INTRODUCTION
Idiopathic pulmonary fibrosis (IPF) is a chronic fibrotic lung disease of unknown etiology characterized by usual interstitial pneumonia (UIP) pattern on histopathology or radiology (1). Central to the diagnosis of IPF is the exclusion of a defined connective tissue disorder (CTD), as a UIP histopathologic and radiologic pattern can be seen in patients with CTD, in particular among those with rheumatoid arthritis and scleroderma (2-6).
Published guidelines suggest that all patients with suspected IPF be screened with rheumatoid factor (RF), anti-cyclic citrullinated peptide (CCP), and anti-nuclear antibody (ANA) testing, even in the absence of CTD symptoms. Further, it is recommended that those patients with positive screening results be carefully evaluated for an occult CTD, often in consultation with a rheumatologist (1, 7). Importantly, circulating autoantibodies have been described in patients with IPF who have no evidence of a defined CTD (2, 8-10). However, the clinical relevance of these circulating autoantibodies remains unknown.
In this study, we investigated the frequency and clinical significance of commonly measured circulating autoantibodies in a well-characterized cohort of patients with IPF. We sought to determine if the frequency and type of circulating autoantibodies in patients with IPF differed from two control populations, healthy, age-similar controls and patients with undifferentiated connective tissue disease (UCTD). In addition, we examined whether patients with IPF and circulating autoantibodies comprised a distinct clinical phenotype and whether the presence of circulating autoantibodies in IPF influenced survival.
MATERIALS AND METHODS
Study Population
Patients with IPF seen between October 2005 and May 2010 were identified from an ongoing cohort study of interstitial lung disease (ILD) at the University of California San Francisco (UCSF). Signs and symptoms of CTD were systematically and prospectively collected on all patients. This Institutional Review Board-approved cohort included informed consent for the use of clinical data and banked serum for future studies. Patients were included in the current study if they had a diagnosis of IPF based on the previous guidelines and had banked serum available (11).
The primary control population was healthy volunteers, ages 50 to 80 years old. We also identified all patients in the UCSF database who had banked serum available and a diagnosis of undifferentiated connective tissue disease-associated (UCTD) ILD. Patients were given a multidisciplinary diagnosis of UCTD-ILD if they have at least one clinical manifestation suggestive of a CTD (e.g. Raynaud's, arthralgias, dry eyes and mouth), at least one positive serology, and absence of sufficient American College of Rheumatology criteria for a defined CTD (12).
Radiological Evaluation
Available chest high-resolution computed tomography (HRCT) scans for patients with IPF were re-evaluated by a thoracic radiologist blinded to the clinical course and autoantibody status of all patients. UIP pattern (yes or no) was identified based on current criteria (1).
Autoantibody Profile
An extensive autoantibody evaluation was performed on banked serum from all patients. The laboratory was blinded to the diagnosis and other clinical features of all patients. ANA by immunofluorescence assay (IFA) and RF by nephelometry were performed by the UCSF Clinical Laboratory according to standard protocols. Additional autoantibody testing was performed using the BioPlex™ 2200 System (Bio-Rad Laboratories, Hercules, CA). This system is an FDA-cleared fully-automated random-access analyzer, which employs multiplexed fluoromagnetic bead technology to simultaneously perform measurements of multiple autoantibodies in a single sample (13). The following circulating autoantibodies were tested using the BioPlex system: anti-cyclic citrullinated peptides (CCP), Smith (Sm), ribonucleoprotein (RNP), SmRNP, Scl-70, Jo-1, anti-Ro (SS-A), anti-La (SS-B), double stranded deoxyribonucleic acid (dsDNA), chromatin, ribosomal P, centromere B, proteinase 3 (PR3), myeloperoxidase (MPO), and glomerular basement membrane (GBM).
Statistical Analysis
Patients were categorized as autoantibody positive if they had one or more circulating autoantibody levels above the established reference values, except for ANA and RF. An ANA titer of ≥ 1:320 and a RF value of ≥ 60 IU/ml were considered positive (14).
Intergroup comparisons were performed using an unpaired t-test, Chi-squared test, or Fisher's Exact test, as appropriate. Transplant-free survival time was defined as time from serum collection to transplant, death or censoring as determined by review of clinic records and the Social Security Death Index. Kaplan–Meier survival estimate curves were generated and compared using the log-rank test. Two adjusted Cox regression approaches were used to determine the predictive value of autoantibody status on transplant-free survival in IPF. The first model adjusted for covariates that were considered a priori by the investigators to be important potential confounders of the relationship between autoantibody positivity and survival (i.e. age, gender, and smoking status). The second model adjusted for covariates that were found to have a p value < 0.15 on unadjusted analysis. All statistical analyses were performed using STATA version 11 (College Station, TX). Significance was defined as a p-value of <0.05.
RESULTS
Patient Characteristics
Sixty-seven patients with IPF and banked serum were identified from the longitudinal cohort. There was no significant difference in baseline characteristics or survival time between IPF patients with and without available serum (Supplementary Table 1, Supplementary Figure 1). The vast majority of patients had their blood drawn at the time of their initial clinic visit (median time between initial clinic visit and blood draw 0 days, inter-quartile range 0, 0 days). Only four patients had their blood drawn more than 6 months from their initial clinic visit.
Patients with IPF were predominantly male with a mean age of 69 (Table 1). Seventy-five percent were current or former smokers. The mean forced vital capacity (FVC) was 68% predicted and the mean diffusion capacity for carbon monoxide (DLCO) was 45% predicted. Eighteen percent reported long-term oxygen therapy.
Table 1.
Baseline Demographics*
| IPF (n=67) | UCTD-ILD (n=22) | p value | |
|---|---|---|---|
| Age, years | 69 (8) | 60 (11) | 0.04 |
| Female gender | 16 (24%) | 18 (82%) | <0.01 |
| Ever smoker | 50 (75%) | 9 (41%) | 0.09 |
| Dyspnea score† | 9.4 (6.1) | 10.1 (5.9) | 0.57 |
| Joint pain or stiffness | 27 (43%) | 9 (41%) | 0.46 |
| Dry eyes or mouth | 14 (23%) | 6 (27%) | 0.36 |
| Raynaud's | 4 (7%) | 3 (14%) | 0.15 |
| Reflux symptoms | 29 (44%) | 9 (41%) | 0.31 |
| Current prednisone therapy | 18 (29%) | 16 (73%) | <0.01 |
| Current azathioprine therapy | 6 (10%) | 0 (0%) | 0.15 |
| Long-term oxygen therapy | 12 (18%) | 4 (18%) | 0.64 |
| Forced vital capacity, % predicted | 68 (17) | 66 (17) | 1.00 |
| Diffusing capacity for carbon monoxide, % predicted | 45 (18) | 46 (14) | 0.83 |
| Definite UIP pattern on HRCT(1)‡ | 27 (52%) | 5 (24%) | 0.13 |
| Surgical lung biopsy performed | 30 (45%) | 9 (41%) | 0.71 |
IPF – idiopathic pulmonary fibrosis; UCTD – undifferentiated connective tissue disease; ILD – interstitial lung disease; UIP – usual interstitial pneumonia; HRCT – high-resolution computed tomography
Data are presented as n (%) or mean (SD)
Score from Clinical-Radiographic-Physiologic (CRP) Dyspnea Score (23)
HRCT available for re-review in 52 patients
The healthy control population included 52 people (26 women and 26 men), ages 50-80 years old. The UCTD-ILD cohort included 22 patients (Table 1, see below for further description). Some of these patients have been previously reported (12).
Frequency of Circulating Autoantibodies
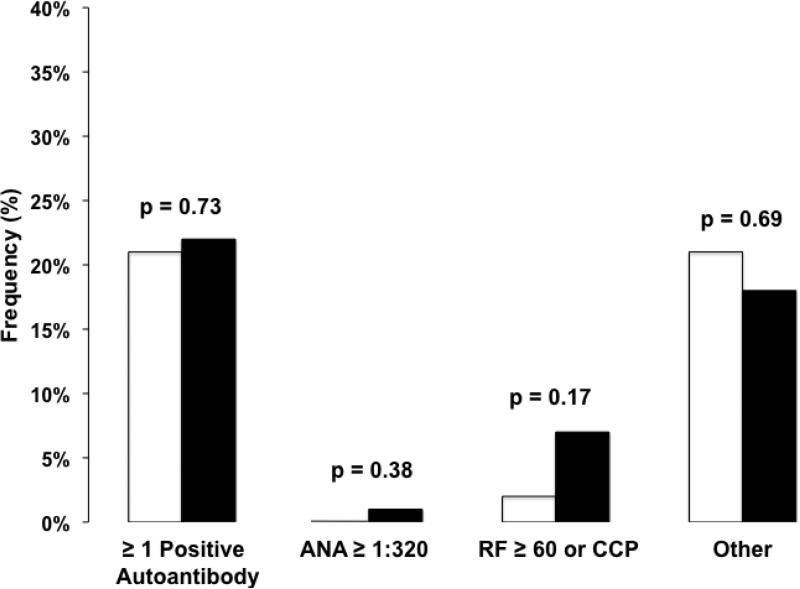
One or more positive circulating autoantibodies were detected in 22% patients with IPF and 21% of healthy controls (p = 0.73, Figure 1).
Figure 1.
The frequency of circulating autoantibody positivity in healthy controls (white bars) compared to idiopathic pulmonary fibrosis (IPF, black bars). There were no significant differences in frequency of autoantibodies between healthy controls and IPF. “Other” includes double stranded deoxyribonucleic acid (dsDNA), chromatin, ribosomal P, anti-Ro (SS-A), anti-La (SS-B), centromere B, Smith (Sm), ribonucleoprotein (RNP), SmRNP, Scl-70, Jo-1, proteinase 3 (PR3), myeloperoxidase (MPO) and glomerular basement membrane (GBM). Abbreviations: ANA – anti-nuclear antibody, RF – rheumatoid factor, CCP – cyclic citrullinated peptide.
The majority of IPF patients and healthy controls had a negative ANA by IFA (73% in both groups, p = 0.99, Table 2). Low-level ANA titer positivity (≥ 1:40 and < 1:320) was present in 25% of patients with IPF and 27% of healthy controls (p = 0.84). Only one IPF patient had a high ANA titer. This was an 81 year-old man with no rheumatologic signs or symptoms and an ANA titer of 1:640 (mixed speckled and diffuse pattern). Overall, the ANA patterns in the IPF patients and healthy controls with detectable ANA titers were similar; only one IPF patient had a nucleolar staining pattern. This was a 50 year-old woman with no rheumatologic signs or symptoms and an ANA titer of 1:40.
Table 2.
Frequency of Circulating Autoantibodies in IPF, Healthy Controls, and UCTD-ILD
| IPF (n=67) | Healthy Controls (n=52) | p value (healthy vs. IPF) | UCTD-ILD (n=22) | p value (UCTD vs. IPF) | |
|---|---|---|---|---|---|
| ANA titer by IFA | |||||
| < 1:40 | 49 (73%) | 38 (73%) | 0.99 | 6 (27%) | 0.07 |
| ≥ 1:40 and < 1:320 | 17 (25%) | 14 (27%) | 0.84 | 5 (23%) | 0.32 |
| ≥ 1:320 | 1 (1%) | 0 (0%) | 0.38 | 11 (50%) | <0.01 |
| ANA pattern | |||||
| Speckled | 17 (25%) | 13 (25%) | 0.85 | 14 (64%) | 0.11 |
| Diffuse | 11 (16%) | 3 (6%) | 0.03 | 2 (9%) | 0.18 |
| Cytoplasmic | 1 (1%) | 1 (2%) | 0.70 | 0 (0%) | 0.24 |
| Nucleolar | 1 (1%) | 0 (0%) | 0.37 | 6 (27%) | 0.10 |
| Centromere | 0 (0%) | 1 (2%) | 0.25 | 0 (0%) | n/a |
| Rheumatoid factor > 60 | 4 (6%) | 0* (0%) | 0.09 | 5 (31%) | 0.35 |
| IU/mL | |||||
| CCP | 1 (1%) | 1 (2%) | 0.86 | 2 (10%) | 0.72 |
| Centromere | 0 (0%) | 1 (2%) | 0.25 | 1 (5%) | 0.39 |
| Chromatin | 1 (1%) | 0 (0%) | 0.38 | 3 (14%) | 0.46 |
| dsDNA | 1 (1%) | 1 (2%) | 0.86 | 1 (5%) | 0.82 |
| Jo-1 | 0 (0%) | 0 (0%) | n/a | 0 (0%) | n/a |
| RNP | 4 (6%) | 1 (2%) | 0.28 | 5 (23%) | 0.87 |
| Scl-70 | 2 (3%) | 3 (6%) | 0.45 | 0 (0%) | 0.09 |
| Smith | 0 (0%) | 0 (0%) | n/a | 3 (14%) | 0.12 |
| Sm/RNP | 0 (0%) | 1 (2%) | 0.25 | 3 (14%) | 0.12 |
| GBM | 0 (0%) | 0 (0%) | n/a | 0 (0%) | n/a |
| Ribosomal P | 0 (0%) | 0 (0%) | n/a | 0 (0%) | n/a |
| SSA | 3 (4%) | 2 (4%) | 0.87 | 7 (32%) | 0.37 |
| SSB | 0 (0%) | 2 (4%) | 0.11 | 1 (5%) | 0.39 |
| MPO | 0 (0%) | 0 (0%) | n/a | 3 (14%) | 0.12 |
| PR3 | 1 (1%) | 0 (0%) | 0.38 | 0 (0%) | 0.24 |
IPF – idiopathic pulmonary fibrosis; UCTD – undifferentiated connective tissue disease; ILD – interstitial lung disease; ANA – anti-nuclear antibody; IFA – immunofluorescence assay; CCP – anti-cyclic citrullinated peptide; dsDNA – double stranded deoxyribonucleic acid; RNP – ribonucleoprotein; GBM – glomerular basement membrane; SSA – anti-Ro; SSB – anti-La; MPO – myeloperoxidase; PR3 – proteinase 3 Data are presented as n (%)
RF tested in only 48 controls
The rheumatoid factor was positive in 6% of patients with IPF and none of the healthy controls (p = 0.09). There was no difference in CCP antibody positivity between patients with IPF and healthy controls (p = 0.86). There were no significant differences between IPF patients and healthy controls in the frequency of any of the other individual autoantibodies.
Circulating Autoantibodies and Clinical Phenotype in IPF
There were no significant differences in baseline demographics, pulmonary function test values, or the presence of definite UIP pattern on HRCT between patients with IPF with and without circulating autoantibodies (Table 3). Similarly, there were no significant differences in CTD symptoms or the use of immunosuppressive medications (i.e. prednisone and azathioprine), between these two groups. Upon re-review of the IPF autoantibody positive subgroup, only one IPF patient, a 75 year-old man with symptoms of gastroesophageal reflux and arthralgias, was found to have an associated circulating antibody detected on the analysis of banked serum - an isolated positive RNP. This patient had definite UIP pattern on HRCT and serologic evaluation at the time of diagnosis, including ANA, RF, SSA, SSB, aldolase, and Scl70, were negative. Follow-up on this patient could not be performed because he had died by the time this information was available.
Table 3.
Comparison of Autoantibody Positive and Autoantibody Negative IPF
| IPF Autoantibody Positive* (n = 15) | IPF Autoantibody Negative* (n = 52) | p value | |
|---|---|---|---|
| Age, years | 67 (9) | 70 (8) | 0.15 |
| Female gender | 3 (20%) | 13 (25%) | 0.69 |
| Ever smoker | 10 (67%) | 40 (77%) | 0.42 |
| Mean dyspnea score† | 9.4 (6.0) | 9.4 (6.2) | 0.99 |
| Joint pain or stiffness | 7 (50%) | 20 (41%) | 0.54 |
| Dry eyes or mouth | 2 (15%) | 12 (24%) | 0.49 |
| Raynaud's | 0 (0%) | 4 (9%) | 0.27 |
| Reflux | 4 (27%) | 25 (49%) | 0.13 |
| Current prednisone therapy | 4 (31%) | 14 (28%) | 0.84 |
| Current azathioprine therapy | 0 (0%) | 6 (12%) | 0.19 |
| Long-term oxygen therapy | 2 (13%) | 10 (20%) | 0.58 |
| Forced vital capacity, % predicted | 65 (19) | 70 (17) | 0.38 |
| Diffusing capacity for carbon monoxide, % predicted | 44 (18) | 47 (18) | 0.69 |
| Definite UIP pattern on HRCT(1)‡ | 5 (45%) | 22 (54%) | 0.63 |
| Surgical lung biopsy performed | 7 (47%) | 23 (45%) | 0.92 |
| Lung transplantation | 1 (7%) | 5 (10%) | 0.73 |
| Number of deaths | 4 (27%) | 26 (50%) | 0.11 |
| Median follow-up, days | 754 (323, 1143) | 1033 (269, 1316) | 0.15 |
IPF – idiopathic pulmonary fibrosis; UIP – usual interstitial pneumonia; HRCT – high-resolution computed tomography
Data are presented as n (%), mean (SD), median (interquartile range)
Patients were categorized as autoantibody positive if they had one or more circulating autoantibody levels above the established reference values, except for ANA and RF. An ANA titer of ≥ 1:320 and a RF value of ≥ 60 IU/ml were considered positive.
Score from Clinical-Radiographic-Physiologic (CRP) Dyspnea Score (23)
HRCT available for re-review in 52 patients
Comparison of IPF Autoantibody Positive Patients to UCTD-ILD Patients
Compared to the IPF autoantibody positive group (n=15), the UCTD-ILD patients (n=22) were younger (60 vs. 67 years, p = 0.04), more likely to be female (82% vs. 20%, p < 0.01), and less likely to have a history of smoking (41% vs. 67%, p=0.09). There was no difference in CTD symptoms, FVC % predicted or DLCO % predicted between these two groups. There was a trend towards fewer patients with definite UIP pattern in the UCTD-ILD group (24% vs. 45%, p = 0.13). The UCTD-ILD patients had higher ANA titers compared to the IPF autoantibody positive subgroup (ANA ≥ 1:320 in 50% vs. 7 %, p < 0.01). There were no significant differences in the frequency of the other autoantibodies between these two groups.
Circulating autoantibodies and survival
On unadjusted survival analysis, there was a trend towards longer transplant-free survival time in patients with positive circulating autoantibodies compared to those without positive circulating autoantibodies (HR 0.43, p = 0.11, Supplementary Figure 2, Supplementary Table 2). On adjusted analysis, the presence of positive circulating autoantibodies appeared to be associated with longer transplant-free survival time, however the statistical significance varied depending on which statistical model was used (HR 0.22 – 0.47, p = 0.03 – 0.17, Table 4). Additional sensitivity analyses, including exclusion of patients with blood drawn > 6 months after initial diagnosis (n=4) and censoring at lung transplant, yielded similar results (data not shown).
Table 4.
Adjusted Predictors of Survival Time in IPF
| Method 1* Hazard Ratio (95% CI) | p value | Method 2† Hazard Ratio (95% CI) | p value | |
|---|---|---|---|---|
| Age | 1.02 (0.97 – 1.08) | 0.39 | --- | |
| Female gender | 0.88 (0.30 – 2.55) | 0.81 | --- | |
| Ever smoker | 1.26 (0.51 – 3.10) | 0.62 | --- | |
| FVC % predicted | --- | 0.97 (0.94 – 1.00) | 0.01 | |
| DLCO % predicted | --- | 0.97 (0.94 – 1.00) | 0.02 | |
| Positive autoantibody* | 0.47 (0.16 – 1.39) | 0.17 | 0.23 (0.07 – 0.80) | 0.02 |
IPF – idiopathic pulmonary fibrosis; CI – confidence interval; FVC – forced vital capacity; DLCO – diffusing capacity for carbon monoxide
Patients were categorized as autoantibody positive if they had one or more circulating autoantibody levels above the established reference values, except for ANA and RF. An ANA titer of ≥ 1:320 and a RF value of ≥ 60 IU/ml were considered positive.
Method 2 – This model adjusted for covariates that were found to have a p value < 0.15 on unadjusted analysis.
Method 1 – This model adjusted for covariates (age, gender, smoking history) that were identified as important confounders in the relationship between autoantibody positivity and survival.
DISCUSSION
The type and frequency of circulating autoantibodies is not different in patients with IPF compared to healthy, age-similar controls. In addition, among IPF patients, there are no differences in clinical characteristics between those with and without circulating autoantibodies. Interestingly, the presence of circulating autoantibodies may be associated with longer transplant-free survival time.
The significance of circulating autoantibodies in patients with IPF has been of clinical and scientific interest for many years. The primary concern is that these IPF autoantibody positive patients do not have IPF, but some unrecognized and occult CTD. Surprisingly, there are very few studies have systematically examined the clinical relevance of this relationship using the current definition of IPF. Fischer et al reported on ANA positivity in a cohort of 285 patients with IPF and found that 34% had a positive ANA (defined as ≥ 1:40) (10). They found no survival difference between IPF patients who were ANA positive vs. negative. Vij et al studied an IPF cohort of 58 patients and found that 41% had an ANA titer ≥ 1:160 (8). They found other circulating autoantibodies in their IPF cohort, including 7% with RF and 5% with SSA. They found no difference in survival based on ANA titer among IPF patients with a positive ANA.
Our study expands on these earlier observations in several important ways. First, we included a more comprehensive and uniform assessment of circulating autoantibodies beyond the ANA by IFA. Second, we directly compared the phenotype of IPF patients with and without circulating autoantibodies. Last, we compared IPF patients to subjects from two control populations. Importantly, by including healthy controls, we have shown that circulating autoantibodies are no more common in patients with IPF than in healthy, similarly aged adults.
Our finding of similar autoantibody positivity between IPF patients and health, age-similar patients argues that the presence of circulating autoantibodies and the presence of IPF in these patients may be unrelated; in other words, that the presence of circulating autoantibodies in patients with IPF may not be pathobiologically relevant. Other investigators have reported a similar prevalence of autoantibodies in the healthy elderly population. A study of 64 healthy individuals (32 men and 32 women, mean age 81) reported positive RF in 14.1%, ANA in 31.3% and SSA in 1.6% (15). A study measuring RF and ANA in 279 healthy individuals with a mean age of 71 years found a prevalence of 14% and 18%, respectively (16). A third study of 300 healthy subjects (mean age 79, 137 men and 163 women), found that 17% had a positive ANA, 7.6% had a positive dsDNA, and 40% had a positive RF (17). The lack of RF positivity in our healthy controls is interesting and may be due to the screening process of these donors (exclusion of patients who have hepatitis B or C, which can lead to RF positivity that is not driven by rheumatoid arthritis (18, 19)).
Our group and others have previously described a population of ILD patients with circulating autoantibodies and signs or symptoms common to CTD who do not meet criteria for a defined CTD (8, 12, 14, 20). This population has been referred to by several names including UCTD-ILD, autoimmune-featured (AIF) ILD, and lung-dominant CTD-ILD (8, 12, 14). We do not believe our population of IPF patients with circulating autoantibodies represents this condition. The demographics of our IPF autoantibody positive population are different from our UCTD-ILD population. Consistent with previous studies, our UCTD-ILD patients were younger, mostly female, never-smokers with a predominant radiographic pattern that was not UIP (12, 20). Our UCTD-ILD patients also had higher ANA titers compared to IPF autoantibody positive patients. Similarly, the AIF-ILD population described by Vij et al has a different autoantibody profile than we found in our IPF autoantibody positive patients, with the majority (92%) having a positive ANA and 40% having an ANA titer ≥ 1:320 (8). In fact, the autoantibody profile in our IPF autoantibody positive group is similar to their IPF cohort.
The survival findings of this study are provocative, but are inconsistent based on model choice and contradict previous reports (8, 10). Additional study of this issue will be important. Our survival findings may be due to differences in patient populations or methods of analysis. For example, previous studies comparing the influence of autoantibody positivity and survival in IPF used a different ANA titer cut-off (>1:40) (10). If we analyze our data without using cut-offs based on titer for ANA and RF, the prevalence of autoantibody positivity increases but remains similar between IPF and healthy controls (40% vs. 42%). There remain no significant differences in baseline demographics between autoantibody positive IPF patients compared to autoantibody negative IPF patients with the exception of slightly younger age at diagnosis. Finally, all of the survival associations (unadjusted and both adjusted methods) are qualitatively similar but become less statistically significant. We believe our cutoff of 1:320 is more clinically meaningful based on the published literature on this topic (14, 21).
Although the diagnosis of IPF in this cohort was made prospectively using multidisciplinary review, we do not know for certain if any of these patients developed a defined CTD in the years following their diagnosis. In some cases, autoantibody status may have been known at the time of diagnosis and could have influenced the multidisciplinary diagnosis. Also, we identified patients as autoantibody positive regardless of which autoantibody was positive. It could be that specific autoantibodies have more or less importance in IPF. Given the small numbers of patients with positive autoantibodies, it was not possible to address this issue. There were a few instances where the BioPlex bead-based test yielded positive results for specific autoantibodies despite the ANA by IFA demonstrating a negative or low titer. This suggests a higher sensitivity and/or lower specificity of the BioPlex system. When such discrepancies occur, we generally place more clinical weight on the ANA titer. Finally, there were autoantibodies that were not included in the BioPlex panel including several scleroderma-specific antibodies (Th/To, U3-RNP, and U11/U12) and myositis antibodies (22, 23).
The results of this study show that circulating autoantibody positivity is no more common in patients with IPF than in healthy, age-similar adults, and that patients with IPF and circulating autoantibodies do not represent a distinct clinical phenotype. This suggests that the presence of circulating autoantibodies in patients with IPF may be a reflection of aging rather than representing an occult CTD. However, given that we do not know if these patients developed a CTD in follow-up, these findings should be validated in a separate cohort and future research should focus on prospective, comprehensive evaluation of patients with IPF to further investigate the significance of autoantibodies in IPF and their relationship to survival. If validated, our findings support treating patients with IPF and circulating autoantibodies similarly to those without circulating autoantibodies, including enrollment in clinical trials of novel IPF therapies.
Supplementary Material
Acknowledgements
The authors wish to thank Bio-Rad for donating the assays and the UCSF Clinical Laboratory and Ryk Sheppard for performing the laboratory tests, Sally McLaughlin and Jane Berkeley for assistance with patient identification and data retrieval, the physician members of the UCSF Interstitial Lung Disease Consortium for referring their patients for evaluation by our program, and the many patients who generously agreed to participate in our longitudinal cohort study.
Funding: This work was supported by the National Institutes of Health [Grants NHLBI HL086516, HL097383]
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, et al. An Official ATS/ERS/JRS/ALAT Statement: Idiopathic Pulmonary Fibrosis: Evidence-based Guidelines for Diagnosis and Management. Am J Respir Crit Care Med. 2011 Mar 15;183(6):788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Song JW, Do KH, Kim MY, Jang SJ, Colby TV, Kim DS. Pathologic and radiologic differences between idiopathic and collagen vascular disease-related usual interstitial pneumonia. Chest. 2009 Jul;136(1):23–30. doi: 10.1378/chest.08-2572. [DOI] [PubMed] [Google Scholar]
- 3.Parambil JG, Myers JL, Lindell RM, Matteson EL, Ryu JH. Interstitial lung disease in primary Sjogren syndrome. Chest. 2006 Nov;130(5):1489–95. doi: 10.1378/chest.130.5.1489. [DOI] [PubMed] [Google Scholar]
- 4.Kim EJ, Collard HR, King TE., Jr Rheumatoid arthritis-associated interstitial lung disease: the relevance of histopathologic and radiographic pattern. Chest. 2009 Nov;136(5):1397–405. doi: 10.1378/chest.09-0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim DS, Yoo B, Lee JS, Kim EK, Lim CM, Lee SD, et al. The major histopathologic pattern of pulmonary fibrosis in scleroderma is nonspecific interstitial pneumonia. Sarcoidosis Vasc Diffuse Lung Dis. 2002 Jun;19(2):121–7. [PubMed] [Google Scholar]
- 6.Fischer A, Swigris JJ, Groshong SD, Cool CD, Sahin H, Lynch DA, et al. Clinically significant interstitial lung disease in limited scleroderma: histopathology, clinical features, and survival. Chest. 2008 Sep;134(3):601–5. doi: 10.1378/chest.08-0053. [DOI] [PubMed] [Google Scholar]
- 7.Bradley B, Branley HM, Egan JJ, Greaves MS, Hansell DM, Harrison NK, et al. Interstitial lung disease guideline: the British Thoracic Society in collaboration with the Thoracic Society of Australia and New Zealand and the Irish Thoracic Society. Thorax. 2008 Sep;63(Suppl 5):v1–58. doi: 10.1136/thx.2008.101691. [DOI] [PubMed] [Google Scholar]
- 8.Vij R, Noth I, Strek ME. Autoimmune-Featured Interstitial Lung Disease: A Distinct Entity. Chest. 2011 May 12; doi: 10.1378/chest.10-2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haslam PL, Thompson B, Mohammed I, Townsend PJ, Hodson ME, Holborow EJ, et al. Circulating immune complexes in patients with cryptogenic fibrosing alveolitis. Clin Exp Immunol. 1979 Sep;37(3):381–90. [PMC free article] [PubMed] [Google Scholar]
- 10.Fischer A, Pfalzgraf FJ, Feghali-Bostwick CA, Wright TM, Curran-Everett D, West SG, et al. Anti-th/to-positivity in a cohort of patients with idiopathic pulmonary fibrosis. J Rheumatol. 2006 Aug;33(8):1600–5. [PubMed] [Google Scholar]
- 11.American Thoracic Society Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS). Am J Respir Crit Care Med. 2000 Feb;161(2 Pt 1):646–64. doi: 10.1164/ajrccm.161.2.ats3-00. [DOI] [PubMed] [Google Scholar]
- 12.Kinder BW, Collard HR, Koth L, Daikh DI, Wolters PJ, Elicker B, et al. Idiopathic nonspecific interstitial pneumonia: lung manifestation of undifferentiated connective tissue disease? Am J Respir Crit Care Med. 2007 Oct 1;176(7):691–7. doi: 10.1164/rccm.200702-220OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fu Q, Zhu J, Van Eyk JE. Comparison of multiplex immunoassay platforms. Clin Chem. 2010 Feb;56(2):314–8. doi: 10.1373/clinchem.2009.135087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fischer A, West SG, Swigris JJ, Brown KK, du Bois RM. Connective tissue disease-associated interstitial lung disease: a call for clarification. Chest. 2010 Aug;138(2):251–6. doi: 10.1378/chest.10-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manoussakis MN, Tzioufas AG, Silis MP, Pange PJ, Goudevenos J, Moutsopoulos HM. High prevalence of anti-cardiolipin and other autoantibodies in a healthy elderly population. Clin Exp Immunol. 1987 Sep;69(3):557–65. [PMC free article] [PubMed] [Google Scholar]
- 16.Goodwin JS, Searles RP, Tung KS. Immunological responses of healthy elderly population. Clin Exp Immunol. 1982 May;48(2):403–10. [PMC free article] [PubMed] [Google Scholar]
- 17.Ruffatti A, Rossi L, Calligaro A, Del Ross T, Lagni M, Marson P, et al. Autoantibodies of systemic rheumatic diseases in the healthy elderly. Gerontology. 1990;36(2):104–11. doi: 10.1159/000213183. [DOI] [PubMed] [Google Scholar]
- 18.Shiraishi H, Alter HJ, Feinstone SM, Purcell RH. Rheumatoid factor-like reactants in sera proven to transmit non-A, non-B hepatitis: a potential source of false-positive reactions in non-A, non-B assays. Hepatology. 1985 Mar-Apr;5(2):181–7. doi: 10.1002/hep.1840050204. [DOI] [PubMed] [Google Scholar]
- 19.Briantais MJ, Grangeot-Keros L, Pillot J. Specificity and sensitivity of the IgM capture immunoassay: studies of possible factors inducing false positive or false negative results. J Virol Methods. 1984 Aug;9(1):15–26. doi: 10.1016/0166-0934(84)90079-x. [DOI] [PubMed] [Google Scholar]
- 20.Corte TJ, Copley SJ, Desai SR, Zappala CJ, Hansell DM, Nicholson AG, et al. Significance of connective tissue disease features in idiopathic interstitial pneumonia. Eur Respir J. 2012 Mar;39(3):661–8. doi: 10.1183/09031936.00174910. [DOI] [PubMed] [Google Scholar]
- 21.Tan EM, Feltkamp TE, Smolen JS, Butcher B, Dawkins R, Fritzler MJ, et al. Range of antinuclear antibodies in “healthy” individuals. Arthritis Rheum. 1997 Sep;40(9):1601–11. doi: 10.1002/art.1780400909. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi T, Wada I, Ohtsuka Y, Munakata M, Homma Y, Kuroki Y. Autoantibody to alanyl-tRNA synthetase in patients with idiopathic pulmonary fibrosis. Respirology. 2007 Sep;12(5):642–53. doi: 10.1111/j.1440-1843.2007.01140.x. [DOI] [PubMed] [Google Scholar]
- 23.Fischer A, Swigris JJ, du Bois RM, Lynch DA, Downey GP, Cosgrove GP, et al. Anti-synthetase syndrome in ANA and anti-Jo-1 negative patients presenting with idiopathic interstitial pneumonia. Respir Med. 2009 Nov;103(11):1719–24. doi: 10.1016/j.rmed.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.