Abstract
In Arabidopsis thaliana the CPC-like MYB transcription factors [CAPRICE (CPC), TRIPTYCHON (TRY), ENHANCER OF TRY AND CPC 1, 2, 3/CPC-LIKE MYB 3 (ETC1, ETC2, ETC3/CPL3), TRICHOMELESS 1, 2/CPC-LIKE MYB 4 (TCL1, TCL2/CPL4)] and the bHLH transcription factors [GLABRA3 (GL3) and ENHANCER OF GLABRA 3 (EGL3)] are central regulators of trichome and root-hair development. We identified TRY and GL3 homologous genes from the tomato genome and named them SlTRY and SlGL3, respectively. Phylogenic analyses revealed a close relationship between the tomato and Arabidopsis genes. Real-time reverse transcription PCR analyses showed that SlTRY and SlGL3 were predominantly expressed in aerial parts of developing tomato. After transformation into Arabidopsis, CPC::SlTRY inhibited trichome formation and enhanced root-hair differentiation by strongly repressing GL2 expression. On the other hand, GL3::SlGL3 transformation did not show any obvious effect on trichome or non-hair cell differentiation. These results suggest that tomato and Arabidopsis partially use similar transcription factors for epidermal cell differentiation, and that a CPC-like R3 MYB may be a key common regulator of plant trichome and root-hair development.
Introduction
Epidermal cell differentiation, including trichome and root-hair formation, in Arabidopsis thaliana is a popular model system for studying cell fate determination. Several regulatory factors are known to be involved in this event. The CAPRICE (CPC) gene encodes an R3 type MYB transcription factor that has been identified as a key regulator of root-hair differentiation [1]. Arabidopsis has several additional CPC-like MYB genes in its genome, including TRYPTICHON (TRY), ENHANCER OF TRY AND CPC1 and 2 (ETC1 and ETC2), ENHANCER OF TRY AND CPC3/CPC-LIKE MYB3 (ETC3/CPL3), and TRICHOMELESS1 and 2/CPC-LIKE MYB4 (TCL1 and TCL2/CPL4) [2]–[10]. The TRY protein has a regulatory role mainly in trichome differentiation [2], [11]. ETC1 and ETC2 enhance the functions of CPC and TRY [3]–[5]. TCL1 and TCL2/CPL4 negatively regulate trichome formation on the inflorescence stems and pedicels [8]–[10].
The GLABRA3 (GL3) gene encodes a bHLH transcription factor that is also involved in trichome and root-hair differentiation in Arabidopsis [12]. A GL3 homologous gene, ENHANCER OF GLABRA3 (EGL3), functions in a redundant manner with GL3 in Arabidopsis [13]. The GLABRA2 (GL2) gene, which encodes a homeodomain leucine zipper protein, is thought to act farthest downstream in the epidermal cell fate regulatory pathway in Arabidopsis [1], [14]–[17]. Transcription of GL2 is controlled by a protein complex that includes the WEREWOLF (WER), GL3/EGL3 and TRANSPARENT TESTA GLABRA1 (TTG1) proteins [18]. WER encodes an R2R3 type MYB transcription factor and promotes the differentiation of Arabidopsis root epidermal cells into non-hair cells [16]. The TTG1 gene, which encodes a WD-40 protein, is also required for the formation of non-hair cells [14]. Two bHLH proteins, GL3 and EGL3, interact with WER [13] and TTG1 [12], [19], [20]. The WER homologous gene GLABRA1 (GL1) is also thought to form a transcriptional complex with GL3/EGL3 and TTG1 to promote GL2 expression [12], [20]–[22]. The CPC and CPC-like MYB proteins interact with GL3/EGL3 and may serve as epidermal cell fate determinants [7].
Although both tomato and Arabidopsis have trichomes, tomato trichomes are distinct from Arabidopsis trichomes. Arabidopsis has non-glandular three-branched unicellular trichomes that form on stem and leaf surfaces to an extent that depends on the ecotype [23], [24]. On the other hand, tomato trichomes are highly diverse in morphology and chemistry [25]–[27]. Tomato trichomes are classified into types I–VII, with types I, IV, VI and VII being glandular, and types II, III and V being non-glandular [26], [28]. Glandular trichomes contain various sticky or toxic chemicals that may resist herbivores [27], whereas non-glandular trichomes may function in defense by physically limiting herbivores [29].
Trichome morphology and root-hair patterning are different in tomato and Arabidopsis. Arabidopsis root-hair cells are located over two underlying cortical cells, whereas non-hair cells are positioned over a single cortical cell [14], [30]. This position-dependent pattern results in rows of root-hair cells along the longitudinal root axis and has been found in Brassicaceae and other eudicot families [31]–[34]. This striped root-hair pattern (Type 3) is one of three types of root-hair cell distribution patterns [31]–[33], [35]–[38]. Tomato belongs to the Type 1 root-hair pattern group, in which all the root epidermal cells have the potential to produce root-hairs. This type of pattern appears to be the most widespread in plants [39].
In this study, we have identified Arabidopsis TRY and GL3 homologous genes from tomato. Transformants expressing the tomato TRY homologous gene (SlTRY) in Arabidopsis had no trichomes and a greater number of root-hairs, a phenotype similar to that seen in over-expressors of CPC-like MYB genes. On the other hand, transformants expressing the tomato GL3 homologous gene (SlGL3) in Arabidopsis had no obvious GL3-like effects on trichome and non-hair cell differentiation. We concluded that tomato and Arabidopsis use similar transcription factors for trichome and root-hair cell differentiation and that the SlTRY-like R3 MYB may be a key common regulator of plant trichome and root-hair development.
Materials and Methods
Plant Materials and Growth Conditions
Tomato, Solanum lycopersicum L. cv. Micro-Tom, was used. Seeds were surface-sterilized with 10% commercial bleach including a detergent (Kitchen Haiter, Kao, Tokyo, Japan), for 20 min and then rinsed with sterilized water three times for 5 min each and sown on 1.5% agar plates containing 0.5xMS medium [40]. Seeded plates were kept at 4°C for 2 d and then incubated at 25°C under constant white light (50–100 µmol m−2 s−1) for 7 days to produce seedlings for DNA and RNA extraction. Some 7-day-old seedlings were transplanted into soil and grown in a photoperiod of 16 h light at 25°C for 4 additional weeks to produce mature plant tissues for RNA extraction.
Arabidopsis thaliana ecotype Columbia (Col-0) and, cognate cpc-2 [41] and gl3-7454 [42] mutant plants were used. Seeds were surface-sterilized, sown on 1.5% agar plates as described previously [43] and propagated to observe seedling phenotypes. Seeded plates were kept at 4°C for 2 d and then incubated at 22°C under constant white light (50–100 µmol m−2 s−1). For each transgenic line, at least ten individual 5-day-old seedlings were assayed for root-hair number, and at least five individual 2-week-old third leaves were assayed for trichome number.
Gene Constructs
Primers
All primer sequences used in this paper are listed in Table 1.
Table 1. Primer sequences used in this study.
| Primer Name | Sequence (5′ to 3′) |
| RTSlTRY-F | 5′-CGATGTTGCAGCCAATGAAGA-3′ |
| RTSlTRY-R | 5′-TGTGCAAACCCATCACTGTGTC-3′ |
| RTSlGL3-F | 5′-AATGTTGGCCAAGGGTTACCAG-3′ |
| RTSlGL3-R | 5′-AAAGACTTTACTCTCGGCTTGGTGA-3′ |
| LeActin-F | 5′-TGTCCCTATTTACGAGGGTTATGC-3′ |
| LeActin-R | 5′-CAGTTAAATCACGACCAGCAAGAT-3′ |
| GL2-F | 5′-ATCGTCACACCACCGATCAGA-3′ |
| GL2-R | 5′-CCAGCCCTAGTTGCTTGCTCA-3′ |
| GFP-F | 5′-CAGTCCGCCCTGAGCAAAGAC-3′ |
| GFP-R | 5′-CCCTTGCTCACCATGGACTTGTA-3′ |
| Act2-F | 5′-CTGGATCGGTGGTTCCATTC-3′ |
| Act2-R | 5′-CCTGGACCTGCCTCATCATAC-3′ |
| SlTRY-F01 | 5′-TGAAACCGGTCTCGAGATGTGGTTAAGC-3′ |
| SlTRY-R01 | 5′-TTTGATCCATCGAACTAATCTGAAGACACG-3′ |
| SlTRY-F02 | 5′-GATTAGTTCGATGGATCAAAATCTCCATCAC-3′ |
| SlTRY-R02 | 5′-CGGCGGCTGTAGGTGGTAGACTTTTCTTAATTG-3′ |
| SlTRY-F03 | 5′-TCTACCACCTACAGCCGCCGCCGCCATGGTGAG -3′ |
| SlTRY-R03 | 5′-ACGAATTCGAGCTCGGTACCCGGGGATCCTC-3′ |
| SlGL3-F01 | 5′-GGGGGAACTCCTCGAGGCCAAAC-3′ |
| SlGL3-R01 | 5′-CCATAGCCATTGTTTCTTCATCCCTATATC-3′ |
| SlGL3-F02 | 5′-TGAAGAAACAATGGCTATGGGACACCAAG-3′ |
| SlGL3-R02 | 5′-GGTGGATGGGAGATTTCCATACTACTCTCTG-3′ |
| SlGL3-F03 | 5′-ATGGAAATCTCCCATCCACCATTTACGAACG-3′ |
| SlGL3-R03 | 5′-ACGAATTCGAGCTCGGTACC-3′ |
| SlTRY-P2 | 5′-CAAATGTTTGAACGATCTGC-3′ |
| SlTRY-P3 | 5′-GAATGAACTTGTTGGCCCTAC-3′ |
| SlTRY-P4 | 5′-CATAGAAGGGACATACTGGT-3′ |
| SlTRY-VP1 | 5′-GTATACAACAAATGTGCTTC-3′ |
| SlTRY-VP2 | 5′-GAGTTAGCTCACTCATTAGG-3′ |
| SlGL3-P1 | 5′-CTAATGGTATTCTAGTCAAC-3′ |
| SlGL3-P4 | 5′-CACTGACTGACCTACATATG-3′ |
| SlGL3-P5 | 5′-CACCTTGAACCCGTCTATTG-3′ |
| SlGL3-VP1 | 5′-CTATAGGGAGAATCAACGTC-3′ |
| SlGL3-VP2 | 5′-CAATTAATGTGAGTTAGCTC-3′ |
| SlGL3-F1 | 5′-TCAGGCGGGGGAAGTTAATG-3′ |
| SlGL3-F2 | 5′-AATCCTCTTTGCCTCACCAG-3′ |
| SlGL3-F3 | 5′-ATTAACTTTGGGACCACATT-3′ |
| SlGL3-R1 | 5′-AAATTACCCTTGGCCAACAT-3′ |
| SlGL3-R2 | 5′-TCCATTTGACATATTTTAGG-3′ |
| SlGL3-R3 | 5′-GTCATCAACTTCTGGTCTCC-3′ |
CPC::SlTRY Construct
A 1.0-kb PCR-amplified linear CPC promoter sequence (primers SlTRY-F01/R01) from the Arabidopsis genome, a 0.8-kb PCR-amplified linear SlTRY tomato genomic fragment (primers SlTRY-F02/R02) and a 1.8-kb PCR-amplified 2xGFP fragment [41] (primers SlTRY-F03/R03) using PrimeSTAR HS DNA Polymerase and TaKaRa LA Taq (Takara, Tokyo, Japan) were ligated into the XhoI and KpnI sites of pJHA212K binary vector [44] using an In-Fusion HD Cloning Kit (Takara, Tokyo, Japan) to create CPC::SlTRY. PCR-generated constructs were completely sequenced following isolation of the clones to check for amplification-induced errors. The plasmid of CPC::SlTRY was sequenced using the SlTRY-P2, -P3, -P4, -F02, -F03, -VP1 and -VP2 primers.
GL3::SlGL3 Construct
A 1.3-kb PCR-amplified linear GL3 promoter sequence (primers SlGL3-F01/R01) from the Arabidopsis genome, a 4.3-kb PCR-amplified linear SlGL3 tomato genomic fragment (primers SlGL3-F02/R02) and a 1.0-kb PCR-amplified GFP fragment [41] (primers SlGL3-F03/R03) using PrimeSTAR HS DNA Polymerase and PrimeSTAR GXL DNA Polymerase (Takara, Tokyo, Japan) were ligated into the XhoI and KpnI sites of pJHA212K binary vector [44] using an In-Fusion HD Cloning Kit (Takara, Tokyo, Japan) to create CPC::SlGL3. PCR-generated constructs were completely sequenced following isolation of the clones to check for amplification-induced errors. The plasmid of CPC::SlGL3 was sequenced using the SlGL3-P1, -P4, -P5, -F1, -F2, -F3, -F03, -R1, -R2, -R3, -VP1 and -VP2 primers.
Transgenic Plants
Gene constructs were introduced into Agrobacterium tumefaciens C58C1. Arabidopsis plants (wild-type Col-0, cpc-2, and gl3-7454) were transformed by the floral dipping method [45] and screened on 0.8% agar plates containing diluted (50% v/v) Murashige and Skoog medium and 50 mg/L (for Col-0, and gl3-7454 background) or 100 mg/L (for cpc-2 background) kanamycin sulfate. Homozygous transgenic lines were selected based on kanamycin resistance. We isolated at least twenty T1 lines for each construct and selected at least ten T2 and five T3 lines on the basis of their segregation ratios for kanamycin resistance.
Real-time Reverse Transcription PCR Analysis
Total RNA from tomato or Arabidopsis tissues was extracted with MagDEA RNA 100 (GC) (PSS, Chiba, Japan) using a Magtration System 12 GC (PSS, Chiba, Japan). To remove contaminating genomic DNA, RNA samples were treated with DNase I (Ambion, Austin, TX, USA) according to the Magtraction System protocol. Plant tissue (100 mg) was homogenized using a TissueLyser II (Qiagen, Valencia, CA, USA) with 100 µl of RLT buffer (Qiagen, Valencia, CA, USA). Sample supernatants were applied to the instrument, and RNA was eluted with 50 ml of sterile distilled water.
First-strand cDNA was synthesized from 1 µg total RNA in a 20 µl reaction mixture using the Prime Script RT Master Mix (Perfect Real Time) (Takara, Tokyo, Japan). Real-time PCR was performed using a Chromo4 Real-Time IQ5 PCR Detection System (Bio-Rad, Hercules, CA, USA) with SYBR Premix Ex Taq II (Takara, Tokyo, Japan). PCR amplification employed a 30 s denaturing step at 95°C, followed by 5 s at 95°C and 30 s at 60°C with 40 cycles for SlTRY, SlGL3, LeActin, GL2, GFP and ACT2. Real-time PCR was used to analyze the mRNA expression level of each transcript encoding SlTRY and SlGL3 in tomato, and GL2 and GFP in Arabidopsis transformants. The relative expression of each transcript was calculated by the ΔΔCT method [46]. The expression levels of SlTRY and SlGL3 were estimated after being normalized to the endogenous control gene LeActin (TC116322). The expression levels of GL2 and GFP were estimated after being normalized to the endogenous control gene ACT2 (AB026654). The primers were: RTSlTRY-F and RTSlTRY-R for SlTRY; RTSlGL3-F and RTSlGL3-R for SlGL3; LeActin-F and LeActin-R for LeActin [47]; GL2-F and GL2-R for GL2 [48]; GFP-F and GFP-R for GFP; and Act2-F and Act2-R for ACT2 [49].
Light Microscopy
To observe trichomes, images were recorded with a VC4500 3D digital fine microscope (Omron, Kyoto, Japan) or a digital microscope (VH-8000; Keyence, Osaka, Japan). At least five 2-week-old third true leaves were analyzed for trichome number for each transgenic line. Root phenotypes were observed using an Olympus Previs AX70 microscope and an Olympus SZH binocular microscope. For each transgenic line, at least ten individual 5-day-old seedlings were analyzed for root-hair number.
Results
Identification of the SlTRY and SlGL3 Genes
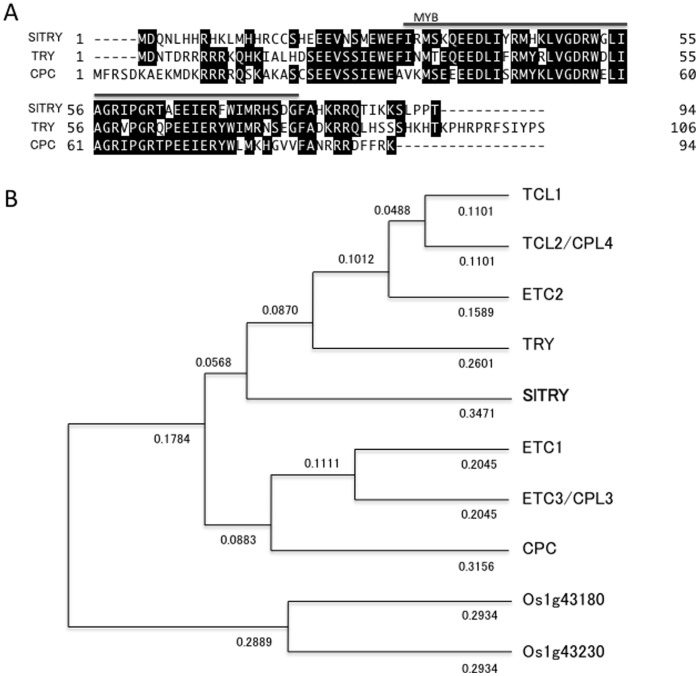
To find transcription factors regulating trichome and root-hair differentiation of tomato epidermis, we searched a tomato genome database (http://solgenomics.net/). We identified tomato homologs of the Arabidopsis CPC and GL3 genes and named them SlTRY (Solyc01g095640.1.1) and SlGL3 (Solyc08g081140.2.1), respectively. Members of the CPC family encode R3 type MYB transcription factor proteins in Arabidopsis [1]–[10]. The SlTRY encoded protein is more closely related to TRY than CPC (Figure 1A). Alignment of the amino acid sequences showed that the full length SlTRY protein shares 53% amino acid identity with TRY, and 50% with CPC. The R3 MYB motif of SlTRY shares 73% amino acid identity with that of TRY, and 71% with that of CPC.
Figure 1. Amino acid sequence and phylogenic tree of CPC-like R3 MYB proteins.
(A) Sequence alignment of SlTRY (Solyc01g095640.1.1), TRY (AC007288) and CPC (FJ268773). Shaded letters indicate identical residues. R3 MYB domains are indicated by a line above the sequences. (B) Phylogenic tree based on deduced amino acid sequences of CPC-like R3 MYB proteins [SlTRY, TRY, CPC, ETC1 (NM100020), ETC2 (FJ972652), ETC3/CPL3 (AB264292), TCL1 (FJ972675), TCL2/CPL4 (FJ972681), Os1g43180 and Os1g43230] were aligned with a multiple alignment program (Genetyx ver. 16.0.2 software, Genetyx, Tokyo, Japan), and a dendrogram was created using clustering with the Unweighted Pair Group Method with Arithmetic Mean (UPGMA). Branch length indicates relative evolutionary distances. Numbers above branches are genetic distances based on 10,000 bootstrap replicates. Distances are shown as the p-distance.
To provide a framework for examining R3 MYB transcription factor evolution, we estimated the phylogeny of CPC-like R3 MYB transcription factor proteins from Arabidopsis (CPC, TRY, ETC1, ETC2, ETC3/CPL3, TCL1 and TCL2/CPL4), rice (Oryza sativa) (Os1g43180 and Os1g43230) and tomato (SlTRY) based on their deduced amino acid sequences (Figure 1B). SlTRY was more closely related to TRY than CPC, which belongs to a cluster that includes TCL1, TCL2/CPL4, ETC2, and TRY (Figure 1B). ETC1, ETC3/CPL3 and CPC belong to another cluster branching from the TRY subgroup. Consistent with previous reports, phylogenic analyses using the entire amino acid sequence showed that the CPC-like MYB family can be divided into two groups: TRY, ETC2, TCL1 and TCL2/CPL4 in one group and CPC, ETC1 and ETC3 in the other [6]–[9]. As previously described, the two rice orthologs, Os01g43180 and Os01g43230, form a distinct clade from the Arabidopsis CPC-like R3 MYB family (Figure 1B) [7].
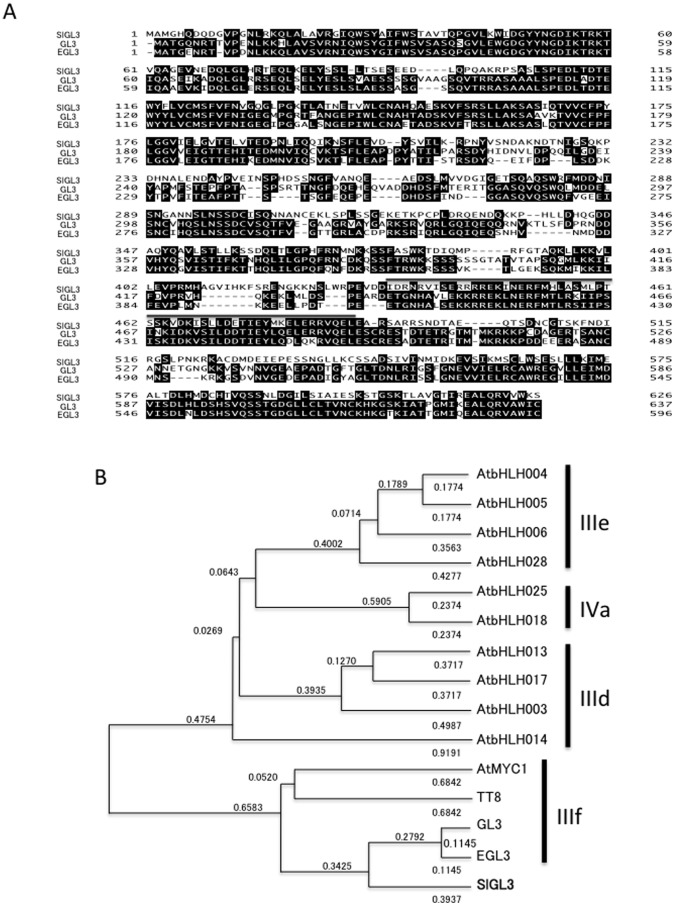
The SlGL3 encoded protein is closely related to the Arabidopsis bHLH transcription factor proteins encoded by GL3 and EGL3 (Figure 2A). Alignment of the amino acid sequences showed that the full length SlGL3 protein shares 45% amino acid identity with GL3, and 46% with EGL3. The bHLH motif of SlGL3 shares 46% amino acid identity with that of GL3, and 50% with that of EGL3. Based on the amino acid sequences of the bHLH regions, the Arabidopsis bHLH transcription factors are classified into 12 groups (I-XII) [50]. Group III contains 6 subgroups (IIIa-f), and GL3 and EGL3 belong to the IIIf subgroup [50].
Figure 2. Amino acid sequence and phylogenic tree of bHLH proteins.
(A) Sequence alignment of SlGL3 (Solyc08g081140.2.1), GL3 (AF246291) and EGL3 (NM20235). Shaded letters indicate identical residues. bHLH regions are indicated as line above the sequences. (B) Phylogenic tree based on deduced amino acid sequences of bHLH proteins [SlGL3, GL3, EGL3, TT8 (AJ277509), AtMYC1 (AF251697), AtbHLH003 (AF251688), AtbHLH004 (AF251689), AtbHLH005 (AF251690), AtbHLH006 (X99548), AtbHLH013 (AY120752), AtbHLH014 (AJ619812), AtbHLH017 (AY094399), AtbHLH018 (AF488562), AtbHLH025 (AF488567) and AtbHLH028 (AF252636)] aligned with a multiple alignment program (Genetyx ver. 16.0.2 software, Genetyx, Tokyo, Japan). The dendrogram was created using clustering with the Unweighted Pair Group Method with Arithmetic Mean (UPGMA). Branch length indicates relative evolutionary distances. Numbers above branches are genetic distances based on 10,000 bootstrap replicates. Distances are shown as the p-distance. Subdivision groups of Arabidopsis bHLH proteins (Group IIId, IIIe, IIIf and IVa) are shown to the right of the gene names.
To characterize SlGL3, we evaluated the phylogeny of bHLH transcription factor proteins (Figure 2B). Clustering in a phylogenic tree constructed from subgroups IIId (AtbHLH003, AtbHLH013, AtbHLH014 and AtbHLH017), IIIe (AtbHLH004, AtbHLH005, AtbHLH006 and AtbHLH028), IIIf (GL3, EGL3, TT8 and AtMYC1) and IVa (AtbHLH018, AtbHLH020 and AtbHLH025) was similar to the clustering in previously reported phylogenic trees [50]–[52] (Figure 2B). SlGL3 belongs to the IIIf subgroup and is more closely related to GL3 and EGL3 than AtMYC1 and TT8 (Figure 2B).
Expression Patterns of the SlTRY and SlGL3 Genes in Tomato
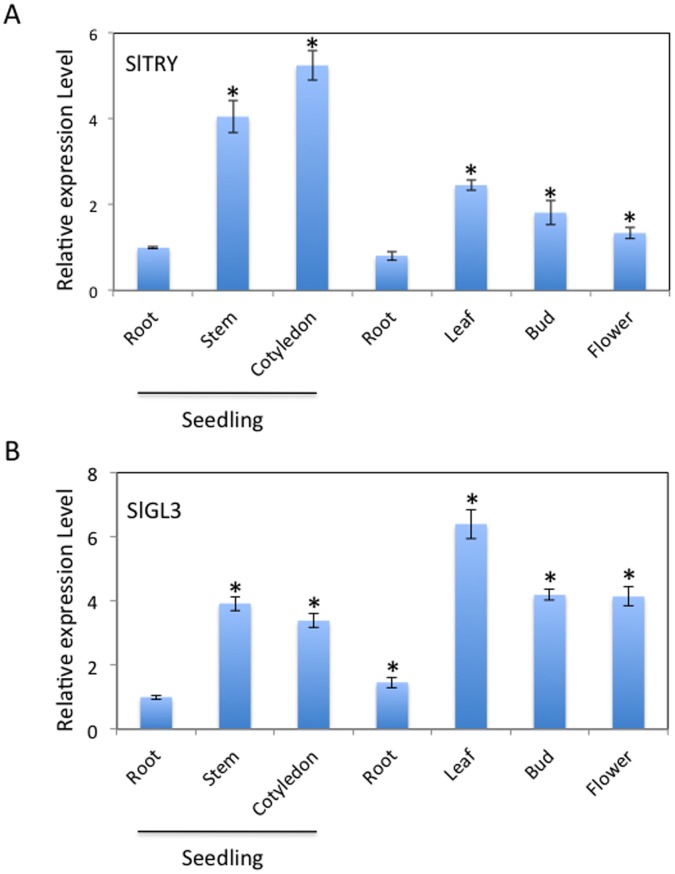
Expression of SlTRY and SlGL3 was examined in tomato tissues using real-time reverse transcription PCR. SlTRY was strongly expressed in stem and cotyledons of 7-day-old seedlings (Figure 3A). The relative expression level of SlTRY in cotyledon tissues was approximately 5 times greater than that in seedling root tissues (Figure 3A). The strongest expression of SlGL3 was observed in 5-week-old plant leaves (Figure 3B). The relative expression level of SlGL3 in true leaf tissues was approximately 6 times greater than that in seedling root tissues (Figure 3B). Both SlTRY and SlGL3 were more strongly expressed in aerial tissues (including stem, cotyledon, leaf, bud and flower) than in roots. These results suggest that both SlTRY and SlGL3 act in both shoot and root tissues and might have relatively strong functions in the aerial parts of plants.
Figure 3. Tomato SlTRY and SlGL3 gene expression.
(A) Real-time reverse transcription PCR analysis of SlTRY gene expression in tomato organs. (B) Real-time reverse transcription PCR analysis of SlGL3 gene expression in tomato organs. Total RNA was isolated from the indicated tissues from 7-day-old seedlings and 5-week-old plants. Expression levels of SlTRY and SlGL3 in each organ relative to those in the seedling root were shown. The experiments were repeated three times. Error bars indicate the standard error. Bars marked with asterisks indicate a significant difference between the seedling root and the other organs by Student’s t-test (P<0.050).
SlTRY Gene Functions in Trichome and Root-hair Development in Arabidopsis
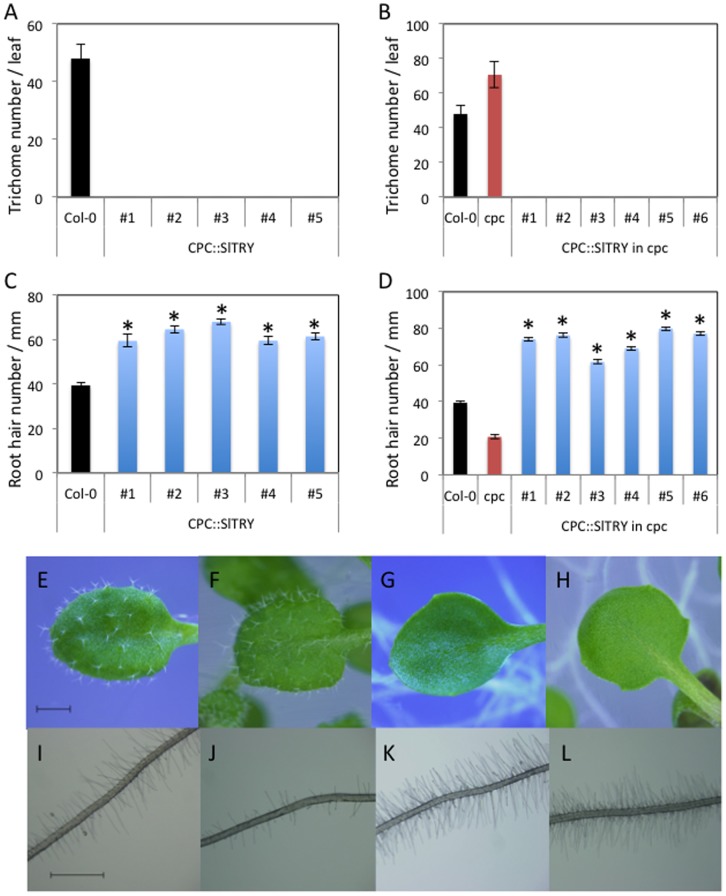
To see if SlTRY is functionally similar to the CPC family of MYB transcription factors, we introduced SlTRY into Arabidopsis wild-type Col-0 plants under the control of the CPC promoter (CPC::SlTRY). The CPC-like MYB genes are thought to function redundantly in trichome and root-hair formation. For example, 35S::CPC, 35S::ETC1, and 35S::ETC3 transgenic plants are all trichome-deficient and have a greater number of root-hairs [1], [3], [4], [7]. Consistent with these previous observations, all homozygous CPC::SlTRY transgenic lines (#1–#5) have the no-trichome phenotype, although wild-type Col-0 produces approximately 50 trichomes on the adaxial surface of the third true leaf (Figure 4A, E, G). To see a SlTRY function more clearly, we introduced CPC::SlTRY into the cpc-2 mutant. As previously reported, the cpc-2 mutant has a greater number of trichomes than wild-type [7] (Figure 4B, F). All homozygous CPC::SlTRY in cpc-2 transgenic lines (#1–#6) show the no-trichome phenotype (Figure 4A, G) as observed in CPC::SlTRY in a wild-type background (Figure 4A). These results indicate that the tomato SlTRY protein has a function similar to the Arabidopsis CPC-like MYB proteins in regulating trichome development.
Figure 4. Trichome and root hair phenotypes of CPC::SlTRY transgenic plants.
(A) Trichome formation on 2-week-old Arabidopsis third leaves of wild-type Col-0 and CPC::SlTRY (#1, #2, #3, #4 and #5). (B) Trichome formation on 2-week-old Arabidopsis third leaves of wild-type Col-0, cpc-2 mutant and CPC::SlTRY in cpc-2 (#1, #2, #3, #4 and #5). Number of trichomes per leaf was determined by counting a minimum of five 2-week-old third leaves from each line. (C) Root hair formation in 5-day-old Arabidopsis seedlings of wild-type Col-0 and CPC::SlTRY (#1, #2, #3, #4 and #5). (D) Root hair formation in 5-day-old Arabidopsis seedlings of wild-type Col-0, cpc-2 mutant and CPC::SlTRY in cpc-2 (#1, #2, #3, #4 and #5). The number of root hairs per mm was determined by counting a minimum of ten 5-day-old seedlings from each line. Error bars indicate the standard error. Bars marked with asterisks indicate a significant difference between the wild-type Col-0 and the transgenic lines (C), or the CPC-2 mutant and the transgenic lines (D) by Student’s t-test (P<0.050). Trichome phenotypes of wild-type Col-0 (E), cpc-2 (F), CPC::SlTRY (G) and CPC::SlTRY in cpc-2 (H). Root hair phenotypes of wild-type Col-0 (I), cpc-2 (J), CPC::SlTRY (K) and CPC::SlTRY in cpc-2 (L). Scale bars: 1 mm.
On the other hand, all homozygous CPC::SlTRY transgenic lines (#1–#6) produced greater numbers of root-hairs compared with wild-type Col-0, which produces approximately 40 root-hairs per mm (Figure 4C, I, K). This result is similar to the root-hair numbers of CPC-like MYB over-expressors [1], [3], [4], [7]. Homozygous CPC::SLTRY in cpc-2 transgenic lines (#1–#6) also showed a greater number of root-hairs compared with wild-type and cpc-2, a mutant that produces a decreased number of root-hairs. These results are similar to the previously reported results using CPC::CPC in cpc-2 or GL2::CPC in cpc-1 transgenic plants [53], [54] (Figure 4D, J, L). These results indicate that the tomato protein SlTRY has a function similar to Arabidopsis CPC-like MYB proteins in regulating root-hair development. Together, our results show that SlTRY functions similar to CPC-like MYBs both in trichome and root-hair formation in Arabidopsis.
SIGL3 does not Function in Trichome and Root-hair Development in Arabidopsis
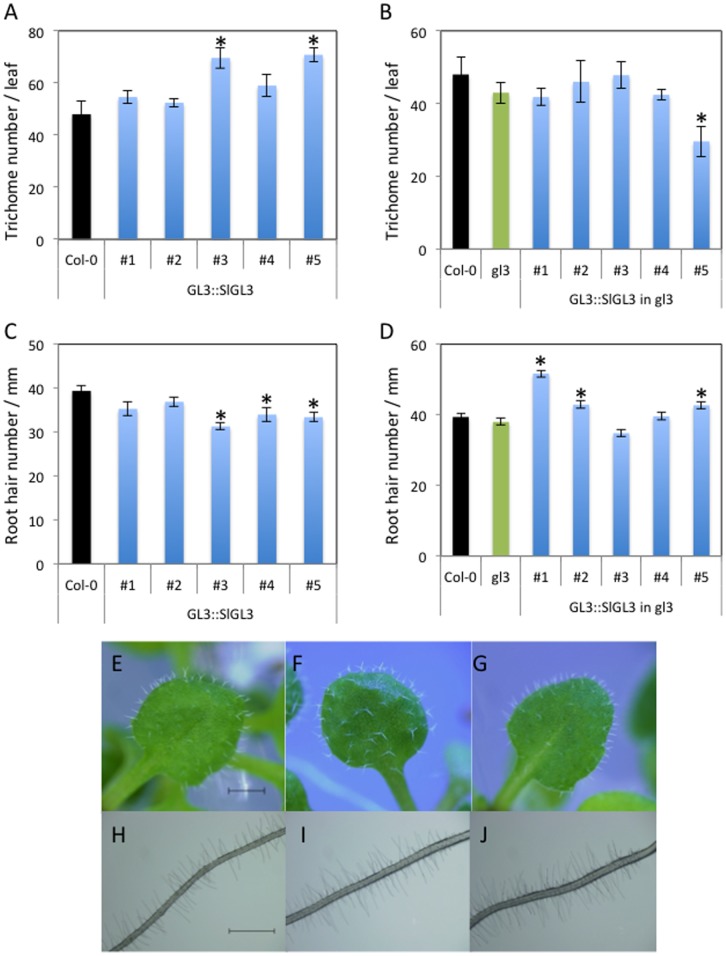
To see if SlGL3 is functionally similar to Arabidopsis GL3 or EGL3, we introduced SlGL3 into Arabidopsis wild-type Col-0 under the control of the GL3 promoter (GL3::SlGL3). The GL3 and EGL3 genes are thought to be involved in trichome and root-hair formation. GL3 or EGL3 over-expressors produce greater numbers of trichomes and reduced numbers of root-hairs [12], [13]. Most of homozygous GL3::SlGL3 transgenic lines produced the similar number of trichomes as that of wild-type (Figure 5A, F). Unlike the previous observation from complementation analysis of the gl3-1 mutant by GL3::GL3 [12], homozygous GL3::SlGL3 in gl3-7454 transgenic plants did not have an increased number of trichomes compared with the gl3-7454 mutant (Figure 5B, E, G). On the contrary, one of the GL3::SlGL3 in gl3-7454 transgenic lines (#5) had significantly fewer trichomes in comparison with the gl3-7454 mutant (Figure 5B). These results suggest that tomato SlGL3 may not have a function same to Arabidopsis GL3.
Figure 5. Trichome and root hair phenotypes of GL3::SlGL3 transgenic plants.
(A) Trichome formation on 2-week-old Arabidopsis third leaves of wild-type Col-0 and GL3::SlGL3 (#1, #2, #3, #4 and #5). (B) Trichome formation on 2-week-old Arabidopsis third leaves of wild-type Col-0, gl3-7454 mutant and CPC::SlTRY in gl3-7454 (#1, #2, #3, #4 and #5). Number of trichomes per leaf was determined by counting a minimum of five 2-week-old third leaves from each line. (C) Root hair formation in 5-day-old Arabidopsis seedlings of wild-type Col-0 and GL3::SlGL3 (#1, #2, #3, #4 and #5). (D) Root hair formation in 5-day-old Arabidopsis seedlings of wild-type Col-0, gl3-7454 mutant and CPC::SlTRY in gl3-7454 (#1, #2, #3, #4 and #5). The number of root hairs per mm was determined by counting a minimum of ten 5-day-old seedlings from each line. Error bars indicate the standard error. Bars marked with asterisks indicate a significant difference between the wild-type Col-0 and the transgenic lines [(A), (C)], or the gl3-7454 mutant and the transgenic lines [(B), (D)] by Student’s t-test (P<0.050). Trichome phenotypes of gl3-7454 (E), GL3::SlGL3 (F), and GL3::SlGL3 in gl3-7454 (G). Root hair phenotypes of gl3-7454 (H), GL3::SlGL3 (I), and GL3::SlGL3 in gl3-7454 (J). Scale bars: 1 mm.
In addition to the effects on trichome number, GL3 is known to affect the trichome branching phenotype [12]. As observed in the gl3-1 mutant (Ler background) [12], trichomes of the gl3-7454 mutant (Col-0 background) had fewer branches than wild-type Col-0 (Table 2). Introduction of the GL3::SlGL3 gene did not rescue the decreased branch number phenotype of the gl3-7454 mutant trichomes (Table 2). This result suggests that the SlGL3 gene does not have a function similar to GL3 in the induction of trichome branching.
Table 2. Trichome branch numbers.
| branches (br)/trichome (%) | ||||
| Genotype | 1 br | 2 br | 3 br | 4 br |
| Col-0 | 0 | 12±2 | 86±4 | 2±1 |
| gl3 | 26±9 | 71±10 | 3±2 | 0 |
| GL3::SlGL3 | 1±1 | 40±14 | 57±13 | 2±1 |
| GL3::SlGL3 in gl3 | 30±13 | 64±12 | 6±4 | 0 |
Data, including s.d., were obtained from at least 10 two-week-old third leaves from each line.
Three of five homozygous GL3::SlGL3 transgenic lines (#3–#5) produced significantly fewer root-hairs compared with wild-type (Figure 5C, I). This result is similar to the tendency for fewer root-hairs in the GL3 or EGL3 over-expressors [13], but the effect of SlGL3 was weaker than that of GL3 and EGL3. In contrast, three of five homozygous GL3::SlGL3 plants in gl3-7454 transgenic lines (#3–#5) produced significantly higher numbers of root-hairs compared with wild-type Col-0 and gl3-7454 (Figure 5D, H, J).
Expression of the GL2 Gene in SlTRY expressing Plants
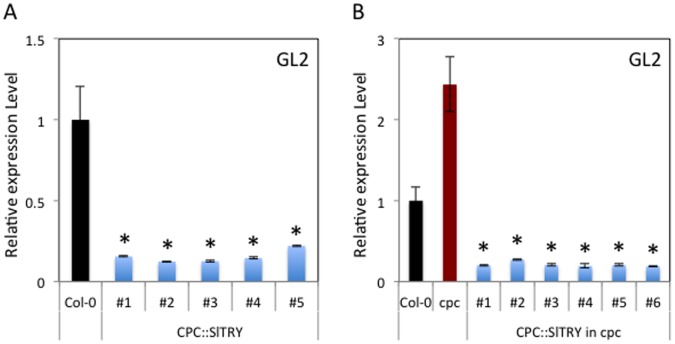
To determine whether CPC::SlTRY functions (Figure 4) were due to epistatic effects of SlTRY on GL2 activity, we carried out real-time reverse transcription PCR analyses using GL2 primers (Figure 6). The GL2 gene is thought to act downstream of the MYB-bHLH transcriptional complex to promote trichome formation and inhibit root-hair formation [1], [14]–[17]. Consistent with the CPC::SlTRY transgene phenotype (Figure 4A), GL2 expression was strongly repressed in all CPC::SlTRY transgenic lines (Figure 6A). In the CPC::SlTRY in cpc-2 transgenic lines, GL2 expression was also strongly repressed compared with wild-type and cpc-2 as was the case in the wild-type background (Figure 6B). To compare gene expression levels of the introduced gene among transgenic lines, we checked GFP expression since GFP was fused to the C-terminal region of SlTRY (Figure S1A). Although the transgene expression levels varied depending on the lines (Figure S1A), expression in all lines was strong enough to repress GL2 expression.
Figure 6. GL2 expression in the CPC::SlTRY transgenic plants.
Real-time reverse transcription PCR analyses of the GL2 gene in wild-type Col-0 and CPC::SlTRY (#1, #2, #3, #4 and #5) (A), and wild-type Col-0, cpc-2 mutant and CPC::SlTRY in cpc-2 (#1, #2, #3, #4 and #5) (B). Expression levels were normalized to Act2 expression. An expression level of GL2 in each line relative to that in wild-type was indicated. The experiments were repeated three times. Error bars indicate the standard error. Bars marked with asterisks indicate a significant difference between the wild-type Col-0 and the transgenic lines (A), or the cpc-2 mutant and the transgenic lines (B) by Student’s t-test (P<0.050).
Expression of the GL2 Gene in SlGL3 expressing Plants
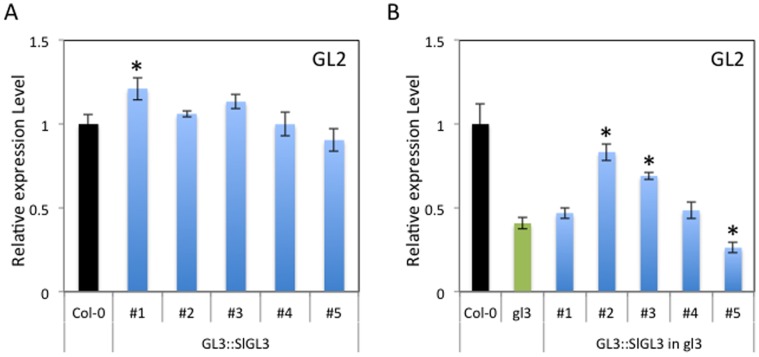
To determine whether CPC::SlGL3 functions (Figure 5) were due to epistatic effects of SlGL3 on GL2 activity, we also carried out real-time reverse transcription PCR analyses using GL2 primers (Figure 7). Inconsistent with the GL3::SlGL3 transgene phenotypes (Figure 5A), significant GL2 expression changes were observed only in GL3::SlGL3 line #1 compared with wild-type Col-0 (Figure 7A). Apparently, SlGL3 does not have a remarkable effect on GL2 expression. In GL3::SlGL3 in gl3-7454 transgenic plants, a significant increase in GL2 expression was observed in lines #2 and #3 compared with that in the gl3-7454 mutant; however, these GL2 expression levels did not reach similar expression levels of GL2 in wild-type Col-0 (Figure 7B). A significant decrease in GL2 expression was observed in line #5 compared with that in the gl3-7454 mutant (Figure 7B). Thus, we checked GFP expression that should reflect the introduced SlGL3 expression levels since the SlGL3 construct was fused to GFP (Figure S1B). The GFP expressions varied greatly among GL3::SlGL3 in gl3-7454 transgenic lines (Figure S1B). In addition, the relative expression levels of GFP in GL3::SlGL3 in gl3-7454 lines were lower than that in the GL3::SlGL3 lines (Figure S1B). These results suggest that SlGL3 expression was unstable in the gl3-7454 mutant background.
Figure 7. GL2 expression in CPC::SlGL3 transgenic plants.
Real-time reverse transcription PCR analyses of the GL2 gene in wild-type Col-0 and CPC::SlGL3 (#1, #2, #3, #4 and #5) (A), and wild-type Col-0, gl3-7454 mutant and CPC::SlGL3 in gl3-7454 (#1, #2, #3, #4 and #5) (B). Expression levels were normalized to Act2 expression. An expression level of GL2 in each line relative to that in wild-type was indicated. The experiments were repeated three times. Error bars indicate the standard error. Bars marked with asterisks indicate a significant difference between the wild-type Col-0 and the transgenic lines (A), or the gl3-7454 mutant and the transgenic lines (B) by Student’s t-test (P<0.050).
Discussion
In this study, we identified tomato SlTRY and SlGL3 genes that were orthologous to the Arabidopsis TRY and GL3 genes, respectively. Recently, a high-quality genome sequence of tomato was released by the Tomato Genome Consortium [55]. Since tomatoes are very distantly related to Arabidopsis evolutionarily, these sequence data may offer important information about plant evolution in the future. The functional analyses of the SlTRY and SlGL3 genes in this study provide insights into tomato trichome and root-hair evolution. Branching of the SlTRY and CPC clusters from a common trunk in the phylogenic tree suggests that the evolution of tomato and Arabidopsis CPC-like R3 MYB genes began with duplication of a single common ancestor after divergence from rice (Figure 1B). Based on the functions of known members of the Arabidopsis bHLH transcription factor family, it was hypothesized that different members participate in distinct developmental processes [50]. Among the genes, members of the IIIf subgroup, including AtMYC1, TT8, GL3 and EGL3 function in trichome and root-hair development, flavonoid/anthocyanin metabolism, and/or mucilage biosynthesis [13], [19], [20], [56]–[59]. Phylogenic analyses predicted that tomato SlGL3 evolved from a common ancestor to Arabidopsis GL3 and EGL3 after divergence of the IIIf subgroup from other bHLH subgroups (Figure 2B). Thus, we expected that SlTRY and SlGL3 would have similar functions to TRY/CPC and GL3/EGL3 in trichome and root-hair differentiation. Both SlTRY and SlGL3 were shown to be expressed in all the tissues examined, especially in the aerial parts (Figure 3), suggesting that these genes function in nearly the entire tomato plant body.
In our experiment, SlTRY was demonstrated to function quite similarly to the CPC-like MYB transcription factors in Arabidopsis trichome and root-hair formation (Figure 4). Both CPC::SlTRY and CPC::SlTRY in cpc-2 transgenic plants showed the no-trichome and increased root-hair phenotypes (Figure 4). Previously, an R3-type MYB of TRY and an R2R3-type MYB of GL1 were reported to compete for a GL3 binding site to form different types of MYB-bHLH complexes involved in Arabidopsis trichome differentiation [60]. TRY prevents the interaction between GL1 and GL3 [19], and CPC physically interacts with GL3/EGL3 [13], suggesting a competition model for CPC and WER [13], [16]. The CPC protein was proposed to disrupt the WER-GL3/EGL3 protein complex by competitive binding with WER, leading to repression of GL2 expression [18], [53], [54]. In this study, we showed that SlTRY also repressed GL2 expression (Figure 6). These results suggest that the SlTRY protein may also disrupt the MYB-bHLH complex of GL1/WER-GL3/EGL3, leading to repression of GL2 expression.
In contrast to SlTRY, SlGL3 did not show clear GL3/EGL3-like functions for trichome and root-hair differentiation in Arabidopsis (Figure 5). Overexpression of GL3 and/or EGL3 induced a notable increase in trichome number and a decrease in root-hair number in Arabidopsis [12], [13]. However, in our experiment, only two of five and three of five GL3::SlGL3 transgenic lines showed a significant increase in trichome number and a significant decrease in root-hair number compared with wild-type, respectively (Figure 5A and B). It is thus possible that tomato SlGL3 has an evolutionarily conserved, highly homologous amino acid sequence and only a partially similar function to Arabidopsis GL3/EGL3. Since trichome and root-hair structure differs between Arabidopsis and tomato, the SlGL3 gene may have acquired another function from GL3/EGL3 during evolution. The functional difference between SlGL3 and GL3/EGL3 may be derived from the relatively low amino acid homology region in the bHLH motifs (Figure 2A). Only one of five GL3::SlGL3 transgenic lines showed a significant increase in the GL2 expression level compared with wild-type Col-0 (Figure 7A). Thus, two possibilities exist. First, a low affinity of SlGL3 protein to WER/GL1 proteins may result in the formation of an incomplete MYB-bHLH protein complexthat cannot activate GL2 expression. Recently, Zhao et al. reported that a single amino acid substitution in another GL3 homologous gene AtMYC1, leads to trichome and root-hair patterning defects by abolishing its interaction with partner proteins in Arabidopsis [61]. Arginine (R173) in the AtMYC1 protein is an essential amino acid residue for interaction with MYB proteins for proper functions [61]. We confirmed that there is a conserved Arg in the SlGL3 protein as in the GL3, EGL3 and AtMYC1 proteins (Figure 2A). Thus, some amino acid substitution other than Arg may contribute to the functional difference between SlGL3 and GL3/EGL3. Second, SlGL3 might have lost either the DNA binding ability to the GL2 promoter region or the ability to activate the GL2 promoter. For example, we previously reported that WER loses its DNA binding ability by at least two amino acid substitutions [53]. Tomato SlGL3 may have lost its DNA binding ability to the GL2 promoter region during evolution.
In order to compare our results in the same Col-0 background, we used the gl3-7454 mutant for the complementary experiment. The gl3-7454 mutant shows only a mild phenotype compared with the gl3-1 mutant (Ler:Landsberg erecta background) [12]. The gl3-7454 mutant shows no significant difference in trichome number or in root-hair number compared with wild-type Col-0 (Figure 5B and D). Unexpectedly, one of five GL3::SlGL3 in gl3-7454 transgenic lines showed significant decreases in trichome number compared with gl3-7454 (Figure 5B), and three of five GL3::SlGL3 in gl3-7454 transgenic lines showed significant increases in root-hair number compared with gl3-7454 (Figure 5D). Consistent with these unexpected phenotypes of GL3::SlGL3 in gl3-7454 transgenic plants, the relative expression levels of GL2 varied (Figure 7B). Two of five GL3::SlGL3 in gl3-7454 transgenic lines showed significantly higher GL2 expression levels compared with that in gl3-7454, but the level did not reach that in the wild-type Col-0 (Figure 7B). One of five GL3::SlGL3 in gl3-7454 transgenic lines showed significantly lower GL2 expression levels compared with that in the gl3-7454 mutant (Figure 7B). As checked by fusion of SlGL3 to GFP, expression of introduced SlGL3 was unstable and fluctuated depending on the lines (Figure S1). In addition, SlGL3 did not rescue the reduced number of trichome branches phenotype of gl3-7454 (Table 2). These data strongly suggest the functional divergence between tomato SlGL3 and Arabidopsis GL3/EGL3. There are 158 bHLH genes in Arabidopsis [52], [62]. Tomato should have the similar or more number of the bHLH genes when the full annotations of tomato genes are determined. We concluded that there is other GL3 ortholog(s) in the unannotated tomato genomes or tomato uses other pathways to regulate the epidermal cell differentiation.Additional investigations to further determine the functions of R3-MYB and bHLH in trichome and root-hair differentiation in tomato are necessary.
Supporting Information
GFP expression in the transgenic plants. Real-time reverse transcription PCR analyses of the GFP gene in CPC::SlTRY (#1, #2, #3, #4 and #5) (A), CPC::SlTRY in cpc-2 (#1, #2, #3, #4 and #5) (B), CPC::SlGL3 (#1, #2, #3, #4 and #5) (C), and CPC::SlGL3 in gl3-7454 (#1, #2, #3, #4 and #5) (D). Expression levels were normalized to Act2 expression. Relative expression levels: expression levels of GFP in each line relative to each transgenic line #1. The experiment was repeated three times. Error bars indicate the standard error.
(TIFF)
Acknowledgments
We thank Tetsuya Ishida, Ryosuke Sano and Tetsuya Kurata for useful suggestions, and Mineko Iwata for technical supports.
Funding Statement
This work was financially supported by the program "Improvement of Research Environment for Young Researchers" from the Ministry of Education, Culture, Sports, Science and Technology, a grant for Scientific Research on Priority Areas from the University of Miyazaki, a Grant-in-Aid for Scientific Research (C) from the Japan Society for the Promotion of Science (No. 23570057) and a Grant-in-Aid for Scientific Research on Priority Areas from the Ministry of Education, Culture, Sports, Science and Technology (No. 23012035). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Wada T, Tachibana T, Shimura Y, Okada K (1997) Epidermal cell differentiation in Arabidopsis determined by a Myb homolog, CPC. Science 277: 1113–1116. [DOI] [PubMed] [Google Scholar]
- 2. Schellmann S, Schnittger A, Kirik V, Wada T, Okada K, et al. (2002) TRIPTYCHON and CAPRICE mediate lateral inhibition during trichome and root hair patterning in Arabidopsis. EMBO J 21: 5036–5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kirik V, Simon M, Huelskamp M (2004) Schiefelbein J (2004) The ENHANCER OF TRY AND CPC1 gene acts redundantly with TRIPTYCHON and CAPRICE in trichome and root hair cell patterning in Arabidopsis. Dev Biol 268: 506–513. [DOI] [PubMed] [Google Scholar]
- 4. Kirik V, Simon M, Wester K (2004) Schiefelbein J, Hulskamp M (2004) ENHANCER of TRY and CPC 2 (ETC2) reveals redundancy in the region-specific control of trichome development of Arabidopsis. Plant Mol Biol 55: 389–398. [DOI] [PubMed] [Google Scholar]
- 5. Esch JJ, Chen MA, Hillestad M, Marks MD (2004) Comparison of TRY and the closely related At1g01380 gene in controlling Arabidopsis trichome patterning. Plant J 40: 860–869. [DOI] [PubMed] [Google Scholar]
- 6. Simon M, Lee MM, Lin Y, Gish L (2007) Schiefelbein J (2007) Distinct and overlapping roles of single-repeat MYB genes in root epidermal patterning. Dev Biol 311: 566–578. [DOI] [PubMed] [Google Scholar]
- 7. Tominaga R, Iwata M, Sano R, Inoue K, Okada K, et al. (2008) Arabidopsis CAPRICE-LIKE MYB 3 (CPL3) controls endoreduplication and flowering development in addition to trichome and root hair formation. Development 135: 1335–1345. [DOI] [PubMed] [Google Scholar]
- 8. Wang S, Kwak SH, Zeng Q, Ellis BE, Chen XY, et al. (2007) TRICHOMELESS1 regulates trichome patterning by suppressing GLABRA1 in Arabidopsis. Development 134: 3873–3882. [DOI] [PubMed] [Google Scholar]
- 9. Gan L, Xia K, Chen JG, Wang S (2011) Functional Characterization of TRICHOMELESS2, a New Single-Repeat R3 MYB Transcription Factor in the Regulation of Trichome Patterning in Arabidopsis. BMC Plant Biol 11: 176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tominaga-Wada R, Nukumizu Y (2012) Expression Analysis of an R3-Type MYB Transcription Factor CPC-LIKE MYB4 (TRICHOMELESS2) and CPL4-Related Transcripts in Arabidopsis. Int J Mol Sci 13: 3478–3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hulskamp M, Misra S, Jurgens G (1994) Genetic dissection of trichome cell development in Arabidopsis. Cell 76: 555–566. [DOI] [PubMed] [Google Scholar]
- 12. Payne CT, Zhang F, Lloyd AM (2000) GL3 encodes a bHLH protein that regulates trichome development in arabidopsis through interaction with GL1 and TTG1. Genetics 156: 1349–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bernhardt C, Lee MM, Gonzalez A, Zhang F, Lloyd A, et al. (2003) The bHLH genes GLABRA3 (GL3) and ENHANCER OF GLABRA3 (EGL3) specify epidermal cell fate in the Arabidopsis root. Development 130: 6431–6439. [DOI] [PubMed] [Google Scholar]
- 14. Galway ME, Masucci JD, Lloyd AM, Walbot V, Davis RW, et al. (1994) The TTG gene is required to specify epidermal cell fate and cell patterning in the Arabidopsis root. Dev Biol 166: 740–754. [DOI] [PubMed] [Google Scholar]
- 15. Rerie WG, Feldmann KA, Marks MD (1994) The GLABRA2 gene encodes a homeo domain protein required for normal trichome development in Arabidopsis. Genes Dev 8: 1388–1399. [DOI] [PubMed] [Google Scholar]
- 16. Lee MM (1999) Schiefelbein J (1999) WEREWOLF, a MYB-related protein in Arabidopsis, is a position-dependent regulator of epidermal cell patterning. Cell 99: 473–483. [DOI] [PubMed] [Google Scholar]
- 17. Bernhardt C, Zhao M, Gonzalez A, Lloyd A (2005) Schiefelbein J (2005) The bHLH genes GL3 and EGL3 participate in an intercellular regulatory circuit that controls cell patterning in the Arabidopsis root epidermis. Development 132: 291–298. [DOI] [PubMed] [Google Scholar]
- 18. Koshino-Kimura Y, Wada T, Tachibana T, Tsugeki R, Ishiguro S, et al. (2005) Regulation of CAPRICE Transcription by MYB Proteins for Root Epidermis Differentiation in Arabidopsis. Plant Cell Physiol 46: 817–826. [DOI] [PubMed] [Google Scholar]
- 19. Esch JJ, Chen M, Sanders M, Hillestad M, Ndkium S, et al. (2003) A contradictory GLABRA3 allele helps define gene interactions controlling trichome development in Arabidopsis. Development 130: 5885–5894. [DOI] [PubMed] [Google Scholar]
- 20. Zhang F, Gonzalez A, Zhao M, Payne CT, Lloyd A (2003) A network of redundant bHLH proteins functions in all TTG1-dependent pathways of Arabidopsis. Development 130: 4859–4869. [DOI] [PubMed] [Google Scholar]
- 21. Szymanski DB, Jilk RA, Pollock SM, Marks MD (1998) Control of GL2 expression in Arabidopsis leaves and trichomes. Development 125: 1161–1171. [DOI] [PubMed] [Google Scholar]
- 22. Lee MM (2001) Schiefelbein J (2001) Developmentally distinct MYB genes encode functionally equivalent proteins in Arabidopsis. Development 128: 1539–1546. [DOI] [PubMed] [Google Scholar]
- 23.Hülskamp M, Kirik V (2000) Trichome differentiation and morphogenesis in Arabidopsis. In: Hallahan DL, Gray JC, editors. Advances in Botanical Research. New York: Academic Press. 37–75.
- 24.Werker E (2000) Trichome diversity and development. In: Hallahan DL, Gray JC, editors. Advances in Botanical Research. New York: Academic Press. 37–75.
- 25. Kang JH, Liu G, Shi F, Jones AD, Beaudry RM, et al. (2010) The tomato odorless-2 mutant is defective in trichome-based production of diverse specialized metabolites and broad-spectrum resistance to insect herbivores. Plant Physiol 154: 262–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kang JH, Shi F, Jones AD, Marks MD, Howe GA (2010) Distortion of trichome morphology by the hairless mutation of tomato affects leaf surface chemistry. J Exp Bot 61: 1053–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schilmiller A, Shi F, Kim J, Charbonneau AL, Holmes D, et al. (2010) Mass spectrometry screening reveals widespread diversity in trichome specialized metabolites of tomato chromosomal substitution lines. Plant J 62: 391–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Luckwill LC (1943) The genus Lycopersicon: a historical, biological and taxonomic survey of the wild and cultivated tomato. Aberd Univ Stud 120: 1–44. [Google Scholar]
- 29. Baur R, Binder S, Benz G (1991) Non-glandular leaf trichomes as short-term inducible defense of the grey alder, Alnus incana (L.), against the chrysomelid beetle, Agelastica alni L. Oecologia. 87: 219–226. [DOI] [PubMed] [Google Scholar]
- 30. Dolan L, Janmaat K, Willemsen V, Linstead P, Poethig S, et al. (1993) Cellular organisation of the Arabidopsis thaliana root. Development 119: 71–84. [DOI] [PubMed] [Google Scholar]
- 31. Cormack RGH (1947) A comparative model of developing epidermal cells in white mustard and tomato roots. American Journal of Botany 34: 310–314. [Google Scholar]
- 32. Clowes FAL (2000) Pattern in root meristem development in angiosperms. New Phytologist 146: 83–94. [Google Scholar]
- 33. Dolan L, Costa S (2001) Evolution and genetics of root hair stripes in the root epidermis. J Exp Bot 52: 413–417. [DOI] [PubMed] [Google Scholar]
- 34. Kim DW, Lee SH, Choi SB, Won SK, Heo YK, et al. (2006) Functional conservation of a root hair cell-specific cis-element in angiosperms with different root hair distribution patterns. Plant Cell 18: 2958–2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Leavitt RG (1904) Trichomes of the root in vascular cryptograms and angiosperms. Proc Boston Soc Nat Hist 31: 273–313. [Google Scholar]
- 36. Cormack RGH (1937) The development of root hair by Elodea canadensis. New Phytol 36: 19–25. [Google Scholar]
- 37. Cutter EG, Hung CY (1972) Symmetric and asymmetric mitosis and cytokinesis in the root tip of Hydrocharis morsus-ranae L. J Cell Sci. 11: 723–737. [DOI] [PubMed] [Google Scholar]
- 38. Dolan L (1996) Pattern in the root epidermis: an interplay of diffusible signals and cellular geometry. Annals of Botany 77: 547–553. [Google Scholar]
- 39. Pemberton LM, Tsai SL, Lovell PH, Harris PJ (2001) Epidermal patterning in seedlings roots of eudicotyledons. Annals of Botany 87: 649–654. [Google Scholar]
- 40. Murashige T, Skoog F (1962) A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiologia Plantarum 15: 473–497. [Google Scholar]
- 41. Kurata T, Ishida T, Kawabata-Awai C, Noguchi M, Hattori S, et al. (2005) Cell-to-cell movement of the CAPRICE protein in Arabidopsis root epidermal cell differentiation. Development 132: 5387–5398. [DOI] [PubMed] [Google Scholar]
- 42. Ishida T, Hattori S, Sano R, Inoue K, Shirano Y, et al. (2007) Arabidopsis TRANSPARENT TESTA GLABRA2 is directly regulated by R2R3 MYB transcription factors and is involved in regulation of GLABRA2 transcription in epidermal differentiation. Plant Cell 19: 2531–2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Okada K, Shimura Y (1990) Reversible Root Tip Rotation in Arabidopsis Seedlings Induced by Obstacle-Touching Stimulus. Science 250: 274–276. [DOI] [PubMed] [Google Scholar]
- 44. Yoo SY, Bomblies K, Yoo SK, Yang JW, Choi MS, et al. (2005) The 35S promoter used in a selectable marker gene of a plant transformation vector affects the expression of the transgene. Planta 221: 523–530. [DOI] [PubMed] [Google Scholar]
- 45. Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743. [DOI] [PubMed] [Google Scholar]
- 46. Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25: 402–408. [DOI] [PubMed] [Google Scholar]
- 47. Girardi CL, Bermudez K, Bernadac A, Chavez A, Zouine M, et al. (2006) The mitochondrial elongation factor LeEF-Tsmt is regulated during tomato fruit ripening and upon wounding and ethylene treatment. Postharvest Biology and Technology 42: 1–7. [Google Scholar]
- 48. Tominaga-Wada R, Iwata M, Sugiyama J, Kotake T, Ishida T, et al. (2009) The GLABRA2 homeodomain protein directly regulates CESA5 and XTH17 gene expression in Arabidopsis roots. Plant J 60: 564–574. [DOI] [PubMed] [Google Scholar]
- 49. Yoshizumi T, Tsumoto Y, Takiguchi T, Nagata N, Yamamoto YY, et al. (2006) Increased level of polyploidy1, a conserved repressor of CYCLINA2 transcription, controls endoreduplication in Arabidopsis. Plant Cell 18: 2452–2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Heim MA, Jakoby M, Werber M, Martin C, Weisshaar B, et al. (2003) The basic helix-loop-helix transcription factor family in plants: a genome-wide study of protein structure and functional diversity. Mol Biol Evol 20: 735–747. [DOI] [PubMed] [Google Scholar]
- 51. Tominaga-Wada R, Iwata M, Nukumizu Y, Wada T (2011) Analysis of IIId, IIIe and IVa group basic-helix-loop-helix proteins expressed in Arabidopsis root epidermis. Plant Sci 181: 471–478. [DOI] [PubMed] [Google Scholar]
- 52. Pires N, Dolan L (2009) Origin and diversification of basic-helix-loop-helix proteins in plants. Mol Biol Evol 27: 862–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tominaga R, Iwata M, Okada K, Wada T (2007) Functional analysis of the epidermal-specific MYB genes CAPRICE and WEREWOLF in Arabidopsis. Plant Cell 19: 2264–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wada T, Kurata T, Tominaga R, Koshino-Kimura Y, Tachibana T, et al. (2002) Role of a positive regulator of root hair development, CAPRICE, in Arabidopsis root epidermal cell differentiation. Development 129: 5409–5419. [DOI] [PubMed] [Google Scholar]
- 55. Consortium TTG (2012) The tomato genome sequence provides insights into fleshy fruit evolution. Nature 485: 635–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhao M, Morohashi K, Hatlestad G, Grotewold E, Lloyd A (2008) The TTG1-bHLH-MYB complex controls trichome cell fate and patterning through direct targeting of regulatory loci. Development 135: 1991–1999. [DOI] [PubMed] [Google Scholar]
- 57. Baudry A, Heim MA, Dubreucq B, Caboche M, Weisshaar B, et al. (2004) TT2, TT8, and TTG1 synergistically specify the expression of BANYULS and proanthocyanidin biosynthesis in Arabidopsis thaliana. Plant J 39: 366–380. [DOI] [PubMed] [Google Scholar]
- 58. Nesi N, Debeaujon I, Jond C, Pelletier G, Caboche M, et al. (2000) The TT8 gene encodes a basic helix-loop-helix domain protein required for expression of DFR and BAN genes in Arabidopsis siliques. Plant Cell 12: 1863–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Baudry A, Caboche M, Lepiniec L (2006) TT8 controls its own expression in a feedback regulation involving TTG1 and homologous MYB and bHLH factors, allowing a strong and cell-specific accumulation of flavonoids in Arabidopsis thaliana. Plant J 46: 768–779. [DOI] [PubMed] [Google Scholar]
- 60. Marks MD, Esch JJ (2003) Initiating inhibition. Control of epidermal cell patterning in plants. EMBO Rep 4: 24–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zhao H, Wang X, Zhu D, Cui S, Li X, et al. (2012) A single amino acid substitution in IIIf subfamily of basic helix-loop-helix transcription factor AtMYC1 leads to trichome and root hair patterning defects by abolishing its interaction with partner proteins in Arabidopsis. J Biol Chem 287: 14109–14121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Pires N, Dolan L (2010) Early evolution of bHLH proteins in plants. Plant Signal Behav 5: 911–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
GFP expression in the transgenic plants. Real-time reverse transcription PCR analyses of the GFP gene in CPC::SlTRY (#1, #2, #3, #4 and #5) (A), CPC::SlTRY in cpc-2 (#1, #2, #3, #4 and #5) (B), CPC::SlGL3 (#1, #2, #3, #4 and #5) (C), and CPC::SlGL3 in gl3-7454 (#1, #2, #3, #4 and #5) (D). Expression levels were normalized to Act2 expression. Relative expression levels: expression levels of GFP in each line relative to each transgenic line #1. The experiment was repeated three times. Error bars indicate the standard error.
(TIFF)