Abstract
Many advances in the cosmetic industry have increased our ability to enhance youth and beauty. Hair-coloring products are one such innovation. Over the past several decades, a significant amount of work has been dedicated to understanding the possible long-term side effects associated with hair-dye use, specifically looking at cancer risk. This paper describes the hair-coloring process, highlights the potentially carcinogenic ingredients in various hair-dying products, and reviews the epidemiological evidence relating personal hair-dye use to the risk of developing several types of malignancies.
Introduction
Historically, hair has functioned as a social indicator of attractiveness, femininity, masculinity, health, and beauty. Maintaining the color of one’s hair has become of critical importance, especially in a society focusing more and more on youthfulness. In essence, gray hair is equated with old age. As such, dyeing one’s hair is popular among consumers attempting to project a more youthful look.
The association between the application of hair dye and the development of cancer has been a topic of debate over the past several decades. Despite these controversies, it has been estimated that 66 to 74 percent of women use hair-coloring products.1 Therefore, the causal relationship between cancer and hair-dye application raises an important public health concern.
This article critically examines the potential risks of hair-dye coloring. The different types of hair dyes available to consumers on the market and the process involved in hair coloring are reviewed. In an attempt to debunk the myth relating hair-dye exposure to cancer development, the available meta-analytic studies for bladder, hematological, breast, skin, ovarian, and cervical malignancies are reviewed. Meta-analyses questioning the use of hair-dye risk to offspring of pregnant women were not found, but given that many pregnant patients frequently ask questions on the use of hair-dye exposure and fetal risk, all known studies are reviewed.
Historical perspectives
In the United States, the United States Food and Drug Administration (FDA) regulates the safety of cosmetic products. Additionally, the Cosmetic Ingredient Review (CIR) program was established in 1976 by the Cosmetic, Toiletry, and Fragrance Association (CTFA) to provide an independent expert assessment of product safety. It is now known as the Personal Care Products Council.
Chemicals contained in hair dyes, particularly aromatic amines, are mutagenic in vitro,2–26 carcinogenic to animals,18–31 and able to penetrate human skin.32–41 In 1980, the FDA required label warning on hair dyes containing 4-methoxy-m-phenylenediamine (4-MMPD) stating the following: “contains an ingredient that can penetrate your skin and has been determined to cause cancer in laboratory animals.”42 In addition, hair dye products containing 4-chloro-m-phenylenediamine, 2,4-toluenediamine, 2-nitro-p-phenylenediamine, and 4-amino-2-nitrophenol have also been found to exhibit dermal penetration in humans and have produced carcinogenic effects on laboratory animals.42 In response, the cosmetic industry has stopped incorporating such ingredients into hair dyes, resulting in drastic formulary changes post-1980s. At this time, commercially available hair-dye formulations contain chemical ingredients that are similar to the aforementioned banned ingredients (Table 1).
TABLE 1.
Types of hair dyes and ingredients in hair dye formulations
| HAIR DYE TYPE | INGREDIENTS |
|---|---|
| Temporary | Azo derivatives |
| Azine derivatives | |
| Thiazine derivatives | |
| Indoamines | |
| Triphenylmethane | |
| Semi-permanent | Nitroanilines |
| Nitrophenylenediamines | |
| Nitroaminophenols | |
| Azo derivatives | |
| Anthraquinone | |
| Permanent | Para-phenylenediamine |
| Para-toluylenediamine | |
| Substituted para-diamines | |
| Ortho- or para-aminophenols |
Hair shaft anatomy and the science behind hair-dye coloring
Hair should be thought of as two distinct anatomical sections, namely, that which grows below the skin’s surface and that which is seen externally. The first section of hair is located beneath the skin surface. This portion consists of various cellular layers that give rise to the second section of hair known as the hair shaft. The hair shaft is a proteinaceous substance that grows above the skin’s surface and is the actual section of hair that is dyed.
The hair shaft is made up of three major components. From outermost layer to innermost, these consist of the hair shaft cuticle, the hair shaft cortex, and the hair shaft medulla. The hair shaft cuticle is made of several thin, flat, enucleated keratinocytes, similar to the keratinocytes that form the stratum corneum of the epidermis. Thus, the cuticle forms a protective barrier around the entire hair shaft. The cortex of the hair shaft contains bundles of keratin fibers, arranged in a vertical, rod-like fashion, that actually give shape and support to the hair structure. Finally, the medulla is an air-filled space at the center of the hair shaft that currently has no known biological function. All hair shafts have a cuticle and cortex, but very few actually have a medulla.
Hair color is attributed to the presence of melanin pigment in the keratinocytes of the hair shaft cortex.43 Modification of hair color can be achieved by the following two methods: removal of cortical melanin pigment via bleaching or addition of artificial pigment via application of a hair dye. Commercial hair dyes are classified into the following three main subtypes according to color permanence: temporary, semi-permanent, and permanent.1 For the purpose of this manuscript, a reference to hair dye will include temporary, semi-permanent, and permanent dyes unless otherwise specified.
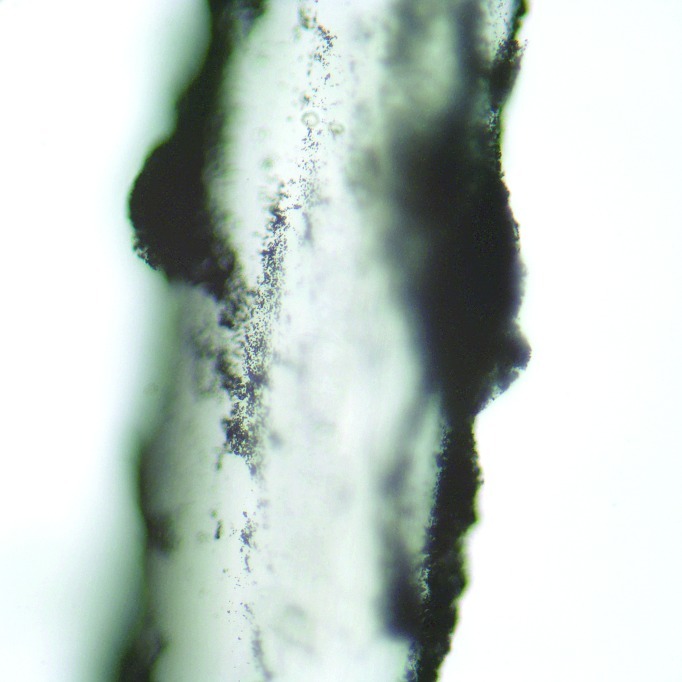
Temporary dyes weakly adhere to the hair shaft cuticle via van der Waals forces, but do not penetrate the hair cortex.44–45 Van der Waals forces are relatively weak attractions between atoms, molecules, and surfaces that rely on fluctuating polarizations of nearby particles. Figure 1 demonstrates the very superficial attachment of temporary hair dyes to the outermost layer of the hair shaft. In direct contrast, permanent dyes penetrate deeper into the hair shaft, passing through the hair cuticle and are deposited completely into the cortex, as captured in Figure 2. This process requires the formation of both covalent and ionic bonds, the process of which is explained below. Lastly, semi-permanent dyes coat the hair shaft cuticle in a similar fashion to temporary dyes while also partially infiltrating the cortex.45 The semi-permanent dyes are retained within the hair shaft by van der Waals forces.
Figure 1.
Microscopic view at 400x of a gray hair stained with temporary hair dye. The temporary black hair dye has a superficial absorption of the gray hair's outer root sheath and can be seen coating the outside of the hair shaft as black clumps.
Figure 2.
Microscopic view at 400x of a gray hair stained with permanent hair dye. The permanent black hair dye has deep penetration into the cortex and cuticle of the gray hair, as seen here as the intense dark band in the center of the hair shaft.
Permanent hair dyes differ from temporary and semi-permanent dyes in several ways. First, as the name implies, this type of hair dye is permanent and is not removable by shampooing, whereas temporary dyes are easily washed out in one shampoo rinse,44–45 and semi-permanent dyes are removed in 4 to 12 shampoos.44–45 Second, temporary and semi-permanent products are direct dyes that do not require chemical reactions to impart color and rely on van der Waals forces for adhesion. Permanent dyes are actually colorless precursors and require a developer to impart color.1,44–45 Developers contain hydrogen peroxide, which serves several functions—it causes swelling of the hair cuticle, allowing for diffusion of the colorless precursor into the hair cortex; it bleaches the natural melanin pigment; and it catalyzes the oxidation of the colorless precursor into large colored molecules, which become trapped inside the hair cortex.1,44–45 Color is infused into the hair shaft rather than just painted over it. The hair shaft sustains oxidative damage with permanent hair-dye use. The damage is accentuated with the use of dark-colored dyes (black, dark-brown) because darker shades need higher concentrations of precursors.1 The destructive nature of permanent hair dyes, especially dark-colored dyes, is reflected in the epidemiological evidence demonstrating its potential association to human malignancy.
Review of epidemiolgical data on association with malignancy
Bladder malignancy. In the United States, the incidence of bladder cancer has increased more than 50 percent during the past two decades, while the mortality rate has shown steady decline.46 In 2008, the United States reported an estimated 69,000 new cases with 14,000 deaths.46 Known risk factors for transitional bladder carcinoma include cigarette smoking, occupational exposure to aromatic amines, and urinary tract infection with Schistosoma haematobium.47 Structural similarities between the aromatic amines used in industrial dyes and the commercially available hair dyes, especially those sold before the 1980s, instigated numerous epidemiological studies into this subject.47 To date, three published meta-analyses have examined the epidemiological evidence relating personal hair dye use to the risk of bladder cancer. Of the three, one study found a significant relationship to hair-dye use and bladder cancer, whereas two did not.
Huncharek et al48 combined data from six case-controlled studies and one cohort between the years 1980 and 2001 and did not find an association between bladder cancer and hair dye.48 Initial pooling of the data from the seven studies yielded a nonsignificant relative risk (RR) of 1.01 (95% confidence interval [CI]: 0.92–1.11) for any type of hair dye use. However, the data were re-evaluated after excluding three studies. Studies by Altekruse et al,60 Stavraky et al,61 and Howe et al62 were eliminated for three reasons. First, the cohort by Altekruse et al was eliminated because cancer mortality rather than cancer incidence was used as the study endpoint. Superficial bladder cancer is often nonfatal and cancer mortality may not reflect the total number of cancers associated with hair-dye use. Second, the study by Stavraky et al was eliminated because urinary tract cancers were not stratified based on anatomic location. As a result, the data represented combined risk of bladder cancer and other neoplasms of the kidney. Finally, the study by Howe et al was eliminated due to small study population. Pooled analysis excluding these studies produced a statistically significant RR of 1.50 (95% CI: 1.30–1.98) for any type of hair-dye use. Based on these results, Huncharek et al concluded that a positive association existed between personal use of hair dye and bladder malignancy.
In direct contrast to the findings above, there have been two additional investigations that negate the association between hair-dye use and bladder malignancy. Takkouche et al49 combined the data from nine case-controlled studies and one cohort between the years 1977 and 2004. Ultimately, they included three case-controlled studies that were not incorporated into the meta-analysis by Huncharek et al. In addition, the cohort study was updated with a longer follow up. They did not observe an association between hair-dye use and risk of bladder cancer (RR 1.01, 95% CI: 0.89–1.14). Furthermore, RR remained nonsignificant despite stratification based on permanent hair-dye application and intense dye exposures, defined as greater than 200 lifetime exposures to hair dyes.
A more recent meta-analysis by Kelsh et al50 combined data from 11 case-controlled studies and one cohort study between the years 1977 and 2006. An association could not be substantiated based on the pooled data (RR 0.97, 95% CI: 0.87–1.08). RR remained nonsignificant despite stratification of the data based on permanent hair-dye use, dark-colored hair-dye use, duration of hair-dye use, and lifetime applications of hair dye.
Despite the positive correlation concluded by Huncharek et al, after selectively disregarding results from certain case-controlled studies, the bulk of the meta-analysis data does not support an association between personal hair-dye use and bladder malignancies.
Hematological malignancies. In the United States, the incidence of leukemia, lymphoma, and multiple myeloma have been on the rise. The role of hair dye as an etiological agent has been explored in several epidemiological studies. One meta-analysis, combining data from 31 case-controlled studies and nine cohorts, has revealed a slight increase in risk of hematopoietic cancers for any user of hair dye (RR 1.15, 95% CI: 1.05–1.27).49 The increased risk was primarily attributed to the data in the 31 case-controlled studies (RR 1.23, 95% CI: 1.09–1.39), and the risk was more specific to men (RR 1.57, 95% CI: 1.33–1.84).
Non-Hodgkin’s lymphoma. In the United States, the incidence of non-Hodgkin’s lymphoma (NHL) has risen by 80 percent from 1973 to 1997, with a three-percent increase annually. This large increase in incidence is undefined and cannot be attributed to improved diagnosis or known risk factors.51–52 Known risks include genetic susceptibility, primary or acquired immunosuppression, and infectious agents, such as the Epstein-Barr virus, human T-cell lymphotropic virus-I, human herpes virus 8, hepatitis C, and Helicobacter pylori.51–52 Lifestyle factors, such as cigarette smoking, hair-dye use, sunlight exposure, and dietary intake, have also been studied.51–52 Highlighting the epidemiological studies on hair dyes, two meta-analyses have demonstrated elevated risk of NHL in hair-dye users; however, this elevated risk was refuted by two recent case-controlled studies that show no such association.
Results of one meta-analysis indicate an increased risk of NHL in any hair-dye user (RR 1.23, 1.07–1.42, 95% CI).49 Correspondingly, Zhang et al53 demonstrated that any hair-dye use before 1980 had a positive association to NHL (OR 1.2, 95% CI: 1.0–1.3), primarily in women. Furthermore, the results revealed increased risk of a NHL subtype, follicular lymphoma, regardless of the year of application (OR 1.3, 95% CI: 1.0–1.6). This risk was further increased in individuals who dyed hair before 1980 and with the use of permanent or dark-colored dyes. Future research may seek to further explore these variables, namely, the use of dark and permanent hair-coloring products and the use of hair-coloring products prior to 1980, all of which have been hypothesized to be potently carcinogenic.
A case-controlled study examining the association between NHL and genetic variations in the enzyme utilized to metabolize aromatic amines, N-acetyltransferase (NAT), yielded positive results.54 Risk of NHL was not increased in users of hair dye after 1980, but prior to 1980 risk of NHL was increased four-fold in women who used permanent, dark-colored dyes for greater than 15 years (OR 3.9, 95% CI: 1.2–12.5). However, stratification based on NAT subtype revealed an increased risk in women who were NAT2 rapid or intermediate acetylators. Among the NAT2 rapid/intermediate acetylators, the highest risk was observed in subjects exposed to dark-colored, permanent dye with greater than five applications per year, in subjects with greater than five years of hair-dye application, and in those with more than 25 cumulative hair-dye applications. In addition, permanent hair dye used before 1980 in women who are homozygous or heterozygous for the NAT1*10 allele revealed an increased risk of NHL compared to women without the NAT1*10 allele. This risk was more pronounced with dark-colored permanent dyes. These results suggest that there may be a genetic predisposition toward development of NHL in certain subsets of hair-dye users.
Hodgkin’s lymphoma. In the United States, the incidence of Hodgkin’s lymphoma (HL) is on the decline and has decreased more than 16 percent from 1973 to 1996.55 The mortality rate has plummeted even more with a 65-percent decline over the same period, and an annual decrease of four percent, secondary to the advent of effective therapeutic regimens.55 Known risk factors for HL are genetic susceptibility, Epstein Barr virus, and acquired immunodeficiency syndrome.55 Postulated associations with occupational exposures have not been verified. On personal hair-dye use, the meta-analysis by Takkouche et al49 did not reveal a significant increased risk when stratifying for HL (RR 0.88, 95% CI: 0.54–1.42).
Leukemia. In the United States, the incidence of leukemia has been stable.52 Known risk factors include exposure to ionizing radiation, electromagnetic fields, chemotherapeutic agents, and viral agents. Occupational exposures to formaldehyde, benzene, and dioxins as well as agricultural pesticides and herbicides have also been associated with increased risk.52
The risk of personal exposure to hair dye was assessed by a meta-analysis by Takkouche et al.49 Stratification of data on leukemia did not reveal a significant increased risk (RR 1.12, 95% CI: 0.94–1.34).
Multiple myeloma. Multiple myeloma is observed most often in the elderly. In the United States, the incidence of multiple myeloma is on the rise due to an increasing elderly population.52 Occupational exposure to chemicals, such as pesticides, fertilizers, petrochemicals, and wood dust, have been associated with increased risk.52 On personal hair-dye use, the meta-analysis by Takkouche et al demonstrated no increased risk of multiple myeloma in ever users of hair dye (RR 1.11, 95% CI: 0.95–1.31).49
In summary, the authors can extrapolate that there is likely an increased risk of developing NHL in hair-dye users. Also, the data show that the number of hair-dye applications and the darker colored dyes increase risk of malignancy.
Breast malignancy. Breast cancer is the most common noncutaneous female malignancy in the United States with 182,460 new cases diagnosed each year. The rising incidence of breast cancer is thought to be due to increased detection with screening mammography, and the incidence of mortality has drastically declined over the past three decades. Risk factors for breast cancer are multifactorial and include age, gender, ethnicity, history of benign breast disease, previous history of breast cancer, family history, genetic influences, reproductive and hormonal factors, exposure to ionizing radiation, lifestyle and dietary habits, and environmental exposures. The association between hair dye and breast cancer was explored in the meta-analysis by Takkouche et al.49 Data pooled from 12 case-controlled studies and two cohort studies did not reveal a significant association between female breast cancer and any hair-dye users (RR 1.06, 95% CI: 0.95–1.18), permanent dye users (RR 1.00, 95% CI: 0.94–1.05), or users with more than 200 lifetime exposures to hair dye (RR 0.99, 95% CI: 0.89–1.11).
Interestingly, a population-based, case-controlled study by Ambrosome et al,56 which sought to detect the presence of carcinogenic-DNA adducts in exfoliated breast ductal epithelial cells, showed a positive association between the presence of carcinogenic adducts and the use of light-colored hair dye (OR 18.12, 95% CI: 1.45–226.83). This is a surprising finding because light-colored hair dye is theoretically less carcinogenic because it causes less destruction to the hair shaft than darker shades. The use of hair dye at least once in the last six months was also an independent risk factor.
Miscellaneous malignancies. A meta-analysis by Takkouche et al49 pooled data on brain tumors, skin cancer, ovarian cancer, and cervical cancer. Two studies per site were analyzed.49 The risk was nonsignificant for skin cancer (0.89, 95% CI: 0.53–1.9), ovarian cancer (RR 0.74, 95% CI: 0.51–1.07), and cervical cancer (RR 0.89, 95% CI: 0.53–1.9), but significance was observed for brain tumors (RR 1.83, 95% CI: 1.16–2.89) and ovarian cancer (RR 1.71, 95% CI: 1.15-2.53). These significant findings are questionable because they are based on small case-controlled studies.
Parental exposure and risk of malignancy in the unborn child. Understanding the malignant potential of hair-dye products when used during pregnancy is of great importance due to the possible risk of exposure to the unborn child. Of the four case-controlled studies examined, three demonstrate a positive relationship between cancer development and hair-dye exposure. McCall et al57 explored the association between maternal hair-dye use and the risk of neuroblastoma in the offspring. They concluded that maternal hair-dye use a month before conception and throughout pregnancy increased risk of neuroblastoma in the offspring (OR 1.6, 95% CI: 1.2–2.22). Risk stratification based on hair-dye type revealed a greater risk for maternal use of temporary hair dyes, rather than use of permanent hair dyes. This is a surprising finding, as the use of permanent hair dye has been more consistently associated with carcinogenesis, and temporary hair dye does not penetrate the hair shaft.
Another study showed significant risk of childhood brain tumors in offspring of mothers using hair dye within five years of childbearing after 1980.63 Once again, this finding contradicts previous data, which has shown increased risk with dye exposure prior to 1980. These studies suggest there may be harm to the baby in utero and therefore pregnant patients should be warned about possible carcinogenesis. Thus, it is the authors’ current recommendation that pregnant patients avoid all hair coloring products.
Finally, Chen et al58 conducted a case-controlled study examining the relationship between exposure to pesticides, chemicals, dusts, fumes, metals, hair dye, and the development of childhood germ-cell tumors.58 Maternal use of hair dye during breastfeeding increased risk of childhood germ-cell tumors (OR 1.5, 95% CI: 1.0–2.2), primarily in girls. Maternal use of hair dye one month prior to pregnancy was associated with an increased risk of tumors in boys.
In 1994, Bunin et al59 published a population-based case-control study of 321 cases of childhood astrocytic glioma or primitive neuroectodermal tumors of the brain with 321 age-matched controls. Maternal use of hair-coloring products during pregnancy was not associated with childhood development of astrocytoma (OR 0.7, 95% CI: 0.3–1.6) or primitive neuroectodermal tumors of the brain (1.1, 95% CI: 0.4–2.6).
Conclusion
Hair coloring products have proven to be potent carcinogens in animal models. Epidemiological evidence of hair-coloring products as human oncogenic agents remains largely inconclusive due to mixed results. However, enough evidence exists to help counsel questioning patients.
The first step is to explain that epidemiological evidence attempts to calculate risk, but in no way represents direct correlations. With that said, the strongest evidence points toward an increased risk of NHL, especially of the follicular subtype. Patients with a personal or a first-degree family history of NHL should be cautious in utilizing hair dye because there may be a cumulative risk.
The studies, for the most part, document that risk is further increased with darker colors and increased number of exposures. Alarming data points toward a link between hair-dye use in pregnancy and the development of several childhood malignancies in offspring. Concerned pregnant women should avoid all hair coloring.
Finally, the authors counsel their patients to apply a petroleum-based ointment to the scalp skin prior to hair-dye application to minimize direct cutaneous exposure to the dye. They also advise their patients to reduce the time of dye application by 25 percent for each hair-dying session. Although these methods have not been studied and no known investigation differentiates risk of malignancy based on the time of exposure or the route of chemical administration via cutaneous diffusion, these may be good anecdotal practices for patients who dye their hair.
Footnotes
Disclosures:The authors report no relevant conflicts of interest.
References
- 1.Rollison DE, Helzlsouer KJ, Pinney SM. Personal hair dye use and cancer: a systematic literature review and evaluation of exposure assessment in studies published since 1992. J Toxicol Environ Health B Crit Rev. 2006 Sep–Oct;;9(5):413–439. doi: 10.1080/10937400600681455. [DOI] [PubMed] [Google Scholar]
- 2.Ames BN, Kammen HO, Yamasaki E. Hair dyes are mutagenic: identification of a variety of mutagenic ingredients. Proc Natl Acad Sci U S A. 1975 Jun;;72(6):2423–2427. doi: 10.1073/pnas.72.6.2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garrigue JL, Ballantyne M, Kumaravel T, et al. In vitro genotoxicity of para-phenylenediamine and its N-monoacetyl or N,N'-diacetyl metabolites. Mutat Res. 608(1):58–71. doi: 10.1016/j.mrgentox.2006.05.001. 200619. Epub 2006 Jun 30. [DOI] [PubMed] [Google Scholar]
- 4.Ferguson LR, Roberton AM, Berriman J. Direct-acting mutagenic properties of some hair dyes used in New Zealand. Mutat Res. 1990;245(1):41–46. doi: 10.1016/0165-7992(90)90023-d. [DOI] [PubMed] [Google Scholar]
- 5.Hill LE, Parton JW, Probst GS, Garriott ML. Mutagenicity evaluation of HC Blue No. 1 and HC Blue No. 2, II. Effect on the in vitro induction of unscheduled DNA synthesis in rat, mouse, rabbit, hamster, and monkey primary hepatocytes. Mutat Res. 1990;241(2):145–150. doi: 10.1016/0165-1218(90)90118-l. [DOI] [PubMed] [Google Scholar]
- 6.Oberly TJ, Kokkino AJ, Bewsey BJ, Richardson KK. Mutagenicity evaluation of HC Blue No. 1 and HC Blue No. 2. III. Effects in the Salmonella typhimurium/Escherichia coli reversion assay and the mouse lymphoma L5178Y TK+/- forward mutation assay. Mutat Res. 1990;241(2):151–159. doi: 10.1016/0165-1218(90)90119-m. [DOI] [PubMed] [Google Scholar]
- 7.Palmer KA, Denunzio A, Green S. The mutagenic assay of some hair dye components, using the thymidine kinase locus of L5178Y mouse lymphoma cells. J Environ Pathol Toxicol. 1978 Sep–Oct;;1(1):87–91. [PubMed] [Google Scholar]
- 8.Cosmetic Ingredient Review Expert Panel. Final report on the safety assessment of disperse Blue 7. Int J Toxicol. 2007;26(Suppl 2):65–77. doi: 10.1080/10915810701351210. [DOI] [PubMed] [Google Scholar]
- 9.Murata M, Nishimura T, Chen F, Kawanishi S. Oxidative DNA damage induced by hair dye components ortho-phenylenediamines and the enhancement by superoxide dismutase. Mutat Res. 2006;607(2):184–191. doi: 10.1016/j.mrgentox.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 10.Watanabe T, Hirayama T, Fukui S. Mutagenicity of commercial hair dyes and detection of 2,7-diaminophenazine. Mutat Res. 1990;244(4):303–308. doi: 10.1016/0165-7992(90)90077-w. [DOI] [PubMed] [Google Scholar]
- 11.Watanabe T, Hirayama T, Fukui S. Phenazine derivatives as the mutagenic reaction product from o- or m-phenylenediamine derivatives with hydrogen peroxide. Mutat Res. 1989;227(3):135–145. doi: 10.1016/0165-7992(89)90037-7. [DOI] [PubMed] [Google Scholar]
- 12.Venitt S, Crofton-Sleigh C, Osborne MR. The hair-dye reagent 2-(2',4'-diaminophenoxy) ethanol is mutagenic to Salmonella typhimurium. Mutat Res. 1984;135(1):31–47. doi: 10.1016/0165-1218(84)90145-9. [DOI] [PubMed] [Google Scholar]
- 13.Albano G, Carere A, Crebelli R, Zito R. Mutagenicity of commercial hair dyes in Salmonella typhimurium TA98. Food Chem Toxicol. 1982;20(2):171–175. doi: 10.1016/s0278-6915(82)80243-3. [DOI] [PubMed] [Google Scholar]
- 14.Prival MJ, Mitchell VD, Gomez YP. Mutagenicity of a new hair dye ingredient: 4-ethoxy-m-phenylenediamine. Science. 1980;207(4433):907–908. doi: 10.1126/science.6986651. [DOI] [PubMed] [Google Scholar]
- 15.Matsuki Y, Fukuhara K, Inoue Y, et al. Characterization of aminoindamines and aminoindoanilines formed by oxidative hair dyeing and their mutagenicity. J Pharmacobiodyn. 1981;4(4):269–274. doi: 10.1248/bpb1978.4.269. [DOI] [PubMed] [Google Scholar]
- 16.Loprieno N, Barale R, Mariani L, Zaccaro L. The hair-dye reagent 2-(2',4'-diaminophenoxy) ethanol is mutagenic to. Salmonella typhimurium. Mutat Res. 1982;102(4):331–346. doi: 10.1016/0165-1218(82)90095-7. [DOI] [PubMed] [Google Scholar]
- 17.National Toxicology Program. NTP Toxicology and Carcinogenesis Studies of HC Blue No. 2 [2,2'-((4-((2-Hydroxyethyl)amino)-3-nitrophenyl)imino)bis(ethanol)] (CAS No. 33229-34-4) in F344/N Rats and B6C3F 1 Mice (Feed Studies) Natl Toxicol Program Tech Rep Ser. 1985;293:1–192. [PubMed] [Google Scholar]
- 18.Venitt S, Searle CE. Mutagenicity and possible carcinogenicity of hair colourants and constituents. IARC Sci Publ. 1976;(13):263–271. [PubMed] [Google Scholar]
- 19.National Toxicology Program. NTP Toxicology and Carcinogenesis Studies of C I. Acid Orange 3 (CAS No. 6373-74-6) in F344/N Rats and B6C3F1 Mice (Gavage Studies) Natl Toxicol Program Tech Rep Ser. 1988 Dec;;335:1–157. [PubMed] [Google Scholar]
- 20.National Toxicology Program. NTP Toxicology and Carcinogenesis Studies of 2-Amino-4-Nitrophenol (CAS No. 99-57-0) in F344/N Rats and B6C3F1 Mice (Gavage Studies) Natl Toxicol Program Tech Rep Ser. 1988 Jun;;339:1–170. [PubMed] [Google Scholar]
- 21.National Toxicology Program. NTP Toxicology and Carcinogenesis Studies of 2-Amino-5-Nitrophenol (CAS No. 121-88-0) in F344/N Rats and B6C3F1 Mice (Gavage Studies) Natl Toxicol Program Tech Rep Ser. 1988 Feb;;334:1–158. [PubMed] [Google Scholar]
- 22.National Toxicology Program. NTP Toxicology and Carcinogenesis Studies of C I. Disperse Blue 1 (A commercial dye containing approximately 50% 1,4,5,8-tetraaminoanthraquinone, and 20% water) (CAS No. 2475-45-8) in F344/N Rats and B6C3F1 Mice (Feed Studies) Natl Toxicol Program Tech Rep Ser. 1986 May;;299:1–241. [PubMed] [Google Scholar]
- 23.National Toxicology Program. NTP Toxicology and Carcinogenesis Studies of HC Red No. 3 [2,((Amino-2-nitrophenyl)amino)ethanol] (CAS No. 2871-01-4) in F344/N Rats and B6C3F1 Mice (Gavage Studies) Natl Toxicol Program Tech Rep Ser. 1986 Jan;;281:1–184. [PubMed] [Google Scholar]
- 24.National Toxicology Program. NTP Toxicology and Carcinogenesis Studies of HC Blue No. 1 (CAS No. 2784-94-3) in F344/N Rats and B6C3F1 Mice (Feed Studies) Natl Toxicol Program Tech Rep Ser. 1985 Aug;;271:1–192. [PubMed] [Google Scholar]
- 25.National Toxicology Program. NTP Toxicology and Carcinogenesis Studies of HC Yellow 4 (CAS No. 59820-43-8) in F344 Rats and B6C3F1 Mice (Feed Studies) Natl Toxicol Program Tech Rep Ser. 1992 Jun;;419:1–223. [PubMed] [Google Scholar]
- 26.Rojanapo W, Kupradinun P, Tepsuwan A, Chutimataewin S, Tanyakaset M. Carcinogenicity of an oxidation product of p-phenylenediamine. Carcinogenesis. 1986;7(12):1997–2002. doi: 10.1093/carcin/7.12.1997. [DOI] [PubMed] [Google Scholar]
- 27.Sontag JM. Carcinogenicity of substituted-benzenediamines (phenylenediamines) in rats and mice. J Natl Cancer Inst. 1981;66(3):591–602. [PubMed] [Google Scholar]
- 28.Burnett CM, Corbett JF. Failure of short-term in vitro mutagenicity tests to predict the animal carcinogenicity of hair dyes. Food Chem Toxicol. 1987 Sep;;25(9):703–707. doi: 10.1016/0278-6915(87)90104-9. [DOI] [PubMed] [Google Scholar]
- 29.Haws LC, Jackson BA, Turnbull D, Dressler WE. Two approaches for assessing human safety of disperse blue 1. Regul Toxicol Pharmacol. 1994;19(1):80–96. doi: 10.1006/rtph.1994.1007. [DOI] [PubMed] [Google Scholar]
- 30.Rojanapo W, Kupradinun P, Tepsuwan A, et al. Carcinogenicity of an oxidation product of p-phenylenediamine. Carcinogenesis. 1986;7(12):1997–2002. doi: 10.1093/carcin/7.12.1997. [DOI] [PubMed] [Google Scholar]
- 31.Van Duuren BL. Carcinogenicity of hair dye components. J Environ Pathol Toxicol. 1980;3(4 Spec No):237–251. [PubMed] [Google Scholar]
- 32.Wolfram LJ, Maibach HI. Percutaneous penetration of hair dyes. Arch Dermatol Res. 1985;277(3):235–241. doi: 10.1007/BF00404323. [DOI] [PubMed] [Google Scholar]
- 33.Lademann J, Richter H, Jacobi U, et al. Human percutaneous absorption of a direct hair dye comparing in vitro and in vivo results: implications for safety assessment and animal testing. Food Chem Toxicol. 2008;46(6):2214–2223. doi: 10.1016/j.fct.2008.02.018. Epub 2008 Feb 29. [DOI] [PubMed] [Google Scholar]
- 34.Maibach HI, Leaffer MA, Skinner WA. Percutaneous penetration following use of hair dyes. Arch Dermatol. 1975;111(11):1444–1445. [PubMed] [Google Scholar]
- 35.Marzulli FN, Watlington PM, Maibach HI. Exploratory skin penetration findings relating to the use of lead acetate hair dyes. Hair as a test tissue for monitoring uptake of systemic lead. Curr Probl Dermatol. 1978;7:196–204. [PubMed] [Google Scholar]
- 36.Tsomi V, Kalopissis G. Cutaneous penetration of some hairdyes in the hairless rat. Toxicol Eur Res. 1982;4(3):119–127. [PubMed] [Google Scholar]
- 37.Hofer H, Hruby E. Skin penetration by 2,4-diaminoanisole in the rat. Food Chem Toxicol. 1983;21(3):331–334. doi: 10.1016/0278-6915(83)90069-8. [DOI] [PubMed] [Google Scholar]
- 38.Genina EA, Bashkatov AN, Sinichkin YP, et al. In vitro and in vivo study of dye diffusion into the human skin and hair follicles. J Biomed Opt. 2002;7(3):471–477. doi: 10.1117/1.1486247. [DOI] [PubMed] [Google Scholar]
- 39.Yourick JJ, Bronaugh RL. Percutaneous penetration and metabolism of 2-nitro-p-phenylenediamine in human and fuzzy rat skin. Toxicol Appl Pharmacol. 2000;166(1):13–23. doi: 10.1006/taap.2000.8962. [DOI] [PubMed] [Google Scholar]
- 40.Steiling W, Kreutz J, Hofer H. Percutaneous penetration/dermal absorption of hair dyes in vitro. Toxicol In Vitro. 2001;15(4–5):565–570. doi: 10.1016/s0887-2333(01)00062-5. [DOI] [PubMed] [Google Scholar]
- 41.Ammenheuser MM, Warren ME. Detection of mutagens in the urine of rats following topical application of hair dyes. Mutat Res. 1979;66(3):241–245. doi: 10.1016/0165-1218(79)90084-3. [DOI] [PubMed] [Google Scholar]
- 42.Product and ingredient safety: hair dye products. U.S. Food and Drug Administration. [June 28, 2009]. http://www.fda.gov/Cosmetics/ProductandIngredientSafety/ProductInformation/ucm143066.htm
- 43.Wolfram LJ. Human hair: a unique physicochemical composite. J Am Acad Dermatol. 2003;48(6 Suppl):S106–S114. doi: 10.1067/mjd.2003.276. [DOI] [PubMed] [Google Scholar]
- 44.Correa A, Mohan A, Jackson L, et al. Use of hair dyes, hematopoietic neoplasms, and lymphomas: a literature review. I. Leukemias and myelodysplastic syndromes. Cancer Invest. 2000;18(4):366–380. doi: 10.3109/07357900009012180. [DOI] [PubMed] [Google Scholar]
- 45.Draelos ZD. Boca Raton: Taylor & Frances; 2005. Hair Care: An Illustrated Dermatologic Handbook. [Google Scholar]
- 46.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 47.Pelucchi C, Bosetti C, Negri E, et al. Mechanisms of disease: the epidemiology of bladder cancer. Nat Clin Pract Urol. 2006;3(6):327–340. doi: 10.1038/ncpuro0510. [DOI] [PubMed] [Google Scholar]
- 48.Huncharek M, Kupelnick B. Personal use of hair dyes and the risk of bladder cancer: results of a meta-analysis. Public Health Rep. 2005;120(1):31–38. doi: 10.1177/003335490512000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takkouche B, Etminan M, Montes-Martinez A. Personal use of hair dyes and risk of cancer: a meta-analysis. JAMA. 2005;293(20):2516–2525. doi: 10.1001/jama.293.20.2516. [DOI] [PubMed] [Google Scholar]
- 50.Kelsh MA, Alexander DD, Kalmes RM, Buffler PA. Personal use of hair dyes and risk of bladder cancer: a meta-analysis of epidemiologic data. Cancer Causes Control. 2008;19(6):549–558. doi: 10.1007/s10552-008-9123-z. [DOI] [PubMed] [Google Scholar]
- 51.Rodriguez-Abreu D, Bordoni A, Zucca E. Epidemiology of hematological malignancies. Ann Oncol. 2007;18(Suppl 1):i3–i8. doi: 10.1093/annonc/mdl443. [DOI] [PubMed] [Google Scholar]
- 52.Baris D, Zahm SH. Epidemiology of lymphomas. Curr Opin Oncol. 2000;12(5):383–394. doi: 10.1097/00001622-200009000-00002. [DOI] [PubMed] [Google Scholar]
- 53.Zhang Y, Sanjose SD, Bracci PM, et al. Personal use of hair dye and the risk of certain subtypes of non-Hodgkin lymphoma. Am J Epidemiol. 20081;167(11):1321–1331. doi: 10.1093/aje/kwn058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morton LM, Bernstein L, Wang SS, et al. Hair dye use, genetic variation in N-acetyltransferase 1 (NAT1) and 2 (NAT2), and risk of non-Hodgkin lymphoma. Carcinogenesis. 2007;28(8):1759–1764. doi: 10.1093/carcin/bgm121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Benavente Y, Garcia N, Domingo-Domenech E, et al. Regular use of hair dyes and risk of lymphoma in Spain. Int J Epidemiol. 2005;34(5):1118–1122. doi: 10.1093/ije/dyi109. [DOI] [PubMed] [Google Scholar]
- 56.Ambrosone CB, Abrams SM, Gorlewska-Roberts K, Kadlubar FF. Hair dye use, meat intake, and tobacco exposure and presence of carcinogen-DNA adducts in exfoliated breast ductal epithelial cells. Arch Biochem Biophys. 2007;464(2):169–175. doi: 10.1016/j.abb.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 57.McCall EE, Olshan AF, Daniel JL. Maternal hair dye use and risk of neuroblastoma in offspring. Cancer Causes Control. 2005;16(6):743–748. doi: 10.1007/s10552-005-1229-y. [DOI] [PubMed] [Google Scholar]
- 58.Chen Z, Robison L, Giller R, et al. Environmental exposure to residential pesticides, chemicals, dusts, fumes, and metals, and risk of childhood germ cell tumors. Int J Hyg Environ Health. 2006;209(1):31–40. doi: 10.1016/j.ijheh.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 59.Bunin GR, Buckley JD, Boesel CP, et al. Risk factors for astrocytic glioma and primitive neuroectodermal tumor of the brain in young children: a report from the Children’s Cancer Group. Cancer Epidemiol Biomarkers Prev. 1994;3(3):197–204. [PubMed] [Google Scholar]
- 60.Altekruse SH, Henley ST, Thun MT. Deaths from hematopoietic and other cancers in relation to permanent hair dye use in a large prospective study. Cancer Causes Control. 1999;10:617–635. doi: 10.1023/a:1008926027805. [DOI] [PubMed] [Google Scholar]
- 61.Stavraky KM, Clarke EA, Dunner A. A case control study of hair-dye use and cancers of various sites. Br J Cancer. 43:236–239. doi: 10.1038/bjc.1981.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Howe GR, Burch JD, Miler AB, et al. Tabacco use, occupation, coffee, various nutrients, and bladder cancer. J Natl Cancer Inst. 1980;64:701–713. [PubMed] [Google Scholar]
- 63.Efird ET, Holly EA, Cordier S, et al. Beauty product-related exposures and childhood brain tumors in seven countries: results from the SEARCH international brain tumor study. J Neuro-Onc. 2005;72:133–147. doi: 10.1007/s11060-004-3121-0. [DOI] [PubMed] [Google Scholar]