Abstract
Although amnestic mild cognitive impairment (aMCI; often considered a prodromal phase of Alzheimer’s disease, AD) is most recognized by its implications for decline in memory function, research suggests that deficits in attention are present early in aMCI and may be predictive of progression to AD. The present study used functional magnetic resonance imaging to examine differences in the brain during the attention network test between 8 individuals with aMCI and 8 neurologically healthy, demographically matched controls. While there were no significant behavioral differences between groups for the alerting and orienting functions, patients with aMCI showed more activity in neural regions typically associated with the networks subserving these functions (e.g., temporoparietal junction and posterior parietal regions, respectively). More importantly, there were both behavioral (i.e., greater conflict effect) and corresponding neural deficits in executive control (e.g., less activation in the prefrontal and anterior cingulate cortices). Although based on a small number of patients, our findings suggest that deficits of attention, especially the executive control of attention, may significantly contribute to the behavioral and cognitive deficits of aMCI.
Introduction
Alzheimer’s disease (AD) first presents as mild cognitive impairment (MCI) in terms of memory loss or decline in other cognitive functions (e.g., attention). Studies suggest that the conversion rate of MCI to AD is 41% over a 1-year period and 64% over a 2-year period [1]. Amnestic MCI (aMCI) has such a high conversion rate to AD that it is considered by some as a prodromal phase of AD [2], [3]. While the economic burden attributable to MCI is quite small [2], the annual cost of patient care in AD is more than $100 billion in the United States alone [4]. Global projections suggest that delaying the progression and onset of AD by as little as one year could have a massive impact on the global economic burden of the disease [5]. Although AD is primarily characterized by memory impairments [6], there is accumulating evidence that attentional deficits occur during relatively early stages of the disease [7]–[11]. In fact, some research has shown that efficiency of attentional processes discriminate between patients with mild AD and the healthy elderly [12]. Further, other studies have shown that attentional impairment is a predictor of cognitive decline in early stages of probable AD [13]. Thus alterations in attentional function may be a useful diagnostic marker, prognostic indicator, and potential point of intervention, among those with prodromal AD.
Attention refers to the activity of a set of brain networks that can influence the priority of the computations of other brain networks for access to consciousness [14]. Impairments of attention may contribute to functional decline in other cognitive domains, such as memory in aging and dementia [15]. Although deficits in attention [16] and executive control of attention [17] are usually the initial deficits observed following emergence of amnestic symptoms during early stages of AD [17], [18], little is known about the pathophysiological basis of these deficits relative to memory impairments. Behavioral studies of attention mechanisms, in combination with new technologies such as functional neuroimaging, may assist in better identifying the pathophysiology of deficits associated with AD [19], as well as its precursor, aMCI [3].
One attentional network theory has conceptualized attention as comprised of three functionally and anatomically defined brain networks of alerting, orienting, and executive control [20]–[22]. The alerting network involves tonically maintaining the alert state and phasically responding to a warning signal. It involves the thalamic, frontal, and parietal regions, and temporoparietal junction [23]. The orienting network subserves the functions of endogenous and exogenous selecting of information from among numerous sensory inputs. The key neural substrates for the orienting network include the superior parietal lobule and frontal eye fields [23]. The executive control function of attention involves the engagement of more complex mental operations during monitoring and resolving conflict between computations. The anterior cingulate cortex (ACC) and dorsolateral prefrontal cortex (DLPFC) are involved in this network [23]. This attention network theory [20]–[22] can be mapped onto the stimulus-driven and goal-directed model of Corbetta and Shulman [24] by considering the (re)orienting function as the hub of top-down and bottom-up convergence [25]. In this way, the phasic alerting network can be perceived as a potential bottom-up influence, while the executive control network can be perceived as a potential top-down influence on selective attention.
Previous findings have suggested that attention deficits contribute to the symptomatic profile of AD. Deficits have been documented in the alerting and orienting networks [12], [15], [17], [26]–[35], as well as in the executive control of attention among individuals with AD [9], [11], [12], [17], [36]–[42]. Further evidence has shown broad deficits of general executive function in AD [17], [43]–[49]. A behavioral study using the attention network test (ANT) showed selective impairments in executive control and an interaction between orienting and executive control in AD [10]. These various attention deficits, observed in AD, have been previously explained as a disruption of the basal forebrain cholinergic system and cortico-cortical tracts connecting distinct cortical regions [17], [18]. Nonetheless, the neural basis of attention deficits in AD is still not fully understood [17]. One structure of potential interest is the ACC. Converging evidence has indicated that the ACC plays a key role in the network subserving executive control of attention [50], [51]. In AD, several studies have shown deficits of the ACC [52]–[58]. These findings suggest that abnormalities in this structure may underlie deficits in executive control of attention [59]. Deficits of executive control of attention in AD (and its precursor aMCI), implicating neural areas such as the ACC, would fill gaps in the existing literature.
In the present study, we assessed the three attentional functions of alerting, orienting, and executive control, and the corresponding neural networks in patients with aMCI. Participants completed the ANT, which we previously developed and have validated in both healthy controls and psychiatric patients [20], [21], [23], [60], [61], while undergoing functional magnetic resonance imaging (fMRI). We predicted that, compared to healthy age-matched controls, patients with aMCI might show deficits in alerting and orienting, but more likely, less efficient executive control associated with a greater conflict effect and reduced ACC (and other prefrontal cortical) activation.
Materials and Methods
Participants
We recruited 19 individuals with aMCI and 15 healthy controls (HC) via the Alzheimer’s Disease Research Center (ADRC) at Mount Sinai School of Medicine (MSSM). This study was approved by the MSSM institutional review board (IRB) and signed consent forms were collected from the participants. While MCI participants are not typically without capacity as they are not demented, standard MSSM consent procedures in this cohort requires that each participant be given adequate time to ask questions about the study so that they are fully informed with regard to study procedures and participants must demonstrate understanding of procedures by paraphrasing key aspects of the study. If a subject appears to lack understanding, the legally authorized representative provides consent as per MSSM IRB guidelines.
Individuals were assessed and diagnosed through the Clinical Core of the ADRC using the National Alzheimer Coordinating Center’s Uniform Data Sets (UDS). The evaluation includes a semi-structured interview of the participant and an informant regarding clinical symptoms and chronology, as well as medical, neurological and neuropsychiatric examination, and neuropsychological testing. Amnestic MCI was diagnosed according to previously used, and established criteria [62], [63], in the present study this included (but was not limited to) a Mini-Mental State Exam (MMSE [64]) score of 24 or higher, performance on delayed recall of the first paragraph of the Wechsler Memory Scale [65] using age and education adjusted scores, and no significant impairment in social or occupational function. HCs underwent the same evaluations, with Wechsler Memory Scale performance falling within the normal range for age and education. The evaluation also included administration of the Clinical Dementia Rating scale (CDR: [66]). Amnestic MCI patients had a CDR of 0.5 while healthy controls predominantly had a CDR of 0. HCs were not excluded for a CDR = 0.5, since those with ‘mild’ dementia are not necessarily representative of individuals who are likely to progress to AD (as are those with aMCI), and some minimal dementia might be anticipated in a normal geriatric sample. Determination of aMCI or normal control status was accomplished via clinical consensus following complete review by the evaluating physician and an ADRC neuropsychologist. Of the 34 originally recruited individuals, 10 MCIs and 4 HCs could not undergo MRI scans for numerous reasons (e.g., arthritis prevented comfortable position on scanner bed, extreme difficulty seeing the visual display, or metallic implant). Another MCI and 2 HCs were excluded due to excessive head motion (>3 mm within a run). An additional HC was excluded due to reaction time (RT) and accuracy that had an absolute distance from the mean of more than 2 standard deviations (SD). Our final sample size was 8 MCIs and 8 HCs. All participants were right-handed and had normal or corrected-to-normal vision. Corrective lenses were used as necessary and visual acuity was tested in advance to ensure participants could view the arrows clearly. Demographic and diagnostic information is provided in Table 1.
Table 1. Demographic characteristics and statistical comparisons.
| HC (n = 8) | aMCI (n = 8) | ||
| M (SD) | M (SD) | p | |
| Age | 74.6 (9.2) | 77.6 (7.0) | 0.48 |
| Education | 16.9 (2.4) | 14.6 (3.2) | 0.12 |
| MMSE | 28.8 (1.4) | 27.1 (1.8) | 0.06 |
| CDR | 0.13 (0.23) | 0.50 (0.00) | 0 |
| % | % | p | |
| Male | 25 | 50 | 0.6 |
| Race | 0.3 | ||
| White | 100 | 62.5 | |
| African American | 0 | 12.5 | |
| Asian | 0 | 12.5 | |
| No Answer | 0 | 12.5 |
HC = Healthy control; aMCI = amnestic Mild Cognitive Impairment; MMSE = Mini-Mental State Exam; CDR = Clinical Dementia Rating; p = p value resulting from statistical test.
Task and Procedure
Attention Network Test
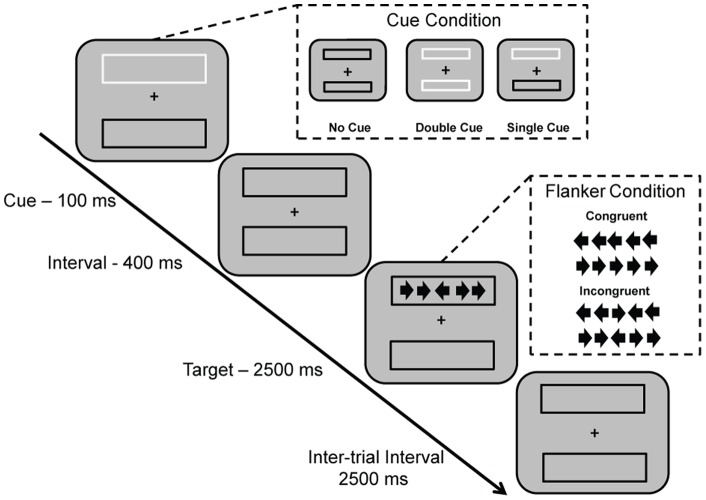
The ANT [14], [20] was re-designed for the present study to optimize attentional contrasts in an elderly population. The ANT, modified for use in a geriatric population (ANT-G) used three cue conditions (no cue, double cue, and spatial cue) with two target conditions (congruent and incongruent). In this version, the cue-to-target interval was held constant at 400 ms and there were no invalid cues (cues were always valid indicators of target location). As with previous versions of the ANT, a central arrowhead points leftward or rightward and there are two arrowheads on either side of the central arrowhead. All four flanking arrowheads can face the same or opposite direction as the central arrowhead, which is the target. The target and flanker were presented for 2500 ms, though the response window was open for an additional 1500 ms, followed by a 2500 ms inter-trial interval.
In the ANT-G, the shape of arrowheads was revised to enlarge the vertical dimension (4× the original dimension). Additionally, the visual angle for orienting (up/down) was enlarged by 30% compared to the original version [20] to make target detection easier for elderly participants. The participants’ task was to identify the direction of the center arrow by pressing a button with their left index finger if the target was pointing to the left and a button with the right index finger for the right direction if the target was pointing right. In the ANT-G, participants completed 3 blocks of 32 trials, for a total of 96 trials. In each block, 16 blank periods (no cue and no target presented) of equivalent length each to a single trial, were used to jitter the presentation of trials. Details of this version of the ANT-G are illustrated in Figure 1.
Figure 1. Schematic of modified Attention Network Test for geriatric samples (ANT-G).
In each trial, depending on the cue condition (no cue, double cue, spatial cue), a box changes from black to white (flashes) for 100 ms. After 400 ms, the target (center arrow) and four flanker arrows (two on either side of center arrow, congruent or incongruent with center arrow) are presented for 2500 ms. The participant makes a response to indicate the direction of the center arrow (left or right). The response window remains open for an additional 1500 ms after the termination of the target (4000 ms in total for the response window), proceeding into the 2500 ms inter-trial interval.
Each of the three attentional networks is operationally defined as a comparison of the performance (RT and error rate) of one condition and the appropriate reference condition, increasing the likely of a positive score for each attentional network. For the alerting network, the effect is defined as RTno cue – RTdouble cue. For the orienting network, the effect is defined as RTdouble cue – RTsingle cue. For the executive control network, the conflict effect is defined as RTflanker incongruent – RTflanker congruent. Performance in error rate was computed using the exact same formulae. Error rates were computed as number of incorrect trials for a given trial type (condition) divided by total number of trials presented for that same trial type.
Prior to implementation in the scanner, participants completed a training session of the ANT-G with step-by-step instructions for 6 trials, followed by a practice block containing 24 trials. This was done on a PC outside the scanner. After participants completed this training session, they then completed 32 trials of the ANT-G in an MRI simulator (Psychology Software Tools, Inc., Pittsburgh, PA), which provided a realistic approximation of the MRI scanner, including simulation of the noises related to the scan sequences, to permit acclimatization to the scanner environment.
fMRI data acquisition and analysis
All MRI data were obtained using a 3 T Siemens Allegra MRI system at MSSM. Foam padding was used to minimize subject head movements. All images were acquired along axial planes parallel to the anterior commissure-posterior commissure line. A high-resolution T2-weighted anatomical volume of the whole brain was acquired with a turbo spin-echo pulse sequence. The fMRI imaging was performed using a gradient-echo echo-planar imaging (GE-EPI) sequence with the following protocol: 40 axial slices, 4 mm-thick, and skip = 0 mm, TR = 2500 ms, TE = 27 ms, flip angle = 82°, FOV = 240 mm, and matrix size = 64×64. Slices were obtained corresponding to the T2-weighted anatomical images. Three series of EPIs corresponding to the three runs were acquired. Each series started with 2 dummy volumes before the onset of the task to allow for equilibration of T1 saturation effects, followed by 165 image volumes. Each of the 3 runs of the ANT-G was preceded and followed by a 30-s fixation period.
Event-related analyses of the fMRI data from the tasks were conducted using the statistical parametric mapping package (SPM8; Wellcome Trust Centre for Neuroimaging, London, UK). Functional scans were adjusted for slice timing, realigned to the first volume, co-registered to the T2 image, normalized to a standard template (MNI, Montreal Neurological Institute), resampled to 2×2×2 mm voxel size, and spatially smoothed with an 8×8×8 mm full-width-at-half-maximum Gaussian kernel. General linear modeling [67] was then conducted for the functional scans from each participant by modeling the observed event-related blood oxygenation level-dependent (BOLD) signals and regressors to identify the relationship between the task event and the BOLD signal. Regressors were created by convolving a train of delta functions representing the sequence of onsets of cues and targets with the default SPM basis function, which consists of a synthetic hemodynamic response function composed of two gamma functions [68].
Regressors were generated for each of the three cue conditions (3 regressors: double cue, single/spatial cue, no cue; all cue locked), as well as their interactions with the congruent and incongruent flanker conditions (6 regressors; all target locked), for a total of 9 regressors. Six parameters generated during motion correction were entered as covariates. The alerting effect was examined by computing the double cue minus no cue contrast, for these cue-locked regressors. The orienting effect was examined by computing the single cue minus double cue contrast, for these cue-locked regressors. The executive control or flanker conflict effect was examined by computing all incongruent minus all congruent conditions for the six target-locked regressors.
Contrast images from all participants were entered into a second-level group analysis conducted with a random-effect model. The group differences represent the “activation” differences rather than the baseline differences. This is consistent with the ANT score computation because the attentional network test is based on cognitive subtraction. Significant activations of interest were identified with voxel-wise p<0.05 in conjunction with an extent threshold of k = 120 (t ≥1.89 for single subject contrasts and t ≥1.76 for group contrasts, resampled voxel size). This threshold was determined using a Monte Carlo simulation that modeled the entire imaging volume iteratively, using an individual voxel type I error rate of p<.05 and 8 mm FWHM smoothing. A cluster extent threshold was determined across 1,000 iterations to set the overall type I error rate to.05 (i.e., p<.05), given the parameters of data acquisition [69].
Results
Group Demographics
Table 1 shows that the aMCI and HC groups did not significantly differ on age (t (14) = 0.73, p = .48, d = 0.39), education (t (14) = 1.60, p = 0.13, d = 0.87), gender, (χ2 (1) = 1.07, p = 0.30), or race (χ2 (3) = 3.69, p = 0.30). Table 1 also shows that the groups differ, at a level near but not reaching significance, on Mini-Mental State Exam [64] scores, at the time of evaluation (t (14) = 2.02, p = 0.06, d = 1.13). Scores ranged from 24 to 30. The groups also differ on the CDR [66], with 100% of individuals in the aMCI group exhibiting scores of 0.5 (very mild dementia) and 25% (2 individuals) exhibiting scores of 0.5 in the HC group, t (14) = 4.58, p<0.001, d = 2.43. Scores did not exceed 0.5.
Behavioral Results
On average, the median RT was 44.4 ms less than the mean RT. Only 2 of 16 individuals showed higher median than mean RTs. Along with an average SD of 276.43 ms, the findings suggested positive skew. Thus, we opted to use median reaction time as the basis for our analyses. Because there were an equal number of trials in each of the experimental conditions, and equal sample sizes in both groups, we were not concerned about bias in median reaction times [70].
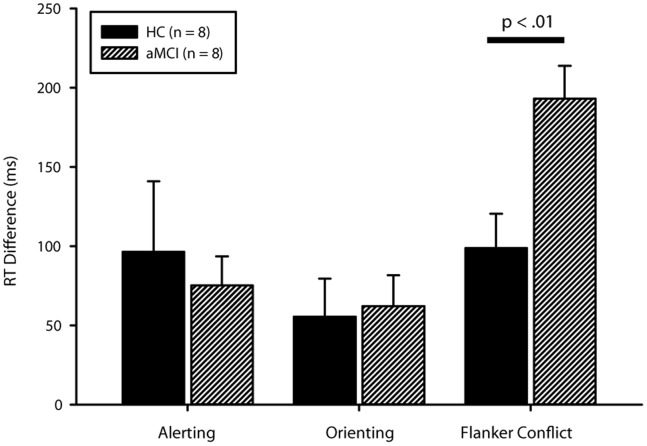
Group differences in the accuracy of alerting approached significance (t (14) = 1.97, p = 0.07), while orienting (t (14) = 0.62, p = 0.55) and executive control (t (14) = 0.10, p = 0.92) did not differ statistically (see Table 2). It is important to note that while the alerting effect on error rate approached significance, there was no statistical difference in error rate on any of the individual trial types or overall performance (see Table 2). This statistical equivalence in terms of accuracy is important because it indicates a comparable number of correct trials to be modeled for the neuroimaging analysis. This lack of difference in accuracy also led us to retain error trials for the imaging contrasts. There were no significant differences between groups in the reaction times of the alerting (t (14) = 0.44, p = 0.67) or orienting functions (t (14) = 0.21, p = 0.84), though there was a large significant difference in the executive function, t (14) = 3.16, p = 0.007, Cohen’s d = 1.7 (see Table 3, Figure 2).
Table 2. Error rates between groups for trial conditions and attentional effects.
| HC (n = 8) | aMCI (n = 8) | Cohen’s d | |
| Cue Condition | |||
| None | 6.00 (11.96) | 8.00 (8.32) | 0.21 |
| Double | 10.00 (15.78) | 6.00 (7.05) | 0.35 |
| Single/Spatial | 9.00 (13.17) | 6.00 (7.09) | 0.3 |
| Flanker Condition | |||
| Congruent | 6.00 (8.43) | 5.00 (3.94) | 0.16 |
| Incongruent | 11.00 (19.08) | 9.00 (10.34) | 0.14 |
| Effect | |||
| Alerting | −3.88 (4.22) | 1.38 (6.26) | 1.05# |
| Orienting | 1.50 (3.33) | 0.00 (6.00) | 0.33 |
| Executive Control | 4.62 (11.26) | 4.12 (7.64) | 0.06 |
| Overall | 5.00 (6.48) | 7.00 (7.11) | 0.31 |
p = 0.07.
Table 3. Reaction time between groups for trial conditions and attentional effects.
| HC (n = 8) | aMCI (n = 8) | Cohen’s d | ||
| Cue Condition | ||||
| None | 1123.13 (210.72) | 1096.75 (178.98) | 0.14 | |
| Double | 1026.75 (148.52) | 1021.38 (194.66) | 0.03 | |
| Single/Spatial | 971.19 (149.60) | 959.19 (202.62) | 0.07 | |
| Flanker Condition | ||||
| Congruent | 989.06 (188.36) | 926.88 (172.67) | 0.37 | |
| Incongruent | 1088.06 (149.48) | 1120.06 (203.13) | 0.19 | |
| Effect | ||||
| Alerting | 96.38 (126.48) | 75.38 (51.31) | 0.23 | |
| Orienting | 55.56 (67.54) | 62.19 (55.49) | 0.11 | |
| Executive Control | 99.00 (60.78) | 193.19 (58.40) | 1.69** | |
| Overall RT | 1049.38 (166.62) | 1020.25 (185.74) | 0.18 | |
p<0.01.
Note: Reaction time (RT) analyses were performed using Median RT due to skew.
Figure 2. Group differences in median reaction time by attentional function.
Only the group difference for the flanker conflict (executive control) effect reached significance at p<0.05 (actual p<0.01). Error bars represent standard error.
Functional Neuroimaging Results
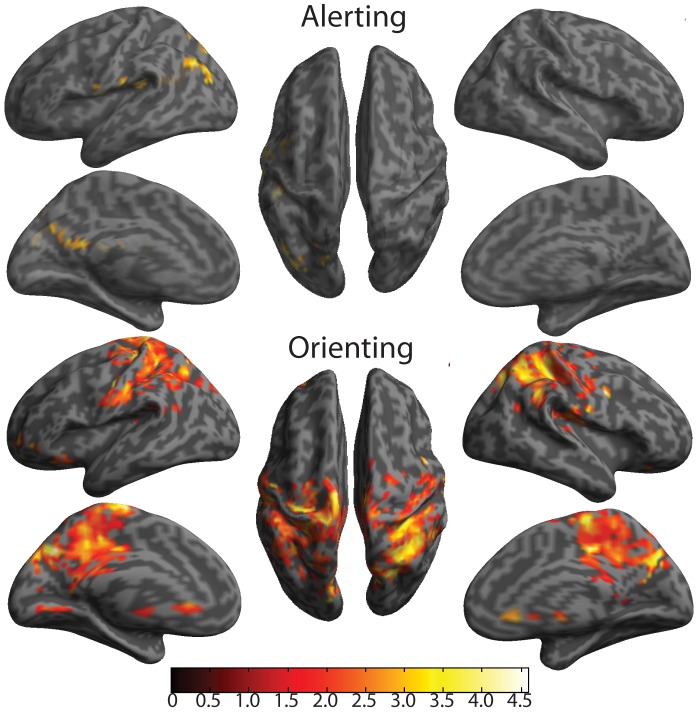
Due to comparable performances between groups in the components of the alerting condition, we conducted analyses examining potentially greater (compensatory) activity in the aMCIs vs. HC. Differences in BOLD activity, related to the alerting effect, were present despite no behavioral differences (see Table 3, Figure 3). Notably, aMCIs showed greater activation of the temporoparietal junction (TPJ; x = −48, y = −36, z = 20), precuneus (x = −2, y = −48, z = 18), and angular gyrus (x = −48, y = −68, z = 34), all in the left hemisphere. Because posterior cingulate cortex (PCC), precuneus, and angular gyrus are prominent nodes of the default mode network (DMN) [71], the difference between aMCI and HC may indicate less deactivation in the aMCI. Greater TPJ activation and left laterality is consistent with our previous findings using the original ANT [23].
Figure 3. Cortical surface maps of Alerting and Orienting effects for aMCI>HC contrast.
All represented activity has been thresholded at p<0.05 for height and k = 120 (p<0.05) for cluster extent to set the nominal alpha level to p<0.05 for multiple comparisons (corresponds to t t ≥1.76), based on Monte Carlo simulation of our data.
Similar to the alerting effect, and due to comparable performances between groups in the orienting condition, we conducted analyses examining potentially greater (compensatory) activity in the aMCIs vs. HC. Differences in BOLD activity, related to the orienting effect, were also present despite no behavioral differences (see Table 4, Figure 3). MCIs predominantly showed greater activation in areas traditionally associated with the orienting function (i.e., posterior parietal regions [23]). Areas of greater activity in aMCIs included the superior parietal lobule and pre- and postcentral gyri, and PCC, all regions bilaterally. For the PCC, the greater activation in aMCI might be related to less deactivation in this brain region.
Table 4. Greater network-related activation in aMCI compared to HC.
| Region | L/R | BA | MNI coordinates | Z | p | k | ||
| x | y | z | ||||||
| Alerting | ||||||||
| Superior temporal gyrus | L | 41 | −48 | −36 | 20 | 3.16 | 0.001 | 191 |
| Postcentral gyrus | L | 43 | −52 | −18 | 18 | 2.39 | 0.008 | |
| Angular gyrus | L | 39 | −48 | −68 | 34 | 2.54 | 0.005 | 335 |
| Middle occipital lobe | L | 19 | −28 | −78 | 42 | 2.54 | 0.006 | |
| Middle occipital lobe | L | 39 | −38 | −80 | 28 | 2.52 | 0.006 | |
| Superior parietal lobule | L | 7 | −26 | −72 | 50 | 2.2 | 0.014 | |
| Superior parietal lobule | L | 7 | −16 | −76 | 50 | 2.1 | 0.018 | |
| Middle occipital lobe | L | 39 | −36 | −70 | 22 | 2.06 | 0.02 | |
| Precuneus | L | 30 | −2 | −48 | 18 | 2.32 | 0.01 | 227 |
| Cuneus | L | 31 | −8 | −64 | 28 | 2.02 | 0.022 | |
| Calcarine | R | 17 | 6 | −66 | 18 | 1.68 | 0.046 | |
| Superior temporal lobe | L | 22 | −58 | −8 | 6 | 2.3 | 0.011 | 125 |
| Insula | L | 13 | −42 | 0 | 12 | 2.01 | 0.022 | |
| Cerebellum 4/5 | R | 30 | 14 | −42 | −16 | 2.11 | 0.018 | 160 |
| Cerebellum 6 | R | 37 | 26 | −50 | −30 | 2.01 | 0.022 | |
| Cerebellum 1 | R | 36 | −58 | −30 | 1.99 | 0.023 | ||
| Orienting | ||||||||
| Paracentral lobule | L | 6 | −4 | −18 | 72 | 3.9 | 0 | 19649 |
| Supplementary motor area | R | 4 | 8 | −20 | 62 | 3.89 | 0 | |
| Precuneus | L | 7 | −8 | −74 | 38 | 3.64 | 0 | |
| Cuneus | R | 7 | 14 | −68 | 34 | 3.54 | 0 | |
| Superior parietal lobule | L | 7 | −18 | −40 | 42 | 3.53 | 0 | |
| Inferior parietal lobule | R | 7 | 28 | −52 | 56 | 3.47 | 0 | |
| Precentral gyrus | R | 6 | 44 | −2 | 36 | 3.41 | 0 | |
| Precuneus | R | 19 | 18 | −68 | 42 | 3.3 | 0 | |
| Postcentral gyrus | R | 3 | 32 | −38 | 56 | 3.22 | 0.001 | |
| Precuneus | L | 3 | −14 | −38 | 72 | 3.2 | 0.001 | |
| Postcentral gyrus | L | 3 | −34 | −30 | 52 | 3.18 | 0.001 | |
| Paracentral lobule | L | 4 | −6 | −26 | 66 | 3.17 | 0.001 | |
| Inferior parietal lobule | L | 40 | −28 | −48 | 40 | 3.15 | 0.001 | |
| Superior parietal lobule | L | 7 | −30 | −64 | 46 | 3.11 | 0.001 | |
| Posterior cingulate gyrus | L | 31 | 0 | −44 | 46 | 3.08 | 0.001 | |
| Angular gyrus | R | 40 | 44 | −44 | 36 | 3.07 | 0.001 | |
| Cuneus | L | 19 | −12 | −84 | 32 | 2.96 | 0.002 | |
| Supramarginal gyrus | R | 40 | 44 | −36 | 42 | 2.87 | 0.002 | |
| Precentral gyrus | L | 6 | −30 | −22 | 62 | 2.7 | 0.003 | |
| Superior parietal lobule | R | 7 | 14 | −68 | 56 | 2.63 | 0.004 | |
| Rolandic operculum | R | 43 | 40 | −14 | 20 | 2.61 | 0.005 | |
| Lingual gyrus | L | 18 | −8 | −58 | 4 | 2.5 | 0.006 | |
| Posterior cingulate gyrus | R | 31 | 8 | −38 | 42 | 2.42 | 0.008 | |
| Supramarginal gyrus | L | 48 | −48 | −26 | 28 | 2.41 | 0.008 | |
| Lingual gyrus | L | 18 | −4 | −68 | 4 | 2.38 | 0.009 | |
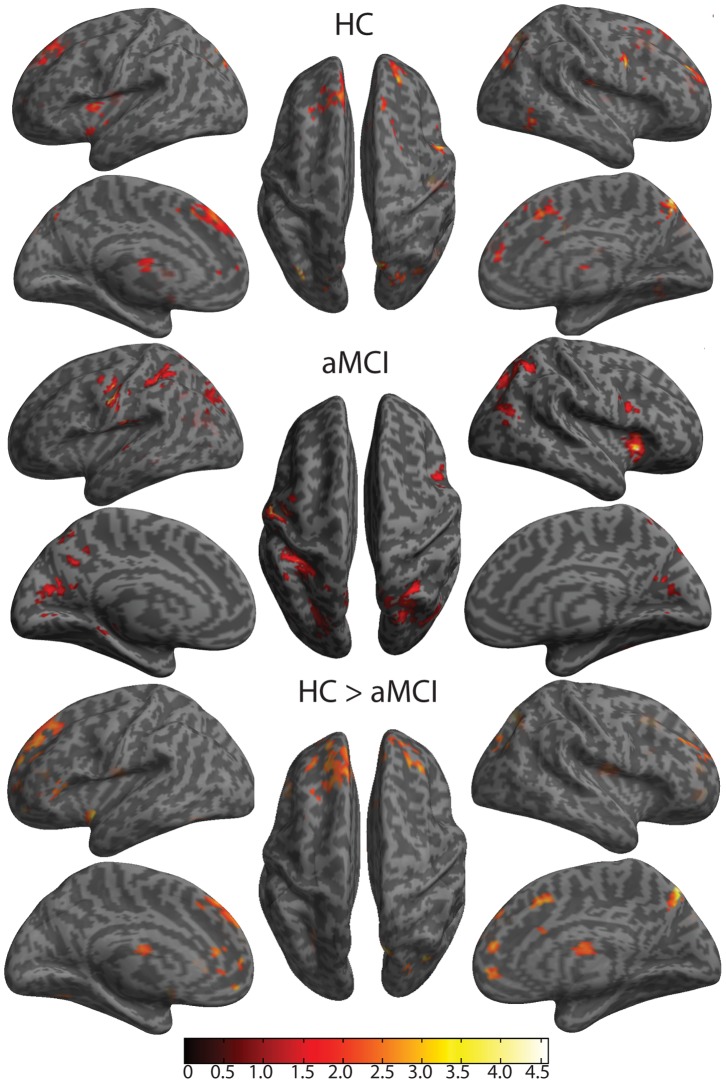
Consistent with the primary hypothesis, there were significant behavioral differences in relation to the flanker conflict effect. Behaviorally, the HC group showed a significantly smaller difference between the congruent and incongruent conditions than the aMCI group (see Figure 2). The aMCI group exhibited corresponding BOLD differences, such that there was less activation in the medial prefrontal regions, especially prefrontal cortex (Brodmann area 10) and ACC, which also extended to the DLPFC (see Table 5, Figure 4). Differences are consistent with previous findings for the flanker conflict effect [23] and with recent discussion about a dual architecture for cognitive control [72].
Table 5. BOLD activation related to executive control in HC, aMCI, and HC>aMCI.
| Region | L/R | BA | MNI coordinates | Z | p | k | ||
| x | y | z | ||||||
| HC | ||||||||
| Precuneus | R | 7 | 10 | −72 | 54 | 3.91 | 0 | 678 |
| Superior occipital gyrus | R | 7 | 26 | −76 | 42 | 2.68 | 0.004 | |
| Cuneus | R | 19 | 20 | −82 | 42 | 2.67 | 0.004 | |
| Precuneus | L | 7 | −4 | −80 | 44 | 2.67 | 0.004 | |
| Superior occipital gyrus | R | 19 | 26 | −74 | 26 | 2.38 | 0.009 | |
| Middle occipital gyrus | R | 19 | 34 | −80 | 38 | 2.35 | 0.009 | |
| Rolandic operculum | R | 43 | 42 | −16 | 16 | 3.73 | 0 | 135 |
| Middle occipital gyrus | L | 19 | −28 | −82 | 38 | 3.14 | 0.001 | 126 |
| Precentral gyrus | R | 44 | 54 | 10 | 32 | 3.1 | 0.001 | 206 |
| Thalamus | L | −12 | −6 | 12 | 3.01 | 0.001 | 523 | |
| Thalamus | R | 6 | −10 | 6 | 2.3 | 0.011 | ||
| Anterior cingulate gyrus | R | 32 | 2 | 50 | 12 | 2.79 | 0.003 | 232 |
| Insula | L | 13 | −36 | 2 | 6 | 2.76 | 0.003 | 312 |
| Putamen | L | −30 | 8 | −2 | 2.39 | 0.008 | ||
| Supplementary motor area | L | 32 | 0 | 16 | 48 | 2.73 | 0.003 | 1614 |
| Superior frontal gyrus, medial | L | 32 | −8 | 36 | 44 | 2.65 | 0.004 | |
| Middle frontal gyrus | L | 8 | −24 | 26 | 42 | 2.29 | 0.011 | |
| Anterior cingulate gyrus | R | 24 | 2 | 22 | 32 | 2.1 | 0.018 | |
| Middle frontal gyrus | L | 9 | −26 | 38 | 34 | 1.96 | 0.025 | |
| Superior frontal gyrus | R | 46 | 24 | 44 | 24 | 2.69 | 0.004 | 194 |
| Superior frontal gyrus | R | 10 | 22 | 56 | 16 | 2.3 | 0.011 | |
| Middle frontal gyrus | R | 46 | 24 | 56 | 24 | 2.19 | 0.014 | |
| Inferior temporal gyrus | R | 37 | 50 | −58 | −4 | 2.46 | 0.007 | 140 |
| Superior frontal gyrus | R | 32 | 16 | 26 | 50 | 2.38 | 0.009 | 121 |
| Middle frontal gyrus | R | 8 | 24 | 12 | 52 | 2.12 | 0.017 | |
| Superior frontal gyrus | R | 6 | 28 | 2 | 56 | 1.91 | 0.028 | |
| aMCI | ||||||||
| Insula/Inferior frontal gyrus | R | 47 | 32 | 18 | 0 | 4.26 | 0 | 390 |
| Insula | R | 13 | 32 | 20 | 10 | 3.24 | 0.001 | |
| Postcentral gyrus | L | 3 | −52 | −14 | 32 | 4.03 | 0 | 376 |
| Postcentral gyrus | L | 3 | −42 | −12 | 38 | 3.45 | 0 | |
| Superior occipital gyrus | L | 23 | −20 | −64 | 26 | 3.72 | 0 | 2255 |
| Superior occipital gyrus | R | 7 | 26 | −74 | 42 | 3.57 | 0 | |
| Calcarine sulcus | L | 17 | −4 | −66 | 12 | 3.54 | 0 | |
| Superior occipital gyrus | R | 19 | 26 | −64 | 24 | 3.26 | 0.001 | |
| Superior occipital gyrus | L | 19 | −24 | −82 | 36 | 3.11 | 0.001 | |
| Middle occipital gyrus | R | 39 | 40 | −72 | 22 | 2.95 | 0.002 | |
| Calcarine sulcus | R | 17 | 4 | −66 | 14 | 2.87 | 0.002 | |
| Precuneus | R | 5 | 12 | −60 | 60 | 2.58 | 0.005 | |
| Middle temporal gyrus | R | 39 | 46 | −66 | 16 | 2.42 | 0.008 | |
| Superior parietal lobule | L | 7 | −22 | −72 | 46 | 2.05 | 0.02 | |
| Inferior frontal gyrus | R | 44 | 44 | 8 | 26 | 3.56 | 0 | 222 |
| Superior parietal lobule | L | 7 | −30 | −48 | 70 | 3.5 | 0 | 1037 |
| Precuneus | L | −14 | −58 | 36 | 3.38 | 0 | ||
| Inferior parietal lobule | L | 40 | −30 | −44 | 40 | 3.24 | 0.001 | |
| Superior parietal lobule | L | 7 | −26 | −50 | 50 | 2.94 | 0.002 | |
| Inferior parietal lobule | L | 40 | −38 | −44 | 54 | 2.77 | 0.003 | |
| Superior parietal lobule | L | 7 | −26 | −56 | 68 | 2.34 | 0.01 | |
| Superior temporal lobe | L | 22 | −60 | −10 | 8 | 3.29 | 0 | 238 |
| Superior temporal lobe | L | 22 | −60 | −18 | 10 | 3.26 | 0.001 | |
| Fusiform gyrus | R | 37 | 42 | −46 | −22 | 2.47 | 0.007 | 135 |
| Inferior temporal gyrus | R | 37 | 44 | −44 | −12 | 2.32 | 0.01 | |
| Parahippocampal gyrus | L | 37 | −22 | −34 | −8 | 2.23 | 0.013 | 172 |
| Vermis 3 | 2 | −36 | −4 | 2.06 | 0.02 | |||
| Cerebellum 4/5 | L | 30 | −8 | −42 | −12 | 1.99 | 0.023 | |
| HC>aMCI | ||||||||
| Precuneus | R | 7 | 8 | −74 | 52 | 3.28 | 0.001 | 224 |
| Anterior cingulate cortex | L | 32 | 0 | 50 | 4 | 3.21 | 0.001 | 2788 |
| Middle frontal gyrus (medial) | L | 10 | −8 | 50 | −6 | 2.99 | 0.001 | |
| Superior frontal gyrus (medial) | L | 8 | −4 | 40 | 52 | 2.87 | 0.002 | |
| Anterior cingulate Gyrus | R | 32 | 6 | 16 | 44 | 2.64 | 0.004 | |
| Middle frontal gyrus | R | 46 | 26 | 58 | 24 | 2.58 | 0.005 | |
| Superior frontal gyrus | L | 46 | −26 | 54 | 22 | 2.46 | 0.007 | |
| Middle frontal gyrus | L | 9 | −24 | 42 | 34 | 2.3 | 0.011 | |
| Middle frontal gyrus | L | 46 | −30 | 40 | 26 | 2.23 | 0.013 | |
| Superior frontal gyrus | L | 9 | −22 | 30 | 44 | 2.2 | 0.014 | |
| Superior frontal gyrus | R | 10 | 14 | 58 | 24 | 1.99 | 0.023 | |
| Anterior cingulate gyrus | L | 32 | −2 | 34 | 30 | 1.92 | 0.028 | |
| Middle occipital lobe | R | 19 | 32 | −80 | 28 | 2.98 | 0.001 | 121 |
| Insula | L | 13 | −34 | 0 | −12 | 2.73 | 0.003 | 124 |
| Thalamus | L | −12 | −12 | 16 | 2.52 | 0.006 | 346 | |
| Thalamus | R | 8 | −8 | 12 | 2.39 | 0.008 | ||
| Inferior frontal gyrus | L | 47 | −46 | 22 | 0 | 2.39 | 0.008 | 125 |
| Middle frontal gyrus | R | 46 | 28 | 40 | 26 | 2.38 | 0.009 | 141 |
| Cerebellum 6 | L | 19 | −32 | −62 | −20 | 2.34 | 0.01 | 130 |
| Fusiform gyrus | L | 37 | −26 | −58 | −14 | 1.98 | 0.024 | |
Figure 4. Cortical surface maps of Executive Control effect for HC, aMCI, and group contrast (HC>aMCI).
The top set is the contrast between flanker incongruent and flanker congruent conditions in Healthy Controls (HC). The middle set is the contrast between flanker incongruent and flanker congruent conditions in patients with amnestic Mild Cognitive Impairment (aMCI). The bottom set is the contrast between HC and aMCI. All represented activity has been thresholded at p<0.05 for height and k = 120 (p<0.05) for cluster extent to set the nominal alpha level to p<0.05 for multiple comparisons, based on Monte Carlo simulation of the data.
Discussion
In the present investigation of a modified version of the attention network test (ANT-G) in healthy controls and individuals with aMCI, there were notable attention deficits among patients with aMCI. While the groups exhibited no significant behavioral differences in the alerting or orienting networks, consistent with some prior work (e.g., [10]), there were significant neural differences for these networks. Since performance was equivalent across groups, but the aMCI group exhibited increased neural activation in the alerting and orienting networks, one might argue that compensatory activity contributed to behavioral performance among the aMCI group comparable to HC (see e.g., [73]). These neural findings are consistent with previous studies that demonstrated deficits in alerting and orienting in MCI and/or AD [12], [15], [17], [26]–[35].
Attentional deficits in aMCI were most notable during the flanker conflict component of the ANT-G (i.e., executive control of attention), where both behavioral and neural differences were evident between groups. One must use caution when considering group differences (between patients and healthy controls) when task performance is not equal; differences in neural activity could reflect different approaches and/or strategies to the task [73]. However, the important role of the ACC in conflict resolution (e.g., [74]), as well as the previously observed hypometabolism of the ACC in those who convert from MCI to AD [52]–[55], lend support to our interpretation of the present findings; deficits of executive control of attention may be due to deficits of ACC function. Deficits in the executive control of attention [9], [11], [12], [17], [36]–[42] and executive function, more generally [17], [43]–[49], have previously been documented, though the neural substrates have not been well elucidated. The findings of the present examination suggest that in addition to the other deficits characteristic of aMCI (e.g., [2]), there may be substantial deficits in the executive control network (corresponding to less activation in the medial prefrontal cortex).
Given the behavioral and neural deficits in executive control network observed herein, it is interesting to consider plausible mechanisms for changes to the neural substrates, especially the ACC. Although only a few studies have directly investigated abnormalities in the ACC related to the executive control of attention in AD using functional neuroimaging (e.g., [56]–[58]), there is much indirect evidence that ACC dysfunction underlies the observed behavioral deficits in this population. For example, aberrant activation as well as deactivation of the ACC has been observed when subjects with AD or at risk for AD perform non-attentional tasks that necessitate the involvement of attentional functions [75], [76]. Further, a recent study of grey matter density and white matter integrity found grey matter atrophy in the cingulate cortex, and more interestingly, that deafferentation in the cingulate cortex, along with grey matter integrity in hippocampal and parahippocampal areas is predictive of impairment in cognitive function among patients with AD [59]. A recent longitudinal study has also shown that individuals who convert from MCI to AD show decreased metabolic activity in regions of the ACC [77]. Abnormalities in ACC-related functional networks have also been reported in patients with AD and MCI under various task conditions, though with somewhat inconsistent findings [78]–[83]. Increases in ACC functional connectivity have been attributed to the engagement of alternative networks for task performance (i.e., the plasticity argument [79]), while decreases in connectivity among patients with AD has been explained as a breakdown of the memory [83], default mode [80], [84], and attentional networks [82].
In conjunction with previous findings regarding the potential importance of the ACC in MCI and AD, the present study suggests that behavioral deficits in attentional conflict resolution may be due to hypoactivity during conflict resolution in the medial prefrontal cortex among individuals with aMCI. While there are certainly limitations to the present study, we attempted to simultaneously examine multiple attentional functions while also acquiring fMRI data in a population with aMCI. Given that executive control of attention is critical to determining what information reaches conscious awareness, the present findings might suggest that deficits in the executive control of attention are characteristic of aMCI.
Another important consideration is that of breakdown in the DMN among individuals with aMCI and AD [80], [84], [85]. Prefrontal, lateral temporal, and lateral parietal regions, along with the precuneus show amyloid depositions, altered metabolism, and atrophy in AD progression, as well as having a prominent role in the DMN [85]. Given activation of a task-positive network, in conjunction with deactivation of the DMN, during task demands, some of the present findings might best be explained in the context of greater activation of DMN regions in alerting by the aMCI group and more differentiation between the cue conditions of orienting by the aMCI group. This pattern needs further exploration.
Beyond the ACC and the executive control of attention, our findings suggest more broad deficits of attention. One interesting implication of the current findings, though their preliminary basis cannot be overlooked, is that deficits in memory among those with aMCI and AD [6] may in fact be related to deficits in attention (e.g., [86]). There is an intimate relationship between attention and memory such that the two processes mutually constrain one another [87]. Impaired attentional function, as is evident in early stages of AD [7]–[11], may partially contribute to the notable declines in memory function. If this is accurate, one way to identify those individuals with aMCI who are mostly likely to progress to AD may be to evaluate attentional function using well-validated tasks like the ANT. This hypothesis is supported by the fact that attentional function is predictive of cognitive decline among those in early stages of probable AD [13]. Another potential implication of the relationship between attention and memory among those with aMCI is that attentional and/or cognitive training interventions could potentially delay the conversion to AD (e.g., [88]). Delay in conversion might have profound implications for both the individual and for society, especially given the economic burden of AD [5].
The primary limitation of the current study is the small sample size. Although there were no differences between our larger sample and those for whom we were able to collect fMRI data, one thing to consider is whether the current sample is representative of a particular subclass of individuals with aMCI. Those individuals willing to participate in a research study and undergo an MRI scan may be more functional than their peers who are not so inclined. This may be one reason that our MMSE scores were so similar between the two groups. However, this finding may actually lead to an underestimate of the potential differences between the HC and aMCI groups.
Furthermore some of the neural activation observed may be due to Type I error, even though correction for multiple comparisons was conducted using Monte Carlo simulation methods. Despite these limitations, our observations do suggest some interesting patterns. The neural activity associated with the aMCI minus HC contrast for alerting (e.g., TPJ) and orienting (e.g., posterior parietal regions) is consistent with previous findings showing the involvement of these regions in alerting and orienting [23], despite the absence of behavioral differences. This may suggest impairments and compensatory neural activity in the alerting and orienting networks among individuals with aMCI. Neurobehavioral activity related to alerting and orienting in aMCI necessitates further research. There were both behavioral and corresponding neural deficits in executive control corresponding to the flanker conflict condition of the ANT-G. These findings are consistent with known deficits to the executive control of attention [17] in aMCI and AD. Although preliminary, our findings suggest that deficits in attention, particularly in the executive control network, may have important contributions in the clinical presentation of aMCI and potentially its progression to AD.
Acknowledgments
The authors thank all the physicians, researchers, and staff that are affiliated with the Mount Sinai Alzheimer’s Disease Research Center for their help on this project. The authors also thank the individual participants for making this study possible.
Funding Statement
The present study was funded as a pilot investigation to J.F. by National Institute on Aging (of the National Institutes of Health) grant P50 AG005138 (M.S., Sam Gandy, P.R.H.). Authors J.F., P.R.H., & M.S. conceived the research design. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Geslani DM, Tierney MC, Herrmann N, Szalai JP (2005) Mild cognitive impairment: an operational definition and its conversion rate to Alzheimer’s disease. Dementia and Geriatric Cognitive Disorders 19: 383–389. [DOI] [PubMed] [Google Scholar]
- 2. Gauthier S, Reisberg B, Zaudig M, Petersen RC, Ritchie K, et al. (2006) Mild cognitive impairment. The Lancet 367: 1262–1270. [DOI] [PubMed] [Google Scholar]
- 3. Morris JC, Storandt M, Miller JP, McKeel DW, Price JL, et al. (2001) Mild cognitive impairment represents early-stage Alzheimer disease. Arch Neurol 58: 397–405. [DOI] [PubMed] [Google Scholar]
- 4. Rafii MS, Aisen PS (2009) Recent developments in Alzheimer’s disease therapeutics. BMC medicine 7: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM (2007) Forecasting the global burden of Alzheimer’s disease. Alzheimer’s and Dementia 3: 186–191. [DOI] [PubMed] [Google Scholar]
- 6. Welsh KA, Butters N, Hughes JP, Mohs RC, Heyman A (1992) Detection and staging of dementia in Alzheimer’s disease. Use of the neuropsychological measures developed for the Consortium to Establish a Registry for Alzheimer’s Disease. Arch Neurol 49: 448–452. [DOI] [PubMed] [Google Scholar]
- 7. Haxby JV, Grady CL, Koss E, Horwitz B, Heston L, et al. (1990) Longitudinal study of cerebral metabolic asymmetries and associated neuropsychological patterns in early dementia of the Alzheimer type. Arch Neurol 47: 753–760. [DOI] [PubMed] [Google Scholar]
- 8. Perry RJ, Watson P, Hodges JR (2000) The nature and staging of attention dysfunction in early (minimal and mild) Alzheimer’s disease: relationship to episodic and semantic memory impairment. Neuropsychologia 38: 252–271. [DOI] [PubMed] [Google Scholar]
- 9. Pignatti R, Rabuffetti M, Imbornone E, Mantovani F, Alberoni M, et al. (2005) Specific impairments of selective attention in mild Alzheimer’s disease. J Clin Exp Neuropsychol 27: 436–448. [DOI] [PubMed] [Google Scholar]
- 10. Fernandez-Duque D, Black SE (2006) Attentional networks in normal aging and Alzheimer’s disease. Neuropsychology 20: 133–143. [DOI] [PubMed] [Google Scholar]
- 11. Castel AD, Balota DA, Hutchison KA, Logan JM, Yap MJ (2007) Spatial attention and response control in healthy younger and older adults and individuals with Alzheimer’s disease: evidence for disproportionate selection impairments in the Simon task. Neuropsychology 21: 170–182. [DOI] [PubMed] [Google Scholar]
- 12. Gorus E, De Raedt R, Lambert M, Lemper JC, Mets T (2006) Attentional processes discriminate between patients with mild Alzheimer’s disease and cognitively healthy elderly. Int Psychogeriatr 18: 539–549. [DOI] [PubMed] [Google Scholar]
- 13. Marra C, Silveri MC, Gainotti G (2000) Predictors of cognitive decline in the early stage of probable Alzheimer’s disease. Dement Geriatr Cogn Disord 11: 212–218. [DOI] [PubMed] [Google Scholar]
- 14. Fan J, Gu X, Guise KG, Liu X, Fossella J, et al. (2009) Testing the behavioral interaction and integration of attentional networks. Brain Cogn 70: 209–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rizzo M, Anderson SW, Dawson J, Myers R, Ball K (2000) Visual attention impairments in Alzheimer’s disease. Neurology 54: 1954–1959. [DOI] [PubMed] [Google Scholar]
- 16. Parasuraman R, Greenwood PM, Sunderland T (2002) The apolipoprotein E gene, attention, and brain function. Neuropsychology 16: 254–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Perry RJ, Hodges JR (1999) Attention and executive deficits in Alzheimer’s disease. A critical review. Brain 122 (Pt 3): 383–404. [DOI] [PubMed] [Google Scholar]
- 18. Parasuraman R, Haxby JV (1993) Attention and brain function in Alzheimer’s disease: A review. Neuropsychology 7: 242–272. [Google Scholar]
- 19. Sano M (2006) Neuropsychological testing in the diagnosis of dementia. J Geriatr Psychiatry Neurol 19: 155–159. [DOI] [PubMed] [Google Scholar]
- 20. Fan J, McCandliss BD, Sommer T, Raz A, Posner MI (2002) Testing the efficiency and independence of attentional networks. J Cogn Neurosci 14: 340–347. [DOI] [PubMed] [Google Scholar]
- 21.Fan J, Raz A, Posner MI (2003) Attentional Mechanisms. In: Aminoff MJ, Daroff RB, editors. Encyclopedia of Neurological Sciences. San Diego: Academic Press. pp. 292–299.
- 22. Posner MI, Petersen SE (1990) The attention system of the human brain. Annu Rev Neurosci 13: 25–42. [DOI] [PubMed] [Google Scholar]
- 23. Fan J, McCandliss BD, Fossella J, Flombaum JI, Posner MI (2005) The activation of attentional networks. Neuroimage 26: 471–479. [DOI] [PubMed] [Google Scholar]
- 24. Corbetta M, Shulman GL (2002) Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci 3: 201–215. [DOI] [PubMed] [Google Scholar]
- 25. Corbetta M, Patel G, Shulman GL (2008) The reorienting system of the human brain: from environment to theory of mind. Neuron 58: 306–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nebes RD, Brady CB (1989) Focused and divided attention in Alzheimer’s disease. Cortex 25: 305–315. [DOI] [PubMed] [Google Scholar]
- 27. Parasuraman R, Greenwood PM, Haxby JV, Grady CL (1992) Visuospatial attention in dementia of the Alzheimer type. Brain 115: 711–733. [DOI] [PubMed] [Google Scholar]
- 28. Oken BS, Kishiyama SS, Kaye JA, Howieson DB (1994) Attention deficit in Alzheimer’s disease is not simulated by an anticholinergic/antihistaminergic drug and is distinct from deficits in healthy aging. Neurology 44: 657–662. [DOI] [PubMed] [Google Scholar]
- 29. Faust ME, Balota DA (1997) Inhibition of return and visuospatial attention in healthy older adults and individuals with dementia of the Alzheimer type. Neuropsychology 11: 13–29. [DOI] [PubMed] [Google Scholar]
- 30. Ballard C, O’Brien J, Gray A, Cormack F, Ayre G, et al. (2001) Attention and fluctuating attention in patients with dementia with Lewy bodies and Alzheimer disease. Arch Neurol 58: 977–982. [DOI] [PubMed] [Google Scholar]
- 31. Tales A, Muir JL, Bayer A, Jones R, Snowden RJ (2002) Phasic visual alertness in Alzheimer’s disease and ageing. Neuroreport 13: 2557–2560. [DOI] [PubMed] [Google Scholar]
- 32. Festa-Martino E, Ott BR, Heindel WC (2004) Interactions between phasic alerting and spatial orienting: effects of normal aging and Alzheimer’s disease. Neuropsychology 18: 258–268. [DOI] [PubMed] [Google Scholar]
- 33. Berardi AM, Parasuraman R, Haxby JV (2005) Sustained attention in mild Alzheimer’s disease. Dev Neuropsychol 28: 507–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tales A, Snowden RJ, Brown M, Wilcock G (2006) Alerting and orienting in Alzheimer’s disease. Neuropsychology 20: 752–756. [DOI] [PubMed] [Google Scholar]
- 35.Festa EK, Ott BR, Heindel WC (2006) Considering phasic alerting in Alzheimer’s disease: comment on Tales et al. (2006). Neuropsychology 20: 757–760; discussion 761–752. [DOI] [PubMed]
- 36. Baddeley AD, Baddeley HA, Bucks RS, Wilcock GK (2001) Attentional control in Alzheimer’s disease. Brain 124: 1492–1508. [DOI] [PubMed] [Google Scholar]
- 37. Sebastian MV, Menor J, Elosua MR (2006) Attentional dysfunction of the central executive in AD: evidence from dual task and perseveration errors. Cortex 42: 1015–1020. [DOI] [PubMed] [Google Scholar]
- 38. Rapp MA, Reischies FM (2005) Attention and executive control predict Alzheimer disease in late life: results from the Berlin Aging Study (BASE). Am J Geriatr Psychiatry 13: 134–141. [DOI] [PubMed] [Google Scholar]
- 39. Backman L, Jones S, Berger AK, Laukka EJ, Small BJ (2005) Cognitive impairment in preclinical Alzheimer’s disease: a meta-analysis. Neuropsychology 19: 520–531. [DOI] [PubMed] [Google Scholar]
- 40. Foster JK (2001) Selective attention in Alzheimer’s disease. Front Biosci 6: D135–153. [DOI] [PubMed] [Google Scholar]
- 41. Foster JK, Behrmann M, Stuss DT (1999) Visual attention deficits in Alzheimer’s disease: simple versus conjoined feature search. Neuropsychology 13: 223–245. [DOI] [PubMed] [Google Scholar]
- 42. Levinoff EJ, Li KZ, Murtha S, Chertkow H (2004) Selective attention impairments in Alzheimer’s disease: evidence for dissociable components. Neuropsychology 18: 580–588. [DOI] [PubMed] [Google Scholar]
- 43. Grady CL, Haxby JV, Horwitz B, Sundaram M, Berg G, et al. (1988) Longitudinal study of the early neuropsychological and cerebral metabolic changes in dementia of the Alzheimer type. J Clin Exp Neuropsychol 10: 576–596. [DOI] [PubMed] [Google Scholar]
- 44. Lafleche G, Albert M (1995) Executive function deficits in mild Alzheimer’s disease. Neuropsychology 9: 313–320. [Google Scholar]
- 45. Binetti G, Magni E, Padovani A, Cappa SF, Bianchetti A, et al. (1996) Executive dysfunction in early Alzheimer’s disease. J Neurol Neurosurg Psychiatry 60: 91–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Collette F, Van der Linden M, Salmon E (1999) Executive dysfunction in Alzheimer’s disease. Cortex 35: 57–72. [DOI] [PubMed] [Google Scholar]
- 47. Royall DR, Lauterbach EC, Cummings JL, Reeve A, Rummans TA, et al. (2002) Executive control function: a review of its promise and challenges for clinical research. A report from the Committee on Research of the American Neuropsychiatric Association. J Neuropsychiatry Clin Neurosci 14: 377–405. [DOI] [PubMed] [Google Scholar]
- 48. Baudic S, Barba GD, Thibaudet MC, Smagghe A, Remy P, et al. (2006) Executive function deficits in early Alzheimer’s disease and their relations with episodic memory. Arch Clin Neuropsychol 21: 15–21. [DOI] [PubMed] [Google Scholar]
- 49. Woo BK, Harwood DG, Melrose RJ, Mandelkern MA, Campa OM, et al. (2010) Executive deficits and regional brain metabolism in Alzheimer’s disease. International Journal of Geriatric Psychiatry 25: 1150–1158. [DOI] [PubMed] [Google Scholar]
- 50. Bush G, Luu P, Posner MI (2000) Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Science 4: 215–222. [DOI] [PubMed] [Google Scholar]
- 51. Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, et al. (2011) The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat Rev Neurosci 12: 154–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Johnson KA, Jones K, Holman BL, Becker JA, Spiers PA, et al. (1998) Preclinical prediction of Alzheimer’s disease using SPECT. Neurology 50: 1563–1571. [DOI] [PubMed] [Google Scholar]
- 53. El Fakhri G, Kijewski MF, Johnson KA, Syrkin G, Killiany RJ, et al. (2003) MRI-guided SPECT perfusion measures and volumetric MRI in prodromal Alzheimer disease. Arch Neurol 60: 1066–1072. [DOI] [PubMed] [Google Scholar]
- 54. Mosconi L, Perani D, Sorbi S, Herholz K, Nacmias B, et al. (2004) MCI conversion to dementia and the APOE genotype: a prediction study with FDG-PET. Neurology 63: 2332–2340. [DOI] [PubMed] [Google Scholar]
- 55.Salmon E, Lekeu F, Garraux G, Guillaume B, Magis D, et al.. (2007) Metabolic correlates of clinical heterogeneity in questionable Alzheimer’s disease. Neurobiol Aging. [DOI] [PubMed]
- 56. Johannsen P, Jakobsen J, Bruhn P, Gjedde A (1999) Cortical responses to sustained and divided attention in Alzheimer’s disease. Neuroimage 10: 269–281. [DOI] [PubMed] [Google Scholar]
- 57. Hao J, Li K, Li K, Zhang D, Wang W, et al. (2005) Visual attention deficits in Alzheimer’s disease: an fMRI study. Neurosci Lett 385: 18–23. [DOI] [PubMed] [Google Scholar]
- 58. Dannhauser TM, Walker Z, Stevens T, Lee L, Seal M, et al. (2005) The functional anatomy of divided attention in amnestic mild cognitive impairment. Brain 128: 1418–1427. [DOI] [PubMed] [Google Scholar]
- 59. Bozzali M, Giulietti G, Basile B, Serra L, Spanò B, et al. (2012) Damage to the cingulum contributes to alzheimer’s disease pathophysiology by deafferentation mechanism. Human Brain Mapping 33: 1295–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Fan J, Posner M (2004) Human attentional networks. Psychiatr Prax 31 Suppl 2210–214. [DOI] [PubMed] [Google Scholar]
- 61. Wang K, Fan J, Dong Y, Wang CQ, Lee TM, et al. (2005) Selective impairment of attentional networks of orienting and executive control in schizophrenia. Schizophr Res 78: 235–241. [DOI] [PubMed] [Google Scholar]
- 62. Petersen RC, Thomas RG, Grundman M, Bennett D, Doody R, et al. (2005) Vitamin E and donepezil for the treatment of mild cognitive impairment. New England Journal of Medicine 352: 2379–2388. [DOI] [PubMed] [Google Scholar]
- 63. Sano M, Raman R, Emond J, Thomas RG, Petersen R, et al. (2011) Adding delayed recall to the Alzheimer Disease Assessment Scale is useful in studies of mild cognitive impairment but not Alzheimer disease. Alzheimer Disease and Associated Disorders 25: 122–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research 12: 189–198. [DOI] [PubMed] [Google Scholar]
- 65.Wechsler D (1997) Wechsler Memory Scale - 3rd Edition (WMS-III). Sant Antonio, TX: Harcourt Assessment.
- 66. Morris JC (1993) The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 43: 2412–2414. [DOI] [PubMed] [Google Scholar]
- 67. Friston KJ, Holmes AP, Poline JB, Grasby PJ, Williams SC, et al. (1995) Analysis of fMRI time-series revisited. Neuroimage 2: 45–53. [DOI] [PubMed] [Google Scholar]
- 68. Friston KJ, Fletcher P, Josephs O, Holmes A, Rugg MD, et al. (1998) Event-Related fMRI: Characterizing Differential Responses. Neuroimage 7: 30–40. [DOI] [PubMed] [Google Scholar]
- 69. Slotnick SD, Moo LR, Segal JB, Hart Jr J (2003) Distinct prefrontal cortex activity associated with item memory and source memory for visual shapes. Cognitive Brain Research 17: 75–82. [DOI] [PubMed] [Google Scholar]
- 70. Miller J (1988) A warning about median reaction time. Journal of Experimental Psychology: Human Perception and Performance 14: 539–543. [DOI] [PubMed] [Google Scholar]
- 71. Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL (2010) Functional-anatomic fractionation of the brain’s default network. Neuron 65: 550–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Dosenbach NU, Fair DA, Cohen AL, Schlaggar BL, Petersen SE (2008) A dual-networks architecture of top-down control. Trends Cogn Sci 12: 99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Jeyakumar SLE, Warriner EM, Raval VV, Ahmad SA (2004) Balancing the Need for Reliability and Time Efficiency: Short Forms of the Wechsler Adult Intelligence Scale-III. Educational and Psychological Measurement 64: 71–87. [Google Scholar]
- 74. Fan J, Guise KG, Liu X, Wang HB (2008) Searching for the Majority: Algorithms of Voluntary Control. PLoS One 3: e3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Stern Y, Moeller JR, Anderson KE, Luber B, Zubin NR, et al. (2000) Different brain networks mediate task performance in normal aging and AD: defining compensation. Neurology 55: 1291–1297. [DOI] [PubMed] [Google Scholar]
- 76. Rombouts SA, Barkhof F, Goekoop R, Stam CJ, Scheltens P (2005) Altered resting state networks in mild cognitive impairment and mild Alzheimer’s disease: an fMRI study. Hum Brain Mapp 26: 231–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Fouquet M, Desgranges B, Landeau B, Duchesnay E, Mezenge F, et al. (2009) Longitudinal brain metabolic changes from amnestic mild cognitive impairment to Alzheimer’s disease. Brain : a journal of neurology 132: 2058–2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Han Y, Wang J, Zhao Z, Min B, Lu J, et al. (2011) Frequency-dependent changes in the amplitude of low-frequency fluctuations in amnestic mild cognitive impairment: a resting-state fMRI study. Neuroimage 55: 287–295. [DOI] [PubMed] [Google Scholar]
- 79. Grady CL, Furey ML, Pietrini P, Horwitz B, Rapoport SI (2001) Altered brain functional connectivity and impaired short-term memory in Alzheimer’s disease. Brain 124: 739–756. [DOI] [PubMed] [Google Scholar]
- 80. Greicius MD, Srivastava G, Reiss AL, Menon V (2004) Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A 101: 4637–4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Bokde AL, Lopez-Bayo P, Meindl T, Pechler S, Born C, et al. (2006) Functional connectivity of the fusiform gyrus during a face-matching task in subjects with mild cognitive impairment. Brain 129: 1113–1124. [DOI] [PubMed] [Google Scholar]
- 82. Celone KA, Calhoun VD, Dickerson BC, Atri A, Chua EF, et al. (2006) Alterations in memory networks in mild cognitive impairment and Alzheimer’s disease: an independent component analysis. J Neurosci 26: 10222–10231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wang L, Zang Y, He Y, Liang M, Zhang X, et al. (2006) Changes in hippocampal connectivity in the early stages of Alzheimer’s disease: evidence from resting state fMRI. Neuroimage 31: 496–504. [DOI] [PubMed] [Google Scholar]
- 84. Zhou J, Greicius MD, Gennatas ED, Growdon ME, Jang JY, et al. (2010) Divergent network connectivity changes in behavioural variant frontotemporal dementia and Alzheimer’s disease. Brain 133: 1352–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, et al. (2005) Molecular, structural, and functional characterization of Alzheimer’s Disease: Evidence for a relationship between default activity, amyloid, and memory. The Journal of Neuroscience 25: 7709–7717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Castel AD, Balota DA, McCabe DP (2009) Memory efficiency and the strategic control of attention at encoding: impairments of value-directed remembering in Alzheimer’s disease. Neuropsychology 23: 297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Cowan N (1988) Evolving conceptions of memory storage, selective attention, and their mutual constraints within the human information-processing system. Psychological Bulletin 104: 163–191. [DOI] [PubMed] [Google Scholar]
- 88. Simon SS, Yokomizo JE, Bottino CM (2012) Cognitive intervention in amnestic Mild Cognitive Impairment: A systematic review. Neuroscience and Biobehavioral Reviews 36: 1163–1178. [DOI] [PubMed] [Google Scholar]