Abstract
Purpose
To evaluate the associations of high sensitivity Troponin T (Hs-TnT), N-terminal pro-brain natriuretic peptide (NT-proBNP), and high sensitivity C-reactive protein (Hs-CRP) with mortality from any cause, cardiovascular disease (CVD), coronary heart disease (CHD), stroke, cancer, and respiratory disease in the Atherosclerosis Risk in Communities (ARIC) cohort.
Methods
11193 participants aged 54-74 years, initially free of the conditions being studied, had biomarkers measured and were followed for a mean of 9.9 years.
Results
Hazard ratios (HR), adjusted for multiple risk factors, for mortality in participants in the highest Hs-TnT category compared to those with undetectable levels were: total 3.42 (95% Confidence Interval: 2.75-4.26), CVD 7.34 (4.64-11.6), CHD 6.06 (2.91-12.6), stroke 3.31 (1.26-8.66), cancer 1.60 (1.08-2.38) and respiratory 3.85 (1.39-10.7). Comparing the highest NT-proBNP quintile to those in the lowest quintile, the adjusted HRs for mortality were: total 3.05 (2.46-3.77), CVD 7.48 (4.67-12.0), CHD 4.07 (2.07-7.98) and stroke 10.4 (2.26-47.7). Comparing extreme Hs-CRP quintiles, the adjusted HRs for mortality were: total 1.61 (1.32-1.97), CVD 1.76 (1.19-2.62) and respiratory 3.36 (1.34-8.45). Having multiple markers elevated simultaneously greatly increased cause-specific mortality risks.
Conclusions
Greater levels of Hs-TnT, NT-proBNP and Hs-CRP are associated with increased risk of death, not just from cardiovascular disease but also from some non-cardiovascular causes.
Keywords: biomarkers, troponin T, B natriuretic peptide, C- reactive protein, mortality
INTRODUCTION
Identification of novel biomarkers has helped increase understanding of the pathophysiology of diseases and improved the accuracy of diagnosis and disease prognosis. High sensitivity Troponin T (Hs-TnT), N-terminal pro-brain natriuretic peptide (NT-proBNP) and high sensitivity C-reactive protein (Hs-CRP) are distinct biomarkers that reflect cardiovascular disease (CVD) or inflammation. Troponin T elevation in plasma is usually considered to be due to cardiomyocyte necrosis. Recently, a Hs-TnT assay was developed to detect a 10- fold lower concentration than the conventional Troponin T assay. Hs-TnT has been shown to be positively associated with incident coronary heart disease (CHD), (1) heart failure, (1-2) cardiovascular mortality,(1-2) and all-cause mortality(1,3). NT-proBNP is synthesized in response to cardiac wall stress in conditions associated with volume overload. Plasma NT-proBNP is a strong predictor of heart failure, (4) and CVD mortality, (5) and long term mortality (6-7) in patients with acute coronary syndromes. Multimarker models have shown that NT-proBNP improves risk stratification of cardiovascular event in individuals with prevalent CVD.(8) Also a similar study with NT-proBNP, Troponin I, cystatin C and Hs-CRP combined improved risk stratification for CVD death in individuals with or without CVD.(9) Hs-CRP is a sensitive but non-specific marker of inflammation positively associated with cardiovascular disease incidence (10,11) and mortality (12-15) and all-cause mortality.(12-14) Though CRP is being used for risk stratification in the general population, there is still a lot of debate and limited information supporting its predictive role. (9,10,16) There are non-cardiac conditions where NT-proBNP and TnT elevation have been noted: for example, in patients with chronic hypercapnic respiratory failure in the absence of heart failure (17) and in intracranial hemorrhage or stroke,(18) respectively. Although there is some information on Hs-CRP and cause-specific mortality, there is little information for Hs-TnT and NT-proBNP and cause-specific mortality in the general population.
We therefore aimed to determine the independent association of Hs-TnT, NT-proBNP, and Hs-CRP with mortality from any cause, all CVD, CHD, stroke, cancer, and respiratory disease in participants in the Atherosclerosis Risk in Communities (ARIC) study. In addition, we evaluated the joint associations of elevated Hs-TnT, NT-proBNP, and Hs-CRP with cause-specific mortality.
METHODS
Study design and population
The ARIC study includes a prospective cohort of 15,792 participants, aged 45-64 years when recruited between 1987 and 1989, via probability sampling from four US communities (Jackson, MS; suburban Minneapolis, MN; Forsyth County, NC; and Washington County, MD). Participants were required to be residents of the communities with no definite plans to leave the area, mentally and physically able to participate, and free of language barriers to participation. ARIC examined the participants four times: 1987-89, 1990-92 (return rate=93%), 1993-95 (return rate=86%) and 1996-98 (return rate=80%, n=11668), with a fifth visit ongoing. Participants were also contacted yearly by telephone to inquire about their health status. This present analyses used visit 4 as their start point.
Measurement of biomarkers (independent variables)
Assays were recently performed on visit 4 (1996-98) plasma samples stored at -70°C. Troponin T levels were determined on a Cobas e411 analyzer using the Elecys Troponin T, a novel high sensitivity assay (Roche Diagnostics, Indianapolis, IN). The lower limit of detection is 0.003 ug/L. The reliability coefficient for blinded quality control replicate measurements (n=418 pairs) of Hs-TnT from single blood draws was 0.98. Plasma NT-proBNP was measured on a Cobas e411 analyzer using the Elecys proBNP II immunoassay (Roche Diagnostics, Indianapolis, IN). The range of detection was 5- 35,000 pg/ml. The reliability coefficient for blind replicate measurements was 0.99 (N=418 pairs). CRP levels were assessed by the immunoturbidimetric CRP-Latex (II) high sensitivity assay from Denka Seiken (Tokyo, Japan) using a Hitachi 911 analyzer (Roche Diagnostics, Indianapolis). The reliability coefficient for blind replicate measurements was 0.99 (N=55 pairs).
Measurement of other exposures
At visit 4, estimated glomerular filtration rate (eGFR) was calculated with the Chronic Kidney Epidemiology Collaboration Formula.(19) Plasma cholesterol (total and high density lipoprotein) and triglycerides were measured with standardized Monotest Cholesterol and Glycerolphosphate-oxidase Triglyceride procedures, respectively, using reagents from Boehringer Mannheim on a Roche Cobas Fara II analyzer.
Body mass index at visit 4 was calculated as weight in kilograms divided by the square of height in meters. Hypertension was defined as a systolic blood pressure of 140 mmHg or higher, a diastolic blood pressure of 90 mmHg or higher, or use of antihypertensive medication in the past 2 weeks. Diabetes was defined as a fasting blood glucose of 126 mg/dl or higher, non-fasting blood glucose of 200 mg/dl or higher, use of antidiabetic medication in the past 2 weeks, or a physician diagnosis of diabetes. Standardized, questionnaires were used to ascertain medical history, cigarette smoking, and alcohol consumption at visit 4.
A few possible confounding variables were unavailable at visit 4 and were taken at earlier visits. Sports participation (20) and dietary intake were obtained at ARIC visit 3. Principal components-derived ‘Prudent’ and ‘Western’ dietary pattern scores were estimated using a 66-item food frequency questionnaire.(21) ‘Western’ diet was characterized by greater consumption of refined grains, processed meat, fried food, eggs, red meat, and soda, while the ‘Prudent’ diet was characterized by greater intake of vegetables, fruits, fish, seafood, poultry, whole grains, and low-fat dairy products. Forced expiratory volume in 1 second was determined at ARIC visit 2 with a Collins Survey II water-seal spirometer and was analyzed as a percentage of predicted FEV1.
Measurement of prevalent disease and mortality outcomes
Depending on the mortality outcome of interest, we excluded certain prevalent diseases at visit 4 from the at-risk cohort, and the definitions of the prevalent diseases were as follows. Prevalent CHD at visit 4 was defined as (a) a self-reported myocardial infarction (MI), ECG evidence of MI, or coronary revascularization reported at visit 1 or (b) incident MI (22), silent MI, or revascularization procedure before visit 4. Prevalent stroke at visit 4 was defined as (a) reporting a physician diagnosed stroke at visit 1 or (b) a definite or probable incident stroke (23) before visit 4. Prevalent heart failure (HF) at visit 4 was defined as (a) taking medications for HF or having HF by Gothenburg criteria (24) at visit 1 or (b) incident hospitalized heart failure (hospital discharge codes ICD-9 CM 428) before visit 4. Individuals who reported at visit 4 that a physician ever told them that they had cancer or lung disease represented those with prevalent cancer or respiratory disease, respectively.
Deaths from visit 4 (1996-98) through 2008 were identified through contacts with next of kin, searching hospital records and state death records, and linkage to the National Death Index. Cause- specific mortality was defined using the underlying cause of death on the death certificate (ICD-9 code or ICD-10 code). “All CVD deaths” were defined as death with ICD-9 code 401-459 or ICD-10 code I10-I99; “CHD deaths” were those with ICD-9 code 410-414 or ICD-10 code I20-I25; “stroke deaths”: ICD-9 code 430-438 or ICD-10 code I60-I69; “cancer deaths”: ICD-9 code 140-239 or ICD-10 code C00-D48 and “respiratory disease deaths”: ICD-9 code 460-519 or ICD-10 code J00- J98.
Statistical analysis
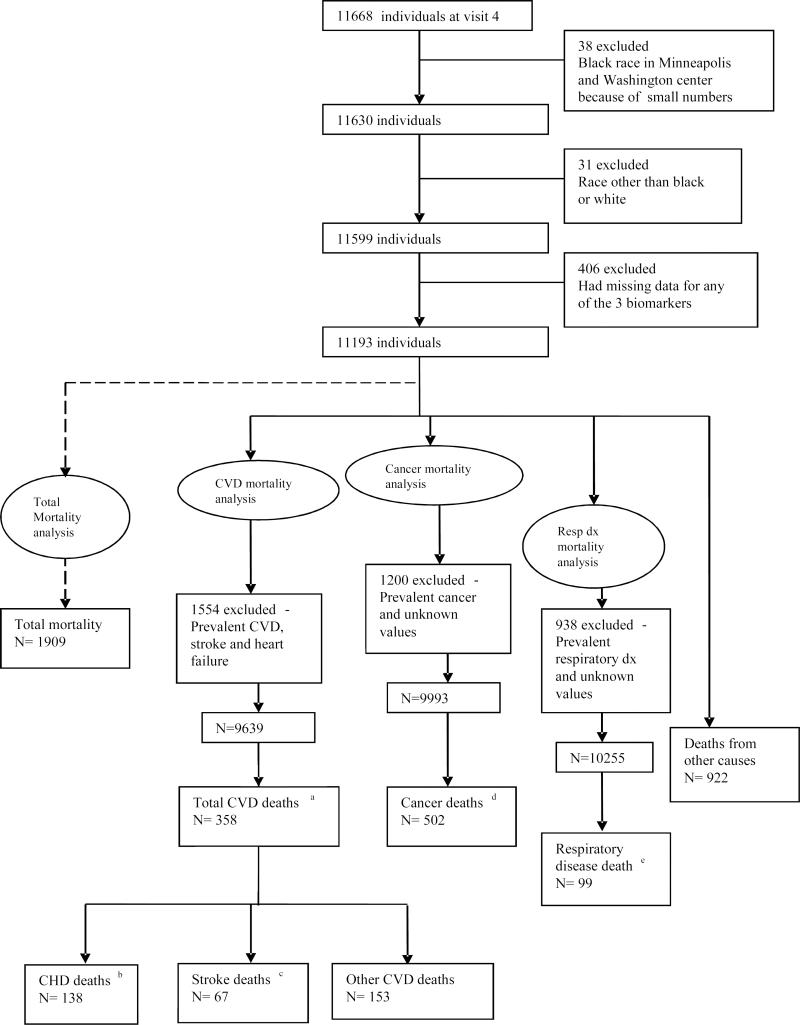
From the 11,668 who attended ARIC visit 4, we made exclusions as shown in Figure 1. For analyses of all CVD, CHD and stroke deaths, we excluded 1554 participants with prevalent CHD, stroke or heart failure at visit 4. We excluded 1200 participants with a history of cancer from cancer mortality analyses and 938 participants who had a known history of respiratory diseases from respiratory disease mortality analyses.
Figure 1.
Participant Flow Chart
a Total Cardiovascular disease deaths: ICD 9 code 401- 459 or ICD-10 code I10- I99
b Coronary Heart disease deaths: ICD 9 code 410- 414 or ICD-10 code I20- I25
c Stroke deaths: ICD 9 code 430- 438 or ICD-10 code I60- I69
d Cancer deaths: ICD 9 code 140- 239 or ICD-10 code C00- D48
The association between the three biomarkers and mortality outcomes were determined with Cox proportional hazard models. We modeled Hs-TnT, Hs-CRP and NT-proBNP at visit 4 as both categorical and continuous variables. For Hs-TnT categorical analysis, the 31.7% with undetectable levels were the reference group. The remaining 68.3% were split into four groups : 0.003-0.005ug/L, 0.006-0.008ug/L, 0.009-0.013ug/L and ≥ 0.014ug/L, which corresponds to the 99th percentile value for Hs-TnT in a healthy subpopulation.(25) Hs-CRP and NT-proBNP were categorized as quintiles. The p for linear trend were determined by fitting a slope to biomarker categories. The distributions for Hs-TnT, NT-proBNP and Hs-CRP were right skewed and therefore were natural logarithm transformed for continuous analyses. Hazard ratios (HR) for continuous biomarkers were reported per standard deviation (SD) increment of the log-transformed biomarker.
Proportional hazard model 1 adjusted for age, sex and race. Model 2 adjusted for all components of model 1; body mass index, total cholesterol, high-density lipoprotein cholesterol, systolic blood pressure, diet, sport index, forced expiratory volume in 1 second, and estimated glomerular filtration rate as continuous variables; smoking status (current, former and never), drinking status (3 categories), hormone use (estrogen, estrogen and progestin, former and never), , antihypertensive medication (yes/no) and diabetes (yes/no) as categorical variables. Model 3 adjusted for all components of model 2 plus the other two biomarkers.
We tested two-way multiplicative interactions of each biomarker with race or sex and found none statistically significant at p<0.10, after Bonferroni correction for multiple interaction testing. Therefore, we pooled men and women, whites and African Americans for all final analyses. The proportional hazards assumption was tested by including time by biomarker interaction terms in the unadjusted models.
The Cox proportional hazard model was also used to assess the joint association with mortality of having zero, one, two, or three of the biomarkers elevated. The 80th percentile of each biomarker was used to dichotomize high and low.
RESULTS
As shown in Supplemental Tables 1-3, Hs-TnT, NT-proBNP, and Hs-CRP were associated with most risk factors at p < 0.05, given ARIC's large sample size. During a mean of 9.9 years follow up, there were 1909 total deaths, 358 all CVD deaths, 138 CHD deaths, 67 stroke deaths, 502 cancer deaths and 99 respiratory disease deaths (Figure 1).
As shown in Table 1, Hs-TnT was associated positively with risk of every cause-specific mortality outcome, after adjustment for CV risk factors (Model 2). Hazard ratios for the highest group versus the lowest in model 2 were: total mortality (HR: 3.42, 95% CI: 2.75-4.26), all CVD mortality (HR: 7.34, 95% CI: 4.64- 11.6), CHD mortality (HR: 6.06, 95% CI: 2.91-12.6), stroke mortality (HR: 3.31, 95% CI: 1.26-8.66), cancer mortality (HR: 1.60, 95% CI: 1.08- 2.38), and respiratory disease mortality (HR: 3.85, 95% CI: 1.39-10.7). The p value for trend for cancer mortality was not significant. Further adjustment for the other biomarkers (Model 3) somewhat attenuated the Hs-TnT associations, though they remained significant except for stroke mortality.
Table 1.
Hazard Ratios for associations between Troponin T and mortality, ARIC, 1996-2008
| Hazard ratio (95% Confidence Interval) |
||||||
|---|---|---|---|---|---|---|
| <3ng/L | 3- ≤5ng/L | 6-≤ 8ng/L | 9- ≤13ng/L | ≥14ng/L | P for trend | |
| N | 3544 | 2779 | 2269 | 1535 | 1066 | |
| Total mortality (n=1909) Events | 306 | 349 | 359 | 394 | 501 | |
| Model 1 | 1 | 1.26 (1.07- 1.47) | 1.37 (1.16- 1.61) | 2.02 (1.71- 2.38) | 4.34 (3.68- 5.12) | <0.0001 |
| Model 2 | 1 | 1.38 (1.13- 1.67) | 1.53 (1.25- 1.86) | 1.91 (1.55- 2.35) | 3.42 (2.75- 4.26) | <0.0001 |
| Model 3 | 1 | 1.32 (1.09- 1.61) | 1.42 (1.16- 1.74) | 1.73 (1.40- 2.13) | 2.82 (2.26- 3.53) | <0.0001 |
| All CVD mortality (n=358 ) Events | 51 | 63 | 64 | 77 | 103 | |
| Model 1 | 1 | 1.42 (0.98- 2.07) | 1.67 (1.14- 2.44) | 2.88 (1.97- 4.20) | 7.71 (5.31- 11.2) | <0.0001 |
| Model 2 | 1 | 1.50 (0.96- 2.36) | 1.89 (1.20- 2.97) | 3.01 (1.91- 4.75) | 7.34 (4.64- 11.6) | <0.0001 |
| Model 3 | 1 | 1.41 (0.90- 2.22) | 1.70 (1.08- 2.66) | 2.54 (1.60- 4.01) | 5.09 (3.18- 8.15) | <0.0001 |
| CHD mortality (n= 138) Events | 19 | 24 | 30 | 28 | 37 | |
| Model 1 | 1 | 1.37 (0.74- 2.51) | 1.89 (1.04- 3.43) | 2.42 (1.29- 4.51) | 6.22 (3.37- 11.5) | <0.0001 |
| Model 2 | 1 | 1.28 (0.61- 2.68) | 2.17 (1.09- 4.35) | 2.42 (1.16- 5.06) | 6.06 (2.91- 12.6) | <0.0001 |
| Model 3 | 1 | 1.25 (0.60- 2.63) | 2.03 (1.01- 4.07) | 2.22 (1.06- 4.64) | 4.65 (2.19- 9.89) | <0.0001 |
| Stroke mortality (n= 67) Events | 17 | 15 | 5 | 14 | 16 | |
| Model 1 | 1 | 0.98 (0.48- 1.97) | 0.38 (0.14- 1.06) | 1.53 (0.71- 3.32) | 3.87 (1.78- 8.41) | 0.003 |
| Model 2 | 1 | 1.00 (0.43- 2.29) | 0.31 (0.09- 1.14) | 1.89 (0.78- 4.58) | 3.31 (1.26- 8.66) | 0.02 |
| Model 3 | 1 | 0.91 (0.39- 2.09) | 0.28 (0.08- 1.02) | 1.57 (0.64- 3.85) | 2.34 (0.86- 6.33) | 0.09 |
| Cancer Mortality (n= 502) Events | 127 | 119 | 105 | 81 | 70 | |
| Model 1 | 1 | 0.99 (0.76- 1.28) | 0.87 (0.66- 1.14) | 0.90 (0.66-1.23) | 1.28 (0.91- 1.80) | 0.60 |
| Model 2 | 1 | 1.15 (0.85- 1.54) | 1.04 (0.75- 1.43) | 1.02 (0.71- 1.46) | 1.60 (1.08- 2.38) | 0.17 |
| Model 3 | 1 | 1.14 (0.84- 1.53) | 1.02 (0.74- 1.41) | 0.99 (0.69- 1.43) | 1.52 (1.01- 2.28) | 0.27 |
| Respiratory disease mortality (n= 99) Events | 15 | 14 | 24 | 23 | 23 | |
| Model 1 | 1 | 0.91 (0.43- 1.95) | 1.69 (0.85- 3.38) | 2.11 (1.02- 4.35) | 3.84 (1.82- 8.10) | <0.0001 |
| Model 2 | 1 | 1.15 (0.42- 3.12) | 2.34 (0.94- 5.78) | 2.48 (0.96- 6.45) | 3.85 (1.39- 10.7) | 0.004 |
| Model 3 | 1 | 1.12 (0.41- 3.03) | 2.26 (0.91- 5.62) | 2.48 (0.95- 6.51) | 3.80 (1.35- 10.7) | 0.004 |
CHD- indicates coronary heart disease, CVD- indicates cardiovascular disease, P for linear trend across categories
Model 1- adjusted for age, sex and race
Model 2- adjusted for model 1 + body mass index, total cholesterol, high density lipoprotein cholesterol, diet, sport index, smoking status, drinking status, hormone use, systolic blood pressure, antihypertensive medication, diabetes, forced expiratory volume in 1 second/forced vital capacity predicted %, estimated glomerular filtration rate. In addition, total mortality was adjusted for history of cancer, CVD, stroke, heart failure and respiratory disease
Model 3- adjusted for model 2 + high sensitivity C- reactive protein (Hs-CRP) and N terminal pro B natriuretic peptide (NT-proBNP)
NT-proBNP groups were associated positively with total, all CVD, CHD, and stroke mortality (Table 2). Model 2 HRs for quintiles 5 versus 1 were: total (HR: 3.05, 95% CI: 2.46-3.77), all CVD (HR: 7.48, 95% CI: 4.67- 12.0), CHD (HR: 4.07, 95% CI: 2.07-7.98) and stroke mortality (HR: 10.4, 95%CI: 2.26-47.7). Associations were attenuated but remained significant after adjustment for the other two biomarkers (Model 3). In contrast, there was no significant association of NT-proBNP group with cancer or respiratory disease mortality after Model 2 adjustment.
Table 2.
Hazard Ratios for associations between N Terminal pro-B natriuretic peptide and mortality, ARIC, 1996-2008
| Hazard ratio (95% Confidence Interval) |
||||||
|---|---|---|---|---|---|---|
| ≤ 27.4pg/ml | 27.5-52.3pg/ml | 52.4-88.6pg/ml | 88.7-158.0pg/ml | ≥159pg/ml | P for trend | |
| N | 2240 | 2244 | 2239 | 2233 | 2237 | |
| Total mortality (n=1909) Events | 208 | 256 | 342 | 350 | 753 | |
| Model 1 | 1 | 1.28 (1.06- 1.55) | 1.80 (1.51- 2.16) | 1.92 (1.60- 2.30) | 3.87 (3.27- 4.58) | <0.0001 |
| Model 2 | 1 | 1.33 (1.07- 1.65) | 1.78 (1.43- 2.20) | 1.92 (1.55- 2.38) | 3.05 (2.46- 3.77) | <0.0001 |
| Model 3 | 1 | 1.26 (1.01- 1.57) | 1.63 (1.32- 2.02) | 1.74 (1.40- 2.16) | 2.46 (1.98- 3.05) | <0.0001 |
| All CVD mortality (n= 358) Events | 33 | 51 | 67 | 62 | 145 | |
| Model 1 | 1 | 1.77 (1.14- 2.75) | 2.57 (1.68- 3.93) | 2.70 (1.75- 4.18) | 7.33 (4.92- 10.9) | <0.0001 |
| Model 2 | 1 | 1.99 (1.20- 3.29) | 3.03 (1.87- 4.92) | 2.77 (1.67- 4.60) | 7.48 (4.67- 12.0) | <0.0001 |
| Model 3 | 1 | 1.81 (1.09- 2.99) | 2.55 (1.57- 4.14) | 2.27 (1.37- 3.78) | 5.10 (3.16- 8.22) | <0.0001 |
| CHD mortality (n= 138) Events | 18 | 25 | 28 | 15 | 52 | |
| Model 1 | 1 | 1.52 (0.82- 2.80) | 1.86 (1.01- 3.42) | 1.12 (0.55- 2.27) | 4.44 (2.50- 7.88) | <0.0001 |
| Model 2 | 1 | 1.55 (0.78- 3.11) | 2.01 (1.02- 3.98) | 1.11 (0.49- 2.47) | 4.07 (2.07- 7.98) | 0.0002 |
| Model 3 | 1 | 1.43 (0.71- 2.86) | 1.73 (0.87- 3.43) | 0.93 (0.41- 2.07) | 2.81 (1.41- 5.60) | 0.01 |
| Stroke mortality (n= 67) Events | 3 | 9 | 13 | 13 | 29 | |
| Model 1 | 1 | 2.95 (0.79- 11.0) | 4.38 (1.23- 15.6) | 4.64 (1.29- 16.7) | 12.0 (3.52- 41.1) | <0.0001 |
| Model 2 | 1 | 3.87 (0.81- 18.4) | 5.91 (1.29- 27.1) | 4.50 (0.93- 21.8) | 10.4 (2.26- 47.7) | 0.001 |
| Model 3 | 1 | 3.61 (0.76- 17.2) | 5.34 (1.17- 24.5) | 4.02 (0.83- 19.5) | 7.76 (1.67- 36.2) | 0.01 |
| Cancer Mortality (n= 502) Events | 86 | 98 | 103 | 99 | 116 | |
| Model 1 | 1 | 1.13 (0.84- 1.52) | 1.24 (0.92- 1.68) | 1.20 (0.88- 1.63) | 1.41 (1.04- 1.93) | 0.04 |
| Model 2 | 1 | 1.13 (0.82- 1.56) | 1.21 (0.87- 1.70) | 1.25 (0.89- 1.76) | 1.39 (0.97- 2.00) | 0.07 |
| Model 3 | 1 | 1.12 (0.81- 1.55) | 1.19 (0.85- 1.66) | 1.22 (0.86- 1.72) | 1.30 (0.90- 1.88) | 0.16 |
| Respiratory disease mortality (n= 99) Events | 8 | 13 | 24 | 23 | 31 | |
| Model 1 | 1 | 1.52 (0.62- 3.68) | 2.98 (1.32- 6.77) | 2.78 (1.20- 6.44) | 3.36 (1.46- 7.72) | 0.001 |
| Model 2 | 1 | 1.48 (0.49- 4.48) | 2.45 (0.86- 7.04) | 2.99 (1.05- 8.49) | 2.02 (0.67- 6.09) | 0.12 |
| Model 3 | 1 | 1.33 (0.44- 4.06) | 2.15 (0.75- 6.17) | 2.59 (0.91- 7.38) | 1.45 (0.48- 4.43) | 0.37 |
CHD- indicates coronary heart disease, CVD- indicates cardiovascular disease, P for linear trend across quintiles
Model 1- adjusted for age, sex and race
Model 2- adjusted for model 1 + body mass index, total cholesterol, high density lipoprotein cholesterol, diet, sport index, smoking status, drinking status, hormone use, systolic blood pressure, antihypertensive medication, diabetes, forced expiratory volume in 1 second/forced vital capacity predicted %, estimated glomerular filtration rate. In addition, total mortality was adjusted for history of cancer, CVD, stroke, heart failure and respiratory disease
Model 3- adjusted for model 2 + high sensitivity C- reactive protein (Hs-CRP) and troponin T
As shown in Table 3, Hs-CRP levels in Model 2 were positively significantly associated with total mortality, all CVD mortality and respiratory disease mortality but not with CHD, stroke or cancer mortality when comparing quintile 5 versus 1. After adjustment for NT-proBNP and Hs-TnT (Model 3), greater Hs-CRP level was no longer associated with all-CVD mortality.
Table 3.
Hazard Ratios for associations between C reactive protein and mortality, ARIC, 1996-2008
| Hazard ratio (95% Confidence Interval) |
||||||
|---|---|---|---|---|---|---|
| ≤0.9mg/L | 0.917-1.761 mg/L | 1.762-3.389mg/L | 3.390-6.578mg/L | ≥6.579mg/L | P for trend | |
| N | 2238 | 2239 | 2239 | 2239 | 2238 | |
| Total mortality (n=1909) Events | 302 | 328 | 331 | 430 | 518 | |
| Model 1 | 1 | 1.07 (0.91- 1.26) | 1.13 (0.96- 1.32) | 1.63 (1.40- 1.90) | 2.14 (1.84- 2.49) | <0.0001 |
| Model 2 | 1 | 1.05 (0.87- 1.27) | 1.14 (0.94- 1.38) | 1.48 (1.22- 1.79) | 1.61 (1.32- 1.97) | <0.0001 |
| Model 3 | 1 | 1.07 (0.88- 1.29) | 1.12 (0.93- 1.36) | 1.40 (1.16- 1.70) | 1.46 (1.20- 1.78) | <0.0001 |
| All CVD mortality (n= 358) Events | 62 | 68 | 50 | 77 | 101 | |
| Model 1 | 1 | 1.12 (0.79- 1.58) | 0.85 (0.59- 1.24) | 1.44 (1.03- 2.02) | 2.09 (1.51- 2.90) | <0.0001 |
| Model 2 | 1 | 1.07 (0.73- 1.59) | 0.85 (0.56- 1.30) | 1.26 (0.85- 1.88) | 1.76 (1.19- 2.62) | 0.004 |
| Model 3 | 1 | 1.11 (0.75- 1.65) | 0.85 (0.56- 1.31) | 1.15 (0.77- 1.72) | 1.49 (1.00- 2.21) | 0.06 |
| CHD mortality (n= 138) Events | 21 | 25 | 20 | 36 | 36 | |
| Model 1 | 1 | 1.22 (0.68- 2.18) | 1.03 (0.56- 1.90) | 2.10 (1.22- 3.62) | 2.43 (1.40- 4.22) | 0.0002 |
| Model 2 | 1 | 1.08 (0.57- 2.03) | 0.98 (0.50- 1.91) | 1.66 (0.89- 3.09) | 1.88 (0.98- 3.59) | 0.02 |
| Model 3 | 1 | 1.12 (0.59- 2.12) | 0.98 (0.50- 1.91) | 1.52 (0.81- 2.84) | 1.66 (0.87- 3.18) | 0.08 |
| Stroke mortality (n= 67) Events | 16 | 14 | 9 | 8 | 20 | |
| Model 1 | 1 | 0.88 (0.43- 1.80) | 0.58 (0.26- 1.32) | 0.56 (0.24- 1.31) | 1.58 (0.80- 3.12) | 0.45 |
| Model 2 | 1 | 1.17 (0.50- 2.74) | 0.90 (0.35- 2.31) | 0.90 (0.33- 2.44) | 2.24 (0.90- 5.53) | 0.21 |
| Model 3 | 1 | 1.15 (0.49- 2.73) | 0.93 (0.36- 2.38) | 0.85 (0.31- 2.34) | 1.91 (0.77- 4.71) | 0.33 |
| Cancer Mortality (n= 502) Events | 82 | 99 | 103 | 110 | 108 | |
| Model 1 | 1 | 1.23 (0.92- 1.66) | 1.35 (1.01- 1.82) | 1.61 (1.20- 2.16) | 1.72 (1.27- 2.33) | <0.0001 |
| Model 2 | 1 | 1.12 (0.81- 1.56) | 1.31 (0.94- 1.81) | 1.35 (0.96- 1.91) | 1.38 (0.95- 2.00) | 0.05 |
| Model 3 | 1 | 1.14 (0.82- 1.59) | 1.31 (0.94- 1.82) | 1.34 (0.95- 1.89) | 1.35 (0.93- 1.96) | 0.07 |
| Respiratory disease mortality (n= 99) Events | 14 | 15 | 15 | 20 | 35 | |
| Model 1 | 1 | 1.01 (0.47- 2.19) | 1.20 (0.56- 2.56) | 2.02 (1.00- 4.07) | 4.01 (2.08- 7.73) | <0.0001 |
| Model 2 | 1 | 1.57 (0.63- 3.87) | 1.34 (0.52- 3.45) | 2.08 (0.83- 5.22) | 3.36 (1.34- 8.45) | 0.01 |
| Model 3 | 1 | 1.65 (0.66- 4.11) | 1.41 (0.54- 3.66) | 2.09 (0.83- 5.26) | 3.38 (1.34- 8.50) | 0.01 |
CHD- indicates coronary heart disease, CVD- indicates cardiovascular disease, P for linear trend across quintiles
Model 1- adjusted for age, sex and race
Model 2- adjusted for model 1 + body mass index, total cholesterol, high density lipoprotein cholesterol, diet, sport index, smoking status, drinking status, hormone use, systolic blood pressure, antihypertensive medication, diabetes, forced expiratory volume in 1 second/forced vital capacity predicted %, estimated glomerular filtration rate. In addition, total mortality was adjusted for history of cancer, CVD, stroke, heart failure and respiratory disease
Model 3- adjusted for model 2 + troponin T and N terminal pro B natriuretic peptide (NT-proBNP)
When we evaluated binary cutoffs in supplemental table 4-6 for each biomarker, the associations remained the same. However, the association of Hs-TnT and cancer mortality was now significant in Model 3.
The more markers elevated, the higher the cause specific mortality rate (Table 4). For example in Model 2, having all three of Hs-TnT, Hs-CRP and NT-proBNP elevated compared to having none carried a 5-fold increased risk of total mortality (HR: 4.31, 95% CI: 3.31-5.63), over a 10-fold risk of all CVD death (HR: 10.5, 95% CI: 6.38-17.3), a 6-fold risk of CHD mortality (HR: 6.18, 95% CI: 2.68- 14.3), a 12-fold risk of stroke mortality (HR: 12.0, 95% CI: 3.61-40.2) and a 5 fold risk of respiratory disease deaths (HR: 5.14, 95% CI: 1.61-16.4). Although the corresponding HRs was also elevated for cancer mortality (HR: 1.99, 95% CI: 1.00-3.98) this was not statistically significant. Notably, over 64% (i.e., 178/278) of those with all three biomarkers elevated died during the follow-up period.
Table 4.
Hazard Ratios for Joint associations of elevated Troponin T, NT-proB natriuretic peptide, or C reactive protein with mortality, ARIC 1996-2008
| Hazard ratio (95% Confidence Interval) according to number elevated a |
||||
|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |
| N | 5899 | 3789 | 1227 | 278 |
| Total mortality (n=1909) | 569 | 692 | 470 | 178 |
| Model 2 | 1 | 1.50 (1.31- 1.72) | 2.31 (1.94- 2.74) | 4.31 (3.31- 5.63) |
| All CVD mortality (n=358) | 93 | 135 | 99 | 31 |
| Model 2 | 1 | 2.06 (1.51- 2.81) | 5.12 (3.61- 7.25) | 10.5 (6.38- 17.3) |
| CHD mortality (n= 138) | 44 | 44 | 41 | 9 |
| Model 2 | 1 | 1.22 (0.75- 1.99) | 4.11 (2.45- 6.90) | 6.18 (2.68- 14.3) |
| Stroke mortality (n=67) | 19 | 25 | 15 | 8 |
| Model 2 | 1 | 2.09 (1.04- 4.22) | 4.87 (2.18- 10.9) | 12.0 (3.61- 40.2) |
| Cancer Mortality (n= 502) | 227 | 194 | 62 | 19 |
| Model 2 | 1 | 1.13 (0.90- 1.42) | 1.14 (0.80- 1.63) | 1.99 (1.00- 3.98) |
| Respiratory disease mortality (n= 99) | 28 | 41 | 19 | 11 |
| Model 2 | 1 | 1.27 (0.69- 2.34) | 1.70 (0.78- 3.74) | 5.14 (1.61- 16.4) |
CHD- indicates coronary heart disease, CVD- indicates cardiovascular disease, model 1- adjusted for age, sex and race
elevated was defined by being above versus below the 80th percentile for each biomarker
Adjusted for model 1+ body mass index, total cholesterol, high density lipoprotein cholesterol, diet, sport index, smoking status, drinking status, hormone use, systolic blood pressure, antihypertensive medication, diabetes, forced expiratory volume in 1 second/forced vital capacity predicted %, estimated glomerular filtration rate. In addition, total mortality was adjusted for history of cancer, CVD, stroke, heart failure and respiratory disease
There was some evidence that the proportional hazards assumption was violated for all three biomarkers for total mortality (p<0.001) and for NT-proBNP for CVD and CHD mortality (p=0.01). We therefore compared hazard ratios for the first and second halves of follow-up. For total mortality, associations with the three biomarkers tended to be stronger in later compared with earlier follow-up. The associations of NT-proBNP with CVD and CHD mortality tended to be stronger in earlier compared with later follow-up.
DISCUSSION
Our primary aim was to determine the association of Hs-TnT, NT-proBNP and Hs-CRP with cause-specific mortality in a population-based prospective study. The analyses after adjusting for cardiovascular risk factors suggest that all CVD and stroke mortality were associated most strongly with elevated NT-proBNP levels compared with Hs-TNT and Hs-CRP levels. The risk of CHD mortality was most strongly associated with elevated Hs-TNT, then NT-proBNP, and least for Hs-CRP. All three biomarkers showed only weak associations with cancer mortality. Our most novel finding may be that greater levels of Hs-TnT was associated with increased respiratory disease mortality. Risk of total, all CVD, CHD and stroke mortality were particularly elevated when all three biomarkers were elevated simultaneously, with over 60% of such participants dying during the follow up period.
The high sensitivity assay for TnT can detect a 10-fold lower concentration than detectable with the TnT conventional assay. With the high sensitivity TnT assay, approximately 25-67% of adults from the general population have detectable troponin levels.(1-3) In a study by Daniels et al, TnT was detectable in 4% of apparently healthy participants using a conventional assay, and they were at increased risk of all-cause and cardiovascular death.(26) The low prevalence of detection with conventional assays limited the utility of troponin measurement for predicting future cardiovascular disease.(3) Although there are few direct comparisons made between the conventional and high sensitivity assays,(15,27) Hs-TnT seems to increase the predictive power of troponin measurement. The Cardiovascular Health Study and Dallas Heart Study have shown greater levels of Hs-TnT are associated with incident cardiovascular events and all-cause or cardiovascular mortality;(1-3,26). ARIC showed similar association with incident cardiovascular events and all-cause mortality.(1) We now extend these findings to mortality from CVD in ARIC, CHD, stroke, and respiratory disease. The strong association of low detectable troponin T with all CVD and CHD mortality is consistent with the presence of subclinical chronic myocardial injury causing increased risk of death.(28-30) Recent studies have suggested that the association of TnT levels with cardiac outcomes in previously asymptomatic individuals may be mediated through mechanisms independent of atherosclerosis.(1) Though these mechanisms remain unclear, cardiac myocytes may release troponin T due to asymptomatic ischemia, coronary microvascular dysfunction, apoptosis, or subclinical cardiac structural or functional abnormalities. In addition, the Prevention of Events with Angiotensin Converting Enzyme Inhibition (PEACE) trial revealed that Hs-TnT was associated with heart failure and death but not myocardial infarction in patients with chronic coronary artery disease.(31) However, the Heart Outcomes Prevention Evaluation study (HOPE) showed association of high sensitivity Troponin I with myocardial infarction in a high risk population. (32)
It is of interest that elevated Hs-TnT is associated with increased respiratory disease mortality especially if troponin is released as a result of myocardial damage. In our analyses, patients with chronic obstructive pulmonary disease (COPD) may have had CHD (33) or acute exacerbations of COPD may cause subclinical myocardial damage via right ventricular overload. Also COPD could lead to hypoxia and tachycardia, which could affect cardiac oxygen demand, and delivery, causing myocardial ischemia which may cause release of TnT.(34) A recent study has shown that elevated Hs-TnT during acute exacerbation of COPD is more frequent among patients with tachycardia than among those with normal heart rate, and this is associated with decreased survival.(34)
Brain natriuretic peptide (BNP) is synthesized as an inactive pro-hormone primarily from the cardiac ventricles as a result of myocardial wall stress, it splits into an active BNP and inactive but stable NT-proBNP. They both serve as biomarkers for diagnosis, prognosis, and management in patients with cardiovascular disease and heart failure.(7,35) In this study, greater levels of NT-proBNP were positively associated with total mortality, all CVD mortality, CHD and stroke mortality. The Framingham Heart Study (FHS), Prevention of Renal and Vascular End-stage Disease (PREVEND) study and two other European studies, have documented a positive association of plasma B type natriuretic peptide with cardiovascular events and all-cause mortality.(36-40) Linssen et al reported NT-proBNP is associated crudely with non-CVD mortality, but not after adjustment for risk factors, as seen in our analysis for cancer and respiratory disease mortality.(36) Elevated NT-proBNP levels in the general population may reflect the presence of structural heart disease or cardiac remodeling resulting from increased cardiac stretch from pressure or volume overload,(15,36,41) or they also may reflect coronary atherosclerosis.(42-45)
Our finding of NT-proBNP associated with stroke mortality might be explained by strokes from cardio-embolic causes, such as atrial fibrillation, and concomitant NT-proBNP elevation.(46) Immunochemical studies also have suggested that cerebral ischemia induces natriuretic peptide secretion in brain tissue;(47) elevated BNP levels are not uncommon in acute stroke and are associated with reduced survival.(48) Cerebral ischemia and neurologic deficits have been found to be associated with high concentration of plasma BNP, and occult cardiac dysfunction increases the risk of severe strokes in patients.(48) Although the association of NT-proBNP with stroke mortality was statistically significant, the confidence interval of the HR estimates was wide due relatively few stroke deaths. Therefore, larger investigations may be needed to address the relation of NT-proBNP and stroke incidence and mortality.
Since Hs-CRP serves as a sensitive but nonspecific marker of systemic inflammation,(10) it is not surprising that we found CRP associated with multiple mortality outcomes, because inflammation plays a major role in the pathophysiology of the disease related mortality. The Emerging Risk Factors Collaboration carried out a meta-analysis assessing the association of Hs-CRP with vascular and non-vascular mortality in people without a history of vascular disease from long-term prospective studies (ARIC included).(12) Every three-fold higher increment of CRP approximately doubled risk of all vascular, non-vascular, cancer and respiratory disease deaths, after adjustment for age and sex.(12) The significant association of Hs-CRP and CVD mortality was lost after adjusting for the other two biomarkers. This may also question the need of CRP as a predictor of future cardiovascular events.
Individuals with cancer often die from infection, respiratory failure, cardiovascular insufficiency, hemorrhagic and thromboembolic phenomena,(49) which could possibly be related to the effects of treatment-surgery(50) or chemotherapy.(51) Some of these conditions are associated with elevated TnT(51), which could possibly explain a weak association with cancer mortality. Although CRP is an inflammatory marker, there is still limited and less consistent evidence of its association with cancer.(52)
The strengths of our study include the large cohort of middle aged to older adults, and the use of highly sensitive assays. We studied mortality because information relating TnT and NT-proBNP to cause-specific mortality was limited, and it is useful to know if these cardiac biomarkers only relate to cardiovascular causes of death or whether they relate non-specifically to other causes. There are some drawbacks to our study. We did not perform a validation study for causes of death but rather used the ICD code for underlying cause. We had only a single assessment of biomarkers at the beginning of follow-up, and changes likely would have occurred during follow-up. We excluded individuals who were initially free of the disease whose mortality was being studied, but many people likely had co-morbid conditions, often subclinical, that contributed to death over the next decade. A final drawback of our study was that, despite its size, the numbers of stroke and respiratory deaths were modest.
In summary, greater levels of Hs-TnT, NT-proBNP and Hs-CRP are associated with increased risk of death, not just from cardiovascular disease but also from some non-cardiovascular causes.
Supplementary Material
Acknowledgement
The authors thank the staff and participants of the Atherosclerosis Risk in Communities study for their important contributions.
Sources of Funding
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C).
The authors thank the staff and participants of the ARIC study for their important contributions.
Abbreviation and Acronym
- Hs-TnT
High sensitivity Troponin T
- NT-proBNP
N-terminal pro-brain natriuretic peptide
- Hs-CRP
High sensitivity C-reactive protein
- ARIC
Atherosclerosis Risk in Communities
- CVD
Cardiovascular disease
- CHD
Coronary heart disease
- eGFR
Estimated glomerular filtration rate
- FEV1
Forced expiratory volume in 1 second
- MS
Mississippi
- MN
Minnesota
- NC
North Carolina
- MD
Maryland
- MI
Myocardial infarction
- HF
Heart failure
- HR
Hazard ratio
- PEACE
Prevention of Events with Angiotensin Converting Enzyme Inhibition
- COPD
Chronic obstructive pulmonary disease
- FHS
Framingham Heart Study
- PREVEND
Prevention of Renal and Vascular End-stage Disease
- HOPE
Heart Outcomes Prevention Evaluation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Saunders JT, Nambi V, de Lemos JA, Chambless LE, Virani SS, Boerwinkle E, et al. Cardiac troponin T measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the Atherosclerosis Risk in Communities Study. Circulation. 2011 Apr 5;123(13):1367–1376. doi: 10.1161/CIRCULATIONAHA.110.005264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.deFilippi CR, de Lemos JA, Christenson RH, Gottdiener JS, Kop WJ, Zhan M, et al. Association of serial measures of cardiac troponin T using a sensitive assay with incident heart failure and cardiovascular mortality in older adults. JAMA. 2010 Dec 8;304(22):2494–2502. doi: 10.1001/jama.2010.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Lemos JA, Drazner MH, Omland T, Ayers CR, Khera A, Rohatgi A, et al. Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA. 2010 Dec 8;304(22):2503–2512. doi: 10.1001/jama.2010.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCullough PA, Nowak RM, McCord J, Hollander JE, Herrmann HC, Steg PG, et al. B-type natriuretic peptide and clinical judgment in emergency diagnosis of heart failure: analysis from Breathing Not Properly (BNP) Multinational Study. Circulation. 2002 Jul 23;106(4):416–422. doi: 10.1161/01.cir.0000025242.79963.4c. [DOI] [PubMed] [Google Scholar]
- 5.Marz W, Tiran B, Seelhorst U, Wellnitz B, Bauersachs J, Winkelmann BR, et al. N-terminal pro-B-type natriuretic peptide predicts total and cardiovascular mortality in individuals with or without stable coronary artery disease: the Ludwigshafen Risk and Cardiovascular Health Study. Clin Chem. 2007 Jun;53(6):1075–1083. doi: 10.1373/clinchem.2006.075929. [DOI] [PubMed] [Google Scholar]
- 6.Omland T, Persson A, Ng L, O'Brien R, Karlsson T, Herlitz J, et al. N-terminal pro-B-type natriuretic peptide and long-term mortality in acute coronary syndromes. Circulation. 2002 Dec 3;106(23):2913–2918. doi: 10.1161/01.cir.0000041661.63285.ae. [DOI] [PubMed] [Google Scholar]
- 7.Kragelund C, Gronning B, Kober L, Hildebrandt P, Steffensen R. N-terminal pro-B-type natriuretic peptide and long-term mortality in stable coronary heart disease. N Engl J Med. 2005 Feb 17;352(7):666–675. doi: 10.1056/NEJMoa042330. [DOI] [PubMed] [Google Scholar]
- 8.Zethelius B, Berglund L, Sundstrom J, Ingelsson E, Basu S, Larsson A, et al. Use of multiple biomarkers to improve the prediction of death from cardiovascular causes. N Engl J Med. 2008 May 15;358(20):2107–2116. doi: 10.1056/NEJMoa0707064. [DOI] [PubMed] [Google Scholar]
- 9.Blankenberg S, McQueen MJ, Smieja M, Pogue J, Balion C, Lonn E, et al. Comparative impact of multiple biomarkers and N-Terminal pro-brain natriuretic peptide in the context of conventional risk factors for the prediction of recurrent cardiovascular events in the Heart Outcomes Prevention Evaluation (HOPE) Study. Circulation. 2006 Jul 18;114(3):201–208. doi: 10.1161/CIRCULATIONAHA.105.590927. [DOI] [PubMed] [Google Scholar]
- 10.Ridker PM. High-sensitivity C-reactive protein: potential adjunct for global risk assessment in the primary prevention of cardiovascular disease. Circulation. 2001 Apr 3;103(13):1813–1818. doi: 10.1161/01.cir.103.13.1813. [DOI] [PubMed] [Google Scholar]
- 11.Buckley DI, Fu R, Freeman M, Rogers K, Helfand M. C-reactive protein as a risk factor for coronary heart disease: a systematic review and meta-analyses for the U.S. Preventive Services Task Force. Ann Intern Med. 2009 Oct 6;151(7):483–495. doi: 10.7326/0003-4819-151-7-200910060-00009. [DOI] [PubMed] [Google Scholar]
- 12.Emerging Risk Factors Collaboration. Kaptoge S, Di Angelantonio E, Lowe G, Pepys MB, Thompson SG, et al. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet. 2010 Jan 9;375(9709):132–140. doi: 10.1016/S0140-6736(09)61717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koenig W, Khuseyinova N, Baumert J, Meisinger C. Prospective study of high-sensitivity C-reactive protein as a determinant of mortality: results from the MONICA/KORA Augsburg Cohort Study, 1984-1998. Clin Chem. 2008 Feb;54(2):335–342. doi: 10.1373/clinchem.2007.100271. [DOI] [PubMed] [Google Scholar]
- 14.Ridker PM. High-sensitivity C-reactive protein as a predictor of all-cause mortality: implications for research and patient care. Clin Chem. 2008 Feb;54(2):234–237. doi: 10.1373/clinchem.2007.099465. [DOI] [PubMed] [Google Scholar]
- 15.Wannamethee SG, Welsh P, Lowe GD, Gudnason V, Di Angelantonio E, Lennon L, et al. N-terminal pro-brain natriuretic Peptide is a more useful predictor of cardiovascular disease risk than C-reactive protein in older men with and without pre-existing cardiovascular disease. J Am Coll Cardiol. 2011 Jun 28;58(1):56–64. doi: 10.1016/j.jacc.2011.02.041. [DOI] [PubMed] [Google Scholar]
- 16.Kistorp C. Risk stratification in secondary prevention: advances in multimarker profiles, or back to basics? Circulation. 2006 Jul 18;114(3):184–186. doi: 10.1161/CIRCULATIONAHA.106.639732. [DOI] [PubMed] [Google Scholar]
- 17.Budweiser S, Luchner A, Jorres RA, Heinemann F, Hitzl AP, Schmidbauer K, et al. NT-proBNP in chronic hypercapnic respiratory failure: a marker of disease severity, treatment effect and prognosis. Respir Med. 2007 Sep;101(9):2003–2010. doi: 10.1016/j.rmed.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 18.Jeremias A, Gibson CM. Narrative review: alternative causes for elevated cardiac troponin levels when acute coronary syndromes are excluded. Ann Intern Med. 2005 May 3;142(9):786–791. doi: 10.7326/0003-4819-142-9-200505030-00015. [DOI] [PubMed] [Google Scholar]
- 19.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009 May 5;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982 Nov;36(5):936–942. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 21.Lutsey PL, Steffen LM, Stevens J. Dietary intake and the development of the metabolic syndrome: the Atherosclerosis Risk in Communities study. Circulation. 2008 Feb 12;117(6):754–761. doi: 10.1161/CIRCULATIONAHA.107.716159. [DOI] [PubMed] [Google Scholar]
- 22.White AD, Folsom AR, Chambless LE, Sharret AR, Yang K, Conwill D, et al. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) Study: methods and initial two years’ experience. J Clin Epidemiol. 1996 Feb;49(2):223–233. doi: 10.1016/0895-4356(95)00041-0. [DOI] [PubMed] [Google Scholar]
- 23.Rosamond WD, Folsom AR, Chambless LE, Wang CH, McGovern PG, Howard G, et al. Stroke incidence and survival among middle-aged adults: 9-year follow-up of the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke. 1999 Apr;30(4):736–743. doi: 10.1161/01.str.30.4.736. [DOI] [PubMed] [Google Scholar]
- 24.Avery CL, Mills KT, Chambless LE, Chang PP, Folsom AR, Mosley TH, et al. Long-term association between self-reported signs and symptoms and heart failure hospitalizations: the Atherosclerosis Risk In Communities (ARIC) Study. Eur J Heart Fail. 2010 Mar;12(3):232–238. doi: 10.1093/eurjhf/hfp203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Agarwal SK, Avery CL, Ballantyne CM, Catellier D, Nambi V, Saunders J, et al. Sources of variability in measurements of cardiac troponin T in a community-based sample: the atherosclerosis risk in communities study. Clin Chem. 2011 Jun;57(6):891–897. doi: 10.1373/clinchem.2010.159350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Daniels LB, Laughlin GA, Clopton P, Maisel AS, Barrett-Connor E. Minimally elevated cardiac troponin T and elevated N-terminal pro-B-type natriuretic peptide predict mortality in older adults: results from the Rancho Bernardo Study. J Am Coll Cardiol. 2008 Aug 5;52(6):450–459. doi: 10.1016/j.jacc.2008.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reichlin T, Hochholzer W, Bassetti S, Steuer S, Stelzig C, Hartwiger S, et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med. 2009 Aug 27;361(9):858–867. doi: 10.1056/NEJMoa0900428. [DOI] [PubMed] [Google Scholar]
- 28.de Lemos JA, Grundy SM. Low levels of circulating troponin as an intermediate phenotype in the pathway to heart failure. J Am Coll Cardiol. 2012 Jan 31;59(5):490–492. doi: 10.1016/j.jacc.2011.10.874. [DOI] [PubMed] [Google Scholar]
- 29.Rubin J, Matsushita K, Ballantyne CM, Hoogeveen R, Coresh J, Selvin E. Chronic hyperglycemia and subclinical myocardial injury. J Am Coll Cardiol. 2012 Jan 31;59(5):484–489. doi: 10.1016/j.jacc.2011.10.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wallace TW, Abdullah SM, Drazner MH, Das SR, Khera A, McGuire DK, et al. Prevalence and determinants of troponin T elevation in the general population. Circulation. 2006 Apr 25;113(16):1958–1965. doi: 10.1161/CIRCULATIONAHA.105.609974. [DOI] [PubMed] [Google Scholar]
- 31.Omland T, de Lemos JA, Sabatine MS, Christophi CA, Rice MM, Jablonski KA, et al. A sensitive cardiac troponin T assay in stable coronary artery disease. N Engl J Med. 2009 Dec 24;361(26):2538–2547. doi: 10.1056/NEJMoa0805299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kavsak PA, Xu L, Yusuf S, McQueen MJ. High-sensitivity cardiac troponin I measurement for risk stratification in a stable high-risk population. Clin Chem. 2011 Aug;57(8):1146–1153. doi: 10.1373/clinchem.2011.164574. [DOI] [PubMed] [Google Scholar]
- 33.Brekke PH, Omland T, Smith P, Soyseth V. Underdiagnosis of myocardial infarction in COPD - Cardiac Infarction Injury Score (CIIS) in patients hospitalised for COPD exacerbation. Respir Med. 2008 Sep;102(9):1243–1247. doi: 10.1016/j.rmed.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 34.Hoiseth AD, Neukamm A, Karlsson BD, Omland T, Brekke PH, Soyseth V. Elevated high-sensitivity cardiac troponin T is associated with increased mortality after acute exacerbation of chronic obstructive pulmonary disease. Thorax. 2011 Sep;66(9):775–781. doi: 10.1136/thx.2010.153122. [DOI] [PubMed] [Google Scholar]
- 35.de Lemos JA, Morrow DA, Bentley JH, Omland T, Sabatine MS, McCabe CH, et al. The prognostic value of B-type natriuretic peptide in patients with acute coronary syndromes. N Engl J Med. 2001 Oct 4;345(14):1014–1021. doi: 10.1056/NEJMoa011053. [DOI] [PubMed] [Google Scholar]
- 36.Linssen GC, Bakker SJ, Voors AA, Gansevoort RT, Hillege HL, de Jong PE, et al. N-terminal pro-B-type natriuretic peptide is an independent predictor of cardiovascular morbidity and mortality in the general population. Eur Heart J. 2010 Jan;31(1):120–127. doi: 10.1093/eurheartj/ehp420. [DOI] [PubMed] [Google Scholar]
- 37.Laukkanen JA, Kurl S, Ala-Kopsala M, Vuolteenaho O, Ruskoaho H, Nyyssonen K, et al. Plasma N-terminal fragments of natriuretic propeptides predict the risk of cardiovascular events and mortality in middle-aged men. Eur Heart J. 2006 May;27(10):1230–1237. doi: 10.1093/eurheartj/ehi878. [DOI] [PubMed] [Google Scholar]
- 38.Olsen MH, Hansen TW, Christensen MK, Gustafsson F, Rasmussen S, Wachtell K, et al. N-terminal pro-brain natriuretic peptide, but not high sensitivity C-reactive protein, improves cardiovascular risk prediction in the general population. Eur Heart J. 2007 Jun;28(11):1374–1381. doi: 10.1093/eurheartj/ehl448. [DOI] [PubMed] [Google Scholar]
- 39.Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Omland T, et al. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med. 2004 Feb 12;350(7):655–663. doi: 10.1056/NEJMoa031994. [DOI] [PubMed] [Google Scholar]
- 40.Kistorp C, Raymond I, Pedersen F, Gustafsson F, Faber J, Hildebrandt P. N-terminal pro-brain natriuretic peptide, C-reactive protein, and urinary albumin levels as predictors of mortality and cardiovascular events in older adults. JAMA. 2005 Apr 6;293(13):1609–1616. doi: 10.1001/jama.293.13.1609. [DOI] [PubMed] [Google Scholar]
- 41.Nilsson JC, Groenning BA, Nielsen G, Fritz-Hansen T, Trawinski J, Hildebrandt PR, et al. Left ventricular remodeling in the first year after acute myocardial infarction and the predictive value of N-terminal pro brain natriuretic peptide. Am Heart J. 2002 Apr;143(4):696–702. doi: 10.1067/mhj.2002.120293. [DOI] [PubMed] [Google Scholar]
- 42.Abdullah SM, Khera A, Das SR, Stanek HG, Canham RM, Chung AK, et al. Relation of coronary atherosclerosis determined by electron beam computed tomography and plasma levels of n-terminal pro-brain natriuretic peptide in a multiethnic population-based sample (the Dallas Heart Study). Am J Cardiol. 2005 Nov 1;96(9):1284–1289. doi: 10.1016/j.amjcard.2005.06.073. [DOI] [PubMed] [Google Scholar]
- 43.Doi Y, Ninomiya T, Hata J, Hirakawa Y, Mukai N, Ikeda F, et al. N-terminal pro-brain natriuretic peptide and risk of cardiovascular events in a Japanese community: the Hisayama study. Arterioscler Thromb Vasc Biol. 2011 Dec;31(12):2997–3003. doi: 10.1161/ATVBAHA.111.223669. [DOI] [PubMed] [Google Scholar]
- 44.D'Souza SP, Baxter GF. B Type natriuretic peptide: a good omen in myocardial ischaemia? Heart. 2003 Jul;89(7):707–709. doi: 10.1136/heart.89.7.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morita E, Yasue H, Yoshimura M, Ogawa H, Jougasaki M, Matsumura T, et al. Increased plasma levels of brain natriuretic peptide in patients with acute myocardial infarction. Circulation. 1993 Jul;88(1):82–91. doi: 10.1161/01.cir.88.1.82. [DOI] [PubMed] [Google Scholar]
- 46.Takahashi T, Nakamura M, Onoda T, Ohsawa M, Tanno K, Itai K, et al. Predictive value of plasma B-type natriuretic peptide for ischemic stroke: a community-based longitudinal study. Atherosclerosis. 2009 Nov;207(1):298–303. doi: 10.1016/j.atherosclerosis.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 47.Nogami M, Shiga J, Takatsu A, Endo N, Ishiyama I. Immunohistochemistry of atrial natriuretic peptide in brain infarction. Histochem J. 2001 Feb;33(2):87–90. doi: 10.1023/a:1017996113871. [DOI] [PubMed] [Google Scholar]
- 48.Idris I, Hill R, Ross I, Sharma JC. N-terminal probrain natriuretic peptide predicts 1-year mortality following acute stroke: possible evidence of occult cardiac dysfunction among patients with acute stroke. Age Ageing. 2010 Nov;39(6):752–755. doi: 10.1093/ageing/afq098. [DOI] [PubMed] [Google Scholar]
- 49.Ambrus JL, Ambrus CM, Mink IB, Pickren JW. Causes of death in cancer patients. J Med. 1975;6(1):61–64. [PubMed] [Google Scholar]
- 50.Cardinale D, Colombo A, Sandri MT, Lamantia G, Colombo N, Civelli M, et al. Increased perioperative N-terminal pro-B-type natriuretic peptide levels predict atrial fibrillation after thoracic surgery for lung cancer. Circulation. 2007 Mar 20;115(11):1339–1344. doi: 10.1161/CIRCULATIONAHA.106.647008. [DOI] [PubMed] [Google Scholar]
- 51.Dolci A, Dominici R, Cardinale D, Sandri MT, Panteghini M. Biochemical markers for prediction of chemotherapy-induced cardiotoxicity: systematic review of the literature and recommendations for use. Am J Clin Pathol. 2008 Nov;130(5):688–695. doi: 10.1309/AJCPB66LRIIVMQDR. [DOI] [PubMed] [Google Scholar]
- 52.Lee S, Choe JW, Kim HK, Sung J. High-sensitivity C-reactive protein and cancer. J Epidemiol. 2011 May 5;21(3):161–168. doi: 10.2188/jea.JE20100128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.