Abstract
Early life exposure to specific environmental factors can increase risk for developing psychopathology including major depression in adulthood. However, the molecular pathways and epigenetic mechanisms that mediate the effects of early environments on adult mood remain poorly understood. We examined the effects of different gestational and rearing conditions on adult anxiety- and depression-like behavior using a combined reciprocal out-crossing and cross-fostering design in Balb/cJ (cJ) and C57BL/6J (B6) mouse strains. First filial (F1) hybrid offspring, which were gestated by B6 or cJ dams and then reared by either strain, were evaluated for behavior and whole-genome hippocampal gene expression during adulthood. Adult hybrid mice gestated by B6 dams showed increased depression-like behavior in the forced swim and sucrose preference tests, increased hippocampal expression of alpha calcitonin gene-related peptide (αCGRP) transcripts, and decreased methylation of the αCGRP promoter compared to those gestated by cJ dams. Differential expression of αCGRP in adulthood did not result from genomic imprinting, and differences between B6 and cJ mitochondrial DNA were not responsible for behavioral phenotypes observed. Lastly, central administration of αCGRP to adult hybrid mice increased depression-like behavior, while the CGRP1 receptor antagonist CGRP8–37 reduced depression-like behavior in the FST. Our findings suggest that gestational factors influence adult depression-like behavior through methylation of the αCGRP gene.
Keywords: depression, forced swim test, sucrose preference, epigenetic, αCGRP, hippocampus
Introduction
Major depressive disorder is a common and disabling psychiatric illness with a lifetime prevalence of 10–15%1. Population based twin studies indicate that environmental factors account for approximately 60% of the variance in adult depression2. Environmental factors acting in early development are thought to exert a strong influence on the development of depression later in life3,4. For example, early adverse experience such as parental maltreatment and neglect have been implicated in the development of depressive phenotypes during adulthood in humans and animals5–7. Furthermore, prenatal factors including maternal malnutrition8,9, stress exposure10–13, mood state14,15, and endocrine factors6,16 have also been implicated in the development of adult depressive phenotypes in humans and rodent species. However, the pathophysiological mechanisms by which early environmental factors confer susceptibility to adult depression are poorly understood.
The long-term effects of environmental factors on physiology and behavior are mediated by epigenetic mechanisms, which alter gene expression without changing DNA sequence. Epigenetic modifications include methylation of DNA and posttranslational modification of histones, both of which alter gene transcription17. Epigenetic modifications have been correlated with behavioral phenotypes induced by early life environmental factors in rodents. For example, exposure to early maltreatment induces long-lasting changes in methylation of the BDNF gene in the prefrontal cortex and increases adult anxiety levels in mice18.
We aimed to identify novel molecular pathways on which epigenetic mechanisms act to program adult emotional behavior. We used a reciprocal outcross design to investigate the role of early environmental factors on adult emotional behavior in female F1 mice, which are genetically identical except for mitochondrial DNA. We reciprocally outcrossed C57Bl/6J (B6) and BALB/cJ (cJ) mice, which vary dramatically in stress reactivity and maternal behavior, to expose their F1 offspring to different early environments19,20. Specifically, cJ mice exhibit greater stressor-provoked activation of the HPA axis and less arch-backed nursing and licking/grooming of pups compared to B6 dams20–22. However, B6 mice exhibit more depression-like behavior than cJ mice in several behavioral paradigms. For example, B6 mice show increased depression-like behavior in the forced swim test (FST)23–26, and exhibit increased anhedonia in the sucrose and fructose preference tests compared to many other mouse strains27, including cJ.
We used an unbiased approach to identify genes that regulate adult emotional behavior and are epigenetically modified by the early environment. First, we assessed F1 offspring for anxiety- and depression-like behavior and the response to chronic antidepressant treatment. Second, we cross-fostered F1 offspring to determine whether gestational factors or maternal care altered depression-like behavior in adult F1 offspring. Third, we assessed genome-wide hippocampal gene expression in adult F1 mice, since the hippocampus has been strongly implicated in the regulation of mood28,29. After identifying the alpha calcitonin gene-related peptide (αCGRP) gene as differentially expressed between F1 strains, we assessed αCGRP gene methylation. We also assessed hippocampal αCGRP gene expression and methylation on postnatal day 1 to determine the onset of these effects. Additionally, we performed allelic expression studies to evaluate potential genomic imprinting of the αCGRP gene, and behavioral studies using conplastic strains to assess the potential influence of mitochondrial DNA on depression-like behavior. Finally, we explored a causal role for αCGRP in depression-like behavior by centrally administering αCGRP or the CGRP1 receptor antagonist CGRP8–37 and examining FST behavior.
Methods
Animals
BALB/cJ (cJ), C57BL/6J (B6), A/J, C57BL/6J-mtA/J/NaJ, and PWD/phJ mice were obtained from Jackson Laboratory (Bar Harbor, Maine, USA). Mice were 12–16 week old females for all studies, except for neonatal studies which used postnatal day 1 mice. We studied only females to hold genetics constant across F1 strains (except for mitochondrial DNA), and avoid genetic differences introduced by differential sex chromosome inheritance in males. Mice used for molecular studies were experimentally naive. Animals were housed and maintained on a 12-hour light/dark schedule with food and water provided ad libitum. Behavioral testing occurred during the light phase. Experiments were conducted in accordance with the National Institutes of Health Laboratory Animal Care guidelines and with Institutional Animal Care and Use Committee approval.
Drugs
Fluoxetine (ANAWA, Zurich, Switzerland) was administered in the drinking water at a dose of 18 mg/kg/day (160 mg/L)30, which reduces anxiety- and depression-like behavior in mice, and results in serum fluoxetine levels within the range observed in patients taking therapeutic doses30. Rat αCGRP or the rat CGRP1 receptor antagonist (CGRP8–37)(Tocris Bioscience, Ellisville, MI) were administered i.c.v. See Supplement 1 for details of central drug administration.
Reciprocal outcrossing
10–12 week old B6 and cJ mice were reciprocally outcrossed to generate two F1 hybrid strains. Breeding pairs were housed together throughout pregnancy and separated when pups were weaned at 21 days of age. To minimize potential litter effects, only two mice from each litter were assigned to an experimental group. F1 mice used for i.c.v. infusion studies were 12 week old female offspring from cJ dams × B6 sires, and were purchased from Jackson Laboratory.
Cross fostering
Newborn F1 offspring of B6 or cJ dams were cross-fostered to B6 or cJ dams during the first postnatal 48 hours, yielding four experimental groups: B6-gestation and cJ-rearing (B6-cJ), B6-gestation and -rearing (B6-B6), cJ-gestation and B6-rearing (cJ-B6), and cJ-gestation and -rearing (cJ-cJ). Each dam had all pups fostered to another female and received 6–8 novel pups.
Behavioral studies
Information regarding the performance of the open field test, light/dark test, FST, and chronic mild stress (CMS) paradigm, and the analysis of behavioral data are provided in Supplement 1.
Microarray study
Whole genome hippocampal gene expression levels were assessed in B6-cJ, B6-B6, cJ-B6, and cJ-cJ F1 mice. RNA samples were hybridized onto the Illumina MouseRef-8 v2.0 expression chip. Beadchips were scanned using an Illumina BeadStation 500 G - BeadArray Reader and microarray data were analyzed by the ‘lumi’ bioconductor package. Data were transformed, normalized, and differential expression was determined using the limma bioconductor package by fitting a linear model. For details, see Supplement 1.
Quantitative real-time PCR
Quantitative real-time polymerase chain reaction (qPCR) was used to confirm a subset of genes fulfilling the B-statistics criterion (B>0.95) from the microarray study. TaqMan qPCR reactions were run using an Applied Biosystems Sequence Detection System 7500. For details, see Supplement 1.
Allelic-specific gene expression and mtDNA studies
Methods for assessing allelic-specific gene expression of the αCGRP gene and potential behavioral effects of B6 versus cJ mtDNA are provided in Supplement 1.
Bisulfite sequencing PCR
Hippocampal DNA was treated with bisulfite reagent to convert cytosine to uracil while leaving methylated 5’-cytosines intact. Bisulfite-modified DNA samples were amplified using PCR, and sequenced. The percentage methylation at each CpG site was quantified as the ratio of peak values of guanine (G) and adenine (A) (G/[G+A]). For more details, see Supplement 1.
Statistical analysis
ANOVAs were applied and significant interactions were resolved using Newman-Keuls post-hoc tests for between subjects factors and post-hoc ANOVAs for within subjects factors. P values for post-hoc ANOVAs were adjusted for multiple comparisons using the Bonferroni procedure. P was set at .05.
Results
Early environment programs adult depression-like behavior
To examine genetic versus environmental influences on adult depression-like behavior and the antidepressant response, we compared cJ- and B6-gestated F1 hybrid strains. B6-gestated F1 mice showed higher immobility compared to cJ-gestated F1 mice in the FST [F(1,56)=4.39;P<0.05](Figure 1a). No differences in swimming or climbing were found (Figure 1b and c). No difference between F1 strains was found in the light/dark test (Figure 1e,f), in time spent in the center of the open field (Figure 1f), or on locomotor activity (Figure 1g).
Figure 1.
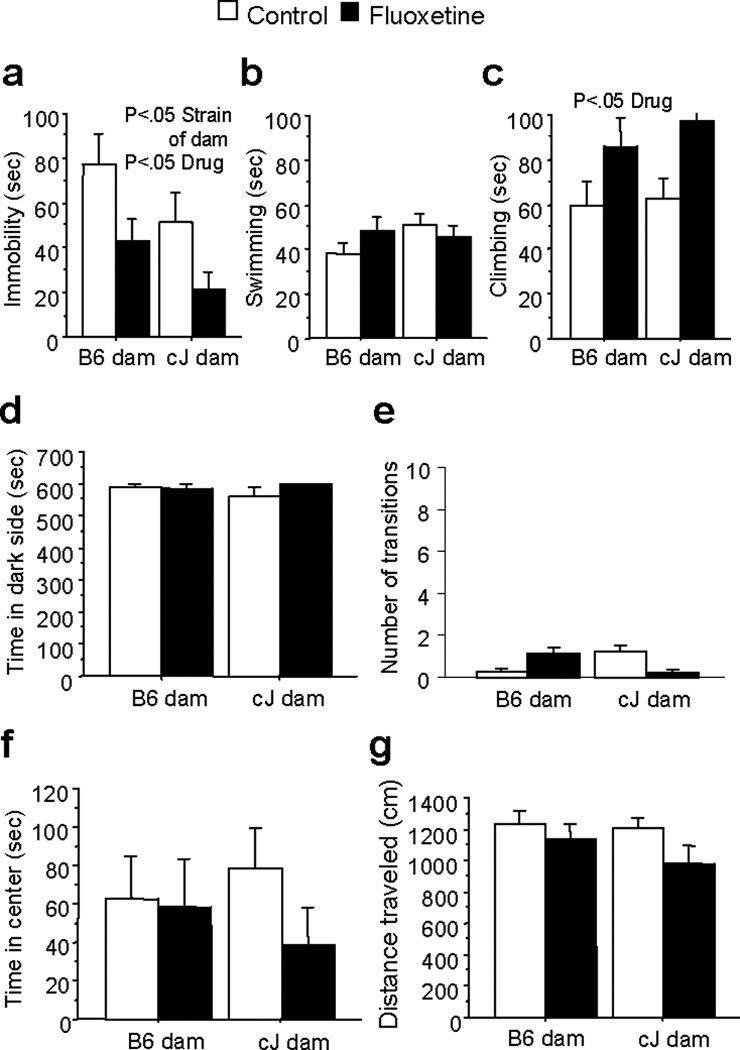
Effects of maternal strain and chronic fluoxetine treatment on depression- and anxiety-like behavior of F1 mice. F1 mice were generated from reciprocal cJ × B6 outcrosses. F1 mice with cJ dams showed significantly less immobility in the FST than F1 mice with B6 dams, and chronic fluoxetine (18mg/kg/day) treatment reduced immobility across F1 strains (a). Neither dam strain nor fluoxetine treatment altered swimming behavior in the FST (b). Fluoxetine treatment increased climbing behavior across F1 strains in the FST (c). Neither dam strain nor fluoxetine treatment altered time in the dark side (d), or transitions (e) in the light/dark test, or center time (f) or distance traveled (g) in the open field test. Bars represent mean ± SEM (n=15 mice per group).
We have previously shown that cJ, but not B6, mice respond to chronic SSRI treatment in the FST23,30,31. Here, we evaluated whether genetic or environmental factors contribute to the behavioral response to chronic SSRI treatment. Chronic fluoxetine treatment reduced immobility [F(1,56)=8.04;P<0.01] across F1 strains (Figure 1a), and the effect size was comparable within each F1 strain (Cohen’s D=.76 for F1s with B6 dams; .70 for F1s with cJ dams). Chronic fluoxetine treatment also increased climbing [F(1,56)=8.41;P<0.01] across F1 strains (Figure 1c). However, chronic fluoxetine treatment had no effect on any anxiety-like behavior (Figure 1d-f), or locomotor activity (Figure 1g).
We also assessed depression-like behavior using the chronic mild stress (CMS) paradigm. Under baseline conditions, no differences in sucrose preference were found between F1 strains (Figure 2a). However, an interaction of strain of dam and stress condition [F(1,45)=6.96;P<0.05] and post-hoc tests revealed that CMS reduced sucrose preference in B6-gestated F1s, but not cJ-gestated F1s (Figure 2b). Furthermore, B6-gestated F1s preferred sucrose less than cJ-gestated F1s under control (non-stress) conditions (Figure 2b).
Figure 2.
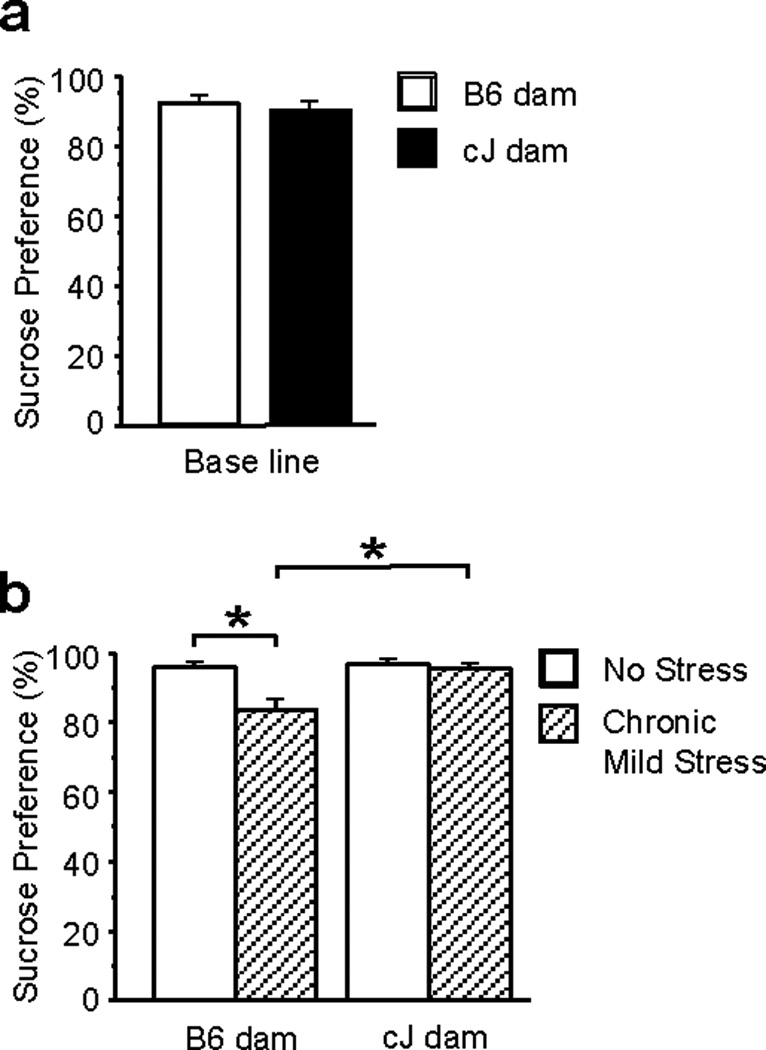
Effects of maternal strain on depression-like behavior in the chronic mild stress (CMS) paradigm of F1 mice. F1 mice were generated from reciprocal cJ×B6 outcrosses. F1 strains showed no differences in sucrose preference at baseline (a). CMS-exposed F1 mice with B6 dams showed significantly less sucrose preference than control-exposed F1 mice with B6 dams, and CMS-exposed F1 mice with cJ dams (b). Bars represent mean ± SEM (n=10–15 mice per group).
Gestational environment programs adult depression-like behavior
We evaluated whether gestation or rearing programs depression-like behavior in F1 mice. F1 mice were generated through reciprocal outcrossing, and then cross-fostered. Analysis revealed a significant main effect of gestational condition [F(1,56)=9.52;P<0.01], but no effects of rearing condition, on immobility in FST with B6-gestated F1 mice showing increased immobility (Figure 3a). Only a trend for rearing condition to affect climbing behavior was found [F(1,56)=4.00;P=0.05], with F1 mice reared by B6 dams showing less climbing behavior than F1 mice reared by cJ dams (Figure 3c).
Figure 3.
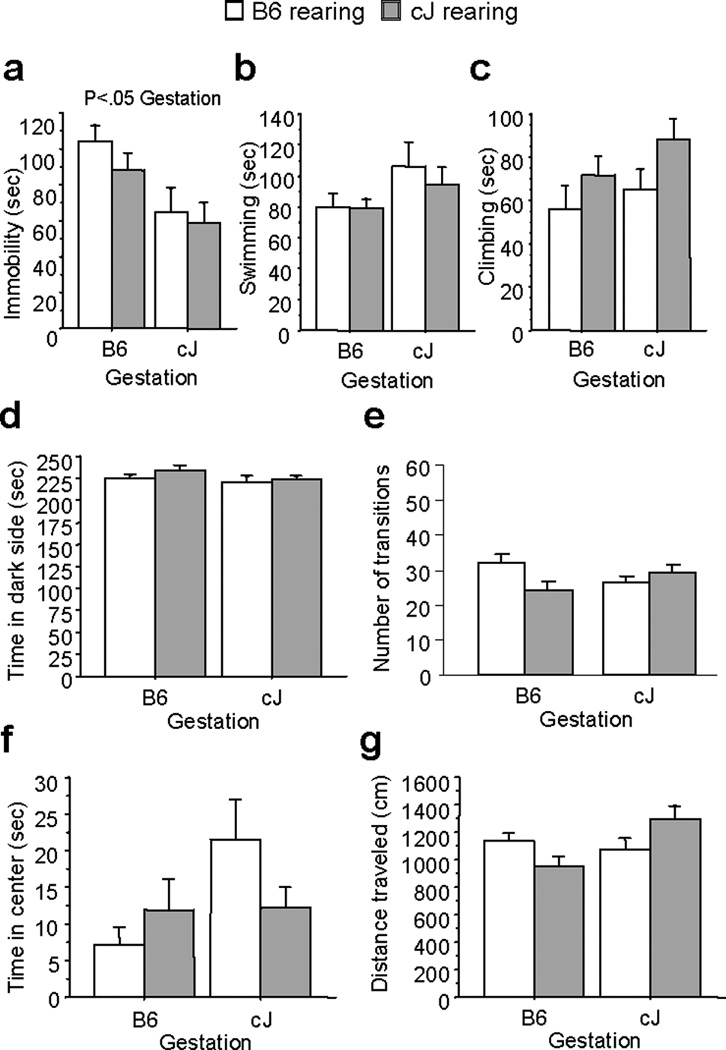
Effects of gestational and rearing conditions on FST behavior are shown for F1 mice cross fostered to Balb/cJ (cJ) or C57BL/6J (B6) dams. F1 mice gestated by cJ dams showed significantly less immobility than F1 mice gestated by B6 dams (a). Neither gestational or rearing condition altered swimming (b) or climbing behavior (c). Neither gestational or rearing condition altered time in the dark side (d), or transitions (e) in the light/dark test, or center time (f) or distance traveled (g) in the open field test. Bars represent mean ± SEM (n=15 mice per group).
No effect of gestational or rearing condition was found on time spent in the dark chamber or number of transitions in the light/dark test (Figure 3d-e). Similarly, neither condition altered time spent in the center of the open field (Figure 3f) or locomotor activity (Figure 3g).
F1 hybrid strains differentially express αCGRP
We compared whole genomic hippocampal mRNA expression of cross-fostered F1 adult offspring using the Illumina mouse Ref-8 v2.0 expression chip. A total of 25,676 transcripts or 19,000 unique genes levels were compared among the four experimental groups (B6-cJ, B6-B6, cJ-B6, cJ-cJ). Three genes were differentially expressed between gestational conditions (q<0.05) and met the B statistic criteria value (>0.95)(Table 1): necdin (Ndn), ubiquitin specific peptidase 29 (Usp29), and αCGRP. All three genes were expressed at higher levels in F1s gestated by B6 dams versus cJ dams. Rearing condition had no effect on expression levels of any genes, and no significant interaction of rearing×gestation was found.
Table 1.
| Gene Symbol |
Gene name | Q value | P value | B statistic | Fold Change (B6 vs. cJ gestation) |
|---|---|---|---|---|---|
| Ndn | necdin | 1.75E-06 | 1.23E-10 | 2.5 | 1.03 |
| Usp29 | ubiquitin specific peptidase 29 |
1.8E-04 | 2.56E-08 | 2.2 | 2.01 |
| αCGRP | calcitonin-related polypeptide, alpha |
0.106 | 2.23E-05 | 1.7 | 1.18 |
Of the three genes identified, only αCGRP gene has been previously associated with depression32–34. Since the Ndn and Usp29 genes were likely differentially expressed due to being imprinting, we focused on the potential role of αCGRP in depression-like behavior. A qPCR study revealed higher expression of αCGRP [F(1,16)=6.70;P<0.05] mRNA in hippocampal tissue of B6-gestated F1 mice, and no effect of maternal care on αCGRP expression. Although microarray results indicated a 1.18 fold change in αCGRP gene expression between B6- versus cJ-gestated mice, qPCR results indicated a 1.82 fold change (Supplemental Figure 1).
We found no difference in hippocampal αCGRP expression between F1 strains on postnatal day 1. However, cJ-gestated F1s showed significantly higher expression levels of brain-derived neurotrophic factor (BDNF) than B6-gestated F1s [F(1,14)=7.72;P<0.05](Supplemental Figure 2)
αCGRP allelic expression
Genomic imprinting confers non-equivalent parental contribution to gene expression, is prevalent in the brain, and can differ across brain regions35. We examined whether the differential αCGRP expression between the F1 strains resulted from genomic imprinting. We reciprocally bred B6 and PWD/phj mice to generate F1 hybrid strains, and examined the allelic-specific expression pattern of αCGRP in hippocampal tissue of the F1 strains. Expression of αCGRP mRNA from the B6 chromosome was substantially higher than that from the PWD chromosome across both F1 strains [F(1,7)=110.0;P<0.001]. However, no preferential αCGRP expression from paternally versus maternally inherited chromosomes was found, indicating that the αCGRP gene is not genomically imprinted (Supplemental Figure 3).
Effects of mtDNA on depression-like behavior
Mammalian mitochondrial DNA (mtDNA) is transmitted maternally. We used conplastic strains, which contain the mtDNA of one inbred strain and the genomic DNA of another, to explore the potential contribution of cJ versus B6 mtDNA to the observed differences in depression-like behavior. A conplastic strain with B6 genomic DNA and cJ mtDNA was not available; thus, we compared N15 generation C57BL/6J-mtA/J conplastic mice to identical mice with B6 mitochondria reintroduced by a previous breeding step. A/J mtDNA is identical to cJ mtDNA at all coded amino acids36. B6 mice have one amino acid change within the cytochrome oxidase subunit III gene compared to cJ and A/J mice36. We found no differences in FST behavior or locomotor activity between C57BL/6J-mtC57BL/6J and C57BL/6J-mtA/J mice (Supplemental Figure 4).
Differential methylation of the αCGRP promoter in F1 strains
We examined whether differential αCGRP expression between F1 strains was associated with differential αCGRP gene methylation. We assessed percent methylation of 48 CpGs upstream of the αCGRP translation start site in exon 2. These CpG sites are located in exon 1, intron 1, and a promoter region 0 – 800 bp upstream of the αCGRP transcription start site37 (Figure 4a). This promoter region encompasses a cyclic adenosine monophosphate (cAMP) response element (TGATGTCA). Sequence analysis of bisulfite-converted DNA isolated from the hippocampus of the F1 strains revealed that percent methylation was higher in the promoter region compared to intron 1 and exon 1 [F(1,26)=12.47;P<0.01](Figure 4b). Within the promoter region, cJ-gestated F1 mice had higher percent methylation than B6-gestated F1 mice [F(1,253)=6.46;P<0.05](Figure 4c). Methylation levels at four specific CpGs sites in the promoter region were increased in B6-gestated F1 mice. One of these sites, CpG site −232, is located 18 bp away from a cAMP-responsive element (CRE) nucleotide sequence. The differences in methylation at this CpG site may lead to differential CREB-induced gene transcription between F1 strains. Rearing condition had no effect on methylation of the promoter region (Figure 4d). Furthermore, neither gestation nor rearing had any effect on methylation levels in exon 1 or intron 1 (Supplemental Figure 5).
Figure 4.
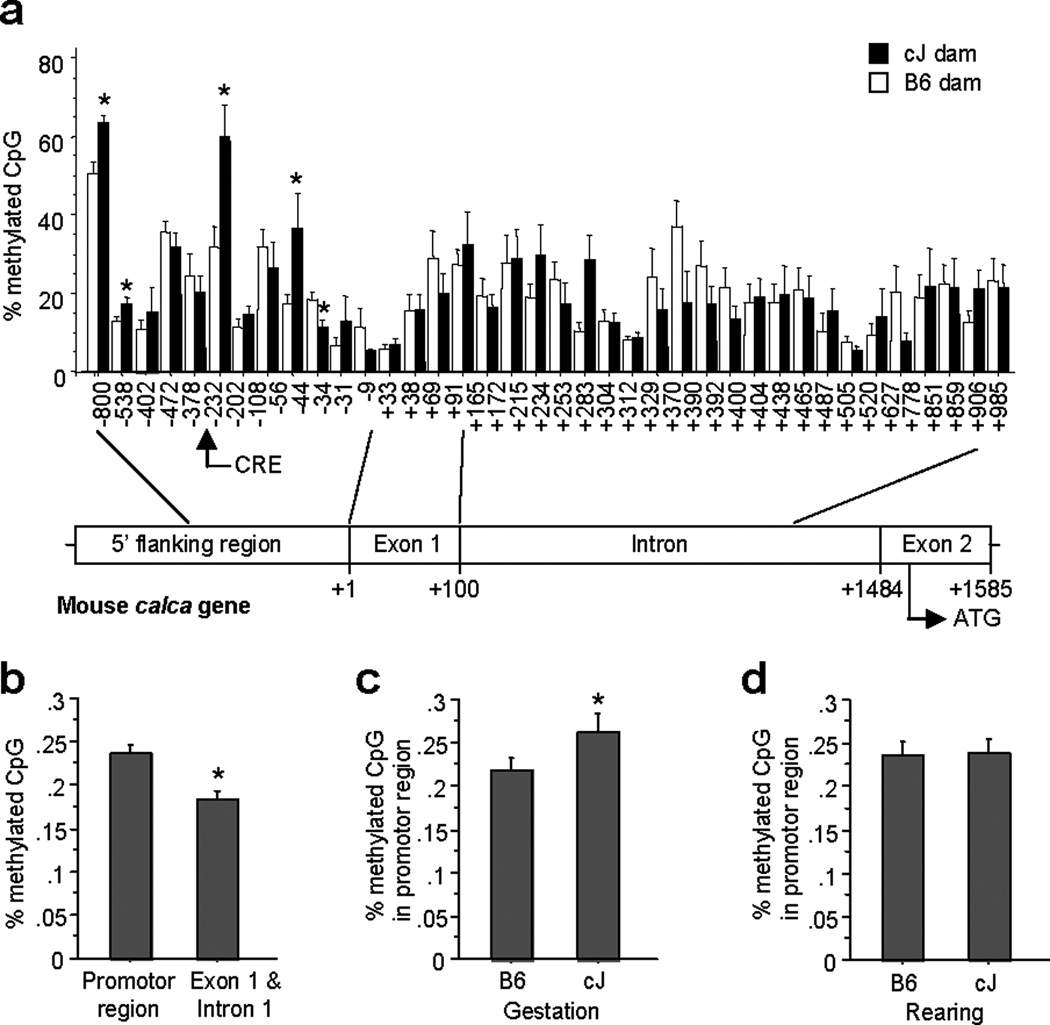
Differential methylation of the αCGRP gene in F1 hybrid strains. Analysis of DNA methylation at the mouse αCGRP locus by sequencing of clones obtained from sodium bisulfate-treated genomic DNA extracted from the hippocampus (a). The percent methylation of the promoter region spanning 0 – 800 bp upstream of the transcription initiation site was higher than in exon 1 and intron 1 (b). The percent methylation of the αCGRP promoter region was higher in F1 mice gestated by cJ dams (c). Post hoc tests indicated that percent methylation of four specific CpG sites in the promoter region were higher in F1 mice gestated by cJ dams (a). Rearing condition had no effect on methylation of the αCGRP promoter region (d). Bars represent mean ± SEM (n=15 mice per group).
We also examined hippocampal αCGRP gene methylation on postnatal day 1. A higher percent of methylation was found in the promoter region than in intron 1 and exon 1 [F(1,15)=9.05;P<0.01](Supplemental Figure 6a). However, no difference in percent methylation of the promotor region, exon 1, or intron 1 was found between B6- or cJ-gestated F1 neonates [F(1,14)=1.99;P=0.18](Supplemental Figure 6b,c,d).
αCGRP modulates depression-like behavior
To examine a causal role for αCGRP expression on adult depression-like behavior, we centrally administered αCGRP or the CGRP1 receptor antagonist CGRP8–37 and assessed FST behavior. Mice receiving 4 µg CGRP8–37 showed reduced immobility compared to mice receiving saline or 1.5 µg αCGRP [F(2,33)=5.88;P<0.01](Figure 5a). Mice treated with 1.5 µg αCGRP exhibited reduced climbing compared to saline- or CGRP8–37-treated mice [F(2,33)=5.37;P<0.01](Figure 5c). Neither αCGRP nor CGRP8–37 altered locomotor activity (Figure 5d).
Figure 5.
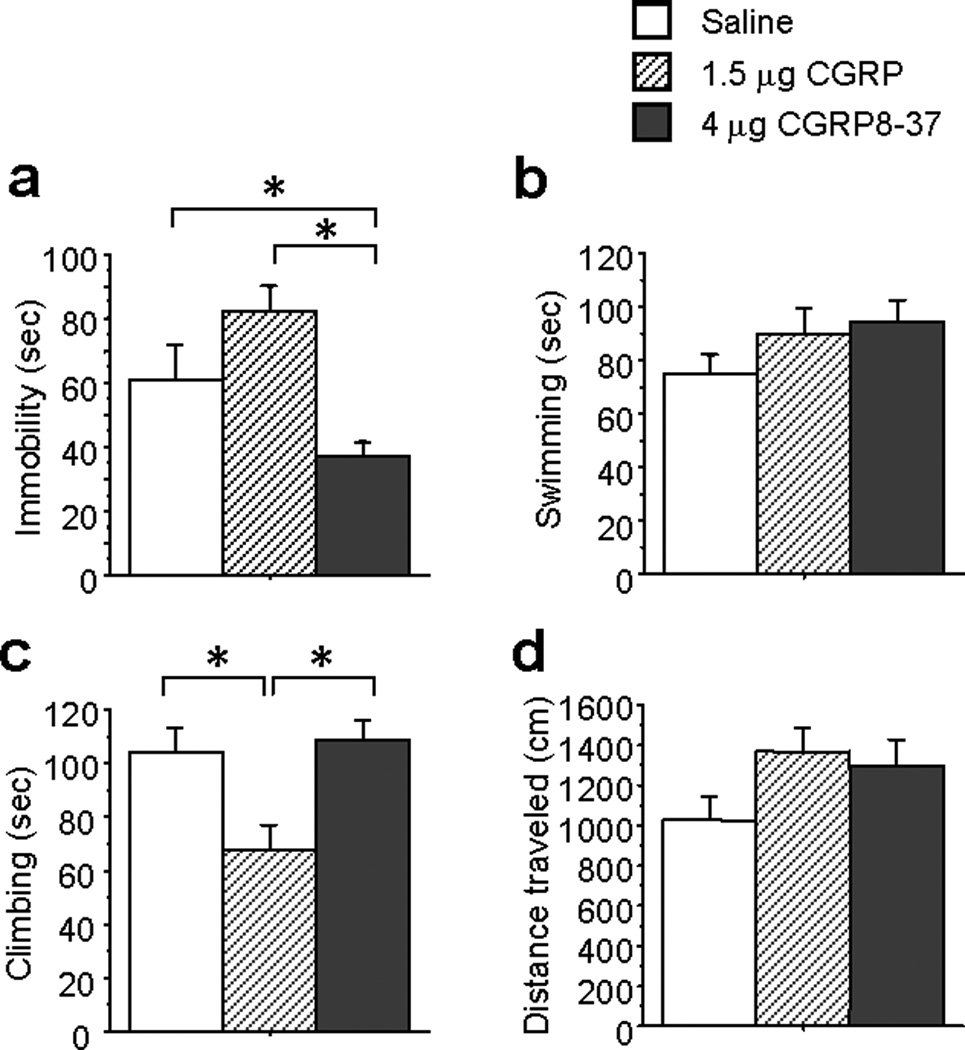
Central administration of αCGRP increases, and CGRP8–37 decreases, depression-like behavior in the FST in F1 mice bred from cJ females and B6 males. CGRP8–37 reduced immobility compared to vehicle (a). Neither αCGRP nor CGRP8–37 altered swimming (b). αCGRP reduced climbing compared to vehicle and CGRP8–37 (c). Bars represent mean ± SEM (n=8–12 per group).
Discussion
Our present findings show that gestational environment programs adult depression-like behavior through epigenetic modification of the αCGRP gene. Specifically, we found that differences between B6 and cJ dam gestational environments, but not maternal behavior, affect adult depression-like behavior of F1 offspring. B6-gestated F1 mice showed increased depression-like behavior, reduced methylation of the αCGRP promoter, and increased hippocampal αCGRP gene expression compared to cJ-gestated F1 mice. These F1 strain differences in αCGRP gene methylation and expression were observed during adulthood, but not on postnatal day 1. Therefore, altered αCGRP gene regulation is a downstream, rather than an initial, effect of exposure to B6 versus cJ gestational environments. In addition, we found reduced hippocampal BDNF expression in B6-gestated F1 mice on postnatal day 1, which might contribute to the alterations in αCGRP gene regulation during adulthood. Finally, intracerebroventricular infusion of αCGRP increased depression-like behavior, while infusion of CGRP8–37 produced antidepressant effects in adult F1 mice. F1 strain differences in hippocampal αCGRP mRNA expression did not result from genomic imprinting of the αCGRP gene, and F1 strain differences in mitochondrial DNA did not affect depression-like behavior. In summary, gestational environment influences adult depression-like behavior by altering αCGRP gene methylation and expression.
Our findings that both F1 strains respond to chronic fluoxetine treatment suggest that a dominant genetic factor in cJ mice underlies this effect. This finding is consistent with our previous report that cJ and three closely related mouse strains (BALB/cByJ, SEA/J, A/J) respond to chronic treatment with SSRIs, while other more distantly related strains including B6 do not23,30. Both F1 strains showed reductions in immobility (Figure 1a) and increases in climbing (Figure 1c) following chronic fluoxetine treatment. Evidence suggests that SSRIs increase swimming behavior, while noradrenergic reuptake inhibitors (NRIs) increase climbing behavior, following acute treatment38. However, chronic treatment with SSRIs has been reported to increase climbing behavior in BALB/cJ mice23,39, and rats selectively bred for learned helplessness40. Increased climbing might result from the rise in cortical and hippocampal norepinephrine levels induced by chronic, but not acute, SSRI treatment41,42. However, others have reported that chronic SSRI treatment increases swimming, rather than climbing, in rats43. Effects of chronic SSRI treatment are likely species and strain dependent.
Gestation by B6 dams increased depression-like behavior in adult F1 offspring compared to gestation by cJ dams. B6-gestated F1s showed increased immobility in the FST (Figures 1a and 3a) and reduced sucrose preference in the CMS paradigm (Figure 2). In contrast, cross-fostering did not reveal any effects of B6 versus cJ maternal care on depression-like behavior (Figure 3). However, we cannot rule out that the brief exposure to B6 versus cJ maternal care prior to cross-fostering (the first 48 hours of life) caused the differential phenotypes between the F1 strains. B6 and cJ dams used in the present studies exhibited the previously reported differences in maternal care (Supplemental Figure 7). Numerous factors potentially differing between cJ and B6 gestational environments could be responsible for the effects on depression-like behavior. Gestational factors implicated in later life affective phenotypes in humans and rodents include endocrine44–46, nutritional9,47–49, and emotional46,50 variables. Few studies have compared B6 and cJ gestational environments. One study reported higher progesterone content in cJ-B6 than B6-cJ (dam strain is first) fetuses on day 7 of pregnancy51. Although baseline corticosterone levels are comparable between B6 and cJ mice19, intrauterine corticosterone levels have not been evaluated during pregnancy. B6 mice exhibit more depression-like behavior in the FST and sucrose and fructose preference tests than cJ mice, and these differential phenotypes could be associated with endocrine, hormonal, or behavioral traits that affect the gestational environment. More work will be required to identify the gestational factors that induce epigenetic modification of the αCGRP gene.
Although gestational environment affected hippocampal αCGRP gene methylation and expression in adult F1 strains (Figure 4), this difference was absent at postnatal day 1 (Supplementary Figures 2a and 6b). B6 versus cJ gestational environments likely induced a cascade of molecular events resulting in differential αCGRP gene regulation by adulthood. Consistent with this idea, central infusion of αCGRP during adulthood increased depression-like behavior in F1 mice (Figure 5). In addition, we identified increases in hippocampal BDNF mRNA expression in cJ- versus B6-gestated F1s at postnatal day 1(Supplemental Figure 2b). Hippocampal BDNF has been strongly implicated in the regulation of depression-like behavior 52,53, and might contribute to the downstream changes in αCGRP gene regulation observed during adulthood.
Our results show that central administration of αCGRP increases, while CGRP8–37 decreases, depression-like behavior in the FST. These results are consistent with our findings that hippocampal αCGRP levels are positively associated with depression-like behavior in F1 strains. Together, these findings suggest that the differential αCGRP expression is responsible for the differences in depression-like behavior between the F1 strains, at least in part. However, it should be noted that the exogeneous administration of αCGRP may not precisely model the endogenous differences in αCGRP levels between F1 strains, since the timepoint at which their αCGRP levels diverge remains unknown. Our present findings are consistent with reports that CGRP is increased in the cerebrospinal fluid33,34 and plasma32 of depressed patients. Only one previous report has evaluated the effects of αCGRP and CGRP8–37 on depression-like behavior in rodents. αCGRP and CGRP8–37 were reported to increase, and not alter, active behavior in the FST, respectively54. These conflicting findings could be due to mouse strain differences, sex differences, or different behavioral scoring techniques.
αCGRP is one of the most abundant peptides in the periphery and central nervous system with multiple functions including regulation of cardiovascular homeostasis and nociception55. Recently, αCGRP has been implicated in phenotypes relevant to psychiatric disorders, including anxiety in rats56. However, our present findings suggest that hippocampal αCGRP expression selectively modulates depression-like, and not anxiety-like, behavior. This discrepancy could result from species differences, or differences in the anxiety paradigms used. The specific brain regions into which hippocampal neurons release αCGRP remains unclear. More work will be required to identify the specific brain regions in which αCGRP modulates depression-like behavior.
Results of allelic expression studies and experiments using conplastic strains showed that F1 strain differences in αCGRP gene methylation, expression, and depression-like behavior did not result from genomic imprinting of the αCGRP gene or mitochondrial DNA polymorphisms. However, we cannot rule out that differential expression of imprinted genes between the two F1 strains could be responsible for the phenotypic differences observed. Furthermore, signaling pathways downstream of imprinted genes differentially expressed between the F1 strains could influence αCGRP gene methylation, expression, and ultimately behavior. However, we detected only two other genes, Ndn and Usp29, which were differentially expressed between the F1 strains. Ndn and Usp29 are genomically imprinted genes57,58, and are likely differentially expressed in the F1 strains due to genomic imprinting rather than exposure to different gestational environments. Neither Ndn nor Usp29 regulates αCGRP expression, to our knowledge. Therefore, the differential αCGRP expression between the F1 strains likely results from exposure to different gestational environments.
In summary, the present findings indicate that gestational environment influences adult depression-like behavior in mice through epigenetic modification of the αCGRP gene. Our findings provide a novel mechanism by which variation in the gestational environment can influence adult affective phenotype. Finally, our results identify the αCGRP signaling pathway as an important modulator of depression-like behavior during adulthood, and a potential target for novel treatments for depression.
Supplementary Material
Acknowledgments
This work was funded by National Institutes of Health Grants MH079424 and a NARSAD Young Investigator Award to SD.
Footnotes
The authors of this article have no biomedical financial interests or potential conflicts of interest to report.
References
- 1.Hirschfeld R, Weisssman M. Risk factors for major depression and bipolar disorder. In: Davis K, Charney D, Coyle J, Nemeroff C, editors. Neuropsychopharmacology:The fifth generation of progress. Philadelphia: Lippincott Williams & Wilkins; 2002. pp. 1018–1025. [Google Scholar]
- 2.Kendler KS, Gatz M, Gardner CO, Pedersen NL. A Swedish national twin study of lifetime major depression. Am J Psychiatry. 2006;163(1):109–114. doi: 10.1176/appi.ajp.163.1.109. [DOI] [PubMed] [Google Scholar]
- 3.Pryce CR, Ruedi-Bettschen D, Dettling AC, Weston A, Russig H, Ferger B, et al. Long-term effects of early-life environmental manipulations in rodents and primates: Potential animal models in depression research. Neurosci Biobehav Rev. 2005;29(4–5):649–674. doi: 10.1016/j.neubiorev.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 4.Nemeroff CB. Neurobiological consequences of childhood trauma. J Clin Psychiatry. 2004;65(Suppl 1):18–28. [PubMed] [Google Scholar]
- 5.Murgatroyd C, Wu Y, Bockmuhl Y, Spengler D. Genes learn from stress: How infantile trauma programs us for depression. Epigenetics. 2011;5(3) doi: 10.4161/epi.5.3.11375. [DOI] [PubMed] [Google Scholar]
- 6.de Kloet ER, Sibug RM, Helmerhorst FM, Schmidt MV. Stress, genes and the mechanism of programming the brain for later life. Neurosci Biobehav Rev. 2005;29(2):271–281. doi: 10.1016/j.neubiorev.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 7.Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, et al. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277(5332):1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- 8.Roseboom TJ, Painter RC, van Abeelen AF, Veenendaal MV, de Rooij SR. Hungry in the womb: What are the consequences? Lessons from the Dutch famine. Maturitas. 2011 doi: 10.1016/j.maturitas.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 9.Jaiswal AK, Upadhyay SN, Satyan KS, Bhattacharya SK. Behavioural effects of prenatal and postnatal undernutrition in rats. Indian J Exp Biol. 1996;34(12):1216–1219. [PubMed] [Google Scholar]
- 10.Behan AT, van den Hove DL, Mueller L, Jetten MJ, Steinbusch HW, Cotter DR, et al. Evidence of female-specific glial deficits in the hippocampus in a mouse model of prenatal stress. Eur Neuropsychopharmacol. 2011;21(1):71–79. doi: 10.1016/j.euroneuro.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 11.Behan AT, van den Hove DL, Mueller L, Jetten MJ, Steinbusch HW, Cotter DR, et al. Evidence of female-specific glial deficits in the hippocampus in a mouse model of prenatal stress. Eur Neuropsychopharmacol. 21(1):71–79. doi: 10.1016/j.euroneuro.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 12.Alonso SJ, Damas C, Navarro E. Behavioral despair in mice after prenatal stress. J Physiol Biochem. 2000;56(2):77–82. doi: 10.1007/BF03179902. [DOI] [PubMed] [Google Scholar]
- 13.Weinstock M. Alterations induced by gestational stress in brain morphology and behaviour of the offspring. Prog Neurobiol. 2001;65(5):427–451. doi: 10.1016/s0301-0082(01)00018-1. [DOI] [PubMed] [Google Scholar]
- 14.Oberlander TF, Weinberg J, Papsdorf M, Grunau R, Misri S, Devlin AM. Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant cortisol stress responses. Epigenetics. 2008;3(2):97–106. doi: 10.4161/epi.3.2.6034. [DOI] [PubMed] [Google Scholar]
- 15.Oberlander TF, Papsdorf M, Brain UM, Misri S, Ross C, Grunau RE. Prenatal effects of selective serotonin reuptake inhibitor antidepressants, serotonin transporter promoter genotype (SLC6A4), and maternal mood on child behavior at 3 years of age. Arch Pediatr Adolesc Med. 2010;164(5):444–451. doi: 10.1001/archpediatrics.2010.51. [DOI] [PubMed] [Google Scholar]
- 16.O'Reilly EJ, Mirzaei F, Forman MR, Ascherio A. Diethylstilbestrol exposure in utero and depression in women. Am J Epidemiol. 2010;171(8):876–882. doi: 10.1093/aje/kwq023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsankova N, Renthal W, Kumar A, Nestler EJ. Epigenetic regulation in psychiatric disorders. Nat Rev Neurosci. 2007;8(5):355–367. doi: 10.1038/nrn2132. [DOI] [PubMed] [Google Scholar]
- 18.Roth TL, Lubin FD, Funk AJ, Sweatt JD. Lasting epigenetic influence of early-life adversity on the BDNF gene. Biol Psychiatry. 2009;65(9):760–769. doi: 10.1016/j.biopsych.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Priebe K, Romeo RD, Francis DD, Sisti HM, Mueller A, McEwen BS, et al. Maternal influences on adult stress and anxiety-like behavior in C57BL/6J and BALB/cJ mice: a cross-fostering study. Dev Psychobiol. 2005;47(4):398–407. doi: 10.1002/dev.20098. [DOI] [PubMed] [Google Scholar]
- 20.Tarantino LM, Sullivan PF, Meltzer-Brody S. Using animal models to disentangle the role of genetic, epigenetic, and environmental influences on behavioral outcomes associated with maternal anxiety and depression. Front Psychiatry. 2011;2:44. doi: 10.3389/fpsyt.2011.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anisman H, Zaharia MD, Meaney MJ, Merali Z. Do early-life events permanently alter behavioral and hormonal responses to stressors? Int J Dev Neurosci. 1998;16(3–4):149–164. doi: 10.1016/s0736-5748(98)00025-2. [DOI] [PubMed] [Google Scholar]
- 22.Carlier M, Roubertoux P, Cohen-Salmon C. Differences in patterns of pup care in Mus musculus domesticus l-Comparisons between eleven inbred strains. Behav Neural Biol. 1982;35(2):205–210. doi: 10.1016/s0163-1047(82)91213-4. [DOI] [PubMed] [Google Scholar]
- 23.Jiao J, Nitzke AM, Doukas DG, Seiglie MP, Dulawa SC. Antidepressant response to chronic citalopram treatment in eight inbred mouse strains. Psychopharmacology (Berl) 2010;213(2–3):509–520. doi: 10.1007/s00213-010-2140-0. [DOI] [PubMed] [Google Scholar]
- 24.Sugimoto Y, Kajiwara Y, Hirano K, Yamada S, Tagawa N, Kobayashi Y, et al. Mouse strain differences in immobility and sensitivity to fluvoxamine and desipramine in the forced swimming test: analysis of serotonin and noradrenaline transporter binding. Eur J Pharmacol. 2008;592(1–3):116–122. doi: 10.1016/j.ejphar.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 25.Lucki I, Dalvi A, Mayorga AJ. Sensitivity to the effects of pharmacologically selective antidepressants in different strains of mice. Psychopharmacology (Berl) 2001;155(3):315–322. doi: 10.1007/s002130100694. [DOI] [PubMed] [Google Scholar]
- 26.Alcaro A, Cabib S, Ventura R, Puglisi-Allegra S. Genotype- and experience-dependent susceptibility to depressive-like responses in the forced-swimming test. Psychopharmacology (Berl) 2002;164(2):138–143. doi: 10.1007/s00213-002-1161-8. [DOI] [PubMed] [Google Scholar]
- 27.Pinhas A, Aviel M, Koen M, Gurgov S, Acosta V, Israel M, et al. Strain differences in sucrose- and fructose-conditioned flavor preferences in mice. Physiol Behav. 2011;105(2):451–459. doi: 10.1016/j.physbeh.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malykhin NV, Carter R, Seres P, Coupland NJ. Structural changes in the hippocampus in major depressive disorder: contributions of disease and treatment. J Psychiatry Neurosci. 2010;35(5):337–343. doi: 10.1503/jpn.100002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frodl T, Meisenzahl EM, Zetzsche T, Born C, Groll C, Jager M, et al. Hippocampal changes in patients with a first episode of major depression. Am J Psychiatry. 2002;159(7):1112–1118. doi: 10.1176/appi.ajp.159.7.1112. [DOI] [PubMed] [Google Scholar]
- 30.Dulawa SC, Holick KA, Gundersen B, Hen R. Effects of chronic fluoxetine in animal models of anxiety and depression. Neuropsychopharmacology. 2004;29(7):1321–1330. doi: 10.1038/sj.npp.1300433. [DOI] [PubMed] [Google Scholar]
- 31.Holick KA, Lee DC, Hen R, Dulawa SC. Effects of chronic fluoxetine in BALB/cJ mice do not require adult hippocampal neurogenesis or the serotonin 1A receptor. Neuropsychopharmacology. 2008;33(2):406–417. doi: 10.1038/sj.npp.1301399. [DOI] [PubMed] [Google Scholar]
- 32.Cizza G, Marques AH, Eskandari F, Christie IC, Torvik S, Silverman MN, et al. Elevated neuroimmune biomarkers in sweat patches and plasma of premenopausal women with major depressive disorder in remission: the POWER study. Biol Psychiatry. 2008;64(10):907–911. doi: 10.1016/j.biopsych.2008.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mathe AA, Agren H, Lindstrom L, Theodorsson E. Increased concentration of calcitonin gene-related peptide in cerebrospinal fluid of depressed patients. A possible trait marker of major depressive disorder. Neurosci Lett. 1994;182(2):138–142. doi: 10.1016/0304-3940(94)90782-x. [DOI] [PubMed] [Google Scholar]
- 34.Mathe AA, Agren H, Wallin A, Blennow K. Calcitonin gene-related peptide and calcitonin in the CSF of patients with dementia and depression: possible disease markers. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26(1):41–48. doi: 10.1016/s0278-5846(01)00219-6. [DOI] [PubMed] [Google Scholar]
- 35.Sittig LJ, Herzing LB, Shukla PK, Redei EE. Parent-of-origin allelic contributions to deiodinase-3 expression elicit localized hyperthyroid milieu in the hippocampus. Mol Psychiatry. 2011 doi: 10.1038/mp.2011.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bayona-Bafaluy MP, Acin-Perez R, Mullikin JC, Park JS, Moreno-Loshuertos R, Hu P, et al. Revisiting the mouse mitochondrial DNA sequence. Nucleic Acids Res. 2003;31(18):5349–5355. doi: 10.1093/nar/gkg739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Amara SG, Evans RM, Rosenfeld MG. Calcitonin/calcitonin gene-related peptide transcription unit: tissue-specific expression involves selective use of alternative polyadenylation sites. Mol Cell Biol. 1984;4(10):2151–2160. doi: 10.1128/mcb.4.10.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cryan JF, Lucki I. Antidepressant-like behavioral effects mediated by 5-Hydroxytryptamine(2C) receptors. J Pharmacol Exp Ther. 2000;295(3):1120–1126. [PubMed] [Google Scholar]
- 39.Wang L, Jiao J, Dulawa SC. Infant maternal separation impairs adult cognitive performance in BALB/cJ mice. Psychopharmacology (Berl) 2011 doi: 10.1007/s00213-011-2209-4. (in press). [DOI] [PubMed] [Google Scholar]
- 40.Shumake J, Colorado RA, Barrett DW, Gonzalez-Lima F. Metabolic mapping of the effects of the antidepressant fluoxetine on the brains of congenitally helpless rats. Brain Res. 2010;1343:218–225. doi: 10.1016/j.brainres.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thomas DN, Nutt DJ, Holman RB. Sertraline, a selective serotonin reuptake inhibitor modulates extracellular noradrenaline in the rat frontal cortex. J Psychopharmacol. 1998;12(4):366–370. doi: 10.1177/026988119801200406. [DOI] [PubMed] [Google Scholar]
- 42.Page ME, Abercrombie ED. An analysis of the effects of acute and chronic fluoxetine on extracellular norepinephrine in the rat hippocampus during stress. Neuropsychopharmacology. 1997;16(6):419–425. doi: 10.1016/S0893-133X(96)00281-3. [DOI] [PubMed] [Google Scholar]
- 43.Cryan JF, Page ME, Lucki I. Differential behavioral effects of the antidepressants reboxetine, fluoxetine, and moclobemide in a modified forced swim test following chronic treatment. Psychopharmacology (Berl) 2005;182(3):335–344. doi: 10.1007/s00213-005-0093-5. [DOI] [PubMed] [Google Scholar]
- 44.Brummelte S, Lieblich SE, Galea LA. Gestational and postpartum corticosterone exposure to the dam affects behavioral and endocrine outcome of the offspring in a sexually-dimorphic manner. Neuropharmacology. 2012;62(1):406–418. doi: 10.1016/j.neuropharm.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 45.Van den Bergh BR, Van Calster B, Smits T, Van Huffel S, Lagae L. Antenatal maternal anxiety is related to HPA-axis dysregulation and self-reported depressive symptoms in adolescence: a prospective study on the fetal origins of depressed mood. Neuropsychopharmacology. 2008;33(3):536–545. doi: 10.1038/sj.npp.1301450. [DOI] [PubMed] [Google Scholar]
- 46.Weinstock M. The long-term behavioural consequences of prenatal stress. Neurosci Biobehav Rev. 2008;32(6):1073–1086. doi: 10.1016/j.neubiorev.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 47.Brown AS, Susser ES, Lin SP, Neugebauer R, Gorman JM. Increased risk of affective disorders in males after second trimester prenatal exposure to the Dutch hunger winter of 1944–45. Br J Psychiatry. 1995;166(5):601–606. doi: 10.1192/bjp.166.5.601. [DOI] [PubMed] [Google Scholar]
- 48.Muskiet FA, Kemperman RF. Folate and long-chain polyunsaturated fatty acids in psychiatric disease. J Nutr Biochem. 2006;17(11):717–727. doi: 10.1016/j.jnutbio.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 49.Herrick K, Phillips DI, Haselden S, Shiell AW, Campbell-Brown M, Godfrey KM. Maternal consumption of a high-meat, low-carbohydrate diet in late pregnancy: relation to adult cortisol concentrations in the offspring. J Clin Endocrinol Metab. 2003;88(8):3554–3560. doi: 10.1210/jc.2003-030287. [DOI] [PubMed] [Google Scholar]
- 50.Davis EP, Glynn LM, Schetter CD, Hobel C, Chicz-Demet A, Sandman CA. Prenatal exposure to maternal depression and cortisol influences infant temperament. J Am Acad Child Adolesc Psychiatry. 2007;46(6):737–746. doi: 10.1097/chi.0b013e318047b775. [DOI] [PubMed] [Google Scholar]
- 51.Gerlinskaya LA, Evsikov VI. Influence of genetic dissimilarity of mother and fetus on progesterone concentrations in pregnant mice and adaptive features of offspring. Reproduction. 2001;121(3):409–417. doi: 10.1530/rep.0.1210409. [DOI] [PubMed] [Google Scholar]
- 52.Voleti B, Duman RS. The roles of neurotrophic factor and Wnt signaling in depression. Clinical pharmacology and therapeutics. 2012;91(2):333–338. doi: 10.1038/clpt.2011.296. [DOI] [PubMed] [Google Scholar]
- 53.Duman RS. Role of neurotrophic factors in the etiology and treatment of mood disorders. Neuromolecular medicine. 2004;5(1):11–25. doi: 10.1385/NMM:5:1:011. [DOI] [PubMed] [Google Scholar]
- 54.Schorscher-Petcu A, Austin JS, Mogil JS, Quirion R. Role of central calcitonin gene-related peptide (CGRP) in locomotor and anxiety- and depression-like behaviors in two mouse strains exhibiting a CGRP-dependent difference in thermal pain sensitivity. J Mol Neurosci. 2009;39(1–2):125–136. doi: 10.1007/s12031-009-9201-z. [DOI] [PubMed] [Google Scholar]
- 55.Recober A, Russo AF. Calcitonin gene-related peptide: an update on the biology. Curr Opin Neurol. 2009;22(3):241–246. doi: 10.1097/wco.0b013e32832b2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sink KS, Walker DL, Yang Y, Davis M. Calcitonin gene-related peptide in the bed nucleus of the stria terminalis produces an anxiety-like pattern of behavior and increases neural activation in anxiety-related structures. J Neurosci. 2011;31(5):1802–1810. doi: 10.1523/JNEUROSCI.5274-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim J, Noskov VN, Lu X, Bergmann A, Ren X, Warth T, et al. Discovery of a novel, paternally expressed ubiquitin-specific processing protease gene through comparative analysis of an imprinted region of mouse chromosome 7 and human chromosome 19q13.4. Genome Res. 2000;10(8):1138–1147. doi: 10.1101/gr.10.8.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.MacDonald HR, Wevrick R. The necdin gene is deleted in Prader-Willi syndrome and is imprinted in human and mouse. Hum Mol Genet. 1997;6(11):1873–1878. doi: 10.1093/hmg/6.11.1873. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


