrier S, Baba K, Hsu GL, Padma-Nathan H, Nunes L, Lue TF. Structural alterations in the tunica albuginea of the penis: Impact of Peyronie’s disease, ageing and impotence. Br J Urol. 1997;79:47–53. doi: 10.1046/j.1464-410x.1997.26511.x. [DOI] [PubMed] [Google Scholar]
- 14.Deree J, Martins J, de Campos T, Putnam JG, Loomis WH, Wolf P, Coimbra R. Pentoxifylline attenuates lung injury and modulates transcription factor activity in hemorrhagic shock. J Surg Res. 2007;143:99–108. doi: 10.1016/j.jss.2007.03.083. [DOI] [PubMed] [Google Scholar]
- 15.Hung KY, Huang JW, Chiang CK, Tsai TJ. Preservation of peritoneal morphology and function by pentoxifylline in a rat model of peritoneal dialysis: Molecular studies. Nephrol Dial Transplant. 2008;23:3831–40. doi: 10.1093/ndt/gfn369. [DOI] [PubMed] [Google Scholar]
- 16.Lin SL, Chen RH, Chen YM, Chiang WC, Lai CF, Wu KD, Tsai TJ. Pentoxifylline attenuates tubulointerstitial fibrosis by blocking Smad3/4-activated transcription and profibrogenic effects of connective tissue growth factor. J Am Soc Nephrol. 2005;16:2702–13. doi: 10.1681/ASN.2005040435. [DOI] [PubMed] [Google Scholar]
- 17.Ng YY, Chen YM, Tsai TJ, Lan XR, Yang WC, Lan HY. Pentoxifylline inhibits transforming growth factor-beta signaling and renal fibrosis in experimental crescentic glomerulone-phritis in rats. Am J Nephrol. 2009;29:43–53. doi: 10.1159/000150600. [DOI] [PubMed] [Google Scholar]
- 18.Valente EG, Vernet D, Ferrini MG, Qian A, Rajfer J, Gonzalez-Cadavid NF. l-arginine and phosphodiesterase (PDE) inhibitors counteract fibrosis in the Peyronie’s fibrotic plaque and related fibroblast cultures. Nitric Oxide. 2003;9:229–44. doi: 10.1016/j.niox.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 19.Brant WO, Dean RC, Lue TF. Treatment of Peyronie’s disease with oral pentoxyfylline. Nat Clin Pract Urol. 2006;3:111–5. doi: 10.1038/ncpuro0409. [DOI] [PubMed] [Google Scholar]
- 20.Lin GT, Wang Z, Liu BC, Lue TF, Lin CS. Identification of potential biomarkers of Peyronie’s disease. Asian J Androl. 2005;7:237–43. doi: 10.1038/aja.2005.45. [DOI] [PubMed] [Google Scholar]
- 21.Mulhall JP, Nicholson B, Pierpaoli S, Lubrano T, Shankey TV. Chromosomal instability is demonstrated by fibroblasts derived from the tunica of men with Peyronie’s disease. Int J Impot Res. 2004;16:288–93. doi: 10.1038/sj.ijir.3901170. [DOI] [PubMed] [Google Scholar]
- 22.Lin G, Chow S, Lin J, Wang G, Lue TF, Lin CS. Effect of cell passage and density on protein kinase G expression and activation in vascular smooth muscle cells. J Cell Biochem. 2004;92:104–12. doi: 10.1002/jcb.20043. [DOI] [PubMed] [Google Scholar]
- 23.ten Dijke P, Hill CS. New insights into TGF-beta-Smad signalling. Trends Biochem Sci. 2004;29:265–73. doi: 10.1016/j.tibs.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 24.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–84. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 25.Hirai M, Horiguchi M, Ohbayashi T, Kita T, Chien KR, Nakamura T, Latent TG. F-beta-binding protein 2 binds to DANCE/fibulin-5 and regulates elastic fiber assembly. EMBO J. 2007;26:3283–95. doi: 10.1038/sj.emboj.7601768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hassoba H, El-Sakka A, Lue T. Role of increased transforming growth factor beta protein expression in the pathogenesis of Peyronie’s disease. Egypt J Immunol. 2005;12:1–8. [PubMed] [Google Scholar]
- 27.Bivalacqua TJ, Diner EK, Novak TE, Vohra Y, Sikka SC, Champion HC, Kadowitz PJ, Hellstrom WJ. A rat model of Peyronie’s disease associated with a decrease in erectile activity and an increase in inducible nitric oxide synthase protein expression. J Urol. 2000;163:1992–8. [PubMed] [Google Scholar]
- 28.Mulhall JP, Anderson MS, Lubrano T, Shankey TV. Peyronie’s disease cell culture models: Phenotypic, genotypic and functional analyses. Int J Impot Res. 2002;14:397–405. doi: 10.1038/sj.ijir.3900874. [DOI] [PubMed] [Google Scholar]
- 29.Del Carlo M, Cole AA, Levine LA. Differential calcium independent regulation of matrix metalloproteinases and tissue inhibitors of matrix metalloproteinases by interleukin-1beta and transforming growth factor-beta in Peyronie’s plaque fibroblasts. J Urol. 2008;179:2447–55. doi: 10.1016/j.juro.2008.01.093. [DOI] [PubMed] [Google Scholar]
- 30.Szardening-Kirchner C, Konrad L, Hauck EW, Haag SM, Eickelberg O, Weidner W. Upregulation of mRNA expression of MCP-1 by TGF-beta1 in fibroblast cells from Peyronie’s disease. World J Urol. 2009;27:123–30. doi: 10.1007/s00345-008-0320-x. [DOI] [PubMed] [Google Scholar]
- 31.Hoff CR, Perkins DR, Davidson JM. Elastin gene expression is upregulated during pulmonary fibrosis. Connect Tissue Res. 1999;40:145–53. doi: 10.3109/03008209909029110. [DOI] [PubMed] [Google Scholar]
- 32.Cantor JO, Keller S, Mandl I, Turino GM. Increased synthesis of elastin in amiodarone-induced pulmonary fibrosis. J Lab Clin Med. 1987;109:480–5. [PubMed] [Google Scholar]
- 33.Costa WS, Rebello SB, Cardoso LE, Cavalcanti AG, Sampaio FJ. Stereological and biochemical analysis of muscular and connective tissue components in the penile corpus cavernosum adjacent to the fibrous plaque of Peyronie’s disease. BJU Int. 2009;103:212–6. doi: 10.1111/j.1464-410X.2008.08023.x. [DOI] [PubMed] [Google Scholar]
- 34.Gonzalez-Cadavid NF, Rajfer J. Experimental models of Peyronie’s disease. Implications for new therapies. J Sex Med. 2009;6:303–13. doi: 10.1111/j.1743-6109.2008.01104.x. [DOI] [PubMed] [Google Scholar]
- 35.Beermann B, Ings R, Mansby J, Chamberlain J, McDonald A. Kinetics of intravenous and oral pentoxifylline in healthy subjects. Clin Pharmacol Ther. 1985;37:25–8. doi: 10.1038/clpt.1985.6. [DOI] [PubMed] [Google Scholar]
- 36.Nicklasson M, Bjorkman S, Roth B, Jonsson M, Hoglund P. Stereoselective metabolism of pentoxifylline in vitro and in vivo in humans. Chirality. 2002;14:643–52. doi: 10.1002/chir.10121. [DOI] [PubMed] [Google Scholar]