Abstract
Several new sulfonebiscompounds having a biologically active 1,2-dihydropyridine-2-one 3–19, acrylamide 20, chromene 21, 22 and chromenopyridine 23, 24 moieties were synthesized and evaluated as potential anticancer agents. The structures of the products were confirmed via elemental analyses and spectral data. The screening tests showed that many of the biscompounds obtained exhibited good anticancer activity against human breast cell line (MCF7) comparable to doxorubicin which was used as reference drug. Compounds 11, 17 and 24 showed IC50 values 35.40 μM, 29.86 μM and 30.99 μM, respectively. In order to elucidate the mechanism of action of the synthesized compounds as anticancer agents, docking on the active site of farnesyltransferase and arginine methyltransferase was also performed and good results were obtained.
Keywords: Sulfone, Pyridines, Chromenes, Pyridnochromenes, Anticancer activity
Background
Many naturally occurring and synthetic compounds containing the 2-pyridone scaffold possess interesting pharmacological properties [1]. The pyridine derivative I, for example, has been identified as specific non-nucleuoside reverse transcriptase inhibitor in treatment of HIV-1 [2,3]. While the pyridine derivatives, Milirinone II and Amrinone III, and their analouges are used as cardiotonic agents in the treatment of heart failure [4-7]. Also, Pirfeidione (PFD) IV, a pyridine derivative which demonstrated antifibrotic activity in several organs in experimental animals, including lung, kidney and uterus has proven beneficial cure for a range of fibrotic conditions through both anti-inflammtory and and antifibrotic mechanisms [8]. A phase II clinical study showed PFD to be promising agent for the treatment of idiopathic pulmonary fibrosis, initiated in mice treated with cyclophosamide [9], amiodarone [10] or belomycin [11-16]. The reported antifbrotic activity of PFD prompted us to synthesize a new series of sulfonebiscompounds carrying biologically active 1,2-dihydropyridine-2-one, chromene and chromenopyridine as analoges to PFD. In addition, some 2-pyridones are also reported to possess antitumor, antibacterial [17] and other biological activities [18-20]. On the otherhand, sulfone derivatives have been found to exhibit a wide variety of pharmacological activities [21-25]. In addition, the bisheterocyclic compounds chromenes and chromenopyridine derivatives are well known as anticancer agents [26-29]. Also, diphenylsulfones and bisheterocyclic compounds are reported to have a broad spectrum of biological activities. Some are endowed with antitumor or antifungal properties [30]. On the other hand, some pyridine and isoquinoline derivatives have various biological properties such as antimicrobial [31], anticancer [32-35] activities.
Recent studies have proved the remarkable effect of Dapson on inhibiting cell growth in glioblastoma by acting as anti-VEGF and anti-angiogenic agent via depriving glioblastoma of neutrophil-mediated growth promoting effects [36]. Allantodapson V, a Dapson derivative showed high activity as anticancer through inhibition of arginine methyltranseferase (PRMT1) an enzyme which plays an important role in hormone dependent cancers. A series of acylated diarylsulfone derivatives were evaluated for the same activity and compound VI exihibited good activity as (PRMT1) inhibitor [37].
In view of these findings, and in continuation to our work in the synthesis of novel anticancer agents [38-42] we undertook the synthesis of bisheterocyclicsulfone compounds analogues for 2-pyridones incorporating biologically active 1,2-dihydropyridine-2-one, chromene, and chromenopyridone in one molecule to explore the promising anticancer compounds.
Results and discussion
Chemistry
Several compounds were designed with the aim of exploring anticancer properties (Scheme 1, Scheme 2, Scheme 3). Scheme 1 outlines the synthetic pathway used to obtain compounds 3–16. The starting material N,N’-(4,4’-sulfonylbis(4,1-phenylene))bis(2-cyanoacetamid) 2 was obtained via reaction of Dapson 1 with ethyl cyanoacetate. Compound 2 was established by elemental analysis and spectral data. Thus, IR spectrum of 2 revealed bands at 3448, 3363 cm-1 (2NH), 2256 cm-1 (2 C ≡ N), 1701 cm-1 (2 C = O) and 1342, 1180 cm-1 (SO2). 1 H-NMR spectrum of 2 in (DMSO-d6) exhibited signals at 4.0 ppm due to CH2 group, 7.4-7.9 ppm corresponding to aromatic protons and 10.7 ppm due to 2 NH groups. Treatment of compound 2 with appropriate aldehyde and malononitrile in the presence of catalytic amounts of pipredine compounds 3–16, respectively. These compounds were verified on the basis of elemental analyses, IR, 1 H-NMR and 13 C-NMR. Thus, IR spectra of compounds 3–16 exhibited the presence of NH2, C ≡ N, C = O and SO2 bands. 1 H-NMR spectra of compounds 3–16 in (DMSO-d6) revealed the presence of NH2 at 6.0-6.8 ppm and aromatic protons at 6.9-8.7 ppm (Scheme 1).
Scheme 1.
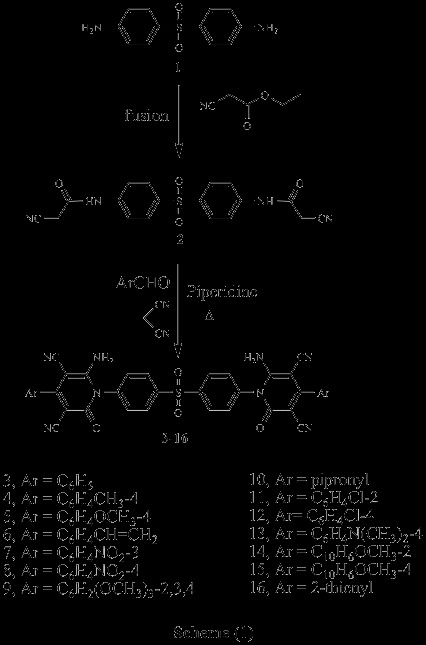
Synthetic pathways used to obtain compounds 2-16.
Scheme 2.
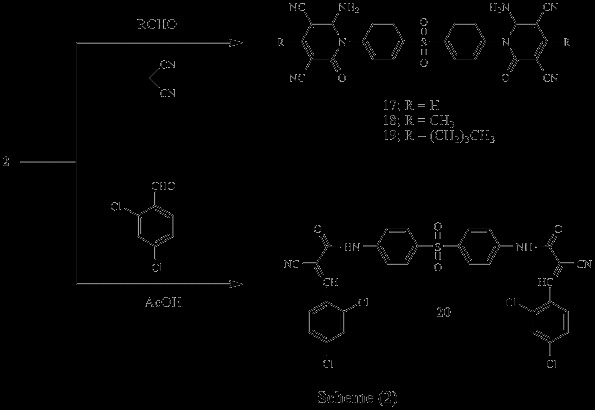
Synthetic pathways used to obtain compounds 17-20.
Scheme 3.
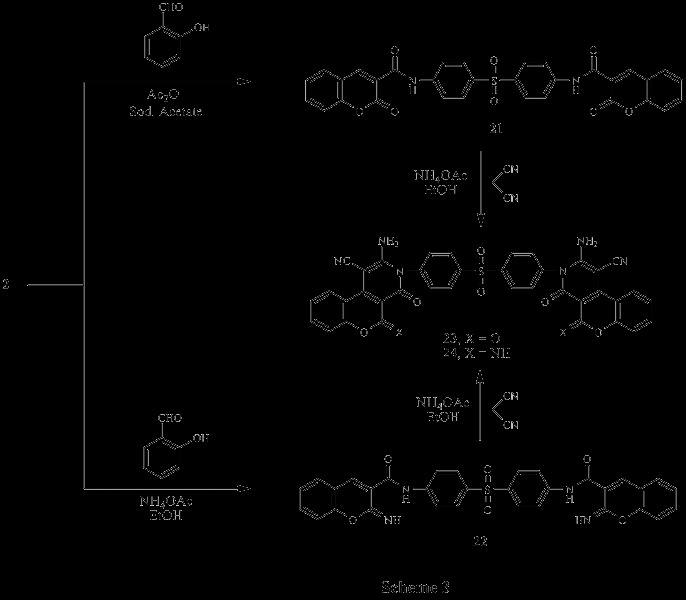
Synthetic pathways used to obtain compounds 21-24.
Similarly, interaction of 2 with aliphatic aldehyde and malononitrile in ethanol containing catalytic amount of piperidine afforded the corresponding 1,2-dihydropyridine-2-one derivatives 17–19. IR spectra of compounds 17–19, exhibited the presence of characteristic bands of NH2, C ≡ N, C = O and SO2 groups 1H-NMR spectrum of 17 in (DMSO-d6) revealed signals at 6.8 ppm due to 2NH2, while 1 H-NMR spectrum of 18 in (DMSO-d6) revealed signals at 1.8 ppm corresponding to 2CH3. On the other hand, 1 H-NMR spectrum of 19 in (DMSO-d6) triplet signal at 1.1 ppm for CH3 and a multiplet one at 1.3-2.0 ppm corresponding to CH2 groups. Interaction of 2 with 2,4-dichlorobenzaldehyde in acetic acid gave the corresponding acrylamide derivative 20. IR spectrum of 20 revealed bands at 3372 cm-1 (2NH), 2203 cm-1 (2 C ≡ N), 1652 cm-1 (2 C = O) and 829 cm-1 (C-Cl). 1 H-NMR spectrum of 20 in (DMSO-d6) showed signals at 8.0 ppm due to CH groups, 10.0 ppm corresponding to NH groups (Scheme 2).
Furthermore, Perkin reaction was carried out by reacting compound 2 with salicylaldehyde in acetic anhydride containing catalytic amount of anhydrous sodium acetate to give the corresponding chromene derivative 21, while reaction of 2 with salicylaldehyde in ammonium acetate afforded 2-iminochromene derivative 22 (Scheme 3).
Molecular docking
The zinc-metalloenzyme farnesyl transferase (FTase) catalyzes the transfer of a farnesyl group to a cysteine thiol group contained in the C-terminal tetra peptide signal sequence of Ras, frequently referred to as aCAAX motif. Farnesylation causes membrane localization of Ras which, in turn, determines the switch from an inactive to an active Ras-GTP-bound form [43-45]. Among the Ras isoforms H-ras, N-ras, and K-ras, mutations in the K-ras isoform are most relevant to human cancers in particular pancreatic, colon, and lung cancers, which exhibit approximately 90, 40, and 25% incidence of Kras mutations, respectively. Inhibitors of FTase prevent membrane localization of the Ras oncogene and have the ability to revert the transformed phenotype, providing the rationale for the development of farnesyl transferase inhibitors (FTIs) as anticancer drugs [46-49].
On the other hand, the relative levels of arginine methyltransferase (PRMT1) isoforms are altered between normal and cancerous breast issue, with two of the isoforms down-regulated [50]. Therefore, it appears that PRMT1expression in cancer cells may be altered depending on the tumor type. Studies are beginning to examine the specific role of PRMT1in cancer. PRMT1 is an essential component of a Mixed Line age Leukaemia (MLL) transcriptional complex that modifies histones by methylation, at H4R3, and acetylation [51]. This serves as the first demonstration of a direct role for PRMT1-mediated transcriptional up regulation during cancer progression.
Thus, the present investigation is concerned with the synthesis of novel anticancer agents and trying to understand their mechanism of action. In order to perform the aim of the present investigations the authors have performed molecular docking of the synthesized compounds on the active sites of both farnesyl transferase and arginine methyltransferase (PRMT1) which may lead to understanding of their effect as antitumor agents.
Molecular docking on the active site of farnesyl transferase
The protein data bank file (PDB:3E30) was selected for this purpose. The file contains farnesyl transferase enzyme co-crystallized with a sulfone ligand. All docking procedures were achieved by MOE (Molecular Operating Environment) software 10.2008 provided by chemical computing group, Canada. Docking on the active site of farnesyl transferase enzyme was performed for all synthesized compounds 2–24.
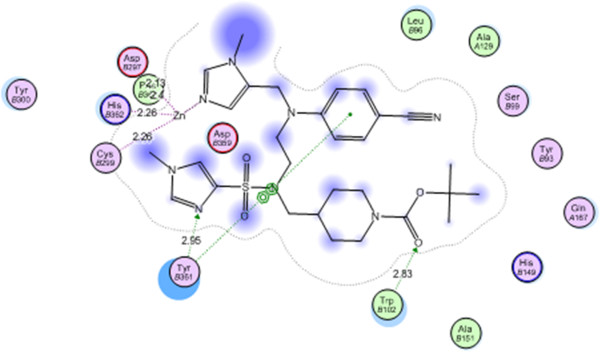
Docking protocol was verified by redocking of the co-crystallized ligand in the vicinity of the active site of the enzyme with energy score (S) = −25.6345 Kcal/ mol and root mean standard deviation (RMSD) = 2.8268 (Figure 1).
Figure 1.
Co-crystallized sulfone ligand on the active site of farnesyltransferase.
The sulfone ligand interacts with the active site of farnesyl transferase by four interactions: Try B361 with a hydrogen bond of 2.95 Ao and arene-arene interaction, Trp 102 with a hydrogen bond of 2.83 and with Zn by the lone pair of imidazole nitrogen. All synthesized compounds were fit to the active site of farnesyl transferase enzyme with good energy scores (S) suggesting activity as farnesyl transferase inhibitors. Energy scores (S) and amino acid interactions for synthesized compounds were listed in (Table 1).
Table 1.
Binding scores and amino acid interactions of the docked compounds on the active site of farnesyltransferase (FT)
| Compound no. | S Kcal/Mol | Amino acid interactions | H bond length Ao | Interaction with Zn |
|---|---|---|---|---|
| 2 |
-22.2685 |
Leu B295, Lys B294 |
3.37, 2.76 |
No interaction |
| 3 |
-37.4155 |
Lys A164, Arg B202 |
3.39, 2.53-3.14 |
CN |
| 4 |
-25.1368 |
Lys B234, Tyr B334, LysB358, Arg B202 |
3.08, 2.75, 3.28, 2.73 |
SO2 |
| 5 |
-22.9916 |
Lys B294, Lys A164, Gln A167, Arg B202 |
3.47, 2.84, 3.00, 3.09 |
C = O |
| 6 |
-31.4218 |
Lys A164, Arg B202 |
2.49, 3.28 |
SO2 |
| 7 |
-30.3616 |
Arg B291, Arg B202 |
3.19, 2.47-2.96 |
CN |
| 8 |
-26.5141 |
Arg B291, Lys B294 |
3.55, 2.94 |
CN |
| 9 |
-25.5855 |
Lys B294, Lys A168, His B362 |
2.58, 2.76, 3.19 |
CN |
| 10 |
-27.1374 |
Ser B99, Ser B367, Arg B291 |
3.30, 3.05, 2.56 |
No interaction |
| 11 |
-23.4085 |
Trp B102, Lys A168 |
2.75, 2.80 |
C = O |
| 12 |
-28.7413 |
Lys A164, Ser B99 |
3.00, 3.25 |
CN |
| 13 |
-27.1676 |
Lys A164, Arg B202 |
2.48, 2.76 |
SO2 |
| 14 |
-28.8232 |
Lys A164, Arg B202 |
2.81, 2.89-3.25 |
C = O |
| 15 |
-32.2519 |
Tyr B300, Asn A165 |
3.10, 3.32 |
CN |
| 16 |
-38.0536 |
Arg B202, Arg B291, Lys B294 |
2.57, 3.01, 3.39 |
CN |
| 17 |
-19.9521 |
Lys B353, Gly B290, Lys B294, Arg B202 |
2.78, 3.29, 2.67, 3.13 |
No interaction |
| 18 |
-23.0290 |
Leu B295, Lys B294 |
3.05, 2.61 |
No interaction |
| 19 |
-32.9232 |
Arg B291 |
3.81 |
CN |
| 20 |
-24.4073 |
Arg B202 |
2.35 |
C = O |
| 21 |
-29.7807 |
Tyr B300 |
2.85 |
C = O |
| 22 |
-38.6191 |
Arg B202, Asp B352 |
2.92, 1.96 |
C = O, NH |
| 23 |
-38.8898 |
Lys A164, Arg B202 |
2.81, 2.49-2.55 |
C = O, C = O |
| 24 | -45.9317 | Lys A164, Arg B202 | 2.83, 2.46-2.45 | C = O, NH |
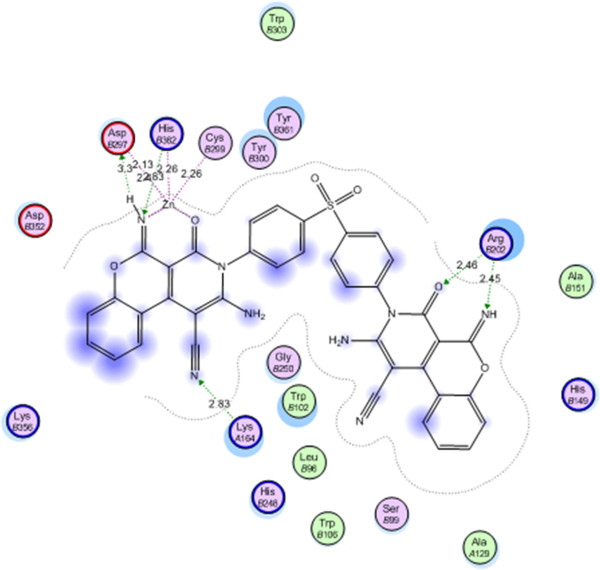
Compound 24 showed the best energy score (S) = −45.9317 Kcal/mol and interacted with Lys A146 with a hydrogen bond of 2.83 Ao, with Arg B202 with two hydrogen bonds of 2.45, 2.46 Ao and with Zn through its C = O and NH (Figure 2).
Figure 2.
Compound 24 on the active site of farnesyltransferase.
Molecular docking on the active site of arginine methyltransferase (PRMT1)
The protein data bank file (PDB:3Q7E) was selected for this purpose. The file contains arginine methyltransferase co-crystallized with its ligand (S-adenosyl methionine). All docking procedures were achieved by MOE (Molecular Operating Environment)software 10.2008 provided by chemical computing group, Canada. Docking on the active site of arginine methyltransferase enzyme was performed for all synthesized compounds 2–24.
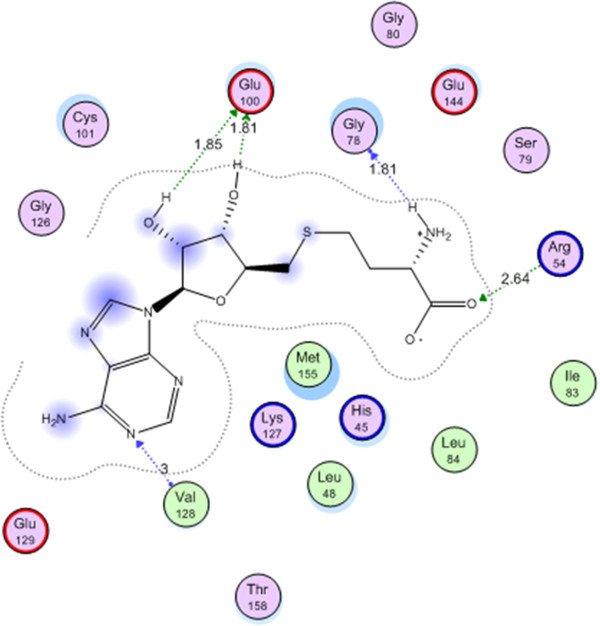
Docking protocol was verified by redocking of the co-crystallized ligand in the vicinity of the active site of the enzyme with energy score (S) = −18.5932 Kcal/ mol and root mean standard deviation (RMSD) = 0.3523. The ligand interacts with the active site of arginine methyltransferase by five interactions: Val 128 with a hydrogen bond of 3.00 Ao, with Arg 54 with a hydrogen bond of 2.64, with Gly 78 with a hydrogen bond of 1.81 Ao and with Glu 100 with two hydrogen bonds of 181, 186 Ao (Figure 3).
Figure 3.
Co-crystallized S-adenosyl methionine ligand on the active site of arginine methyltransferase (PRMT1).
All synthesized compounds were fit to the active site of arginine methyltransferase enzyme with good energy scores (S) except compounds 7, 18 and 19 suggesting good activity as arginine methyltransferase inhibitors for most of the synthesized compounds. Energy scores (S) and amino acid interactions for the synthesized compounds were listed in (Table 2).
Table 2.
Binding scores and amino acid interactions of the docked compounds on the active site of arginine methyltransferase (PRMT1)
| Compound no. | S Kcal/Mol | Amino acid interactions | H bond length Ao |
|---|---|---|---|
| 2 |
-20.0584 |
Lys 127, His 293 |
2.65, 2.81 |
| 3 |
-13.8464 |
Lys 127, Arg 327 |
2.39, 2.96 |
| 4 |
-17.2063 |
Lys 127, Arg 327 |
2.42-2.39, 2.45 |
| 5 |
-13.6909 |
Lys 127, His 45, Arg 327 |
2.57, 2.95, 2.36 |
| 6 |
-18.0294 |
Arg 327 |
2.45-3.02 |
| 7 |
11.0959 |
------------ |
------------ |
| 8 |
-15.9006 |
Lys 127, Arg 327 |
2.40, 2.30 |
| 9 |
-5.1052 |
His 45, Glu 153, Arg 327 |
2.75, 1.65, 2.36 |
| 10 |
-17.1347 |
Lys 127, Glu 153, His 45 |
2.75, 1.58, 2.87 |
| 11 |
-12.0837 |
Asn 167 |
2.65 |
| 12 |
-19.6261 |
Lys 127, Arg 327 |
2.59-2.84, 2.85 |
| 13 |
-15.7402 |
Lys 127, Glu 153, Arg 327 |
2.47, 1.93, 2.44 |
| 14 |
-20.4078 |
Asn 157, Lys 127 |
3.18, 2.66-2.79 |
| 15 |
-18.8629 |
Gln 163, Lys 127 |
2.22, 2.42-3.23 |
| 16 |
14.8212 |
------------ |
------------ |
| 17 |
-20.6494 |
Asn 157, His 45, Lys 127 |
3.24, 3.21, 2.68 |
| 18 |
6.1835 |
------------ |
------------ |
| 19 |
10.1989 |
------------ |
------------ |
| 20 |
-17.2838 |
Lys 127 |
2.51 |
| 21 |
-17.6535 |
Lys 127 |
2.51, 2.86 |
| 22 |
-15.4395 |
Arg 327, Glu 144 |
2.79, 1.47 |
| 23 |
-19.4615 |
Lys 127 |
2.54, 2.52 |
| 24 | -23.0582 | Arg 327, Lys 127, Glu 130 | 2.51-2.46, 2.75, 1.36 |
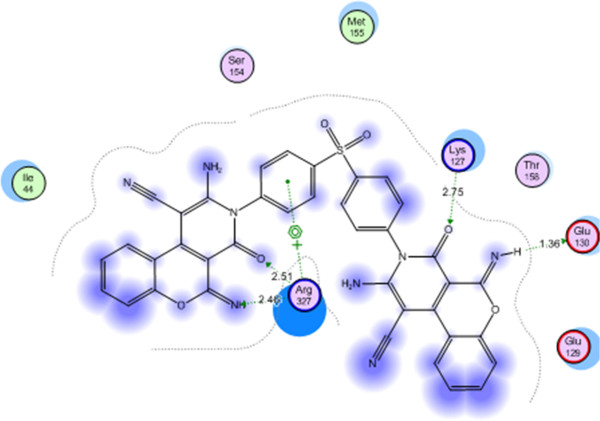
Compound 24 showed the best energy score (S) = −23.0582 Kcal/mol and interacted with Arg 327 with two hydrogen bonds of 2.51, 2.46 Ao, with Lys 127 with a hydrogen bond of 2.75 Ao and with Glu 130 with a hydrogen bond of 1.36 Ao (Figure 4).
Figure 4.
Compound 24 on the active site of arginine methyltransferase (PRMT1).
In vitro antitumor activity
The newly synthesized compounds were evaluated for their in vitro cytotoxic activity against human breast cancer cell line; MCF7. Doxorubicin which is one of the most effective anticancer agents was used as the reference drug in this study. The relationship between surviving fraction and drug concentration was plotted to obtain the survival curve of breast cancer cell line (MCF7).The response parameter calculated was the IC50 value, which corresponds to the concentration required for 50% inhibition of cell viability. Table 3 shows the in vitro cytotoxic activity of the synthesized compounds where all compounds exhibited significant activity compared to the reference drug.
Table 3.
In vitro anticancer screening of the synthesized compounds against human breast cell line (MCF7)
| Comp NO. |
Compound concentration (μM) |
IC50 (μM) | |||
|---|---|---|---|---|---|
|
10 μM |
25 μM |
50 μM |
100 μM |
||
| Surviving fraction (Mean ± S.E.)* | |||||
| Doxorubicin |
0.721 ± 0.02 |
0.546 ± 0.02 |
0.461 ± 0.01 |
0.494 ± 0.03 |
71.80 |
| 2 |
0.727 ± 0.134 |
0.427 ± 0.055 |
0.307 ± 0.029 |
0.317 ± 0.021 |
46.57 |
| 3 |
0.793 ± 0.055 |
0.454 ± 0.097 |
0.292 ± 0.008 |
0.332 ± 0.050 |
52.45 |
| 4 |
0.840 ± 0.063 |
0.435 ± 0.035 |
0.403 ± 0.015 |
0.335 ± 0.082 |
54.37 |
| 5 |
0.906 ± 0.021 |
0.642 ± 0.059 |
0.428 ± 0.038 |
0.547 ± 0.046 |
81.22 |
| 6 |
0.732 ± 0.333 |
0.584 ± 0.046 |
0.406 ± 0.069 |
0.229 ± 0.097 |
49.65 |
| 7 |
0.761 ± 0.190 |
0.546 ± 0.123 |
0.254 ± 0.031 |
0.297 ± 0.048 |
47.83 |
| 8 |
0.830 ± 0.124 |
0.399 ± 0.082 |
0.199 ± 0.021 |
0.272 ± 0.005 |
42.56 |
| 9 |
0.649 ± 0.028 |
0.394 ± 0.339 |
0.207 ± 0.027 |
0.261 ± 0.049 |
37.29 |
| 10 |
0.609 ± 0.059 |
0.479 ± 0.095 |
0.332 ± 0.058 |
0.316 ± 0.064 |
45.45 |
| 11 |
0.747 ± 0.197 |
0.359 ± 0.052 |
0.153 ± 0.020 |
0.189 ± 0.002 |
35.40 |
| 12 |
0.604 ± 0.075 |
0.232 ± 0.019 |
0.376 ± 0.089 |
0.312 ± 0.029 |
40.12 |
| 13 |
0.650 ± 0.184 |
0.401 ± 0.016 |
0.253 ± 0.021 |
0.401 ± 0.017 |
45.77 |
| 14 |
0.875 ± 0.066 |
0.580 ± 0.046 |
0.336 ± 0.049 |
0.467 ± 0.047 |
65.58 |
| 15 |
0.886 ± 0.047 |
0.423 ± 0.024 |
0.259 ± 0.054 |
0.389 ± 0.047 |
52.48 |
| 16 |
0.669 ± 0.114 |
0.539 ± 0.088 |
0.276 ± 0.064 |
0.259 ± 0.080 |
44.62 |
| 17 |
0.509 ± 0.235 |
0.230 ± 0.139 |
0.300 ± 0.134 |
0.279 ± 0.065 |
29.86 |
| 18 |
0.865 ± 0.057 |
0.615 ± 0.048 |
0.232 ± 0.046 |
0.286 ± 0.071 |
50.74 |
| 19 |
0.815 ± 0.042 |
0.545 ± 0.109 |
0.264 ± 0.044 |
0.336 ± 0.096 |
51.48 |
| 20 |
0.703 ± 0.189 |
0.427 ± 0.194 |
0.251 ± 0.026 |
0.374 ± 0.085 |
46.26 |
| 21 |
0.941 ± 0.020 |
0.472 ± 0.209 |
0.199 ± 0.090 |
0.278 ± 0.108 |
47.49 |
| 22 |
0.653 ± 0.291 |
0.574 ± 0.180 |
0.337 ± 0.116 |
0.359 ± 0.044 |
52.74 |
| 23 |
0.878 ± 0.032 |
0.563 ± 0.065 |
0.276 ± 0.031 |
0.389 ± 0.058 |
56.37 |
| 24 | 0.648 ± 0.329 | 0.280 ± 0.154 | 0.174 ± 0.105 | 0.194 ± 0.065 | 30.99 |
* Each value is the mean of three values ± Standard Error.
All the synthesized compounds showed better cytotoxic activity than Doxorubicin except compound 5 which showed IC50 value 81.22 μM. The 1,2-dihdropyridine-2-one derivatives 3–19 showed IC50 values in the rang 29.86-81.22 μM. Compound 17 which showed IC50 value 29.86 μM was the most active compound. Compound 17 also showed good scoring energy S = −19.9521 kcal/Mol. and the good amino acid interactions upon docking on the active site of farnesyl transferase enzyme. It also showed good energy score S = −20.9464 kcal/Mol. and good amino acid interactions upon docking on the active site of arginine methyl transferase enzyme. Upon substitution on position 4 of compound 17 with several substitutions the activity drops. However, 2,3,4-trimethoxy phenyl substitution, 2-chloro phenyl substitution and 4-chloro phenyl substitution did not decrease the activity in the same way substitution with 4-CH3 phenyl, 4-OCH3 phenyl and 2-OCH3 naphthyl did. This was clearly illustrated by the values of IC50 of the 1,2-dihdropyridine-2-one derivatives 9, 11 and 12 with IC50 values of 37.29 μM, 35.40 μM and 40.12 respectively. On the other hand, the IC50 values for the 1,2-dihdropyridine-2-one derivatives in which the substitution was with 4-CH3 phenyl, 4-OCH3 phenyl and 2-OCH3 naphthyl were much higher indicating less activity. This was clearly shown in the 1,2-dihydropyridine derivatives 4,5 and 14 with IC50 values of 54.37 μM, 81.22 μM and 65.58 μM, respectively.
Compounds 20–24 showed cytotoxic activity with IC50 values in the range of 30.99 to 56.37 μM with cytotoxic activity better than that of Doxorubicin. The chromenopyridine derivative 24 was with the best IC50 = 30.99 μM among these compounds while compound 23 showed the highest IC50 value 56.37 μM among these compounds. Compound 24 also showed the best scoring energy S = −45.9317 kcal/Mol. and the best amino acid interactions upon docking on the active site of farnesyl transferase enzyme. It also showed the best energy score S = −23.0582 kcal/Mol. and the best amino acid interactions upon docking on the active site of arginine methyl transferase enzyme.
The promising results of cytotoxic activity of the synthesized compounds especially compounds 17, 24 urge more investigations for their mechanism of action. The trial in the present investigation to predict an assumption of the mechanism of action of the synthesized compounds was conducted through molecular docking on the active site of two enzymes based on the similarities between the synthesized compounds and the enzyme inhibitors of these enzymes.
Experimental
Chemistry
Melting points (°C, uncorrected) were determined in open capillaries on a Gallenkemp melting point apparatus (Sanyo Gallenkemp, Southborough, UK) and were uncorrected. Precoated silica gel plates (silica gel 0.25 mm, 60 G F254; Merck, Germany) were used for thin layer chromatography, dichloromethane/methanol (9.5:0.5) mixture was used as a developing solvent system and the spots were visualized by ultraviolet light and/or iodine. Infra-red spectra were recorded in KBr discs using IR-470 Shimadzu spectrometer (Shimadzu, Tokyo, Japan). NMR spectra (in DMSO-d6) were recorded on Bruker AC-300 Ultra Shield NMR spectrometer (Bruker, Flawil, Switzerland, δ ppm) at 300 MHz using TMS as internal Standard and peak multiplicities are designed as follows: s, singlet; d, doublet; t, triplet; m, multiplet. Elemental analyses were performed on Carlo Erba 1108 Elemental Analyzer (Heraeus, Hanau, Germany).
N,N'-(4,4'-sulfonylbis(4,1-phenylenebis (2-cyanoacetamide) 2
A mixture of Dapsone (2.48 g, 0.01 mol.) and ethyl cyanoacetate (1.13 g, 0.01 mol.) was refluxed for 3 h, concentrated and cooled. The obtained solid was filtered and crystallized from ethanol to give 2. Yield 92%, melting point 137.5-139°C. IR:υmax./cm-1 3448, 3363 (2 NH), 3062 (CH aromatic), 2960, 2931 (CH aliphatic), 2256 (CN), 1701 ( 2 C = O),1342, 1180 (SO2). 1 H-NMR (DMSO-d6, D2O):δ 4.0 (s, 4 H, 2CH2), 7.4-7.9 (m, 8 H, Ar-H), 10.7 (s, 2 H, 2NH exch.). 13 C-NMR(DMSO-d6, D2O): 24.4(2), 115.6(2), 119.2(2), 119.3(2), 128.1(2), 129.2(2), 137.8(2), 142.7(2), 162.2(2). Anal. Calcd. for C18H14N4O4S (382.39): C, 56.54; H, 3.69; N, 14.65. Found: C, 56.81; H, 3.84; N, 14.29.
General procedure for compounds 3–16 and 17–19
A mixture of the starting material 2 (6.86 g, 0.01 mol.), appropriate aldehydes (0.01 mol.) and malononitrile (0.66 g, 0.01 mol.) in ethanol (50 mL) containing catalytic amount of piperidine in ethanol (50 mL) was heated under reflux for 5 h. The obtained solid was crystallized from dioxane to give 3–19, respectively.
1,1'-(4,4'-sulfonylbis(4,1-phenylene))bis(6-amino-2-oxo-4-phenyl-1,2-dihydropyridine-3,5-dicarbonitrile) 3
Yield 68%, melting point 257.7-259°C. IR:υmax./cm-1 3448, 3371 (2 NH2), 3077 (CH aromatic), 2210 (2 C ≡ N), 1670 (2 C = O),1290 (2 C = S), 1399, 1149 (SO2).1 H-NMR (DMSO-d6, D2O):δ 6.2 (s, 4 H, 2NH2, exch.), 7.4-7.9 (m, 18 H, Ar-H). Anal. Calcd. for C38H22N8O4S(686.70): C, 66.46; H, 3.23; N, 16.32. Found: C, 66.71; H, 3.20; N, 16.00.
1,1'-(4,4'-sulfonylbis(4,1-phenylene))bis(6-amino-2-oxo-4-p-tolyl-1,2-dihydropyridine-3,5-dicarbonitrile) 4
Yield 76%, melting point 234.2°C. IR:υmax./cm-1 3371, 3209 (2 NH2), 3100 (CH aromatic), 2960, 2870 (CH aliphatic),2218 (2 C ≡ N), 1674 (2 C = O), 1400, 1149 (SO2). 1 H-NMR (DMSO-d6, D2O):δ 2.4 (s, 6 H, 2 CH3), 6.6 (s, 4 H, 2NH2, exch.), 7.3-8.1 (m, 16 H, Ar-H). 13 C-NMR(DMSO-d6, D2O): 17.6 (2), 80.2 (2), 114.2 (2), 115.6 (4), 128.0 (4), 128.6 (4), 130.1 (4), 131.0 (4), 133.8 (4), 134.7 (4), 152.8 (2), 153.9 (2), 163.4 (2). Anal. Calcd. forC40H26N8O4S(714.75): C, 67.22; H, 3.67; N, 15.68. Found: C, 67.56; H, 3.44; N, 15.50.
1,1'-(4,4'-sulfonylbis(4,1-phenylene))bis(6-amino-4-(4-methoxyphenyl)-2-oxo-1,2-dihydropyridine-3,5-dicarbonitrile) 5
Yield 71%, melting point >340°C.IR:υmax./cm-1 3390, 3210 (NH, NH2),3100 (CH aromatic), 2962, 2839 (CH aliphatic), 2214 (2 C ≡ N), 1680 (2 C = O), 1400, 1180 (SO2).1 H-NMR (DMSO-d6, D2O):δ 3.8 (s, 6 H, 2 OCH3), 6.3 (s, 4 H, 2NH2, exch.), 7.0-8.1 (m, 16 H, Ar-H). 13 C-NMR(DMSO-d6, D2O): 55.2 (2), 77.9 (2), 113.7 (4), 117.6 (2), 117.9 (4), 127.5 (4), 129.1 (2), 129.5 (4), 130.8 (4), 140.6 (2), 141.2 (2), 157.7 (2), 159.6 (2), 160.3 (2), 160.8 (2). Anal. Calcd. forC40H26N8O6S(746.75): C, 64.34; H, 3.51; N, 15.01. Found: C, 64.24; H, 3.19; N, 15.38.
1,1'-(4,4'-sulfonylbis(4,1-phenylene))bis(6-amino-2-oxo-4-styryl-1,2-dihydropyridine-3,5-dicarbonitrile) 6
Yield 79%, melting point 235.8°C. IR:υmax./cm-1 3448, 3370 (2NH2),2939,2860 (CH aliph.), 2218 (2 C ≡ N), 1685 (2 C = O), 1390, 1149 (SO2). 1 H-NMR (DMSO-d6, D2O):δ 6.6, 6.9 (2d, 4 H, 2 CH = CH, J = 7.4,7.3 Hz), 7.0-7.8 (m, 22 H, Ar-H + 2NH2, exchangable). 13 C-NMR(DMSO-d6): 62.9(2), 101.8(2), 113.0(4), 120.7(4), 125.7(2), 126.8(4), 128.1(2), 128.5(4), 129(4), 131.9(2), 133.7(2), 137.2(2), 138.8(2), 154.3(2), 155.9(2), 176.1(2). Anal. Calcd. for C42H26N8O4S(738.77): C, 68.28; H, 3.55; N, 15.17. Found: C, 68.53; H, 3.61; N, 14.92.
1,1'-(4,4'-sulfonylbis(4,1-phenylene))bis(6-amino-4-(3-nitrophenyl)-2-oxo-1,2-dihydropyridine-3,5-dicarbonitrile) 7
Yield 81%, melting point 167.2°C. IR:υmax./cm-1 3375, 3213 (2 NH2), 3093 (CH arom.), 2218 (2 C ≡ N), 1674 (2 C = O), 1350, 1149 (SO2), 1593, 1330 (NO2).1 H-NMR (DMSO-d6, D2O):δ 6.6 (s, 4 H, 2NH2, exchangable), 7.5-8.4 (m, 16 H, Ar-H). 13 C-NMR(DMSO-d6): 75.6(2), 113.0(2), 116.7(4), 122.8(2), 124.8(4), 125.1(2), 128.8(4), 129.8(2), 134.5(2), 136.0(2), 137.2(2), 138.4(2), 147.7(2), 156.9(2), 159.1(2), 165.5(2). Anal. Calcd. for C38H20N10O8S(776.69): C, 58.76; H, 2.60; N, 18.03. Found: C, 58.90; H, 2.91; N, 17.89.
1,1'-(4,4'-sulfonylbis(4,1-phenylene))bis(6-amino-4-(4-nitrophenyl)-2-oxo-1,2-dihydropyridine-3,5-dicarbonitrile) 8
Yield 72%, melting point 208.0°C. IR:υmax./cm-1 3371, 3210 (2 NH2), 3100 (CH arom.), 2194 (2 C ≡ N), 1674 (2 C = O), 1346, 1149 (SO2), 1593, 1332 (NO2). 1 H-NMR (DMSO-d6, D2O):δ 6.7 (s, 4 H, 2NH2, exchangable), 7.8-8.4 (m, 16 H, Ar-H). 13 C-NMR(DMSO-d6): 73.7(2), 114.4(2), 114.9(4), 124.5(4), 127.9(4), 129.1(4), 130.2(4), 137.6(2), 138.9(2), 141.1(2), 144.6(2), 156.8(2), 157.3(2), 166.9(2). Anal. Calcd. for C38H20N10O8S(776.69): C, 58.76; H, 2.60; N, 18.03. Found: C, 59.00; H, 2.78; N, 18.09.
1,1'-(4,4'-sulfonylbis(4,1-phenylene))bis(6-amino-2-oxo-4-(2,3,4-trimethoxyphenyl)-1,2-dihydropyridine-3,5-dicarbonitrile) 9
Yield 70%, melting point 286.4°C. IR:υmax./cm-1 3378, 3210 (2 NH2), 3097 (CH arom.), 2943, 2839(CH aliph.), 2218 (2 C ≡ N), 1674 (2 C = O), 1390, 1157 (SO2).1 H-NMR (DMSO-d6, D2O):δ 3.7, 3.8 (2 s, 18 H, 6OCH3), 6.7 (s, 4 H, 2NH2, exchangable), 6.9-8.0 (m, 12 H, Ar-H). 13 C-NMR(DMSO-d6): 55.8(2), 55.9(2), 60.5(2), 76.7(2), 107.7(2), 113.3(2), 115.4(2), 116.1(4), 121.1(2), 124.9(4), 129.8(4), 130.6(2), 138.9(2), 141.8(2), 150.1 (2), 153.5(2), 156.4(2), 159.6(2), 165.9(2). Anal. Calcd. for C44H34N8O10S(866.85): C, 60.96; H, 3.95; N, 12.93. Found: C, 60.72; H, 4.03; N, 12.81.
1,1'-(4,4'-sulfonylbis(4,1-phenylene))bis(6-amino-4-(benzo[d][1,3]dioxol-5-yl)-2-oxo-1,2-dihydropyridine-3,5-dicarbonitrile) 10
Yield 77%, melting point 291.6°C. IR:υmax./cm-1 3313, 3197 (2 NH2), 3100 (CH arom.), 2912, 2836(CH aliph.), 2214 (2 C ≡ N), 1686 (2 C = O), 1390, 1195 (SO2).1 H-NMR (DMSO-d6, D2O):δ 6.1 (s, 4 H, 2CH2), 6.3 (s, 4 H, 2NH2, exchangable), 7.0-8.0 (m, 14 H, Ar-H). 13 C-NMR(DMSO-d6): 79.1(2), 102.4(2), 109.1(2), 113.9(2), 115.7(2), 116.2(4), 120.5(2), 125.7(4), 128.3(2), 128.4(4), 136.0(4), 148.1(4), 151.2(2), 161.3(2), 164.9 (2). Anal. Calcd. for C40H22N8O8S(774.72): C, 62.01; H, 2.86; N, 14.46. Found: C, 61.88; H, 2.94; N, 14.30.
1,1'-(4,4'-sulfonylbis(4,1-phenylene))bis(6-amino-4-(2-chlorophenyl)-2-oxo-1,2-dihydropyridine-3,5-dicarbonitrile)11
Yield 69%, melting point 251.4°C. IR:υmax./cm-1 3380, 3213 (2 NH2), 3097 (CH arom.), 2222 (2 C ≡ N), 1678 (2 C = O), 1380, 1153 (SO2), 740 (C-Cl).1 H-NMR (DMSO-d6, D2O):δ 6.6 (s, 4 H, 2NH2, exchangable), 7.4-7.9 (m, 16 H, Ar-H). 13 C-NMR(DMSO-d6): 76.2(2), 114.8(2), 115.4(4), 124.6(4), 128.7(2), 129.8(2), 130.1(4), 130.3(2), 130.6(2), 131.7(2), 133.8(2), 138.7(2), 141.9(2), 153.7(2), 156.7(2), 159.4(2). Anal. Calcd. for C38H20Cl2N8O4S(755.59): C, 60.40; H, 2.67; N, 14.83. Found: C, 60.32; H, 2.79; N, 14.61.
1,1'-(4,4'-sulfonylbis(4,1-phenylene))bis(6-amino-4-(4-chlorophenyl)-2-oxo-1,2-dihydropyridine-3,5-dicarbonitrile)12
Yield 86%, melting point 313.1°C. IR:υmax./cm-1 3387, 3329 (2 NH2), 3093 (CH arom.), 2187 (2 C ≡ N), 1660 (2 C = O), 1370, 1161 (SO2), 771 (C-Cl). 1 H-NMR (DMSO-d6, D2O):δ 6.6 (s, 4 H, 2NH2, exchangable), 7.5-8.1 (m, 16 H, Ar-H). 13 C-NMR(DMSO-d6): 80.2(2), 114.2(2), 115.6(4), 123.6(4), 128.7(4), 128.9(4), 130.0(4), 130.1(4), 134.8(4), 158.8(2), 153.9(2), 163.4(2). Anal. Calcd. for C38H20Cl2N8O4S(755.59): C, 60.40; H, 2.67; N, 14.83. Found: C, 60.71; H, 2.38; N, 15.08.
1,1'-(4,4'-sulfonylbis(4,1-phenylene))bis(6-amino-4-(4-(dimethylamino)phenyl)-2-oxo-1,2-dihydropyridine-3,5-dicarbonitrile)13
Yield 71%, melting point 277.4°C. IR:υmax./cm-1 3464, 3367 (2 NH2), 3097 (CH arom.), 2908, 2870 (CH aliph.), 2210 (2 C ≡ N), 1678 (2 C = O), 1381, 1168 (SO2).1 H-NMR (DMSO-d6, D2O):δ 3.0 (s, 12 H, 4 CH3), 6.8 (s, 4 H, 2NH2, exchangable), 7.5-8.0 (m, 16 H, Ar-H). 13 C-NMR(DMSO-d6): 40.1(4), 78.1(2), 112.9(4), 117.8(2), 118.4(4), 120.2(2), 125.8(4), 128.2(4), 129.2(4), 127.6(2), 143.2(2), 151.4(2), 153.4(2), 162.2(2), 162.3(2). Anal. Calcd. for C42H32N10O4S(772.83): C, 65.27; H, 4.17; N, 18.12. Found: C, 65.50; H, 4.00; N, 18.41.
1,1'-(4,4'-sulfonylbis(4,1-phenylene))bis(6-amino-4-(2-methoxyphenyl)-2-oxo-1,2-dihydropyridine-3,5-dicarbonitrile)14
Yield 70%, melting point 269.6°C. IR:υmax./cm-1 3448, 3367 (2 NH2), 3066 (CH arom.), 2935, 2870 (CH aliph.), 2183 (2 C ≡ N), 1678 (2 C = O), 1350, 1149 (SO2).1 H-NMR (DMSO-d6, D2O):δ 3.9 (s, 6 H, 2 OCH3), 6.6 (s, 4 H, 2NH2, exchangable), 7.3-8.1 (m, 16 H, Ar-H). Anal. Calcd. for C40H26N8O6S(746.75): C, 64.34; H, 3.51; N, 15.01. Found: C, 64.77; H, 3.31; N, 15.36.
1,1'-(4,4'-sulfonylbis(4,1-phenylene))bis(6-amino-4-(4-methoxyphenyl)-2-oxo-1,2-dihydropyridine-3,5-dicarbonitrile)15
Yield 76%, melting point 303.8°C. IR:υmax./cm-1 3317, 3197 (2 NH2), 3070 (CH arom.), 2339, 2843 (CH aliph.), 2214 (2 C ≡ N), 1686 (2 C = O), 1370, 1149 (SO2).1 H-NMR (DMSO-d6, D2O):δ 3.9 (s, 6 H, 2 OCH3), 6.3 (s, 4 H, 2NH2, exchangable), 7.0-8.3 (m, 16 H, Ar-H). 13 C-NMR(DMSO-d6): 56.0(2), 80.2(2), 105.4(2), 114.2(2), 115.6(4), 123.4(4), 123.8(4), 128.6(4), 131.3(4), 133.1(4), 133.6(2), 134.2(4), 134.8(2), 151.3(2), 152.8(2), 153.8(2), 163.4(2). Anal. Calcd. for C40H26N8O6S(746.75): C, 64.34; H, 3.51; N, 15.01. Found: C, 64.48; H, 3.70; N, 14.92.
1,1'-(4,4'-sulfonylbis(4,1-phenylene))bis(6-amino-2-oxo-4-(thiophen-2-yl)-1,2-dihydropyridine-3,5-dicarbonitrile)16
Yield 81%, melting point 187.7°C. IR:υmax./cm-1 3375, 3213 (2 NH2), 3100 (CH arom.), 2214 (2 C ≡ N), 1676 (2 C = O), 1390, 1149 (SO2).1 H-NMR (DMSO-d6, D2O):δ 6.6 (s, 4 H, 2NH2, exchangable), 7.2-8.7 (m, 14 H, Ar-H). 13 C-NMR(DMSO-d6): 75.3(2), 116.2(4), 120.5(2), 127.2(2), 127.7(2), 128.7(2), 129.8(4), 130.4(2), 135.6(2), 138.1(2), 144.6(2), 153.5(2), 162.1(2), 171.9(2). Anal. Calcd. for C34H18N8O4S3(698.75): C, 58.44; H, 2.60; N, 16.04. Found: C, 58.19; H, 2.90; N, 16.32.
1,1'-(4,4'-sulfonylbis(4,1-phenylene))bis(6-amino-2-oxo-1,2-dihydropyridine-3,5-dicarbonitrile)17
Yield 66%, melting point 308.9°C. IR:υmax./cm-1 3371, 3206 (2 NH2), 2187 (2 C ≡ N), 1680 (2 C = O), 1377, 1145 (SO2).1 H-NMR (DMSO-d6, D2O):δ6.8 (s, 4 H, 2NH2, exchangable), 7.6-7.8 (m, 10 H, Ar-H + 2CH pyridone). 13 C-NMR(DMSO-d6): 62.9(2), 100.2(2), 116.2(4), 122.9(4), 130.2(4), 136.9(2), 139.6(2), 151.7(2), 154.2(2), 158.7(2). Anal. Calcd. for C26H14N8O4S(534.51): C, 58.42; H, 2.64; N, 20.96. Found: C, 58.60; H, 2.88; N, 20.71.
1,1'-(4,4'-sulfonylbis(4,1-phenylene))bis(6-amino-4-methyl-2-oxo-1,2-dihydropyridine-3,5-dicarbonitrile)18
Yield 68%, melting point 230.2°C. IR:υmax./cm-1 3410, 3394 (2 NH2), 2935, 2860 (CH aliph.), 2198 (2 C ≡ N), 1686 (2 C = O), 1390, 1149 (SO2).1 H-NMR (DMSO-d6, D2O):δ 1.6 (s,6 H, 2 CH3), 6.7 (s, 4 H, 2NH2, exchangable), 7.3-8.0 (m, 8 H, Ar-H). 13 C-NMR(DMSO-d6): 8.4(2), 56.2(2), 114.9(2), 116.2(4), 119.5(4), 128.1(4), 136.7(2), 136.9(2), 151.2(2), 152.8(2), 166.9(2). Anal. Calcd. for C28H18N8O4S(562.56): C, 59.78; H, 3.23; N, 19.92. Found: C, 59.54; H, 3.40; N, 19.69.
1,1'-(4,4'-sulfonylbis(4,1-phenylene))bis(6-amino-4-butyl-2-oxo-1,2-dihydropyridine-3,5-dicarbonitrile)19
Yield 62%, melting point 236.3°C. IR:υmax./cm-1 3380, 3367 (2 NH2), 2954, 2840 (CH aliph.), 2195 (2 C ≡ N), 1650 (2 C = O), 1399, 1149 (SO2).1 H-NMR (DMSO-d6, D2O):δ 1.1 (t,6 H, 2 CH3), 1.3-2(m,12 H,6 CH2), 6.6 (s, 4 H, 2NH2, exchangable), 7.3-7.9 (m, 8 H, Ar-H). 13 C-NMR(DMSO-d6): 13.5(2), 21.7(2), 22.3(2), 29.1(2), 62.6(2), 113.5(2), 113.9(4), 120.8(4), 129.1(4), 133.9(2), 142.3(2), 153.6(2), 161.6(2), 175.8(2). Anal. Calcd. for C34H30N8O4S(646.72): C, 63.14; H, 4.68; N, 17.33. Found: C, 63.00; H, 4.90; N, 17.01.
(2E,2'E)-N,N'-(4,4'-sulfonylbis(4,1-phenylene))bis(2-cyano-3-(2,4-dichlorophenyl)acrylamide) 20
A mixture of 2 (3.82 g, 0.01 mol.) and 2,4-dichlorobenzaldehyde (3.50 g, 0.02 mol.) in acetic acid was refluxed for 8 h, the obtained solid was filtered and recrystallized from acetic acid to give 20. Yield 88%, melting point 253.4°C. IR:υmax./cm-1 3372 (2 NH), 3986 (CH arom.), 2940, 2860 (CH aliph.), 2203 (2 C ≡ N), 1652 (2 C = O), 1390, 1145 (SO2), 829 (C-Cl).1 H-NMR (DMSO-d6, D2O):δ6.9-7.8 (m, 14 H, Ar-H), 10.0 (s, 2 H, 2NH, exchangable). 13 C-NMR(DMSO-d6): 112.8(2), 119.0(2), 122.8(4), 127.9(2), 128.5(4), 129.0(2), 130.1(2), 130.4(2), 132.6(2), 134.6(2), 135.9(2), 142.8(2), 153.3(2), 163.3(2). Anal. Calcd. for C32H18Cl4N4O4S(696.39): C, 55.19; H, 2.61; N, 8.05. Found: C, 55.36; H, 2.50; N, 7.99.
N,N'-(4,4'-sulfonylbis(4,1-phenylene))bis(2-oxo-2 H-chromene-3-carboxamide) 21
To a solution of 2 (3.82 g, 0.01 mol.) in acetic anhydride (30 mL), salicylaldehyde (2.44 g, 0.02 mol.) and fused Na acetate (1.6 g, 0.02 mol.) were added, the reaction mixture was refluxed for 3 h, cooled and the solid obtained was crystallized from dioxane to give 21. Yield 59%, melting point 195.5°C. IR:υmax./cm-1 3433 (2 NH), 3097 (CH arom.), 1762, 1720 (4 C = O), 1396, 1157 (SO2).1 H-NMR (DMSO-d6, D2O):δ 7.3-7.6 (m, 16 H, Ar-H), 8.2 (s, 2 H, 2CH), 10.4 (s, 2 H, 2NH, exchangable). 13 C-NMR(DMSO-d6): 118.9(2), 121.8(2), 123.3(2), 124.4(4), 126.4(2), 127.7(2), 128.8(2), 130.7(4), 137.6(2), 141.2(2), 144.3(2), 152.1(2), 169.1(2), 171.9(2). Anal. Calcd. for C32H20N2O8S(592.57):C, 64.86; H, 3.40; N, 4.73. Found: C, 64.76; H, 3.31; N, 5.00.
N,N'-(4,4'-sulfonylbis(4,1-phenylene))bis(2-imino-2 H-chromene-3-carboxamide) 22
A mixture of compound 2 (3.82 g, 0.01 mol.), salicylaldehyde (2.44 g, 0.02 mol.) and anhydrous ammounium acetate (2.30 g, 0.03 mol.) was refluxed in ethanol (50 mL) for 2 h. The solid obtained was crystallized from ethanol to give 22.Yield 64%, melting point 256.1°C. IR:υmax./cm-1 3383, 3259 (4 NH), 1686 (2 C = O), 1377, 1149 (SO2).1 H-NMR (DMSO-d6, D2O):δ 7.2-8.0 (m, 16 H, Ar-H), 8.6 (s, 2 H, 2CH), 10.4 (s, 2 H, 2NH, exchangable), 13.1 (s, 2 H, 2NH, exchangable),. 13 C-NMR(DMSO-d6): 114.9(2), 119.7(2), 122.2(2), 122.5(4), 125.7(2), 127.9(2), 128.8(2), 129.4(4), 134.1(2), 141.6(2), 142.1(2), 153.7(2), 160.2(2), 165.5(2). Anal. Calcd. for C32H22N4O6S(590.61): C, 65.08; H, 3.75; N, 9.49. Found: C, 65.23; H, 3.60; N, 9.29.
General procedure for synthesis of compound 23 and 24
Equimolar amount of compound 21or 22 (0.01 mol.) and malononitrile (1.32 g, 0.02 mol.) and anhydrous ammonium acetate (2.30 g, 0.03 mol.) were refluxed in ethanol (50 mL) for 5 h. The obtained solid was crystallized from dioxane to give 23 and 24, respectively.
3,3'-(4,4'-sulfonylbis(4,1-phenylene))bis(2-amino-4,5-dioxo-4,5-dihydro-3 H-chromeno[3,4-c]pyridine-1-carbonitrile) 23
Yield 75%, melting point 267.8°C. IR:υmax./cm-1 3444, 3344 (2 NH2), 3100 (CH arom.), 2199 (2 C ≡ N), 1690, 1660 (4 C = O), 1373, 1153 (SO2). 1 H-NMR (DMSO-d6, D2O):δ 6.7 (s, 4 H, 2NH2, exchangable), 7.1-8.0 (m, 16 H, Ar-H). 13 C-NMR(DMSO-d6): 76.7(2), 115.8(2), 119.0(2), 121.8(2), 122.6(4), 123.9(2), 124.8(2), 128.3(4), 129.0(2), 135.0(4), 141.1(2), 143.5(2), 158.0(2), 158.4(2), 164.8(2), 169.1(2), 170.3(2). Anal. Calcd. for C38H20N6O8S(720.67): C, 63.33; H, 2.80; N, 11.66. Found: C, 63.11; H, 2.96; N, 11.49.
3,3'-(4,4'-sulfonylbis(4,1-phenylene))bis(2-amino-5-imino-4-oxo-4,5-dihydro-3 H-chromeno[3,4-c]pyridine-1-carbonitrile) 24
Yield 77%, melting point >360°C. IR:υmax./cm-1 3441, 3348, 3236, 3186 (2NH, 2NH2), 2203 (2 C ≡ N), 1680 (2 C = O), 1381, 1153 (SO2).1 H-NMR (DMSO-d6, D2O):δ 6.6 (s, 4 H, 2NH2, exchangable), 7.1-7.9 (m, 16 H, Ar-H), 8.9(s, 2 H, 2NH, exchangable). 13 C-NMR(DMSO-d6): 78.1(2), 112.6(2), 116.7(4), 122.4(2), 122.8(2), 124.6(4), 128.1(2), 128.9(4), 129.1(2), 137.4(2), 155.6(2), 156.1(2), 158.7(2), 159.6(2), 163.5(2). Anal. Calcd. for C38H22N8O6S(718.70):C, 63.50; H, 3.09; N, 15.59. Found: C, 63.44; H, 3.18; N, 15.75.
Molecular docking
All the molecular modeling studies were carried out on an Intel Pentium 1.6 GHz processor, 512 MB memory with Windows XP operating system using Molecular Operating Environment (MOE, 10.2008) software. All the minimizations were performed with MOE until a RMSD gradient of 0.05 kcal mol-1Ao-1 with MMFF94X force field and the partial charges were automatically calculated. The X-ray crystallographic structure of franesyltransferase and arginine methyltransferase (PRMT1) complexes with their ligands (PDB ID: 3E30, 3Q7E) were obtained from the protein data bank. The enzymes were prepared for docking studies where: (i) Ligand molecule was removed from the enzyme active site. (ii) Hydrogen atoms were added to the structure with their standard geometry. (iii) MOE Alpha Site Finder was used for the active sites search in the enzyme structure and dummy atoms were created from the obtained alpha spheres. (iv) The obtained model was then used in predicting the ligand enzymes interactions at the active site.
In vitro antitumor activity
Human tumor breast cell line (MCF7) was used in this study. The cytotoxic activity was measured in vitro for the newly synthesized compounds using the Sulfo-Rhodamine-B stain (SRB) assay using the method of Skehan et al. [52]. The in vitro anticancer screening was done by the pharmacology unit at the National Cancer Institute, Cairo University.
Cells were plated in 96-multiwell plate (104 cells/well) for 24 h before treatment with the compound(s) to allow attachment of cell to the wall of the plate. Test compounds were dissolved in dimethyl sulfoxide. Different concentrations of the compound under test (10, 25, 50, and 100 μM) were added to the cell monolayer. Triplicate wells were prepared for each individual concentration. Monolayer cells were incubated with the compound(s) for 48 h at 37°C and in atmosphere of 5% CO2. After 48 h, cells were fixed, washed and stained for 30 min with 0.4% (wt/vol) SRB dissolved in 1% acetic acid. Excess unbound dye was removed by four washes with 1% acetic acid and attached stain was recovered with Trise-EDTA buffer. Color intensity was measured in an ELISA reader. The relation between surviving fraction and drug concentration is plotted to get the survival curve for breast tumor cell line after the specified time. The molar concentration required for 50% inhibition of cell viability (IC50) was calculated and compared to the reference drug Doxorubicin (CAS, 25316-40-9). The surviving fractions were expressed as means ± standard error and the results are given in Table 3.
Conclusions
Diarylsulfone derivatives may serve as good candidates in the search for novel anticancer agents as illustrated by the IC50 values of the investigated compounds. These values were better than that of Doxorubicin. The mechanism of action as anticancer of the synthesized compounds was investigated through molecular docking on the active site of farnesyl transferase and arginine methyltransferase. Both enzymes could be the target of action of these compounds based on the good energy scores and amino acid interactions in the active sites of enzymes however, the exact mechanism of action still needs more investigation to be clarified.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
M.Al-Said, M.Ghorab designed the synthetic schemes for all synthesized compounds. All authors contributed in the chemical synthesis. Y.Nissan carried out molecular docking and interpretation of its results as well as interpretation of the biological results. All authors read and approved the final manuscript. All authors read and approved the final manuscript.
Contributor Information
Mansour S Al-Said, Email: msalsaid@ksu.edu.sa.
Mostafa M Ghorab, Email: mmsghorab@yahoo.com.
Yassin M Nissan, Email: yassin.nissan@hotmail.com.
Acknowledgement
The authors are grateful to the sponsorship of the College of Pharmacy Research Centre and the Deanship of the Scientific Research, King Saud University, Riyadh, Saudi Arabia.
References
- Cocco MT, Congiu C, Onnis V. Synthesis and antitumour activity of 4-hydroxy-2-pyridone derivatives. Euro J of Med Chem. 2000;35:545–552. doi: 10.1016/S0223-5234(00)00149-5. [DOI] [PubMed] [Google Scholar]
- Fan N, Evans DB, Rank KB, Thomas RC, Tarpley WG, Sharma SK. Mechanism of resistance to U-90152 S and sensitization to L-697,661 by a proline to leucine change at residue 236 of human immunodeficiency virus type 1 (HIV-1) reverse transcriptase. FEBS Lett. 1995;359:233–238. doi: 10.1016/0014-5793(95)00051-A. [DOI] [PubMed] [Google Scholar]
- Emini EA, Staszewski S, Schneider CL, Waterbury JA, Schleif WA, Goehler R, Deussen A, Duerr S, Massari FE, Calandra GB, Hoffstedt B, Byrnes VW. 94 Combination therapy with AZT prevents selection of HIV-1 variants that are highly resistant to the nonnucleoside reverse transcriptase inhibitor L-697, 661. Antiviral Res. 1993;20:94. [Google Scholar]
- Van Der Zypp A, Rechtman M, Majewski H. The role of cyclic nucleotides and calcium in the relaxation produced by amrinone in rat aorta. Gen Pharmacol. 2000;34:245–253. doi: 10.1016/S0306-3623(00)00071-9. [DOI] [PubMed] [Google Scholar]
- Rechtman MP, Van Der Zypp A, Majewski H. Amrinone reduces ischaemia-reperfusion injury in rat heart. Eur J Pharmacol. 2000;402:255–262. doi: 10.1016/S0014-2999(00)00443-X. [DOI] [PubMed] [Google Scholar]
- Jeremy JY, Gill J, Mikhailidis D. Effect of milrinone on thromboxane A2 synthesis, cAMPphosphodiesterase and 45Ca2+ uptake by human platelets. Eur J Pharmacol. 1993;245:67–73. doi: 10.1016/0922-4106(93)90171-5. [DOI] [PubMed] [Google Scholar]
- Raffaeli S, Ferroni C, Spurgeon HA, Capogrossi MC. Milrinone enhances cytosolic calcium transient and contraction in rat cardiac myocytes during beta-adrenergic stimulation. Int J Cardiol. 1989;25:S63–S69. doi: 10.1016/0167-5273(89)90095-8. [DOI] [PubMed] [Google Scholar]
- Card JW, Racz WJ, Brien JF, Margolin SB, Massey TE. Differential effects of pirfenidone on acute pulmonary injury and ensuing fibrosis in the hamster model of amiodarone-induced pulmonary toxicity. Toxicological sciences an official journal of the Society of Toxicology. 2003;75:169–180. doi: 10.1093/toxsci/kfg167. [DOI] [PubMed] [Google Scholar]
- Kehrer JP, Margolin SB. Pirfenidone diminishes cyclophosphamide-induced lung fibrosis in mice. Toxicol Lett. 1997;90:125–132. doi: 10.1016/S0378-4274(96)03845-3. [DOI] [PubMed] [Google Scholar]
- Card JW, Lalonde BR, Rafeiro E, Tam AS, Racz WJ, Brien JF, Bray TM, Massey TE. Amiodarone-induced disruption of hamster lung and liver mitochondrial function: lack of association with thiobarbituric acid-reactive substance production. Toxicol Lett. 1998;98:41–50. doi: 10.1016/S0378-4274(98)00097-6. [DOI] [PubMed] [Google Scholar]
- Giri SN, Schwartz LW, Hollinger MA, Freywald ME, Schiedt MJ, Zuckerman JE. Biochemical and structural alterations of hamster lungs in response to intratracheal administration of bleomycin. Exp Mol Pathol. 1980;33:1–14. doi: 10.1016/0014-4800(80)90002-7. [DOI] [PubMed] [Google Scholar]
- Iyer SN, Wild JS, Schiedt MJ, Hyde DM, Margolin SB, Giri SN. Dietary intake of pirfenidone ameliorates bleomycin-induced lung fibrosis in hamsters. J Lab Clin Med. 1995;125:779–785. [PubMed] [Google Scholar]
- Iyer SN, Gurujeyalakshmi G, Giri SN. Effects of pirfenidone on transforming growth factor-beta gene expression at the transcriptional level in bleomycin hamster model of lung fibrosis. J Pharmacol Exp Ther. 1999;291:367–373. [PubMed] [Google Scholar]
- Iyer SN, Hyde DM, Giri SN. Anti-inflammatory effect of pirfenidone in the bleomycin-hamster model of lung inflammation. Inflamm. 2000;24:477–491. doi: 10.1023/A:1007068313370. [DOI] [PubMed] [Google Scholar]
- Selman M, King TE, Pardo A. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med. 2001;134:136–151. doi: 10.7326/0003-4819-134-2-200101160-00015. [DOI] [PubMed] [Google Scholar]
- Kakugawa T, Mukae H, Hayashi T, Ishii H, Abe K, Fujii T, Oku H, Miyazaki M, Kadota J, Kohno S. Pirfenidone attenuates expression of HSP47 in murine bleomycin-induced pulmonary fibrosis. Eur Respir J. 2004;24:57–65. doi: 10.1183/09031936.04.00120803. [DOI] [PubMed] [Google Scholar]
- Pemberton N, Pinkner JS, Jones JM, Jakobsson L, Hultgren SJ, Almqvist F. Bicyclic 2-pyridone targeting pilus biogenesis in uropathogenic E coli. Tetrahedron Lett. 2007;48:4543–4546. doi: 10.1016/j.tetlet.2007.04.142. [DOI] [Google Scholar]
- Hamdy NA, Gamal-Eldeen AM. New pyridone, thioxopyridine, pyrazolopyridine and pyridine derivatives that modulate inflammatory mediators in stimulated RAW 264.7 murine macrophage. Eur J Med Chem. 2009;44:4547–4556. doi: 10.1016/j.ejmech.2009.06.023. [DOI] [PubMed] [Google Scholar]
- Li Q, Wang W, Berst KB, Claiborne A, Hasvold L, Raye K, Tufano M, Nilius A, Shen LL, Flamm R, Alder J, Marsh K, Crowell D, Chu DT, Plattner J. Synthesis and structure-activity relationships of 2-pyridones: II. 8-(Fluoro-substituted pyrrolidinyl)-2-pyridones as antibacterial agents. Bioorg Med Chem Lett. 1998;8:1953–1958. doi: 10.1016/S0960-894X(98)00355-2. [DOI] [PubMed] [Google Scholar]
- Vegi SR, Boovanahalli SK, Patro B, Mukkanti K. SPF32629A and SPF32629B: enantioselective synthesis, determination of absolute configuration, cytotoxicity and antibacterial evaluation. Eur J Med Chem. 2011;44:1803–1812. doi: 10.1016/j.ejmech.2011.02.039. [DOI] [PubMed] [Google Scholar]
- Dominguez JN, Leon C, Rodrigues J, Gamboa De Dominguez N, Gut J, Rosenthal PJ. Synthesis of chlorovinylsulfones as structural analogs of chalcones and their antiplasmodial activities. Eur J Med Chem. 2009;44:1457–1462. doi: 10.1016/j.ejmech.2008.09.044. [DOI] [PubMed] [Google Scholar]
- Szilágyi G, Somorai T, Bozó É, Langó J, Nagy G, Reiter J, Janáky J. Preparation and antiarthritic activity of new 1,5-diaryl-3-alkylthio-1 H-1,2,4-triazoles and corresponding sulfoxides and sulfones. H-1,2,4-triazoles and corresponding sulfoxides and sulfones. Eur J Med Chem. 1990;25:95–101. doi: 10.1016/0223-5234(90)90015-U. [DOI] [Google Scholar]
- Santelli-Rouvier C, Barret JM, Farrell CM, Sharples D, Hill BT, Barbe J. Synthesis of 9-acridinyl sulfur derivatives: sulfides, sulfoxides and sulfones. Comparison of their activity on tumour cells. Eur J Med Chem. 2004;39:1029–1038. doi: 10.1016/j.ejmech.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Murafuji T, Fujiwara Y, Yoshimatsu D, Miyakawa I, Migita K, Mikata Y. Bismuth heterocycles based on a diphenylsulfone scaffold: synthesis and substituent effect on the antifungal activity against Saccharomyces cerevisiae. Eur J Med Chem. 2011;46:519–525. doi: 10.1016/j.ejmech.2010.11.031. [DOI] [PubMed] [Google Scholar]
- Usera AR, Dolan P, Kensler TW, Posner GH. Novel alkyl side chain sulfone 1alpha,25-dihydroxyvitamin D3 analogs: a comparison of in vitro antiproliferative activities and in vivo calcemic activities. Bioorg Med Chem. 2009;17:5627–5631. doi: 10.1016/j.bmc.2009.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badal S, Williams SA, Huang G, Francis S, Vendantam P, Dunbar O, Jacobs H, Tzeng TJ, Gangem J, Delgoda R. Cytochrome P450 1 enzyme inhibition and anticancer potential of chromene amides from Amyrisplumieri. Fitoterapia. 2011;82:230–236. doi: 10.1016/j.fitote.2010.10.003. [DOI] [PubMed] [Google Scholar]
- Endo S, Matsunaga T, Kuwata K, Zhao HT, El-Kabbani O, Kitade Y, Hara A. Chromene-3-carboxamide derivatives discovered from virtual screening as potent inhibitors of the tumour maker, AKR1B10. Bioorg Med Chem. 2010;18:2485–2490. doi: 10.1016/j.bmc.2010.02.050. [DOI] [PubMed] [Google Scholar]
- Heo SJ, Kim KN, Yoon WJ, Oh C, Choi YU, Affan A, Lee YJ, Lee HS, Kang DH. Chromene induces apoptosis via caspase-3 activation in human leukemia HL-60 cells. Food Chem Toxico. 2011;49:1998–2004. doi: 10.1016/j.fct.2011.05.011. [DOI] [PubMed] [Google Scholar]
- Rao RN, Suman P, Yogeeswari P, Sriram D, Basha T, Vardhan S. Synthesis, structure-activity relationship of novel substituted 4 H-chromen-1,2,3,4-tetrahydropyrimidine-5-carboxylates as potential anti-mycobacterial and anticancer agents. Bioorg & Med Chem Lett. 2011;21:2855–2859. doi: 10.1016/j.bmcl.2011.03.079. [DOI] [PubMed] [Google Scholar]
- Santelli-Rouvier C, Barret JM, Farrell CM, Sharples D, Hill BT, Barbe J. Synthesis of 9-acridinyl sulfur derivatives: sulfides, sulfoxides and sulfones. Comparison of their activity on tumour cells. Eur J Med Chem. 2004;39:1029–1038. doi: 10.1016/j.ejmech.2004.06.015. [DOI] [PubMed] [Google Scholar]
- El-Sayed AT. Synthesis of some novel pyrazolo[3,4-b]pyridine and pyrazolo[3,4-d]pyrimidine derivatives bearing 5,6-diphenyl-1,2,4-triazine moiety as potential antimicrobial agents. Eur J Med Chem. 2009;44:4385–92. doi: 10.1016/j.ejmech.2009.05.031. [DOI] [PubMed] [Google Scholar]
- Onnis V, Cocco MT, Fadda R, Congiu C. Synthesis and evaluation of anticancer activity of 2-arylamino-6-trifluoromethyl-3-(hydrazonocarbonyl)pyridines. Bioorg Med Chem. 2009;17:6158–65. doi: 10.1016/j.bmc.2009.07.066. [DOI] [PubMed] [Google Scholar]
- Karki R, Thapa P, Kang MJ, Jeong TC, Nam JM, Kim HL, Na Y, Cho WJ, Kwon Y, Lee ES. Synthesis, topoisomerase I and II inhibitory activity, cytotoxicity, and structure-activity relationship study of hydroxylated 2,4-diphenyl-6-aryl pyridines. Bioorg Med Chem. 2010;18:3066–77. doi: 10.1016/j.bmc.2010.03.051. [DOI] [PubMed] [Google Scholar]
- Gomez-Monterrey I. New benzo[g]isoquinoline-5,10-diones and dihydrothieno [2,3-b]naphtho-4,9-dione derivatives Synthesis and biological evaluation as potential antitumoral agents. Bioorg Med Chem. 2003;11:3769–3775. doi: 10.1016/S0968-0896(03)00310-9. [DOI] [PubMed] [Google Scholar]
- Valderrama JA, González MF, Pessoa-Mahana D, Tapia RA, Fillion H, Pautet F, Rodriguez JA, Theoduloz C, Schmeda-Hirschmann G. Studies on quinones. Part 41: synthesis and cytotoxicity of isoquinoline-containing polycyclic quinones. Bioorg Med Chem. 2006;14:5003–5011. doi: 10.1016/j.bmc.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Kast RE, Scheuerle A, Wirtz CR, Karpel-Massler G. The Rationale of Targeting Neutrophils with Dapsone during Glioblastoma Treatment. Anticancer Agents Med Chem. 2011;11:756–761. doi: 10.2174/187152011797378805. [DOI] [PubMed] [Google Scholar]
- Bissinger EM, Heinke R, Spannhoff A, Eberlin A, Metzger E, Cura V, Hassenboehler P, Cavarelli J, Schüle R, Bedford MT, Sippl W, Jung M. Acyl derivatives of p-aminosulfonamides and dapsone as new inhibitors of the arginine methyltransferase hPRMT1. Bioorg Med Chem. 2011;19:3717–3731. doi: 10.1016/j.bmc.2011.02.032. [DOI] [PubMed] [Google Scholar]
- Ghorab MM, Radwan MAA, Taha NMH, Amin NE, Shehab MA, Faker IMI. Dapson in Heterocyclic Chemistry, Part I: Novel Synthesis of SulfoneBiscompounds for Antimicrobial and Antitumor Activities. Phosphorus, Sulfur, and Silicon and the Related Elements. 2008;183:2891–2905. doi: 10.1080/10426500802505408. [DOI] [Google Scholar]
- Ghorab MM, Amin NE, El Gaby MSA, Taha NMH, Shehab MA, Faker IMI. Dapson in Heterocyclic Chemistry, Part III: Synthesis, Antimicrobial, and Antitumor Activities of Some New Bisheterocyclic Compounds Containing Biologically Active Diphenylsulfone Moiety. Phosphorus, Sulfur, and Silicon and the Related Elements. 2008;183:2918–2928. doi: 10.1080/10426500802505440. [DOI] [Google Scholar]
- Ghorab MM, Amin NE, El Gaby MSA, Taha NMH, Shehab MA, Faker IMI. Dapson in Heterocyclic Chemistry, Part IV: Synthesis of Some Novel Diphenylsulfones Containing Acetamide, Pyrrolidine, Piperazine, and Thiomorpholine Moieties as Antimicrobial and Antitumor Agents. Phosphorus, Sulfur, and Silicon and the Related Elements. 2008;183:2929–2942. doi: 10.1080/10426500802505457. [DOI] [Google Scholar]
- Ghorab MM, Radwan MAA, Taha NMH, Amin NE, Shehab MA, Faker IMI. Dapson in Heterocyclic Chemistry, Part II: Antimicrobial and Antitumor Activities of Some Novel SulfoneBiscompounds Containing Biologically Active Thioureido, Carbamothioate, Quinazoline, Imidazolidine, and Thiazole Moieties. Phosphorus, Sulfur, and Silicon and the Related Elements. 2008;183:2906–2917. doi: 10.1080/10426500802505424. [DOI] [Google Scholar]
- Al-Said MS, Bashandy MS, Al-Qasoumi SI, Ghorab MM. Anti-breast cancer activity of some novel 1,2-dihydropyridine, thiophene and thiazole derivatives. Eur J Med Chem. 2011;46:137–41. doi: 10.1016/j.ejmech.2010.10.024. [DOI] [PubMed] [Google Scholar]
- Vojtek AB, Der CJ. Increasing complexity of the Rassignaling pathway. The Journal of Biological Chemistry. 1998;273:19925–19928. doi: 10.1074/jbc.273.32.19925. [DOI] [PubMed] [Google Scholar]
- Liang PH, Ko TP, Wang AHJ. Structure, mechanism and function of prenyltransferases. The Federation of European Biochemical Societies Journal. 2002;269:3339–3354. doi: 10.1046/j.1432-1033.2002.03014.x. [DOI] [PubMed] [Google Scholar]
- Long SB, Casey PJ, Beese LS. Reaction path of protein farnesyltransferase at atomic resolution. Nat. 2002;419:645–650. doi: 10.1038/nature00986. [DOI] [PubMed] [Google Scholar]
- Mazieres J, Pradines A, Favre G. Perspectives on farnesyltransferase inhibitors in cancer therapy. Cancer Lett. 2004;206:159–167. doi: 10.1016/j.canlet.2003.08.033. [DOI] [PubMed] [Google Scholar]
- Hunt JT, Lee VG, Leftheris K, Seizinger B, Carboni J, Mabus J, Ricca C, Yan N, Manne V. Potent, cell active, non-thioltetrapeptide inhibitors of farnesyltransferase. J Med Chem. 1996;39:353–358. doi: 10.1021/jm9507284. [DOI] [PubMed] [Google Scholar]
- Adjei AA. Farnesyltransferase inhibitors. Cancer Chemother Biol Response Modif. 2001;3:161–162. [PubMed] [Google Scholar]
- Crul M, De Klerk GJ, Beijnen JH, Schellens JH. Ras biochemistry and farnesyltransferase inhibitors: a literature survey. Anticancer drugs. 2001;12:163–184. doi: 10.1097/00001813-200103000-00001. [DOI] [PubMed] [Google Scholar]
- Scorilas A, Black MH, Talieri M, Diamandis EP. Genomic organization, physical mapping, and expression analysis of the human protein arginine methyltransferase 1 gene. Biochem Biophys Res Commun. 2000;260:466–474. doi: 10.1006/bbrc.2000.3807. [DOI] [PubMed] [Google Scholar]
- Cheung N, Chan LC, Thompson A, Cleary ML, So CWE. Protein arginine-methyltransferase-dependent oncogenesis. Nat Cell Biol. 2007;9:1208–1215. doi: 10.1038/ncb1642. [DOI] [PubMed] [Google Scholar]
- Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S, Boyd MR. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst. 1990;82:1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]