Abstract
Background
Filariasis, caused by Brugia malayi, is a public health problem in Thailand. Currently, at least two locations in southern Thailand are reported to be active endemic areas. Two and four Mansonia species are primary and secondary vectors, respectively, of the nocturnally subperiodic race, whereas, Coquillettidia crassipes is a vector of the diurnally subperiodic race. Although several Anopheles species have been incriminated extensively as natural and/or suspected vectors of B. malayi, little is known about vector competence between indigenous Anopheles and this filaria in Thailand. Thus, the susceptibility levels of eight species members in the Thai An. hyrcanus group to nocturnally subperiodic B. malayi are presented herein, and the two main refractory factors that affect them in different degrees of susceptibility have been elucidated.
Methods
Aedes togoi (a control vector), An. argyropus, An. crawfordi, An. nigerrimus, An. nitidus, An. paraliae, An. peditaeniatus, An. pursati and An. sinensis were allowed to feed artificially on blood containing B. malayi microfilariae, and dissected 14 days after feeding. To determine factors that take effect at different susceptibility levels, stain-smeared blood meals were taken from the midguts of Ae. togoi, An. peditaeniatus, An. crawfordi, An. paraliae, An. sinensis and An. nitidus immediately after feeding, and their dissected-thoraxes 4 days post blood-feedings were examined consecutively for microfilariae and L1 larvae.
Results
The susceptibility rates of Ae. togoi, An. peditaeniatus, An. crawfordi, An. nigerrimus, An. argyropus, An. pursati, An. sinensis, An. paraliae and An. nitidus to B. malayi were 70–95%, 70–100%, 80–85%, 50–65%, 60%, 60%, 10%, 5%, and 0%, respectively. These susceptibility rates related clearly to the degrees of normal larval development in thoracic muscles, i.e., Ae. togoi, An. peditaeniatus, An. crawfordi, An. paraliae, An. sinensis and An. nitidus yielded normal L1 larvae of 93.15%, 96.34%, 97.33%, 23.60%, 15.38% and 0%, respectively.
Conclusions
An. peditaeniatus, An. crawfordi, An. nigerrimus, An. argyropus and An. pursati were high potential vectors. An. paraliae and An. sinensis were low potential vectors, while An. nitidus was a refractory vector. Two refractory mechanisms; direct toxicity and/or melanotic encapsulation against filarial larval were involved in the refractoriness of development in the thoracic muscles of the mosquito.
Keywords: Anopheles hyrcanus group, Brugia malayi, Susceptibility level, Refractory factor, Thailand
Background
Lymphatic filariasis, due to Wuchereria bancrofti, Brugia malayi and B. timori, is a major health problem in many tropical and sub-tropical countries. At present, 1.3 billion people worldwide are at risk of lymphatic filariasis infection, with approximately 120 million affected in 72 countries [1-4]. In Thailand, at least two endemic areas of lymphatic filariasis have been reported, i.e., B. malayi in the south and W. bancrofti on the southwest to northwest Thai-Myanmar border [5,6].
So far, at least two physiological races of B. malayi, i.e., nocturnally subperiodic and diurnally subperiodic have been discerned in southern Thailand. The nocturnally subperiodic race is located in endemic areas of five provinces, i.e., Nakhon Si Thammarat, Phattalung, Pattani, Yala and Narathiwat. These regions are rural and semi-forested, and Mansonia uniformis and Ma. bonneae are the primary vectors in open swamp and swamp-forested areas, respectively, whereas Ma. dives, Ma. indiana, Ma. annulata and Ma. annulifera are considered as secondary vectors. The endemic area for the diurnally subperiodic race is confined to Surat Thani province, and Coquillettidia crassipes is an important vector [6-8]. When comparing these six provinces, Narathiwat is the highest endemic area, with more than half of the filariasis cases reported there each year. This may result from suitable microhabitats or large areas of swamp for Mansonia breeding-places; the existence of cats as animal reservoir hosts; or local insurgence that is considered a main factor in bringing about control failure in this province [8,9]. Regarding control measures, the reduction of microfilariae in the peripheral blood of carriers interrupts the mosquito-transmitted cycle by using a microfilaricide (diethylcarbamazine, [DEC]), which was established in 2002 by the Division of Filariasis, Department of Communicable Disease Control, Ministry of Public Health, Thailand. Consequently, the provinces of Surat Thani and Narathiwat are considered active endemic areas of diurnally and nocturnally subperiodic B. malayi, respectively [10]. Despite the control program succeeding at satisfactory levels, as determined by the reduction of microfilaraemic cases to 0% in four provinces (Nakhon Si Thammarat, Phattalung, Pattani and Yala), the two active endemic areas (Surat Thani and Narathiwat provinces) are still regarded as a source of microfilaria. The transmitting cycle has the potential to generate infection not only in these two active endemic areas, but also in adjacent provinces, due to migration of microfilaraemic carriers and long-term settlements as well as inadequate control of animal reservoir-hosts. In addition, this endemic disease could re-emerge at any time, even in thoroughly controlled endemic regions, where the environmental factor(s) favors suitable conditions for the transmission-cycle. This was reported recently in other mosquito-borne diseases, e.g., re-emergence of malaria due to Plasmodium vivax in South Korea [11-13].
In southern Thailand, only one and six mosquito species, which have been incriminated as natural vectors of B. malayi, belong to the genera Coquillettidia and Mansonia, respectively. Besides, at least one anopheline species of the subgenus Cellia (An. minimus) and five of the subgenus Anopheles (An. barbirostris, An. campestris, An. donaldi, An. lesteri and An. sinensis) were reported and incriminated as natural and/or suspected vectors of this filarial nematode in southeast and/or east Asian regions [14]. This information clearly emphasizes that knowledge of the vector competence of Anopheles mosquitoes to B. malayi is lacking, particularly according to data on the susceptibility level of indigenous Anopheles species to a local strain of B. malayi. Hence, this study reports the susceptibility of eight species members of the indigenous Thai An. hyrcanus group (An. argyropus, An. crawfordi, An. nigerrimus, An. nitidus, An. paraliae, An. peditaeniatus, An. pursati and An. sinensis) to nocturnally subperiodic B. malayi (Narathiwat province, southern Thailand strain). Additionally, the possible factor(s) affecting the different degrees in susceptibility of these anopheline species to nocturnally subperiodic B. malayi was elucidated.
Methods
Mosquito species and strains
As B. malayi is endemic, eight species members of the An. hyrcanus group were collected mainly in southern Thailand. This location comprised: (1) former endemic provinces [Chumphon (CP) and Nakhon Si Thammarat (NS)], and (2) provinces adjacent to former and/or current endemic provinces [Phang Nga (PG), Songkhla (SK), and Trang (TG)]. In addition, two provinces free from B. malayi infection in western [Ratchaburi (RB)] and northeastern [Ubon Ratchathani (UR)] Thailand were included in this study. The species and strains of the An. hyrcanus group were as follows: An. argyropus (NS strain), An. crawfordi (CP and TG strains), An. nigerrimus (UR, NS and SK strains), An. nitidus (UR and PG strains), An. paraliae (RB strain), An. peditaeniatus (CP and SK strains), An. pursati (RB strain) and An. sinensis (CP strain). Wild-caught, fully engorged females of the 8 An. hyrcanus species were collected from cow-baited traps and established successfully for many consecutive generations in the insectary of the Department of Parasitology, Faculty of Medicine, Chiang Mai University, Thailand, using the techniques described previously [15,16]. These colonies were used for studies on susceptibility to nocturnally subperiodic B. malayi throughout the experiments. Regarding the control vector, autogenous Ae. togoi (Chanthaburi province, eastern Thailand) was selected as a proven efficient laboratory vector for a wide-range of genera and species of filarial nematodes, including the nocturnally subperiodic B. malayi [17,18].
Nocturnally subperiodic B. malayi
This filarial parasite originated from a 20-year-old women, who was a resident of Bang Paw district, Narathiwat province, southern Thailand. Domestic cats were later infected experimentally with the parasite, which was maintained at the Department of Medical Entomology, Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand, from 1982 to 1986, when it was transferred to Mongolian jirds (Meriones unguiculatus) and then maintained at the animal house of the Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand [19].
Preparation of blood containing B. malayi microfilariae
The jirds were intraperitoneally inoculated for at least 3 months with infective larvae of nocturnally subperiodic B. malayi [20] and anesthetized deeply with ethylene ether. The microfilariae were collected by injecting 3 ml of Hank’s Balanced Salt Solution (HBSS, pH 7.2-7.4) into the peritoneal cavity before withdrawing by peritoneal washing. The 0.05 ml of peritoneal-washed-rich microfilariae was mixed with 10 ml of human-heparinized blood (10 units of heparin/ml of blood), taken from human volunteers who had signed the consent form. Then, the adjusted microfilarial density ranged from approximately 200 to 300 microfilariae (mf)/20 μl by using the human-heparinized blood for artificially feeding all of the mosquito species. The reason for adjusting microfilarial density in blood to range from 200 to 300 mf/20 μl was based on several proven experiments that yielded satisfactorily susceptible Ae. togoi to nocturnally subperiodic B. malayi (susceptibility rates: 70–95%). This agreed with experiments reporting susceptibility of An. sinensis to periodic B. malayi, i.e., using a microfilarial density of 5, 10, 20 and 50 mf/20 μl, with a susceptibility rate of 30, 65, 93 and 100%, respectively [21].
Infection of mosquitoes with B. malayi microfilariae
Five-day-old adult female Ae. togoi, An. argyropus, An. crawfordi, An. nigerrimus, An. nitidus, An. paraliae, An. peditaeniatus, An. pursati and An. sinensis fasted for 24 hrs and then were allowed artificial feeding simultaneously on blood-containing B. malayi microfilariae (microfilarial density = 312, 208, 256 and 283 mf/20 μl in experiment 1, 2, 3 and 4, respectively), using the techniques and apparatus previously described [22]. Fourteen days after feeding, all infected mosquitoes were dissected in normal saline solution and examined under a dissecting microscope. The number of mosquitoes with one or more infective stage larvae in any part of the body (head, thorax or abdomen) was recorded.
Determination of the possible factor(s) affecting the level of susceptibility
Five-day-old adult female mosquitoes, i.e., an efficient laboratory vector (Ae. togoi), high potential vectors [An. peditaeniatus (CP strain) and An. crawfordi (TG strain)], low potential vectors [An. paraliae (RB strain) and An. sinensis (CP strain)] and a refractory vector [An. nitidus (PG strain)] were allowed artificial feeding simultaneously on blood containing B. malayi microfilariae, as mentioned above. The infected mosquitoes were divided into 2 groups, i.e., (1) those with their midgut extracted immediately after full engorgement. The ingested blood meals were then made into thick blood films, dried out, de-hemoglobinized, fixed with methanol, stained with Giemsa (pH 7.2) and counted for microfilariae under a compound microscope; and (2) those with their thorax severed, torn in a drop of normal saline solution and examined under a compound microscope 4 days after feeding. The first stage (L1) larvae were counted and scored as normal L1 larvae if alive with intact morphology. The larvae were scored as melanized L1 if they had evidence of a retained stage and melanotic encapsulation; and scored as degenerated L1 if they demonstrated vacuolated internal organs without any evidence of melanotic encapsulation.
Ethical clearance
The protocols were approved by the Animal Ethics Committee of Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand.
Results
Details of the infective rates and parasite loads of Ae. togoi, An. argyropus, An. crawfordi, An. nigerrimus, An. nitidus, An. paraliae, An. peditaeniatus, An. pursati and An. sinensis 14 days after feeding on blood containing B. malayi microfilariae are shown in Table 1. The 95%, 70%, 80% and 80% infective rates corresponded to an average of 19.05, 7.50, 10.56 and 11.81 infective (L3) larvae per infected Ae. togoi in experiment 1, 2, 3 and 4, respectively, which indicated that all feeding experiments were under conditions of sufficient B. malayi microfilarial densities in infected blood.
Table 1.
Infective rates and parasite loads of 8 species in the An. hyrcanus group after feeding on blood containing B. malayi microfilariae (microfilarial density = 312, 208, 256 and 283 mf/20 μl in experiment 1, 2, 3 and 4, respectively), with all mosquitoes dissected 14 days after feeding
| Mosquito species | Infective rates (No.)* | Average No. L3 per infected mosquito (range)+ |
L3-distribution |
||
|---|---|---|---|---|---|
| % head (No.) | % thorax (No.) | % abdomen (No.) | |||
| Experiment 1 |
|
|
|
|
|
|
Ae. togoi |
95 (19/20) |
19.05 (1–49) |
59.39 (215) |
21.27 (77) |
19.34 (70) |
|
An. crawfordi (CP) |
85 (17/20)a |
6.24 (1–27)n |
52.83 (56) |
38.68 (41) |
8.49 (9) |
|
An. nigerrimus (NS) |
65 (13/20)b |
9.77 (1–32)o |
44.88 (57) |
38.58 (49) |
16.54 (21) |
|
An. nigerrimus (SK) |
65 (13/20)c |
6.69 (1–15)p |
45.98 (40) |
19.54 (17) |
34.48 (30) |
|
An. nitidus (PG) |
0 (0/20)d |
- |
- |
- |
- |
| Experiment 2 |
|
|
|
|
|
|
Ae. togoi |
70 (14/20) |
7.50 (1–36) |
83.81 (88) |
9.52 (10) |
6.67 (7) |
|
An. argyropus (NS) |
60 (12/20)e |
2.92 (1–6)q |
68.57 (24) |
22.86 (8) |
8.57 (3) |
|
An. nigerrimus (UR) |
50 (10/20)f |
4.20 (1–9)r |
69.05 (29) |
16.66 (7) |
14.29 (6) |
|
An. nitidus (UR) |
0 (0/20)g |
- |
- |
- |
- |
|
An. pursati (RB) |
60 (12/20)h |
3.83 (1–11)s |
67.39 (31) |
19.57 (9) |
13.04 (6) |
| Experiment 3 |
|
|
|
|
|
|
Ae. togoi |
80 (16/20) |
10.56 (1–32) |
73.37 (124) |
16.57 (28) |
10.06 (17) |
|
An. paraliae (RB) |
5 (1/20)i |
1.00 (0–1)t |
100.00 (1) |
- |
- |
|
An. peditaeniatus (CP) |
100 (20/20)j |
7.75 (1–23)u |
57.42 (89) |
14.19 (22) |
28.39 (44) |
| Experiment 4 |
|
|
|
|
|
|
Ae. togoi |
80 (16/20) |
11.81 (2–28) |
77.25 (146) |
15.87 (30) |
6.88 (13) |
|
An. crawfordi (TG) |
80 (16/20)k |
6.06 (1–19)v |
79.38 (77) |
11.34 (11) |
9.28 (9) |
|
An. peditaeniatus (SK) |
70 (14/20)l |
8.00 (2–22)w |
80.36 (90) |
10.71 (12) |
8.93 (10) |
| An. sinensis (CP) | 10 (2/20)m | 1.50 (1–2)x | 100.00 (3) | - | - |
*Fisher exact test: a, j, k vs. control, P > 0.05; b, c vs. control, P < 0.05.
*Chi-square test: e, f, h, l vs. control, P > 0.05; d, g, i, m vs. control, P < 0.05.
+t-test (two-sided); q, r, s, u, w vs. control: P > 0.05; n, o, p, t, v, x vs. control: P < 0.05.
The infective rates (IR) and average number of L3 larvae per infected mosquito (AL3) of An. crawfordi [experiment 1 (CP strain: IR = 85%, AL3 = 6.24) and experiment 4 (TG strain: IR = 80%, AL3 = 6.06)], An. nigerrimus [experiment 1 (NS strain: IR = 65%, AL3 = 9.77; SK strain: IR = 65%, AL3 = 6.69) and experiment 2 (UR strain: IR = 50%, AL3 = 4.20)], An. nitidus [experiment 1 (PG strain: IR = 0%, AL3 = 0%) and experiment 2 (UR strain: IR = 0%, AL3 = 0%)], An. argyropus [experiment 2 (NS strain: IR = 60%, AL3 = 2.92)], An. pursati [experiment 2 (RB strain: IR = 60%, AL3 = 3.83)], An. paraliae [experiment 3 (RB strain: IR = 5%, AL3 = 1.00)], An. peditaeniatus [experiment 3 (CP strain: IR = 100%, AL3 = 7.75) and experiment 4 (SK strain: IR = 70%, AL3 = 8.00)] and An. sinensis [experiment 4 (CP strain: IR = 10%, AL3 = 1.50)] were mostly lower than those in Ae. togoi, an efficient control vector. This was the case in all experimental studies, except for the infective rate of An. peditaeniatus (100%), which was higher than that of Ae. togoi (80%) in experiment 3. Comparative statistical analyses of the infective rates and average number of L3 larvae per infected mosquito were carried out between Ae. togoi and all An. hyrcanus species. The results revealed that the infective rates between Ae. togoi and An. hyrcanus species in experiment 1 [An. crawfordi (CP strain)], 2 [An. argyropus (NS strain), An. nigerrimus (UR strain) and An. pursati (RB strain)], 3 [An. peditaeniatus (CP strain)] and 4 [An. crawfordi (TG strain) and An. peditaeniatus (SK strain)], and average number of L3 larvae per infected mosquito between Ae. togoi and An. hyrcanus species in experiment 2 [An. argyropus (NS strain), An. nigerrimus (UR strain) and An. pursati (RB strain)] and 3 [An. peditaeniatus (CP strain) and 4 (SK strain)] did not differ significantly (P > 0.05). It is noteworthy that all infective larvae obtained from the four experimental feedings were very active and found to distribute in all regions of the head, thorax and abdomen, and their behavior was similar, with more than 44% of infective larvae migrating from the thorax to the head and proboscis.
Parasite loads dissected immediately and 4 days after feeding on blood containing B. malayi microfilariae in Ae. togoi, An. peditaeniatus, An. crawfordi, An. paraliae, An. sinensis and An. nitidus are detailed in Table 2. Investigative results on stain-smeared blood meals from the midguts of mosquitoes fed immediately, and fully engorged, indicated that all the mosquito species were successful in taking a considerable number of microfilariae from infected blood, with an average number of microfilariae per infected midgut of 25.20, 27.40, 22.00, 32.40, 26.40 and 25.80 in Ae. togoi, An. peditaeniatus, An. crawfordi, An. paraliae, An. sinensis and An. nitidus, respectively. Likewise, a satisfactory average number of 14.60, 16.40, 15.00, 17.80, 15.60 and 10.80 L1 larvae were recovered in the thoracic muscles of Ae. togoi, An. peditaeniatus, An. crawfordi, An. paraliae, An. sinensis and An. nitidus, respectively. However, variations in degrees of normal and abnormal L1 larval development in the thoracic muscles of six mosquito species were observed clearly. Ae. togoi, An. peditaeniatus, An. crawfordi, An. paraliae, An. sinensis and An. nitidus yielded normal, melanized and degenerated L1 larvae of 93.15%, 0% and 6.85%; 96.34%, 0% and 3.66%; 97.33%, 0% and 2.67%; 23.60%, 47.19% and 29.21%; 15.38%, 32.05% and 52.57%; 0%, 94.44% and 5.56%, respectively (Figure 1).
Table 2.
Parasite loads in Ae. togoi, An. peditaeniatus, An. crawfordi, An. paraliae, An. sinensis and An. nitidus dissected immediately and 4 days after feeding on blood containing B. malayi microfilariae (microfilarial density = 247 mf/20 μl)
| Mosquito species | Average No. mf per infected midgut (range)* | Average No. L1 per infected thorax (range)+ | % normal L1 (No.) | % melanized L1 (No.) | % degenerated L1 (No.) |
|---|---|---|---|---|---|
|
Ae. togoi |
25.20 (26–65) |
14.60 (14–30) |
93.15 (68) |
0 (0/73) |
6.85 (5) |
|
An. peditaeniatus (CP) |
27.40 (22–94) |
16.40 (12–47) |
96.34 (79) |
0 (0/82) |
3.66 (3) |
|
An. crawfordi (TG) |
22.00 (15–41) |
15.00 (8–25) |
97.33 (73) |
0 (0/75) |
2.67 (2) |
|
An. paraliae (RB) |
32.40 (20–37) |
17.80 (10–17) |
23.60 (21) |
47.19 (42) |
29.21 (26) |
|
An. sinensis (CP) |
26.40 (23–72) |
15.60 (13–36) |
15.38 (12) |
32.05 (25) |
52.57 (41) |
| An. nitidus (PG) | 25.80 (13–29) | 10.80 (5–11) | 0 (0/54) | 94.44 (51) | 5.56 (3) |
*Dissected from 5 midguts; + Dissected from 5 thoraxes.
Figure 1.
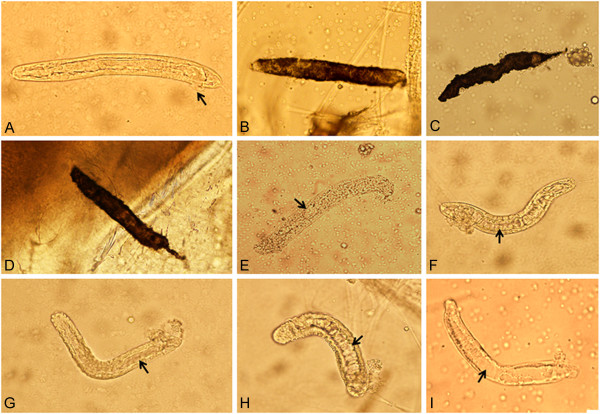
L1 larvae recovered from thoracic muscles of mosquitoes 4 days after infected blood meals. (A) Normal live larva with intact cuticle and internal organs (small arrow: protuberance of anal plug at the anal pore) recovered from Ae. togoi. (B, C, D) Completely melanotic encapsulated larvae obtained from An. paraliae, An. sinensis and An. nitidus. (E, F, G, H, I) Degenerated and vacuolated internal organs (small arrow) acquired from An. peditaeniatus, An. crawfordi, An. sinensis, An. paraliae and An. nitidus, respectively.
Discussion
To incriminate a mosquito vector in an endemic area of filariasis, it is necessary to confirm the susceptibility rate in a laboratory-bred, clean mosquito colony, which has been fed on carrier blood containing microfilariae. By using this criterion, the susceptibility test in an experimental laboratory is an efficient classical tool when suspecting the potential vector of a certain mosquito species. Nonetheless, susceptibility alone does not imply an important role in the transmission of disease in nature, whereas a refractory one can rule out the significance of a vector entirely [14].
Investigation on the susceptibility of eight species members of the Thai An. hyrcanus group to nocturnally subperiodic B. malayi indicated that An. peditaeniatus, An. crawfordi, An. nigerrimus, An. argyropus and An. pursati were high potential vectors. An. paraliae and An. sinensis were low potential vectors, while An. nitidus was a refractory vector. However, a crucial question regarding the susceptibility level determined in this study might be raised, due to the artificial feeding of mosquitoes on blood containing B. malayi microfilariae, which was not as natural as direct feeding on cat- and/or jird-infected B. malayi. Nevertheless, previous reports [23] confirmed that these two feeding techniques could be used robustly for routine screening of potential mosquito vectors of filarial parasites, since they did not differ significantly. This was despite the artificial feeding technique yielding slightly higher infective rates and parasite loads than the direct feeding method, presumably due to the effect of anticoagulant (10 units of heparin/1 ml of blood).
Among the five high potential vectors, An. peditaeniatus, An. crawfordi and An. nigerrimus were found to be abundant and widely distributed in Thailand and other countries [India (Assam, Bihar and Punjab), Sri Lanka, Bangladesh, China (Hainan Island), Myanmar, Cambodia, Vietnam, Malaysia (Malaysian Peninsular, Sabah and Sarawak), Indonesia (Java and Sumatra) and Brunei], and were proven as outdoor-biters of humans in certain localities of Thailand [24,25]. Regarding vector competence, An. peditaeniatus and An. nigerrimus have been incriminated so far as suspected vectors of P. vivax in Thailand [26-28], as well as An. nigerrimus as a potential natural vector of W. bancrofti in Phang Nga province, southern Thailand [8], and An. peditaeniatus as a secondary vector of Japanese encephalitis virus in China and India [29,30]. Beneficial results reported herein emphasize the potential role of An. peditaeniatus, An. crawfordi, An. nigerrimus, An. argyropus and An. pursati in transmitting nocturnally subperiodic B. malayi in southern Thailand as well as other countries, in which these anopheline species and filarial parasite were found sympatrically and/or co-endemic with malaria and Japanese encephalitis. The list of these potential vector-species could be used as a promising guideline for the field approach to incriminate natural vectors in endemic areas of Brugian filariasis. Remarkably, An. sinensis has been incriminated as an important vector of nocturnally periodic B. malayi in China, Korea and Japan [14], but in this study, it was proven as a low potential vector of nocturnally subperiodic B. malayi. It is interesting to note that the An. sinensis strain from Korea and China was compatible genetically and/or nearly identical to that from Thailand, based on the crossing experiments and comparative sequence analyses of the ribosomal DNA (rDNA) internal transcribed spacer 2 (ITS2), and mitochondrial cytochrome c oxidase subunit I (COI) and subunit II (COII) [31]. This evidence appeared to support the high specificity between B. malayi physiological races and the An. sinensis vector.
It has been known for refractoriness of certain mosquito species towards filarial parasites to occur in the forgut (cibarial and pharyngeal amartures), midgut (fast blood coagulation) or thoracic muscles (direct toxicity and melanotic encapsulation) [32-34]. Regarding refractoriness in the thoracic muscle, large numbers of B. malayi and B. pahangi microfilariae exsheathed in refractory Ae. albopictus after gaining entry into the mosquitoes, and subsequently migrated to the thoracic muscles without further development [35,36]. The results revealed that the factor(s) in the thoracic muscles of Ae. albopictus conferred with the refractoriness. Evidence of refractoriness to B. pahangi microfilariae infection is of additional interest, as it could be induced in normally susceptible Ae. tabu by rearing female mosquitoes on sugar solution containing thoracic homogenate of refractory Ae. malayansis mosquitoes [33]. This result agreed with a subsequent study in that the high inhibition of B. pahangi larval development could be induced in the thoracic muscle of susceptible Ae. togoi. This was performed by intrathoracic injection of crude thoracic homogenate (CTH) from refractory Ae. albopictus into susceptible Ae. togoi prior to feeding on blood containing B. pahangi microfilariae [37]. Thus, these two pieces of evidence seem to reflect the inhibitory effect that might be due to direct toxicity of the homogenate on developing larvae. Furthermore, the melanization of immune responses in various insects against a wide-range of invading pathogens and parasites has been documented [34,38-40]. The immune response of mosquitoes is put into effect through the plasma components of both the hemolymph, i.e., the humoral response, and hemocytes, the cellular response [34]. The authors also suggested that the intracellular melanotic encapsulation of filarial developing stages, as observed in specific mosquito organs, may be caused by exposure to low-molecular-weight immune molecules, which are carried in the hemolymph (plasma) and can penetrate the basement membrane covering the cells of specific organs. This concept suggested that the same mechanisms controlling melanotic encapsulation reactions (immune response) extracellularly in the hemocoel also control them intracellularly in specific organs of the host in which the parasite develops. Subsequent evidence from using RNAi methodology to knock-down PAH (phenylalanine hydroxylase) expression in the mosquitoes, Ae. aegypti and Armigeres subalbatus, demonstrated that limitation in the amount of tyrosine, available for tyrosinase-mediated hydroxylation, significantly reduces the effectiveness of melanization reactions against inoculated filarial parasites [41]. Additionally, at least four specific enzymes [DCE (dopachrome conversion enzyme), DDC (dopa decarboxylase), PO (phenoloxidase) and TH (tyrosine hydroxylase)] were concerned in the biosynthesis of melanin [39]. Current studies on the possible factors affecting the difference in susceptibility levels of eight An. hyrcanus species to nocturnally subperiodic B. malayi revealed that at least two refractory mechanisms (direct toxicity and/or melanotic encapsulation) were involved in the refractoriness of thoracic muscles for parasite development. Variations in the percentages of melanotic encapsulation and degenerated L1 larvae recovered in the thoracic muscles of Ae. togoi (0% and 6.85%), An. peditaeniatus (0% and 3.66%), An. crawfordi (0% and 2.67%), An. paraliae (47.19% and 29.21%), An. sinensis (32.05% and 52.57%) and An. nitidus (94.44% and 5.56%), were good supportive evidence.
Conclusions
Eight species members of the An. hyrcanus group, i.e., An. argyropus, An. crawfordi, An. nigerrimus, An. nitidus, An. paraliae, An. peditaeniatus, An. pursati and An. sinensis were tested for susceptibility to nocturnally subperiodic B. malayi. They were allowed to feed artificially on blood containing B. malayi microfilariae, and dissected 14 days after feeding. The susceptibility rates were 70-100%, 80-85%, 50-65%, 60%, 60%, 10%, 5% and 0% in An. peditaeniatus, An. crawfordi, An. nigerrimus, An. argyropus, An. pursati, An. sinensis, An. paraliae and An. nitidus, respectively. As determined by levels of susceptibility, results indicated that An. peditaeniatus, An. crawfordi, An. nigerrimus, An. argyropus and An. pursati were high potential vectors when compared with the control vector, Aedes togoi. An. paraliae and An. sinensis were low potential vectors, while An. nitidus was a refractory vector. In order to determine the possible factor(s) affecting different degrees of susceptibility, stained-smears of blood meals from midguts immediately after fully engorged and dissected-thoraxes 4 days post blood-feeding from the control vector (Ae. togoi), high potential vectors (An. peditaeniatus and An. crawfordi), low potential vectors (An. paraliae and An. sinensis) and refractory vector (An. nitidus) were examined for microfilariae and L1 larvae, respectively. The results revealed that an appreciable number of microfilaria obtained in the ingested blood meals and L1 larvae recovered in thoracic muscles was similar in appearance to those in all infected mosquitoes. Nonetheless, the marked variations in degrees of normal development of L1 larvae in thoracic muscles were observed clearly from the four vector-groups, i.e., the control vector: 93.15%, high potential vectors: 96.34–97.33%, low potential vectors: 15.38–23.60% and refractory vector: 0%. At least, two refractory mechanisms, direct toxicity and melanotic encapsulation, were involved in the inhibition of L1 larval development in thoracic muscles.
Competing interests
The authors declare no competing interests.
Authors’ contributions
All the authors contributed significantly to this study. AS participated in the study design, field and laboratory experiments, data analysis and writing of the manuscript. CH, ST and KT carried out field and laboratory experiments. VB participated in data analysis, and criticized the manuscript. NJ and UC helped with data analyses. WC designed the experiments, carried out field and laboratory experiments, interpreted the results, and edited the manuscript. All authors read and approved the final version of the manuscript.
Contributor Information
Atiporn Saeung, Email: atsaeung@mail.med.cmu.ac.th.
Chayanit Hempolchom, Email: huggie_you@hotmail.com.
Visut Baimai, Email: visut.bai@mahidol.ac.th.
Sorawat Thongsahuan, Email: sorawat_ton@hotmail.com.
Kritsana Taai, Email: smileant_na@hotmail.com.
Narissara Jariyapan, Email: njariyapan@gmail.com.
Udom Chaithong, Email: uchaitho@mail.med.cmu.ac.th.
Wej Choochote, Email: wchoocho@mail.med.cmu.ac.th.
Acknowledgements
This work was supported by funding from the Thailand Research Fund (TRF Senior Research Scholar: RTA5480006) and Diamond Research Grant of the Faculty of Medicine, Chiang Mai University, awarded to W. Choochote and A. Saeung.
References
- World Health Organization. Global programme to eliminate lymphatic filariasis: progress report on mass drug administration, 2010. Wkly Epidemiol Rec. 2011;86:377–388. [PubMed] [Google Scholar]
- Deribe K, Meribo K, Gebre T, Hailu A, Ali A, Assefa A, Davey G. The burden of neglected tropical diseases in Ethiopia, and opportunities for integrated control and elimination. Parasit Vectors. 2012;5:240. doi: 10.1186/1756-3305-5-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza DK, Koudou B, Kelly-Hope LA, Wilson MD, Bockarie MJ, Boakye DA. Diversity and transmission competence in lymphatic filariasis vectors in West Africa, and the implications for accelerated elimination of Anopheles-transmitted filariasis. Parasit Vectors. 2012;5:259. doi: 10.1186/1756-3305-5-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontes G, Leite AB, Vasconcelos de Lima AR, Freitas H, Ehrenberg JP, Mauricio da Rocha EM. Lymphatic filariasis in Brazil: epidemiological situation and outlook for elimination. Parasit Vectors. 2012;5:272. doi: 10.1186/1756-3305-5-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harinasuta C, Sucharit S, Deesin T, Surathin K, Vutikes S. Bancroftian filariasis in Thailand, a new endemic area. Southeast Asian J Trop Med Public Health. 1970;1:233–245. [Google Scholar]
- Guptavanij P, Harinasuta C, Surathin K, Vutikes S, Deesin T. Studies on the prevalence of Malayan filariasis in South Thailand. Southeast Asian J Trop Med Public Health. 1977;8:42–52. [PubMed] [Google Scholar]
- Guptavanij P, Harinasuta C, Sucharit S, Vutikes S. Studies on subperiodic Brugia malayi in Southern Thailand. Southeast Asian J Trop Med Public Health. 1971;2:44–50. [PubMed] [Google Scholar]
- Division of Filariasis, Department of Communicable Disease Control, Ministry of Public Health. 1998. pp. 1–33.
- Kanjanopas K, Choochote W, Jitpakdi A, Suvannadabba S, Loymak S, Chungpivat S, Nithiuthai S. Brugia malayi in a naturally infected cat from Narathiwat province, southern Thailand. Southeast Asian J Trop Med Public Health. 2001;32:585–587. [PubMed] [Google Scholar]
- Bureau of Vector-Borne Disease, Department of Disease Control, Ministry of Public Health. Lymphatic Filariasis. 2012. http://www.thaivbd.org.
- Chai IH, Lim GI, Yoon SN, Oh WL, Kim SJ, Chai JY. Ocurrence of tertian malaria in a male patient who never been abroad. Korean J Parasitol. 1994;32:195–200. doi: 10.3347/kjp.1994.32.3.195. [DOI] [PubMed] [Google Scholar]
- Park JW, Son JI, Hur JP, Jong JS, Hwangbo Y, Lee SW, Kee MK, Shin YH, Yang BK. An outbreak of vivax malaria in Republic of Korea in 1999. Korean J Infec Dis. 2000;32:335–339. [Google Scholar]
- Shim JC, Shin EH. Malaria in Korea 2002. Korean J Infec Dis. 2002;34:104–135. [Google Scholar]
- Sasa M. Human filariasis: A global survey of epidemiology and control. Tokyo: University of Tokyo Press; 1976. [Google Scholar]
- Choochote W, Sucharit S, Abeywickreme W. A note on adaptation of Anopheles annularis Van Der Wulp, Kanchanaburi, Thailand to free mating in a 30 × 30 × 30 cm cage. Southeast Asian J Trop Med Public Health. 1983;14:559–560. [PubMed] [Google Scholar]
- Kim SJ, Choochote W, Jitpakdi A, Junkum A, Park SJ, Min GS. Establishment of a self-mating mosquito colony of Anopheles sinensis from Korea. Korean J Entomol. 2003;33:267–271. doi: 10.1111/j.1748-5967.2003.tb00080.x. [DOI] [Google Scholar]
- Choochote W, Keha P, Sukhavat K, Khamboonruang C, Sukontason K. Aedes (Finlaya) togoi Theobald 1907, Chanthaburi strain. A laboratory vector in studies of filariasis in Thailand. Southeast Asian J Trop Med Public Health. 1987;18:259–260. [PubMed] [Google Scholar]
- Jumkum A, Choochote W, Jitpakdi A, Leemingswat S, Komalamisra N, Jariyapan N, Boonyatakorn C. Comparative studies on the biology and filarial susceptibility of selected blood-feeding and autogenous Aedes togoi sub-colonies. Mem Inst Oswaldo Cruz. 2003;98:481–485. doi: 10.1590/S0074-02762003000400009. [DOI] [PubMed] [Google Scholar]
- Choochote W, Sukhavat K, Somboon P, Khamboonruang C, Maleewong W, Suwanpanit P. The susceptibility of small laboratory animals to nocturnally superiodic Brugia malayi in Thailand. J Parasitol Trop Med Assoc Thailand. 1986;9:35–37. [Google Scholar]
- Choochote W, Chaithong U, Somboon P, Pakdicharoen A, Tookyang B, Likitvong K, Siriprasert V, Sukontasan K, Thitasut P. Small laboratory animal model for nocturnally subperiodic Brugia malayi (Narathiwat province, southern Thailand strain) J Trop Med Parasitol. 1991;14:51–58. [Google Scholar]
- Luo H, Qu FY. Experimental infection index of Anopheles sinensis and melanization of periodic Brugia malayi. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 1990;8:260–263. [PubMed] [Google Scholar]
- Chomcharn Y, Surathin K, Bunnag D, Sucharit S, Harinasuta T. Effects of a single dose of primaquine on a Thai strain of Plasmodium falciparum. Southeast Asian J Trop Med Public Health. 1980;11:408–409. [PubMed] [Google Scholar]
- Insun D, Choochote W, Pitasawat B, Rongsriyam Y, Jitpakdi A, Tippawangkosol P. Comparative susceptibility of mosquito vectors to the filarial parasite after direct and artificial membrane feeding on microfilaremic blood. J Trop Med Parasitol. 1998;21:51–54. [Google Scholar]
- Scanlon JE, Peyton EL, Gould DJ. An annotated checklist of the Anopheles of Thailand. Thai Natl Sci Pap Fauna Ser. 1968;2:1–35. [Google Scholar]
- Harrison BA, Scanlon JE. Medical entomology studies II. The subgenus Anopheles in Thailand (Diptera: Culicidae) Contrib Am Entomol Inst. 1975;12:36–78. [Google Scholar]
- Harbach RE, Gingrich JB, Pang LW. Some entomological observations on malaria transmission in a remote village in northwestern Thailand. J Am Mosq Control Assoc. 1987;3:296–301. [PubMed] [Google Scholar]
- Gingrich J, Weatherhead A, Sattabongkot J, Pilakasiri C, Wirtz RA. Hyperendemic malaria in Thai Village: dependence of year-round transmission on focal and seasonally circumscribed mosquito (Diptera: Culicidae) habitats. J Med Entomol. 1990;27:1016–1026. doi: 10.1093/jmedent/27.6.1016. [DOI] [PubMed] [Google Scholar]
- Rattanarithikul R, Harrison BA, Harbach RE, Panthusiri P, Coleman RE. Illustrated keys to the mosquitoes of Thailand IV. Anopheles. Southeast Asian J Trop Med Public Health. 2006;37(Suppl 2):1–128. [PubMed] [Google Scholar]
- Zhang HL. The natural infection rate of mosquitoes by Japanese encephalitis B virus in Yunnan Province. Zhonghua Yu Fang Yi Xue Za Zhi. 1990;24:265–267. [PubMed] [Google Scholar]
- Kanojia PC, Shetty PS, Geevarghese G. A long-term study on vector abundance & seasonal prevalence in relation to the occurrence of Japanese encephalitis in Gorakhpur district, Uttar Pradesh. Indian J Med Res. 2003;117:104–110. [PubMed] [Google Scholar]
- Park MH, Choochote W, Kim SJ, Somboon P, Saeung A, Tuetan B, Tsuda Y, Takagi M, Joshi D, Ma YJ, Min GS. Nonreproductive isolation among four allopatric strains of Anopheles sinensis in Asia. J Am Mosq Control Assoc. 2008;24:489–495. doi: 10.2987/08-5753.1. [DOI] [PubMed] [Google Scholar]
- Denham DA, McGreevy PB. Brugian Filariasis: epidemiological and experimental studies. Adv Parasitol. 1977;15:243–309. doi: 10.1016/s0065-308x(08)60530-8. [DOI] [PubMed] [Google Scholar]
- Owen RR. Non-development of Brugia pahangi in a refractory mosquito, Aedes malayansis. Ann Trop Med Parasit. 1979;73:193–195. doi: 10.1080/00034983.1979.11687248. [DOI] [PubMed] [Google Scholar]
- Townson H, Chaithong U. Mosquito host influences on development of filariae. Ann Trop Med Parsitol. 1991;85:149–163. doi: 10.1080/00034983.1991.11812541. [DOI] [PubMed] [Google Scholar]
- Ewert A. Comparative migration of microfilariae and development of Brugia pahangi in various mosquitoes. Am J Trop Med Hyg. 1965;14:254–259. doi: 10.4269/ajtmh.1965.14.254. [DOI] [PubMed] [Google Scholar]
- Oda T, Wada Y. Exsheathment and migration of microfilariae of Brugia malayi (Che-ju Strain) in mosquitoes. Trop Med. 1980;22:27–33. [Google Scholar]
- Abeywickreme W, Sucharit S, Choochote W, Chaicumpa W, Tumrasavin W. Alteration in Aedes togoi susceptibility to Brugia pahangi microfilariae induced by Aedes albopictus thoracic homogenate. Southeast Asian J Trop Med Public Health. 1989;11:408–409. [PubMed] [Google Scholar]
- Nayar JK, Knight JW. Comparison of migration and encapsulation of Brugia malayi microfilariae from the midgut to the hemocoel between Anopheles quadrimaculatus and Aedes aegypti. J Invertebr Pathol. 1995;65:295–299. doi: 10.1006/jipa.1995.1045. [DOI] [PubMed] [Google Scholar]
- Christensen BM, Li J, Chen CC, Nappi A. Melanization immune response in mosquito vectors. Trends Parasitol. 2005;21:192–199. doi: 10.1016/j.pt.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Magalhaes T, Oliveira IF, Melo-Santos MAV, Oliveira CMF, Lima CA, Ayres CFJ. Expression of defensin, cecropin, and transferrin in Aedes aegypti (Diptera: Culicidae) infected with Wuchereria bancrofti (Spirurida: Onchocercidae), and the abnormal development of nematodes in the mosquito. Exp Parasitol. 2008;120:364–371. doi: 10.1016/j.exppara.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Infanger LC, Rocheleau TA, Bartholomay LC, Johnson JK, Fuchs J, Higgs S, Chen CC, Christensen BM. The role of phenylalanine hydroxylase in melanotic encapsulation of filarial worms in two species of mosquitoes. Insect Biochem Mol Biol. 2004;34:1329–1338. doi: 10.1016/j.ibmb.2004.09.004. [DOI] [PubMed] [Google Scholar]


