Abstract
Context
Evidence on the health risks associated with short-term exposure to fine particles (particulate matter ≤2.5 μm in aerodynamic diameter [PM2.5]) is limited. Results from the new national monitoring network for PM2.5 make possible systematic research on health risks at national and regional scales.
Objectives
To estimate risks of cardiovascular and respiratory hospital admissions associated with short-term exposure to PM2.5 for Medicare enrollees and to explore heterogeneity of the variation of risks across regions.
Design, Setting, and Participants
A national database comprising daily time-series data daily for 1999 through 2002 on hospital admission rates (constructed from the Medicare National Claims History Files) for cardiovascular and respiratory outcomes and injuries, ambient PM2.5 levels, and temperature and dew-point temperature for 204 US urban counties (population >200 000) with 11.5 million Medicare enrollees (aged >65 years) living an average of 5.9 miles from a PM2.5 monitor.
Main Outcome Measures
Daily counts of county-wide hospital admissions for primary diagnosis of cerebrovascular, peripheral, and ischemic heart diseases, heart rhythm, heart failure, chronic obstructive pulmonary disease, and respiratory infection, and injuries as a control outcome.
Results
There was a short-term increase in hospital admission rates associated with PM2.5 for all of the health outcomes except injuries. The largest association was for heart failure, which had a 1.28% (95% confidence interval, 0.78%–1.78%) increase in risk per 10-μg/m3 increase in same-day PM2.5. Cardiovascular risks tended to be higher in counties located in the Eastern region of the United States, which included the Northeast, the Southeast, the Midwest, and the South.
Conclusion
Short-term exposure to PM2.5 increases the risk for hospital admission for cardiovascular and respiratory diseases.
Numerous epidemiological studies have shown associations of acute and chronic exposures to airborne particles with risk for adverse effects on morbidity and mortality.1,2 The recent evidence on adverse effects of particulate air pollution on public health has led to more stringent standards for levels of particulate matter in outdoor air in the United States and in other countries. In 1997, the US National Ambient Air Quality Standard for airborne particulate matter was revised, maintaining the previous indicator of particulate matter of less than or equal to 10 μm in aerodynamic diameter (PM10) and creating a new indicator for fine particulate matter of less than or equal to 2.5 μm in aerodynamic diameter (PM2.5).3 Following the implementation of the PM2.5 National Ambient Air Quality Standard, a nationwide monitoring system of this pollutant was implemented. Data on PM2.5 are now available for many parts of the United States starting from 1999 through the present.
Although the US Environmental Protection Agency (EPA) added a PM2.5 standard in 1997 based on available evidence that these small particles were particularly damaging, few epidemiological studies on this size range of particulate matter had been reported at that time. The EPA heavily weighted the few studies with available PM2.5 data when it considered the level that should be set for the standard.4 The EPA also considered the dosimetry of particles in the lung. Particles in the size range of PM2.5 have a much greater probability of reaching the small airways and the alveoli of the lung than do larger particles. The availability of the new monitoring network for PM2.5 allows epidemiological analyses at the national level on the health effects of fine particles.
The national data on PM2.5 concentrations were used to assess associations of short-term exposure to PM2.5 with risk for hospitalization regionally and by city among Medicare participants. We followed the model of the National Morbidity, Mortality and Air Pollution Study, which used PM10 data for time-series analyses.5–8 The Medicare cohort covers nearly all members of an elderly population considered to be vulnerable to air pollution; the size of this population allows for assessments of specific cardiac and respiratory diagnostic categories that have been associated with particulate air pollution.
METHODS
This analysis is based on daily counts of hospital admissions for 1999–2002 obtained from billing claims of Medicare enrollees. Because the Medicare data analyzed for this study did not involve individual identifiers, consent was not specifically obtained. This study was reviewed and exempted by the institutional review board at Johns Hopkins Bloomberg School of Public Health. Each billing claim contains the date of service, treatment, disease (International Classification of Diseases, Ninth Revision9 [ICD-9] codes), age, sex, self-reported race, and place of residence (ZIP code and county). The daily counts of each health event within each county were obtained by summing the number of hospital admissions for each of the diseases considered a primary diagnosis. To calculate hospitalization rates, we constructed a time series of the numbers of individuals at risk in each county for each day (defined as the number of individuals enrolled in Medicare on a given day).
Eight outcomes were considered based on the ICD-9 codes for 5 cardiovascular outcomes (heart failure [428], heart rhythm disturbances [426–427], cerebrovascular events [430–438], is-chemic heart disease [410–414, 429], peripheral vascular disease [440–448]), 2 respiratory outcomes (chronic obstructive pulmonary disease [COPD; 490–492], respiratory tract infections [464–466, 480–487]), and hospitalizations caused by injuries and other external causes (800–849). The county-wide daily hospitalization rates for each outcome for 1999–2002 appear in Table 1.
Table 1.
Percentage Change in Hospitalization Rate per 10-μg/m3 Increase in PM2.5 on Average Across 204 Counties
| Reason for Hospital Admission | Lag Day No.* | Daily Hospitalization Rates for 1999–2002, Median (IQR) per 100 000 Individuals | National Average Relative Rate, PE (95% PI)† | All Counties, PE (95% PI)‡ | ||
|---|---|---|---|---|---|---|
| All Medicare Enrollees (Aged >65 y) | Aged 65–74 y | Aged ≥75 y | ||||
| Injury | 0 | 4.1 (3.7 to 4.5) | −0.41 (−1.00 to 0.18) | 0.22 (−1.01 to 1.45) | −0.46 (−1.16 to 0.24) | 1.47 (0.33 to 2.48) |
| Cerebrovascular disease | 0 | 5.4 (4.8 to 6.0) | 0.81 (0.30 to 1.32) | 0.91 (0.01 to 1.82) | 0.80 (0.21 to 1.38) | 1.24 (0.35 to 2.05) |
| Peripheral vascular disease | 0 | 1.7 (1.5 to 1.9) | 0.86 (−0.06 to 1.79) | 1.21 (−0.26 to 2.67) | 0.86 (−0.39 to 2.11) | 2.11 (0.79 to 3.40) |
| Ischemic heart disease | 2 | 8.1 (7.1 to 9.4) | 0.44 (0.02 to 0.86) | 0.37 (−0.22 to 0.96) | 0.52 (−0.01 to 1.04) | 1.15 (0.40 to 1.77) |
| Heart rhythm | 0 | 3.8 (3.3 to 4.2) | 0.57 (−0.01 to 1.15) | 0.46 (−0.63 to 1.54) | 0.72 (0.02 to 1.42) | 1.05 (0.26 to 1.91) |
| Heart failure | 0 | 5.5 (4.7 to 6.6) | 1.28 (0.78 to 1.78) | 1.21 (0.35 to 2.07) | 1.36 (0.78 to 1.94) | 1.09 (0.42 to 1.78) |
| COPD | 0 | 2.6 (2.1 to 3.2) | 0.91 (0.18 to 1.64) | 0.42 (−0.64 to 1.48) | 1.47 (0.54 to 2.40) | 1.61 (0.56 to 2.66) |
| Respiratory tract infection | 2 | 5.4 (4.7 to 6.2) | 0.92 (0.41 to 1.43) | 0.93 (0.04 to 1.82) | 0.92 (0.32 to 1.53) | 1.39 (0.60 to 2.09) |
Abbreviations: COPD, chronic obstructive pulmonary disease; IQR, interquartile range; PE, point estimate; PI, posterior interval; PM2.5, particulate matter of less than or equal to 2.5 μm in aerodynamic diameter.
Results are reported for the lag at which the greatest effect of PM2.5 was estimated.
Percentage change in hospital admission rates per 10-μg/m3 increase in PM2.5 concentration.
The SD of the true relative rates among counties (heterogeneity).
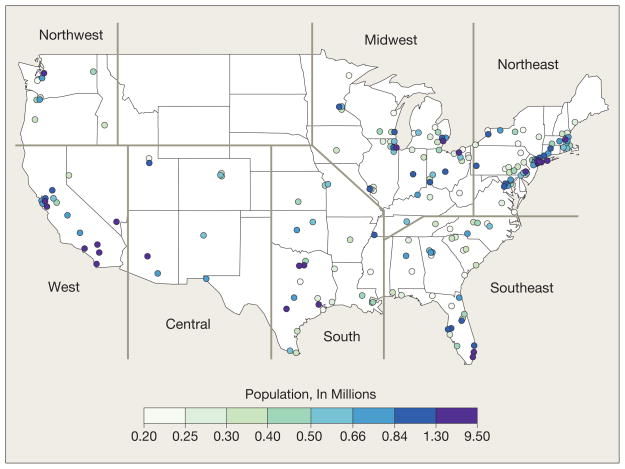
The study population includes 11.5 million Medicare enrollees residing an average of 5.9 miles from a PM2.5 monitor. The analysis was restricted to the 204 US counties with populations larger than 200 000. Of these 204 counties, 90 had daily PM2.5 data across the study period and the remaining counties had PM2.5 data collected once every 3 days for at least 1 full year. The locations of the 204 counties appear in Figure 1. The counties were clustered into 7 geographic regions by applying the K-means clustering algorithm to longitude and latitude for the counties.10,11
Figure 1.
US Counties With Populations Larger Than 200 000 Included in Analysis
The PM2.5 and ozone data were obtained from the EPA’s Aerometric Information Retrieval Service (now referred to as the Air Quality System database). Temperature and dew-point temperature data were gathered from the National Climatic Data Center on the Earth-Info CD database.12 To protect against consequences of outliers, we used a 10% trimmed mean to average across monitors after correcting for yearly averages for each monitor.
County names and location, air pollution data, weather data, county-specific estimates of health risk, and software developed to construct county-specific time-series data are available online (http://www.biostat.jhsph.edu/MCAPS). Billing claims of Medicare enrollees are not publicly available. Calculations were implemented using R statistical software version 2.2.0.13
We applied Bayesian 2-stage hierarchical models14–16 to estimate county-specific, region-specific, and national average associations between day-to-day variation of PM2.5 (at lags 0, 1, and 2 days) and day-to-day variation in the county-level hospital admission rates, accounting for weather, seasonality, and long-term trends. A lag of 0 days corresponds to the association between PM2.5 concentration on a given day and the risk of hospitalization on the same day. We also applied distributed lag models17–20 to the 90 counties with daily PM2.5 data available to estimate the relative rate (RR) of hospitalization associated with cumulative exposure over the current day and the 2 previous days. Significance is assessed by the posterior probability that the RR is larger than zero. Values greater than .95 are considered significant.
In the first stage, single lag and distributed lag overdispersed Poisson regression models21,22 were used for estimating county-specific RRs of hospital admissions associated with ambient levels of PM2.5. These county-specific models include as explanatory variables: (1) the logarithm of the daily number of individuals at risk; (2) indicator variables for the day of the week to allow for different baseline hospital admission rates for each day; (3) smooth functions of calendar time (natural cubic splines) with 8 degrees of freedom per year to adjust for seasonality and for other time-varying influences on admissions (eg, influenza epidemics and longer-term trends due to changes in medical practice patterns); and (4) smooth functions of temperature (6 degrees of freedom) and dew-point temperature (3 degrees of freedom) on the same day and of the 3 previous days’ temperature and dew-point temperature to control for the potential confounding effect of weather.
For the smooth functions of calendar time, we chose 8 degrees of freedom per year so that little information at the time scales of longer than 2 months would be retained in estimating the risks. For temperature, we chose 6 degrees of freedom so that the model has sufficient flexibility to take account of potential nonlinearity in the relationship of temperature with hospitalization.23
This modeling approach was developed for the National Morbidity, Mortality and Air Pollution Study analyses22,24 and applied to national databases for estimating short-term effects of PM10 and ozone on mortality.5,12 Statistical properties of this modeling approach and alternative modeling specifications for confounding adjustment are reported elsewhere.7,25
In the second stage, to produce a national average estimate of the short-term association between PM2.5 and hospital admissions, we used Bayesian hierarchical models14–16,26 to combine RRs across counties accounting for within-county statistical error and for between-county variability of the “true” RRs (also called heterogeneity). To produce regional estimates, we used the same 2-stage hierarchical model described above but separately within each of the 7 regions.
To explore effect modification of air pollution risks by location-specific characteristics, we fitted a weighted linear regression model with the dependent variable as the location-specific RR estimate and the independent variable as the location-specific characteristic. The observations were weighted inversely to the statistical variance of the location-specific estimate.
The county and regional averages of PM2.5 concentration, ozone concentration, and temperature for 2000 through 2002 were calculated as potential modifiers. A regional average was calculated by using all of the county-specific concentrations within the region.
Finally, the annual reduction in hospital admissions (H) attributable to a 10-μg/m3 reduction in the daily PM2.5 level for the 204 counties by cause-specific admissions were calculated. H is defined as
where β is the national RR estimate for a 1-μg/m3 increase in PM2.5, Δx is 10-μg/m3, and N is the number of hospital admissions across the 204 counties for 2002.
The sensitivity of key findings was examined with respect to the lag of exposure; degrees of freedom in the smooth functions of time; and degrees of freedom in the smooth functions of temperature and dew-point temperature.
RESULTS
More than 2 years of PM2.5 data were available for most of the 204 counties. The average of the county mean annual values for 1999–2002 was 13.4 μg/m3 (interquartile range [IQR], 11.3–15.2 μg/m3). There was substantial homogeneity of fine particulate matter concentrations across geographic areas. The median of pairwise correlations among PM2.5 monitors within the same county for 2000 was 0.91 (IQR, 0.81–0.95).
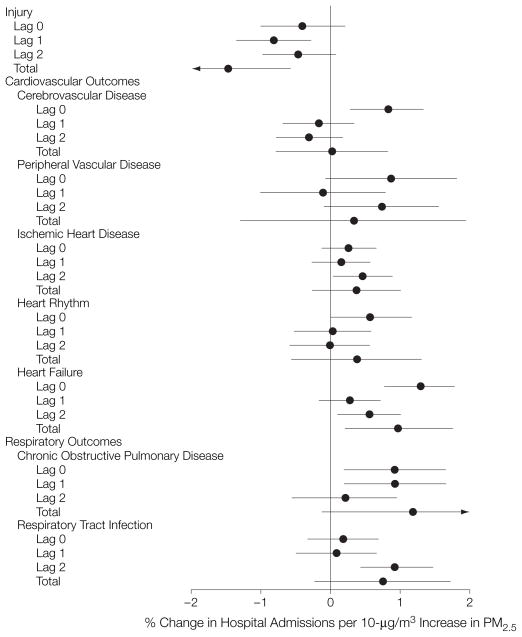
The point estimates and 95% posterior intervals (PIs) for the percentage increase in daily admission rates per 10-μg/m3 increase in PM2.5 concentration (national average RRs) for single lags of 0, 1, and 2 days and the distributed lag models for lags 0 through 2 for all disease outcomes (total) appear in Figure 2. The single lag model estimates the effect of exposure on 1 day only, lagged by 0, 1, or 2 days, while the total estimate from the distributed lag model summarizes the effect of 3 days of exposure (lag 0, 1, and 2 days). We found evidence of positive associations between day-to-day variation in PM2.5 concentration and hospital admissions for all outcomes, except injuries, for at least 1 exposure lag. The largest effect was found at lag 0 for all of the cardiovascular outcomes except ischemic heart disease, for which the largest effect was at lag 2. For respiratory outcomes, the largest effects occurred at lags 0 and 1 for COPD and at lag 2 for respiratory tract infections. Distributed lag estimates were statistically significant for heart failure. Compared with the single lag estimates, the wider 95% PIs for the distributed lag estimates reflect the restriction of the analysis to 90 of the 204 counties with daily data. The results for the single lag models were also stratified by age group at the lag with the greatest effect (Table 1). The national average RR estimates were larger for the oldest group for some outcomes including ischemic heart disease, heart rhythm disturbances, heart failure, and COPD.
Figure 2. Percentage Change in Hospitalization Rate by Cause per 10-μg/m3 Increase in PM2.5 on Average Across 204 US Counties.
Point estimates and 95% posterior intervals of the percentage change in admission rates per 10 μg/m3 (national average relative rates) for single lag (0,1, and 2 days) and distributed lag models for 0 to 2 days (total) for all outcomes. PM2.5 indicates particulate matter of less than or equal to 2.5 μm in aerodynamic diameter.
Several analyses were conducted as internal checks. Analyses for lag −1 were run to predict today’s outcome by using the next day’s pollution and for hospitalizations caused by injuries and other external causes. Positive associations were not found for injuries or for other external causes, which was expected. When lag −1 PM2.5 was used as the exposure indicator, positive associations also were not found. The main results were robust to the number of degrees of freedom used to adjust for temporal confounding, to the adjustment for weather, and to adjust for the prior distributions used for the analysis.
The point estimates and 95% PIs of the heterogeneity parameter, defined as the between-county SD of the “true” county-specific rates in relation to their mean, appear in Table 1. For example, the estimate of the heterogeneity parameter for COPD is 1.61. This value indicates that with a national average RR of 0.91% per 10-μg/m3 increase in PM2.5, 95% of the “true” county-specific RRs are within the interval of 0.91 to 1.96×1.61=−2.24% and 0.91×1.96×1.61=4.06%. To determine the strength of evidence supporting the null hypothesis of no heterogeneity, we calculated the posterior probability that the heterogeneity parameter is smaller than .05 (the Bayesian analogue of a P value) and this was found to be close to 0 for all outcomes.
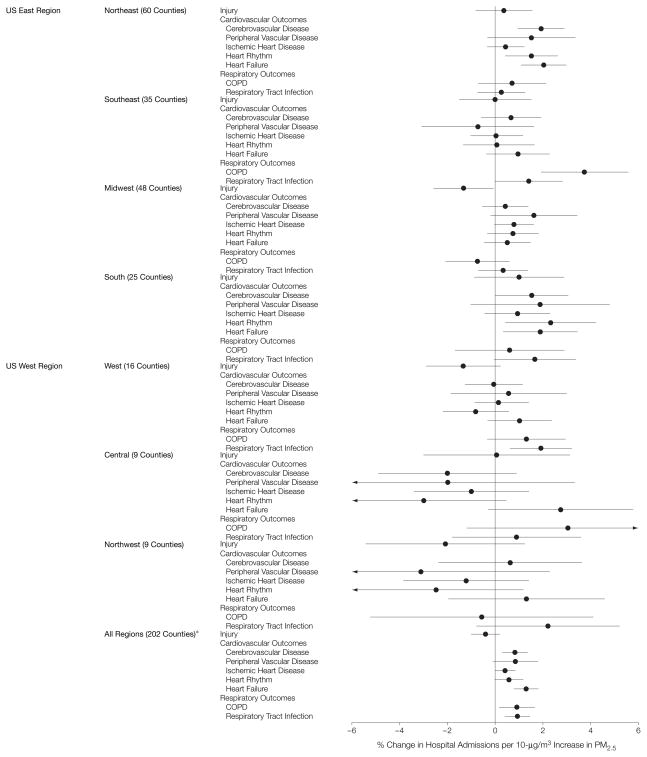
To determine whether there was significant variation of risks across the 7 geographic regions, the RR for each outcome was estimated separately within the regions, which excluded Honolulu, Hawaii, and Anchorage, Alaska. The point estimates and 95% PIs of the regional RRs for each outcome at the lag with the greatest estimated RR appear in Figure 3 and Table 1. For the 2 groups of outcomes (cardiovascular and respiratory), the estimated RRs have distinct regional patterns. For cardiovascular diseases, all estimates in the Midwestern, Northeastern, and Southern regions were positive, while estimates in the other regions were close to 0. Compared with cardiovascular diseases, there was greater consistency between the regions for respiratory diseases. However, there were larger effects in the Central, Southeastern, Southern, and Western regions than in the other regions.
Figure 3. Percentage Change in Hospitalization Rate by Region and Cause per 10-μg/m3 Increase in PM2.5 Within Each Region.
Point estimates and 95% posterior intervals of the percentage change in admission rates per 10 μg/m3 (regional relative rates). PM2.5 indicates particulate matter of less than or equal to 2.5 μm in aerodynamic diameter; COPD, chronic obstructive pulmonary disease. *Honolulu, Hawaii, and Anchorage, Alaska, were excluded.
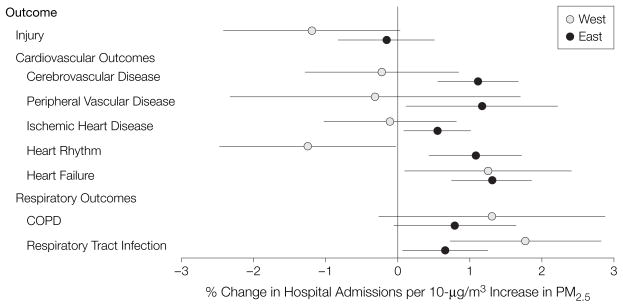
Regional differences were investigated by dividing the United States into an Eastern region (Northeast, Southeast, Midwest, and South) and a Western region (West, Central, and Northwest). The average effect estimates and 95% PIs of the RRs for each outcome and for the lags with the greatest estimated national average effects appear in Figure 4. There were 168 counties included in the Eastern region and 34 counties included in the Western region. Using analysis of variance, the differences in risk of hospitalization between the 2 regions were statistically significant for outcomes except for heart failure and COPD. All RR estimates for cardiovascular outcomes were positive in the US Eastern region but not in the US Western region. The RR estimates for respiratory tract infections were larger in the Western region than in the Eastern region.
Figure 4. Percentage Change in Hospitalization Rate by Cause per 10-μg/m3 Increase in PM2.5 for the US Eastern and Western Regions for all Outcomes.
Point estimates and 95% posterior intervals of the percentage change in admission rates per 10 μg/m3. PM2.5 indicates particulate matter of less than or equal to 2.5 μm in aerodynamic diameter; COPD, chronic obstructive pulmonary disease.
Effect modification of short-term effects of PM2.5 on hospital admission rates was investigated by using both county and regional averages of PM2.5 concentrations, temperature, and ozone. Both county and regional average temperature positively modified the association between PM2.5 and hospital admission rates for the 2 respiratory outcomes. For example, comparing 2 regions that differ by 1°C, there would be an estimated 18 additional hospital admissions per 10 000 individuals for COPD and 9 additional hospital admissions per 10 000 individuals for respiratory tract infections per 10-μg/m3 increase in PM2.5 in the warmer region. We did not find evidence of the effect modification by average concentrations of either PM2.5 or ozone.
The yearly hospital admissions attributable to a 10-μg/m3 reduction in the daily PM2.5 also were calculated (Table 2). For example, a 10-μg/m3 reduction in PM2.5 would reduce the number of hospitalizations for heart failure by 3156 for the 204 urban counties in 2002.
Table 2.
Annual Reduction in Admissions Attributable to a 10-μg/m3 Reduction in the Daily PM2.5 Level for the 204 Counties in 2002
| Cause-Specific Hospital Admissions | Annual No. of Admissions | Annual Reduction in Admissions (95% PI)* |
|---|---|---|
| Cerebrovascular disease | 226 641 | 1836 (680 to 2992) |
| Peripheral vascular disease | 70 061 | 602 (−42 to 1254) |
| Ischemic heart disease | 346 082 | 1523 (69 to 2976) |
| Heart rhythm | 169 627 | 967 (−17 to 1951) |
| Heart failure | 246 598 | 3156 (1923 to 4389) |
| COPD | 108 812 | 990 (196 to 1785) |
| Respiratory tract infection | 226 620 | 2085 (929 to 3241) |
Abbreviations: COPD, chronic obstructive pulmonary disease; PI, posterior interval; PM2.5, particulate matter of less than or equal to 2.5 μm in aerodynamic diameter.
Per 10-μg/m3 reduction in PM2.5.
COMMENT
The Medicare National Claims History Files were used in this study to estimate the short-term effects of PM2.5 on cause-specific hospitalization rates. Data obtained from national databases on health were combined with data on air pollution and weather.5,27 This is a replicable approach that can be applied periodically for air pollutants or other environmental factors as a component of a national health surveillance system to track adverse health effects. This approach also has the strength of analyzing the national data uniformly, avoiding the potential for publication bias that occurs when data from only 1 or several counties are analyzed and positive findings are selectively reported.27
In interpreting the findings, consideration needs to be given to the inherent limitations of the data analyzed and to the possibility that even the complex statistical models used are not adequate to eliminate all bias. Medicare data are collected for administrative purposes and diagnoses are known to be subject to some degree of misclassification28–30 and to vary geographically.31,32 The resulting misclassification and geographic variability would introduce a bias in daily time-series analyses only if patterns of diagnosis and coding were associated with level of PM2.5. We used only primary diagnosis, an approach that should reduce misclassification of outcomes. To investigate whether geographic differences in diagnosis rates could modify the risks, a second-stage analysis was performed using county-specific hospital admission rates (number of admissions per 100 000 individuals) as an independent variable and county-specific RR estimates as a dependent variable. This analysis did not find such evidence of effect modification by underlying diagnosis rates. While we relied on monitors cited for regulatory purposes, the average distance from the centroid of a ZIP code to the monitor was only 5.9 miles and PM2.5 levels tend to be uniform across such distances.
The modeling approach used in this study enabled extensive exploration of the sensitivity of the findings. At the first stage of the hierarchical model, we specified the same number of degrees of freedom in the smooth functions of time and temperature used to control for confounding for all the locations. This approach does not necessarily lead to a similar degree of control for confounding across counties, but it does give similar flexibility to the smooth functions, allowing their shape to vary across counties. An alternative is to allow a different number of degrees of freedom across counties, an approach used in multisite time-series studies in Europe.33–36 Recently we have compared these 2 modeling strategies and found that national estimates of PM10 risks were robust to the choice of method.19 We also have explored the sensitivity of the estimated RRs to different degrees of adjustment for weather and seasonality and found the results to be robust. Statistical challenges inherent to the adjustment for temporal confounding have been explored elsewhere.19,25,37
Overall, we found evidence of an association between recently measured PM2.5 concentrations and daily hospitalizations on a national scale. Our findings complement substantial evidence on particulate matter and hospitalization for respiratory or cardiovascular causes using exposure measures other than PM2.5 and the more limited evidence using PM2.5 specifically. While mechanisms underlying the adverse effects of particulate matter on the respiratory and cardiac systems remain a focus of research, the leading hypotheses emphasize inflammatory responses in the lung and release of cytokines with local and systemic consequences.1,38–40 In the lung, particulate matter may promote inflammation and thereby exacerbate underlying lung disease and reduce the efficacy of lung-defense mechanisms. Cardiovascular effects may reflect neurogenic and inflammatory processes.40 Experimental studies of atherosclerosis using genetically susceptible mice also suggest that particulate matter may accelerate the development of atherosclerosis41; parallel human findings also were found.42
Although many time-series studies have used PM10 as an exposure indicator, only a few studies have specifically assessed associations of PM2.5 with hospitalization or other morbidity measures.43 Lippmann et al44 and Ito et al45 used Medicare admission data for Detroit, Mich, for 1992–1994, along with size-fractionated particle concentration data from a nearby monitoring station in Windsor, Ontario. As reported by Ito et al,45 updated analyses of these data showed positive associations of PM2.5 for hospitalization for pneumonia, COPD, ischemic heart disease, and heart failure. In comparison with the present study, the reported risk estimates were several-fold higher. Moolgavkar46,47 used data for Los Angeles County, California, for 1987–1995 and found that PM2.5 was significantly associated with risk for hospital admission for cardiovascular disease in persons aged 65 years or older. Sheppard et al48,49 reported a positive association of PM2.5 with risk for hospital admission for asthma in Seattle, Wash, for 1987–2004, but elderly persons were excluded. Finally, Burnett et al50 assessed risk for hospitalization for cardiorespiratory diseases in relation to particulate air pollution over 3 summers in Toronto, Ontario. Positive associations were found in univariate models that were attenuated with consideration of gaseous pollutants in bivariate models.
There is much more literature on PM10 and risk for hospitalization, which generally shows positive associations.2,51 In most urban locations across the United States, PM2.5 accounts for at least half of the PM10 mass, and a scaling factor of 0.55 has been used to convert PM10 concentrations to PM2.5. With this assumption, our quantitative findings for PM2.5 are quite similar to those for both PM10 and for PM2.5 as recently summarized by the EPA.43 The comparability of the PM10 and PM2.5 estimates suggests that the effect of PM10 on hospital admissions largely reflects its PM2.5 component.
The sources of particles contributing to the observed risks need to be identified so that control strategies can be targeted efficiently. Because the source mix for PM2.5 varies across locations, we explored spatial variation of the effect of PM2.5 on risk for hospitalization. Strong evidence for spatial heterogeneity in the effect of PM2.5 on risk for hospitalization was found. The pattern and degree of heterogeneity tended to vary by outcome measure. Because the magnitude of the effects contrasted greatly in the comparisons between the 7 regions, counties were grouped into an Eastern region and a Western region. There are known differences in the composition of particles at this geographic scale, including a greater sulfate component in the East and a greater nitrate component in the West.2 There are also well-characterized differences in the mix of sources across these broad areas that may be relevant, including power generation and the smokestack industry in the East and a larger contribution from transportation sources in parts of the West.
With clear and continuing indication that inhaled particles affect public health adversely, the emphasis of research should shift toward the difficult issue of identifying those characteristics of particles that determine their toxicity.1 The EPA’s Speciation Trends Network, which is now providing extensive data on characteristics of PM2.5 at selected sites, offers a needed resource for this research.52
Under the Clean Air Act,53 the EPA is required to set a particulate matter National Ambient Air Quality Standard that protects public health with an “adequate margin of safety.” Our findings indicate an ongoing threat to the health of the elderly population from airborne particles and provide a rationale for setting a PM2.5 National Ambient Air Quality Standard that is as protective of their health as possible. Our national approach offers a method for continuing to search for the characteristics of particles that determine their toxicity.53
Acknowledgments
Funding/Support: Funding for Drs Dominici, McDermott, Zeger, and Samet was provided by the US Environmental Protection Agency (RD-83241701). Funding for Drs Dominici, Peng, and Zeger and Mr Pham was also provided by the National Institute for Environmental Health Sciences (ES012054-03) and by the National Institute of Environmental Health Sciences’ Center in Urban Environmental Health (P30 ES 03819). Funding for Dr Bell was provided by the Health Effects Institute, an organization jointly funded by the Environmental Protection Agency and automotive manufacturers through the Walter A. Rosenblith New Investigator Award (4720-RFA04-2/04-16).
Role of the Sponsor: The funding agencies/sponsors listed above had no involvement in the design and conduct of the study; in the collection, management, analysis, or interpretation of the data; or in the preparation, review, or approval of the manuscript.
We thank Charlotte Gerczak, MLA, for her editorial input and Keita Ebisu, MS, for his aid in collecting the particulate matter data. Neither Ms Gerczak nor Mr Ebisu received any financial compensation for their work.
Footnotes
Financial Disclosures: None reported.
Author Contributions: Drs Dominici, Peng, and McDermott and Mr Pham had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Dominici, Peng, Zeger, Samet.
Acquisition of data: Dominici, McDermott.
Analysis and interpretation of data: Dominici, Peng, Bell, Pham, McDermott, Zeger.
Drafting of the manuscript: Dominici, Peng, Pham, Samet.
Critical revision of the manuscript for important intellectual content: Dominici, Peng, Bell, McDermott, Zeger, Samet.
Statistical analysis: Dominici, Peng, Bell, Pham, McDermott, Zeger.
Obtained funding: Dominici, Bell, Samet.
Administrative, technical, or material support: Dominici.
Study supervision: Dominici.
Disclaimer: The research described in this article was funded wholly or in part by the US Environmental Protection Agency through a grant to Johns Hopkins University but it has not been subjected to the Environmental Protection Agency’s required peer and policy review and therefore does not necessarily reflect the views of the Environmental Protection Agency and no official endorsement should be inferred. The contents of this article do not necessarily reflect the views and policies of the Health Effects Institute.
References
- 1.National Research Council; Committee on Research Priorities for Airborne Particulate Matter. Research Priorities for Airborne Particulate Matter, IV: Continuing Research Progress. Washington, DC: National Academies Press; 2004. [Google Scholar]
- 2.National Center for Environmental Assessment. Air Quality Criteria for Particulate Matter. Research Triangle Park, NC: US Environmental Protection Agency; 2004. [Google Scholar]
- 3.US Environmental Protection Agency. National ambient air quality standards for particulate matter. [Accessed February 9, 2006.];Fed Regist. 1997 62:138. Available at: http://www.epa.gov/ttn/caaa/t1/fr_notices/pmnaaqs.pdf. [Google Scholar]
- 4.US Environmental Protection Agency; Office of Research and Development. Air Quality Criteria for Particulate Matter. Washington, DC: US Government Printing Office; 1996. [Google Scholar]
- 5.Samet JM, Dominici F, Curriero FC, Coursac I, Zeger SL. Fine particulate air pollution and mortality in 20 U.S. cities, 1987–1994. N Engl J Med. 2000;343:1742–1749. doi: 10.1056/NEJM200012143432401. [DOI] [PubMed] [Google Scholar]
- 6.Dominici F, Daniels M, Zeger SL, Samet JM. Air pollution and mortality. J Am Stat Assoc. 2002;97:100–111. [Google Scholar]
- 7.Dominici F, McDermott A, Daniels M, Zeger SL, Samet JM. Revised Analyses of Time-Series Studies of Air Pollution and Health: Mortality Among Residents of 90 Cities. Boston, Mass: Health Effects Institute; 2003. [Google Scholar]
- 8.Peng RD, Dominici F, Pastor-Barriuso R, Zeger SL, Samet JM. Seasonal analyses of air pollution and mortality in 100 US cities. Am J Epidemiol. 2005;161:585–594. doi: 10.1093/aje/kwi075. [DOI] [PubMed] [Google Scholar]
- 9.International Classification of Diseases, Ninth Revision (ICD-9) Geneva, Switzerland: World Health Organization; 1977. [Google Scholar]
- 10.MacQueen JB. Some Methods for Classification and Analysis of Multivariate Observations. Berkeley: University of California Press; 1967. [Google Scholar]
- 11.Hartigan JA. Clustering Algorithms. New York, NY: Wiley; 1975. [Google Scholar]
- 12.Bell ML, McDermott A, Zeger SL, Samet JM, Dominici F. Ozone and short-term mortality in 95 US urban communities, 1987–2000. JAMA. 2004;292:2372–2378. doi: 10.1001/jama.292.19.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2005. [Google Scholar]
- 14.Lindley DV, Smith AFM. Bayes estimates for the linear model. J R Stat Soc [Ser B] 1972;34:1–41. [Google Scholar]
- 15.Du Mouchel WH, Harris JE. Bayes methods for combining the results of cancer studies in humans and other species. J Am Stat Assoc. 1983;78:293–315. [Google Scholar]
- 16.Gelman A, Carlin JB, Stern HS, Rubin DB. Bayesian Data Analysis. 2. New York, NY: Chapman & Hall; 2003. [Google Scholar]
- 17.Almon S. The distributed lag between capital appropriations and expenditures. Econometrica. 1965;33:178–196. [Google Scholar]
- 18.Schwartz J. The distributed lag between air pollution and daily deaths. Epidemiology. 2000;11:320–326. doi: 10.1097/00001648-200005000-00016. [DOI] [PubMed] [Google Scholar]
- 19.Welty LJ, Zeger SL. Are the acute effects of particulate matter on mortality in the National Morbidity, Mortality, and Air Pollution Study the result of inadequate control for weather and season? Am J Epidemiol. 2005;162:80–88. doi: 10.1093/aje/kwi157. [DOI] [PubMed] [Google Scholar]
- 20.Zanobetti A, Wand M, Schwartz J. Generalized additive distributed lag models: quantifying mortality displacement. Biostatistics. 2000;1:279–292. doi: 10.1093/biostatistics/1.3.279. [DOI] [PubMed] [Google Scholar]
- 21.McCullagh P, Nelder JA. Generalized Linear Models. 2. New York, NY: Chapman & Hall; 1989. [Google Scholar]
- 22.Kelsall JE, Samet JM, Zeger SL, Xu J. Air pollution and mortality in Philadelphia, 1974–1988. Am J Epidemiol. 1997;146:750–762. doi: 10.1093/oxfordjournals.aje.a009351. [DOI] [PubMed] [Google Scholar]
- 23.Curriero FC, Heiner KS, Samet JM, et al. Temperature and mortality in eleven cities of the eastern United States. Am J Epidemiol. 2002;155:80–87. doi: 10.1093/aje/155.1.80. [DOI] [PubMed] [Google Scholar]
- 24.Dominici F, Samet J, Zeger SL. Combining evidence on air pollution and daily mortality from the largest 20 U.S. cities: a hierarchical modeling strategy. J R Stat Soc [Ser A] 2000;163:263–302. [Google Scholar]
- 25.Peng RD, Dominici F, Louis TA. Model choice in time series studies of air pollution and mortality. J R Stat Soc [Ser A] 2006;169(part 2):179–203. [Google Scholar]
- 26.Everson P, Morris C. Inference for multivariate normal hierarchical models. J R Stat Soc [Ser B] 2000;62:399–412. [Google Scholar]
- 27.Bell ML, Dominici F, Samet JM. A meta-analysis of time-series studies of ozone and mortality with comparison to the national morbidity, mortality, and air pollution study. Epidemiology. 2005;16:436–445. doi: 10.1097/01.ede.0000165817.40152.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Losina E, Barrett J, Baron JA, Katz JN. Accuracy of Medicare claims data for rheumatologic diagnoses in total hip replacement recipients. J Clin Epidemiol. 2003;56:515–519. doi: 10.1016/s0895-4356(03)00056-8. [DOI] [PubMed] [Google Scholar]
- 29.Fisher ES, Whaley FS, Krushat WM, et al. The accuracy of Medicare’s hospital claims data: progress has been made, but problems remain. Am J Public Health. 1992;82:243–248. doi: 10.2105/ajph.82.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kiyota Y, Schneeweiss S, Glynn RJ, et al. Accuracy of Medicare claims-based diagnosis of acute myocardial infarction. Am Heart J. 2004;148:99–104. doi: 10.1016/j.ahj.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 31.Baicker K, Chandra A, Skinner JS, Wennberg JE. Who you are and where you live. [Accessed February 3, 2006.];Health Aff (Millwood) 2004 23:VAR33–VAR44. doi: 10.1377/hlthaff.var.33. [DOI] [PubMed] [Google Scholar]
- 32.Havranek EP, Wolfe P, Masoudi FA, Rathore SS, Krumholz HM, Ordin DL. Provider and hospital characteristics associated with geographic variation in the evaluation and management of elderly patients with heart failure. Arch Intern Med. 2004;164:1186–1191. doi: 10.1001/archinte.164.11.1186. [DOI] [PubMed] [Google Scholar]
- 33.Samoli E, Analitis A, Touloumi G, et al. Estimating the exposure-response relationships between particulate matter and mortality within the APHEA multicity project. Environ Health Perspect. 2005;113:88–95. doi: 10.1289/ehp.7387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Touloumi G, Samoli E, Quenel P, et al. Short-term effects of air pollution on total and cardiovascular mortality. Epidemiology. 2005;16:49–57. doi: 10.1097/01.ede.0000142152.62400.13. [DOI] [PubMed] [Google Scholar]
- 35.Le Tertre A, Medina S, Samoli E, et al. Short-term effects of particulate air pollution on cardiovascular diseases in eight European cities. J Epidemiol Community Health. 2002;56:773–779. doi: 10.1136/jech.56.10.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Touloumi G, Katsouyanni K, Zmirou D, et al. Short-term effects of ambient oxidant exposure on mortality: a combined analysis within the APHEA project. Am J Epidemiol. 1997;146:177–185. doi: 10.1093/oxfordjournals.aje.a009249. [DOI] [PubMed] [Google Scholar]
- 37.Dominici F, McDermott A, Hastie TJ. Improved semi-parametric time series models of air pollution and mortality. J Am Stat Assoc. 2004;99:938–948. [Google Scholar]
- 38.Pope CA, III, Hansen ML, Long RW, et al. Ambient particulate air pollution, heart rate variability, and blood markers of inflammation in a panel of elderly subjects. Environ Health Perspect. 2004;112:339–345. doi: 10.1289/ehp.6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pope CA, III, Burnett RT, Thurston GD, et al. Cardiovascular mortality and long-term exposure to particulate air pollution. Circulation. 2004;109:71–77. doi: 10.1161/01.CIR.0000108927.80044.7F. [DOI] [PubMed] [Google Scholar]
- 40.Brook RD, Franklin B, Cascio W, et al. Air pollution and cardiovascular disease. Circulation. 2004;109:2655–2671. doi: 10.1161/01.CIR.0000128587.30041.C8. [DOI] [PubMed] [Google Scholar]
- 41.Sun Q, Wang A, Jin X, et al. Long-term air pollution exposure and acceleration of atherosclerosis and vascular inflammation in an animal model. JAMA. 2005;294:3003–3010. doi: 10.1001/jama.294.23.3003. [DOI] [PubMed] [Google Scholar]
- 42.Kunzli N, Jerrett M, Mack WJ, et al. Ambient air pollution and atherosclerosis in Los Angeles. Environ Health Perspect. 2005;113:201–206. doi: 10.1289/ehp.7523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clean Air Scientific Advisory Committee. Review of the National Ambient Air Quality Standards for Particulate Matter: Policy Assessment of Scientific and Technical Information. Research Triangle Park, NC: US Environmental Protection Agency; 2005. [Google Scholar]
- 44.Lippmann M, Ito K, Nadas A, Burnett RT. Association of particulate matter components with daily mortality and morbidity in urban populations. Res Rep Health Eff Inst. 2000;95:5–72. [PubMed] [Google Scholar]
- 45.Ito K, De Leon SF, Lippmann M. Associations between ozone and daily mortality: analysis and meta-analysis. Epidemiology. 2005;16:446–457. doi: 10.1097/01.ede.0000165821.90114.7f. [DOI] [PubMed] [Google Scholar]
- 46.Moolgavkar SH. Air pollution and hospital admissions for chronic obstructive pulmonary disease in three metropolitan areas in the United States. Inhal Toxicol. 2000;12(suppl 4):75–90. doi: 10.1080/089583700750019512. [DOI] [PubMed] [Google Scholar]
- 47.Moolgavkar SH. Air pollution and daily mortality in three U.S. counties. Environ Health Perspect. 2000;108:777–784. doi: 10.1289/ehp.00108777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sheppard L, Levy D, Norris G, Larson TV, Koenig JQ. Effects of ambient air pollution on nonelderly asthma hospital admissions in Seattle, Washington, 1987–1994. Epidemiology. 1999;10:23–30. [PubMed] [Google Scholar]
- 49.Sheppard L. Revised Analyses of the National Morbidity, Mortality, and Air Pollution Study, Part II. Boston, Mass: Health Effects Institute; 2003. Reanalysis of effects of ambient air pollution on nonelderly asthma hospital admissions in Seattle, WA, 1987–1994; pp. 227–230. [Google Scholar]
- 50.Burnett RT, Cakmak S, Brook JR, Krewski D. The role of particulate size and chemistry in the association between summertime ambient air pollution and hospitalization for cardiorespiratory diseases. Environ Health Perspect. 1997;105:614–620. doi: 10.1289/ehp.97105614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stieb DM, Judek S, Burnett RT. Meta-analysis of time-series studies of air pollution and mortality. J Air Waste Manag Assoc. 2003;53:258–261. doi: 10.1080/10473289.2003.10466149. [DOI] [PubMed] [Google Scholar]
- 52.Office of Air Quality Planning and Standards. Quality Assurance Project Plan: PM2.5 Speciation Trends Network Field Sampling. Research Triangle Park, NC: Environmental Protection Agency; 2000. [Google Scholar]
- 53. [Accessibility verified February 15, 2006.];US Environmental Protection Agency Clean Air Act (amended in 1990) Available at: http://www.epa.gov/oar/caa/contents.html.