Abstract
Modifications of both DNA and protein by methylation are key factors in normal T and B cell immune responses as well as in the development of autoimmune disease. For example, the failure to maintain the methylation status of CpG dinucleotides in DNA triggers T cell autoreactivity. Methylated proteins are known targets of autoimmunity, including the symmetrical dimethylarginine residues of SmD1 and SmD3 in SLE. Herein, we demonstrate that altering the metabolism of S-adenosylmethionine (SAM), the major methyl donor for transmethylation reactions, can suppress T cell immunity. A by-product of SAM metabolism, 5′-Deoxy-5′-methylthioadenosine (MTA), and an indirect inhibitor of methyltransferases, inhibits T cell responses including T cell activation markers, Th1/Th2 cytokines and TCR-related signaling events. Moreover, treatment of the lupus-prone MRL/lpr mouse with MTA markedly ameliorates splenomegaly, lymphadenopathy, autoantibody titers as well as IgG deposition and cellular infiltration in the kidney. Incubation of cells with SAM, which increases intracellular MTA levels, inhibits both TCR-mediated T cell proliferation and BCR (anti-IgM)-triggered B cell proliferation in a dose-dependent manner. These studies define the central role of MTA and SAM in immune responses and provide a simple approach to altering lymphocyte transmethylation and T cell mediated autoimmune syndromes.
Keywords: Transmethylation, methylthioadenosine, S-adenosylmethionine, CD4+ T cells, SLE
Introduction
Methylation modifications influence a number of immunologic responses ranging from intracellular signaling and transcriptional regulation to protein processing and antigen presentation. Gene expression in immune cells is regulated via methylation of histone proteins and DNA CpG dinucleotides, controlling expression of immune response proteins. Important immune response mediators controlled by methylation include INFγ, IL-2, IL-4, CD11a, CD70, and protein phosphatase 2A (PP2A), among others [1,2]. The protein arginine methyltransferase (PRMT) family of enzymes has emerged as a key regulator in T cell activation including modulating the DNA binding ability of phosphorylated STAT1 and the promoter activity of NFAT [3,4]. Of interest, CD28 costimulation with TCR signaling increases PRMT activity and Vav1 methylation in T cells [5].
Recent studies have linked transmethylation reactions to the onset and development of autoimmune diseases. For example, methylated ribonucleoproteins (snRNPs) have been demonstrated as specific targets of autoantibodies. Symmetric dimethylarginine residues in the snRNP proteins, SmD1 and SmD3, are recognized by anti-Sm autoantibodies and a diagnostic marker in human lupus [6]. Mice deficient in protein L-isoaspartate O-methyltransferase (PIMT) develop anti-DNA autoantibodies and kidney pathology typical of that found in systemic lupus erythematosus (SLE) [7]. Moreover, the substrates of PIMT, isoaspartate-containing proteins, accumulate in the lupus murine model and mediates abnormal T cell hyperproliferation [8].
Finally, arginine-methylated myelin basic protein provokes an autoimmune response in multiple sclerosis (MS) and anti-myelin basic protein antibodies have been found in both human and murine model of MS [9]. These observations support the notion that methylation modifications play a critical role in maintaining T cell function and/or immune dysfunction in inflammation and autoimmune disease.
All transmethylation reactions depend on the availability of an intracellular methyl donor group. S-adenosylmethionine (SAM) serves as the major intracellular methyl donor and is formed via the conversion of methionine (Met) in an ATP-consuming reaction (Figure 1). Upon the transfer of methyl groups catalyzed by methyltransferases, SAM converts to S-adenosylhomocysteine (SAH). Thereafter, SAH hydrolase converts SAH into homocysteine (HCys) that can be recycled back to methionine. SAM and SAH are the substrate and product of methyltransferase reactions with SAH serving as a potent inhibitor of methyltransferases. In addition, methylthioadenosine (MTA), which is the by-product of polyamine biosynthesis or the spontaneous breakdown of SAM, can also be converted back to methionine in a series of reactions. Importantly, MTA is known as a potent inhibitor of SAH hydrolase [10–12]. In general, a higher level of MTA is usually associated with a higher level of SAH, thus indirectly inhibiting transmethylation reactions.
Figure 1.
The intracellular methylation metabolic cycle. SAM is formed from methionine (Met) by ATP-consuming reaction. Methyltransferase (➀) catalyzes the methylation of intracellular substrates including DNA, lipid and protein in the presence of SAM, which serves as the intracellular methyl donor. S-adenosylhomocysteine (SAH) is converted to homocysteine (Hcys) by SAH hydrolase (➁) and subsequently into Met in this cycle. MTA is a methylation inhibitor via its inhibition of SAH hydrolase. The major source of MTA is formed as a product of spermindine biosynthesis. But, MTA also can be breakdown from SAM spontaneously (➂ non-enzymatic reaction). Finally, MTA is converted to Met in a series of steps requiring MTA phosphorylase (➃).
In the present study, we demonstrate that disruption of the SAM metabolic cycle by incubation of cells with either SAM or MTA results in profound alteration of TCR signaling, impaired Th1/Th2 cytokine release, and decreased T cell activation and proliferation. Treatment of MRL/lpr mice, the murine model of human SLE, with MTA reduces auto-immune manifestations and disease pathology. Taken together, the manipulation of SAM-dependent methylation can modulate T cell function that may provide a basis for therapeutic strategies to treat T cell-mediated disorders.
Materials and Methods
Mice
B10.BR and MRL/Mp-Fas lpr (MRL/lpr) mice were obtained from The Jackson Laboratory (Bar Harbor, ME). AND transgenic mice expressing an α/β TCR (Vα11+, Vβ3+) which recognizes the amino acids 88–104 of pigeon cytochrome C (PCC 88–104) were maintained and screened as described previously [13]. All animals utilized were female and ≥6 weeks of age and maintained in specific pathogen-free facilities at the Yale Animal Resources Center.
T and B Cell purification
Pooled axillary, inguinal, brachial and popliteal lymph nodes and spleens were used for purification of CD4+ T cells and B cells. T cells and B cells were purified by negative selection using mouse CD4+ T cell enrichment kit and mouse B cell enrichment kit according to the manufacturer’s instructions respectively (StemCell Technologies, Vancouver, Canada). The resulting B and T cell populations were >95% pure as determined by flow cytometry.
Cell proliferation assays
Mice were immunized subcutaneously with 50 μg of ovalbumin (OVA, Sigma) in PBS emulsified 1:1 in complete Freund’s adjuvant (Sigma) at the base of the tail and hind footpad. MTA (Sigma, D5011; 96 μmol/kg body weight) or SAM (p-toluenesulfonate salt form, Sigma, A2408; 96 μmol/kg body weight) or placebo (PBS containing 0.4% dimethylsulfoxide) was administered by daily intraperitoneal (i.p.) injection starting after immunization. Ten days later, draining lymph nodes were excised and single cell suspensions were prepared for thymidine incorporation assay as described previously [8].
In brief, 5 × 105 cells were cultured in 96-well plates in the presence of different concentrations of OVA or anti-CD3 (5 μg/ml) and anti-CD28 (1 μg/ml) for three days. For AND transgenic mice, purified CD4+ T cells (2 × 105) and mitomycin C-treated CH 27 B lymphoblastoid cells (8 × 105) as antigen presenting cells (APC) were cultured with 0.1 μM PCC 88–104 in the absence or presence of MTA. In some experiments, purified CD4+ T cells (4 × 105) or B cells (1 × 105) were cultured with coated mouse anti-CD3 (5 μg/ml) and anti-CD28 (1 μg/ml) antibodies or anti-IgM antibody (50 μg/ml) in the presence or absence of titrating concentrations of MTA or SAM (see figure legends). In addition, cell viability was analyzed by flow cytometry (FACS Aria, Becton Dickinson Immunocytometry Systems) by using To-Pro3 iodide in conventional staining methodology.
B cell antibody class-switching assay
IgG1 class-switching was measured as described previously [14]. In brief, purified MRL/lpr B cells were labeled by CFSE (5 μM; Molecular Probes) for 5 min and then 4 × 105 B cells were cultured with LPS (40 μg/ml; Sigma) and recombinant murine IL-4 (20 ng/ml; R&D Systems) in the presence of various concentrations of MTA or SAM for 3–4 days. Proliferating B cells was monitored by diluted CFSE staining and switching to IgG1 is detected by surface staining with biotin rat anti-mouse IgG1 (A85-1; generous gift from Dr. Mark Shlomchik) and streptavidin Cy-chrome (BD Pharmingen).
Th1/Th2 cytokine detection
Supernatants from triplicate T cell activation cultures were harvested before pulsing with [3H] thymidine. Concentrations of IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12 (p70), TNFα, INFγ and GM-CSF (Beadlyte® Mouse Multi-Cytokine Detection System 2, Millipore) were determined by Luminex technology (Bio-Plex; Bio-Rad, Inc.).
T cell and B cell activation marker expression
Purified T or B cells were stimulated with anti-CD3 (5 μg/ml) and anti-CD28 (1 μg/ml) mAb or anti-IgM (25 μg/ml), respectively, in the presence of 50 μM of MTA or SAM dissolved in tissue culture medium. T cells were analyzed with antibodies specific for CD4 (RM4-5), CD69 (H1.2F3), CD25 (PC61), CD44 (IM7) and CD62 ligand (CD62L, MEL-14) and analyzed by flow cytometry (FACS Aria, Becton Dickinson Immunocytometry Systems). B cell activation markers were similarly analyzed with antibodies to CD19 (1D3), CD69, CD25, CD80 (B7.1; 16-10A1) and CD86 (B7.2; GL1).
Phosphoprotein signaling cascade analysis
Purified CD4+T cells were stimulated with anti-CD3 (5 μg/ml) and anti-CD28 (1 μg/ml) mAb in the absence or presence of different concentrations of MTA or SAM for 2 min, 1 hr or 1 day. Cell were lysed and the levels of phosphoproteins including c-Jun, CREB, ERK1/2, JNK, MEK1, p38 MAPK, p70 S6 kinase, p90RSK, STAT3 (Tyr705) and STAT6 (Tyr641) (customized Bio-Plex Phosphoproteins multiplex Kit, Bio-Rad) were analyzed according to the manufacturer’s instructions.
Measurement of MTA phosphorylase activity
MTA phosphorylase activity was measured by a modification of the method described by Savarese et al. [15]. Briefly, the liberation of adenine by MTA phosphorylase is monitored by adding xanthine oxidase to convert into 2,8-dihydroadenine, which has a maximum extinction at 305 nm measured by using SpectramaxPLUS384 (Molecular Devices) at 37°C. One unit of MTA phosphorylase activity is defined as the formation of 1 μmol of adenine/min under the above conditions.
Treatment of MRL/lpr mice with MTA or SAM and clinical assessment of lupus phenotypes
Mice (10 mice/group) were i.p. injected daily with MTA or SAM (96 μmol/kg body weight) or PBS (containing 0.4% dimethylsulfoxide). Initially, ten mice per group of 6-week-old MRL/lpr mice were treated and there were 8 mice in PBS and SAM group and 7 mice in MTA group still survived at the end point experiment (the age of 20 weeks). Animals were weighed weekly and there was no difference of body weight between groups during the period of treatment (data not shown). Blood urea nitrogen was measured using the Roche COBAS Mira Plus automated chemistry analyzer at the Yale University Mouse Metabolic Phenotyping Center metabolic testing core. Sera were collected for quantification of anti-nuclear (ANA), anti-dsDNA and anti-snRNP autoantibodies as described previously [16]. For renal pathology, one kidney of each mouse was prepared in haemotoxylineosin (H&E) stained slides (Yale Pathology Tissue Services) and inflammatory cell infiltration was assessed. For IgG deposition, the other kidney was prepared in 5-μm-thick sections after being frozen and then stained with 1:100 diluted fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG (Sigma).
Statistical analysis
All statistics were performed using a Student’s unpaired two-tailed t-test except anti-nuclear antibody score (ANA) by using Mann–Whitney test. A value of p < 0.05 was regarded as significant.
Results
Disruption of cellular methyl donor metabolism suppresses T and B cell proliferation
T and B cell proliferation was examined in the presence of SAM or MTA, two critical metabolites in cellular methylation reactions [17–19]. The baseline proliferation index was arbitrarily set at 100% when cells were activated by anti-CD3 plus anti-CD28 mAbs for T cells or by anti-IgM mAb for B cells in the absence of MTA or SAM. As shown in Figure 2A, SAM has similar inhibitory effects on T and B cell proliferation. In concentrations as low as 1 μM of SAM, proliferative responses were reduced by 20%. Above 10 μM of SAM, there was a sharp decrease of TCR engaged-T cell and anti-IgM-stimulated B cell proliferation.
Figure 2.
MTA and SAM inhibit T cell and/or B cell proliferation. (A) Purified T and B cells from B10.BR mice were stimulated in the presence of various concentrations of MTA or SAM (see Materials and Methods). Six independent results are represented as the percentage of the stimulation control response (% ± S.D. of the mean). (B) CD4+ T cells were purified from placebo-, MTA-treated and SAM-treated OVA immunized B10.BR mice (n = 6 for each group). The proliferative response against immunizing antigen (OVA) or general TCR engagement (anti-CD3 plus anti-CD28 mAb) was evaluated by [3H] thymidine incorporation as described in Materials and Methods. Positive control PPD responses were >68,000 cpm. ★, p < 0.05 for comparison to placebo group.
In contrast, we observed somewhat different effects of MTA on B cell vs. T cell proliferation. No significant inhibition of T cell responses were detected at 1 and 10 μM MTA. Above 10 μM of MTA, there was a sharp decrease of T cell proliferation, similar to that observed with SAM. At 50 μM of MTA, greater than 90% proliferative responses was inhibited, yet viability remained relatively unchanged (Table 1). Of interest, T cell proliferation was inhibited by 50% at 30 μM MTA, a concentration virtually identical to the Ki of MTA in inhibiting SAH hydrolase (36 μM) [11]. This observation suggests that the effect of interfering T cell responses of MTA is likely due to inhibition of transmethylation mediated by SAH hydrolase inhibition. However, we note that the pathway by which MTA inhibits proliferation is complex, and can involve the rate of transport of MTA into cells and the levels of SAH that accumulate upon the hydrolase inhibition in addition to the Ki of MTA for the hydrolase. In anti-IgM-stimulated B cells, MTA had no effect on proliferation. Taken together, SAM has potent inhibitory effects for both T and B cell proliferation while MTA has selective suppression on T cell proliferation.
Table 1.
MTA does not affect T cell viability.
| MTA (μM) | Viability (% ± S.D.) |
|---|---|
| 0 | 65.6 ± 7.9 |
| 10 | 75.6 ± 5.0 |
| 20 | 70.7 ± 7.6 |
| 40 | 71.1 ± 3.1 |
| 50 | 69.2 ± 3.1 |
| 100 | 62.5 ± 10.3 |
Viability assay was determined by To-Pro 3 staining. Values in the table represent the percentage of T cell viability under the stimulation of anti-CD3 and anti-CD28 mAb in the presence of various concentrations of MTA for 3 days from 2 independent experiments.
We next assessed the effect of MTA and SAM on the generation of specific T cells in vivo and the overall T cell responses in B10.BR mice sensitized with ovalbumin (OVA). Mice were administered MTA, SAM or placebo via daily i.p. injection for 10 days upon immunization with OVA. Purified CD4+ T cells from draining lymph node were then restimulated with OVA antigen. As shown in Figure 2B, OVA specific-proliferative response was significantly reduced in both MTA or SAM-treated mice. In addition, proliferation of CD4+ T cells stimulated by anti-CD3/CD28 engagement were also diminished in MTA or SAM-treated mice compared to placebo group. Our observations illustrate that MTA and SAM suppress T cell response in a receptor-mediated manner and/or downstream of TCR or CD28 activation.
The expression of T and B cell activation markers in the presence of MTA or SAM
T and B cell development and activation is marked by a succession of changes in specific surface macromolecules. We examined CD62L (L-selectin) and CD44 in T cells and CD80 (B7.1) and CD86 (B7.2) in B cells. We found that the major populations of anti-CD3/28 stimulated T cells were activated cells (CD4+CD44+CD62L+) and memory cells (CD4+CD44+CD62L−) while naïve populations approach control (unstimulated) levels (Figure 3, top panel). Activated and memory populations of T cells were significantly diminished in the presence of either MTA or SAM. For B cells, the expression of CD86 (Figure 3, bottom panel) and CD80 (data not shown) did not significantly change in the presence of SAM and was slightly increased in the presence of MTA. This observation is consistent with the effect of MTA on B cell proliferation at 50 μM dose (Figure 2). As noted above, in contrast to B cell responses, both T cell proliferation and activation was suppressed in the presence of MTA and SAM.
Figure 3.
MTA and SAM activation marker expression in Tand B cells. T cells and B cells were stimulated by anti-CD3 and anti-CD28 mAb or anti-IgM in the presence or absence of MTA or SAM. T cells were stained for CD4, CD44 and CD62L and B cells were stained for CD19 and CD86 surface proteins. The expression of CD44 and CD62L in CD4-gated T cells are shown in top panel. The expression of CD86 in CD19-gated B cells was shown in bottom panel. Data are representative of three independent experiments.
Two other surface molecules, IL-2Rα (CD25) and C-type lectin receptor (CD69) are typically induced within 24 hr in activated T and B cells. In T cells, the expression of CD25 was significantly reduced in the presence of MTA in anti-CD3/28 treated T cells although the expression of CD25 remained similar in the presence of SAM in anti-CD3/28 treated T cells (data not shown). However, the inhibition of CD25 expression was not seen in B cells after treatment of MTA or SAM. In addition, neither MTA nor SAM altered CD69 expression in T and B cells (data not shown).
Differences in MTA phosphorylase explains altered methylation in T and B cells
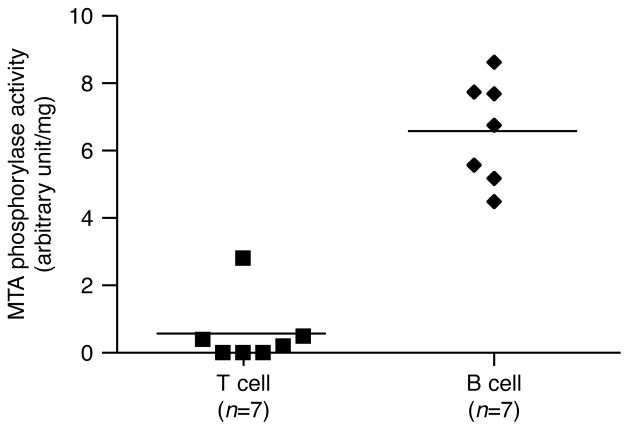
MTA phosphorylase is the major MTA metabolizing enzyme that catalyzes the phosphorylation of MTA to methylthioribose-1-phosphate (MTRP) with the release of adenine. Subsequently, adenine is utilized in purine salvage pathways to form ATP while MTRP is recycled to methionine (Figure 1). It was previously reported that there is little or no MTA phosphorylase activity in human leukemic cells such as T cell acute lymphoblastic leukemia (T-ALL) and adult T cell leukemia (ATL) [20–22]. In contrast, MTA phos-phorylase activity in PBMC from patients with B-chronic lymphocytic leukemia (B-CLL) was higher than from control subjects [23].
These observations suggest that MTA metabolism in T cells differs from that of B cells. As expected, MTA phosphorylase activity in B cells (6 units/mg) was more than 10-fold higher than in T cells (0.5 units/mg) either from normal B10.BR mice (Figure 4) or from lupus-prone MRL/lpr mice (data not shown). In addition, MTA phosphorylase remains the same in populations of both resting and receptor-activated (anti-IgM) B cells (data not shown). These data suggest intracellular MTA concentrations may be limited in B cells but not in T cells as a result of MTA phosphorylase activity. Importantly, these differences provide a mechanistic explanation for why we observe a much larger immunosuppressive effect of MTA for T cells compared to B cells (Figure 2A and Figure 3).
Figure 4.
MTA phosphorylase activity is higher in B cells compared to T cells. T and B cells were purified by negative selection from spleen and MTA phosphorylase activity was measured as described in the Materials and Methods section. One unit of MTA phosphorylase activity is defined as the formation of 1 μmol of adenine per min. The MTA phosphorylase activity between T and B cell population is highly statistically significant ( p < 0.0001).
Th1/Th2 cytokine production is suppressed by MTA and SAM
As noted earlier, there was no decrease in T cell proliferation at 10 μM MTA (Figure 2). However, we surmised that other biological properties of T cells, such as cytokine production, may be altered even when proliferation, per se, is unchanged. Indeed, IL-2, IL-10, TNFα and IFNγ production was decreased in activated T cells in the presence of 10 μM MTA for 48 h (Figure 5). Moreover, the suppression of these cytokines was observed as early as 24 hr with MTA (data not shown). Taken together, IL-2, IL-4, IL-5, IL-6, IL-10, TNFα, INFγ and GM-CSF secretion from anti-CD3/CD28 stimulated T cells were inhibited by MTA and SAM in a dose-dependent manner (Figure 5). As noted, some cytokines (IL-4, IL-5, IL-6, and GM-CSF) are only significantly inhibited at higher concentrations of MTA and/or SAM.
Figure 5.
Th1/2 cytokine production is suppressed by MTA and SAM. Cytokines, as illustrated, were assessed from anti-CD3/CD28 stimulated T cell cultures (as defined for Figure 2) in the presence of MTA or SAM. Then Th1/2 cytokine secretion was assessed by Luminex technology as described in the Materials and Methods section. The error bars indicate S.D. of the mean (n = 3 for each bar). ★, p < 0.05 relative to control cultures.
The effect of MTA and SAM on TCR Signaling pathways
A number of signaling proteins are phosphorylated in T cells within 2 min of stimulation, including MEK 1, p70 S6 kinase and JNK. The presence of MTA or SAM for only 2 min could significantly suppress the phosphorylation of MEK 1 and JNK (Figure 6). Moreover, the phosphorylation of ERK1/2, c-Jun, p38 MAPK and P90RSK were inhibited to varying degrees by both MTA and SAM. In contrast, the phosphorylation of p70 S6 kinase, Stat3, and Stat6 remained unchanged in the presence of MTA or SAM (data not shown) while CREB was only slightly decreased. These data suggest that a combination of the TCR signaling proteins are affected by the alteration of cellular methylation and contributed the immunosuppressive effect of MTA and SAM on T cell functions.
Figure 6.
TCR signaling pathways are altered by MTA and SAM. Cell lysates were collected from purified CD4+ T cells at different time point after stimulation by anti-CD3 plus anti-CD28 mAb in the presence or absence of 10 μM MTA or SAM. The phosphorylation status of different TCR signaling pathway proteins were simultaneously analyzed by Luminex as described in the Materials and Methods section. The phosphorylation status of each protein was arbitrarily set at 100% when cells were activated by anti-CD3 plus anti-CD28 mAbs. Data are given as mean ± S.D. (n = 3 for each bar) and represent two independent experiments. Representative time course of TCR stimulation for each signaling protein is 2 min for MEK1 and JNK and 1 hr for ERK1/2, CREB, c-Jun, p38MAPK and p90RSK. ★, p < 0.05 for comparison to anti-CD3/CD28 only-treated T cells.
MTA ameliorates the lupus-like phenotypes in MRL/lpr mice
The MRL/lpr model resembles human SLE, in that murine disease is marked by renal and skin pathology, autoantibody production, and T cell hyperproliferation. Given the profound immunosuppressive effects of MTA and SAM via modulation of T cell function, we next examined if MTA and/or SAM may alter the progression of murine SLE. Indeed, both lymphadenopathy and splenomegaly were reduced upon treatment with MTA for a period of 14 weeks in lupus-prone MRL/lpr mice. (Figure 7, panels A and B). No discernable change in spleen or lymph node weight or cellularity was observed in parallel MRL/lpr animals treated with SAM. In vitro effects of MTA were examined with T cells specific for PCC 88–104 (B10.AND or MRL.AND mice). Remarkably, specific T cell responses to antigen were completely ameliorated in MTA treated T cells (Figure 7, panel C). Importantly, MRL.AND T cells were highly sensitive to low concentrations of MTA (10 μM) as compared to B10.AND T cells.
Figure 7.
MTA treatment ameliorates splenomegaly and lymphadenopathy in MRL/lpr mice. Representative spleen (A) or lymph nodes (B) from MRL/lpr mice treated daily with i.p. MTA, SAM, or placebo, as illustrated. Splenomegaly and lymphadenopathy were significantly reduced in MTA treatment MRL/lpr mice as shown in the bar graph (n = 8 in PBS and SAM group; n = 7 in MTA group). ★, p < 0.05. N.S., no significant difference. (C) Anti-proliferative effect of MTA in B10.AND and lupus-prone MRL.AND transgenic CD4T cells. Purified AND transgenic CD4+ T cells were stimulated in vitro by APCs with 0.1μM PCC 88–104 in the absence or presence of various concentration of MTA. Proliferation was measured at 72 hr by [3H] thymidine incorporation. (D) Immunoglobulin class-switching was suppressed by MTA. CFSE-labeled MRL/lpr B cells were stimulated in vitro by LPS and IL-4 in the presence of MTA or SAM. IgG1 class switching was determined at 90 hr by surface IgG1/CFSE staining. Data are representative of two independent experiments.
Autoantibodies against nuclear components are hallmarks of both human and murine models of lupus model contribute to tissue pathology. The major function of B cells is in making antibody against antigen. As expected in Figure 7D, MRL/lpr B cell proliferation and IgG1 class-switching were inhibited by SAM. In contrast, MTA did not inhibit B cell proliferation (Figures 2A and 7D), however, B cell antibody class-switching was suppressed by 100μM MTA in vitro (Figure 7D).
Moreover, MTA treatment significantly reduced production of antinuclear antibodies (ANA) starting at 10 weeks of age until the mice were sacrificed at 20 weeks (data not shown). Representative ANA data from 15-week-old mice are illustrated (Figure 8). Finally, kidney dysfunction is one key clinical phenotype in both MRL/lpr models and in human SLE. Blood urea nitrogen, another marker of kidney pathology, was reduced in MTA-treated MRL animals (data not shown). IgG deposition in glomeruli (Figure 9, top panel) and lymphocyte infiltration (Figure 9, bottom panel) were remarkably decreased in MTA-treated animals. Perivascular thickening and glomerular pathology were significantly less in all MTA-treated animals.
Figure 8.
MTA treatment suppresses autoantibody levels in the circulation of MRL/lpr mice. Sera collected from 15-week-old placebo, MTA- or SAM-treated MRL/lpr mice were examined for antinuclear antibodies (ANA) as described in the Materials and Methods section. Representative microscopy views of each group as shown in (A) and the grading scores for ANA as shown in (B) (n = 10 in PBS; n = 9 in MTA and SAM group). ★, p < 0.0005.
Figure 9.

MTA treatment reduces kidney pathology and lymphocyte infiltration in MRL/lpr mice. Representative microscopy of renal IgG deposition (A) and renal H&E staining slides (B) from each group (magnification × 40).
Discussion
Many posttranslational protein modifications, methylation in particular, are key regulators of lymphocyte activation and subsequent immune responses [24]. Recently, protein methylation has been emerged as a specific target for design of immunosuppressive and anti-inflammatory agents. Blockade of SAH hydrolase resulting in the reduction of intracellular protein methylation leads to a decrease in both humoral and cell-mediated immune responses [25–28]. To date, several SAH hydrolase inhibitors are demonstrated to exhibit immunomodulatory effects, including DZ2002, DHCaA and MDL 28,842 [29]. All these compounds have been shown to inhibit T cell proliferation in vitro or in vivo [25,28,30]. Moreover, DHCaA can suppress delayed type hypersensitivity ear swelling and peptidoglycan polysaccharide-induced arthritis [30]. MDL 28,842 can slow the pathology of collagen-induced arthritis [28]. DZ2002 can ameliorate experimental autoimmune encephalomyelitis (EAE) by suppressing T cell activation [26,31]. Taken together, compounds with the capability of inhibiting SAH hydrolase may be effective therapeutic candidates for T cell mediated auto-immune diseases such as SLE, MS, RA and type1 diabetes.
A natural metabolite, MTA is an effective inhibitor of SAH hydrolase [10–12]. MTA is produced in cells in a spontaneous degradation process from SAM and as a by-product of enzymatic reactions, including polyamine synthesis [17]. Importantly, MTA has no observed cellular toxicity in mouse models [18,32]. Recently, MTA was reported to have therapeutic potential in treating MS [33–35]. Administration of MTA in a murine model of multiple sclerosis was found to reverse chronic-relapsing disease with amelioration of brain inflammation. Effects of MTA were thought to be due to a suppression of T cell activation, the prevention of inhibitors of kappa B (IκB-α) degradation and inhibition of cytokine production (including INFγ, TNFα and IL-2). Although MTA is a methylation inhibitor and SAM is an intracellular methyl donor, studies have shown that both compounds have similar effect on cellular functions. These effects include the inhibition of lipopolysaccharide (LPS)-induced TNFα expression, suppressing the binding affinity of methylated histone (H3K4) to TNFα promoter, and inhibiting the activity of ornithine decarboxylase [36–38].
Although the precise details and mechanistic pathways affected by MTA and SAM have not been elucidated, it has been suggested that SAM’s conversion to MTA and the subsequent inhibition of SAH hydrolase can function to raise the intracellular level of SAH, a potent product inhibitor of most methyltransferases [17]. However, our study demonstrated that SAM could inhibit B cell proliferation, yet MTA could not (Figure 2A). One possible explanation is that the bioavailability of MTA is reduced in B cells due to higher MTA phosphorylase activity in B cells compared to T cells (Figure 4). Nonetheless, we cannot rule out an MTA-independent pathway for SAM to interfere with B cell immune response. At least one additional pathway exists for SAM to inhibit methyltransferases. SAM can be slowly and spontaneously hydrolyzed to the methyltransferase inhibitor SAH, with a half-life estimated at 80 days under physiological conditions [19]. However, it is unclear whether any of the SAH formed in such a reaction while lymphocytes were incubated with SAM in our study.
In the present study, we demonstrated that both MTA and SAM could modulate T cell immune response including T cell proliferation, the expression of T cell activation markers, Th1/Th2 cytokines and TCR-related signaling events. However, only MTA administration ameliorates the clinical (splenomegaly and lymphadenopathy), serologic (autoantibodies) and histologic (renal IgG deposition and lymphocyte infiltration) parameters of lupus-like phenotypes in lupus-prone MRL/lpr murine model.
T cell hyperproliferation is a prominent characteristic in many T cell mediated-autoimmune diseases such as SLE, MS and type 1 diabetes [39–41]. Enhanced TCR/CD3-mediated T cell proliferation is found in both patients with SLE [40] and in lupus-prone mice [41,42]. In the MRL lupus model, over 70% of T cells are typically activated (CD44+) in the resident CD4 population [8]. Increased expression of CD69, an early activation marker in T cell populations, is observed in type 1 diabetes [43], lupus [44] and RA patients [45]. Herein, we demonstrated that lupus-prone T cells treated with MTA have significantly reduced antigen-specific proliferation compared to untreated cells.
Aberrant Th1/Th2 cytokine profiles and altered TCR signaling pathways have been described in several models of autoimmune disease [46]. In human SLE, serum levels of IL-4, IL-6, IL-10, IL-12, IL-17, TNFα and IFNγ are significantly elevated while IL-2 and TGFβ are reduced [47,48]. Moreover, altered patterns of phosphorylation in protein tyrosine kinase (PTK) and ERK are found in activated T cells from human lupus patients and MRL mice [49,50]. These pathways are dependent on accurate protein methylation for their cellular biology. Recent evidence demonstrated that MTA or SAM alters protein methylation and subsequently affects gene expression in immune responses [18,37,51,52]. For example, MTA mediated inhibition of arginine methylation reduces IL-2 gene expression by suppression of NFAT driven promoter activity in Jurkat T cells [4].
MTA can inhibit STAT1-mediated IFN responses due to the absence of STAT1 methylation on Arg31 [3]. MTA and/or SAM pretreatment inhibit LPS induced gene expression via modulation of histone 3 lysine 4 (H3K4) methylation in RAW macrophage cells [37]. In our ongoing studies, anti-proliferative effects are also found in primary human T cells treated with either MTA or SAM (data not shown). Taken together, our studies demonstrate the central role of transmethylation in immune responses and provide a simple approach to altering lymphocyte metabolism and T cell-mediated autoimmune syndromes.
Acknowledgments
We are grateful to the Abnova Corp. for providing the MTA phosphorylase recombinant protein for our present studies. We also thank Professor Mark Shlomchik for valuable suggestions and generous gift of anti-IgG1 antibody for B cell antibody class-switching assays.
Abbreviations
- MTA
5′-deoxy-5′-methylthioadenosine
- SAM
S-adenosylmethionine
- SAH
S-adenosylhomocysteine
- Met
methionine
- SLE
systemic lupus erythematosus
- snRNP
small nuclear ribonucleoprotein particle
- TCR
T cell receptor
Footnotes
Declaration of interests: The authors report no conflicts of interest. This work was supported by National Institutes of Health Grant (AI-48120) and the Alliance for Lupus Research to M.J.M., Arthritis Foundation Postdoctoral Fellowship to M-L. Y. and Research Engineering Apprenticeship Program from Department of Defense to A.J.P.G. The authors are responsible for the content and the writing of this paper.
References
- 1.Renaudineau Y, Youinou P. Epigenetics and autoimmunity, with special emphasis on methylation. Keio J Med. 2011;60:10–16. doi: 10.2302/kjm.60.10. [DOI] [PubMed] [Google Scholar]
- 2.Sunahori K, Juang YT, Kyttaris VC, Tsokos GC. Promoter hypomethylation results in increased expression of protein phosphatase 2A in T cells from patients with systemic lupus erythematosus. J Immunol. 2011;186:4508–4517. doi: 10.4049/jimmunol.1000340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mowen KA, Tang J, Zhu W, et al. Arginine methylation of STAT1 modulates IFNalpha/beta-induced transcription. Cell. 2001;104:731–741. doi: 10.1016/s0092-8674(01)00269-0. [DOI] [PubMed] [Google Scholar]
- 4.Richard S, Morel M, Cleroux P. Arginine methylation regulates IL-2 gene expression: a role for protein arginine methyltransferase 5 (PRMT5) Biochem J. 2005;388:379–386. doi: 10.1042/BJ20040373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blanchet F, Cardona A, Letimier FA, Hershfield MS, Acuto O. CD28 costimulatory signal induces protein arginine methylation in T cells. J Exp Med. 2005;202:371–377. doi: 10.1084/jem.20050176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brahms H, Raymackers J, Union A, et al. The C-terminal RG dipeptide repeats of the spliceosomal Sm proteins D1 and D3 contain symmetrical dimethylarginines, which form a major B-cell epitope for anti-Sm autoantibodies. J Biol Chem. 2000;275:17122–17129. doi: 10.1074/jbc.M000300200. [DOI] [PubMed] [Google Scholar]
- 7.Doyle HA, Gee RJ, Mamula MJ. A failure to repair self-proteins leads to T cell hyperproliferation and autoantibody production. J Immunol. 2003;171:2840–2847. doi: 10.4049/jimmunol.171.6.2840. [DOI] [PubMed] [Google Scholar]
- 8.Yang ML, Doyle HA, Gee RJ, et al. Intracellular protein modification associated with altered T cell functions in autoimmunity. J Immunol. 2006;177:4541–4549. doi: 10.4049/jimmunol.177.7.4541. [DOI] [PubMed] [Google Scholar]
- 9.Pritzker LB, Joshi S, Gowan JJ, Harauz G, Moscarello MA. Deimination of myelin basic protein. 1. Effect of deimination of arginyl residues of myelin basic protein on its structure and susceptibility to digestion by cathepsin D. Biochemistry. 2000;39:5374–5381. doi: 10.1021/bi9925569. [DOI] [PubMed] [Google Scholar]
- 10.Della Ragione F, Pegg AE. Effect of analogues of 5′-methylthioadenosine on cellular metabolism. Inactivation of S-adenosylhomocysteine hydrolase by 5′-isobutylthioade-nosine. Biochem J. 1983;210:429–435. doi: 10.1042/bj2100429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferro AJ, Vandenbark AA, MacDonald MR. Inactivation of S-adenosylhomocysteine hydrolase by 5′-deoxy-5′-methylthioadenosine. Biochem Biophys Res Commun. 1981;100:523–531. doi: 10.1016/s0006-291x(81)80208-2. [DOI] [PubMed] [Google Scholar]
- 12.Fox IH, Palella RD, Thompson D, Herring C. Adenosine metabolism: modification by S-adenosylhomocysteine and 5′-methylthioadenosine. Arch Biochem Biophys. 1982;215:302–308. doi: 10.1016/0003-9861(82)90308-3. [DOI] [PubMed] [Google Scholar]
- 13.Peng SL, Fatenejad S, Craft J. Induction of nonpathologic, humoral autoimmunity in lupus-prone mice by a class II-restricted, transgenic alpha beta T cell. Separation of autoantigen-specific and -nonspecific help. J Immunol. 1996;157:5225–5230. [PubMed] [Google Scholar]
- 14.Kracker S, Radbruch A. Immunoglobulin class switching: in vitro induction and analysis. Meth Mol Biol. 2004;271:149–159. doi: 10.1385/1-59259-796-3:149. [DOI] [PubMed] [Google Scholar]
- 15.Savarese TM, Crabtree GW, Parks RE., Jr 5′-Methylthioadenosine phosphorylase-L. Substrate activity of 5′-deoxyadenosine with the enzyme from Sarcoma 180 cells. Biochem Pharmacol. 1981;30:189–199. doi: 10.1016/0006-2952(81)90077-0. [DOI] [PubMed] [Google Scholar]
- 16.Mamula MJ, Gee RJ, Elliott JI, et al. Isoaspartyl post-translational modification triggers autoimmune responses to self-proteins. J Biol Chem. 1999;274:22321–22327. doi: 10.1074/jbc.274.32.22321. [DOI] [PubMed] [Google Scholar]
- 17.Clarke SG. Inhibition of mammalian protein methyltransferases by 5′-methylthioadenosine (MTA): A mechanism of action of dietary SAMe? In Protein Methyltransferases. The Enzymes. 2006;XXIV:467–493. doi: 10.1016/S1874-6047(06)80018-1. [DOI] [PubMed] [Google Scholar]
- 18.Li TW, Yang H, Peng H, et al. Effects of S-adenosylmethionine and methylthioadenosine on inflammation-induced colon cancer in mice. Carcinogenesis. 2012;33:427–435. doi: 10.1093/carcin/bgr295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lakowski TM, Frankel A. Sources of S-adenosyl-L-homocysteine background in measuring protein arginine N-methyltransferase activity using tandem mass spectrometry. Anal Biochem. 2010;396:158–160. doi: 10.1016/j.ab.2009.08.043. [DOI] [PubMed] [Google Scholar]
- 20.Kamatani N, Yu AL, Carson DA. Deficiency of methylthioadenosine phosphorylase in human leukemic cells in vivo. Blood. 1982;60:1387–1391. [PubMed] [Google Scholar]
- 21.Batova A, Cottam H, Yu J, et al. EFA (9-beta-D-erythrofuranosyladenine) is an effective salvage agent for methylthioadenosine phosphorylase-selective therapy of T-cell acute lymphoblastic leukemia with L-alanosine. Blood. 2006;107:898–903. doi: 10.1182/blood-2005-06-2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harasawa H, Yamada Y, Kudoh M, et al. Chemotherapy targeting methylthioadenosine phosphorylase (MTAP) deficiency in adult T cell leukemia (ATL) Leukemia. 2002;16:1799–1807. doi: 10.1038/sj.leu.2402570. [DOI] [PubMed] [Google Scholar]
- 23.Vertongen F, Mandelbaum I. Methylthioadenosine phosphorylase and purine nucleoside phosphorylase in B-chronic lymphocytic leukemia. Thymus. 1984;6:359–364. [PubMed] [Google Scholar]
- 24.German DC, Bloch CA, Kredich NM. Measurements of S-adenosylmethionine and L-homocysteine metabolism in cultured human lymphoid cells. J Biol Chem. 1983;258:10997–11003. [PubMed] [Google Scholar]
- 25.Wu QL, Fu YF, Zhou WL, et al. Inhibition of S-adenosyl-L-homocysteine hydrolase induces immunosuppression. J Pharmacol Exp Ther. 2005;313:705–711. doi: 10.1124/jpet.104.080416. [DOI] [PubMed] [Google Scholar]
- 26.Fu YF, Wang JX, Zhao Y, et al. S-adenosyl-L-homocysteine hydrolase inactivation curtails ovalbumin-induced immune responses. J Pharmacol Exp Ther. 2006;316:1229–1237. doi: 10.1124/jpet.105.093369. [DOI] [PubMed] [Google Scholar]
- 27.Wolos JA, Frondorf KA, Babcock GF, Stripp SA, Bowlin TL. Immunomodulation by an inhibitor of S-adenosyl-L-homocysteine hydrolase: inhibition of in vitro and in vivo allogeneic responses. Cell Immunol. 1993;149:402–408. doi: 10.1006/cimm.1993.1165. [DOI] [PubMed] [Google Scholar]
- 28.Wolos JA, Frondorf KA, Davis GF, et al. Selective inhibition of T cell activation by an inhibitor of S-adenosyl-L-homocysteine hydrolase. J Immunol. 1993;150:3264–3273. [PubMed] [Google Scholar]
- 29.Lawson BR, Eleftheriadis T, Tardif V, et al. Transmethylation in immunity and autoimmunity. Clin Immunol. 2011 doi: 10.1016/j.clim.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saso Y, Conner EM, Teegarden BR, Yuan CS. S-Adenosyl-L-homocysteine hydrolase inhibitor mediates immunosuppressive effects in vivo: suppression of delayed type hypersensitivity ear swelling and peptidoglycan polysaccharide-induced arthritis. J Pharmacol Exp Ther. 2001;296:106–112. [PubMed] [Google Scholar]
- 31.Lawson BR, Manenkova Y, Ahamed J, et al. Inhibition of transmethylation down-regulates CD4 T cell activation and curtails development of autoimmunity in a model system. J Immunol. 2007;178:5366–5374. doi: 10.4049/jimmunol.178.8.5366. [DOI] [PubMed] [Google Scholar]
- 32.Wolford RW, Riscoe MK, Johnson L, Ferro AJ, Fitchen JH. Effect of 5′-methylthioadenosine (a naturally occurring nucleoside) on murine hematopoiesis. Exp Hematol. 1984;12:867–871. [PubMed] [Google Scholar]
- 33.Moreno B, Hevia H, Santamaria M, et al. Methylthioadenosine reverses brain autoimmune disease. Ann Neurol. 2006;60:323–334. doi: 10.1002/ana.20895. [DOI] [PubMed] [Google Scholar]
- 34.Moreno B, Fernandez-Diez B, Di Penta A, Villoslada P. Preclinical studies of methylthioadenosine for the treatment of multiple sclerosis. Mult Scler. 2010;16:1102–1108. doi: 10.1177/1352458510375968. [DOI] [PubMed] [Google Scholar]
- 35.Law RE, Stimmel JB, Damore MA, et al. Lipopolysaccharide-induced NF-kappa B activation in mouse 70Z/3 pre-B lymphocytes is inhibited by mevinolin and 5′-methylthioadenosine: roles of protein isoprenylation and carboxyl methylation reactions. Mol Cell Biol. 1992;12:103–111. doi: 10.1128/mcb.12.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Veal N, Hsieh CL, Xiong S, et al. Inhibition of lipopolysaccharide-stimulated TNF-alpha promoter activity by S-adenosylmethionine and 5′-methylthioadenosine. Am J Physiol Gastrointest Liver Physiol. 2004;287:G352–G362. doi: 10.1152/ajpgi.00316.2003. [DOI] [PubMed] [Google Scholar]
- 37.Ara AI, Xia M, Ramani K, Mato JM, Lu SC. S-adenosylmethionine inhibits lipopolysaccharide-induced gene expression via modulation of histone methylation. Hepatology. 2008;47:1655–1666. doi: 10.1002/hep.22231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berasain C, Hevia H, Fernandez-Irigoyen J, et al. Methylthioadenosine phosphorylase gene expression is impaired in human liver cirrhosis and hepatocarcinoma. Biochim Biophys Acta. 2004;1690:276–284. doi: 10.1016/j.bbadis.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 39.t Hart BA, Hintzen RQ, Laman JD. Multiple sclerosis—a response-to-damage model. Trends Mol Med. 2009;15:235–244. doi: 10.1016/j.molmed.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 40.Stekman IL, Blasini AM, Leon-Ponte M, et al. Enhanced CD3-mediated T lymphocyte proliferation in patients with systemic lupus erythematosus. Arthritis Rheum. 1991;34:459–467. doi: 10.1002/art.1780340411. [DOI] [PubMed] [Google Scholar]
- 41.Vratsanos GS, Jung S, Park YM, Craft J. CD4(+) T cells from lupus-prone mice are hyperresponsive to T cell receptor engagement with low and high affinity peptide antigens: a model to explain spontaneous T cell activation in lupus. J Exp Med. 2001;193:329–337. doi: 10.1084/jem.193.3.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kong PL, Odegard JM, Bouzahza F, et al. Intrinsic T cell defects in systemic autoimmunity. Ann NY Acad Sci. 2003;987:60–67. doi: 10.1111/j.1749-6632.2003.tb06033.x. [DOI] [PubMed] [Google Scholar]
- 43.Gessl A, Waldhausl W. Increased CD69 and human leukocyte antigen-DR expression on T lymphocytes in insulin-dependent diabetes mellitus of long standing. J Clin Endocrinol Metab. 1998;83:2204–2209. doi: 10.1210/jcem.83.6.4889. [DOI] [PubMed] [Google Scholar]
- 44.Portales-Perez D, Gonzalez-Amaro R, Abud-Mendoza C, Sanchez-Armass S. Abnormalities in CD69 expression, cytosolic pH and Ca2 + during activation of lymphocytes from patients with systemic lupus erythematosus. Lupus. 1997;6:48–56. doi: 10.1177/096120339700600107. [DOI] [PubMed] [Google Scholar]
- 45.Hernandez-Garcia C, Fernandez-Gutierrez B, Morado IC, Banares AA, Jover JA. The CD69 activation pathway in rheumatoid arthritis synovial fluid T cells. Arthritis Rheum. 1996;39:1277–1286. doi: 10.1002/art.1780390803. [DOI] [PubMed] [Google Scholar]
- 46.Crispin JC, V, Kyttaris C, Juang YT, Tsokos GC. How signaling and gene transcription aberrations dictate the systemic lupus erythematosus T cell phenotype. Trends Immunol. 2008;29:110–115. doi: 10.1016/j.it.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 47.Akahoshi M, Nakashima H, Tanaka Y, et al. Th1/Th2 balance of peripheral T helper cells in systemic lupus erythematosus. Arthritis Rheum. 1999;42:1644–1648. doi: 10.1002/1529-0131(199908)42:8<1644::AID-ANR12>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 48.Tucci M, Stucci S, Strippoli S, Silvestris F. Cytokine overproduction, T-cell activation, and defective T-regulatory functions promote nephritis in systemic lupus erythematosus. J Biomed Biotechnol. 2010;2010:457146. doi: 10.1155/2010/457146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liossis SN, Ding XZ, Dennis GJ, Tsokos GC. Altered pattern of TCR/CD3-mediated protein-tyrosyl phosphorylation in T cells from patients with systemic lupus erythematosus. Deficient expression of the T cell receptor zeta chain. J Clin Invest. 1998;101:1448–1457. doi: 10.1172/JCI1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yi Y, McNerney M, Datta SK. Regulatory defects in Cbl and mitogen-activated protein kinase (extra-cellular signal-related kinase) pathways cause persistent hyperexpression of CD40 ligand in human lupus T cells. J Immunol. 2000;165:6627–6634. doi: 10.4049/jimmunol.165.11.6627. [DOI] [PubMed] [Google Scholar]
- 51.Tomasi ML, Tomasi I, Ramani K, et al. S-adenosyl methionine regulates ubiquitin-conjugating enzyme 9 protein expression and sumoylation in murine liver and human cancers. Hepatology. 2012;56:982–993. doi: 10.1002/hep.25701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yamaji S, Droggiti A, Lu SC, et al. Adenosylmethionine regulates connexins sub-types expressed by hepatocytes. Euro J Cell Biol. 2011;90:312–322. doi: 10.1016/j.ejcb.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]