Abstract
Studies on environmental exposures during pregnancy often have limited residential history (e.g., at delivery), potentially introducing exposure misclassification. We reviewed studies reporting residential mobility during pregnancy to summarize current evidence and discuss research implications. A meaningful quantitative combination of results (e.g., meta-analysis), was infeasible owing to variation in study designs. Fourteen studies were identified, of which half were from the US. Most were case-control studies examining birth defects. Residential history was typically assessed after delivery. Overall mobility rates were 9–32% and highest in the second trimester. Mobility generally declined with age, parity, and socioeconomic status, although not consistently. Married mothers moved less frequently. Findings were dissimilar by race, smoking, or alcohol use. On the basis of the few studies reporting distance moved, most distances were short (median often <10 km). Results indicate potential misclassification for environmental exposures estimated with incomplete residential information. This misclassification could be associated with potential confounders, such as socioeconomics, thereby affecting risk estimates. As most moves were short distances, exposures that are homogenous within a community may be well estimated with limited residential data. Future research should consider the implications of residential mobility during pregnancy in relation to the exposure’s spatial heterogeneity and factors associated with the likelihood of moving and distance moved.
Keywords: residential mobility, pregnancy, air pollution
INTRODUCTION
Environmental conditions in the area of mother’s residence during pregnancy have been investigated in relation to risk of adverse pregnancy and childhood health outcomes. These include whether mother’s exposure to ambient air pollutants during pregnancy affects fetal growth and risk of birth defects and preterm delivery1–3 and whether proximity to traffic is associated with risk of allergic disorders4 and pregnancy outcomes.5,6 Drinking water quality at mother’s residence has been investigated for risk of birth defects,7 spontaneous abortion,8 and fetal growth impairment.9,10 Residence has been used to asses exposure to pesticides in relation to growth restriction and childhood cancer,11,12 birth defects,13 and autism spectrum disorders.14 Other studies examined proximity during pregnancy to mobile phone stations in a study of childhood cancer,15 nuclear power plants in relation to congenital defects,16 and hazardous waste sites for risk of fetal death.17 To assess environmental exposures, these and similar studies require data or assumptions regarding mother’s location throughout pregnancy.
A key challenge in research of how environmental exposures affect pregnancy and early childhood outcomes is lack of information on residential mobility during pregnancy. Some studies have specific information (e.g., exact address at various time periods over pregnancy) through cohort follow-up or distinctive national datasets. The Norway Statistics database, for example, can be linked to birth certificate registry data to identify residences throughout pregnancy through the mother’s personal identification number.18 More often, researchers rely on approximate measures, such as residence at delivery. Even in cohort studies, full residential history may be unknown, with residences recorded at specific time points (e.g., medical visits). The assessment of exposure to environmental conditions is subject to potential misclassification; however, studies of exposure over longer timeframes, such as pregnancy, have the additional challenge of residential mobility as subjects may not have lived at the same address throughout pregnancy. This misclassification could introduce random variation, reducing the power to detect associations, or could be differential if some segments of the population are more likely to move than others.19
The distance moved will affect the degree of exposure misclassification, as a local move within the same community may not alter exposure estimates depending on the environmental exposure of interest. The impact of residential mobility is also a function of the spatial heterogeneity of the exposure of concern, as shorter moves may introduce misclassification for pollutants with large spatial variation.20 For studies assessing exposure based on the residence at delivery, if the probability of moving differs by timing in pregnancy, exposures by trimester may be differentially affected, with larger misclassification in the first trimester compared with the third trimester.
Residential mobility has been studied in a variety of settings including the general adult US population,21 persons with mental illness,22 children with leukemia,23 and children in general.24 As the probability of moving likely differs for pregnant versus non-pregnant women, general population studies may not be applicable. Research on moves by couples in the Netherlands found that persons were more likely to move a short distance (<40 km) during pregnancy than those without children.25 Differential exposure misclassification may occur, if residential mobility patterns vary by risk of outcome or study subject characteristics, such as socioeconomic status (SES). For example, a study of changes in residence for a mentally ill cohort found links between mobility and several factors including substance abuse and marital status.22
A limited number of studies examined residential mobility of pregnant women. Here we review these studies and synthesize their evidence with respect to the frequency, distance, and timing of moves during pregnancy. We summarize findings regarding the relationship between mobility and population characteristics. Implications for studies of environmental exposure during pregnancy are discussed.
MATERIALS AND METHODS
We identified research on residential mobility during pregnancy using a medical literature database, PubMed, and an academic literature database, Scopus, for studies indexed through August 2011. Searches were conducted for articles with each of the following in the title and/or abstract: (1) “pregnancy,” “pregnant,” “prenatal,” or “maternal;” (2) “mobility;” and (3) “residence,” “residences,” or “residential.” Articles were limited to those published in English. Only peer-reviewed research was included. We also examined references of identified articles as a source of additional studies.
Key features of each study were identified including: location and time period, data sources, number of study subjects, and the nature of population (e.g., pregnant women in the general population or cases from a case-control study). We recorded, where possible, the method used to assess residential histories as well as the times within pregnancy for which residences were recorded (e.g., addresses at conception and birth versus full residential history with times and locations for all moves). Results were summarized with respect to overall mobility rates and distance moved. We evaluated results regarding how the probability of moving during pregnancy varied by demographic factors such as mother’s age, marital status, and SES; smoking and alcohol use during pregnancy; timing in pregnancy; urbanicity; and other factors. We also reviewed results from studies that assessed how residential mobility affects estimates of exposure to environmental conditions. A meaningful quantitative combination of results, such as meta-analysis, was not feasible because of variation in study designs.
RESULTS
Studies on residential mobility during pregnancy
The database searches identified 153 studies, of which only 14 examined residential mobility during pregnancy, and, for most of these studies, the mobility research was not the study’s main focus. Table 1 summarizes the 14 studies’ datasets, time periods, and locations. Datasets from some studies overlapped. Half of the studies (seven) were based in the US, with three UK studies, and one each in Canada, the Netherlands, Norway, and Australia. The number of study subjects ranged from 71 to 25,229. Among studies of, or similar to, the general pregnant population (e.g., control groups), the number of study subjects ranged from 71 to 25,229, with only three studies with >600 subjects. The four largest studies (7,919 to 25,229 subjects) were in the UK and Norway.
Table 1.
Summary of studies on residential mobility during pregnancy.
| Study | Data source | Study area and timeframe | Size and type of population | Nature of data on mobility |
|---|---|---|---|---|
| Canfield et al.26 | Case-control: National Birth Defects Prevention Study (subset for Texas) linked with Texas Birth Defects Registry and birth certificates. | Texas, U.S. 1997–2000 | n =1085. Cases (n =836) with birth defects, controls (n =249). Results for controls presented separately. | Method to assess residence: Computer-assisted 1-hour telephone interview completed median 338 days after delivery. Nature of residence data: All addresses from conception to delivery. Data include street, city, state, zip code, and county. Time of move included the month and year; date of move assigned as the 15th of the month. Notes: Dates of conception estimated. Controls are normal, live births. Also examined residential mobility for the 3 months before pregnancy. |
| Chen et al.27 | Case-control nested in a cohort: National Birth Defects Prevention Study for New York (subset with expected delivery Oct. 1997-Dec. 2002). | Hudson Valley and western regions of New York, U.S. 1997–2002 | n =1324. Cases (n =912) with birth defects, controls (n =412). Results for controls presented separately. | Method to assess residence: Computer-assisted telephone interview after delivery. Nature of residence data: Time of move included the month and year; date of move assigned as the 15th of the month. Notes: Controls are live births. |
| Fell et al.31 | Case-control study of still births. | Nova Scotia and Eastern Ontario, Canada Born July 1999 – Dec. 2001. | n =398. Control subjects of a case-control study. | Method to assess residence: Telephone interview about 6 months after delivery. Nature of residence data: All moves from conception to delivery. Notes: Live births only. |
| Hodgson et al.32 | Prospective registry: Northern Congenital Abnormality Survey (NorCAS) | Northern England 1985–2003 | n =7919. All observations were births with congenital anomalies. | Method to assess residence: Medical records. Nature of residence data: Addresses assessed for two time points in pregnancy: (1) first official pregnancy medical appointment, typically at 13 weeks gestation, and (2) delivery. Addresses at the postcode level (grouping of approximately 15 households). Notes: All observations had gestation >24 weeks. Area-level SES assessed with Index of Multiple Deprivation and Townsend Deprivation scores. |
| Khoury et al.33,34 | Case-control: Maryland Birth Defects Reporting and Information System | Maryland, U.S. 1984 | n =295. All subjects had birth defects. | Method to assess residence: At delivery, address recorded and an obstetric nurse interviewed the mother regarding the address at conception. Nature of residence data: Address assessed for two time points in pregnancy: (1) conception, and (2) delivery. |
| Lupo et al.28 | Case-control: National Birth Defects Prevention Study (subset for Texas) | Texas, U.S. 1997–2004 | n =732. Cases (n =141) with neural tube defect, controls (n =591). Results for controls presented separately. | Method to assess residence: Computer-assisted telephone interview with mothers with 64% participation rate for cases and 57% for controls. Interviews after delivery. Nature of residence data: Data includes residence dates that were used to estimate addresses for conception, pregnancy periods, and delivery. Notes: Conception dates estimated. |
| Madsen et al.38 | Registry: Medical Birth Registry of Norway and Statistics Norway | Oslo, Norway 1999–2002 | n =25,229. | Method to assess residence: Birth registry datasets linked to national statistics datasets at the individual level. Nature of residence data: Data include address and time of change in residence. Notes: Singleton, live, term births >1000gm. |
| Michielin and Mulder25 | Surveys: Stichting Sociaal-Culturele Wetenschappen survey, the Netherlands Family survey | Netherlands 1992–1993 | n =2722. Couples ages 15–45 years who were cohabitating or in their first marriage. | Method to assess residence: Retrospective survey. Nature of residence data: Data include dates of moves and life events (e.g., pregnancy) in months and calendar years and address of previous residences. Notes: Calculated likelihood of short moves <40 km for pregnant women or men with pregnant partners, compared to those with no children, adjusting for gender and other factors. |
| Miller et al.29 | Case-control: Atlanta Birth Defects Risk Factor Surveillance Study | Atlanta, GA, U.S. 1993–1997 | n =991. Cases with birth defects (n =656), controls (n =335). Results for controls presented separately. | Method to assess residence: Telephone survey conducted after delivery with 66% participation rate. Nature of residence data: All addresses from conception to delivery. Notes: Residential history analyzed for 3 months before conception to delivery. A minimum of 4 weeks at a residence was required to qualify as a move. |
| Pearce et al.39 | Cohort: UK Millennium Cohort Study | U.K. 2000–2002 | n =18,234. | Method to assess residence: Survey, usually to mother, at infant’s age of 9 months. Nature of residence data: Time spent at current address used to determine if the last move occurred during pregnancy. Notes: Data stratified to over-represent the smaller three UK countries, disadvantaged areas, and ethnic minorities. |
| Raynes-Greenow et al.36 | Cohort: Randomized controlled trial. | Inner city Sydney, Australia Sep. 2004 to April 2006 | n =585. | Method to assess residence: Cohort subjects enrolled late in pregnancy (~37 weeks gestation). Address ascertained at in-person enrollment and during follow-up through in-person and phone follow-up and other means including residential phone disconnection and returned mail. Nature of residence data: Address compared for two time points in pregnancy: (1) enrollment in cohort, and (2) delivery. Notes: Also assessed mobility up to 12 months after delivery. |
| Shaw and Malcoe30 | Case-control/registry: California Birth Defects Monitoring Program, birth certificate database | Santa Clara County, CA, U.S.1981–1983 | n =403. Cases with birth defects (n =193), controls (n =210). Results for controls presented separately. | Method to assess residence: Residential history from conception through first trimester obtained from phone or in-person interview after delivery. Residence at birth obtained from birth certificate data. Nature of residence data: All addresses from conception to end of first trimester. Address at delivery. Notes: A minimum of 3 weeks at a residence was required to qualify as a move. Controls were live births. |
| Tunstall et al.35 | Cohort: Millennium Cohort Study, Centre for Longitudinal Studies | U.K. Born 2000–2002. | n =18,197. | Method to assess residence: Home interview with main respondent, usually mother, and their partner, at infant’s age of 9 months. Nature of residence data: All addresses with month and year of move. Addresses assessed for two time points in pregnancy: (1) conception, and (2) delivery. Notes: Singleton births only. Data stratified by electoral ward to over-represent wards with minorities (>30% Black or Asian in 1991 Census). Also assessed moves during infancy as moves between delivery and date of interview. |
| Zender et al37 | Recruitment from cross-sectional study | Colorado, U.S. 1996–1997 | n =71 pregnant women. An additional 43 non-pregnant women. | Method to assess residence: In-person or telephone interview at enrollment. Nature of residence data: All subjects were asked whether they were pregnant when they moved into their current residence. Pregnant women were asked whether they have moved during their current pregnancy. Notes: Recruitment from Well Infant and Children program clinics and classes for pregnant women. |
Five studies used data from case-control studies on birth defects,26–30 one study was based on case-control research on stillbirths,31 and two additional studies presented results for a population with birth defects.32–34 For case-control studies, we present findings from the control subjects, when available, as this group better represents the general population. For studies that provided results for both cases and controls, findings comparing these groups are presented in the Supplementary Material.
Not all studies specified when residential history was assessed in relation to pregnancy, such as whether mothers were asked to recall residential history on the day of delivery or whether moves were recorded in near real-time throughout pregnancy. In general, residential mobility was assessed through surveys conducted 6 months to over 1 year after delivery.26,31,35 One study interviewed subjects on the day of delivery to inquire about the residence at conception.32,33 Another study involved a retrospective survey of life-event histories, including moves during previous pregnancy years.25 Of the 14 studies, only four collected residential information during pregnancy: through cohort follow-up36; at the first pregnancy medical appointment (typically 13 weeks gestation)32; at initial enrollment37; and through unique national databases that record residences through time in Norway.38 Many studies have information on addresses at specific points in time (e.g., conception and delivery), although a few incorporated full residential histories.
Overall mobility rates and timing during pregnancy
The percentage of the population who moved during pregnancy ranged from 9%32 to 32%37 with a median of 20% across the studies presenting this information. The study with the lowest mobility rate32 assessed mobility from first prenatal visit to delivery rather than conception to delivery, so this value (9%) is an underestimate of the true mobility from conception to delivery. Of the 12 studies that estimated mobility during the full pregnancy, seven were in the US with a median of 24% moved (range 14–32%), two were in the UK with mobility rates of 15% and 16%, and the remaining studies were in Australia (19% moved), Canada (12%), and Norway (28%). For the US studies, no temporal trends in mobility were observed (Supplementary Figure 1).
Figure 1 shows mobility rates for pregnancy and each trimester. All four studies that present results by trimester found highest mobility during the second trimester. The lowest mobility was observed in the first trimester for two studies28,31 and the third trimester for two studies.27,29 For controls in this study, of those who moved during pregnancy, 74% moved once, 21% moved twice, and 5% moved three to 5 times. One study asked subjects about the timing of their last move when the infants were 9 months of age.39 Results indicate that 15.1% had last moved during pregnancy. This underestimates the total percent that moved during pregnancy, as this measure omits those who moved both during pregnancy and the first 9 months after birth; however, only a small number of mothers had their last move after birth (0.2%). Supplementary Figure 2 shows the percent of mothers who moved during pregnancy and by trimester for cases and controls from case-control studies.
Figure 1.
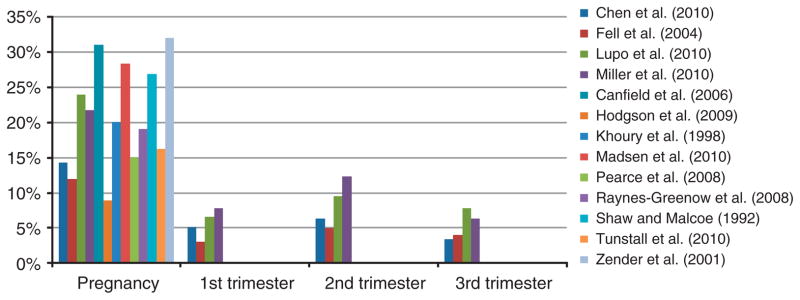
Percent of mothers who moved during pregnancy and by trimester. Based on control study subjects for case-control studies.26–31 Based on cases only (subjects with birth defects).32–34 Mobility during pregnancy from first prenatal visit to delivery rather than conception to delivery.32
Mother’s age
Table 2 shows residential mobility by age of mother. Studies used different specifications for age categories; Table 2 has younger ages towards the top of the table and older age categories towards the bottom. Overall, the probability of moving declined with mother’s age, with minor exceptions. The lowest mobility was observed in the oldest age category for six of the eight studies in Table 2. One study found similar likelihood of moving across age categories (15.5 to 18.6%) except those 20–24 years, where 27.7% moved.33,34 Another found different probabilities of moving across age groups (<25, 26–34, and ≥35 years) with the lowest mobility (7%) in the middle age group and the highest mobility (31%) in the youngest group.31 Supplementary Table 1 shows an analogous table for cases and controls from case-control studies.
Table 2.
Percent of mothers who moved during pregnancy, by age.
| Chen et al.27 | Khoury et al.33,34 | Canfield et al.26 | Miller et al.29 | Shaw and Malcoe30 | Raynes-Greenow et al.36 | Fell et al.31 | Tunstall et al.35 | |
|---|---|---|---|---|---|---|---|---|
| 18–24 years | 43.1 | 64.5 | ||||||
| <20 | 37 | 17.3 | 48.1 | 20.6 | 38.8 | |||
| 20–24 | 30 | 27.7 | 28.3 | 39.0 | 37.2 | |||
| <26 | 31 | |||||||
| 25–30 | 19.6 | |||||||
| 25–29 | 19 | 15.5 | 31.3 | 24.7 | 25.4 | |||
| 26–34 | 7 | |||||||
| 31–35 | 15.8 | |||||||
| 30–34 | 11 | 18.6 | 15.1 | |||||
| >30 | 15.1 | 17.0 | 17.1 |
Alcohol use by mother
Two studies examined mobility in relation to alcohol use during pregnancy. One found a 15.1% mobility for women who drank alcohol during pregnancy compared with 13.0% for those who did not.27 The other found higher rates among non-drinkers (24.2%) than those who consumed alcohol during pregnancy (19.5%).29
Smoking by mother
Findings for mobility by smoking status during pregnancy differed by study, with three of the four studies finding that smokers were more likely to move than non-smokers. Mobility rates among smokers were higher than for non-smokers at (21.1% versus 12.3%)27 and (22% versus 10%).31 In another study, those who smoked during pregnancy were 57% (95% confidence interval 42–74%) more likely to move than non-smokers, and those who quit smoking during pregnancy were 59% (40–81%) more likely to move than non-smokers.35 Other research found higher mobility among non-smokers (31.5%) than smokers (19.9%).29 Among non-smokers, those exposed to environmental tobacco smoke (ETS) during pregnancy had a mobility rate of 21% compared with 23% for those not exposed to ETS.31
Race/ethnicity of mother
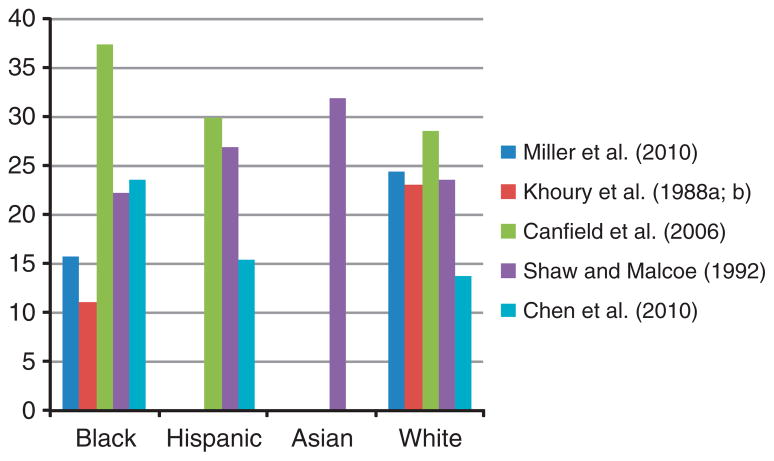
Figure 2 shows residential mobility rates during pregnancy by race. Studies used different categories for race and ethnicity. All studies presented in this figure are based in the US. Whites were more likely to move than blacks in several studies,29,30,33,34 but less likely to move in others.26,27 Mobility rates for Hispanics were slightly higher than for whites. Supplementary Figure 3 provides an analogous figure for cases and controls from case-control studies.
Figure 2.
Percent of mothers who moved during pregnancy, by race/ethnicity. Based on control study subjects for case-control studies.26,27,29,30 Based on cases only (subjects with birth defects).33,34 Black specified as non-Hispanic black; white specified as non-Hispanic White.27 Some studies also presented results for a category of “Other.”
Marital status
Three studies examined marital and living status, finding lower mobility among married women. Whereas 11% of mothers who were married or in common-law marriages moved during pregnancy, 35% of other mothers moved.31 Those cohabitating were 2.28 (2.04–2.55) times more likely to move than married mothers.35 Mothers who were separated or living alone were 1.91 (1.24–2.71) and 1.89 (1.63–2.18) times more likely to move than married mothers, respectively.35 Another study found that 17.2% of mothers living with a partner moved during pregnancy, compared with 42.42% of other mothers.36
Planned pregnancy
Higher mobility was observed for mothers with unplanned pregnancy. In one study, they were 72% (56–90%) more likely to move than those with planned pregnancies.35 In another study, mobility rates were 25.6% for mothers with unplanned pregnancies and 15.3% for those with planned pregnancies.29
Socioeconomic status
Links between SES and residential mobility during pregnancy were assessed in several ways including educational attainment, payment method for medical services, and income, with individual- and area-level data. In general, higher SES was associated with lower mobility.
Table 3 shows mobility rates for pregnant women by educational attainment. As studies differed in specifications of educational categories, results are not directly comparable; however, Table 3 approximates comparison with lower education levels towards the top of the table and higher education levels towards the bottom of the table. Several studies observed lower mobility with increasing education,27,30,31,36 including analysis of paternal education.29 In the two remaining analyses, the highest rate of mobility was for the least educated group (<high school) in one study,29 and, in the other, the highest mobility was observed in the middle group (high school graduate) with the lowest mobility in the most educated (> high school).26 No study found the reverse trend, with higher mobility consistently associated with higher education. In studies based in the UK and Norway, higher mobility was also associated with lower education.35,38 Supplementary Table 2 shows an analogous figure for cases and controls from case-control studies.
Table 3.
Percent of mothers who moved during pregnancy, by education level.
| Raynes-Greenow et al.36 | Miller et al.29
|
Chen et al.27 | Canfield et al.26 | Fell et al.31 | Shaw and Malcoe30 | ||
|---|---|---|---|---|---|---|---|
| Maternal education | Paternal education | ||||||
| Grades 0–8 | 50.2 | ||||||
| Grades 9–11 | 39.9 | ||||||
| Less than high school | 25.8 | 35.7 | 31.0 | ||||
| High school or less | 20.1 | 13 | |||||
| High school | 16.7 | 22.5 | 37.4 | 24.4 | |||
| Secondary | 23.96 | ||||||
| More than high school | 22.9 | 20.6 | 11.4 | 22.7 | 22.8 | ||
| Some/completed college | 14 | ||||||
| Technical/other | 17.20 | ||||||
| Some/completed university | 11 | ||||||
| University | 17.77 | ||||||
Two studies investigated mobility by income. A Canadian study found higher mobility with lower income, with rates of 43% for annual family income <$20,000 and 10% for annual family income ≥$60,000 (Canadian).31 A study based in Texas, USA, observed higher mobility with lower income, with rates of 34.4, 31.0, and 19% for annual family incomes of <$10,000; $10,000–$50,000; and >$50,000 (US).26
Comparisons by source of medical care across countries are difficult as health care systems vary. However, studies found different mobility by type of medical care, within a given region. For example, in the US health insurance coverage relates to SES, with those with lower income or education less likely to have insurance, and those at higher SES more likely to have private health insurance.40,41 In New York, USA, mothers who self-paid for medical care had a mobility rate of 33%, compared with 25.6% for those with Medicaid, 13.1% for those with a health maintenance organization, and 6.9% for those with other private health insurance.27 In an Australian study, mobility rates were 10% for care shared by a general practitioner and midwife or hospital, 18% for private hospitals, 19% for public hospitals, and 24% for birth centers.36
One study found that when both parents were employed, 25.2% of mothers moved during pregnancy, 27.2% moved if one parent was employed, and 36.2% moved if neither parent was employed.30 In other studies, comparison of mobility for unemployed and employed mothers were 17% versus 11% (ref. 31) and 30.2% versus 29.4%.26 Mobility rates by mother’s occupation were 16.2, 16.7, 23.3, 23.5, and 26.2% for managerial, farming/other, service, unemployed, and technical/sales, respectively.29
Area-level SES indicators for mother’s residence were evaluated by several studies, generally indicating higher mobility for lower income areas, but not in all studies. The Index of Multiple Deprivation and Townsend deprivation scores were used to assess overall area-level SES for the community of delivery, finding that movers were more likely to live in areas with higher deprivation than non-movers (P-value <0.01).32 Those living in disadvantaged areas, as defined by child poverty, were 15% (2–29%) more likely to move than those in advantaged areas.35 Some studies found contradictory results. In Norway, no difference in mobility was observed for mothers in neighborhoods above and below mean income levels, perhaps owing to less of an income gradient in that country.38 Another study categorized mothers’ census tracts at conception into SES levels based on the percentage of persons living below census-defined poverty levels.29 In this study, mobility rates were 24.6, 20.0, 24.7, and 8.8% for the high, mid-high, mid-low, and low SES levels, respectively, indicating higher mobility for wealthier communities.
Prenatal Care
Earlier or no prenatal care was associated with lower mobility in several studies. Those with no prenatal care were 18% (8–30%) more likely to move than those with prenatal care.35 In the same study, those who entered care in the second or third trimester had mobility rates only 3% (−0.7–14%) higher than those who received care in the first trimester. In other work, 12.4, 24.9, and 40% of mothers moved for those who received their first prenatal care in months 1–3, 4–6, and 7 or later, respectively.27 Those with prenatal care in the first-to-third month had a mobility rate of 12% compared with 21% for those beginning care later in pregnancy.31
Parity
Table 4 presents mobility rates by parity (i.e., number of previous births). The highest mobility generally was observed in the group with the fewest previous pregnancies but not in all studies.33,34 This study differs from others presented in Table 4 in that all pregnancies resulted in birth defects. In another study, movers were more likely to have a parity of 0 than non-movers.38 Tunstall et al.35 examined a related issue of how many children were present in the household during the pregnancy. Mothers with no children were 2.38 (2.06–2.75) times more likely to move than those with ≥2 children, and those with one child were 1.26 (1.07–1.49) times more likely to move than those with ≥2 children. Supplementary Table 3 shows an analogous figure for cases and controls from case-control studies.
Table 4.
Percent of mothers who moved during pregnancy, by parity.
Health status
Case-control studies of birth defects generally found mobility rates for cases to be slightly higher, but similar to, those for controls (Supplementary Figure 2). The percentage of mothers who moved for cases and controls, respectively, was 22.3% and 21.8%,29 30% and 24%,28 32.9% and 31.3%,26 and 17.4% and 14.3%,27 but 22.4% and 26.9% in another study.30 No differences in mobility rates were observed by type of birth defect among cases.29,33,34 In another study, infants born to movers had birth weights 47.5 g lower than non-movers.38 Children whose mothers moved during pregnancy were 44% (12–85%) more likely to be partially immunized and 42% (−15–234%) more likely to be unimmunized than those who last moved >3 years before birth.39
Mothers’ health was assessed through studies of self-rated health and body mass index (BMI). Mothers with self-rated health of excellent or good had similar mobility to those with fair or poor health ratings.35 Three studies examined mother’s BMI, with inconsistent findings. Of mothers with pre-pregnancy BMI <25 (underweight to normal), 22.1% moved during pregnancy, compared with 20.9% for those with BMI ≥25 (overweight).29 In another study, 15% of those with pre-pregnancy BMI <25 moved, compared with 8% of those with BMI ≥25.31 The reverse trend was observed in another study finding the lowest mobility among those with higher BMI. The percentage of mothers who moved during pregnancy was 24.4% for BMI <18.5 (underweight), 18.7% for BMI 18.5–24.9 (normal), 12.6% for BMI 25–29.9 (overweight), and 17.1% for BMI ≥30 (obese).27
Other factors
Several other factors were examined in relation to residential mobility during pregnancy in a few studies. Mothers who never breastfed were 22% (7–40%) more likely to move than those who breastfed ≥6 months.35 The likelihood of moving for mothers in urban environments compared with those in rural areas was 30.3% versus 29.1%26 and 12% versus 10%.31 Mobility rates for mothers living by the Texas border were 26%, with a rate of 31.1% for those not living by the border.26 Similar mobility was observed based on medication use during pregnancy (12% for users and non-users), exposure to pesticides or chemicals at work or home (yes 10%, no 13%), and complications or illness during pregnancy (yes 8%, no 13%).31 Renters were 3.12 (2.75–3.55) times more likely to move than home owners.35 Of those in English-speaking homes, 19.29% moved compared with 16.88% for others.36 Mothers who were active during pregnancy moved at similar rates (11%) as those with little to moderate activity (13%).31 Similar rates of moving were observed based on season of birth.33,34
Impact of residential mobility on assessment of environmental exposures
Some studies reported the distance between residences, as moves over larger distances are more likely to impact exposure assessments than shorter moves. Median distances in several studies were <10 km, although mean distances were often influenced by extreme observations. Hodgson et al.32 found a median distance of 1.4 km (mean 9.7 km) for moves between first prenatal visit and delivery for pregnancies resulting in birth defects. In a study of 141 subjects with birth defects and 591 subjects without, the median distance moved between conception and delivery was 6.9 km (mean 188.9 km, range 0.2–2,346 km).28 On the basis of 912 cases with birth defects and 412 controls, median distance moved was 4.2 km (mean 16.7, range 0–481.8 km).27 Miller et al.29 found that for movers in the control group with known distances, 19.0% went <4.8 km, with 28.6% moving 4.8 to <13 km, 25.4% moving 13 to <39 km, and 27.0% moving ≥39 km. The percentage of movers that stayed within the same county was 52.1%. Similarly, in another study 69.1% of movers stayed within the same county, and 6.2% stayed within the same census tract.30 In other work, 62.5% of movers stayed within the same municipality and 69% within the same county.31
Lupo et al.28 estimated benzene exposure over pregnancy for a case-control birth defects study in Texas. The National Air Toxics Assessment, Assessment System for Population Exposure Nationwide was used to model pollutant levels for each census track. Quartiles of estimated benzene levels based on addresses at conception were similar to estimates based on address at delivery (P<0.0001). This relationship held for analysis of all subjects, cases, or controls. For example, in the control group, of those assigned to the highest quartile of exposure based on address at delivery, 88% were also assigned to the highest quartile based on address at conception. Of those in the lowest exposure category using address at delivery, 90% also had the lowest exposure using address at conception. The authors concluded that the short distances moved by this population did not significantly impact estimates of benzene exposure over pregnancy.
Ambient air pollution exposure over pregnancy was estimated for subjects in a case-control study of birth defects in New York, NY, USA.27 Addresses at birth were obtained from birth certificates and residential histories by phone interview. Ambient pollutant levels at each residence were estimated with monitoring data for ozone for the full study period and particulate matter with aerodynamic diameter ≤10 μm (PM10) for 1997–1998. The authors divided New York State into 11 ozone regions and 8 PM10 regions with one or more monitors. The size of each region ranged from 152 to 30,536 km2 for ozone and 1,627 to 31,712 km2 for PM10. Daily region-level exposures were calculated from an average of all monitors within a region on that day. Exposures for each pregnancy were estimated for maternal addresses accounting for residential history, and separately for addresses at birth only. The two methods of accounting for residence produced similar ozone and PM10 levels for the 412 control subjects, for exposures at 3–8 weeks gestation, total pregnancy, and each trimester. Results were similar when data were stratified by demographic characteristics or region of birth. The agreement between results decreased slightly with increasing gestational age.
In another birth defects case-control study, residence at delivery was obtained from hospital records and residence at conception was based on nurse interviews at time of delivery.33,34 Authors simulated the effect of residential mobility rates during pregnancy on associations between environmental teratogenesis and birth defects, based on theoretical relative risks of 2, 5, 10, and 100 and theoretical mobility rates of 10, 20, 30, 40, and 50%. The results show the relationship between the true relative risk, the observed relative risk, the frequency of exposure in the population, and the mobility rate. For a given true relative risk, higher frequency of exposure and higher mobility rates were associated with lower observed relative risk. The authors concluded that health risks of teratogens may be underestimated if analysis uses residence at birth.
A simulation study was performed to examine how exposure misclassification due to residential mobility during pregnancy impacts epidemiological studies of congenital malformations.42 Using a case study area of Santa Clara County, California, USA, the authors considered three hypothetical point sources for exposures of interest. They modeled the expected distance from exposure sources to randomly chosen locations for mother’s residence at conception and at birth, with additional parameters to represent the probability that a subject moved during pregnancy (set at 0.15), the distance moved, and direction of the move in relation to the source (i.e., either towards or away from the exposure source). The simulation applied information from a case-control dataset for this region designed to investigate birth defects.30 Four scenarios were explored: (1) both cases and controls move towards the exposure source, (2) both cases and controls move away from the exposure source, (3) cases move towards the source while controls move away, and (4) controls move towards the source while cases move away. Simulations were repeated for each of the three hypothetical exposure points separately. The standardized difference between the distance between subjects and the exposure point was estimated for cases and controls. Results indicate the potential for bias, especially for scenarios in which cases and controls moved in opposite directions, when comparing results based on residence at conception versus at delivery. Although this study explores a specific set of scenarios, it demonstrates the possible impact of residential mobility in assessment of point source exposures.
Madsen et al. estimated associations between ambient exposure to air pollution during pregnancy and term birth weight for 25,229 subjects from 1999 to 2002 in Oslo, Norway.38 Databases from Statistics Norway were linked to the Medical Birth Registry of Norway to identify changes in residence for each mother, including data on the address and time of move. The database provides geographical coordinates for work and residence locations. Birth weights were compared by quartile of gestational exposure for nitrogen dioxide (NO2), PM10, and particulate matter with aerodynamic diameter ≤2.5 μm (PM2.5). Pollutant levels were estimated with a dispersion model. Analyses were conducted using exposures based on residence and work locations, and with residence location only, adjusted for infant sex, parity, and mother’s education, smoking status during pregnancy, and ethnicity. For estimates of exposure based on both residence and work locations, exposure during the first and second trimester used a time-weighted average of levels at work and residence locations for weekdays, levels at residences for weekends, and levels at residences for the third trimester. Table 5 presents a subset of results comparing birth weight for the highest and lowest quartiles of exposure. These findings do not provide evidence of an association between pollutants and birth weight; however, effect estimates differ based on whether work locations were incorporated in estimates of exposure. This study also found that exposures for women who moved were lower than for those who did not move.
Table 5.
Change in birth weight comparing infants in the highest quartile of exposure during pregnancy to the lowest quartile, under various analyses related to residential mobility, from Madsen et al.38
| Pollutant | Highest quartile to lowest quartiles of exposure | Based on exposure assessed with residential and work locations | Based on exposure assessed with residential address only |
|---|---|---|---|
| NO2 | >38.0 to <20.3 g/m3 | 1.8 (−13.7–17.2) gm | 0.9 (−14.6–16.4) gm |
| PM10 | >16.2 to <10.7 g/m3 | 15.9 (0.0–31.9) gm | 11.9 (−3.7–27.4) gm |
| PM2.5 | >14.2 to <9.7 g/m3 | 13.6 (−2.4–29.5) gm | 10.2 (−5.4–25.9) gm |
Authors of a study of residential traffic exposure and pregnancy-related outcomes of 7,339 women in a population-based cohort study performed sensitivity analysis based on residential mobility.6 Associations were estimated for distance-weighted traffic density or distance to major road and pregnancy outcomes (birth weight, small for gestational age, preterm birth, pregnancy-induced hypertension, preeclampsia or hemolysis, elevated liver enzymes and low platelets (HELLP), gestational diabetes). Results for non-movers were similar to those for the total cohort.
DISCUSSION
Results indicate that exposure misclassification may occur in studies assessing environmental exposures with incomplete residential information. Overall, 9 to 32% of mothers moved during pregnancy, with more moves in the second trimester than the first or third trimester in all studies that examined timing within pregnancy. Some studies found a higher probability of moving in the first trimester compared with the third, whereas others found the reverse. This implies that exposure misclassification introduced by residential mobility will differ by population as moves early in pregnancy are more likely to change exposure estimates than later moves, given that many estimates of exposure over pregnancy are based on residential locations at time of delivery. For example, considering a subject with 42-weeks gestation who moves in the first week of pregnancy, exposure based at residence at time of delivery would be inaccurate for only 1 week (2%) of the pregnancy. If a subject moved in the last week of a 42-week pregnancy, exposure based at residence at delivery would be inaccurate for 41 weeks (98%).
Studies that examined distance noted that most moves may not greatly influence exposure estimates, depending on the exposure of concern. The median distance moved was <10 km in most studies, and most mothers stayed within the same general area, such as the same county (52.1–69.1%). The degree of exposure misclassification will be a function of the distance moved in relation to the spatial heterogeneity of the exposure. For example, exposure estimates based on the water supply network at residence would not be affected by local moves within the same system. Analysis of PM2.5 chemical components in the US found spatial heterogeneity for all components considered, but higher variation for elemental carbon and organic carbon matter than for sulfate and nitrate.43 Even for a given pollutant, spatial variation may vary by city or the metric used. A study of São Paulo, Brazil observed a spatially heterogeneous distribution of PM10 and found that heterogeneity depended on the pollutant metric (e.g., daily average versus daily 1-h maximum).44
Studies show that certain types of mothers were more likely to move during pregnancy than others. Higher mobility was generally associated with mothers who were younger, unmarried, had lower parity, and with lower SES based on indicators such as educational attainment or family income. For other factors, findings differed by study, such as whether smokers were more or less likely to move than non-smokers or the relative mobility by race of mother. Although consistent patterns were not observed for these factors, individual studies did identify differences in mobility rates by group, indicating that differential mobility does exist and the direction of such trends may differ by population. Many factors investigated in these studies may be highly correlated such as marital status and planned pregnancy; age and parity; or socioeconomic status and race. Additional variables (e.g., homeownership versus renters) may also covary with socioeconomic status, or with each other, such as smoking or alcohol use in pregnancy, and timing of entry into prenatal care. It is not possible to determine from the published data either the extent of these correlations, or the underlying factor, or set of factors most associated with moving during pregnancy.
Two studies investigated how residential mobility influenced estimates of exposure during pregnancy with actual data.27,28 Results provide evidence that residential mobility does not greatly influence exposure estimates, and presumably subsequent health risk estimates, due to the short distance of most moves. Although useful, these studies are limited. One compared estimates of exposure based on address at delivery to those based on address at conception,28 rather than a comparison between exposure based on address at delivery and full residential histories. The other estimated similar exposures for ozone and PM10 based on full residential histories versus address at delivery.27 However, the exposure regions were larger than those typically used for air pollution studies, and, in both studies, residential history was obtained after delivery. Findings from simulation studies implied that health effect estimates may be underestimated owing to changes in residence over pregnancy.33,34,42
Our review of research on residential mobility during pregnancy found this topic not well studied, with less than a dozen studies and poor representation for non-US countries. Many of the studies reported results for residential mobility, but were focused on other research questions. Our search criteria may have missed some studies that similarly presented mobility results in addition to their main findings. Some studies presented results for unique populations (e.g., pregnancies resulting in birth defects), thus the results are useful but have limited generalizability. Further, studies may present information on a portion of pregnancy and/or a range of the pregnancy period. As an example, a case-control study on traffic and spontaneous abortion noted that 6% of subjects moved between the last menstrual period and an interview that took place within the first 13 weeks of pregnancy.5
In many cases, residential history was assessed after delivery, possibly introducing recall bias. A full understanding of this issue requires assessment of complete residential history during pregnancy (i.e., the times and locations of all moves) as the moves are occurring, or shortly thereafter, in the context of spatial heterogeneity of the exposure of interest. Information on change of residence before pregnancy, as was collected by Canfield et al.,26 may be useful to assess exposure at time of conception. Future studies should consider not only whether the probability of moving differed by population (e.g., mother’s age, SES), but whether the distance moved and timing of moves in pregnancy differ by these factors. Data on why women move may also provide insight into which variables are of most interest. The influence of residential mobility on health risk estimates may differ for those who travel long distances during pregnancy, and, in fact, changing residence may affect pregnancy rates.45,46 Information on mobility is needed for vulnerable populations that may move more often than the general population, such as migrant workers, immigrants, and those without permanent residences.
Ideally, studies of how environmental exposures during pregnancy affect risk of adverse pregnancy and childhood health outcomes would incorporate full residential histories; however, feasibility constraints often require the use of approximations such as the residential location at birth. The design of research studies and the interpretation of results should consider the implications of exposure misclassification from residential mobility. This includes the likelihood that subjects moved during pregnancy, the distance moved, and the spatial heterogeneity of the exposure of interest. Some time frames of exposure will be more influenced by residential mobility than others. For instance, methods using residence at delivery will generate better estimates for the third trimester than the first trimester. Consideration should be given to differential residential mobility in relation to variables important to the study, such as the health outcome. In case-control studies of birth defects, mobility for cases and controls were similar, but were higher for cases in all studies. Potential confounders, such as SES, mother’s health status, and mother’s age, are likely to be associated with the likelihood of moving during pregnancy. The impacts of this bias on study results are difficult to discern; however, if the health impacts of interest are associated with factors that are also related to moving (e.g., SES), the exposure misclassification could be non-differential and could impact the study’s findings.
Supplementary Material
Acknowledgments
This work was supported by funding from the NIEHS (R01ES016317, R01ES015028, and R01ES019587).
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supplementary Information accompanies the paper on the Journal of Exposure Science and Environmental Epidemiology website (http://www.nature.com/jes)
References
- 1.Bell ML, Ebisu K, Belanger K. Ambient air pollution and low birth weight in Connecticut and Massachusetts. Environ Health Perspect. 2007;115:1118–1125. doi: 10.1289/ehp.9759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parker JD, Woodruff TJ, Basu R, Schoendorf KC. Air pollution and birth weight among term infants in California. Pediatrics. 2005;115:121–128. doi: 10.1542/peds.2004-0889. [DOI] [PubMed] [Google Scholar]
- 3.Ritz B, Wilhelm M, Hoggatt KJ, Ghosh JK. Ambient air pollution and preterm birth in the environment and pregnancy outcomes study at the University of California, Los Angeles. Am J Epidemiol. 2007;166:1045–1052. doi: 10.1093/aje/kwm181. [DOI] [PubMed] [Google Scholar]
- 4.Miyake Y, Tanaka K, Fujiwara H, Mitani Y, Ikemi H, Sasaki S, et al. Residential proximity to main roads during pregnancy and the risk of allergic disorders in Japanese infants: the Osaka Maternal and Child Health Study. Pediatr Allergy Immunol. 2010;21:22–28. doi: 10.1111/j.1399-3038.2009.00951.x. [DOI] [PubMed] [Google Scholar]
- 5.Green RS, Malig B, Windham GC, Fenster L, Ostro B, Swan S. Residential exposure to traffic and spontaneous abortion. Environ Health Perspect. 2009;117:1939–1944. doi: 10.1289/ehp.0900943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van den Hooven EH, Jaddoe VW, de Kluizenaar Y, Hofman A, Mackenbach JP, Steegers EA, et al. Residential traffic exposure and pregnancy-related outcomes: a prospective birth cohort study. Environ Health. 2009;8:59. doi: 10.1186/1476-069X-8-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nieuwenhuijsen MJ, Toledano MB, Bennett J, Best N, Hambly P, de Hoogh C, et al. Chlorination disinfection by-products and risk of congenital anomalies in England and Wales. Environ Health Perspect. 2008;116:216–222. doi: 10.1289/ehp.10636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Waller K, Swan SH, DeLorenze G, Hopkins B. Trihalomethanes in drinking water and spontaneous abortion. Epidemiology. 1998;9:134–140. [PubMed] [Google Scholar]
- 9.Hoffman C, Mendola P, Savitz DA, Herring AH, Loomis D, Hartmann KE, et al. Drinking water disinfection by-product exposure and fetal growth. Epidemiology. 2008;19:729–737. doi: 10.1097/EDE.0b013e3181812bd4. [DOI] [PubMed] [Google Scholar]
- 10.Kramer MD, Lynch CF, Isacson P, Hanson JW. The association of waterborne chloroform with intrauterine growth retardation. Epidemiology. 1992;3:407–413. doi: 10.1097/00001648-199209000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Petit C, Chevrier C, Durand G, Monfort C, Rouget F, Garlantezec R, et al. Impact on fetal growth of prenatal exposure to pesticides due to agricultural activities: a prospective cohort study in Brittany, France. Environ Health. 2010;9:71. doi: 10.1186/1476-069X-9-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reynolds P, Von Behren J, Gunier RB, Goldberg DE, Harnly M, Hertz A. Agricultural pesticide use and childhood cancer in California. Epidemiology. 2005;16:93–100. doi: 10.1097/01.ede.0000147119.32704.5c. [DOI] [PubMed] [Google Scholar]
- 13.Bell E, Hertz-Picciotto I, Beaumont JJ. A case-control study of pesticides and fetal death due to congenital anomalies. Epidemiology. 2001;12:148–156. doi: 10.1097/00001648-200103000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Roberts EM, English PB, Grether JK, Windham GC, Somberg L, Wolff C. Maternal residence near agricultural pesticide application and autism spectrum disorders among children in the California Central Valley. Environ Health Perspect. 2007;115:1482–1489. doi: 10.1289/ehp.10168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elliott P, Toledano MB, Bennett J, Beale L, de Hoogh K, Best N, et al. Mobile phone base stations and early childhood cancers: case-control study. Br Med J. 2010;340:c3077. doi: 10.1136/bmj.c3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang SI, Lee LT, Zou ML, Fan CW, Yaung CL. Pregnancy outcome of women in the vicinity of nuclear power plants in Taiwan. Radiat Environ Biophys. 2010;49:57–65. doi: 10.1007/s00411-009-0246-8. [DOI] [PubMed] [Google Scholar]
- 17.Mueller B, Kuehn C, Shapiro-Mendoza C, Tomashek KM. Fetal deaths and proximity to hazardous waste sites in Washington State. Environ Health Perspect. 2007;115:776–780. doi: 10.1289/ehp.9750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blaasaas KG, Tynes T, Lie RT. Residence near power lines and the risk of birth defects. Epidemiology. 2003;14:95–98. doi: 10.1097/00001648-200301000-00023. [DOI] [PubMed] [Google Scholar]
- 19.Bentham G. Migration and morbidity: implications for geographical studies of disease. Soc Sci Med. 1988;26:49–54. doi: 10.1016/0277-9536(88)90044-5. [DOI] [PubMed] [Google Scholar]
- 20.Peng RD, Bell ML. Spatial gnment in time series studies of air pollution and health data. Biostatistics. 2010;11:720–740. doi: 10.1093/biostatistics/kxq017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oishi S, Schimmack U. Residential mobility, well-being, and mortality. J Pers Soc Psychol. 2010;98:980–994. doi: 10.1037/a0019389. [DOI] [PubMed] [Google Scholar]
- 22.Tulloch AD, Fearon P, Fahy T, David A. Residential mobility among individuals with severe mental illness: cohort study of UK700 participants. Soc Psychiatry Psychiatr Epidemiol. 2010;45:767–777. doi: 10.1007/s00127-009-0115-4. [DOI] [PubMed] [Google Scholar]
- 23.Urayama K, Von Behren J, Reynolds P, Hertz A, Does M, Buffler PA. Factors associated with residential mobility in children with leukemia: implications for assigning exposures. Ann Epidemiol. 2009;19:834–840. doi: 10.1016/j.annepidem.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jelleyman T, Spencer N. Residential mobility in childhood and health outcomes: a systematic review. J Epidemiol Community Health. 2008;62:584–592. doi: 10.1136/jech.2007.060103. [DOI] [PubMed] [Google Scholar]
- 25.Michielin F, Mulder CH. Family events and the residential mobility of couples. Environ Planning A. 2008;40:2770–2790. [Google Scholar]
- 26.Canfield MA, Ramadhani TA, Langlois PH, Waller DK. Residential mobility patterns and exposure misclassification in epidemiologic studies of birth defects. J Exp Sci Environ Epidemiol. 2006;16:538–543. doi: 10.1038/sj.jes.7500501. [DOI] [PubMed] [Google Scholar]
- 27.Chen L, Bell EM, Caton AR, Druschel CM, Lin S. Residential mobility during pregnancy and the potential for ambient air pollution exposure misclassification. Environ Res. 2010;110:162–168. doi: 10.1016/j.envres.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 28.Lupo PJ, Symanski E, Chan W, Mitchell LE, Waller DK, Canfield MA, et al. Differences in exposure assignment between conception and delivery: the impact of maternal mobility. Paediatr Perinat Epidemiol. 2010;24:200–208. doi: 10.1111/j.1365-3016.2010.01096.x. [DOI] [PubMed] [Google Scholar]
- 29.Miller A, Siffel C, Correa A. Residential mobility during pregnancy: patterns and correlates. Matern Child Health J. 2010;14:625–634. doi: 10.1007/s10995-009-0492-z. [DOI] [PubMed] [Google Scholar]
- 30.Shaw GM, Malcoe LH. Residential mobility during pregnancy for mothers of infants with or without congenital cardiac anomalies: a reprint. Arch Environ Health. 1992;47:236–238. doi: 10.1080/00039896.1992.9938355. [DOI] [PubMed] [Google Scholar]
- 31.Fell DB, Dodds L, King WD. Residential mobility during pregnancy. Paediatr Perinat Epidemiol. 2004;18:408–414. doi: 10.1111/j.1365-3016.2004.00580.x. [DOI] [PubMed] [Google Scholar]
- 32.Hodgson S, Shirley M, Bythell M, Rankin J. Residential mobility during pregnancy in the north of England. BMC Pregnancy Childbirth. 2009 doi: 10.1186/1471–2393–9–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khoury MJ, Stewart W, Weinstein A, Panny S, Lindsay P, Eibenberg M, Weinstein A, et al. Corrigendum. J Clin Epidemiol. 1988a;41:617. doi: 10.1016/0895-4356(88)90004-2. [DOI] [PubMed] [Google Scholar]
- 34.Khoury MJ, Stewart W, Weinstein A, Panny S, Lindsay P, Eisenberg M. Residential mobility during pregnancy: implications for environmental teratogenesis. J Clin Epidemiol. 1988b;41:15–20. doi: 10.1016/0895-4356(88)90004-2. [DOI] [PubMed] [Google Scholar]
- 35.Tunstall H, Pickett K, Johnsen S. Residential mobility in the UK during pregnancy and infancy: are pregnant women, new mothers and infants “unhealthy migrants”? Soc Sci Med. 2010;71:786–798. doi: 10.1016/j.socscimed.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 36.Raynes-Greenow CH, Nassar N, Roberts CL. Residential mobility in a cohort of primiparous women during pregnancy and post-partum. Aust N Z J Pub Health. 2008;32:131–134. doi: 10.1111/j.1753-6405.2008.00188.x. [DOI] [PubMed] [Google Scholar]
- 37.Zender R, Bachand AM, Reif JS. Exposure to tap water during pregnancy. J Exp Anal Environ Epidemiol. 2001;11:224–230. doi: 10.1038/sj.jea.7500163. [DOI] [PubMed] [Google Scholar]
- 38.Madsen C, Gehring U, Walker SE, Brunekreef B, Stigum H, Naess O, et al. Ambient air pollution exposure, residential mobility and term birth weight in Oslo, Norway. Environ Res. 2010;110:363–371. doi: 10.1016/j.envres.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 39.Pearce A, Elliman D, Bedford H, Law C. Residential mobility and uptake of childhood immunisations: findings from the UK Millenium Cohort Study. Vaccine. 2008;26:1675–1680. doi: 10.1016/j.vaccine.2008.01.031. [DOI] [PubMed] [Google Scholar]
- 40.Lu N, Samuels ME, Wilson R. Socioeconomic differences in health: How much do health behaviors and health insurance coverage account for? J Health Care Poor Underserved. 2004;15:618–630. doi: 10.1353/hpu.2004.0053. [DOI] [PubMed] [Google Scholar]
- 41.Ross CE, Mirowsky J. Does medical insurance contribute to socioeconomic differentials in health? Milibank Q. 2000;78:291–321. doi: 10.1111/1468-0009.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schulman J, Selvin S, Shaw GM, Malcoe LH. Exposure misclassification due to residential mobility during pregnancy in epidemiologic investigations of congenital malformations. Arch Environ Health. 1993;48:114–119. doi: 10.1080/00039896.1993.9938404. [DOI] [PubMed] [Google Scholar]
- 43.Bell ML, Dominici F, Ebisu K, Zeger SL, Samet JM. Spatial and temporal variation in PM2.5 chemical composition in the United States for health effects studies. Environ Health Perspect. 2007;115:989–995. doi: 10.1289/ehp.9621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bravo MA, Bell ML. Spatial heterogeneity of PM10 and O3 in São Paulo, Brazil and implications for human health studies. J Air Waste Manag Assoc. 2011;61:69–77. doi: 10.3155/1047-3289.61.1.69. [DOI] [PubMed] [Google Scholar]
- 45.Chattopadhyay A, White MJ, Debpuur C. Migrant fertility in Ghana: selection versus adaptation and disruption as causal mechanism. Popul Studies. 2006;60:189–203. doi: 10.1080/00324720600646287. [DOI] [PubMed] [Google Scholar]
- 46.Goldstein A, White M, Goldstein S. Migration, fertility, and state policy in Hubei Province, China. Demography. 1997;34:481–491. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.