Abstract
The pathophysiology of sepsis and its accompanying systemic inflammatory response syndrome (SIRS) and the events that lead to multiorgan failure and death are poorly understood. It is known that, in septic humans and rodents, the development of SIRS is associated with a loss of the redox balance, but SIRS can also develop in non-infectious states. In addition, a hyperinflammatory state develops, together with impaired innate immune functions of phagocytes, immunosuppression, and complement activation, collectively leading to septic shock and lethality. Here we discuss recent insights into the signaling pathways in immune and phagocytic cells that underlie sepsis and SIRS and consider how these might be targeted for therapeutic interventions to reverse or attenuate pathways that lead to lethality during sepsis.
Introduction
Sepsis is a huge and expensive medical problem throughout the world. In North America, it is estimated that there are more than 600,000 cases of sepsis annually, with a mortality rate ranging between 30-50% [1]. The costs of medical care are estimated to exceed $17 billion annually in the U.S. [2]. Currently, there are no specific therapeutic interventions that are FDA-approved for treatment in sepsis. Recently, a recombinant human activated protein C (drotrecogin alfa) was withdrawn from the market because of a lack of efficacy (defined by the FDA as survival at 28 days) in septic humans [3, 4]. Many factors have been postulated to trigger SIRS, including products released from bacteria (such as lipoteichoic acid and bacterial lipopolysaccharide (LPS)) as well as products from damaged cells released after ischemia-reperfusion or after blunt trauma) in which no infectious agent is involved [5]. TLR4 signaling, leading to production of inflammatory mediators, has been suggested a key pathway in sepsis pathophysiology. Lipopolysaccharide from Gram negative bacteria reacts with TLR4 to cause phagocytic cells to robustly generate a variety of proinflammatory cytokines. TLR ligands include those derived from bacteria (LPS, lipopeptides, lipoteichoic acid, etc.) in addition to host-derived products such as intracellular proteins, extracellular matrix components and oxidized lipids (reviewed, [5]). There has been speculation that Gram negative bacteria in the gut may gain access to the circulation during septic shock, releasing LPS that reacts with TLR4, leading to SIRS. [6] However, recent clinical trials with a TLR4 antagonist (Eritoran) involving 1,800 septic patients were discontinued due to lack of efficacy of the drug. It is clear that we currently have inadequate knowledge regarding molecular mechanisms associated with the development of SIRS and sepsis in humans.
During sepsis or hemorrhagic shock in both rodents and humans a hyperinflammatory state develops that is referred to as SIRS [7]. This is also associated with enhanced expression of adhesion molecules on blood phagocytes (monocytes and neutrophils) and endothelial cells (reviewed, [8]) that leads to buildup of neutrophils and monocytes in organs. Complement activation products, together with products of neutrophils, monocytes and dendritic cells probably contribute to organ injury and lethality in sepsis [8]. Neutrophil depletion after the onset of sepsis improves survival after CLP and reduces concentrations of proinflammatory cytokines as well as plasma transaminases (indicators of liver injury) and creatinine levels (indicator of renal dysfunction) (reviewed, [8]).
It is possible that the complications developing in experimental sepsis and perhaps in human sepsis may be attenuated by therapeutic interventions that either reduce the levels of proinflammatory mediators or restore degraded adaptive and innate immune responses. In this review we describe studies implying that activation of certain signaling pathways may reverse the loss of the redox balance in sepsis, thus reducing SIRS, multiorgan failure (MOF) and lethality. Also discussed are strategies to reverse the immunosuppression associated with sepsis, the role of C5a and its receptors (C5aR, C5L2) in development of sepsis and how blockade of C5a or its receptors can prevent the progression of SIRS, ROS imbalance and development of septic shock and consequences that lead to MOF and lethality. Finally, we point out how manipulation of the autonomic nervous system can be employed to halt the progression of sepsis, at least in septic animals.
Redox Imbalance, SIRS, and the Hyperinflammatory Response of Sepsis
During sepsis, biochemical changes lead to an imbalance in the redox system leading to formation of an oxidant state, which appears to intensify SIRS and the downstream events (see below) [9]. This imbalance favors the oxidant state and is associated with reduced plasma and tissue levels of the antioxidants such as glutathione (GSH), thioredoxins, selenium (needed for antioxidant enzymes and production of GSH), together with reduced levels of mitochondrial ATP and the presence of oxidized lipids. In clinical trials of sepsis, addition of glutamine (precursor of GSH) and/or selenium to parenteral nutritional fluids improves clinical outcomes and reduces the intensity of the SIRS response [10]. This suggests that there may be a cause and effect relationship between redox imbalance, the intensity of the SIRS response and other downstream events during sepsis. In random, double-blind, placebo-controlled clinical trials in sepsis, administration of antioxidants (N-acetyl cysteine, α-tocopherol, vitamin C) or antioxidant promoting factors (selenium) was associated with clinical improvement, together with an attenuated SIRS response, suggesting a linkage between ROS and SIRS [11]. Strategies for antioxidant interventions in septic humans have been recently reviewed [9, 12]. Larger clinical trials would be needed to substantiate such findings.
During the inflammatory response, activated neutrophils and macrophages generate prodigious amounts of reactive oxygen species (ROS), and in some situations reactive nitrogen species (RNS) [13]. Activated neutrophils produce H2O2 via activation of NADPH oxidase (NOX2) [14]. Neutrophil myeloperoxidase generates derivatives of H2O2 (such as hypochlorous acid), while the hydroxyl radical (HO*), the most reactive of all oxygen radicals, is produced in a manner that requires reduced iron (Fe2+) [15]. RNS products are generated by phagocytic cells and include nitric oxide (*NO) and peroxynitrite anion (ONOO*) which can react with a variety of targets. ROS and RNS products can bring about reversible or irreversible chemical changes (oxidation, nitrosylation and nitrosation) in proteins, lipids, and DNA, resulting in diminished biochemical functions [16]. The greater the amounts of ROS and RNS, the more extensive the chemical changes in these targets. ROS and RNS can induce adducts to DNA, leading to DNA fragmentation [17]. In general, the excessive amount of ROS and RNS products that are produced during sepsis are damaging to cells and organs.
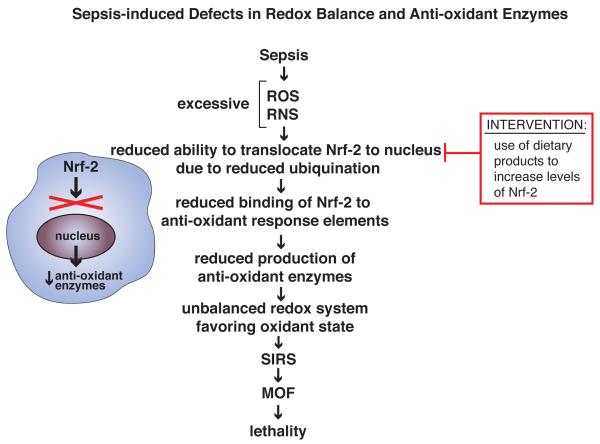
ROS and RNS concentrations at steady-state are tightly regulated by a series of inducible anti-oxidant enzymes (such as NAD(P)H quinone oxidoreductase-1, heme oxygenases, glutamate-cysteine ligase, UDP-glucuronosyltransferase and glutathione-S-transferase). Production of these enzymes is regulated by the nuclear transcription factor, nuclear factor-2 (Nrf2) (Figure 1) [18]. Other important anti-oxidant enzymes include catalase and superoxide dismutase (SOD), which can be induced in various cell types by extracts of medicinal plants, LPS, or lipid peroxides. Excessive production of ROS and RNS impairs the ability of Nrf2 to translocate to the nucleus because of reduced ubiquination, causing a nuclear deficiency of Nrf2. Nrf2 ordinarily binds to the anti-oxidant response elements in nuclear DNA which results in induction of anti-oxidant enzymes listed above [18]. Accordingly, defects in Nrf2 cause an imbalance in the redox system, favoring an oxidant condition. Genetic manipulation of mice resulting in enhancement of Nrf2 levels improved rodent outcomes in sepsis [19, 20] (see Box 1 for discussion of animal models for sepsis). Nrf2 activity can be restored in septic mice by use of compounds such as the anti-oxidant, sulforaphane, which increases intracellular levels of Nrf2, ultimately leading to increased levels of anti-oxidant enzymes, preventing systemic buildup of ROS and RNS [18, 21, 22]. Perhaps intervention with sulforaphane could be done by oral or parenteral administration in septic humans in order to restore reduced Nrf2 nuclear levels during sepsis, thereby reversing imbalances in the redox state during development of sepsis. While these interventions have shown efficacy in vitro [18] and in mice with ischemia [21], it is not known if these interventions would be effective in septic humans.
Figure 1.
Sepsis-induced defects in redox balance and anti-oxidant enzymes. In sepsis, an excessive oxidant state (presence of elevated levels of ROS, RNS) leads to reduced ubiquitination and nuclear translocation of the transcription factor, Nrf2, which in turn leads to reduced activation of genes that encode for anti-oxidant enzymes [19, 20, 22]. This imbalance intensifies the SIRS response and enhances development of MOF and lethality. Restoration of the redox balance by inducers of Nrf2 may reduce the intensity of SIRS and downstream events that would otherwise be lethal, at least based on studies in CLP mice. Dietary products, such as sulforaphane, cause of induction of Nrf2 and may be candidates for reversal of the redox imbalance in sepsis [18].
Text Box 1. Animal Models of Sepsis and Relevance to Sepsis in Humans.
A common experimental sepsis model is cecal ligation and puncture (CLP) in rodents, which has been described as the “gold standard” for study of sepsis [55]. The intensity of the inflammatory response in sepsis can range from “moderate” to “intense”, depending on the number of cecal perforations, the gauge of the needle used, and the amount of the cecum ligated [8, 56]. SIRS commonly develops early in sepsis both in rodents and in humans. It’s development suggests an inability to regulate and confine the inflammatory response, the results of which lead to development of septic shock, multiorgan failure (MOF) and lethality. Alternatively, it is possible that the SIRS response is due to an inability to resolve the infectious pathogenic agent that triggered the sepsis response [4]. Sepsis and its various manifestations are usually caused by an infectious pathogen, but the fact that sepsis SIRS can also develop during conditions of “sterile” inflammation (as in polytrauma in humans) indicates that SIRS may develop in the absence of infectious organisms [57]. Other animal models of sepsis include endotoxemia and peritoneal or airway instillation of live bacteria such as E. coli, Klebsiella sp. or Pseudomonas sp. The assumption that the endotoxemia model is a surrogate for human sepsis has been challenged [58]. Humans are highly susceptible to intravenous infusion of LPS, based on studies in which very small (low ng) quantities of LPS induced strong biological responses, including a febrile state, cytokine storm, neutrophilia, consumptive coagulopathy and many other features of responses occurring early in sepsis [59]. The endotoxemic model in humans has been used for many decades and is still providing useful information that may have relevance to sepsis related to gram negative bacteria, especially involving the gut, lung and urogenital tract. However, it may be difficult to assume that responses to humans infused with LPS can be generalized to encompass all responses in sepsis, including those involving Gram negative bacteria (as in Staph. aureus infections). The advantages and limitations of animal models of sepsis as well as their relevance to human sepsis have been recently reviewed [60-64]. SIRS can be detected in the first few days after CLP as sepsis progresses [8]. This is often followed by cardiac and vascular dysfunction that evolves into septic shock, requiring blood pressure support. During progression of sepsis (in animals and in humans), there is simultaneous development of consumptive coagulopathy (reduction of the various clotting factors in plasma) and other metabolic dysfunctions, all of which can progress to multiorgan failure (MOF), which can include the lungs, liver and kidneys and perhaps the brain. The end result is often death.
Emerging evidence suggests that, in both septic humans and CLP mice recovering from sepsis, long-term defects in innate immune defenses (such as chemotaxis and phagocytosis) develop, as well as immunodeficiency, defective cognition, and diminished skeletal muscle function. In addition, sepsis may create an environment to promote the progression of malignancies as a consequence of diminished tumor surveillance [65]. These observations suggest that sepsis is not only an acute inflammatory disease but may also, after “recovery”, lead to significant complications months or years later [25, 66].The reasons for these delayed, long-lasting complications, which have been referred to as the “lingering consequences of sepsis” [66] are poorly understood. In humans who experienced acute renal failure (acute tubular necrosis, ATN) during sepsis that was successfully treated by hemodialysis, there was a 34.2% mortality rate two years later compared to a a 8.9% mortality rate in recovered ATN patients who had not been septic [67]. Increasingly, it appears that “recovered” septic patients have significantly increased mortality rates compared to age matched individuals who have not previously experienced sepsis [66, 67].
Early in sepsis there is evidence of excessive activation of phagocytes and dendritic cells (as described above), but, as sepsis progresses, there is progressive functional deterioration of these cells [23, 24], which results in degraded innate immune functions (such as phagocytosis, chemotaxis and the ability to kill phagocytized organisms) [8, 25] There are also diminished adaptive immune responses (as described in the section dealing with the immunosuppression of sepsis). The fact that these changes seem to occur sequentially during development of sepsis has implications for therapeutic interventions.
Imbalances in Nuclear Hormone Receptors and Reduced ATP and Antioxidant Enzymes in Sepsis
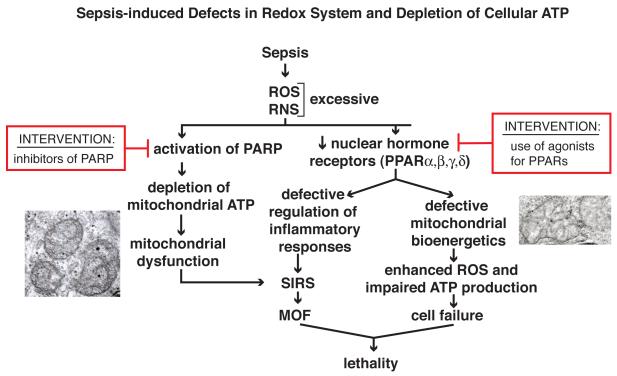
Intranuclear peroxisome proliferator activated receptors (PPARs) are expressed in a variety of cells in many different tissues. They function as transcription factors that negatively regulate the expression of proinflammatory mediators (Figure 2). Interaction of PPARs with their ligands results in anti-inflammatory outcomes in the setting of peritonitis and sepsis, as well as suppressed proinflammatory mediator production in vitro by macrophages [26-30]. Buildup of ROS and RNS during sepsis is accompanied by impaired PPAR signalling [28-30]. Activation of PPARs can be protective in models of sepsis [30, 31], leading to attenuated systemic inflammation (reduced SIRS and diminished buildup of PMNs in organs) and preserved cardiovascular function [27-30, 32]. As sepsis progresses, nuclear levels of PPARs steadily decline. In pediatric patients with septic shock, reduced amounts of mRNA encoding PPARα are detected in blood leukocytes [27]. Bacterial colony forming units in various organs were substantially higher in PPAR-/- mice when compared to Wt mice [30], suggesting that reduced PPAR activity is disadvantageous for survival. Inadequate PPAR activity appears to lessen the ability to control ROS and RNS buildup during sepsis, increasing the intensity of SIRS (Figure 2). The protective role of PPARs in sepsis may be due to their ability to preserve mitochondrial function and bioenergetics. In sepsis, mitochondrial electron transport becomes progressively defective, causing the buildup of ROS within mitochondria, leading to an inability to maintain intracellular levels of ATP which can cause cell and organ failure. PPARγ reduces the expression of nitric oxide synthase (which generates RNS) in sepsis and restrains the production of proinflammatory cytokines [27, 28]. Agonists for PPARγ (such as curcumin or GW0742) attenuate inflammatory responses in experimental sepsis, supporting the protective roles for PPARs in the septic state [20, 30, 33]. Other agonists for PPARs (thiazolidinediones and the cyclopentenone prostaglandin, 15d-PGJ2) may also be useful for restoring the anti-inflammatory activities linked to PPARs [27].
Figure 2.
Sepsis-induced defects in redox system and depletion of cellular ATP. The nuclear hormone peroxisome proliferator activity receptors (PPARs) participate in responses to oxidative stresses, resulting in preservation of mitochondrial function and containment of inflammation [20, 26-30]. Sepsis causes reduced levels of PPARs accompanied by defective mitochondrial function as well as reductions in mitochondrial ATP, and reduced ability to restrain inflammatory responses such as SIRS. The two insets are transmission electron micrographs showing swollen mitochondria (in liver) during sepsis (X12,500). Several agonists for PPARs have been developed [27, 32, 33]. Sepsis and increased ROS and RNS also trigger DNA single strand breaks, which results in activation of poly ADP-ribose polymerase (PARP) for repair of DNA [17]. In the process, depletion of NAD+ levels can occur, accompanied by reductions in cellular and mitochondrial ATP. Inhibitors for PARP have been developed, which may preserve mitochondrial ATP during sepsis.
As described above, elevated levels of ROS and RNS in sepsis can induce defects in DNA (such as adducts or DNA scission) which activate the repair enzyme, poly-ADP ribose polymerase (PARP) (Figure 2). This enzyme consumes ATP, leading to ATP depletion, especially in mitochondria, accompanied by exaggerated SIRS. Collectively, it would appear that, in inflammatory responses to sepsis and to ischemia-reperfusion, activation of PARP compromises homeostasis by causing significant depletion of mitochondrial ATP. Accordingly, blockade of PARP or its reduction by siRNA technology can reduce oxidative stress in endothelial cells [34]. Perhaps similar interventions could be considered for use in humans with sepsis.
Adverse Roles of C5a and C5a Receptors in Sepsis
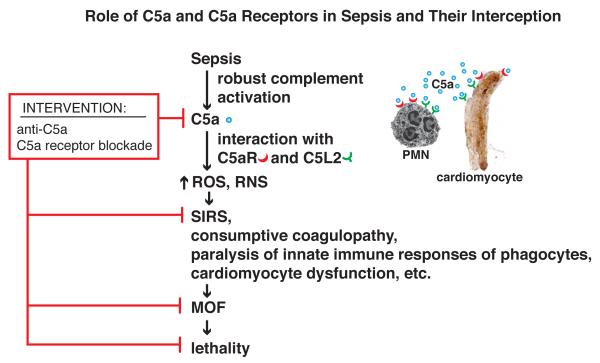
In CLP rodents and in septic humans, robust activation of complement occurs, resulting in generation of the powerful anaphylatoxin, C5a, which is intensely proinflammatory (Figure 3). In CLP rodents, C5a interacting with its receptors (C5aR, C5L2) triggers a cascade of destructive outcomes including inhibition of innate immune signaling pathways, leading to impaired phagocyte function (e.g. phagocytosis, chemotaxis and the oxidative burst) [35]. In vivo blockade of C5a markedly improved survival after CLP accompanied by an attenuation of the consumptive coagulopathy of sepsis as well as reduction of the SIRS response, reduced intensity of MOF involving heart, kidneys, lungs and liver, as well as diminished apoptosis of the lymphoid system and the intensity of septic shock (reviewed, [8]). Genetic ablation of C5a receptors or their blockade with neutralizing antibodies was also protective and substantially improved the rate of survival in CLP mice. Emerging evidence suggests that C5a may, in a dose-dependent manner, also negatively regulate TLR4-dependent macrophage production of proinflammatory mediators via macrophage release of IL-10 [36]. Such data suggest that in CLP mice engagement of both C5a receptors (C5aR, C5L2) is necessary for full expression of SIRS, MOF and lethality. The development of SIRS occurs at a time when CLP animals begin to exhibit an exaggerated proinflammatory phenotype, such as increased expression of adhesion molecules on both endothelial cells and on leukocytes (reviewed, [8]). During development of CLP, neutrophils acquire increased expression of chemokine receptors and β integrins, while endothelial cells generated increased amounts of chemokines and upregulated their surface expression of ICAM-1, all of which represents a “gain of function” phenotype that would be distinctly proinflammatory [37]. These studies emphasize the functional consequences of C5a interacting with its receptors on a variety of cell types to bring about adverse outcomes in polymicrobial sepsis. Based on this information, Phase I (safety) clinical trials have been completed in normal humans, employing a “humanized” mouse monoclonal neutralizing antibody to human C5a. This antibody reacts with C5a but not with the parent molecule, C5. No adverse events were detected in these individuals. Further information can be found at www.inflarx.com/. This may set the stage for application of this antibody in phase II and phase III clinical trials in septic humans.
Figure 3.
C5a and C5a receptors as targets in sepsis. In sepsis, C5a, and its receptors (C5aR, C5L2), are involved in a series of responses that promote development of SIRS, MOF, septic shock and lethality. Early in sepsis, robust activation of complement occurs, generating C5a which interacts with its receptors to promote development of ROS, RNS and onset of SIRS. C5aR and C5L2 are expressed in large amounts by neutrophils, at lower levels in macrophages and monocytes and non-myeloid cells (endothelial cells, alveolar epithelial cells, Kupffer cells, cardiomyocytes, etc.) [35, 36]. Development of SIRS and its complications (immunosuppression, defective innate and adaptive immune responses, consumptive coagulopathy) results in a sequence of events that is often lethal. In vivo neutralization of C5a with antibodies or blockade of C5a receptors (C5aR, C5L2) suppresses this cascade of adverse events and improves survival in CLP rodents. In vivo, blockade of C5a with neutralizing antibody in CLP rodents greatly improves survival, even if given up to 12 h after CLP [8].
Neural Regulation of the Immune/Inflammatory Responses in Sepsis
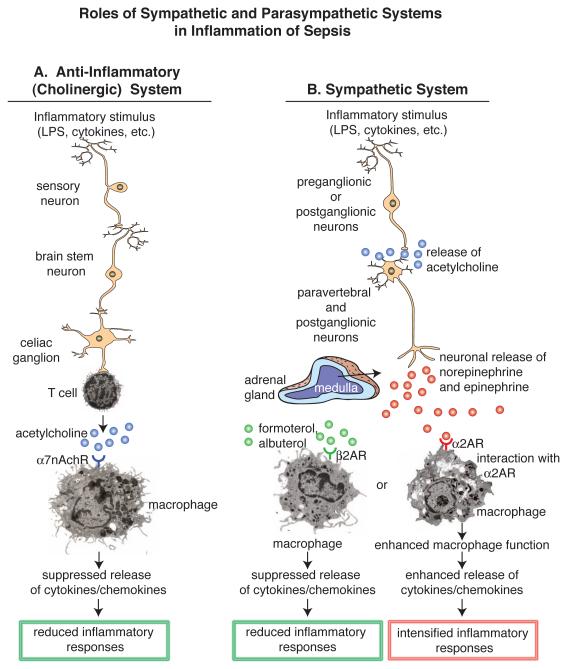
There is accumulating evidence in sepsis and in other inflammatory conditions that immune and inflammatory responses are regulated by the autonomic nervous system, including both the parasympathetic and sympathetic nervous systems (PNS and SNS, respectively) (Figure 4). Most of our understanding of the role of the PNS in sepsis comes from the work of Tracey and colleagues who have coined the term “the inflammatory reflex”, or the “cholinergic anti-inflammatory pathway” [38]. This group has shown in sepsis and in a variety of other inflammatory conditions in animals (e.g. colitis, hemorrhagic shock, ischemia reperfusion injury) that a neural reflex involving the vagus nerve causes T cells to release acetylcholine that interacts with the α7nAch receptor on macrophages to dampen the release of powerful proinflammatory mediators such as TNFα and HMGB-1 (Figure 4A) [39-42]. Stimulation of the vagal nerve suppresses the SIRS response of sepsis and improves survival in CLP mice, as does the use of a selective or a universal synthetic agonist for α7nAchR on macrophages [38] or vagal nerve stimulation [39].
Figure 4.
Role of the parasympathetic and sympathetic nervous systems (PNS, SNS, respectively) in sepsis. a) The PNS, also referred to as the cholinergic system, acts via the vagus nerve to release acetylcholine from T cells, suppressing proinflammatory mediator release form activated macrophages and T cells [38]. b) Depending on which adrenergic receptor is in play, the SNS pathway may either intensify [45] or suppress the lung inflammatory response [46]. The cholinergic pathway can be activated by vagal nerve stimulation or use by chemical antagonists of the acetylcholine receptor, α7nAchR, resulting in suppressed release of proinflammatory mediators from macrophages. The SNS pathways can suppress the inflammatory response in the presence of β2AR agonists (formoterol, albuterol). Alternatively, agonists (epinephrine, norepinephrine) of β2ARs will intensify the lung inflammatory response [45]. Thus there are several strategies to target the PNS or SNS that may be used to suppress inflammatory responses and reduce ROS/RNS formation, the intensity of SIRS and other adverse events in sepsis.
In studies of the SNS, the role of adrenergic receptors (ARs) has been evaluated in a variety of different inflammatory conditions in lungs (Figure 4B). ARs are classified as α(1,2)ARs and β(1,2,3)ARs and interact with catecholamines (epinephrine and norepinephrine), resulting in a variety of biological responses based on the particular AR activated and the cell type expressing the AR (smooth muscle cells, cardiomyocytes, macrophages, etc.). Development of both acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) are common in septic humans. These conditions produce severe problems with gas exchange in lung, and both ALI and ARDS represent harmful inflammatory responses in lung associated with presence of activated neutrophils and the complement activation product, C5a [43, 44]. In human sepsis, pulmonary dysfunction is one of the earliest life-threatening complications, often requiring mechanical ventilation. In the setting of experimental ALI induced either by intra-alveolar deposition of IgG immune complexes (IC) or LPS, pharmacological blockade of α2AR, but not α1, β1 or β2ARs, reduced ALI intensity [45]. In recent studies, use of agonists (formoterol, albuterol) for β2ARs markedly reduced the intensity of ALI (measured by albumin leak into lung, buildup of lung neutrophils and levels of proinflammatory mediators in lung) [46]. It appears that the anti-inflammatory effects of β2AR agonists may be related to their ability to suppress activation of JNK1/2, even though it is known in the upper airways that the same drugs activate adenylate cyclase, producing cAMP, which is a powerful smooth muscle relaxant. β2AR agonists are commonly used in the treatment of human asthmatics (usually together with a corticosteroid). Collectively, it appears that the PNS and SNS pathways modulate inflammatory responses either positively or negatively. It is possible in the setting of sepsis that undesirable inflammatory responses that lead to SIRS and ALI/ARDS as well as to injury in several organs could be manipulated in a manner that suppresses the intensity of the inflammatory responses, taking advantage of new knowledge related to the autonomic nervous system.
Immunosuppression in Sepsis
The onset of sepsis in humans is accompanied by defective innate and adaptive immune responsiveness that can result in immuno-suppression early in sepsis, which is often long-lasting [47-49]. Suppressed immune responsiveness impairs effective removal of infectious organisms that trigger the sepsis response, thus intensifying all of clinical complications of sepsis, as described above. Another outcome is emergence of infective agents (e.g. Mycobacteria sp., pneumocystis fungus) to which healthy individuals are usually resistant. The most obvious in vivo defect in both septic humans and animals is a of loss of delayed type hypersensitivity reactions in the skin, the loss usually occurring a few days after onset of sepsis. Blood lymphocytes isolated from healthy donors produce an array of cytokines (e.g. IL-1β, TNFα, IL-6) in response to in vitro challenge with a variety of antigens or mitogens. Production of these mediators is reduced in cells from septic humans [47]. Intense immunosuppression in septic humans is associated with a worse prognosis, [47, 49].
Sepsis causes immunosuppression by at least three different mechanisms (Figure 5). Extensive apoptotic depletion of T and B cells from lymph nodes, spleen, gut, lung as well as in other organs is an obvious cause for defective immune responsiveness [47]. T and B cell apoptosis is induced by engagement of both the intrinsic pathway (mitochondrial) and by the extrinsic pathway (involving Fas and Fas ligand as well as TNFα and its receptors) [50]. Macrophage and dendritic cell dysfunction and depletion also occur during sepsis [23, 24, 47, 49, 51] resulting in impaired antigen presentation to T cells. Impaired antigen recognition by T cells compromises adaptive immunity. In sepsis innate immune functions of phagocytic cells (such as chemotactic and bactericidal responses) are compromised, which can substantially reduce resistance to infectious organisms. These events can cause inadequate containment of commensal bacteria (e.g. Pseudomonas and Klebsiella sp., E. coli, etc.) and non-commensal bacteria (e.g. Staph. aureus) as well as reduced defenses to fungi (e.g. Candida sp., Aspergillus sp.). This can lead to MOF, septic shock and lethality. It is possible that immunostimulant cytokines, such as IL-7, may restore immunocompetence in the setting of sepsis [52]. IL-7 is produced by a variety of cell types and is a growth factor for both T and B cells, in which it induces increased expression of the anti-apoptotic protein Bcl2. Preliminary studies in CLP mice (see text box 1 on animal models for sepsis) suggest that IL-7 my reverse the immunosuppressive state [52]. In humans with HIV infection, IL-7 has also been shown to improve CD4 and CD8 blood counts [53]. This suggests that IL-7 may be useful for treating immunocompromised patients with sepsis. However, when extensive apoptotic deletion of T and B cells has already occurred as happens in sepsis, the benefit of such strategies might be limited. Another candidate to target immunosuppression during sepsis is IL-15, which like IL-7, seems to prevent apoptosis and reverses the defects in innate and adaptive immunity during sepsis [54].
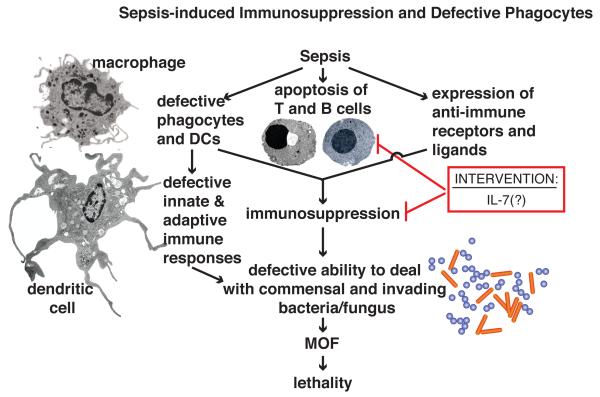
Figure 5.
Sepsis-induced immunosuppression and defective phagocytes. Sepsis leads to immunosuppression and defective phagocytosis, caused by depletion or functional deficiencies in macrophages and DCs, apoptosis of T and B cells, and tissue expression of inhibitory ligands and receptors that suppress immune responses, mostly by targeting T cells. Immunosuppression and defective phagocyte function, leads to failure to contain commensal and invading bacteria and fungi. The immunostimulant, IL-7, which promotes T and B cell proliferation and which has antiapoptotic effects, may be a candidate for clinical trials in humans to reverse the immunosuppression of sepsis [52].
Concluding Remarks
Development of sepsis is associated with a dysregulated inflammatory response, together with a shift in the redox balance toward the oxidant state, followed by development of SIRS, MOF and septic shock, suggesting that regulation of inflammatory responses is defective. Excessive generation of potent proinflammatory cytokines and chemokines soon after the onset of sepsis sets the stage for development of MOF and lethality. Information is developing that may explain why control of the inflammatory responses in sepsis is defective. There may be new interventions that affect signaling pathways and reverse this series of adverse events, restore the redox balance and, possibly, rejuvenate the immune system. Promising strategies for treatment of human sepsis include blockade of C5a, which attenuates the harmful cascade of events in sepsis, or treatment with IL-7 which restores immune responses. This information may have relevance to treatment of humans with sepsis.
Acknowledgements
This work was supported by grants GM-29507, GM-61656 to P.A.W. from the National Institutes of Health and project 571701, BO 3482/1-1 from the Deutsche Forschungsgemeinschaft to M.B. We acknowledge the excellent staff support of Beverly Schumann, Sue Scott and Robin Kunkel.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors have no direct financial or personal interests to declare.
References
- 1.Martin GS, et al. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 2.Coopersmith CM, et al. A comparison of critical care research funding and the financial burden of critical illness in the United States*. Crit Care Med. 2012;40:1072–1079. doi: 10.1097/CCM.0b013e31823c8d03. [DOI] [PubMed] [Google Scholar]
- 3. [Accessed November 18, 2011];Xigris [drotegogin alfa (activated)]: market withdrawal-failure survival benefit. 2011 www.fda.gov/Safety/Medwatch/Safety/Information/SafetyAlertsforHumanMedicalProducts/ucm277143.html. Posted October 25, 2011.
- 4.Angus DC. The search for effective therapy for sepsis: back to the drawing board? JAMA. 2011;306:2614–2615. doi: 10.1001/jama.2011.1853. [DOI] [PubMed] [Google Scholar]
- 5.Erridge C. Endogenous ligands of TLR2 and TLR4: agonists or assistants? J Leukoc Biol. 2010;87:989–999. doi: 10.1189/jlb.1209775. [DOI] [PubMed] [Google Scholar]
- 6.Clark JA, Coopersmith CM. Intestinal crosstalk: a new paradigm for understanding the gut as the “motor” of critical illness. Shock. 2007;28:384–393. doi: 10.1097/shk.0b013e31805569df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cai B, et al. Novel insights for systemic inflammation in sepsis and hemorrhage. Mediators Inflamm. 2010;2010:642462. doi: 10.1155/2010/642462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ward PA. The harmful role of C5a on innate immunity in sepsis. J Innate Immun. 2010;2:439–445. doi: 10.1159/000317194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andrades M, et al. Bench-to-bedside review: Sepsis - from the redox point of view. Critical Care. 2011;15:230. doi: 10.1186/cc10334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berger MM, Chiolero RL. Antioxidant supplementation in sepsis and systemic inflammatory response syndrome. Crit Care Med. 2007;35:S584–590. doi: 10.1097/01.CCM.0000279189.81529.C4. [DOI] [PubMed] [Google Scholar]
- 11.Crimi E, et al. Role of oxidative stress in experimental sepsis and multisystem organ dysfunction. Free Radic Res. 2006;40:665–672. doi: 10.1080/10715760600669612. [DOI] [PubMed] [Google Scholar]
- 12.von Dessauer B, et al. Oxidative stress as a novel target in pediatric sepsis management. J Crit Care. 2011;26:103, e101–107. doi: 10.1016/j.jcrc.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 13.van Berlo D, et al. Neutrophil-derived ROS contribute to oxidative DNA damage induction by quartz particles. Free Radic Biol Med. 2010;49:1685–1693. doi: 10.1016/j.freeradbiomed.2010.08.031. [DOI] [PubMed] [Google Scholar]
- 14.Moore SF, MacKenzie AB. NADPH oxidase NOX2 mediates rapid cellular oxidation following ATP stimulation of endotoxin-primed macrophages. J Immunol. 2009;183:3302–3308. doi: 10.4049/jimmunol.0900394. [DOI] [PubMed] [Google Scholar]
- 15.Lipinski B. Hydroxyl radical and its scavengers in health and disease. Oxid Med Cell Longev. 2011;2011:809696. doi: 10.1155/2011/809696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Valko M, et al. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 17.Martin LJ. DNA damage and repair: relevance to mechanisms of neurodegeneration. J Neuropathol Exp Neurol. 2008;67:377–387. doi: 10.1097/NEN.0b013e31816ff780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zakkar M, et al. Activation of Nrf2 in endothelial cells protects arteries from exhibiting a proinflammatory state. Arterioscler Thromb Vasc Biol. 2009;29:1851–1857. doi: 10.1161/ATVBAHA.109.193375. [DOI] [PubMed] [Google Scholar]
- 19.MacGarvey NC, et al. Activation of mitochondrial biogenesis by heme oxygenase-1-mediated NF-E2-related factor-2 induction rescues mice from lethal Staphylococcus aureus sepsis. Am J Respir Crit Care Med. 2012;185:851–861. doi: 10.1164/rccm.201106-1152OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kong X, et al. Enhancing Nrf2 pathway by disruption of Keap1 in myeloid leukocytes protects against sepsis. Am J Respir Crit Care Med. 2011;184:928–938. doi: 10.1164/rccm.201102-0271OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bell KF, et al. Activation of Nrf2-regulated glutathione pathway genes by ischemic preconditioning. Oxid Med Cell Longev. 2011;2011:689524. doi: 10.1155/2011/689524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kensler TW, Wakabayashi N. Nrf2: friend or foe for chemoprevention? Carcinogenesis. 2010;31:90–99. doi: 10.1093/carcin/bgp231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sirgo G, et al. [Dendritic cells in sepsis: an approach to post-infectious immunosuppression] Med Intensiva. 2010;34:559–566. doi: 10.1016/j.medin.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 24.Scumpia PO, et al. CD11c+ dendritic cells are required for survival in murine polymicrobial sepsis. J Immunol. 2005;175:3282–3286. doi: 10.4049/jimmunol.175.5.3282. [DOI] [PubMed] [Google Scholar]
- 25.Delano MJ, et al. Sepsis induces early alterations in innate immunity that impact mortality to secondary infection. J Immunol. 2011;186:195–202. doi: 10.4049/jimmunol.1002104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Polvani S, et al. PPARgamma and Oxidative Stress: Con(beta) Catenating NRF2 and FOXO. PPAR Res. 2012;2012:641087. doi: 10.1155/2012/641087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaplan JM, Zingarelli B. Novel Therapeutic Agents in Pediatric Sepsis: Peroxisome Proliferator Receptor gamma (PPAR gamma) Agonists. Open Inflamm J. 2011;4:120–124. doi: 10.2174/1875041901104010120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barrera G, et al. The Role of PPAR Ligands in Controlling Growth-Related Gene Expression and their Interaction with Lipoperoxidation Products. PPAR Res. 2008;2008:524671. doi: 10.1155/2008/524671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choi JH, et al. Antidiabetic actions of a non-agonist PPARgamma ligand blocking Cdk5-mediated phosphorylation. Nature. 2011;477:477–481. doi: 10.1038/nature10383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Standage SW, et al. Reduced peroxisome proliferator-activated receptor alpha expression is associated with decreased survival and increased tissue bacterial load in sepsis. Shock. 2012;37:164–169. doi: 10.1097/SHK.0b013e31823f1a00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vachharajani V, et al. Curcumin modulates leukocyte and platelet adhesion in murine sepsis. Microcirculation. 2010;17:407–416. doi: 10.1111/j.1549-8719.2010.00039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun Y, Bennett A. Cannabinoids: a new group of agonists of PPARs. PPAR Res. 2007;2007:23513. doi: 10.1155/2007/23513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khazaei M, et al. Pan-PPAR Agonist, Bezafibrate, Restores Angiogenesis in Hindlimb Ischemia in Normal and Diabetic Rats. Int J Pept. 2012;2012:637212. doi: 10.1155/2012/637212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mathews MT, Berk BC. PARP-1 inhibition prevents oxidative and nitrosative stress-induced endothelial cell death via transactivation of the VEGF receptor 2. Arterioscler Thromb Vasc Biol. 2008;28:711–717. doi: 10.1161/ATVBAHA.107.156406. [DOI] [PubMed] [Google Scholar]
- 35.Bamberg CE, et al. The C5a receptor (C5aR) C5L2 is a modulator of C5aR-mediated signal transduction. J Biol Chem. 2010;285:7633–7644. doi: 10.1074/jbc.M109.092106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bosmann M, et al. Complement activation product C5a is a selective suppressor of TLR4-induced, but not TLR3-induced, production of IL-27(p28) from macrophages. J Immunol. 2012;188:5086–5093. doi: 10.4049/jimmunol.1102914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Speyer CL, Ward PA. Role of endothelial chemokines and their receptors during inflammation. J Invest Surg. 2011;24:18–27. doi: 10.3109/08941939.2010.521232. [DOI] [PubMed] [Google Scholar]
- 38.Andersson U, Tracey KJ. Reflex principles of immunological homeostasis. Annu Rev Immunol. 2012;30:313–335. doi: 10.1146/annurev-immunol-020711-075015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Borovikova LV, et al. Role of vagus nerve signaling in CNI-1493-mediated suppression of acute inflammation. Auton Neurosci. 2000;85:141–147. doi: 10.1016/S1566-0702(00)00233-2. [DOI] [PubMed] [Google Scholar]
- 40.Bonaz B. The cholinergic anti-inflammatory pathway and the gastrointestinal tract. Gastroenterology. 2007;133:1370–1373. doi: 10.1053/j.gastro.2007.08.061. [DOI] [PubMed] [Google Scholar]
- 41.Kamal A. Assessment of autonomic function in patients with rheumatoid arthritis using spectral analysis and approximate entropy method. Neurosciences (Riyadh) 2007;12:136–139. [PubMed] [Google Scholar]
- 42.Stojanovich L, et al. Cardiovascular autonomic dysfunction in systemic lupus, rheumatoid arthritis, primary Sjogren syndrome and other autoimmune diseases. Lupus. 2007;16:181–185. doi: 10.1177/0961203306076223. [DOI] [PubMed] [Google Scholar]
- 43.Pierrakos C, et al. Acute respiratory distress syndrome: pathophysiology and therapeutic options. J Clin Med Res. 2012;4:7–16. doi: 10.4021/jocmr761w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matthay MA, et al. The acute respiratory distress syndrome. J Clin Invest. 2012;122:2731–2740. doi: 10.1172/JCI60331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Flierl MA, et al. Phagocyte-derived catecholamines enhance acute inflammatory injury. Nature. 2007;449:721–725. doi: 10.1038/nature06185. [DOI] [PubMed] [Google Scholar]
- 46.Bosmann M, et al. Anti-inflammatory effects of beta2 adrenergic receptor agonists in experimental acute lung injury. FASEB J. 2012;26:2137–2144. doi: 10.1096/fj.11-201640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boomer JS, et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA. 2011;306:2594–2605. doi: 10.1001/jama.2011.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ward PA. Immunosuppression in sepsis. JAMA. 2011;306:2618–2619. doi: 10.1001/jama.2011.1831. [DOI] [PubMed] [Google Scholar]
- 49.Hotchkiss RS, et al. The sepsis seesaw: tilting toward immunosuppression. Nat Med. 2009;15:496–497. doi: 10.1038/nm0509-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takahashi HK, et al. Activation of survival and apoptotic signaling pathways in lymphocytes exposed to palmitic acid. J Cell Physiol. 2012;227:339–350. doi: 10.1002/jcp.22740. [DOI] [PubMed] [Google Scholar]
- 51.Warner EA, Moldawer LL. Using innate immunity to characterize the host response to microbial invasion in severe sepsis. Future Microbiol. 2008;3:177–189. doi: 10.2217/17460913.3.2.177. [DOI] [PubMed] [Google Scholar]
- 52.Unsinger J, et al. IL-7 promotes T cell viability, trafficking, and functionality and improves survival in sepsis. J Immunol. 2010;184:3768–3779. doi: 10.4049/jimmunol.0903151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sereti I. Where have all the T cells gone? Blood. 2009;114:751–752. doi: 10.1182/blood-2009-04-217091. [DOI] [PubMed] [Google Scholar]
- 54.Inoue S, et al. IL-15 prevents apoptosis, reverses innate and adaptive immune dysfunction, and improves survival in sepsis. J Immunol. 2010;184:1401–1409. doi: 10.4049/jimmunol.0902307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dejager L, et al. Cecal ligation and puncture: the gold standard model for polymicrobial sepsis? Trends Microbiol. 2011;19:198–208. doi: 10.1016/j.tim.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 56.Rittirsch D, et al. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat Protoc. 2009;4:31–36. doi: 10.1038/nprot.2008.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lissauer ME, et al. Differential expression of toll-like receptor genes: sepsis compared with sterile inflammation 1 day before sepsis diagnosis. Shock. 2009;31:238–244. doi: 10.1097/SHK.0b013e3181834991. [DOI] [PubMed] [Google Scholar]
- 58.Remick DG, Ward PA. Evaluation of endotoxin models for the study of sepsis. Shock. 2005;24(Suppl 1):7–11. doi: 10.1097/01.shk.0000191384.34066.85. [DOI] [PubMed] [Google Scholar]
- 59.Schmidt WM, et al. In vivo profile of the human leukocyte microRNA response to endotoxemia. Biochem Biophys Res Commun. 2009;380:437–441. doi: 10.1016/j.bbrc.2008.12.190. [DOI] [PubMed] [Google Scholar]
- 60.Zanotti-Cavazzoni SL, Goldfarb RD. Animal models of sepsis. Crit Care Clin. 2009;25:703–719. vii–viii. doi: 10.1016/j.ccc.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 61.Fink MP. Animal models of sepsis and its complications. Kidney Int. 2008;74:991–993. doi: 10.1038/ki.2008.442. [DOI] [PubMed] [Google Scholar]
- 62.Dyson A, Singer M. Animal models of sepsis: why does preclinical efficacy fail to translate to the clinical setting? Crit Care Med. 2009;37:S30–37. doi: 10.1097/CCM.0b013e3181922bd3. [DOI] [PubMed] [Google Scholar]
- 63.Doi K, et al. Animal models of sepsis and sepsis-induced kidney injury. J Clin Invest. 2009;119:2868–2878. doi: 10.1172/JCI39421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Silva E, et al. Sepsis: from bench to bedside. Clinics (Sao Paulo) 2008;63:109–120. doi: 10.1590/s1807-59322008000100019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cavassani KA, et al. The post sepsis-induced expansion and enhanced function of regulatory T cells create an environment to potentiate tumor growth. Blood. 2010;115:4403–4411. doi: 10.1182/blood-2009-09-241083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Angus DC. The lingering consequences of sepsis: a hidden public health disaster? JAMA. 2010;304:1833–1834. doi: 10.1001/jama.2010.1546. [DOI] [PubMed] [Google Scholar]
- 67.Lopes JA, et al. Long-term risk of mortality after acute kidney injury in patients with sepsis: a contemporary analysis. BMC Nephrol. 2010;11:9. doi: 10.1186/1471-2369-11-9. [DOI] [PMC free article] [PubMed] [Google Scholar]