Abstract
BACKGROUND
Buprenorphine/naloxone (BUP) and methadone (MET) are efficacious treatments for opioid dependence, although concerns about a link between BUP and drug-induced hepatitis have been raised. This study compares the effects of BUP and MET on liver health in opioid-dependent participants.
METHODS
This was a randomized controlled trial of 1269 opioid-dependent participants seeking treatment at 8 federally licensed opioid treatment programs and followed for up to 32 weeks between May 2006 and August 2010; 731 participants met “evaluable” criteria defined as completing 24 weeks of medication and providing at least 4 blood samples for transaminase testing. Participants were randomly assigned to receive BUP or MET for 24 weeks. Shift table analysis determined how many evaluable participants moved between categories of low and elevated transaminase levels. Predictors of moving from low to high transaminase levels were identified.
RESULTS
Changes in transaminase levels did not differ by medication condition. Baseline infection with hepatitis C or B was the only significant predictor of moving from low to elevated transaminase levels; 9 BUP and 15 MET participants showed extreme liver test elevations and were more likely than those without extreme elevations to have seroconverted to both hepatitis B and C during the study, or to use illicit drugs during the first 8 weeks of treatment. MET participants were retained longer in treatment than BUP participants.
CONCLUSIONS
This study demonstrated no evidence of liver damage during the initial 6 months of treatment in either condition. Physicians can prescribe either medication without major concern for liver injury.
Keywords: Liver function, buprenorphine, methadone, treatment outcome
1. INTRODUCTION
The United States is gripped by an epidemic of opioid addiction involving both heroin (Substance Abuse and Mental Health Services Administration, 2009) and diverted prescription opioids (Volkow and McLellan, 2011). To date, the most effective treatment for opioid addiction is opioid agonist therapy with either methadone (MET) or buprenorphine (BUP; Mattick et al., 2008). The use of BUP has expanded considerably since its introduction into the U.S., but data from early studies raised concerns about possible hepatotoxicity (e.g., Berson et al., 2001). As part of The Food and Drug Administration’s (FDA) approval of buprenorphine products in 2002, a Phase IV hepatic safety study to document the relative safety of prolonged exposure to buprenorphine compared to the standard of care for opioid agonist therapy (methadone) was required.
One retrospective study found that patients diagnosed with hepatitis B or C receiving treatment with BUP had significant increases in transminase levels, whereas patients without hepatitis did not (Petry et al., 2000). Several case reports describe patients with hepatitis C who developed severe, acute hepatitis while either misusing BUP by injection or when taking it sublingually as directed, although some of these patients either remained on BUP at a lower dose or were re-challenged with it without further evidence of liver injury (Berson et al., 2001; Herve et al., 2004; Zuin et al., 2009). A potential theoretical mechanism was proposed to explain BUP hepatotoxicity involving disruption of mitochrondrial respiration via proton donation by BUP (Berson et al, 2001).
With increasing numbers of physicians prescribing BUP to patients with underlying liver disease, there is a need to determine if BUP poses any significant risk of hepatotoxicity. Thus, the National Institute on Drug Abuse (NIDA) Clinical Trials Network (CTN) in collaboration with Reckitt Benckiser Pharmaceuticals, designed this prospective, 24-week, open-label, randomized, controlled, phase IV study to examine the comparative effects of BUP (as the combination, buprenorphine/naloxone) and MET on indices of liver health in opioid-dependent patients seeking agonist replacement therapy. Although participants were randomized to medication condition, this study was designed to compare liver results from the two conditions with no specific hypothesis. Liver function, drug use, adverse events, and retention data were collected from participants randomly assigned to BUP or MET.
2. METHODS
2.1 Participants
Individuals were recruited at eight federally licensed opioid treatment programs across the United States. Eligibility criteria included being age 18 or older, meeting DSM-IV-TR criteria for opioid dependence, and not having an alanine amino transferase (ALT) or aspartate amino transferase (AST) value > 5 times, or alkaline phosphatase (ALP) value >3 times the upper limit of normal (ULN). Eligibility criteria were included to ensure that participation would be safe and included exclusion criteria based on medical and psychiatric conditions such as cardiopathy, liver disease, and acute psychosis. Additionally, individuals were excluded who had poor venous access such that venipuncture could not be accomplished.
The FDA required a minimum of 300 evaluable participants on each medication. Because this study was descriptive in design, the target randomization was not based on analysis criteria, and no power analysis was computed. The criteria for “evaluable” were completion of 24 weeks on assigned medication and provision of at least half of the eight liver tests scheduled between weeks 1–24, at weeks 1, 2, 4, 8, 12, 16, 20, and 24. Test windows included −/+ 2 days for weeks 1 and 2, and −/+ 7 days for all other weeks. To reach this goal, the initial randomization scheme of 1:1 (BUP: MET) was changed to 2:1 in December 2007 (18 months after initiation) because of higher dropout in the BUP condition.
2.2 Procedures
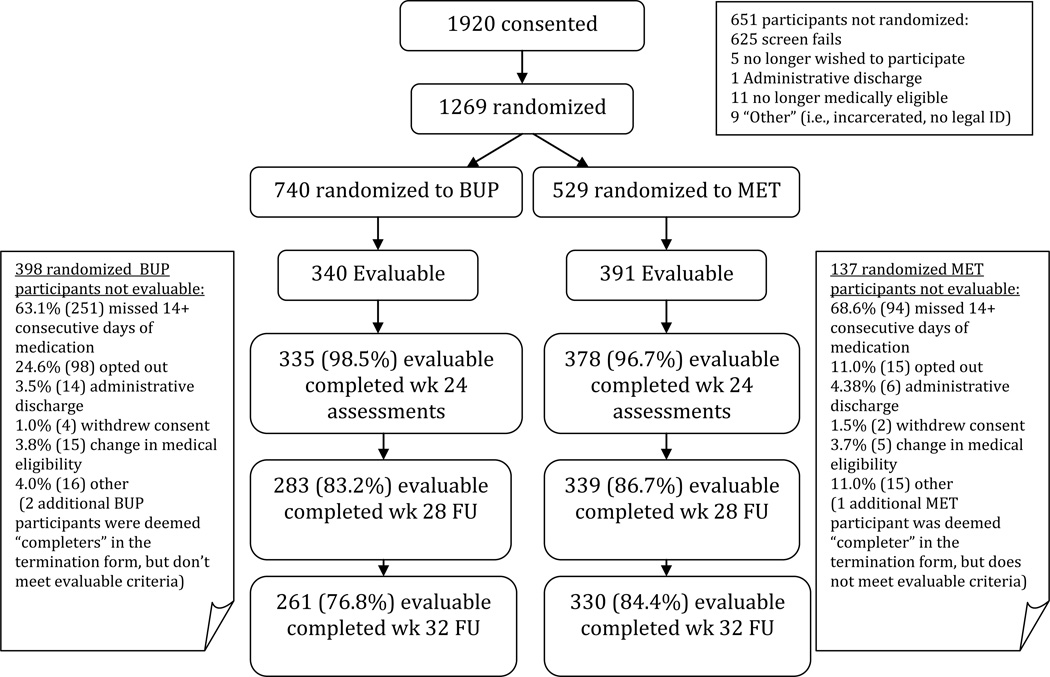
The study was approved by the institutional review boards at participating sites, and participants provided written informed consent. Recruitment occurred between May 2006 and October 2009 with follow-up assessments through August 2010. Oversight was also provided by the NIDA CTN Data Safety and Monitoring Board. Figure 1 depicts the flow of patients through the study. Screening assessments included serum chemistries, ALT, AST, ALP, bilirubin, prothrombin time, albumin, CBC, urinalysis, and pregnancy tests (females). Human immunodeficiency virus (HIV) and hepatitis B and C serologies were obtained within one week following randomization. Participants who tested negative for these viral infections at the beginning of the trial were re-tested at 24 weeks or at early termination when possible to ascertain seroconversion events during the trial.
Figure 1.
Participant Flow
Eligible participants returned to the clinic for induction after abstaining from opioids for 12–24 hours to present in mild opioid withdrawal (Clinical Opiate Withdrawal Scale score ≥8; Wesson and Ling, 2003) or as deemed appropriate by the study physician. Participants were stratified by site and normal versus abnormal transaminase levels and randomized either to open-label MET or BUP and inducted onto medication. Dosing was designed to reflect current dosing standards, and wide variety in both induction dosing and maintenance dosing was allowed, including dose changes across the study duration.
The initial dose of BUP could range from 2–8mg with an additional amount given for persistent withdrawal to a maximum total first day dose of 16mg. BUP could be further increased in subsequent days to a maximum of 32mg. The mean maximum daily BUP dose was 22.1mg (sd = 8.2; median = 24mg).
The maximum initial dose of MET was 30mg with an additional amount given for persistent withdrawal to a maximum total first day dose of 40mg. MET could be increased in subsequent days by 10mg increments. No specific maximum was set for MET. The mean daily maximum MET dose was 93.2mg (sd = 42.2; median = 90mg).
For both medications, study physicians were encouraged to increase doses in response to withdrawal symptoms or opioid use or craving. Participants came to the clinic daily for observed medication administration except Sundays and holidays or when take-home medications were permitted by local regulations. Participants were titrated to an appropriate medication dose typically over the first few medication days for BUP and over several weeks for MET, remained on study medication for the full 24 weeks, and were then tapered off medication over ≤8 weeks or referred for ongoing clinical treatment.
Although not specifically addressed in the study protocol or operations manual, both the BUP and MET groups were scheduled identically for clinic visits. No data were collected to document this, but there is no reason to believe that the groups differed in terms of contact with staff or referrals for ancillary services. Weekly assessments included urine drug screens and adverse event assessments. Self-reported drug use data were collected every four weeks; as noted, liver tests occurred at weeks 1,2,4,8,12,16,20, and 24. The Fagerstrom Test for Nicotine Dependence was obtained at screening to determine rates of heavy smoking (Heatherton et al., 1991). The HIV Risk Behavior Survey was obtained at screening, at week 12, and at week 24 to determine rates of unsafe injection drug use (Needle et al., 1995).
2.3 Outcome Measures and Statistical Analyses
Analyses of transaminase levels via shift tables include data from the evaluable subsample. Other analyses use data from all randomized participants except where specified.
The primary outcome, a shift table analysis of changes in ALT and AST from baseline, sorted evaluable participants into 1 of 5 categories based on a threshold of 2× ULN (chosen because a large proportion of participants would likely have minor elevations in transaminase levels as a consequence of pre-existing liver disease). Shift table categories include:
Baseline transaminases (both ALT and AST) that were ≤ 2× upper limit of normal (ULN) and remained at this level throughout the study;
Baseline transaminases that were ≤ 2× ULN (either ALT or AST) but increased (either ALT or AST) above this level at any time during the study;
Baseline transaminases that were > 2× ULN (either ALT or AST) and decreased and remained at ≤ 2× ULN during the study (both ALT and AST);
Baseline transaminases (both ALT and AST) that were > 2× ULN and remained at this level throughout the study (both ALT and AST);
Baseline transaminases that were > 2× ULN (either ALT or AST) and increased to 2× above this level at any time during the study (either ALT or AST).
Participants with “extreme” liver elevations were defined by any of the following occurring at any point during the 24 weeks of the study: ALT or AST > 10× ULN; total bilirubin >2 mg/dL; direct bilirubin >2 mg/dL; or maximum international normalized ratio >1.5. Comparisons of baseline demographic characteristics, risk behaviors, and HIV and hepatitis test results were conducted between those with and without extreme elevations.
To assess the impact of moderators and mediators (use of shared needles, self-reported alcohol and/or illicit drug use, hepatitis B or C infection, heavy smoking [>20 cigarettes/day]), as well as significant baseline differences (non-heroin opoid use, positive urine drug test for cocaine, and injection drug use) on changes in transaminase values, Cox regression was used to estimate differences between groups. Although weekly urine drug screens were collected during the course of the study, results of drug use will be analyzed and presented in a future paper.
3. RESULTS
3.1 Participant Characteristics
Figure 1 presents participant flow. A total of 1920 participants were screened, and 1269 randomized. The majority of the 651 participants not randomized did not meet study eligibility criteria (n = 625). Additional reasons for non-randomization are shown in Figure 1. Analyses of baseline characteristics between the randomized (n = 1269) and non-randomized (n = 651) participants showed that the randomized group was significantly younger (37.4 [sd = 11.1] vs. 40.4 [sd = 10.6]; t = 5.6, p < 0.0001); included a higher percentage of Whites (74.9% vs. 63.8%;χ2=24.9, p<0.0001); and reported fewer mean days of cocaine (3.3 [sd = 6.6] vs. 4.5 [sd=8.4]; t = 2.5, p 0.014) and amphetamine use in the prior 30 days (1.2 [sd = 3.4] vs. 2.2 [sd = 5.9]; t = 2.3, p = 0.02).
Baseline comparisons of the evaluable (n = 731) and non-evaluable (n = 538) groups indicated that the evaluable group was older (38.8 [sd = 11.3] vs. 35.5 [sd = 10.5]; t=−5.33; p < 0.0001), had a lower percentage of “other” race (12.3% vs. 18.6%, χ2=11.34; p = 0.003), and reported fewer means days of cocaine (3.0, [sd=6.1] vs. 3.8 [sd=7.3]; t=1.99, p = 0.047), amphetamine (0.9 [sd=2.9] vs. 1.6 [sd=3.9]; t=2.45, p = 0.015), and injection heroin use (20.3 [sd = 12.6] vs. 22.2 [sd = 11.8]; t=2.44, p = 0.015). Addressing each treatment group separately, comparisons between the evaluable and non-evaluable BUP groups indicated that a larger percentage of the non-evaluable group had positive hepatitis B surface antibody results compared to the evaluable BUP group (46.4% vs. 32.6%, p = 0.004). For the MET group, a higher percentage of the evaluable group had normal liver function at baseline compared to the non-evaluable group (91.3% vs. 82.6%, p = 0.005).
Significant differences in baseline characteristics between the evaluable treatment groups (Table 1) included days of non-heroin opioid use in the past 30 days (t = 2.0, p = 0.043), with a mean of 9.3 days reported by the BUP group (sd = 12.0) and a mean of 7.3 days (sd = 11.0) reported by the MET group. Also, a lower percentage of evaluable BUP participants tested positive on urine toxicology for cocaine at baseline (29.7%) compared to the evaluable MET group (39.4%)(χ2 = 7.5, p = 0.0062). Last, a larger percentage of those in the MET group reported injection drug use in the past 30 days (69.3%) as compared to the BUP group (61.8%; χ2 =4.6, p = 0.032).
Table 1.
Baseline Characteristics of Evaluable Participants
| Medication Condition | |||
|---|---|---|---|
| BUP (n=340) | MET (n=391) | ||
| Demographic Characteristics (%, n, except as noted) | |||
| Mean Age (sd) | 39.3 (11.3) | 38.4 (11.3) | |
| Gender | |||
| Male | 71.2 (242) | 64.7 (253) | |
| Female | 28.8 (98) | 35.3 (138) | |
| Ethnicity/Race | |||
| Hispanic | 15.6 (53) | 14.3 (56) | |
| White | 72.9 (248) | 79.3 (310) | |
| Black or African American | 12.1 (41) | 10.7 (42) | |
| American Indian/Alaskan Native | 0.9 (3) | 0.5 (2) | |
| Asian | 0.9 (3) | 0.3 (1) | |
| Native Hawaiian/Pacific Islander | 0.3 (1) | 0.0 (0) | |
| Other | 12.7 (43) | 8.7 (34) | |
| Unknown | 0.3 (1) | 0.5 (2) | |
| Self-Reported Mean Days of Drug Use in the Past 30 Days (sd) | |||
| Cocaine | 3.0 (6.3) | 2.9 (5.9) | |
| Heroin | 24.3 (9.9) | 24.2 (9.8) | |
| Non-heroin opioids* | 9.3 (12.0) | 7.3 (11.0) | |
| Amphetamines | 0.8 (2.5) | 1.0 (3.2) | |
| Self-Reported Mean Days of Injection Drug use (sd) | |||
| Heroin by injection | 20.0 (12.9) | 20.6 (12.3) | |
| Non-heroin opioids by injection | 0.8 (4.0) | 0.9 (3.9) | |
| Percent Positive Urine Drug Test (n) | |||
| Opiates (morphine) | 85.3 (290) | 86.7 (339) | |
| Oxycodone | 14.7 (50) | 15.1 (59) | |
| Cocaine** | 29.7 (101) | 39.4 (154) | |
| Benzodiazepines | 19.7 (67) | 18.7 (73) | |
| Cannabis | 25.3 (86) | 20.5 (80) | |
| HIV Risk Behaviors Reported for Last 30 Days | |||
| Mean times injected with shared needles | 2.1 (6.1) | 2.8 (10.8) | |
| % reporting multiple sex partners (n) | 6.8 (23) | 8.2 (32) | |
| % reporting injection drug use*** (n) | 61.8 (210) | 69.3 (271) | |
| Laboratory Results, % (n) | |||
| Hepatitis A Antibody | 0 | 0 | |
| Hepatitis B Surface Antibody | 28.5 (97) | 35.5 (139) | |
| Hepatitis B Core Antibody | 1.5 (5) | 1.5 (6) | |
| Hepatitis B Surface Antigen | 0.3 (1) | 0.5 (2) | |
| Hepatitis C Antibody | 43.5 (148) | 43.5 (170) | |
| HCV RNA | 32.9 (112) | 28.4 (111) | |
| HIV Antibody Screen | 1.2 (4) | 0.5 (2) | |
t= 2.03; p = 0.043;
Chi-square = 7.5, p = 0.0062;
Chi-square=4.60, p=0.0320;
3.2 Liver Outcomes
Of the 731 participants who met “evaluable” criteria: 0.14% (1) provided 4 liver tests, 3.2% (23) provided 5 tests, 6.8% provided 6 tests, 20.0% (146) provided 7 tests, and 70.0% (511) provided all eight possible tests. Table 2 displays the percentage of liver test samples provided by evaluable participants by medication treatment condition.
Table 2.
Percentage of Liver Test Laboratory Samples Provided by Evaluable Participants by Treatment Condition.
| Study Week | 1 | 2 | 4 | 8 | 12 | 16 | 20 | 24 |
|---|---|---|---|---|---|---|---|---|
| MET1 | 91.3 | 92.6 | 96.4 | 95.7 | 94.9 | 96.2 | 96.4 | 94.4 |
| BUP2 | 91.2 | 92.2 | 96.5 | 95.3 | 94.7 | 95.6 | 94.4 | 95.9 |
MET=Methadone treated participants
BUP=buprenorphine/naloxone treated participants
The shift table analysis (Table 3) shows no significant differences between medication groups for liver outcomes. The majority of evaluable participants had both ALT and AST values ≤ 2× ULN throughout the study (category 1) with 113 (15.5%) moving from ≤ 2× ULN at baseline to > 2× ULN at some point during the study (category 2). (As a comparison, 6.5% of the non-evaluable participants met criteria for category 2). Very few participants had baseline liver test values greater than 2× ULN (categories 3−5). Hazard ratios were determined only for the shift from ≤ 2× ULN to > 2× ULN. No significant effect of medication group, alcohol or drug use, sharing needles, or heavy smoking was found. We also adjusted for baseline differences that were found to be significant in Table 1 in our analytical models, and these include non-heroin opioid use, positive urine drug test for cocaine and injection drug use. An effect of hepatitis B or C infection was found (hazard ratio = 2.09; 95% confidence interval 1.09, 4.01).
Table 3.
| Buprenorphine/naloxone (n=340) |
Methadone (n=391) |
|
|---|---|---|
| AST and ALT | n (%) | n (%) |
| Baseline ≤2× ULN;remain ≤2× ULN | 273 (80.3) | 306 (78.3) |
| Baseline ≤2× ULN then ↑ >2× ULN | 43 (12.6) | 70 (17.9) |
| Baseline >2× ULN then ↓ ≤2× ULN and remain ≤2× ULN | 11 (2.4) | 5 (1.3) |
| Baseline>2× ULN do not ↓≤2× ULN or ↑ >2× baseline value | 1 (0.2) | 2 (0.5) |
| Baseline >2× ULN then ↑ >2× baseline value | 9 (2.6) | 6 (1.5) |
AST=aspartate amino transferase
ALT=alanine amino transferase
ULN=upper limit of normal
Evaluable patients completed at least 24 weeks of treatment and provided at least 4 post-baseline transaminase specimens.
some participants did not fit category criteria and are not included in this table.
Extreme elevations in liver tests occurring at any time during the study were exhibited by 9 (2.1%) BUP and 15 (3.6%) MET participants. Among these 24 participants, maximum ALT elevations ranged from 418 to 6280 international units (IU) (n = 15); maximum AST elevations from 493 to 6940 IU (n=8); maximum international normalized ratios from 3.62 to 5.60 (n=7); direct bilirubin from 0.7 to 3.7 mg/dL (n=6); and total bilirubin from 2.8 to 5.0 mg/dL (n=2). These participants did not differ from 821 participants without extreme liver test elevations with sufficient data for comparison, in age, gender, race, ethnicity, use of unsafe injection equipment, or self-reported alcohol use.
Those with extreme elevations were more likely to have both hepatitis C and hepatitis B seroconversion during the study (3/16, 18.8% vs 3/423, 0.7%, p= 0.001), and to have a higher per cent of self-reported illicit drug use for the prior 4 weeks at weeks 4 (median 38.9% vs. 22.2%, p=0.033) and 8 (median 29.6% vs. 11.1%, p=0.034).
3.3 Treatment Exposure
The percentage of dispensed medication doses for those who remained on study medications ranged from 95.4% for week 1 to 84.4% for week 24. Overall, 168.3 dose-years were observed for BUP compared to 187.4 for MET.
3.4 Treatment Retention
Of the randomized sample, the BUP group (n = 740) completed fewer weeks of treatment (mean = 18.5, sd = 12.7) than did the MET group (n = 529; mean = 25.8, sd =10.0, t = −11.47; p < 0.0001).
3.5 Serious Adverse Events (SAEs)
The number of SAEs did not differ by randomized treatment group for the entire randomized sample, with 50 events reported for 38 BUP participants (5.2%), and 59 events reported for 45 MET participants (8.7%). The number of SAEs deemed possibly (n = 13), probably (n = 1), and definitely (n = 3) related to the study included 8 for the BUP group and 9 for the MET group. For the BUP group, these included: persistent headache, non-cardiac chest pain, spontaneous abortion, suicidal ideation, suicidal threat, cholecystitis, accidental benzodiazepine overdose, and suicide plan by heroin overdose. For the MET group, SAEs included two alprazolam overdoses, drug intoxication requiring hospitalization, hospitalization for vomiting, bradycardia, change in mental status, inadvertent methadone overdose, a gastric ulcer, and one death from accidental acute combined use of cocaine and methadone. All SAEs were resolved by the end of the study.
4. DISCUSSION
This study was a systematic, prospective examination of hepatic safety in treatment-seeking opioid-dependent participants randomly assigned to BUP or MET. In this population at high risk for liver disease, relatively low rates of serious exacerbations in indices of liver injury were found. Changes in transaminases over time did not differ between BUP and MET groups, indicating no relative advantage for either medication in terms of liver health. These results accord with a prior small study done in adolescents receiving BUP (Bogenschutz et al., 2010). Because of the risk for liver disease in this population, physicians should continue to monitor laboratory indices of liver health, but, based upon the results of this study, can feel reasonably assured that most patients with liver disease can be safely treated with either medication.
A small number of participants had extreme elevations in transaminases sufficient to merit medical attention. Although the design of this study cannot conclusively show that these elevations were not related to study medications, there was no evidence that these elevations occurred more frequently with either medication. No moderators predicted these events, but these events were significantly mediated by hepatitis C seroconversion and by illicit drug use during the initial 8 weeks of treatment. These findings suggest that extreme elevations in transaminases in participants on opioid agonist treatment are most likely the result of toxic effects of the illicit drugs themselves, impurities in the drugs, or hepatitis B or C.
BUP alone and in combination with naloxone are currently the only available medications other than MET that can be used to treat opioid addiction in federally-licensed opioid treatment programs. These results demonstrate the feasibility of treating patients with BUP in typical, clinically-oriented opioid treatment programs and provide encouragement for more widespread use in these settings.
The BUP group in this open-label trial did have significantly poorer treatment retention than did the MET group, however the 24-week retention rates for the BUP group in this study were in the range seen in prior studies (Johnson et al., 2000; Jones et al., 2010; Ling et al., 1996; Schottenfeld et al., 1997). Further analysis of the data to search for predictors of retention and dropout of the BUP group and the forthcoming results of an associated qualitative study to interview staff and participants about study experiences should help to pinpoint reasons for dropout that might be addressed in future BUP research.
Despite the higher dropout rate, many participants in this and previous studies had excellent clinical responses to BUP. In fact, because of its superior safety profile (Walsh et al., 1994), BUP could be considered as a first line treatment agent. For participants who do not respond well, a switch to MET could be made via an algorithm similar to that in the work of Kakko et al. (2007) in which participants not showing a positive response to BUP were successfully transitioned to MET. In contrast, because of the risk of precipitated withdrawal when switching from MET to BUP (Walsh et al., 1995) attempting to transition poor MET responders to BUP can be difficult.
This study has limitations including the lack of blinding and the differential dropout rate between conditions creating more overall exposure to MET; however, awareness of medication condition would not likely affect liver outcomes. Approximately 44% of participants screened for this study were deemed to be screen fails, which may indicate that those with greater health issues such as liver problems were excluded. Participants deemed ineligible after screening were older, had higher rates of stimulant use, and were less likely to be white compared to those randomized, so the results may not apply to all groups of opioid addicted individuals. Study strengths include observed dosing to assure adequate medication exposure and provision of therapeutic medication doses. Overall, this study suggests that liver injury from BUP occurs rarely, which provides encouragement to physicians to use buprenorphine as an effective treatment option for opioid addiction.
Acknowledgements
Our appreciation to Reckitt Benckiser Pharmaceuticals and personnel, who provided suggestions for initial study design, and supplied Suboxone. Sincere appreciation also to our participating networks: The Pacific Northwest Node (U10 DA01714; PI: Dennis Donovan, PhD) and Evergreen Treatment Services (Study Investigators: Paul Grekin, MD; Andrew Saxon, MD, Ron Jackson, MSW); the Oregon Hawaii Node (U10 DA013036; PI: Dennis McCarty, PhD) and CODA Inc. (Study Investigators: Joshua Boverman, MD, Jeffrey Hayes, MD, Jonathan Berman, MD, Katharina Wiest, PhD); the California/Arizona Node (U10 DA015815; PI: James Sorenson, PhD) and Bi-Valley Medical Clinic (Study Investigators: John McCarty, MD, Esther Billingsley, NP, Lori Matthews, NP); the New England Node (U10 DA13038; PI: Kathleen Carroll, Ph.D.) and Connecticut Counseling Centers (Study Investigators: Hansa Shah, MD, Mark Kraus, MD, Michael Feinberg, MD,) and Yale and Hartford Dispensary (Study Investigators: Gerardo Gonzalez, MD, R. Douglas Bruce, MD, Peter Strong, MD, Cindy Gilligan, PA); the Delaware Valley Node (U10 DA13043PI: George Woody, MD) and NET Steps (Study Investigators: Richard Hellander, MD, Trusandra Elaine Taylor, MD, June Topacio, MD, Angela T. Walker, MD, Bernard Harris, MD, MPH); the Pacific Region Node (U10 DA13045; PI: Walter Ling, MD), and Bay Area Addiction Research & Treatment (Study Investigators: Judith Martin, MD, Laurene Spencer, MD., Audrey Sellers, MD, Carolyn Schuman, MD; Allan Cohen, MA, MFT) and Matrix Institute (Study Investigators: Roger Donovick, MD, Matthew Torrington, MD, Michael McCann, MA); and the New York Node (U10 DA013046; PI: John Rotrosen, MD) and Addiction Research & Treatment Corp (Study Investigators: Lawrence Brown, Jr., MD, MPH, Steven Kritz, MD); the Duke Clinical Research Institute (DSC); EMMES Corporation (CCC); the CCTN and NIDA.
Role of Funding Source
Trial registration was initiated and maintained on clinicaltrials.com (NCT00315341).
Main study funding came from the National Institute on Drug Abuse through the Clinical Trials Network (CTN) through a series of grants provided to each participating node:
The Pacific Northwest Node (U10 DA01714)
The Oregon Hawaii Node (U10 DA013036)
The California/Arizona Node (U10 DA015815)
The New England Node (U10 DA13038)
The Delaware Valley Node (U10 DA13043)
The Pacific Region Node (U10 DA13045)
The New York Node (U10 DA013046)
In addition to funding, NIDA CTN personnel contributed their expertise to the development and conduct of the trial, and Dr. Petra Jacobs participated in the preparation of the main outcome manuscript.
Reckitt Benckiser Pharmaceuticals and personnel provided suggestions for initial study design, and supplied Suboxone.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
All authors contributed to the preparation and/or conduct of the study, and/or analysis of study data. All authors assisted in the preparation and/or review of the final main outcome draft. Additional contributions are noted below
Andrew J. Saxon, M.D.: served as Co-Lead Principal Investigator of the study and participated in the development of the study design and protocol, conduct of the trial, analysis plan, and co-authored the first draft of the main outcome manuscript. Subsequently, Dr. Saxon was also instrumental in revisions of the main outcome manuscript given co-author and CTN feedback.
Walter Ling, M.D.: Dr. Ling was the Principal Investigator for this trial and led all study preparations and conduct efforts, as well as assisting in the preparation of the manuscript.
Maureen Hillhouse, Ph.D.: Dr. Hillhouse was part of the lead team on this project, and provided day-to-day oversight for regulatory efforts and other issues in the conduct of the trial. She oversaw the analysis plan, supervised analysis efforts, and coordinated efforts in the preparation of the final study report. With Dr. Saxon, she prepared the first draft of the manuscript and coordinated co-author contributions.
Christie Thomas, M.P.H.: Served as Lead Team Coordinator for the project, and was intimately involved in all aspects of study development and conduct. She assisted in the preparation of the final report and provided detailed information about aspects of study methods for the main outcome manuscript.
Albert Hasson, M.S.W.; Served as National Study Coordinator for this research and contributed his expertise to the conduct of the trial. Mr. Hasson participated in the preparation of the manuscript.
Geetha Doraimani, M.S.; Served as Lead Team Statistician, providing analyses and written descriptions of the analysis and results sections for the final report and main outcome manuscript.
Gudaye Tasissa, Ph.D.: Provided statistical analyses for the main outcome as well as additional data tables, and reviewed the manuscript providing comments and corrections.
Yuliya Lokhnygina, Ph.D.: Provided statistical analyses for the main outcome as well as additional data tables, and reviewed the manuscript providing comments and corrections.
Jeff Leimberger, Ph.D.: Provided statistical analyses for the main outcome as well as additional data tables, and reviewed the manuscript providing comments and corrections.
R. Douglas Bruce, M.D.: Participated in the conduct of the trial and reviewed the manuscript providing comments and corrections
John McCarthy, M.D.: Participated in the conduct of the trial and reviewed the manuscript providing comments and corrections
Katharina Wiest, Ph.D.: Participated in the conduct of the trial and reviewed the manuscript providing comments and corrections
Paul McLaughlin, M.A.: Participated in the conduct of the trial and reviewed the manuscript providing comments and corrections
Richard Bilangi, M.S.: Participated in the conduct of the trial and reviewed the manuscript providing comments and corrections
Allan Cohen, M.A.: Participated in the conduct of the trial and reviewed the manuscript providing comments and corrections
George Woody, M.D.: Participated in the development and conduct of the trial, and reviewed the manuscript providing comments and corrections
Petra Jacobs, M.D.: Participated in the development and conduct of the trial, and reviewed the manuscript providing comments and corrections
Conflict of Interest
Authors disclosing relevant financial interests, activities, relationships, and affiliations are: Andrew Saxon: Paid consultant to Reckitt Benckiser Pharmaceuticals; Walter Ling: Paid consultant to Reckitt Benckiser Pharmaceuticals; R. Douglas Bruce: Research grant support from Gilead Sciences, Inc., Merck & Co., Bristol Myers Squibb, Boehringer Ingelheim, Reckitt Benckiser Pharmaceuticals, Abbott Laboratories, Pfizer, Inc., and honorarium from Reckitt Benckiser Pharmaceuticals; Yuliya Lokhnygina: Paid consultant to Johnson & Johnson. All other authors report no financial or other possible conflicts of interest.
REFERENCES
- Berson A, Fau D, Fornacciari R, Degove-Goddard P, Sutton A, Descatoire V, Haouzi D, Lettéron P, Moreau A, Feldmann G, Pessayre D. Mechanisms for experimental buprenorphine hepatotoxicity: major role of mitochondrial dysfunction versus metabolic activation. J. Hepatol. 2001;34:261–269. doi: 10.1016/s0168-8278(00)00050-7. [DOI] [PubMed] [Google Scholar]
- Berson A, Gervais A, Cazals D, Boyer N, Durand F, Bernuau J, Marcellin P, Degott C, Valla D, Pessayre D. Hepatitis after intravenous buprenorphine misuse in heroin addicts. J. Hepatol. 2001;34:346–350. doi: 10.1016/s0168-8278(00)00049-0. [DOI] [PubMed] [Google Scholar]
- Bogenschutz M, Abbott PJ, Kushner R, Tonigan JS, Woody GE. Effects of buprenorphine and hepatitis C on liver enzymes in adolescents and young adults. J. Addict. Med. 2010;4:211–216. doi: 10.1097/ADM.0b013e3181c4e27e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom K. The Fagerstrom test for nicotine dependence: a revision of the Fagerstrom Tolerance Questionnaire. Brit. J. Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hervé S, Riachi G, Noblet C, Guillement N, Tanasescu S, Goria O, Thuillez C, Tranvouez JL, Ducrotte P, Lerebours E. Acute hepatitis due to buprenorphine administration. Eur. J. Gastroenterol. Hepatol. 2004;16:1033–1037. doi: 10.1097/00042737-200410000-00013. [DOI] [PubMed] [Google Scholar]
- Johnson RE, Chutuape MA, Strain EC, Walsh SL, Stitzer ML, Bigelow GE. A comparison of levomethadyl acetate, buprenorphine, and methadone for opioid dependence. N. Engl. J. Med. 2000;343:1290–1297. doi: 10.1056/NEJM200011023431802. [DOI] [PubMed] [Google Scholar]
- Jones HE, Kaltenbach K, Heil SH, Stine SM, Coyle MG, Arria AM, O'Grady KE, Selby P, Martin PR, Fischer G. Neonatal abstinence syndrome after methadone or buprenorphine exposure. N. Engl. J. Med. 2010;363:2320–2331. doi: 10.1056/NEJMoa1005359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakko J, Grönbladh L, Svanborg KD, von Wachenfeldt J, Rück C, Rawlings B, Nilsson LH, Heilig M. A stepped care strategy using buprenorphine and methadone versus conventional methadone maintenance in heroin dependence: a randomized controlled trial. Am. J. Psychiatry. 2007;164:797–803. doi: 10.1176/ajp.2007.164.5.797. [DOI] [PubMed] [Google Scholar]
- Ling W, Wesson DR, Charuvastra C, Klett CJ. A controlled trial comparing buprenorphine and methadone maintenance in opioid dependence. Arch. Gen. Psychiatry. 1996;53:401–407. doi: 10.1001/archpsyc.1996.01830050035005. [DOI] [PubMed] [Google Scholar]
- Mattick R, Kimber J, Breen C, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst. Rev. 2008;16:CD002207. doi: 10.1002/14651858.CD002207.pub3. [DOI] [PubMed] [Google Scholar]
- Needle R, Fisher DG, Weatherby N, Chitwood D, Brown B, Cesari H, Booth R, Williams ML, Watters J, Andersen M, Braunstein M. Reliability of self-reported HIV risk behaviors of drug users. Psych. Addict. Behav. 1995;9:242–250. [Google Scholar]
- Petry NM, Bickel WK, Piasecki D, Marsch LA, Badger GJ. Elevated liver enzyme levels in opioid-dependent patients with hepatitis treated with buprenorphine. Am. J. Addict. 2000;9:265–269. doi: 10.1080/10550490050148099. [DOI] [PubMed] [Google Scholar]
- Schottenfeld RS, Pakes JR, Oliveto A, Ziedonis D, Kosten TR. Buprenorphine vs methadone maintenance treatment for concurrent opioid dependence and cocaine abuse. Arch. Gen. Psychiatry. 1997;54:713–720. doi: 10.1001/archpsyc.1997.01830200041006. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration, Office of Applied Studies. Treatment Episode Data Set (TEDS) Rockville, MD: National Admissions to Substance Abuse Treatment Services – Highlights 2007; 2009. [Google Scholar]
- Volkow ND, McLellan TA. Curtailing diversion and abuse of opioid analgesics without jeopardizing pain treatment. JAMA. 2011;305:1346–1347. doi: 10.1001/jama.2011.369. [DOI] [PubMed] [Google Scholar]
- Walsh SL, June HL, Schuh KJ, Preston KL, Bigelow GE, Stitzer ML. Effects of buprenorphine and methadone in methadone-maintained subjects. Psychopharmacology (Berl.) 1995;119:268–276. doi: 10.1007/BF02246290. [DOI] [PubMed] [Google Scholar]
- Walsh SL, Preston KL, Stitzer ML, Cone EJ, Bigelow GE. Clinical pharmacology of buprenorphine: ceiling effects at high doses. Clin. Pharmacol. Ther. 1994;55:569–580. doi: 10.1038/clpt.1994.71. [DOI] [PubMed] [Google Scholar]
- Wesson DR, Ling W. The Clinical Opiate Withdrawal Scale (COWS) J. Psychoactive Drugs. 2003;35:53–59. doi: 10.1080/02791072.2003.10400007. [DOI] [PubMed] [Google Scholar]
- Zuin M, Giorgini A, Selmi C, Battezzati PM, Cocchi CA, Crosignani A, Benetti A, Invernizzi P, Podda M. Acute liver and renal failure during treatment with buprenorphine at therapeutic dose. Dig. Liver Dis. 2009;41:e8–e10. doi: 10.1016/j.dld.2007.12.014. [DOI] [PubMed] [Google Scholar]