Abstract
The increasing incidence of skin cancers and photodamaging effects caused by ultraviolet radiation has increased the use of sunscreening agents, which have shown beneficial effects in reducing the symptoms and reoccurrence of these problems. Many sunscreen compounds are in use, but their safety and efficacy are still in question. Efficacy is measured through indices, such as sun protection factor, persistent pigment darkening protection factor, and COLIPA guidelines. The United States Food and Drug Administration and European Union have incorporated changes in their guidelines to help consumers select products based on their sun protection factor and protection against ultraviolet radiation, whereas the Indian regulatory agency has not yet issued any special guidance on sunscreening agents, as they are classified under cosmetics. In this article, the authors discuss the pharmacological actions of sunscreening agents as well as the available formulations, their benefits, possible health hazards, safety, challenges, and proper application technique. New technologies and scope for the development of sunscreening agents are also discussed as well as the role of the physician in patient education about the use of these agents.
Photoprotective agents protect the skin by preventing and minimizing the damaging effects of ultraviolet (UV) rays of natural light. They can be used as sunblock, which is opaque when applied over the skin and blocks a higher percentage of light as compared to sunscreens, which are translucent and require frequent reapplication for optimum efficacy. Photoaging—manifested as sagging, wrinkling, and photocarcinogenesis—is caused by damage to cells and deoxyribonucleic acid (DNA). It has been observed that sunscreens increase skin's tolerability to UV rays.1
UV radiation has broad spectrum, ranging from 40 to 400nm (30–3eV), which is divided into Vacuum UV (40–190nm), Far UV (190–220nm), UVC (220–290nm), UVB (290–320), and UVA (320–400nm), of which the latter two are medically important. There are two distinct subtypes of UVA radiation. Short-wave UVA (320–340nm) and long-wave UVA (340–400nm), the latter constituting most of UVA radiation. The amount of exposure to UVA usually remains constant, whereas UVB exposure occurs more in the summer.2
Effects of UVA manifest usually after a long duration of exposure, even if doses are low. It has been postulated that UVA up regulates the formation of matrix metalloproteinase (MMPs), enzymes that degrade the matrix protein's elastin and collagen, which, if not prevented, can result in marked reduction in skin elasticity and increased wrinkling. UVA radiation damages skin by penetrating into the layers of skin and producing reactive oxygen resulting in acute and chronic changes.2 UVA radiation can induce polymorphous light eruptions (PMLE) in sensitive skin,3 but in some it has also shown to reduce PMLE.4 UVA can also cause exacerbation of cutaneous lupus erythematoses, whereas solar urticaria can be caused by both UVA and UVB radiation.5
Studies have shown that UVA impairs the antigen presenting cell (APC) activity of the epidermal cells and thereby causes immune suppression, thus contributing to the growth of skin cancer. Sunscreening agents have shown to provide significant protection against epidermal APC activity induced by high UVA dose.6 Mutation occurring in human melanocyte due to damage caused to DNA by UVA radiation is one of the proposed reasons.7 In summary, UVA radiation can cause nuclear and mitochondrial DNA damage, gene mutations and skin cancer, dysregulation of enzymatic chain reactions, immune suppression, lipid peroxidation (membrane damage), and photoallergic and phototoxic effects.
UVB radiation can also cause acute changes, such as pigmentation and sunburn, and chronic changes, such as immune-suppression and photocarcinogenesis. Both UVA and UVB radiation can cause sunburn, photoaging reactions, erythema, and inflammation.2
Sunburn is the most commonly encountered skin damage caused by natural light. Improper sunscreen usage and inadequate application also contribute to the increased prevalence of sunburn, despite the frequent use of sunscreening agents. Available evidence indicates that sunburn is more commonly seen in white-skinned people and young people with sensitive skin. Sunburn is common in the United States with 34.4 percent of adults affected.8 In Sweden, children are frequently affected, and use of sunscreen among children has been found to be protective.9
With the increased incidence in skin cancer cases, such as squamous and basal cell carcinomas, reported worldwide, use of photoprotective agents has increased over the years.10,11 There has been symptomatic improvement and inhibition of reoccurrence of these conditions when photoprotective agents are used either therapeutically or prophylactically, indicating the need to promote and regularize their application.
The authors intend to spread awareness among physicians regarding the amount of sunscreening agents needed, method of application, reapplication, and the importance of patient education in all populations in order to reduce the damaging solar effects on skin.
Composition and Mechanism of Action
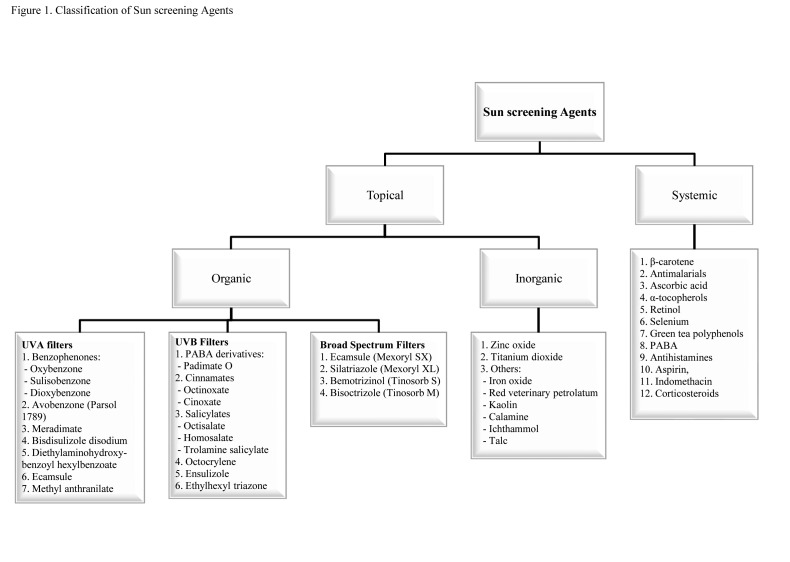
Sunscreening agents contain titanium dioxide (TiO2), kaolin, talc, zinc oxide (ZnO), calcium carbonate, and magnesium oxide. Newer chemical compounds, such as bemotrizinol, avobenzone, bisoctizole, benzophenone-3 (BZ-3, oxybenzone), and octocrylene, are broad-spectrum agents and are effective against a broad range of solar spectrum both in experimental models and outdoor settings. Ecamsule (terephthalylidene dicamphor sulphonic acid), dometrizole trisiloxane, bemotrizinol, and bisoctrizole are considered organic UVA sunscreening agents. Classification12 of sunscreening agents is shown in Figure 1. Commercial preparations available in the market include a combination of these agents to cover a wide range of UV rays.
Figure 1.
Classification of sunscreening agents
Composition and mechanism of action of sunscreening agents vary from exerting their action through blocking, reflecting, and scattering sunlight. Chemical sunscreens absorb high-energy UV rays, and physical blockers reflect or scatter light. Multiple organic compounds are usually incorporated into chemical sunscreening agents to achieve protection against a range of the UV spectrum. Inorganic particulates may scatter the microparticles in the upper layers of skin, thereby increasing the optical pathway of photons, leading to absorption of more photons and enhancing the sun protection factor (SPF), resulting in high efficiency of the compound.13,14
Researchers are postulating that the generation of sunlight-induced free radicals causes changes in skin; use of sunscreens reduces these free radicals on the skin, suggesting the antioxidant property.15 Broad-spectrum agents have been found to prevent UVA radiation-induced gene expression in vitro in reconstructed skin and in human skin in vivo.16
Insect repellents, such as picaridin and N, N-diethyl-3-methylbenzamide (DEET), have been incorporated into sunscreening agents to minimize the risk of developing insect-borne infections. Picardin was found to be a more suitable component than DEET when used along with BZ-3, as it minimizes the penetration of chemicals.17
Ideal sunscreening agents should be safe, chemically inert, nonirritating, nontoxic, photostable, and able to provide complete protection to the skin against damage from solar radiation. They should be formulated in a cosmetically acceptable form and ingredients should remain on the upper layers of the skin even after sweating and swimming. Sunscreening agents should provide efficient scavenging activities against singlet oxygen and other reactive oxygen species.18 They should also effectively block both UVB and UVA rays, which is possible with an agent that has an SPF of 30 or greater. Sunscreens with an SPF of 30 or greater that incorporate photostable or photostabilized UVA filters (labeled as “broad spectrum” in the US) are usually ideal.19 Sunscreens should not only protect the skin from the sun, but also minimize the cumulative health hazards from sun damage caused over time.
Factors Determining Efficacy
SPF and substantivity (the property of continuing therapeutic action despite removal of the vehicle ) are the factors that contribute to the effectiveness of sunscreening agents.20 UVB protection is measured by a product's SPF, which theoretically indicates that products with high SPFs provide more protection against hazardous effects of sunlight than those with low SPFs.21 SPF is measured as the ratio of the amount of UV radiation required to burn the protected skin (with sunscreen) to that required to burn the same unprotected skin (without sunscreen), all other factors being constant. SPF is measured using the following formula:
SPF = MED of protected skin/MED of unprotected skin (MED = minimal erythemal dose).
This means when a product with SPF 50 is applied, it will protect the skin until it is exposed to 50 times more UVB radiation than that is required to burn the unprotected skin.
SPF level, efficacy against a wavelength of UV radiation, and UVA/UVB ratio can be calculated using a computer program or software, such as sunscreen simulators, and can determine if the product meets the regulatory standards.
Bodekaer et al22 studied the reduction in SPF of organic and inorganic sunscreening agents in participants who, over the course of eight hours, performed physical activities, were then exposed to a hot environment, and finally bathed. There was a reduction in SPF of 38 and 41 percent after four hours and of 55 and 58 percent after eight hours of application of organic and inorganic sunscreen, respectively.22 Hence, it is necessary to apply the adequate and recommended amount of sunscreening agent to obtain the claimed benefit (i.e., 2mg/cm2, which is shown to be effective on Asian skin as well). Studies have shown that people apply about a quarter of the recommended dose of sunscreen, which is an inadequate amount.23,24
Protection offered by a sunscreening agent against UVA radiation is measured by the Persistent Pigment Darkening (PPD) Protection Factor. This technique was developed in Japan and has been routinely used by manufacturers. Stanfield25 has discussed its disadvantages. PPD is not done in skin type 1, which is the skin type more prone to solar damage. For wavelengths less than 320nm, action sprectrum is not defined; moreover, clinical significance of PPD is not very clear.
Immediate pigment darkening response is calculated as the dose of UVA required to produce the effect with the sunsceening agent to that produced without an agent.26 Although this test gives rapid results for low doses of UVA, responses have been found to be highly variable and an accurate reproduction of results is difficult. This is usually performed in skin types III and IV and its clinical significance is unknown.27
COLIPA guideline is a new standardized, reproducible, and in-vitro method to measure UVA protection offered by sunscreening products and was developed by “In-Vitro Sun Protection Methods” group. This has been in use by European Union (EU) countries for testing and labeling sunscreen products as it is in line with regulatory recommendation.28
Immunoprotection factor is a measure of a sunscreening agent to prevent UV-induced immunosuppression.12
Regulatory Guidelines
Misguiding information on sunscreen labels has compelled regulatory agencies to make changes in the international regulatory guideline on sunscreens to avoid confusion among the general public, to assist them in selecting a suitable agent, to provide adequate sun protection, and to minimize health hazards of solar damage including the occurrence of skin cancers.
United States Food and Drug Administration (FDA) guidelines. Previously, FDA guidelines29,30 aimed at protection against UVB radiation and sunburns, not toward protection against UVA radiation and prevention of skin cancers. Inappropriate and misguiding labeling with false claims has made the FDA revise its guidelines on sunscreening agents. New, improvised guidelines address such issues as “broad-spectrum designation, use claims, waterproof, sweat proof, sun proof, and water resistance claims and drug facts”. According to the new guidelines, claims about UVA and UVB protection should be made only after the specific tests have proved the same. It is mandatory to test both UVA and UVB radiation. A product can be classified as a broad-spectrum sunscreening agent if it passes the required test, but the reduction in the risk of skin cancer and early skin aging when used as per direction can be stated by only those with SPF 15 or higher. Those with SPF 2 to 14 cannot state the latter.
Labels claiming sunscreens are “waterproof,” “sweat proof,” or “sun blocks” are not legally permitted as these claims overemphasize the product's efficacy. If a product claims to be water resistant, the label should clearly indicate the duration of effectiveness (e.g., 40 minutes or 80 minutes) during activities such as swimming. If the product does not claim to be water resistant, consumers should be instructed to use a water-resistant sunscreen during swimming and those activities that produce sweat. Reapplication for better efficacy has to be mentioned on the label, and manufacturers are not allowed to claim sun protection lasting more than two hours without reapplication. Claims, such as instant protection, are also not permitted. If any such claims are made, supporting data should be submitted to obtain FDA approval.
Labels should also include standard drug facts. Products containing an SPF of more than 50 should mention in the label that there is a lack of evidence to support that sunscreens with an SPF of more than 50 have better efficacy than those containing SPF 50 or below. Manufacturers have to submit supporting data if the formulation is a spray or another dosage form of which comparison with the regular dosage form, such as cream or lotion, is not possible. These new rules became effective June 18, 2012.
EU guidelines. Revised EU guidelines31,32 mandate a minimum level of UVA protection in terms of SPF. The UVA protection factor measured by PPD (in vivo) or COLIPA (in vitro) must be at least one-third of the SPF in-vivo value. Products with SPF 6, 10, 15, 20, 25, 30, 50, 50+ are permitted for consumer use and are categorized as low (SPF 6, 10), medium (SPF 15, 20, 25), high (SPF 30, 50), and very high (SPF 50+). Compounds should provide protection against a minimum critical wavelength of 37nm, which is also under consideration. Products that meet the regulatory standard will have the UVA seal.
Actual protection against UVA is represented by a star system for easy understanding by consumers. This measure was developed by Boots Company in Nottingham, United Kingdom, and was based on Diffey's UVA/UVB ratio. The star system ranges from one to five stars where 1=minimum sun protection, 2=moderate, 3=good, 4=superior, and 5=ultra.
Guidelines from other countries. Japan, Australia, and New Zealand have their own indices on UV protection factor.12 Australian standards define UVA protection in a compound when the transmission of sunlight between a wavelength of 320 and 360nm (at a path length of 8µm) is less than 10% (of the incoming light that is passing through).
New Australian guidelines have set SPF 50+ as a benchmark for sunscreening agents. It has also endorsed the revisions by international standards on terminology, such as “water resistant,” “waterproof,” “sun block,” and “sweat resistant,” as these terms are misleading to consumers. High requirements have been set by the guideline regarding water resistance as per their lifestyle requirement.33
Japanese regulatory guidelines34 describe the method of testing the photoprotection factor of UVA (PFA) as the amount of product to be applied, dose of radiation, and radiation field. These guidelines define minimal persistent pigment darkening (MPPD) dose as the minimum dose of UV rays required to produce slight darkening over the whole radiation area within 2 to 4 hours after exposure. The guidelines also define the time to measure MPPD and who should measure it. PFA is calculated using the following formula:
PFA = MPPD of protected skin/MPPD of unprotected skin. Products are graded based on the PFA value (Table 1).
TABLE 1.
Photoprotection grades according to Japanese cosmetic industry association guidelines
| PHOTOPROTECTION FACTOR OF UVA VALUE | PROTECTION GRADE OF UVA (PA) | PROTECTION LEVEL |
|---|---|---|
| 2 or more, but less than 4 | PA+ | Low |
| 4 or more, but less than 8 | PA++ | Moderate |
| 8 or more | PA+++ | High |
Source: JCIA/persistent pigment darkening protocol
Korea follows Korean measurement standards for UV protection effects (KFDA) and has standards for UVB protection (SPF measurement) and protection grade of UVA (PA). On labels, SPF should be listed for UVB and PA for UVA.35
India guidelines. In India, there are no industry guidelines for standardizing sunscreen agents and there is no detailed list of approved products. The Indian regulatory agency's official website lists only two combination products as approved drugs (Table 2). Many products are classified as cosmetics and are not listed in this section. Apart from routinely used agents, such as BZ-3, ZNO, and TiO2, other agents, such as camphor benzalkonium methosulfate (6%), octyl salicylate (5%), camphor derivatives, and broad-spectrum UV filters (i.e., bis-ethylhexyloxyphenol mcthoxyphcnyl triazine [10%] and methylene bis-benzotriazolyl tetramethylbutylphenol [10%]) are widely used. Table 2 lists some of these agents, which are manufactured by pharmaceutical companies and are available in India. Most of the products available are combination products.
TABLE 2.
Approved sunscreeening agents (combination products) and available preparations in India
| COMBINATION APPROVED BY INDIAN REGULATORY AUTHORITY* | PROTECTION GRADE OF UVA (PA) APPROVED CONCENTRATION (%) | |
|---|---|---|
| Octinoxate + Avobenzone + Oxybenzone + Octocrylene + Zinc Oxide lotion (approved on March 19, 2009) | 7.5+2+3+3+2 | |
| Octinoxate + Avobenzone + Oxybenzone + Titanium dioxide lotion (approved on March 23, 2009) | 7.5+3+3+2 | |
| COMPOUNDS AVAILABLE IN INDIA† | CONCENTRATION (%) | SPF |
| Bis-Ethylhexyloxyphenol Methoxyphenyl Triazine ((Tinosorb S, Bemotrizinol)+ Butyl Methoxydibenzoylmethane ((Tinosorb M- active, Avobenzone)+ Methylene Bis-Benzotriazolyl Tetramethylbutylphenol ( Bisoctrizole )+ Benzophenone-3 (Uvinul M40)+ Octocrylene (Uvinul N539T) | 2+2+5+2+7 | 30 |
| Bis-Ethylhexyloxyphenol Methoxyphenyl Triazine + Butyl Methoxydibenzoylmethane Methylene Bis-Benzotriazolyl Tetramethylbutylphenol ((Tinosorb M, active) + Benzophenone-3 (Uvinul M40)+ Octocrylene (Uvinul N539T) | 5+2+7+3+10 | 50 |
| Tinosorb M; Octinoxate; Octyl triazone [EHT/Uvinul T150] - S3 Complex | 60 | |
| Octinoxate +Avobenzone + Oxybenzone + Octocrylene + Zinc Oxide | 7.5 +2+3+3+2 | 50+ |
| Oxybenzone + OMC + Tinosorb M | 3+5+8 | 30 |
| Tinosorb M +Octinoxate | 30 | |
| Octinoxate +Avobenzone +Oxybenzone + Zinc Oxide | 7.5 +2+3+2 | 26 |
| Octinoxate +Avobenzone +Oxybenzone + Zinc Oxide (micronized) | 7.5 +2+3+6 | NA |
| OMC + Oxybenzone + Titanium Dioxide | 7.5 +3+3 | 20.47 |
| OMC + Oxybenzone + Titanium Dioxide | 8+3+6 | 50+ |
| Zinc Oxide + Octinoxate | 15.5+7.5 | 30 |
| Octylmethoxycinnamate+oxybenzene+Titanium dioxide | 8.5+3+6.5% | 50+ |
| OMC+Avobenzone+Phenyl benzimidazole suphomived | 7.5+2+2 | 30+ |
Source: Central Drugs Standard Control Organization, New Delhi, India
Although many sunscreening products are available in India, composition details are not available, as they are marketed as cosmetics. The authors have mentioned only a few agents for which composition details are available. Most of the products are combination products and a branded product containing one active ingredient other than ZnO is rare in the Indian market.
Pharmacokinetics
It was observed that lipid microparticles loaded with ethylhexyl methoxycinnamate (EHMC), which filters UVB, and butyl methoxydibenzoylmethane (BMDBM), which filters UVA, had reduced skin penetration, thus preserving the UV filter efficacy and limiting potential toxicological risks.36 Gonzalez et al37 studied the percutaneous absorption of BZ-3 after repeated whole-body applications, with and without UV irradiation in 25 volunteers. They observed that large amounts of BZ-3 is absorbed, accumulated in the body, and excreted, even after five days after the last application.37 In another study, pharmacokinetics of BZ-3 was studied in 11 healthy volunteers after topical application. After 48 hours, the average amount of BZ-3 excreted in urine was 11mg (median=9.8mg). In some volunteers, BZ-3 was excreted even after 48 hours. This study showed that BZ-3 undergoes conjugation and converts to a water-soluble compound. The age at which liver attains maturity and is able to metabolize these chemicals and conjugate is unknown. Therefore, it is recommended that physical filters (i.e., zinc oxide, titanium dioxide, ferrous oxide) be used in children.38 BZ-3 is FDA approved for use in children above six months of age.
Pharmacokinetics of the following three chemical UV absorbers—benzophenone-3 (BZ-3), octyl-methoxy-cinnamate (OMC), and 3-(4-methylbenzylidene) camphor (4-MBC)—were studied in 32 healthy volunteers, 15 of whom were young male volunteers and 17 of whom were postmenopausal women. The volunteers were exposed to daily whole-body topical application of 2mg/cm2 of sunscreen formulation at 10% (weight/weight) for four days. Blood and urine concentrations were measured at regular intervals as specified in the protocol. Before the first application of these agents, their concentration was undetectable in plasma and urine, but was detectable 1 to 2 hours after the first application. In female volunteers, the maximum median plasma concentrations of 187ng/mL BP-3, 16ng/mL 4-MBC, and 7ng/mL OMC were seen. In male volunteers, maximum median plasma concentrations were 238ng/mL (BZ-3), 18ng/mL (4-MBC), and 16ng/mL OMC.
The urinary concentration level of BZ-3 was higher in men (81ng/mL) than women (44ng/mL). However, no significant changes were seen with other agents (female volunteers = 4ng/mL of 4-MBC and 6ng/mL OMC; male volunteers = 4ng/mL of 4-MBC and OMC). Men showed a higher concentration of 4-MBC and OMC, whereas women showed a similar pattern in BZ-3 and 4-MBC when 96-hour median concentrations were compared to 24-hour concentrations.39
Janjua et al40 studied the absorption of sunscreens BZ-3, octyl-methoxycinnamate (OMC), and 3-(4-methyl-benzylidene) camphor (4-MBC) from topical application and their effects on the endogenous reproductive hormones in 32 healthy volunteers. After two-week, whole-body application, there was no change in follicle-stimulating hormone (FSH) levels or luteinizing hormone (LH) levels, but there was a minor difference in testosterone levels. In men, a minor difference in serum estradiol and inhibin B levels were observed.40
Formulation also plays a role in the penetration of the compound to the skin. Skin penetration of BZ-3 is faster and greater if formulated as emulsion. However, the rate of penetration is dependent on the concentration of BZ-3 in the formulation.41
Filipe et al42 studied the localization of TiO2 and ZnO nanoparticles and their skin penetration levels and concluded that drug concentration was either undetectable or insufficient under the stratum corneum, indicating minimal systemic absorption with no or minimal penetration into keratinocytes and good skin retention.42–44 Lacatusu et al45 showed that coupling UV absorbers and lipid nanoparticles makes the combination photostable and provides better photoprotection.45
Efficacy
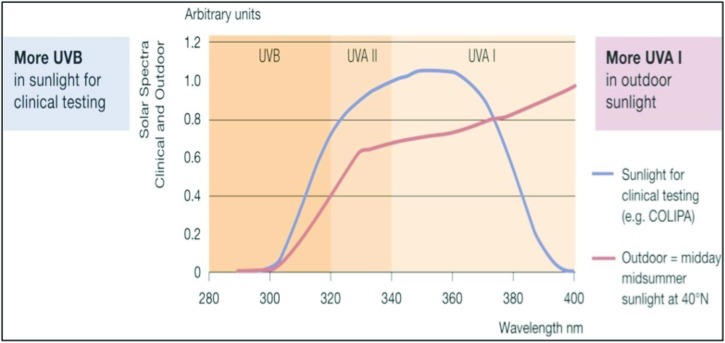
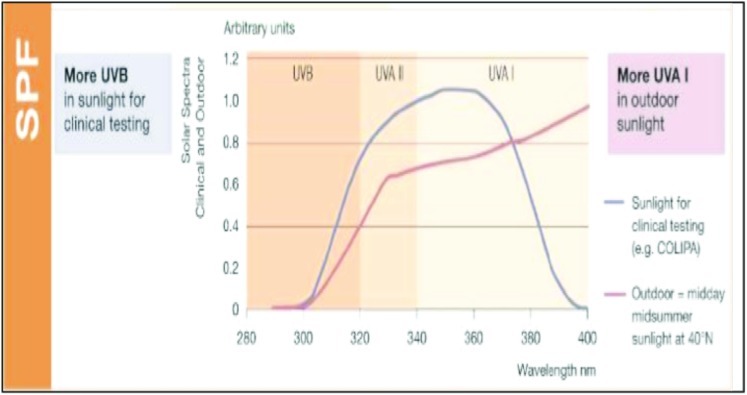
Efficacy of a sunscreen is tested in vitro and in vivo for SPF, UVA indices, and UV protection profile. Figures 2 and 3 show the SPF and UV indices for a product with SPF 30 and Figures 4 and 5 show that for a compound with SPF 50. The ability of a sunscreen to absorb UV radiation is measured in terms of extinction coefficient value. Figure 6 shows the optimal UV protection across the full UV spectrum of various UV filters.
Figure 2.
SPF profile of a product with SPF 30
(Source: Data on file, BASF sunscreen simulator predictions of clinical and outdoor SPF based on UV filter composition. Shadows 30, Dr. Reddy's Laboratories Ltd., India.)
Figure 3.
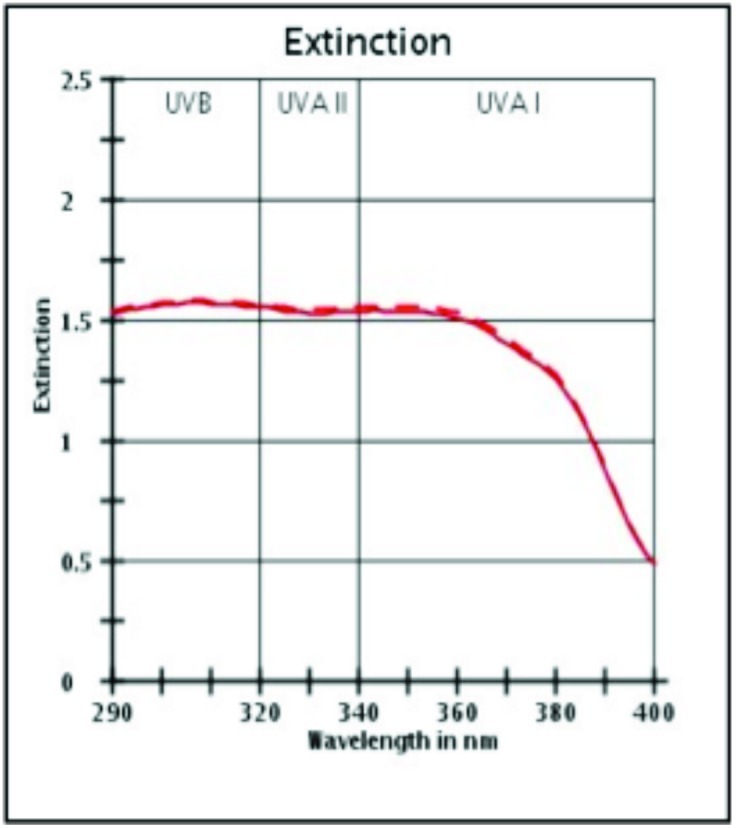
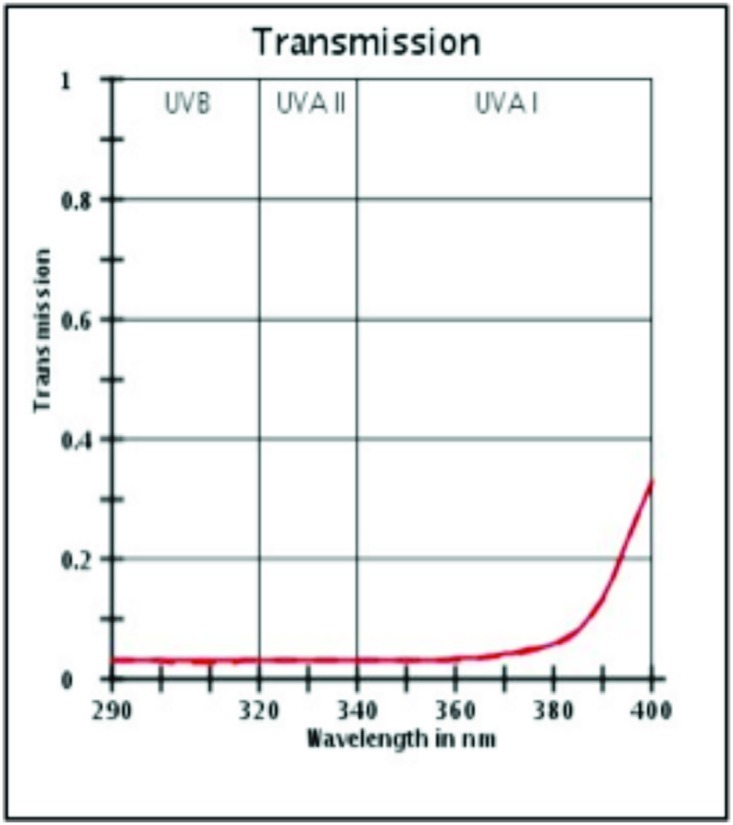
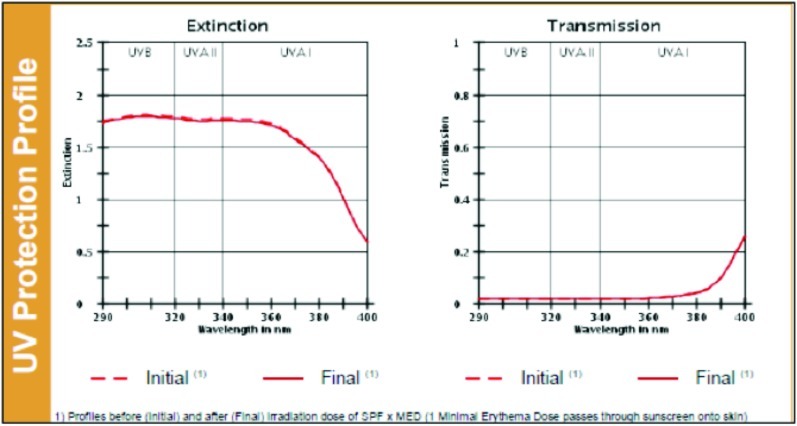
UV protection profile of a product with SPF 30. Profiles before (initial) and after (final) irradiation dose of SPF × MED (1 Minimal Erythema Dose passes through sunscreen onto skin).
(Source: Data on file, BASF sunscreen simulator predictions of clinical and outdoor SPF based on UV filter composition. Dr. Reddy's Laboratories Ltd., India.)
Figure 4.
SPF profile of a product with SPF 50
(Source: Data on file, BASF sunscreen simulator predictions of clinical and outdoor SPF based on UV filter composition. Shadows 50, Dr. Reddy's Laboratories Ltd., India.)
Figure 5.
UV protection profile of a product with SPF 50
(Source: Data on file, BASF sunscreen simulator predictions of clinical and outdoor SPF based on UV filter composition. Shadows 50, Dr. Reddy's Laboratories Ltd., India.)
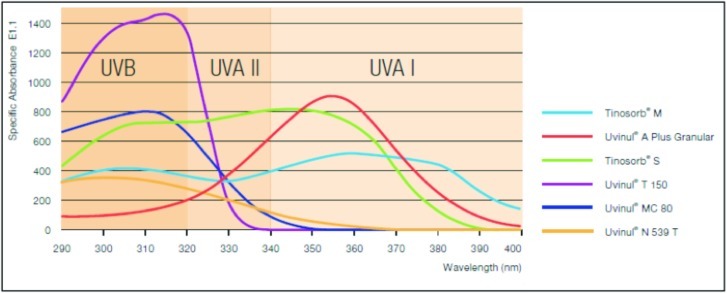
Figure 6.
Optimal UV protection across the full UV spectrum of various UV filters
(Source: Sun Care, Optimal Sun Protection, BASF.)
Studies
Studies are suggesting the use of broad-spectrum sunscreening agents for greater protection.46 Daily application of these agents helps in minimizing solar UV-induced skin changes.47 Results of a study by Diffey11 indicated that regular use of topical photoprotective agents significantly reduces lifetime UV exposure to the face compared to nonuse. The study also emphasized that it is very important to begin regular daily use of topical sunscreens early in life. Sunscreens are used more during the summer season than throughout the year in some regions. Consumers consider SPF, action of sunscreens against UV range, and the usage pattern as less important.11 Green et al48 observed reduction in the incidence of squamous cell carcinoma (40%) and basal cell carcinoma with regular use of sunscreens, supporting their role in the prevention of these skin cancers.
Kuhn et al49 assessed the exclusive use of a broad-spectrum sunscreen in preventing the skin lesions in patients with different subtypes of cutaneous lupus erythematosus (CLE) induced by UV irradiation under standardized conditions in 25 patients. They concluded that use of broad-spectrum sunscreening agents prevents skin lesions in these patients. Efficacy of sunscreens depends upon skin type, amount and frequency of application, exposure to sunlight and time of day, environmental factors, and the amount of product absorbed by the skin.
Safety
The safety of sunscreening agents is determined by toxicity studies, ability to cause irritation, sensitization, phototoxicity, and its impact on environment. Hayden et al50 studied the safety of five commonly used sunscreen agents (avobenzone, octinoxate, octocrylene, BZ-3 [oxybenzone] and padimate O) by determining the penetration of topical agents and found that BZ-3 penetrated the epidermis the most after 24 hours of exposure; however, the concentration in the stratum corneum was too low to cause toxicity. Toxicities have been reported with BZ-3, which has been associated with anaphylaxis.51 The inhalation of spray sunscreens can pose a danger as well. McKinney et al observed pulmonary and cardiovascular changes in rats on inhalation of a product containing TiO2 nanoparticles.
Use of sunscreening agents in Asian skin
Asian skin is classified as type IV,26 which is darker in color, rarely burns, and is more prone to rapid tanning. Asian skin is comparatively smoother, with a slight yellowish tinge and is more prone to pigmentation. Presence of protein melanin in the skin of Asians differentiates it from the skin of Caucasians. It has been observed that melanin equally filters all wavelengths of light, thereby receiving five times less UV radiation. This protein provides photoprotection to a certain extent, minimizing phototoxicity and making the skin less vulnerable to the acute and chronic phototoxic effects.52 Nevertheless, this population shows the effects of photodamage in terms of pigmentation, wrinkling, and sunburn. The formation of freckles in the Asian population is encountered much less frequently. However, overexposure to sunlight can cause photodamaging effects, including skin cancers. Hence, it is advisable for Asians to use sunscreening agents regularly as a preventive measure just as it is in other parts of the world. However, since Asian skin is more prone to hypersensitivity reactions, cosmetic products should be used with care.
Sunscreen Use in Special Populations
Studies have shown that dialysis and organ transplant patients, including renal transplantation patients, should follow photoprotective measures, as they are more prone to develop skin cancers. Use of sunscreening agents have prevented the development of premalignant skin changes in these patients.53,54 Hence, physicians should educate these patients regarding the regular use of preventive measures against sun damage, including the regular use of appropriate sunscreens.
Formulations
Generally, sunscreens are available in the form of creams, lotion, gels, ointments, pastes, oils, butters, sticks, and sprays, which are considered over-the-counter (OTC) products. Less frequently used products include wipes, towelettes, powders, body washes, and shampoos, which are considered non-OTC products by the FDA. Of late, these types of products have been marketed as multifunctional cosmetic formulations incorporated into other cosmetics, such as moisturizers, facial foundations, and foam foundations (mousse). Spray or gel-based sunscreens are preferred in oily skin and acne. New sunscreens with microfine particles are found to be safe and effective in patients with acne and rosacea. Sunscreen filters are also added to hair care products, such as shampoo, to minimize sun damage to hair.
Sunscreen-containing moisturizers usually have SPFs between 15 and 30. Coverage foundations are transparent formulations containing titanium dioxide with an SPF of 2 while moderate coverage foundations are usually translucent with an SPF of 4 to 5.
Gogna et al55 observed that the use of polymethyl-methacrylate (PMMA) microspheres of ethylhexyl methoxycinnamate (EHM) increases the efficacy of the latter by four times and also improves photostability of the preparation. Sprays containing sunscreening agents with high concentrations have been found to retain the medicaments on the top layers of skin, minimizing deeper penetration.56
Studies have shown that microspheres increase the efficacy of sunscreening agents.55 Incorporation of nanoparticles has shown to increase the efficacy of sunscreen agents in terms of superior UV protection and reduced whitening on the skin in comparison with the older generations of sunscreens.57 Currently, formulations containing nanoparticles of TiO2 and ZnO are available. However, studies have shown that nanoparticles of these two compounds cause cytotoxicity, genotoxicity,58,59 and potential photocarcinogenecity.60 In addition, nanoparticles of ZnO, even at a much lower concentration, may induce inflammation by releasing inflammatory mediators, such as cytokines interleukin (IL)-6 and tumor necrosis factor (TNF)-α.61 Sunspheres and microencapsulations are newer technologies in the preparation of sunscreen formulation.12
Health Hazards of Sunscreening Agents
Although considered safe, sunscreening agents are not free from adverse effects. Sensitivity, though rare, can occur in the form of photoallergic reactions, including contact dermatitis. Photopatch testing helps to identify sensitivities.62
There have been reports of increased incidence of melanoma as a result of sunscreen use. Gorham et al63 reviewed the risk of developing melanoma as a result of sunscreen use and opined that those who live in latitudes greater than 40 degress may have an increased risk of melanoma. The reason for this may be because sunscreens absorb UVB almost completely, but transmit large quantities of UVA.63 Sunscreen use may prolong the duration of intentional exposure giving people a false sense of security, especially when using products that have high SPF ratings, thereby increasing the risk of skin cancer.64 A similar trend was observed in European countries as well.65
A product assessment in the United States in January 2011 revealed that retinyl palmitate, a form of vitamin A, which is a widely used compound in cosmetics and sunscreens (as an antioxidant against the aging effects of UV radiation), is thought to increase the rate of the development of skin tumors and lesions. However, Wang et al66 opined that its role in human carcinogenesis is doubtful as there is a lack of evidence. Fourschou et al67 noted an exponential increase in vitamin D levels with the application of thinner layers of sunscreen after UVB exposure, indicating that application of thicker layers can cause a decrease in vitamin D levels resulting in its deficiency.
It is postulated that BZ-3 can disrupt the hormones in the body. The Centers for Disease Control and Prevention has detected BZ-3 in the 97 percent of Americans tested during biomonitoring surveys. Although there have been reports of adverse events with this agent, studies have shown that products formulated with 1 to 6% of BZ-3 do not possess a significant sensitization or irritation potential for the general public.68
Exacerbation of acne and rosacea can also occur with the use of sunscreen agents that contain physical blockers, such as ZnO and TiO2, that are greasy and have large particle sizes, thereby blocking skin pores.
Effective Practice
According to the Environmental Working Group's 2010 Annual Sunscreen Guide, zinc and titanium-based sunscreens are considered more safe and effective than other products available in the United States.69 Powder and spray sunscreens should be avoided, as they may lead to inhalation of particles, which is hazardous. The FDA recommends applying 2mg/cm2 of sunscreen to achieve maximum benefits. Sunscreen should be reapplied every two hours as well as after sweating, toweling off, bathing, and swimming. It is recommended that sunscreen labels highlight the importance of reapplication.70
Avoiding exposure to sunlight during the time of day when UV radiation is at its highest—between 10 am and 3 pm—is recommended. When sun exposure during this time is unavoidable, it is advisable to use sun protection (i.e., umbrella and sun protection clothing).
Causes of Sunscreen Failure
Underapplication and failure to reapply sunscreen every two hours are the main reasons sunscreens fail. Additionally, these agents are unaffordable by many in developing and underdeveloped countries. Sunscreen use year round is expensive,71 which is why some people do not use sunscreen regularly. Another contributing factor to sunscreen failure is the mismatch between the labeled SPF and that delivered on application to skin and exposure to sunlight.
Promoting the Use of Sunscreen Agents
As the rate of sunscreen use is low, education and awareness about the hazards of sun exposure and the benefits of regularly applying sunscreening agents to reduce these effects must be spread.23,72,73 Outdoor activities should be performed before 10 am or after 3 pm.74 Education should target preadolescents so they develop the habit of using sunscreening agents at a young age, particularly as adolescents are more prone to seek sun exposure for intentional tanning purposes.75 Organ transplant patients need to be educated regarding the regular use of sunscreening agents as well.
Lack of awareness among the public regarding the use of sunscreening agents is more evident in the United States, where only about 3 in 10 adults routinely practice sun-protection behaviors. Women and older adults have been found to practice sun protection more than others.8
Role of Physician/Dermatologist
Physicians should be aware of the composition of sunscreen agents and the UVA protection factors of formulations. They should also instruct their patients about the proper application technique and insist on reapplication. Additionally, they should counsel preteens and adolescents regarding the regular and proper use of broad-spectrum sunscreens.
Recent Developments
Newer broad-spectrum chemical agents, such as bis-ethylhexyloxyphenol methoxyphenyl triazine (BEMT), methylene bis-benzotriazolyl tetramethylbutylphenol (MBBT), and butyl methoxy-dibenzoyl methane (BMDBM), have been found to be effective against UVA and UVB rays ranging from 280 to 400nm. These new agents have been formulated to be more fat soluble (oil soluble in cosmetic oils) to aid in efficacy and broad-spectrum activity. They are known to prevent the formation of free radicals induced by UV radiation to a significant level. These agents claim to be photostable, minimize erythema, and provide excellent anti-aging effects as well as protect the skin's antioxidant defense system. In studies, these new agents have shown to provide protection against intentional self tanning. Further, they also claim that there is no bioaccumulation, thereby exhibiting a good safety profile. Figure 6 shows the comparison of photoprotection by these newer compounds.76
These new broad-spectrum sunscreen agents have been found to be compliant with regulatory guidelines in terms of PPD, SPF, COLIPA, and Boots star rating. They also claim to have the following advantages: instant action, longer duration of protection, improved cosmetic appearance of the skin in the form of less wrinkles, suitability for sensitive skin, and suitability for chikdren.
Future Developments/Scope
Bacterial-derived melanin has been shown to provide significant protection to fibroblast cells against UVA radiation. It is a promising product that helps to keep UVA-irradiated skin from pigment darkening, especially in those with photosensitivity.77
The use of antioxidants has shown to minimize the release of oxidants after excessive sun exposure on unprotected skin.15 A compound that is effective in protecting against complete UV spectrum and infrared radiation is welcome.
Challenge
Despite the efforts of physicians and regulatory authorities to spread awareness regarding sunburn, skin cancer, and the benefits of regularly using sunscreening agents, treatment adherence is low.78 Providing cosmetically acceptable preparations and educating people about following the application instructions, is a challenge often faced by treating physicians. Pricing sunscreens reasonably and making them water resistant and non-sticky are a few of the challenges faced by manufacturers. Manufacturers must also consider sensitization reactions, especially in those having eczema or photodermatoses.79 Narrow-spectrum sunscreening agents, especially those that absorb only UVB rays, may contribute to the development of melanoma at latitudes over 40 degrees due to transmission of UVA rays in large amounts.63
Conclusion
Use of sunscreening agents is beneficial in minimizing the occurrence of skin cancers in people with fair skin. However, the same effect on Asian skin is debatable, as this skin type is considered to be resistant to skin cancers. Sunscreen use is advisable in young adults to prevent and minimize other photodamaging effects. Affordability and proper application techniques are the challenges that must be addressed in order to achieve regular sunscreen usage. The authors recommend further comparative studies on sunscreens as well as studies on the Indian population, as there is insufficient data in this population.
Footnotes
DISCLOSURE: Dr. Latha, Ms. Sham Shinde, Dr. Bellary, and Mr. Rao are employed by Dr. Reddy's Laboratories Ltd. and are stakeholders in Dr. Reddy's Laboratories Ltd. Dr. Krishnankutty, Dr. Kumar, and Mr. Varughese were former employees of Dr. Reddy's Laboratories Ltd. and are presently not stakeholders in Dr. Reddy's Laboratories Ltd. Dr. Martis, Dr. Bangera, and Dr. Shobha report no relevant conflicts of interest.
References
- 1.Yuan C, Wang XM, Tan YM, et al. Effects of sunscreen on human skin's ultraviolet radiation tolerance. J Cosmet Dermatol. 2010;9:297–301. doi: 10.1111/j.1473-2165.2010.00525.x. [DOI] [PubMed] [Google Scholar]
- 2.DeBuys HV, Levy SB, Murray JC, et al. Modern approaches to photoprotection. Dermatol Clin. 2000;18:577–590. doi: 10.1016/s0733-8635(05)70208-4. [DOI] [PubMed] [Google Scholar]
- 3.Ortel B, Tanew A, Wolff K, Hönigsmann H. Polymorphous light eruption: action spectrum and photoprotection. J Am Acad Dermatol. 1986;14:748–753. doi: 10.1016/s0190-9622(86)70088-1. [DOI] [PubMed] [Google Scholar]
- 4.Miyamoto C. Polymorphous light eruption: successful reproduction of skin lesions, including papulovesicular light eruption, with ultraviolet B. Photodermatol. 1989;6:69–79. [PubMed] [Google Scholar]
- 5.Ryckaert S, Roelandts R. Solar urticaria. A report of 25 cases and difficulties in phototesting. Arch Dermatol. 1998;134:71–74. doi: 10.1001/archderm.134.1.71. [DOI] [PubMed] [Google Scholar]
- 6.Stoebner PE, Poosti R, Djoukelfit K, Martinez J, Meunier L. Decreased human epidermal antigen-presenting cell activity after ultraviolet A exposure: dose-response effects and protection by sunscreens. Br J Dermatol. 2007;156:1315–1320. doi: 10.1111/j.1365-2133.2007.07895.x. [DOI] [PubMed] [Google Scholar]
- 7.Wang SQ, Setlow R, et al. Ultraviolet A and melanoma: a review. J Am Acad Dermatol. 2001:837–846. doi: 10.1067/mjd.2001.114594. [DOI] [PubMed] [Google Scholar]
- 8.Buller DB, Cokkinides V, Hall HI, et al. Prevalence of sunburn, sun protection, and indoor tanning behaviors among Americans: review from national surveys and case studies of 3 states. J Am Acad Dermatol. 2011;65:S114–S123. doi: 10.1016/j.jaad.2011.05.033. [DOI] [PubMed] [Google Scholar]
- 9.Rodvall YE, Wahlgren CF, Ullén HT, Wiklund KE. Factors related to being sunburnt in 7-year-old children in Sweden. Eur J Cancer. 2010;46:566–572. doi: 10.1016/j.ejca.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 10.Diffey BL. Sunscreens as a preventative measure in melanoma: an evidence-based approach or the precautionary principle? Br J Dermatol. 2009;161:25–27. doi: 10.1111/j.1365-2133.2009.09445.x. [DOI] [PubMed] [Google Scholar]
- 11.Diffey BL. The impact of topical photoprotectants intended for daily use on lifetime ultraviolet exposure. J Cosmet Dermatol. 2011;10:245–250. doi: 10.1111/j.1473-2165.2011.00563.x. [DOI] [PubMed] [Google Scholar]
- 12.Kaimal S, Abraham A. Sunscreens. Indian J Dermatol Venereol Leprol. 2011;77:238–243. doi: 10.4103/0378-6323.77480. [DOI] [PubMed] [Google Scholar]
- 13.Lademann J, Schanzer S, Jacobi U, et al. Synergy effects between organic and inorganic UV filters in sunscreens. J Biomed Opt. 2005;10:14008. doi: 10.1117/1.1854112. [DOI] [PubMed] [Google Scholar]
- 14.Vergou T, Patzelt A, Richter H, et al. Transfer of ultraviolet photon energy into fluorescent light in the visible path represents a new and efficient protection mechanism of sunscreens. J Biomed Opt. 2011;16:105001. doi: 10.1117/1.3631790. [DOI] [PubMed] [Google Scholar]
- 15.Meinke MC, Haag SF, Schanzer S, et al. Radical protection by sunscreens in the infrared spectral range. Photochem Photobiol. 2011;87:452–456. doi: 10.1111/j.1751-1097.2010.00838.x. [DOI] [PubMed] [Google Scholar]
- 16.Marionnet C, Grether-Beck S, Seité S, et al. A broad-spectrum sunscreen prevents UVA radiation-induced gene expression in reconstructed skin in vitro and in human skin in vivo. Exp Dermatol. 2011;20:477–482. doi: 10.1111/j.1600-0625.2011.01265.x. [DOI] [PubMed] [Google Scholar]
- 17.Chen T, Burczynski FJ, Miller DW, Gu X. Percutaneous permeation comparison of repellents picaridin and DEET in concurrent use with sunscreen oxybenzone from commercially available preparations. Pharmazie. 2010;65:835–839. [PubMed] [Google Scholar]
- 18.Giacomoni PU, Teta L, Najdek L. Sunscreens: the impervious path from theory to practice. Photochem Photobiol Sci. 2010;9:524–529. doi: 10.1039/b9pp00150f. [DOI] [PubMed] [Google Scholar]
- 19.Medeiros VL, Lim HW. Sunscreens in the management of photodermatoses. Skin Therapy Lett. 2010;15:1–3. [PubMed] [Google Scholar]
- 20.Rai R, Srinivas CR. Photoprotection. Indian J Dermatol Venereol Leprol. 2007;73(2):73–79. doi: 10.4103/0378-6323.31889. [DOI] [PubMed] [Google Scholar]
- 21.Singhal M, Khanna S, Nasa A. Cosmeceuticals for the skin: an overview. Asian J Pharm Clin Res. 2011;4:16. [Google Scholar]
- 22.Bodekaer M, Faurschou A, Philipsen PA, Wulf HC. Sun protection factor persistence during a day with physical activity and bathing. Photodermatol Photoimmunol Photomed. 2008;24:296–300. doi: 10.1111/j.1600-0781.2008.00379.x. [DOI] [PubMed] [Google Scholar]
- 23.Schalka S, dos Reis VM. Cucé LC. The influence of the amount of sunscreen applied and its sun protection factor (SPF): evaluation of two sunscreens including the same ingredients at different concentrations. Photodermatol Photoimmunol Photomed. 2009;25:175–180. doi: 10.1111/j.1600-0781.2009.00408.x. [DOI] [PubMed] [Google Scholar]
- 24.Kim SM, Oh BH, Lee YW, et al. The relation between the amount of sunscreen applied and the sun protection factor in Asian skin. J Am Acad Dermatol. 2010;62:218–222. doi: 10.1016/j.jaad.2009.06.047. [DOI] [PubMed] [Google Scholar]
- 25.Stanfield JW. Proposed method for assesstng sunscreen photostabtlity and broad spectrum protection. [September 12, 2012]. http://www.fda.gov/ohrms/dockets/dailys/00/Sep00/090700/c000574.pdf Docket No. 78N-0038. Dated 5 Sept 2005.
- 26.Kaidbey K, Barnes A. Determination of WA protection factors by means of immediate pigment darkening in normal skin. J Am Acad Dermatol. 1991;25:262–266. doi: 10.1016/0190-9622(91)70193-6. [DOI] [PubMed] [Google Scholar]
- 27.Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124:869–871. doi: 10.1001/archderm.124.6.869. [DOI] [PubMed] [Google Scholar]
- 28.Matts PJ, Alard V, Brown MW, et al. The COLIPA in vitro UVA method: a standard and reproducible measure of sunscreen UVA protection. Int J Cosmet Sci. 2010;32:35–46. doi: 10.1111/j.1468-2494.2009.00542.x. [DOI] [PubMed] [Google Scholar]
- 29.Guidance for Industry. Enforcement Policy—OTC Sunscreen Drug Products Marketed Without an Approved Application. [September 12, 2012]. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM259001.pdf Updated June 2011.
- 30.Federal Register, Part IV Department of Health and Human Services. Food and Drug Administration. 21 CFR Parts 201, 310, and 352. Sunscreen Drug Products for Over-the-Counter Human Use; Final Rules and Proposed Rules. June 17, 2011. [November 15, 2012]. http://www.gpo.gov/fdsys/pkg/FR-2011-06-17/pdf/2011-14766.pdf
- 31. Commission recommendations of 22 September 2006 on the efficacy of sunscreen products and the claims made relating thereto (2006/647/EC). Official Journal of the European Union. 26.9.2006.265/39-265/43.
- 32.Recommendations from the European Commission. Federal Office of Public Health. Recommendations from European Commission. Updated March 16, 2009. [September 12, 2012]. http://www.bag.admin.ch/themen/lebensmittel/04861/05280/06242/index.html?lang=en
- 33.Standards Australia. Media release. [July 26, 2012]. http://www.standards.org.au/OurOrganisation/News/Documentshttp://www.standards.org.au/OurOrganisation/News/Documents/120613%20Sunscreen%20SPF%2050%20plus%20V2%20CS.pdf Updated June 14, 2012.
- 34.Appendix 3: JCIA persistent pigment darkening protocol. [July 26, 2012]. http://www.fda.gov/ohrms/dockets/dailys/00/Sep00/090600/c000565_appendix_03.pdf
- 35.KFDA: Cosmetics. Evaluation of sun protection effects. [July 26, 2012]. http://www.kfda.go.kr/eng/eng/index.do;jsessionid=mA2u578F9n89nYaF8nXqg88afBkajbKvsalw0ZyzInbbQi79ePrcn0eb31Hs67Tx?nMenuCode=35&searchKeyCode=73&page=1&mode=view&boardSeq=66576
- 36.Scalia S, Mezzena M, Ramaccini D. Encapsulation of the UV filters ethylhexyl methoxycinnamate and butyl methoxydibenzoylmethane in lipid microparticles: effect on in vivo human skin permeation. Skin Pharmacol Physiol. 2011;24:182–189. doi: 10.1159/000324054. [DOI] [PubMed] [Google Scholar]
- 37.Gonzalez H, Farbrot A, Larkö O, Wennberg AM. Percutaneous absorption of the sunscreen benzophenone-3 after repeated whole-body applications, with and without ultraviolet irradiation. Br J Dermatol. 2006;154:337–340. doi: 10.1111/j.1365-2133.2005.07007.x. [DOI] [PubMed] [Google Scholar]
- 38.Gustavsson GH, Farbrot A, Larkö O. Percutaneous absorption of benzophenone-3, a common component of topical sunscreens. Clin Exp Dermatol. 2002;27:691–694. doi: 10.1046/j.1365-2230.2002.01095.x. [DOI] [PubMed] [Google Scholar]
- 39.Janjua NR, Kongshoj B, Andersson AM, Wulf HC. Sunscreens in human plasma and urine after repeated whole-body topical application. J Eur Acad Dermatol Venereol. 2008;22:456–461. doi: 10.1111/j.1468-3083.2007.02492.x. [DOI] [PubMed] [Google Scholar]
- 40.Janjua NR, Mogensen B, Andersson AM, et al. Systemic absorption of the sunscreens benzophenone-3, octyl-methoxycinnamate, and 3-(4-methyl-benzylidene) camphor after whole-body topical application and reproductive hormone levels in humans. J Invest Dermatol. 2004;123:57–61. doi: 10.1111/j.0022-202X.2004.22725.x. [DOI] [PubMed] [Google Scholar]
- 41.Wissing SA, Müller RH. Solid lipid nanoparticles as carrier for sunscreens: in vitro release and in vivo skin penetration. J Control Release. 2002;81:225–233. doi: 10.1016/s0168-3659(02)00056-1. [DOI] [PubMed] [Google Scholar]
- 42.Filipe P, Silva JN, Silva R, et al. Stratum corneum is an effective barrier to TiO 2 and ZnO nanoparticle percutaneous absorption. Skin Pharmacol Physiol. 2009;22:266–275. doi: 10.1159/000235554. [DOI] [PubMed] [Google Scholar]
- 43.Newman MD, Stotland M, Ellis JI. The safety of nanosized particles in titanium dioxide- and zinc oxide-based sunscreens. J Am Acad Dermatol. 2009;61:685–692. doi: 10.1016/j.jaad.2009.02.051. [DOI] [PubMed] [Google Scholar]
- 44.Scalia S, Mezzena M, Iannuccelli V. Influence of solid lipid microparticle carriers on skin penetration of the sunscreen agent, 4-methylbenzylidene camphor. J Pharm Pharmacol. 2007;59:1621–1627. doi: 10.1211/jpp.59.12.0003. [DOI] [PubMed] [Google Scholar]
- 45.Lacatusu I, Badea N, Murariu A, Meghea A. The encapsulation effect of UV molecular absorbers into biocompatible lipid nanoparticles. Nanoscale Res Lett. 2011;6:73. doi: 10.1186/1556-276X-6-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Young AR, Boles J, Herzog B, et al. A sunscreen's labeled sun protection factor may overestimate protection at temperate latitudes: a human in vivo study. J Invest Dermatol. 2010;130:2457–2462. doi: 10.1038/jid.2010.144. [DOI] [PubMed] [Google Scholar]
- 47.Seité S, Fourtanier AM. The benefit of daily photoprotection. J Am Acad Dermatol. 2008;58:S160–S166. doi: 10.1016/j.jaad.2007.04.036. [DOI] [PubMed] [Google Scholar]
- 48.Green A, Williams G, Neale R, et al. Daily sunscreen application and betacarotene supplementation in prevention of basal-cell and squamous-cell carcinomas of the skin: a randomised controlled trial. Lancet. 1999;354:723–729. doi: 10.1016/S0140-6736(98)12168-2. [DOI] [PubMed] [Google Scholar]
- 49.Kuhn A, Gensch K, Haust M, et al. Photoprotective effects of a broad-spectrum sunscreen in ultraviolet-induced cutaneous lupus erythematosus: a randomized, vehicle-controlled, double-blind study. J Am Acad Dermatol. 2011;64:37–48. doi: 10.1016/j.jaad.2009.12.053. [DOI] [PubMed] [Google Scholar]
- 50.Hayden CG, Cross SE, Anderson C, et al. Sunscreen penetration of human skin and related keratinocyte toxicity after topical application. Skin Pharmacol Physiol. 2005;18:170–174. doi: 10.1159/000085861. [DOI] [PubMed] [Google Scholar]
- 51.Spijker GT, Schuttelaar ML, Barkema L, et al. Anaphylaxis caused by topical application of a sunscreen containing benzophenone-3. Contact Dermatitis. 2008;59:248–249. doi: 10.1111/j.1600-0536.2008.01337.x. [DOI] [PubMed] [Google Scholar]
- 52.Kaidbey KH, Agin PP, Sayre RM, Kligman AM. Photoprotection by melanin—a comparison of black and Caucasian skin. J Am Acad Dermatol. 1979;1:249–260. doi: 10.1016/s0190-9622(79)70018-1. [DOI] [PubMed] [Google Scholar]
- 53.Ulrich C, Degen A, Patel MJ, Stockfleth E. Sunscreens in organ transplant patients. Nephrol Dial Transplant. 2008;23:1805–1808. doi: 10.1093/ndt/gfn292. [DOI] [PubMed] [Google Scholar]
- 54.Skiveren J, Mortensen EL, Haedersdal M. Sun protective behaviour in renal transplant recipients. A qualitative study based on individual interviews and the Health Belief Model. J Dermatolog Treat. 2010;21:331–336. doi: 10.3109/09546630903410166. [DOI] [PubMed] [Google Scholar]
- 55.Gogna D, Jain SK, Yadav AK, Agrawal GP. Microsphere based improved sunscreen formulation of ethylhexyl methoxycinnamate. Current Drug Delivery. 2007;4:153–159. doi: 10.2174/156720107780362285. [DOI] [PubMed] [Google Scholar]
- 56.Durand L, Habran N, Henschel V, Amighi K. In vitro evaluation of the cutaneous penetration of sprayable sunscreen emulsions with high concentrations of UV filters. Int J Cosmet Sci. 2009;31:279–292. doi: 10.1111/j.1468-2494.2009.00498.x. [DOI] [PubMed] [Google Scholar]
- 57.Wang SQ, Tooley IR. Photoprotection in the era of nanotechnology. Semin Cutan Med Surg. 2011;30:210–213. doi: 10.1016/j.sder.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 58.Sharma V, Singh SK, Anderson D, et al. Zinc oxide nanoparticle induced genotoxicity in primary human epidermal keratinocytes. J Nanosci Nanotechnol. 2011;11:3782–3788. doi: 10.1166/jnn.2011.4250. [DOI] [PubMed] [Google Scholar]
- 59.Iavicoli I, Leso V, Fontana L, Bergamaschi A. Toxicological effects of titanium dioxide nanoparticles: a review of in vitro mammalian studies. Eur Rev Med Pharmacol Sci. 2011;15:481–508. [PubMed] [Google Scholar]
- 60.Tran DT, Salmon R. Potential photocarcinogenic effects of nanoparticle sunscreens. Australas J Dermatol. 2011;52:1–6. doi: 10.1111/j.1440-0960.2010.00677.x. [DOI] [PubMed] [Google Scholar]
- 61.Heng BC, Zhao X, Tan EC, et al. Evaluation of the cytotoxic and inflammatory potential of differentially shaped zinc oxide nanoparticles. Arch Toxicol. 2011;85:1517–1528. doi: 10.1007/s00204-011-0722-1. [DOI] [PubMed] [Google Scholar]
- 62.Kerr A, Ferguson J. Photoallergic contact dermatitis. Photodermatol Photoimmunol Photomed. 2010;26:56–65. doi: 10.1111/j.1600-0781.2010.00494.x. [DOI] [PubMed] [Google Scholar]
- 63.Gorham ED, Mohr SB, Garland CF, et al. Do sunscreens increase risk of melanoma in populations residing at higher latitudes? Ann Epidemiol. 2007;17:956–963. doi: 10.1016/j.annepidem.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 64.Autier P. Sunscreen abuse for intentional sun exposure. Br J Dermatol. 2009;161:40–45. doi: 10.1111/j.1365-2133.2009.09448.x. [DOI] [PubMed] [Google Scholar]
- 65.Autier P, Boniol M, Doré JF. Sunscreen use and increased duration of intentional sun exposure: Still a burning issue. Int J Cancer. 2007;121:1–5. doi: 10.1002/ijc.22745. [DOI] [PubMed] [Google Scholar]
- 66.Wang SQ, Dusza SW, Lim HW. Safety of retinyl palmitate in sunscreens: a critical analysis. J Am Acad Dermatol. 2010;63:903–906. doi: 10.1016/j.jaad.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 67.Faurschou A, Beyer DM, Schmedes A, et al. The relation between sunscreen layer thickness and vitamin D production after UVB exposure—a randomised clinical trial. Br J Dermatol. 2012;167:391–395. doi: 10.1111/j.1365-2133.2012.11004.x. [DOI] [PubMed] [Google Scholar]
- 68.Agin PP, Ruble K, Hermansky SJ, McCarthy TJ. Rates of allergic sensitization and irritation to oxybenzone-containing sunscreen products: a quantitative meta-analysis of 64 exaggerated use studies. Photodermatol Photoimmunol Photomed. 2008;24:211–217. doi: 10.1111/j.1600-0781.2008.00363.x. [DOI] [PubMed] [Google Scholar]
- 69. [June 12, 2012]. http://www.ewg.org/nanotechnology-sunscreens Nanotechnology and sunscreens: environmental working group. EWG's 2009 Sunscreen Investigation. Washington DC 2009.
- 70.Buller DB, Andersen PA, Walkosz BJ, et al. Compliance with sunscreen advice in a survey of adults engaged in outdoor winter recreation at high-elevation ski areas. J Am Acad Dermatol. 2012;66:63–70. doi: 10.1016/j.jaad.2010.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mahé E, Beauchet A, de Maleissye MF, Saiag P. Are sunscreens luxury products? J Am Acad Dermatol. 2011;65:e73–e79. doi: 10.1016/j.jaad.2010.06.043. [DOI] [PubMed] [Google Scholar]
- 72.Al Robaee AA. Awareness to sun exposure and use of sunscreen by the general population. Bosn J Basic Med Sci. 2010;10:314–318. doi: 10.17305/bjbms.2010.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Olson AL, Gaffney CA, Starr P, Dietrich AJ. The impact of an appearance-based educational intervention on adolescent intention to use sunscreen. Health Educ Res. 2008;23:763–769. doi: 10.1093/her/cym005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Haack RL, Horta BL, Cesar JA. Sunburn in young people: population-based study in Southern Brazil. Rev Saude Publica. 2008;42(1):26–33. doi: 10.1590/s0034-89102008000100004. [DOI] [PubMed] [Google Scholar]
- 75.Robinson NG, White KM, Young RM, et al. Young people and sun safety: the role of attitudes, norms and control factors. Health Promot J Austr. 2008;19:45–51. doi: 10.1071/he08045. [DOI] [PubMed] [Google Scholar]
- 76. Uvinul A Plus [Product information]. BASF, the chemical company; 2012.
- 77.Geng J, Tang W, Wan X, et al. Photoprotection of bacterial-derived melanin against ultraviolet A-induced cell death and its potential application as an active sunscreen. J Eur Acad Dermatol Venereol. 2008;22:852–858. doi: 10.1111/j.1468-3083.2007.02574.x. [DOI] [PubMed] [Google Scholar]
- 78.Armstrong AW, Watson AJ, Makredes M, et al. Text-message reminders to improve sunscreen use: a randomized, controlled trial using electronic monitoring. Arch Dermatol. 2009;145:1230–1236. doi: 10.1001/archdermatol.2009.269. [DOI] [PubMed] [Google Scholar]
- 79.Pustisek N, Lipozencic J, Ljubojevic S. A review of sunscreens and their adverse reactions. Acta Dermatovenerol Croat. 2005;13:28–35. [PubMed] [Google Scholar]