Abstract
Objective
The objective of this study was to assess the predictive value of clinical and exercise test variables in patients with peripheral arterial disease (PAD).
Methods
725 PAD patients referred for exercise testing at the Palo Alto Veterans Hospital between 1997 and 2011 were subjected to a customized symptom-limited ramp treadmill protocol. Detailed clinical and exercise test data were collected at baseline and patients were followed for a mean of 11.3±6.3 years.
Results
During follow up, there were 364 deaths. Baseline exercise capacity was 7.0±2.6 Metabolic equivalents (METs) among survivors and 5.5±2.4 METs in those who died (P<.001). Although several physiologic parameters differed between survivors and non-survivors, age-adjusted Cox regression revealed that exercise capacity was the strongest independent predictor of mortality. Each additional MET achieved was associated with age-adjusted 18% and 20% reductions in all-cause and cardiovascular mortality, respectively (P<.001 for both). This variable surpassed all classical risk factors (including smoking and history of congestive heart failure) as well as all measured exercise test responses (including symptoms and ECG abnormalities).
Conclusions
Amongst PAD patients, reduced exercise capacity is the most powerful harbinger of long term mortality. This factor has predictive power beyond traditional risk factors and confirms the critical importance of fitness in this cohort.
Keywords: Peripheral artery disease, PAD, exercise, stress test, MET, survival
Introduction
Recent efforts have focused on identifying improved prognostic indicators for patients with atherosclerotic disease. The aim of this approach is to identify those who stand to benefit the most from aggressive risk factor reduction and directed therapies. Exercise testing has been an area of intense focus, given its relatively low cost, non-invasive nature, and ability to provide prognostic information beyond traditional risk factor assessment 1. In a number of recent studies, exercise capacity has been a particularly powerful predictor of mortality and other adverse events. In elderly subjects, those with hypertension, and those that are obese, exercise capacity has in fact been shown to be the strongest predictor of both all-cause and cardiovascular mortality2–4. Remarkably, this single variable provided more prognostic information than each of the classical risk factors or even the Framingham risk score 4
To our knowledge, the association between survival and exercise capacity during standardized treadmill exercise testing has not been evaluated in patients with PAD. Accordingly, we aimed to study the association between simple physiological parameters and long term survival in a large cohort of PAD patients from the Veterans Exercise Testing Study (VETS). Ultimately, our goal was to define exercise test parameters which could identify patients at risk, compare these to traditional risk factors and provide important prognostic information to subjects with vascular disease.
Methods
The VETS cohort is an ongoing, prospective evaluation of veteran subjects referred for exercise testing for clinical reasons, designed to address exercise test, clinical, and lifestyle factors and their association with health outcomes 2,5,6. From the VETS cohort, a list of approximately 10,000 male veterans who had undergone a maximal treadmill test for clinical reasons at the VA Palo Alto Health Care System between 1997 and 2011 for clinical indications was formed. This list was used to query the VA computerized database to identify patients with a history of PAD entered at the time the exercise test was performed. A total of 725 subjects were identified as having PAD by history, by claudication during their exercise test, or both. Historical information that was recorded at the time of the exercise test included previous MI by history or Q wave, cardiac procedures, heart failure, hypertension, hypercholesterolemia (>220 mg/dl), intermittent claudication, chronic obstructive pulmonary disease, cancer, end stage renal disease, diabetes, stroke, smoking status (current, past), and use of cardiac medications (categorical only, anticoagulants were not differentiated).
The current study evaluated only data available at the baseline visit as part of a clinically-referred exercise test and the association of these data with cardiovascular and all-cause mortality. Clinical and historical variables were defined in a standard fashion through a history and physical performed at the time of the test by a cardiology fellow, and this historical information was confirmed by computerized records. The exercise test interpretation was over-read by an attending physician.
Exercise testing
Patients underwent symptom-limited treadmill testing using an individualized ramp treadmill protocol such that test duration was targeted to fall within the recommended 8–12 minute range, as previously described 1,7. Electrocardiograms (ECGs) were obtained at rest, each minute during exercise, and for at least 8 minutes during recovery; blood pressure was measured at rest, every minute during exercise and at 1, 2, 5, and 8 minutes during recovery or until symptoms, ECG changes, and blood pressure stabilized. Standard criteria for termination were employed, including moderately severe angina, >2.0 mm abnormal ST depression, a sustained drop in systolic blood pressure or serious rhythm disturbances. In the absence of clinical indications for stopping, participants were encouraged to exercise until volitional fatigue, and the Borg 6 to 20 perceived exertion scale was used to quantify degree of effort8,9. Blood pressure was taken manually and exercise capacity (in metabolic equivalents [METs]) was estimated from peak treadmill speed and grade10. No test was classified as incomplete or indeterminate, medications were not withheld, and age-predicted maximal target heart rates were not used as end points. The exercise tests were performed, analyzed and reported using a standard protocol incorporating a computerized database with all definitions and measurements prospectively defined 11.
Outcomes
The main outcome variable was all-cause mortality; cardiovascular death was also recorded. The California Health Department Service and Social Security Death Indices were used to ascertain the vital status of each patient as of December 31, 2011. Accuracy of deaths was reviewed by two clinicians blinded to exercise test results and confirmed using the Veterans Affairs computerized medical records.
Statistical Analysis
NCSS software (Kayesville, Utah) was used for all statistical analyses. Unpaired t-tests were used for comparisons of continuous variables, and chi-square tests were used to compare dichotomous variables between patients who survived and those who died. Cox proportional hazards analysis was performed to determine which clinical and exercise test variables were independently associated with all-cause and cardiovascular death. Survival analyses were adjusted for age. Hazard ratios were calculated along with their 95% confidence intervals. Receiver-operating-characteristic (ROC) curves were constructed to determine the discriminatory accuracy of exercise capacity and other key variables to predict survival. An exploratory univariate survival analysis was initially performed, and the strongest univariate predictors of risk were entered into a step-wise multivariate analysis. Kaplan-Meier curves were constructed to assess overall survival with time, and to compare survival among subjects with high and low exercise capacity. The log-rank test was used to compare Kaplan Meier curves.
Results
The sample included all 708 males and 17 females PAD patients. Their mean age was 62.0±9.1 years. The mean follow up period was 11.3 years (SD±6.3) and there were 364 deaths (50.2% of the total sample) for an average annual mortality of 4.4%; 36.3% of the deaths were due to cardiovascular causes. Baseline demographic variables, clinical history, and medications in the entire cohort and between those who survived and those who died are presented in Table 1.
Table 1.
Clinical and Demographic Characteristics of the Sample (N±SD of %)
| Demographics | All | Survivors | Non-survivors | p Value |
|---|---|---|---|---|
| n = 725 | n = 361 | n = 364 | ||
| Age (years) | 62.0 ± 9.1 | 59.5 ± 8.4 | 64.6 ± 8.4 | < 0.001 |
| Follow-up Years | 11.3 ± 6.25 | 15.2 ± 4.8 | 7.5 ± 5 | < 0.001 |
| Height (in) | 68.9 ± 3.2 | 68.8 ± 3.3 | 69 ± 3.2 | 0.42 |
| Weight (lbs) | 186.8 ± 36.7 | 191.4 ± 38.5 | 182.2 ± 34.2 | < 0.001 |
| Male | 708 (97.7) | 350 (97) | 358 (98.4) | NS |
| Female | 18 (2.3) | 11 (3) | 6 (1.6) | NS |
| Clinical History | ||||
| Diabetes | 113 (15.6) | 59 (16.3) | 54 (14.8) | 0.58 |
| Hypertension | 468 (64.6) | 238 (65.9) | 230 (63.1) | 0.44 |
| Claudication | 496 (68.4) | 250 (69.3) | 246 (67.6) | 0.63 |
| Heart Failure | 61 (8.4) | 16 (4.4) | 45 (12.4) | < 0.001 |
| Stroke | 49 (6.8) | 20 (5.5) | 29 (8.0) | 0.19 |
| Pulmonary Disease | 102 (14.1) | 50 (13.9) | 52 (14.3) | 0.87 |
| Smoking History | 486 (67) | 259 (71.7) | 227 (62.4) | 0.007 |
| Pack Years Smoking | 32.8 ± 32.3 | 32.3 ± 30.3 | 33.3 ± 34.2 | 0.68 |
| Hyperlipidemia | 283 (39) | 161 (44.6) | 122 (33.5) | < 0.001 |
| Cholesterol (mg/dL) | 205.3 ± 54.5 | 209 ± 57 | 196.4 ± 47.5 | 0.16 |
| HDL (mg/dL) | 43.4 ± 17.3 | 42.8 ± 18.8 | 45.0 ± 13.6 | 0.47 |
| Medications | ||||
| Digoxin | 56 (7.7) | 21 (5.8) | 35 (9.6) | 0.05 |
| Beta Blockers | 183 (25.2) | 103 (28.5) | 80 (22) | 0.04 |
| Calcium Antagonist | 286 (39.4) | 130 (36) | 156 (42.9) | 0.05 |
| Nitrates | 239 (33) | 93 (25.8) | 146 (40.1) | < 0.001 |
| Antihypertensives | 233 (32.1) | 97 (26.9) | 136 (37.4) | 0.002 |
| Antiarrhythmics | 18 (2.5) | 2 (0.6) | 16 (4.4) | <0.001 |
| ACE Inhibitors | 116 (16) | 83 (23) | 33 (9.1) | < 0.001 |
| Anticoagulants | 172 (23.7) | 120 (33.2) | 52 (14.3) | < 0.001 |
A comparison of exercise test responses between those who died and those who survived is presented in Table 2. Maximal heart rate (130± 21 vs. 122± 23, p<0.001) and exercise capacity (7.0±2.6 versus 5.5±2.4 METs, p<0.001) were higher among those who survived, while the proportion of subjects with an abnormal resting ECG was higher among those who died (34.3 vs. 56.9%, p<0.001). A higher proportion of non-survivors was limited by claudication, experienced rhythm abnormalities, and had a hypotensive response to exercise. However, ischemic responses were similar between groups.
Table 2.
Exercise Test Responses
| Variables | All | Survivors | Non-survivors | p-value |
|---|---|---|---|---|
| Resting Heart Rate (bpm) | 77 ± 15 | 77 ± 15 | 77 ± 15 | 0.99 |
| Resting SBP (mm/Hg) | 137 ± 22 | 135 ± 21 | 138 ± 23 | 0.14 |
| Resting DBP (mm/Hg) | 80 ± 12 | 80 ± 12 | 80 ± 11 | 0.88 |
| Maximal Heart Rate (bpm) | 126 ± 22 | 130 ± 21 | 122 ± 23 | < 0.001 |
| Maximal SBP (mm/Hg) | 175 ± 29 | 178 ± 28 | 173 ± 30 | 0.009 |
| Maximal DBP (mm/Hg) | 87 ± 33 | 86 ± 15 | 87 ± 44 | 0.8 |
| Heart Rate Recovery (2 min) | 95 ± 21 | 96 ± 20 | 89 ± 22 | 0.25 |
| Exercise Capacity (METs) | 6.23 ± 2.6 | 7.0 ± 2.6 | 5.5 ± 2.4 | < 0.001 |
| Peak Perceived Exertion | 17 ± 2 | 17 ± 2 | 17 ± 3 | 0.07 |
| Normal Rest ECG | 273 (37.7) | 164 (45.4) | 109 (29.9) | < 0.001 |
| Abnormal Rest ECG | 331 (45.7) | 124 (34.3) | 207 (56.9) | < 0.001 |
| Reasons for Stopping Exercise* | ||||
| Angina | 149 (20.6) | 77 (21.3) | 72 (19.8) | 0.74 |
| Fatigue | 103 (14.2) | 66 (18.3) | 37 (10.2) | 0.001 |
| Shortness of Breath | 82 (11.3) | 54 (15) | 28 (7.7) | 0.002 |
| Leg Fatigue | 76 (10.5) | 48 (13.3) | 28 (7.7) | 0.01 |
| Claudication | 483 (66.6) | 227 (62.9) | 256 (70.3) | 0.03 |
| Other Leg Pain | 77 (10.6) | 45 (12.5) | 32 (8.8) | 0.11 |
| Submaximal Target | 21 (2.9) | 7 (1.9) | 14 (3.8) | 0.13 |
| Dysrhythmias | 61 (8.4) | 17 (4.7) | 44 (12.1) | <0.001 |
| ST Changes | 35 (4.8) | 13 (3.6) | 22 (6) | 0.12 |
| Other Chest Pain | 12 (1.7) | 8 (2.2) | 4 (1.1) | 0.24 |
| Hypotension | 14 (1.9) | 2 (0.6) | 12 (3.3) | 0.007 |
Note that more than one reason for stopping may be recorded.
SBP-systolic blood pressure
DBP–diastolic blood pressure
Univariate predictors of all-cause mortality were age, exercise capacity, pack-years of smoking, history of heart failure, maximal heart rate, and maximal systolic blood pressure. Age-adjusted multivariate proportional hazards analysis for both all-cause and cardiovascular mortality as the outcome is presented in Table 3. Exercise capacity was the strongest predictor of all-cause mortality (hazard ratio [HR] 0.83, 95% CI 0.79–0.88, p<0.001; area under the ROC curve 0.64). Exercise capacity remained the strongest predictor when adjusted for age (HR 0.85, 95% CI 0.81–0.90, p<0.001). The only other predictors of all-cause mortality were pack-years smoking (HR 1.01, 95% CI 1.0–1.01, p=0.001) and history of heart failure (HR 1.92, 95% CI 1.34–2.76, p<0.001; area under the ROC curve 0.54). Similarly, the strongest predictor of cardiovascular mortality was exercise capacity; an age-adjusted 20% reduction cardiovascular mortality was observed for each MET achieved. The only other predictors of cardiovascular mortality were age and history of heart failure.
Table 3.
Multivariate proportional hazards analysis for each predictor alone and then combined.
| All-Cause Mortality | ||||
|---|---|---|---|---|
| Hazard Ratio | 95% Confidence Interval | p value | Regression Coefficient | |
| Model 1 | ||||
| Exercise Capacity | 0.83 | 0.79 – 0.88 | < 0.001 | −0.18 |
| Model 2 | ||||
| Age | 1.03 | 1.01 – 1.04 | < 0.001 | 0.03 |
| Exercise capacity | 0.85 | 0.81 – 0.90 | < 0.001 | −0.15 |
| Model 3 | ||||
| Age | 1.03 | 1.01 – 1.04 | < 0.001 | 0.03 |
| Exercise capacity | 0.85 | 0.81 – 0.90 | < 0.001 | −0.16 |
| Pack-years | 1.01 | 1.00 – 1.01 | 0.001 | 0.006 |
| Model 4 | ||||
| Age | 1.03 | 1.01 – 1.05 | <0.001 | 0.03 |
| Exercise capacity | 0.87 | 0.82 – 0.91 | <0.001 | −0.14 |
| Pack-years | 1.01 | 1.0 – 1.01 | 0.001 | 0.005 |
| Heart failure history | 1.92 | 1.34 – 2.76 | <0.001 | 0.65 |
| Cardiovascular Mortality | ||||
|---|---|---|---|---|
| Hazard Ratio | 95% Confidence Interval | p value | Regression Coefficient | |
| Model 1 | ||||
| Exercise capacity | 0.80 | 0.73 – 0.87 | < 0.001 | −0.22 |
| Model 2 | ||||
| Age | 1.03 | 1.01 – 1.06 | 0.02 | 0.03 |
| Exercise capacity | 0.82 | 0.75 – 0.90 | < 0.001 | −0.19 |
| Model 3 | ||||
| Age | 1.03 | 1.01 – 1.06 | 0.007 | 0.03 |
| Exercise capacity | 0.84 | 0.76 – 0.92 | < 0.001 | −0.18 |
| Heart failure history | 2.24 | 1.3 – 3.9 | 0.004 | 0.81 |
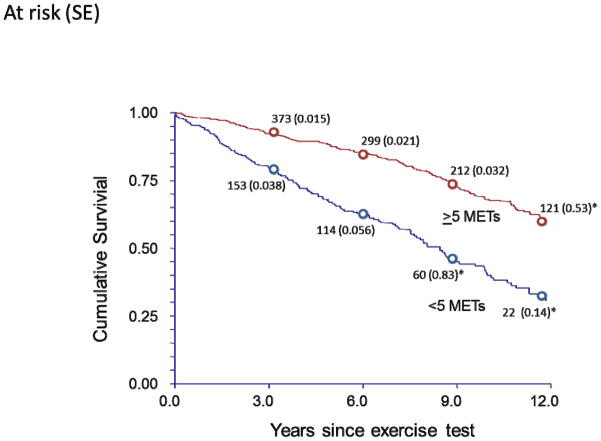
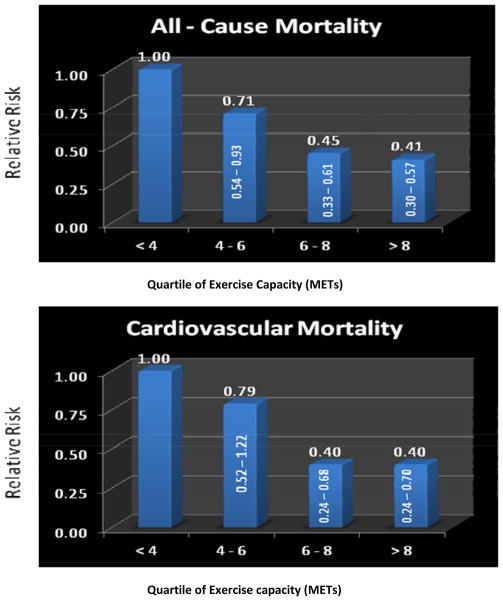
Figure 1 illustrates overall survival across the follow-up, and compares subjects who achieved <5 METs and ≥5 METs for exercise capacity. Survival among those achieving ≥5 METs was significantly better than those with lower exercise capacity p<0.001). Age-adjusted relative risks for cardiovascular and all-cause mortality based on quartiles of exercise capacity are presented in Figure 2. For both outcomes, relative risks decreased as exercise capacity increased (p for trend <0.001); the greatest outcome benefits were observed between the least fit group (<4 METs) and quartiles two and three (4–6 and 6–8 METs, respectively), while the two highest-fit quartiles had similar mortality rates.
Figure 1.
Kaplan-Meier curve showing cumulative survival among subjects achieving >5 METs and those achieving <5 METs. Numbers given along the curves are patients at risk at each time interval; numbers in parentheses are standard errors (p<0.001 between groups).
*indicates standard error >10%.
Figure 2.
Relative risks for all-cause (top) and cardiovascular mortality (bottom) based on quartiles of exercise capacity (p for trend < 0.001).
Discussion
In this report, we show for the first time that total exercise capacity, defined as METs achieved during a treadmill exercise test, is the strongest predictor of survival among clinical and exercise test variables in a large cohort of patients with PAD. We observed that this factor predicts both cardiovascular as well as all-cause mortality, and is independent of other traditional risk factors and measured exercise parameters. Similar to patients with other forms of cardiovascular disease 3, we observed an age-adjusted 17% reduction in total mortality, and an age-adjusted 20% reduction in cardiovascular mortality with each additional MET achieved on a treadmill test. To our knowledge, the prognostic value of this exercise test parameter has not previously been reported in patients with PAD, and these findings support the routine use of exercise capacity when evaluating these patients.
Several prior studies have documented a therapeutic role for exercise in PAD 12–14, but few have assessed the role of exercise capacity as a prognosticator in this patient population. de Liefde and colleagues previously reported that a hypertensive response to exercise was associated with adverse outcomes in PAD patients 15. However, this study employed a single stage submaximal test, and did not assess maximal exercise capacity. In the current study, a hypertensive response to exercise was not found to be a predictive factor. McDermott and colleagues recently showed that several office-based measures of walking could predict survival 16,17, but they did not directly assess overall fitness or exercise capacity, parameters that have been shown to be highly predictive in other cohorts, including patients suspected of having coronary disease, patients with heart failure, and normal subjects 1–6,18. Herein we observed that simply moving from the lowest quartile of cardiovascular fitness into the next lowest category was associated with 20–30% reductions in cardiovascular and all-cause mortality. From an epidemiological perspective, this association between a modest difference in fitness level and higher rates of survival is quantitatively greater than the benefits derived from the best known interventions for PAD. In fact, exercise capacity was superior to all other factors measured in the current study. Based on the significant prognostic power of this single variable, we believe that exercise capacity should be given as much consideration as traditional risk factors, especially when considering the likelihood of long term survival in the PAD patient.
Our results are consistent with recent evidence that has linked PAD to a lifetime of physical inactivity. It is well known that the sedentary state is a risk factor for major adverse cardiovascular events 2,19. Individuals with PAD are known to be relatively sedentary, and it has been assumed that the limitations of impaired limb perfusion were responsible for their reduced physical activity. However, most patients with PAD do not complain of intermittent claudication20, which has been attributed in part to their lack of activity. Most recently, we have found in a cohort of 1381 individuals referred for cardiac catheterization that lifetime physical activity (as assessed by a standardized and validated questionnaire) was positively correlated to the ankle-brachial index 21. Individuals in the quintile with the least lifetime recreational activity had twice the risk of PAD by comparison to those with the most activity [Notably, the reduction in the prevalence of PAD with increasing activity was not linear; the largest reduction occurred between the group with essentially no recreational activity and the next least active group]. Thus, PAD may in part be a manifestation of the sedentary state19. Taken together, it is becoming increasingly clear that identifying those with the lowest exercise capacity is important not only to help predict the presence and severity of PAD, but also to prognosticate the future risk of death.
We did not directly test the hypothesis that raising one’s achievable MET level through exercise training could reclassify a subject into a lower risk category. In addition, fitness is an attribute (not a behavior) that has a strong genetic component 22. However, physical activity is the only known method for developing overall fitness, and prior studies have shown that supervised exercise training has the added benefit of reducing claudication symptoms and extending walking distance amongst PAD patients 12,13,23. Future prospective randomized studies are indicated to measure whether exercise programs can not only improve quality of life14, but also reduce morbidity and mortality in this high risk cohort.
This report has several limitations that warrant consideration. Our cohort was comprised largely of men, and therefore the prognostic utility of exercise capacity in women is not defined. It is worth noting, however, that a recent meta-analysis of exercise testing studies in both men and women showed that achieved METs was equally powerful in healthy subjects of each gender 3. Also, ankle brachial indices (ABIs) were not recorded at the time of exercise testing, and we therefore could not ascertain an interaction between severity of PAD and the prognostic capacity of the exercise test, although ABI has previously been shown to correlate weakly with pain free walking 24. Finally, enrollment for this study began nearly 15 years ago and many subjects were prescribed a regimen that would not be considered maximal medical therapy by today’s standards. Thus, it is not clear if a ‘modern’ medical regimen would attenuate the observed association, or if it would continue to have predictive capacity, as reported in other cohorts.
In conclusion, this study shows the prognostic utility of exercise capacity for the patient with PAD. Exercise capacity on a standardized exercise test is more predictive of long term survival than any traditional risk factor or other measured physiologic parameter. These results raise the hope that event-free survival might be increased in these patients by increasing their exercise capacity, and suggest this as an area of future study.
Acknowledgments
Grant support: This work was supported by grants from the National Institute of Health (K12 HL087746). There are no relationships with industry.
The authors would like to acknowledge the contributions of Shyam Panchal, Joshua Abella and Vic Froelicher for their help with the data collection and treadmill test interpretation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gibbons RJ, Balady GJ, Bricker JT, et al. ACC/AHA 2002 guideline update for exercise testing: summary article. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines) J Am Coll Cardiol. 2002 Oct 16;40(8):1531–1540. doi: 10.1016/s0735-1097(02)02164-2. [DOI] [PubMed] [Google Scholar]
- 2.Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002 Mar 14;346(11):793–801. doi: 10.1056/NEJMoa011858. [DOI] [PubMed] [Google Scholar]
- 3.Kodama S, Saito K, Tanaka S, et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA. 2009 May 20;301(19):2024–2035. doi: 10.1001/jama.2009.681. [DOI] [PubMed] [Google Scholar]
- 4.Balady GJ, Larson MG, Vasan RS, Leip EP, O’Donnell CJ, Levy D. Usefulness of exercise testing in the prediction of coronary disease risk among asymptomatic persons as a function of the Framingham risk score. Circulation. 2004 Oct 5;110(14):1920–1925. doi: 10.1161/01.CIR.0000143226.40607.71. [DOI] [PubMed] [Google Scholar]
- 5.Kokkinos P, Myers J, Faselis C, et al. Exercise capacity and mortality in older men: a 20-year follow-up study. Circulation. 2010 Aug 24;122(8):790–797. doi: 10.1161/CIRCULATIONAHA.110.938852. [DOI] [PubMed] [Google Scholar]
- 6.McAuley P, Pittsley J, Myers J, Abella J, Froelicher VF. Fitness and fatness as mortality predictors in healthy older men: the veterans exercise testing study. J Gerontol A Biol Sci Med Sci. 2009 Jun;64(6):695–699. doi: 10.1093/gerona/gln039. [DOI] [PubMed] [Google Scholar]
- 7.Myers J, Bellin D. Ramp exercise protocols for clinical and cardiopulmonary exercise testing. Sports Med. 2000 Jul;30(1):23–29. doi: 10.2165/00007256-200030010-00003. [DOI] [PubMed] [Google Scholar]
- 8.Borg GB. Human Kinetics. Champaign: 1998. Borg’s Perceived Exertion and Pain Scales. [Google Scholar]
- 9.http://www.cdc.gov/physicalactivity/everyone/measuring/exertion.html.
- 10.American College of Sports Medicine. Metabolic Equations Handbook. Baltimore: Lippincott, Williams, & Wilkins; 2007. pp. 25–30. [Google Scholar]
- 11.Shue PFV. EXTRA: An expert system for exercise test reporting. J Non-Invasive Testing. 1998;II(4):21–27. [Google Scholar]
- 12.Watson L, Ellis B, Leng GC. Exercise for intermittent claudication. Cochrane Database Syst Rev. 2008;(4):CD000990. doi: 10.1002/14651858.CD000990.pub2. [DOI] [PubMed] [Google Scholar]
- 13.Regensteiner JG. Exercise rehabilitation for the patient with intermittent claudication: a highly effective yet underutilized treatment. Curr Drug Targets Cardiovasc Haematol Disord. 2004 Sep;4(3):233–239. doi: 10.2174/1568006043336195. [DOI] [PubMed] [Google Scholar]
- 14.Murphy TP, Cutlip DE, Regensteiner JG, et al. Supervised exercise versus primary stenting for claudication resulting from aortoiliac peripheral artery disease: six-month outcomes from the claudication: exercise versus endoluminal revascularization (CLEVER) study. Circulation. 2012 Jan 3;125(1):130–139. doi: 10.1161/CIRCULATIONAHA.111.075770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de L, II, Hoeks SE, van Gestel YR, et al. Usefulness of hypertensive blood pressure response during a single-stage exercise test to predict long-term outcome in patients with peripheral arterial disease. Am J Cardiol. 2008 Oct 1;102(7):921–926. doi: 10.1016/j.amjcard.2008.05.032. [DOI] [PubMed] [Google Scholar]
- 16.McDermott MM, Tian L, Liu K, et al. Prognostic value of functional performance for mortality in patients with peripheral artery disease. J Am Coll Cardiol. 2008 Apr 15;51(15):1482–1489. doi: 10.1016/j.jacc.2007.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McDermott MM, Liu K, Ferrucci L, et al. Decline in functional performance predicts later increased mobility loss and mortality in peripheral arterial disease. J Am Coll Cardiol. 2011 Feb 22;57(8):962–970. doi: 10.1016/j.jacc.2010.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kokkinos P, Myers J. Exercise and physical activity: clinical outcomes and applications. Circulation. 2010 Oct 19;122(16):1637–1648. doi: 10.1161/CIRCULATIONAHA.110.948349. [DOI] [PubMed] [Google Scholar]
- 19.Franco OH, de Laet C, Peeters A, Jonker J, Mackenbach J, Nusselder W. Effects of physical activity on life expectancy with cardiovascular disease. Arch Intern Med. 2005 Nov 14;165(20):2355–2360. doi: 10.1001/archinte.165.20.2355. [DOI] [PubMed] [Google Scholar]
- 20.Hirsch AT, Criqui MH, Treat-Jacobson D, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001 Sep 19;286(11):1317–1324. doi: 10.1001/jama.286.11.1317. [DOI] [PubMed] [Google Scholar]
- 21.Wilson AM, Sadrzadeh-Rafie AH, Myers J, et al. Low lifetime recreational activity is a risk factor for peripheral arterial disease. J Vasc Surg. 2011 Aug;54(2):427–432. 432 e421–424. doi: 10.1016/j.jvs.2011.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bouchard CMR, Perusse L, editors. Human Kinetics. Champaign: 1997. Genetics of Fitness and Physical Performance. [Google Scholar]
- 23.McDermott MM, Ades P, Guralnik JM, et al. Treadmill exercise and resistance training in patients with peripheral arterial disease with and without intermittent claudication: a randomized controlled trial. JAMA. 2009 Jan 14;301(2):165–174. doi: 10.1001/jama.2008.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Long J, Modrall JG, Parker BJ, Swann A, Welborn MB, 3rd, Anthony T. Correlation between ankle-brachial index, symptoms, and health-related quality of life in patients with peripheral vascular disease. J Vasc Surg. 2004 Apr;39(4):723–727. doi: 10.1016/j.jvs.2003.12.006. [DOI] [PubMed] [Google Scholar]