Abstract
Amphibian metamorphosis involves extensive, but selective, neuronal death and turnover, thus sharing many features with mammalian postnatal development. The antiapoptotic protein Bcl-XL plays an important role in postnatal mammalian neuronal survival. It is therefore of interest that accumulation of the mRNA encoding the Xenopus Bcl-XL homologue, termed xR11, increases abruptly in the nervous system, but not in other tissues, during metamorphosis in Xenopus tadpoles. This observation raises the intriguing possibility that xR11 selectively regulates neuronal survival during postembryonic development. To investigate this hypothesis, we overexpressed xR11 in vivo as a green fluorescent protein (GFP)-xR11 fusion protein by using somatic and germinal transgenesis. Somatic gene transfer showed that the fusion protein was effective in counteracting, in a dose-dependent manner, the proapoptotic effects of coexpressed Bax. When GFP-xR11 was expressed from the neuronal β-tubulin promoter by germinal transgenesis we observed neuronal specific expression that was maintained throughout metamorphosis and beyond, into juvenile and adult stages. Confocal microscopy showed GFP-xR11 to be exclusively localized in the mitochondria. Our findings show that GFP-xR11 significantly prolonged Rohon-Beard neuron survival up to the climax of metamorphosis, even in the regressing tadpole tail, whereas in controls these neurons disappeared in early metamorphosis. However, GFP-xR11 expression did not modify the fate of spinal cord motoneurons. The selective protection of Rohon-Beard neurons reveals cell-specific apoptotic pathways and offers approaches to further analyze programmed neuronal turnover during postembryonic development.
Keywords: transgenesis, fusion protein, green fluorescent protein, Mauthner
Programmed cell death (PCD or apoptosis) is an essential component of animal development and homeostasis. PCD plays a key role in morphogenesis by controlling cell number as well as in the adult by the elimination of extraneous abnormal or nonfunctional cells (1). Amphibian metamorphosis provides a particularly advantageous model to study PCD. Indeed, it was on anuran tadpoles that the first observations of PCD and the associated morphological changes were made (2). Effectively, the switch from a larval to adult form imposes dramatic biochemical and morphological transformations, including limb growth, intestinal shortening, gill resorption, tail loss, and major remodeling of the larval nervous system, which must adapt to a new body plan.
One of the major developmental apoptotic routes is the mitochondrial pathway that involves the Bcl-2 family of regulators (for reviews see refs. 3 and 4). Certain family members are proapoptotic, such as Bax, Bcl-XS, and Bak, whereas others, in particular Bcl-2 and Bcl-XL, are antiapoptotic (5). All share highly conserved domains that allow for various combinations of heterodimers and homodimers (6, 7). An amphipatic C terminus allows some of these proteins (Bax, Bcl-2, and Bcl-XL) to be tail-anchored to intracellular membranes (8, 9). However, Bax is mainly cytoplasmic and only relocalizes to mitochondria under certain conditions (3). In the mitochondria it promotes the release of proapoptotic factors such as cytochrome c, a necessary cofactor for Apaf-1 (apoptotic protease activating factor-1) (10) or SMAC/Diablo proteins that are inhibitors of IAPs (inhibitor of apoptosis proteins) (11). Apaf-1 activates downstream caspases (cysteine-dependent aspartate directed proteases), the central apoptosis executioners that dismantle cells by cleaving cytoskeletal and nuclear proteins as well as components of the replication, transcription, or repair machinery.
Both proapoptotic and antiapoptotic members of the family can act by both heterodimerization-dependent and -independent mechanisms (12, 13). Such cell survival-related activities of Bcl-2 and Bcl-XL implicate their capacity to form ion channels in subcellular membranes including the mitochondrial membrane. Through these properties, Bcl-2 and Bcl-XL are involved in controlling mitochondrial permeability and counteracting the release of proapoptotic factors such as cytochrome c (reviewed in ref. 3).
Various members of the Bcl-2 family are expressed in the nervous system with patterns compatible with potential roles as regulators of neuronal death in vivo. In particular, the roles of two key antiapoptotic proteins, Bcl-2 and Bcl-XL, have been investigated in the developing and adult mouse nervous systems by additive trangenesis and/or homologous recombination (14–17). The phenotype of Bcl-XL null mutants is particularly striking, with mice dying at embryonic day 13 (16). The effects of the Bcl-2 deletion are less damaging, but several peripheric nervous system populations are depleted (17). Moreover, Bcl-XL has a particularly important role in postnatal neuronal development (18), a period that has been equated with amphibian metamorphosis (19). In this light it is interesting to note that mRNA encoding the Xenopus Bcl-XL homologue, xR11, shows an abrupt increase in the brain during midmetamorphosis and postmetamorphosis, whereas no major variations are seen in other tissues (20).
As to other members of the Bcl-2 family, we have shown Bax expression to be increased in a thyroid hormone (3,5,3′-triiodothyronine, T3)-dependent manner in the regressing tail of Xenopus laevis tadpoles (21). Thus it seems that during metamorphosis the balance between proapoptotic and antiapoptotic Bcl-2 family members is modulated in the cascade of events initiated by T3. To explore the potential role of xR11 in the balance between Bcl-2 family members in regulating neuronal survival/death during X. laevis metamorphosis, we generated tadpoles expressing a green fluorescent protein (GFP) fusion protein, GFP-xR11, from a pan-neural promoter. This strategy allowed us to follow expression of xR11 both in toto and in sections, as well as to resolve its subcellular localization in the mitochondria.
At the morphological level, we focused our attention on two classes of neurons that disappear or regress markedly during metamorphosis, respectively, the Mauthner (M) and Rohon-Beard (RB) neurons. M neurons are two giant cells of the hindbrain that are drastically reduced in size during metamorphosis (22), whereas the numerous RB neurons in the spinal cord disappear completely (23). We found that xR11 prolonged survival of RB neurons during metamorphosis and limited morphological changes in M neurons. However, disappearance of certain other neurons, such as spinal cord motoneurons, was not modified by increased xR11 expression. These findings corroborate the hypothesis that cell-specific, mitochondrial-dependent, and -independent neuronal death programs are instigated during postembryonic development.
Methods
Plasmids.
Neuronal β-tubuline (Nβt)-GFP, the promoter of the Nβt, was excised as a 3.8-kb HindIII fragment from plasmid Nβt-CAT (provided by P. A. Krieg, University of Texas, Austin) and inserted in 5′ of GFP at the HindIII site of peGFP-1 (CLONTECH). peGFP-xR11,xR11 was linked in-frame at the C terminal of GFP in peGFP-C2 (CLONTECH). Nβt-GFP-xR11, the ApaLI–AgeI fragment containing the Nβt promoter from the plasmid Nβt-GFP, was linked to the ApaLI–AgeI fragment carrying GFP-xR11 from the plasmid peGFP-xR11.
Somatic Gene Transfer and Restriction Enzyme-Mediated Integration Nuclear Transplantation Transgenesis.
Somatic gene transfer in Xenopus tadpole dorsal muscle and central nervous system were performed as described (24, 25). Restriction enzyme-mediated integration nuclear transplantation was carried out according to Kroll and Amaya (26) with the following modifications: the sperm was purified by centrifugation on a two-layer discontinuous Percoll (Sigma) before the permeabilization step, which was performed with digitonin (Sigma) instead of lysolecithin (27).
Histology, Immunocytochemistry, and Cell Counts.
For histology, animals were killed by decapitation at different stages determined according to Nieuwkoop and Faber (28). Histological analysis was performed as described (29). For GFP observation, sections were counterstained with Hoechst 33342 (Sigma), mounted in Moviol, and observed with epifluorescence by using an Olympus microscope. For immunolabeling, sections were incubated with a mouse monoclonal antimitochondrial (cytochrome c) antibody (1:1,000; a gift from P. Petit, Institut Cochin de Génétique Molécular, Paris), and labeling was visualized by using Cy3 fluorescent secondary antibody (1:400; Amersham Pharmacia). Confocal microscopy was performed on colabeled sections. For histology, counterstaining was carried out with cresyl violet solution. Cell counts (RB neurons and motoneurons) were carried out on adjacent transversal sections along the major part of the spinal cord.
Confocal Microscopy.
Confocal microscopy was performed on a Sarastro 2000 equipped with an argon multiline laser and IMAGESPACE 3D software on Silicon Graphics workstations (Molecular Dynamics), using various Nikon objectives: ×10 numerical aperture (NA) 0.45, ×40 oil NA 1.30, ×100 oil NA 1.40. Cy3 was imaged with 514 nm taking the emission above 570 nm; thereafter, enhanced GFP (eGFP) was imaged with 488 nm and emission above 510 nm. This process avoided crosstalk between channels.
Statistical Analysis of Results.
Student's t test was used to analyze differences between cell counts between GFP- and GFP-xR11-expressing tadpoles.
Results and Discussions
Xenopus xR11, the Homolgoue of Mammalian Bcl-XL, Counteracts Bax-Induced Apoptosis in Tadpole Muscle and Brain.
A crucial first step was to establish in vivo the efficiency of the constructs from which GFP and GFP-xR11 were expressed from either a cytomegalovirus (CMV), a muscle-specific (cardiac β-actin, pCAR), or a pan-neural promoter (Nβt). Tissue specificity of the promoter and functionality of the fusion protein GFP-xR11 in counteracting bax-induced apoptosis were validated by somatic gene transfer into brain (25) and muscle (24) of Xenopus tadpoles.
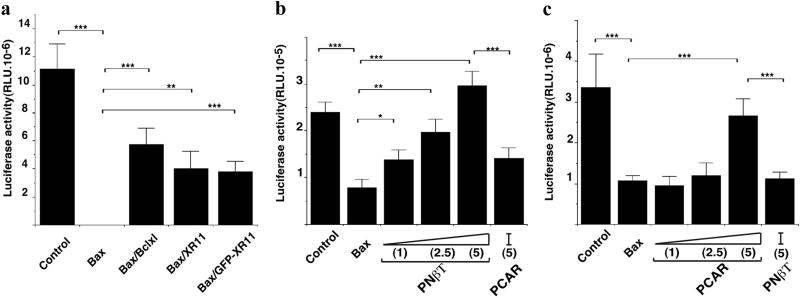
Bax-induced apoptosis was quantified by coinjecting pcDNA3-LUC, which expresses luciferase constitutively (Fig. 1). Previously, we have shown by using the terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling technique that decrease of luciferase activity reflects the degree of apoptosis induced by Bax overexpression in muscle (21). Coinjection of the same amount of either a vector expressing the chicken Bcl-XL (CMV-Bcl-XL), Xenopus xR11 (pcDNA3-xR11), or GFP-xR11 (peGFP-xR11) significantly abrogates Bax-induced apoptosis (Fig. 1a). None of the proteins (Bcl-XL, xR11, or GFP-xR11) tested affected luciferase expression in the absence of Bax, whether expressed in the muscle by direct injection or in the brain by polyethyleneimine-based transfection (see Fig. 7, which is published as supplemental material on the PNAS web site, www.pnas.org).
Figure 1.
Validation of the antiapoptotic effect of xR11 and GFP-xR11 and the promoter specificity of constructs by somatic gene transfer. (a) Cell survival was measured by the luciferase activity (RLU) in muscle extracts 48 h after transfection of 1 μg pcDNA3-LUC into the dorsal muscle of stage 56 tadpoles. The following plasmids were coinjected with pcDNA3-LUC (from left to right): 2 μg pcDNA3 (empty vector, control), 1 μg pcDNA3-Bax, 1 μg pcDNA3-Bax with 1 μg CMV-Bcl-XL, 1 μg pcDNA3-Bax with 1 μg pcDNA3-xR11, 1 μg pcDNA3-Bax with 1 μg peGFP-xR11. All cDNA were CMV-driven. The total amount of DNA injected was brought up to 3 μg by adding control pcDNA3 where necessary. Note that GFP-xR11 is as potent as xR11 and Bcl-XL in counteracting the apoptotic effects of human BAX. (b) Cell survival was measured with tissue-specific promoters in brain extracts 48 h after transfection. The total DNA used was made up to 450 ng, using control vector and complexed with polyethyleneimine. In addition to 150 ng pcDNA3-LUC (control, far left), the following plasmids were coinjected (from left to right): 50 ng pcDNA3-Bax, 50 ng pcDNA3-Bax with 50 ng Nβt-GFP-xR11, 50 ng pcDNA3-Bax with 2.5× Nβt-GFP-xR11, 50 ng pcDNA3-Bax with 5× excess Nβt-GFP-xR11, 50 ng pcDNA3-Bax with 5× excess pCAR-GFP-xR11. Note that in the brain, Bax-dependent apoptosis is only blocked when GFP-xR11 is expressed from the Nβt, but not the pCAR promoter. (c) Cell survival was measured with tissue-specific promoters in muscle extracts 48 h after transfection. Total DNA transfected (as free DNA) was brought up to 2.8 μg, using control vector where necessary. In addition to 1 μg pcDNA3-LUC (control, far left), the following plasmids were coinjected (from left to right): 300 ng pcDNA3-Bax, 300 ng pcDNA3-Bax with 300 ng pCAR-GFP-xR11, 300 ng pcDNA3-Bax with 2.5× excess pCAR-GFP-xR11, 300 ng of pcDNA3-Bax with 5× excess pCAR-GFP-xR11, 300 ng pcDNA3-Bax with 5× excess Nβt-GFP-xR11. Note that in muscle, sufficient GFP-xR11 is expressed from the pCAR, but not the Nβt promoter, to block Bax-dependent apoptosis. Means ± SEM are given; n > 9 in each group. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
In the experiment shown in Fig. 1a, all cDNA were under the transcriptional control of the constitutive CMV promoter. Under these conditions, xR11, GFP-xR11, and mammalian Bcl-XL were equipotent in counteracting Bax-induced apoptosis. When tissue-specific promoters were used, the antiapoptotic effect of GFP-xR11 was both tissue-specific and dose-dependent (Fig. 1 b and c). Fig. 1b shows that cotransfecting increasing quantities of Nβt-GFP-xR11 into the brain gradually reversed the effect of Bax. In contrast, in the brain, 5 μg of the same coding sequence expressed from the muscle-specific promoter was without effect on Bax-induced apoptosis (Fig. 1b). Conversely, in tail muscle (Fig. 1c), cotransfecting increasing quantities of pCAR-GFP-xR11 reversed the effect of Bax, whereas injecting 5 μg of Nβt-GFP-xR11 was without effect in muscle.
GFP and GFP-xR11 Expression in Transgenic Xenopus.
Germinal transgenic animals were obtained by the procedure of Kroll and Amaya (26) using the following plasmids: peGFP-1, peGFP-xR11, Nβt-GFP, and Nβt-GFP-xR11. About 30% of the transgenic Xenopus tadpoles expressed GFP at high levels. Expression was uniform and intense with GFP expressed from the CMV promoter (data not shown). When using the Nβt promoter, both GFP and GFP-xR11 were specifically expressed in the nervous system (Fig. 2). Transgenic animals were fertile and a F1 generation uniformly expressed GFP-xR11 in neuronal tissue. We used broods of F1 generations expressing GFP or GFP-xR11 for quantification, as we have found that transgene expression is more uniform in F1 than in the first generation.
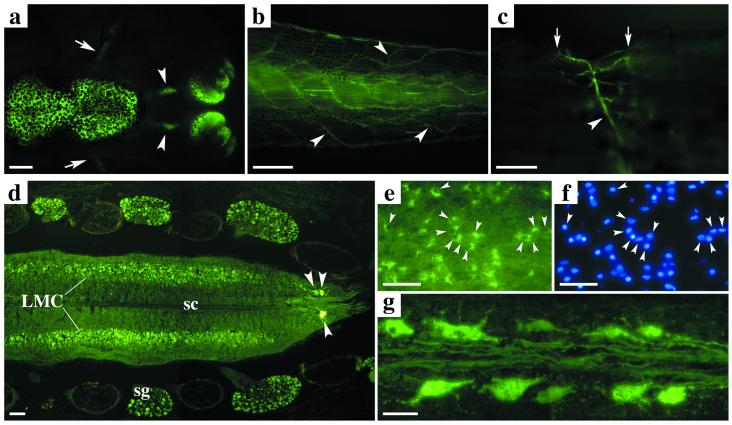
Figure 2.
GFP and GFP-xR11 expression from the Nβt promoter in transgenic Xenopus is limited to the nervous system. (a–c) In toto GFP expression from a Nβt-GFP stage 60 tadpole: (a) brain, olfactory nerves (arrowheads), olfactory bulbs, and optic nerves (arrows); (b) spinal cord and nerves (arrowheads) innervating the tail; (c) neuromuscular junctions (arrows) and axons (arrowhead). (d–g) Longitudinal cryostat sections (20 μm) of spinal cord from a Nβt-GFP-xR11 animal at climax (stage 61): (d) spinal ganglia, interneurons, primary motoneurons (arrowheads), and lateral columns of secondary motoneurons (LMC); (e and f) all interneurons express GFP (compare GFP in e with Hoechst labeling in f); (g) spinal cord primary motoneurons and their axons. LMC: lateral motor column; sg: spinal ganglion; sc: spinal cord. [Scale bars = 1 mm (a and b), 100 μm (c and d), and 50 μm (e-g).]
Expression of GFP-xR11 was stable throughout metamorphosis and in adults. There were no noticeable outward morphological differences between controls (GFP) and those expressing GFP-xR11. GFP expression was strong enough to permit in toto observation (Fig. 2a) of the global metamorphic changes occurring in the nervous system, such as shortening of the olfactory nerves, as well as details of axons and dendrite ramification (Fig. 2 b and c). Neuronal specificity of the Nβt promoter was confirmed on cryostat sections (Fig. 2 d-g), GFP expression being found in all of the major neuronal types in the nervous system. An overall view of tail (Fig. 2d) shows GFP expression throughout the spinal cord. In particular, motoneurons and spinal ganglia neurons are GFP-positive (Fig. 2 d and g), with no signal in muscle or cartilage. Colabeling with a nuclear marker (Fig. 2 e and f) showed that the totality of neurons is GFP-positive.
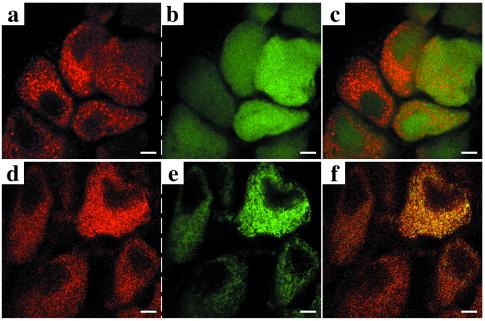
Confocal microscopy showed no specific subcellular localization for GFP alone (Fig. 3 a–c). The GFP signal was diffuse, and most importantly, did not colocalize with mitochondria as revealed by labeling with a specific antimitochondrial antibody against cytochrome c (Fig. 3 a–c). In contrast, the GFP-xR11 signal coincided with the distinct mitochondrial distribution, as seen in spinal ganglia neurons (Fig. 3 d–f) and all other neurons examined (data not shown). This mitochondrial colocalization is important given that the major components of the apoptotic machinery of the cell are found in this organelle (3, 4).
Figure 3.
GFP-xR11, but not GFP alone, is localized in mitochondria. (a–f) Confocal analysis on transversal cryostat sections (30 μm) with a Cy3-coupled anti-cytochrome c (mitochondrial-specific marker) shows a punctuate pattern (a and d) colocalizing with GFP-xR11 (e), but not with GFP alone (b), which gives a homogenous, diffuse signal. Superposition of Cy3 and GFP signal (c) or GFP-xR11 signal (f) is shown in spinal ganglion neurons from a postmetamorphic (stage 66) animal. [Scale bars = 5 μm.]
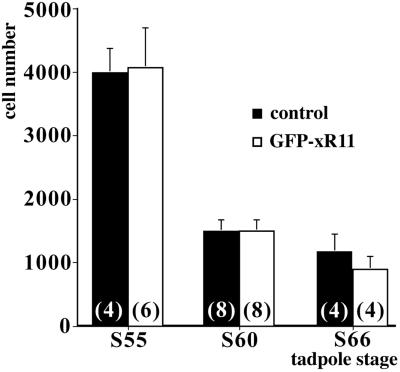
Counts of lumbar lateral motor column neurons in GFP- and GFP-xR11-expressing animals at stages 55, 60, and 66 were carried out. This population of neurons was chosen because it has been studied repeatedly, and the stages at which cell death occur (stages 55–66) are well documented (30). No difference was observed in the numbers of motoneurons in GFP-xR11- versus GFP-expressing tadpoles at any stage up to the end of metamorphosis (Fig. 4). Our counts on control animals corroborate previously published data from other groups (30) and provide evidence that GFP expression per se does not affect neuronal survival. Furthermore, the data comparing neuronal numbers in GFP- and GFP-xR11-expressing tadpoles show that there is no effect of xR11 expression on neuronal fate of this population at any stage.
Figure 4.
GFP-xR11 expression does not abrogate motoneuron loss in the lumbar lateral motor column (L-LMC) during metamorphosis. Motoneurons were counted in the left and right L-LMC in animals expressing GFP or GFP-xR11 at stages (S) 55, 60, and 66. Means ± SD are shown (the n for each group is indicated at the base of the histogram). No differences between GFP or GFP-xR11 animals were seen at any stage (P > 0.05 for each comparison).
In contrast, there were distinct modifications in morphology and survival of RB and M neurons. As regards the M neurons, during prometamorphosis (stage 55), GFP expression levels and the general morphology of M neurons from GFP-xR11 tadpoles were not different from those expressing GFP alone (Fig. 8, which is published as supplemental material). However, as metamorphosis proceeded (stages 61–66) the GFP M neuron underwent morphological changes whereas the GFP-xR11 M neuron did not (Fig. 5). At stage 61 (metamorphic climax), the GFP M neuron showed a distended cytoplasm with numerous small vacuoles and plasma membrane irregularities (Fig. 5c). In contrast, the GFP-xR11 M neuron at the same stage had a compact structure with a marked punctuate GFP-xR11 staining of the cytoplasm (Fig. 5a), reflecting mitochondrial localization of the overexpressed protein. At stage 66, i.e., at the end of metamorphosis, these differences became even more pronounced (Fig. 5 b and d) and were maintained in 2-month-old froglets (Fig. 8). In the case of GFP expression, the cytoplasm was reduced and the nucleus was smaller as compared with those in GFP-xR11 animals (Fig. 8). The changes seen in GFP neurons are reminiscent of apoptotic processes and their absence in GFP-xR11 M neurons suggests that these transient changes implicate pathways dependent on the Bcl-2 family of proteins. However, in neither case does their activation lead to cell death, as M neurons in GFP controls and wild-type animals survived through metamorphosis and were present in adults (data not shown and ref. 31). Thus, in wild-type tadpoles, the M neurons appear to enter an apoptotic process that is then stopped. This effect could be due to activation of caspases that may be involved in the late differentiation process. Indeed, in other systems caspase activation has been shown to be associated not with death but with differentiation (32).
Figure 5.
M neuron morphology and fate are modified by expression of GFP-xR11. M neurons from GFP-xR11 (a and b) or GFP (c and d) animals at stages 61 (a and c) and 66 (b and d). Transversal sections (30 μm) of hindbrain are shown with arrows indicating each M cell nucleus. At metamorphic climax (stage 61) the GFP-xR11 M (a) has a regular appearance whereas the GFP M (c) has a spreading cytoplasm with small vacuoles and an irregular membrane. On completion of metamorphosis (stage 66) these differences are more pronounced. Large vacuoles appear in GFP Ms (d) whereas GFP-xR11 Ms (b) are as at stage 61. A typical neuron from each transgenic series is shown. n = 3 for each stage analyzed. (Scale bars = 50 μm.)
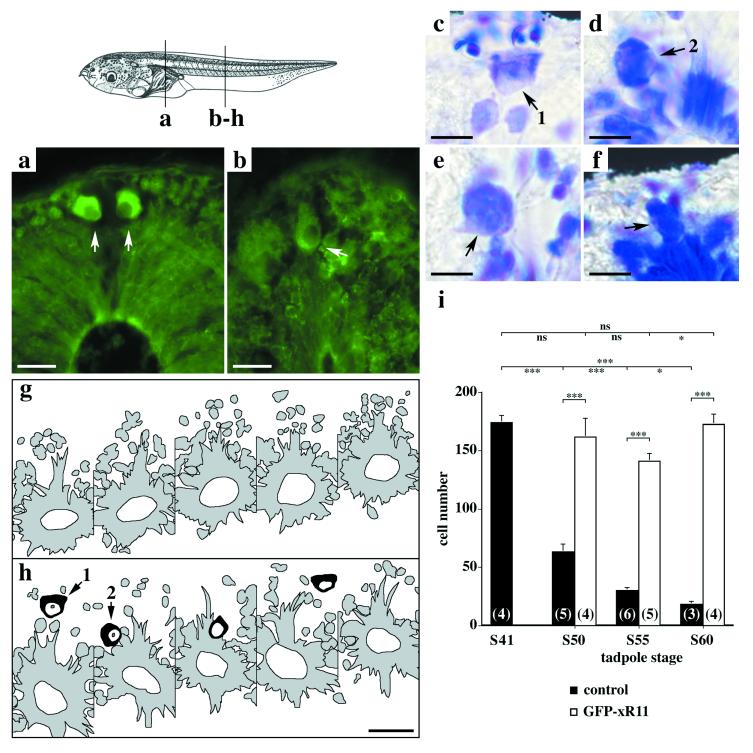
The most striking phenotype was the extended survival of RB neurons. Normally, in very early Xenopus tadpoles (between stages 41 and 46) about 200 RB neurons are found (ref. 33 and Fig. 6i), then over 70% die between stages 50 and 55 at the onset of metamorphosis (ref. 33 and Fig. 6i), when this primary afferent system is replaced by the dorsal root ganglia (23). In tadpoles expressing GFP-xR11, numerous RB neurons were present up to stage 60 just before metamorphic climax (Fig. 6 a–e and h). Comparing successive sections from control or GFP-xR11 tadpoles showed most of the sections from the GFP-xR11 group to possess at least one RB neuron whereas controls did not (Fig. 6 g and h). Some were even found as late as stage 63, the climax of metamorphosis, just before complete regression of the tail (Fig. 6f). Quantification of their distribution (Fig. 6i) showed significantly higher numbers of RB neurons in GFP-xR11-expressing tadpoles than in controls between stages 50 and 60. These results clearly demonstrate that the protective effects of xR11 are cell-specific and that neuronal degeneration is not simply a result of general degenerative changes that are rapidly occurring in the muscle and extracellular matrix surrounding the neurons during metamorphosis. This finding corroborates the hypothesis, suggested by Berry et al. (34), that cell-specific programs govern apoptosis during metamorphosis.
Figure 6.
GFP-xR11 expression significantly increases the number of RB neurons surviving metamorphic climax. (Top Left) Schema of stage 50 tadpole with positions of sections a–h. (a–f) GFP-xR11 expression (a and b) and histology (c–f) show RB cells at different stages (arrows point toward nucleus). (a and b) RB cells from a GFP-xR11 stage 50 tadpole were distinguished by abundant cytoplasm, large nucleus, and position (top of dorsal spinal cord, arrows in a, and top caudal spinal cord, arrow in b). Note punctuate GFP-xR11 distribution, particularly visible in b. (c–f) Histology of RB neurons in caudal region of a GFP-xR11 stage 60 tadpole. (g and h) Schema of serial sections from a GFP stage 60 tadpole (g) showing no RB cells and from a GFP-xR11 stage 60 tadpole (h) showing RB cells (in black) present on all but one section. (i) Quantification of RB cells in GFP and GFP-xR11 tadpoles at premetamorphosis (stage 50), prometamorphosis (stage 55), and metamorphic climax (stage 60). Numbers in parenthesis indicate number of tadpoles examined. [Scale bars = 25 μm (a, b, g, and h) and 10 μm (c–f).] Means ± SEM. ***, P < 0.001; **, P < 0.01; *, P < 0.05; ns: P > 0.05. Number of sections counted varied according to stage and size of animal, from 250 per animal at stage 41 to between 600 and 700 sections per animal at stage 60.
Current thinking holds that two main pathways can lead to cell death: the death receptor pathway and the mitochondrial route (11). The first is activated at the membrane by binding of ligand to a member of the Fas/CD95 family of receptors and initiation of a caspase cascade. The second is triggered at the level of the mitochondria by recrutement of proapoptotic members of the bcl-2 family (3, 4), leading to cytochrome c release and activation of “executioner” caspases. Crosstalk can occur between the two pathways as activation of caspase 8 by death receptors may in some cells lead to oligomerization of Bax and release of cytochrome c (35). Such crosstalk has led to the proposal that two broad types of cells exist: type I, in which expression of Bcl-2 or Bcl-XL does not block death receptor-initiated apoptosis, and type II in which it does (36). Our data on the overexpression of xR11 provides in vivo evidence for the existence of at least two routes leading to neuronal death. First, the kinetics of death of certain neurons, including spinal cord motoneurons and interneurons, are not modified by increased amounts of xR11. This finding suggests that apoptosis in these cells does not involve induction of the mitochondrial pathway and that it proceeds via the death receptor route. In contrast, in RB neurons, overexpression of a Bcl-2 homologue delays cell death right up to the final point of tail regression, indicating that the mitochondrial route is central to induction of apoptosis of these neurons, whether initiated at the mitochondria itself or at the cell membrane. Finally, the M neuron undergoes morphological changes that bear apoptotic features although the process normally is aborted. However, xR11 expression can block the morphological changes, showing again the importance of the mitochondrial route in this program. These interpretations fit with the developmental physiology of these neurons, which is correlated with a high mitochondrial density in both cell types (22, 37).
Finally, this in vivo approach shows that the use of a tissue-specific fluorescent fusion protein in combination with the recently established technique of germinal transgenesis in an amphibian can further enhance the usefulness of metamorphosis for analyzing postembryonic processes. Our present findings also suggest approaches to obtain further insights into developmentally programmed cell death. For example, inducing metamorphosis precociously with exogenous 3,5,3′-triiodothyronine at different developmental stages of Xenopus larvae would allow one to answer the question as to whether or not such apoptosis is determined by relatively narrow time windows during ontogenesis.
Supplementary Material
Acknowledgments
We thank A. de Luze, L. du Pasquier, and G. Levi for advice, O. Randon for production of transgenics, P. Petit for mitochondrial antibody, J. R. Prat for help with confocal microscopy and E. Amaya and O. Bronchain for advice on transgenic techniques. This work was supported by Rhone Poulenc.
Abbreviations
- GFP
green fluorescent protein
- eGFP
enhanced GFP
- M
Mauthner
- RB
Rohon-Beard
- Nβt
neuronal β-tubuline
- CMV
cytomegalovirus
References
- 1.Raff M C, Barres B A, Burne J F, Coles H S, Ishizaki Y, Jacobson M D. Science. 1993;262:695–700. doi: 10.1126/science.8235590. [DOI] [PubMed] [Google Scholar]
- 2.Kerr J F, Harmon B, Searle J. J Cell Sci. 1974;14:571–585. doi: 10.1242/jcs.14.3.571. [DOI] [PubMed] [Google Scholar]
- 3.Gross A, McDonnell J M, Korsmeyer S J. Genes Dev. 1999;13:1899–1911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- 4.Loeffler M, Kroemer G. Exp Cell Res. 2000;256:19–26. doi: 10.1006/excr.2000.4833. [DOI] [PubMed] [Google Scholar]
- 5.Hengartner M O. Nature (London) 2000;407:770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 6.Yin X M, Oltvai Z N, Korsmeyer S J. Nature (London) 1994;369:321–323. doi: 10.1038/369321a0. [DOI] [PubMed] [Google Scholar]
- 7.Conus S, Kaufmann T, Fellay I, Otter I, Rosse T, Borner C. EMBO J. 2000;19:1534–1544. doi: 10.1093/emboj/19.7.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oltvai Z N, Milliman C L, Korsmeyer S J. Cell. 1993;74:609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- 9.Boise L H, Gonzalez-Garcia M, Postema C E, Ding L, Lindsten T, Turka L A, Mao X, Nunez G, Thompson C B. Cell. 1993;74:597–608. doi: 10.1016/0092-8674(93)90508-n. [DOI] [PubMed] [Google Scholar]
- 10.Zou H, Henzel W J, Liu X, Lutschg A, Wang X. Cell. 1997;90:405–413. doi: 10.1016/s0092-8674(00)80501-2. [DOI] [PubMed] [Google Scholar]
- 11.Green D R. Cell. 2000;102:1–4. doi: 10.1016/s0092-8674(00)00003-9. [DOI] [PubMed] [Google Scholar]
- 12.Susin S A, Lorenzo H K, Zamzami N, Marzo I, Snow B E, Brothers G M, Mangion J, Jacotot E, Costantini P, Loeffler M, et al. Nature (London) 1999;397:441–446. doi: 10.1038/17135. [DOI] [PubMed] [Google Scholar]
- 13.Minn A J, Kettlun C S, Liang H, Kelekar A, Vander Heiden M G, Chang B S, Fesik S W, Fill M, Thompson C B. EMBO J. 1999;18:632–643. doi: 10.1093/emboj/18.3.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinou J C, Dubois-Dauphin M, Staple J K, Rodriguez I, Frankowski H, Missotten M, Albertini P, Talabot D, Catsicas S, Pietra C, et al. Neuron. 1994;13:1017–1030. doi: 10.1016/0896-6273(94)90266-6. [DOI] [PubMed] [Google Scholar]
- 15.Farlie P G, Dringen R, Rees S M, Kannourakis G, Bernard O. Proc Natl Acad Sci USA. 1995;92:4397–4401. doi: 10.1073/pnas.92.10.4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Motoyama N, Wang F, Roth K A, Sawa H, Nakayama K, Negishi I, Senju S, Zhang Q, Fujii S, et al. Science. 1995;267:1506–1510. doi: 10.1126/science.7878471. [DOI] [PubMed] [Google Scholar]
- 17.Michaelidis T M, Sendtner M, Cooper J D, Airaksinen M S, Holtmann B, Meyer M, Thoenen H. Neuron. 1996;17:75–89. doi: 10.1016/s0896-6273(00)80282-2. [DOI] [PubMed] [Google Scholar]
- 18.Parsadanian A S, Cheng Y, Keller-Peck C R, Holtzman D M, Snider W D. J Neurosci. 1998;18:1009–1019. doi: 10.1523/JNEUROSCI.18-03-01009.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tata J R. Biochem Soc Symp. 1996;62:123–136. [PubMed] [Google Scholar]
- 20.Cruz-Reyes J, Tata J R. Gene. 1995;158:171–179. doi: 10.1016/0378-1119(95)00159-4. [DOI] [PubMed] [Google Scholar]
- 21.Sachs L M, Abdallah B, Hassan A, Levi G, de Luze A, Reed J C, Demeneix B A. FASEB J. 1997;11:801–808. doi: 10.1096/fasebj.11.10.9271365. [DOI] [PubMed] [Google Scholar]
- 22.Moulton J M, Jurand A, Fox H. J Embryol Exp Morphol. 1968;19:415–431. [PubMed] [Google Scholar]
- 23.Hughes A F W. J Anat. 1957;91:323–338. [PMC free article] [PubMed] [Google Scholar]
- 24.de Luze A, Sachs L, Demeneix B A. Proc Natl Acad Sci USA. 1993;90:7322–7326. doi: 10.1073/pnas.90.15.7322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ouatas T, Le Mevel S, Demeneix B A, de Luze A. Int J Dev Biol. 1998;42:1159–1164. [PubMed] [Google Scholar]
- 26.Kroll K L, Amaya E. Development (Cambridge, UK) 1996;122:3173–3183. doi: 10.1242/dev.122.10.3173. [DOI] [PubMed] [Google Scholar]
- 27.Huang H, Brown D D. Proc Natl Acad Sci USA. 2000;97:190–194. doi: 10.1073/pnas.97.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nieuwkoop P D, Faber J. Normal Table of Xenopus laevis (Daudin): A Systematical and Chronological Survey of the Development from the Fertilized Egg Till the End of Metamorphosis. Amsterdam: Elsevier/North–Holland; 1956. [Google Scholar]
- 29.Coen L, Kissa K, Le Mevel S, Brûlet P, Demeneix B A. Int J Dev Biol. 1999;43:823–830. [PubMed] [Google Scholar]
- 30.Sperry D G. J Comp Neurol. 1987;264:250–267. doi: 10.1002/cne.902640209. [DOI] [PubMed] [Google Scholar]
- 31.Will U. J Comp Neurol. 1986;244:111–120. doi: 10.1002/cne.902440109. [DOI] [PubMed] [Google Scholar]
- 32.Zheng T S, Flavell R A. Exp Cell Res. 2000;256:67–73. doi: 10.1006/excr.2000.4841. [DOI] [PubMed] [Google Scholar]
- 33.Lamborghini J E. J Comp Neurol. 1987;264:47–55. doi: 10.1002/cne.902640105. [DOI] [PubMed] [Google Scholar]
- 34.Berry D L, Schwartzman R A, Brown D D. Dev Biol. 1998;203:12–23. doi: 10.1006/dbio.1998.8974. [DOI] [PubMed] [Google Scholar]
- 35.Kroemer G, Reed J C. Nat Med. 2000;6:513–519. doi: 10.1038/74994. [DOI] [PubMed] [Google Scholar]
- 36.Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli K J, Debatin K M, Krammer P H, Peter M E. EMBO J. 1998;17:1675–1687. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roberts A, Hayes B P. Proc R Soc London Ser B. 1977;196:415–429. doi: 10.1098/rspb.1977.0048. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.