Abstract
A number of behavioral changes occur between late childhood and adulthood, including maturation of social cognition, reward receptivity, impulsiveness, risk-taking and cognitive control. Although some of these abilities show linear improvements with age, some abilities may temporarily worsen, reflecting both the restructuring and/or strengthening of connections within some brain systems. The current study uses resting state functional connectivity to examine developmental differences between late childhood and adulthood in task positive (TP) regions, which play a role in cognitive control functions, and task negative (TN) regions, which play a role in social cognition, self-referential, and internally-directed thought. Within the TP network, developmental differences in connectivity were found with the left dorsolateral prefrontal cortex. Within the TN network, developmental differences in connectivity were found with a broad area of the medial prefrontal cortex and the right parahippocampal gyrus. Connections between the two networks also showed significant developmental differences. Stronger anticorrelations were found in the TN maps of the adult group for the right anterior insula/inferior frontal gyrus, bilateral anterior inferior parietal lobule, bilateral superior parietal lobule and an anterior portion of the right posterior cingulate cortex. There was a significant brain-behavior relationship between the strength of anticorrelation in these regions and inhibitory control performance on two Go/No-go tasks suggesting that the development of anticorrelations between late childhood and adulthood supports mature inhibitory control. Overall, maturation of these networks occurred in specific regions which are associated with cognitive control of goal-directed behavior, including those involved in working memory, social cognition, and inhibitory control.
Keywords: development, resting-state connectivity, network, anticorrelation, response inhibition, cognitive control
1. Introduction
Attention and control function undergoes marked development throughout childhood and adolescence. Developmental improvements occur in a number of goal-directed behaviors including: basic speed of processing and response variability, sustained attention, working memory, set shifting, and response inhibition (Bedard, et al., 2002; Crone, Donohue, Honomichl, Wendelken, & Bunge, 2006; Crone, Wendelken, Donohue, van Leijenhorst, & Bunge, 2006; Geier, Garver, Terwilliger, & Luna, 2009; Geier, Terwilliger, Teslovich, Velanova, & Luna, 2010; Jazbec, et al., 2006; Rueda, et al., 2004; Williams, Ponesse, Schachar, Logan, & Tannock, 1999). While some functions show relatively early maturation, many continue to improve through adolescence. For example, although overall RTs and alerting scores decrease between childhood and adulthood, orienting and conflict scores do not show a difference (Rueda, et al., 2004). These findings suggest that brain systems supporting goal-directed behaviors may not only become more integrated during adolescence, but may undergo specific changes.
Over the course of development, brain regions supporting cognitive control functions become more integrated within their networks and more segregated from other networks. Decreased connectivity between local, adjacent regions and increased connectivity between remote regions supporting distributed mature networks has been well-documented (Fair, et al., 2008; Fair, et al., 2009; Fair, et al., 2007; Kelly, et al., 2009). The angular gyrus, which forms part of the default mode network (DMN), and the adjacent inferior parietal lobule, which form part of the fronto-parietal network, are more strongly connected with each other in children but are distinctly segregated into separate networks in adults (Fair, et al., 2009; Vogel, Power, Petersen, & Schlaggar, 2010). Other long-range connections strengthen over the course of adolescence, such as the one between the posterior cingulate cortex (PCC) and the medial prefrontal cortex (MPFC), which supports integration of the anterior and posterior portions of the DMN in adulthood (Supekar, et al., 2010; Uddin, Supekar, Ryali, & Menon, 2011).
These developmental changes contribute to the formation of mature distributed networks seen in adults, and there is evidence that the basic topology of some of these networks exists early in development. Infants already have identifiable sensorimotor and visual networks within the first year of life, however cognitive systems such as the DMN and fronto-parietal network are at best only partially present (Fransson, Aden, Blennow, & Lagercrantz, 2011; Fransson, et al., 2009; Fransson, et al., 2007). Network maturation involves the strengthening of some preexisting connections, but the reshaping of other connections. Connectivity within task positive and DMN regions increase over the course of development (Anderson, Ferguson, Lopez-Larson, & Yurgelun-Todd, 2011; Fair, et al., 2007) and the strength of voxel connectivity in childhood, predicts how strongly that voxel will affiliate with other voxels within its own network and how weakly that voxel will affiliate with other voxels outside of its network (Anderson, et al., 2011). In addition to changes in the strength of network connectivity, there is evidence that specific restructuring also occurs.
Studies have found that task positive brain networks, which support goal-directed response to external stimuli, undergo reorganization during development. Fair et al. (2007), found that nodes of the dorsal attention network and the fronto-opercular network change affiliation during adolescence. In addition, task-based studies have suggested that adolescents have less-developed prefrontal regions, which may reduce behavioral control and leave them more susceptible to risky behavior (Casey, Getz, & Galvan, 2008; Casey, Jones, & Hare, 2008; Somerville, Hare, & Casey, 2011). Both task-based and resting state functional connectivity studies have found that children and adolescents have immature medial prefrontal regions which may further affect their control processing (Blakemore, den Ouden, Choudhury, & Frith, 2007; Kelly, et al., 2009).
Developmental changes in cognitive control are likely related to these maturational changes within task positive networks. They may also result from increased anti-correlations between task positive (TP) and task negative (TN) regions. Fox et al. (2005) first noted that these two networks, which show opposing activity during a variety of attentionally-demanding and working-memory tasks, also show opposing, anti-correlated time-courses at rest. Further examination has found that this opposing activity is behaviorally relevant (Hampson, Driesen, Skudlarski, Gore, & Constable, 2006; Kelly, Uddin, Biswal, Castellanos, & Milham, 2008; Mennes, et al., 2010; Mennes, et al., 2011). In addition, the anti-correlations between these networks increase over the course of development (Anderson, et al., 2011). However, it is not clear whether developmental behavioral improvements are specifically related to connectivity changes within TP network regions or whether they may also be related to connectivity changes between TP and TN regions.
For the current study, we examined group differences in TP and TN network connectivity between children (8–12 years old) and adults (20–47 years old) and the association of these changes with behavioral measures of cognitive control. We focused on changes in developmental connections with those seed regions of the TP and TN networks that are consistently (de-)activated during attentionally-demanding tasks (Fox, Snyder, Vincent, et al., 2005). This allowed us to identify regions that are important for instantiating mature task control. We examined changes in both positive and negative connections to these two networks. In order to differentiate between those developmental changes that result in stronger connections within a network and those that result in stronger anti-correlations between networks, separate TP and TN network maps were compared between the two groups. Brain-behavior relationships between those connections that show developmental differences and cognitive control on two Go/No-go tasks were examined. One was a “Simple” Go/No-go task in which working memory demands were minimized using a straightforward stimulus-response association (green = Go, red = No-go) and a second “Complex” Go/No-go task in which response selection was associated with higher working memory demand. We hypothesized that regions within the TP and TN networks would show increased connectivity with the seed regions in the adult group and that certain TP and TN regions would become more anti-correlated with the seed regions in the adult group. In addition, we hypothesized that connectivity with these developing regions would be related to cognitive control function as indexed by commission rates on the two Go/No-go tasks.
2. Materials and Methods
2.1 Participants
28 healthy adults (12 male, 16 female) and 63 typically-developing children (36 male, 24 female) participated in the study. All adults and 60 children were right-handed. Two children showed mixed handedness (Edinburgh Handedness Score between 0.5 and −0.5) and one child was left-handed. Adults were between 18–47 years old (mean=27.57, SD=6.22). All adults were screened for any history of mental health or neurological difficulties, including a history of developmental disabilities. Children were between 8 years 0 months and 12 years 11 months of age (mean = 10.20, SD = 1.06). All children had normal Full Scale IQ on the Wechsler Intelligence Scale for Children (mean = 111.00, SD = 10.54) with no history of intellectual disability, developmental language disorder, reading disability, pervasive developmental disorder, visual impairment, neurologic disorder nor psychiatric diagnosis, as confirmed using the DICA-IV (Sala, Granero, & Ezpeleta, 2006). Only subjects with movement of less than 3 mm translation and 3 degrees rotation over the course of the resting scan were included in the current sample.
For the examination of network group-differences, all 28 adults (12 male, 16 female) and 42 gender-matched children (18 male, 24 female, all right-handed) were examined. For the brain-behavior correlations, 27 adults (12 male, 15 female) were included in the analysis. One adult was excluded for poor task performance. This adult had >50% omission error rate on the Complex Go/No-go task and it was not clear whether the subject understood and/or was adequately attending to the task. 25 children had completed the behavioral tasks. Of these, one subject was excluded from further analyses for being left-handed. 24 remaining children were included in the brain-behavior correlation analyses (11 male, 13 female). For this subgroup, mean age was 10.23 years (SD = 1.07).
This study was approved by the Johns Hopkins Medical Institutional Review Board. Written consent was obtained for all participants. For children, written consent was obtained from a parent or legal guardian and verbal assent was obtained from the participating child.
2.2 fMRI Acquisition and Processing
Images were acquired on a Philips 3T scanner. A high-resolution anatomical scan (MPRAGE, 8-channel head coil, TR = 7.99 ms, TE = 3.76 ms, Flip angle = 8°) was acquired for image coregistration, segmentation and normalization processing steps. Resting state scans were acquired in each participant for 5 min 20 sec (2D-SENSE EPI, 8-channel head coil, TR = 2500ms, TE = 30 ms, Flip angle = 70°). Participants were instructed to relax and fixate on a center cross. Preprocessing of functional images was performed using SPM5 and Matlab scripts. This included slice time correction, motion correction, co-registration, segmentation, and normalization. Nuisance variables were removed from each voxel, including cerebrospinal fluid and white matter signals identified using the CompCor method, global mean signal, and six motion parameters. Functional images were spatially smoothed using a 6mm FWHM filter and then temporally filtered (bandpass 0.01–0.1 Hz).
2.3 Data Analysis
6mm-radius 3D seeds were centered at locations taken from a previous study (Fox, Snyder, Vincent, et al., 2005). These seeds were originally centered at peak coordinates for regions showing consistent activation (task positive seeds) and deactivation (task negative seeds) from task-fMRI studies of attention-demanding and working memory tasks (Fox, Snyder, Vincent, et al., 2005). Fox and colleagues had examined full-brain connectivity of task-derived seeds to show that the same regions that show opposing activation during task paradigms also form anti-correlated networks at rest. Those seeds included three task-positive (TP) seeds: intraparietal sulcus (IPS, talairach coordinates: −25, −57, 46), frontal eye field (FEF: 25, −13, 50), and the middle temporal region (MT+: −45, −69, −2); and three task-negative (TN) seeds: medial prefrontal cortex (MPFC: −1, 47, −4), posterior cingulate cortex (PCC: −5, −49, 40), and lateral parietal cortex (LP: −45, −67, 36). The seed coordinates were converted to MNI space using the Lancaster transformation (Lancaster, et al., 2007).
For the current study, full-brain connectivity maps with these seed regions were compared for the child and adult groups. The mean time-course for each seed was extracted from each subject’s resting state scan and full-brain connectivity maps were generated for each of the six seed time-courses. Within each subject, the three TP connectivity maps were averaged to get one TP map and the three TN connectivity maps were averaged to get one TN map. These maps were then entered into second-level analyses. Within each group, one-sample t-tests were performed separately for the TP and TN maps in SPM5 to determine regions with connectivity that significantly differed from zero (both for those connections that were significantly greater than zero and those connections that were significantly less than zero). In addition, the single-subject TP and TN maps were entered into separate two-sample t-tests in SPM5 to test for group differences. Second-level group analyses were thresholded at a voxel-level of p<0.001 and were multiple-comparisons corrected at a cluster-level threshold of p<0.05 according to Random-Field Theory (Kiebel, Poline, Friston, Holmes, & Worsley, 1999). In addition to examining the group differences in the mean TP and TN maps, conjunction analyses were also performed to determine whether the group differences were consistent across all of the network seeds. For this analysis, group differences in the full-brain connectivity maps for each seed region were found using SPM5 and were multiple-comparisons corrected at a cluster-level of p>0.05. Conjunction maps were made of those regions that were significant at a cluster level for all three TP network seeds or for all three TN network seeds.
2.4 Masked Brain-Behavior Relationships
2.4.1 Behavioral Task Procedure
Two Go/No-go tasks were performed in a separate scan session by the subset of participants included in the brain-behavior analyses. For each trial, stimuli were presented for 300 ms followed by a 1500 ms interstimulus interval. 12 sec blocks of rest occurred at the beginning, end and four times throughout each run. The proportion of Go:No-go trials was 3:1, with 78 Go trials and 26 No-go trials occurring in each run. Participants performed two runs each of the two Go/No-go tasks. Each run was preceded by instructions and 20 practice trials. Half of the participants performed the two Simple runs first and the other half performed the two Complex runs first.
The Simple task paradigm utilized well-ingrained stimulus-response associations that minimized the need for working memory to guide response selection. Go stimuli were green while No-go stimuli were red. The Complex task involved inconsistent stimulus-response associations with a one-back format that necessitated increased working memory to guide response selection. Participants had to remember the color of the previous stimulus color to determine the correct response. A change in the stimulus color signaled a Go stimulus, while a repetition of the stimulus color signaled a No-go stimulus. For this task, stimuli were either blue or yellow.
2.4.2 Brain-Behavior Analyses
Brain-behavior relationships were examined between those regions showing developmental changes with the TN maps. To test whether the anti-correlations between developing TN regions were associated with inhibitory control abilities, inter-individual brain-behavior relationships examined the relationship between the strength of connectivity in these regions and performance on two Go/No-go tasks. For these analyses, masks were created for the regions showing significant developmental differences in the TN contrasts. One mask was made for the TN regions that had significantly greater connectivity in the adult than the child group (i.e. TN+ mask, medial prefrontal and parahippocampal DMN regions). A second mask was made for the regions that had greater anticorrelations with the TN network in the adult than the child group (i.e. TN− mask, right IFC/insula, bilateral IPL, and SPL regions). The mean time-course was then extracted for each mask and the two time-courses were correlated to acquire one connectivity (TN+-connectivity) value per subject. Each subject’s TN+-correlation value was then Fisher z-transformed.
27 adults and 24 TD children had performed the Go/No-go paradigm in a separate scan session and were included for the brain-behavior analysis. The Go/No-go paradigm included two runs each of the Simple and Complex Tasks. For each subject, Go/No-go behavioral variables were examined (the percentage of commission errors, Intra-Subject Variability and mean reaction times). Brain-behavior relationships were analyzed using Analysis of Covariance (ANCOVA) with the z-transformed correlation values between the TN+ and TN− regions as the dependent variable, group as a factor, and the behavioral variable as a covariate. This analysis examined inter-individual differences in brain-behavior relationships, while accounting for group differences in the two measures. Since the regions were defined based on group differences, it was important to account for the group effect to ensure that it did not drive the brain-behavior relationship.
In addition to the brain-behavior relationship with developing regions, the brain-behavior relationship with the entire TP and TN networks was examined. For this analysis, two masks were created: one of the adult TP network and one of the adult TN network. As described above, correlation values were obtained for the time-courses between these two sets of regions. Group-by-behavioral covariate ANCOVAs were performed to examine the relationship between behavior on the Go/No-go tasks and the anti-correlation between the entire TP and TN networks.
2.5 Seed-Pairwise Brain-Behavior Relationships
To further examine whether TP-TN anticorrelatons support inhibitory control, brain-behavior relationships were examined with an additional set of a priori-defined response inhibition seeds and two DMN seeds: medial prefrontal cortex (MPFC) and posterior cingulate cortex (PCC). This analysis examined whether anticorrelations with regions commonly implicated in response inhibition are also related to inhibitory behavior. Seeds were placed at peak coordinates found in a separate task-based fMRI study for the No-go contrast using the Simple Go/No-go paradigm (unpublished data). These coordinates overlapped with those regions found in a previous meta-analysis of response inhibition paradigms (Simmonds, Pekar, & Mostofsky, 2008). 9mm seeds were placed in the pre-SMA (MNI coordinates: −6, 8, 54), right anterior insula/inferior frontal cortex (MNI coordinates: 39, 20, 1), and right inferior parietal cortex (MNI coordinates: 60, −32, 38). The latter three seeds overlapped with those regions showing significant developmental differences in the TN− map. In addition, a right dorsolateral prefrontal cortex (DLPFC) seed (MNI coordinates: 45, 25, 27) was examined, which was active during the Complex Go/No-go task, but not the Simple version of the task. For the DMN regions, seeds were placed at peak coordinates in the adult TN maps. 9mm seeds were placed at the Fox coordinates for the MPFC and PCC. For these analyses, the pairwise z-transformed correlations were found between each of the DMN and inhibitory seed pairs. Multivariate Analysis of Covariance (MANCOVA) was used to determine whether the pairwise-region (anti)correlation was consistently related to response inhibition performance across all of the region-pairs examined. Separate ANCOVA analyses were performed to determine whether the DLPFC (anti)correlations were significant in the Complex task.
3. Results
3.1 Within-Group TP and TN network maps
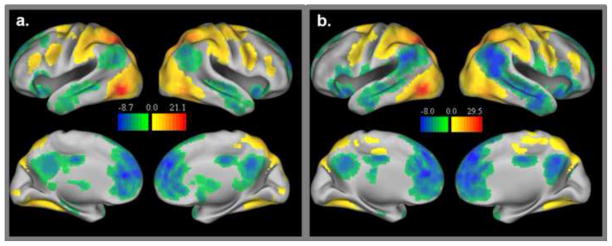
Figure 1 displays the significant TP regions for both the adults and the children. Figure 2 displays the significant TN regions for both groups.
Figure 1.
Task positive network group maps for adults (1a) and children (1b). Both positive (i.e. connections significantly greater than zero) and negative connections (i.e. connections significantly less than zero) with task positive regions are displayed.
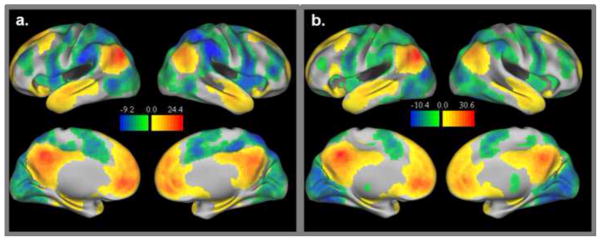
Figure 2.
Task negative network group maps for adults (2a) and children (2b). Both positive (i.e. connections significantly greater than zero) and negative connections (i.e. connections significantly less than zero) with task negative regions are displayed.
3.2 Group-Differences in the TP network
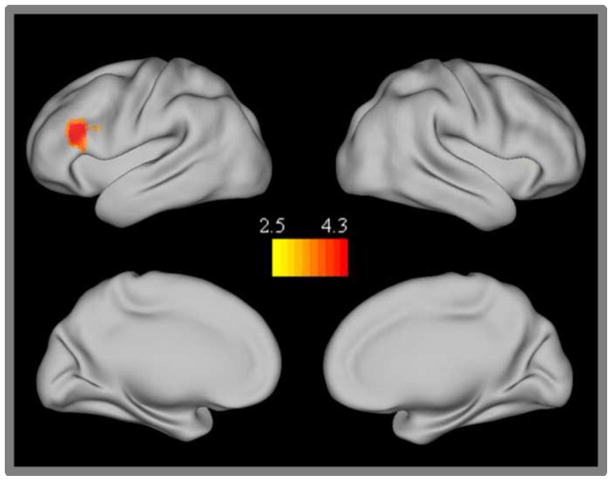
Only one region, the left dorsolateral prefrontal cortex (DLPFC: BA46), showed significant differences in TP network connectivity between children and adults (Figure 3). This region was more strongly connected with the TP network in the adults than in children. Examination of the conjunction map found no common regions that showed significantly different connectivity across all three TP seeds. Further examination of the individual seeds maps revealed that the left dlpfc was only present in the IPS seed map.
Figure 3.
Task positive network group differences. Regions showing stronger connectivity in adults than children.
3.3 Group-Differences with the TN network
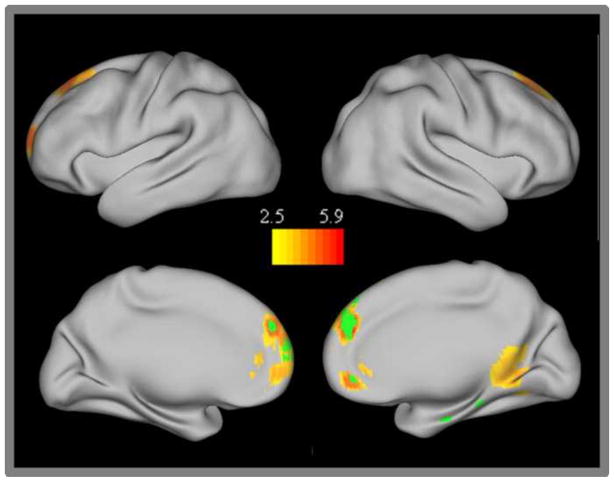
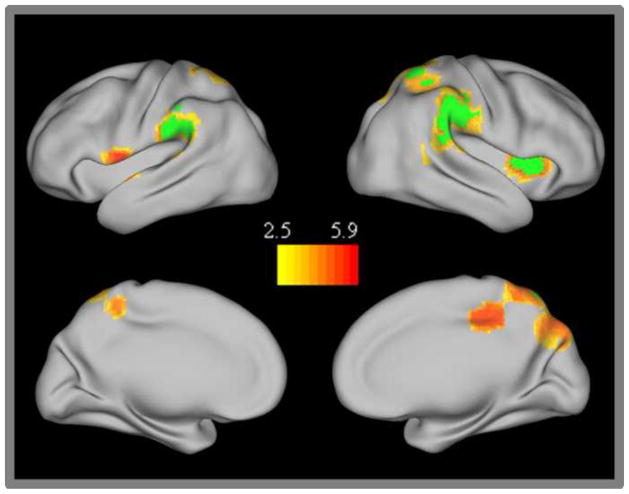
A number of medial frontal regions (BA 9 and 10: including both dorsomedial prefrontal cortex and ventromedial prefrontal cortex) showed significantly greater connectivity with the TN network in adults than in children and were also present in the TN conjunction analysis (Figure 4). One right posterior parahippocampal cortex region also showed significantly increased connectivity with the TN network in adults compared to children. Examination of the individual seed maps revealed that connectivity between this region and the PCC seed was specifically increased in adults.
Figure 4.
Task negative network group differences. Regions showing stronger connectivity in adults than children. The conjunction of those regions showing significant group differences for all three of the individual task negative network seeds are displayed in green.
In addition to those TN regions that became more strongly integrated with the TN network in adulthood, there were a number of attention network regions that were more strongly anti-correlated with the TN network in adults than in children (Figure 5). These regions included right insula/inferior frontal cortex (rIFC BA 13/44), left superior temporal/ premotor cortex (BA 22/6), bilateral inferior parietal lobule (IPL BA 40), bilateral superior parietal lobule (SPL BA 7) and a right posterior cingulate cortex region (rPCC, BA 31). Although many of these regions were bilateral, the developmental changes were stronger on the right side with greater Z-scores and more extensive activation in the right-lateralized ROIs. Activation in the anterior insula was only significant in the right hemisphere; however, there was a small subthreshold region that localized to a nearby premotor location in the left hemisphere with nearby subthreshold activation in the left anterior insula. Many of these regions were also present in the conjunction analysis across all three TN network seeds (i.e. rIFC/anterior insula, right IPL, and right SPL). The left premotor/temporal and the mid-cingulate regions were present in both the PCC and MPFC seed maps, while the left SPL region was specifically present in the LP seed map.
Figure 5.
Task negative network group differences. Regions showing stronger anticorrelation in adults than children. The conjunction of those regions showing significant group differences for all three of the individual task negative network seeds are displayed in green.
3.4 Brain-Behavior Relationships
3.4.1 Behavior
Children made significantly more commission errors for both the Simple (t(49) = −6.38, p<0.001, child mean(SD) = 36.54% (24.90), adult mean(SD) = 4.63% (6.75)) and Complex tasks (t(49) = −11.78, p<0.001,child mean(SD) = 60.34% (19.20), adult mean(SD) = 11.75% (9.01). In addition, they had significantly greater intra-subject variability in reaction time (SD of reaction time/mean of reaction time) for both the Simple (t(49) = −3.20, p=0.002, child mean(SD) = 0.33(0.22), adult mean(SD) = 0.19(0.06)) and the Complex tasks (t(49) = −4.93, p<0.001, child mean(SD)=0.40(0.14), adult mean(SD)=0.25(0.054)). The two groups were not significantly different in their mean RTs for either the Simple (t(49) = −0.47, p=0.64, child mean(SD) = 381.59(101.97), adult mean(SD) = 368.33(100.05)) or for the Complex tasks (t(49) = 1.16, p=0.25, child mean(SD) = 410.07(108.52), adult mean(SD) = 447.78(121.52)).
3.4.2 Developing Regions
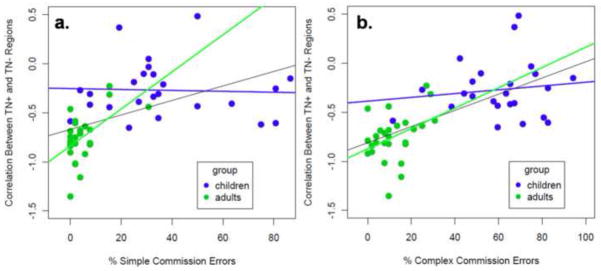
Regions that were more anticorrelated with the TN network in adults are commonly implicated in response inhibition. In particular, right insula/IFC and right IPL are regions that are consistently active during response inhibition paradigms. To test whether inhibitory control function is related to the anti-correlations between these two sets of developing regions, ANCOVAs were performed. These analyses examined the effect of group and response inhibition measures (i.e. % commission errors during the Simple and Complex Tasks) on the z-transformed TN+-correlations. Figure 6 shows the relationship between the TN+-(anti)correlation and inhibitory control performance on the two tasks.
Figure 6.
Brain-behavior Relationship. The strength of anticorrelation in developing TN regions is related to commission error rate on the Simple Go/No-go task (6a) and the Complex Go/No-go task (6b).
For the analysis examining Simple Task inhibitory control, there was a significant effect of group (F(1,47) = 18.6, p<0.001), a significant relationship between percent commission errors and the z-transformed correlation values between the TN+ and TN− regions (F(1,47) = 5.89, p=0.019), and significant relationship for the group x commission rate interaction (F(1,47) = 6.50, p = 0.014). Within each group, simple regression was performed to determine whether there was a significant relationship between Simple Task commission error rate and the TN+-correlation. For adults, there was a significant relationship (R=0.53, p=0.0043), but the relationship did not hold for the children (R=0.041, p=0.85).
For the analysis examining Complex Task inhibitory control, there was a significant effect of group (F(1,47) = 6.18, p=0.016) and a marginal relationship between TN+-correlation and percent commission errors (F(1,47) = 3.85, p=0.053). The group x TN+-correlation was not significant (F(1,47) = 1.80, p=0.19). Within each group, simple regression was performed to determine whether there was a significant relationship between percent of Complex task errors and the TN+-correlation. For adults, there was a modest, but significant relationship (R = 0.39, p = 0.044), but the relationship did not hold for the children (R=0.13, p=0.55).
The associations of these developing TN regions were also examined for ISV since there were group differences in this measure. For these analyses, group x behavioral covariate analyses were performed and the covariate associations are reported. For Simple Task ISV, there was a significant effect of group (F(1,47) = 5.09, p=0.029), but no relationship between ISV and the z-transformed TN+-correlation values (F(1,47) = 1.44, p=0.24), and no relationship for the group x ISV interaction (F(1,47) = 0.25, p = 0.62). For the Complex Task ISV, there was a significant effect of group (F(1,47) = 5.62, p=0.022), but no relationship between ISV and the z-transformed TN+-correlation values (F(1,47) = 0.015, p=0.90), and no relationship for the group x ISV interaction (F(1,47) = 0.53, p = 0.40). Since there were no ISV associations with the TN+-correlation, the remaining analyses focus on associations with commission error rate.
The current analyses identified regions that were developing between the two age groups. In addition, the relationship between age within each group and TN+-connectivity was examined to determine whether development may occur within either of the age groups for both the connectivity measures and the commission error rates. For these analyses, simple regression was performed within each group to test for the association with age. For the children, there was no association between age and TN+-correlation (R = 0.20, p = 0.35) and there was also no association between age and the measures of inhibitory control (Simple commission: R = 0.19, p = 0.39; Complex commission: R = 0.18, p = 0.41). For the adults, there were also no associations with age (TN+-: R = 0.21, p=0.28; Simple Commission: R = 0.34, p = 0.18; Complex Commission: R = 0.27, p = 0.13). Although the two groups had differing connectivity values and differing commission error rates, age within each group was not significantly associated with these variables.
3.4.3 Entire TP and TN networks
Previous studies have found that the anticorrelation across the entire TP and TN networks is behaviorally relevant and therefore may reflect attention and control processing (Kelly, et al., 2008). To determine whether the relationship between inhibitory control is related more generally to anti-correlation across the TP and TN networks, the time-courses for the entire TP network and for the entire TN network were extracted. For this analysis, one mask was created for the adult TP network and one mask was created for the adult TN network. The mean time-course for each of these masks was extracted for each subject and the two time-courses were correlated.
Group x commission rate analyses were performed for both the Simple and Complex Tasks to determine whether these were related to the TP-TN anti-correlation. For the analysis with the Simple Task, there were no significant effects (group: F(1,47) = 2.30, p=0.14; commission rate: F(1,47) = 0.78, p=0.38; group x commission rate (F(1,47) = 0.048, p=0.826). For the Complex Task analysis, there, likewise, were no significant effects as shown by ANCOVA (group: F(1,47) = 1.29, p=0.261; commission rate: F(1,47) = 0.142, p=0.708; group x commission rate: F(1,47) = 0.026, p=0.872).
3.4.4 Pairwise Seed Correlations
To further examine the importance of TP-TN anticorrelations for inhibitory control function, regions that are commonly implicated in response inhibition (i.e. pre-SMA, right IFC, right IPL, and the right DLPFC) were examined. The latter two regions overlap with the regions showing stronger anti-correlation in adults than children. An additional set of pairwise correlation analyses were performed to determine whether the strength of anti-correlation between inhibitory control and DMN regions is related to inhibitory control performance and whether this relationship is consistent for all pairwise connections between these inhibitory control regions and both anterior and posterior nodes of the DMN. For these analyses, the pairwise correlations were found between each of the inhibitory control regions (pre-SMA, right IFC, and right IPL) and each of the DMN regions (PCC and MPFC). In addition, the relationship between right DLPFC and the DMN regions was examined. It was hypothesized that this region would be related to inhibitory control in the Complex Task, but not the Simple Task. Multivariate analysis of variance (MANCOVA) was performed to determine whether group and commission rate are related to the strength of (anti)correlation across this set of regions. The individual ANCOVA analyses were also examined to determine whether the right DLPFC (anti)correlations were significantly associated with commission rate in the Complex task.
For the Simple Task, there was a significant effect for group (Wilkes Lambda = 0.60, F(8,40) = 3.21, p=0.045), a trend toward a significant effect of commission rate (Wilkes Lambda = 0.73, F(8,40) = 1.85, p=0.094), and a marginally significant group x commission rate interaction (Wilkes Lambda = 0.70, F(8,40) = 2.15, p=0.053). For the Complex Task, the effect of group was not significant (Wilkes Lambda = 0.76, F(8,40) = 1.46, p=0.17), the effect of commission rate showed a trend toward significance (Wilkes Lambda = 0.72, F(8,40) = 1.96, p=0.077), and there was a trend toward a significant effect for the group x commission rate interaction (Wilkes Lambda = 0.71, F(8,40) = 2.01, p=0.070).
The relationship between right DLPFC (anti)correlation and inhibitory control performance was specifically examined to determine whether this relationship is task dependent. For this analysis, group x commission rate ANCOVAs were performed for both the PCC and MPFC connections. For the PCC-right DLPFC connections, there was no significant effect of commission rate in the Simple Task (F(1,47) = 0.577, p=0.45, but there was a robust effect in the Complex Task (F(1,47) = 6.70, p=0.013). For the MPFC-right DLPFC connections, there was also no significant effect of commission rate in the Simple Task (F(1,47) = 0.18, p=0.67), and there was a trend toward a significant effect for the Complex Task (F(1,47) = 3.13, p=0.082).
3.5 Motion Effects and Global Signal Regression
Recent examination suggests that some of the connectivity differences between children and adults may be an artifact due to group differences in motion (Power, Barnes, Snyder, Schlaggar, & Petersen, 2012; Satterthwaite, et al., 2012; Van Dijk, Sabuncu, & Buckner, 2012). To determine whether motion may influence group differences in connectivity, four motion scores were generated for each participant. First, the maximum displacement from the first scan over the course of the run was found for both the absolute motion (difference from reference scan 1) and differential motion (difference from previous scan). Second, the mean of the six parameters (x,y,z in mm and roll, pitch yaw rotation converted from degrees to mm) over the course of the run was found for both the absolute and differential motion. There were significant group differences in the amount of motion for all of these scores (max abs motion: t(1,68) = −3.80, p=0.0003; mean abs motion: t(1,68) = −2.71, p=0.008; differential max motion: t(1,68) = −3.24, p=0.002; differential mean motion: (t(1,68) = −3.26, p=0.002).
Since the group differences in motion were robust, group x motion ANCOVAs were examined for each of the region pair correlations to determine whether motion may be related to the strength of (anti)correlation between the TN+-regions. For these analyses, ANCOVAs were performed in which group was a factor, motion was a covariate, and the TN+-(anti)correlation was the dependent variable. For the absolute max motion, there was a significant group effect (F(1,66) = 7.25, p = 0.009), but no significant motion effect (F(1,66) = 0.19, p = 0.67) and no group x motion interaction (F(1,66) = 0.01, p = 0.92). Likewise for the other motion measures, there were significant group effects, but no significant effects of motion and no group x motion interactions (absolute mean motion: group (F(1,66) = 6.89, p = 0.011), motion (F(1,66) = 0.02, p = 0.89), group x motion interaction (F(1,66) = 0.34, p = 0.56; differential max motion: group (F(1,66) = 21.84, p < 0.001), motion (F(1,66) = 0.09, p = 0.76), group x motion interaction (F(1,66) = 0.007, p = 0.93; differential mean motion: group (F(1,66) = 9.98, p = 0.002), motion (F(1,66) = 1.26, p = 0.26), group x motion interaction (F(1,66) = 0.04, p = 0.95). For the region pair correlations between each of the inhibitory control regions and the DMN nodes, there were likewise, no significant relationships between either of the motion scores and any of the region pair correlations.
In addition to the examination of motion as a covariate, we examined the group differences in TN network connectivity for low-movement and high-movement children separately. For this analysis, the full group of 60 right-handed subjects were divided into two groups based on their mean differential movement. The 30 low movement children had similar movement to that of adults (t(1,56) = 0.26,p = 0.60), while the 30 high movement children had significantly greater movement than adults (t(1,56) = 6.93, p<0.001. The network connectivity in the TN maps was compared with that of adults for each of these movement subgroups. Table 1 displays the group differences for each of these movement subgroups. Both groups show developmental group differences in a similar set of regions; however, the differences are more robust and extensive within the high-movement group than within the low-movement group. If high-movement caused interference in network connectivity, then we would expect less robust group differences in the high-movement group or more evidence of distance effects in the high-movement group (i.e. more short-range connectivity and less long-range connectivity in high-movement children than adults). Instead, the high-movement group has more robust group developmental connectivity differences in general, suggesting that these connectivity differences reflect neurological immaturity, rather than disrupted connectivity due to movement itself.
To determine whether the increased TN+-(anti)correlations found in the adult group were an artifact of global signal regression, the TN group analyses were performed without the use of global signal regression. For these analyses, the same set of regions had reduced correlations in the adults compared to the children. In addition, many of the TN-TP connections were still anti-correlated. Examination of the TN+-correlations found that these were on average anti-correlated in adults even without the use of global signal regression mean(SD) = −0.15(0.39) and these values were significantly less than zero (t(26) = 2.10, p = 0.046); while in children the mean(SD) = 0.40(0.35). Examination of the individual regions revealed that the right anterior insula/IFG, bilateral inferior parietal lobule, and a portion of the right superior parietal lobule had connections that were negatively correlated with the original TN seeds in adults while the other regions showing reduced connectivity with the TN seeds in adults were on average, positively correlated.
4. Discussion
In the current study, we identified developmental differences in resting state connectivity that occur both within and between task positive (TP) and task negative (TN) networks. Whole brain connectivity mapping using three separate TP and TN seeds revealed that, in comparison to school-aged children (8–12 years), adults (20–47 years) showed discrete changes in connectivity that occurred between circumscribed regions rather than general increases or decreases in connectivity across the networks. These changes, therefore, are likely to support particular cognitive control functions. Follow-up brain-behavior analyses found a relationship between response inhibition performance and the strength of (anti)correlation between those regions that showed developmental differences in the TN maps. Therefore, the maturation of these antagonistic connections appears important to achieving mature inhibitory control. Overall, the developmental changes identified in the current study are likely to contribute to improvements in task maintenance, cognitive control, social cognition and self-regulation that occur between late childhood and adulthood.
4.1 Developmental Changes in Connections Within the TP Network
Adults showed increased connectivity of the left DLPFC (BA 46) within the TP network as compared with school-aged children. The conjunction analysis and examination of the individual TP seed maps revealed that increased connectivity with the left DLPFC was specific to the IPS seed region. These findings suggest that TP development occurs within fronto-parietal regions. The DLPFC forms part of the TP network at rest, and more specifically has been implicated in a fronto-parietal network (Power, et al., 2011; Vincent, Kahn, Snyder, Raichle, & Buckner, 2008) critical to working memory and goal directed behavior. Animal studies have found that cells within this region maintain information across a delay, even in the presence of intervening, distracting stimuli (Miller, Erickson, & Desimone, 1996). In humans, the DLPFC, along with inferior parietal regions, has consistently been implicated in working memory tasks (Barch, et al., 1997; Braver, et al., 1997; Cohen, et al., 1997; Courtney, Ungerleider, Keil, & Haxby, 1997; D’Esposito, 2007). Although these regions are connected with the TP network at rest, they may change affiliation depending on the nature of the task. During visuospatial planning, the fronto-parietal network is activated along with the dorsal attention network, but during autobiographical planning tasks, it is activated along with default mode network regions (Spreng, Stevens, Chamberlain, Gilmore, & Schacter, 2010). The current findings suggest that the increased connectivity of the DLPFC with the dorsal attention seeds may promote better goal-directed attention in adults.
Abnormal maturation of DLPFC connections during late childhood and adolescence may contribute to impaired development of control processes and may also play a role in the pathophysiology of neuropsychiatric disorders with onset around this developmental period. In schizophrenic patients, the DLPFC shows atypical resting state connectivity with a number of task positive regions and abnormalities are already present at first episode in young adults with the disorder (Zhou, Liang, Jiang, et al., 2007; Zhou, Liang, Tian, et al., 2007). Given that onset often occurs at late adolescence or early adulthood, disrupted development of DLPFC-TP connections during adolescence may play a role in occurrence of the disorder.
4.2 Developmental Changes in Connections Within the TN Network
Adults showed increased connectivity of the right parahippocampal gyrus and a broad area of the MPFC within the TN network as compared to children. These regions make up part of the DMN and have been implicated in social cognition and self-reflective thought (Blakemore, 2008; Blakemore, et al., 2007; Santos, et al., 2010). In addition to social cognition, the parahippocampal gyrus has also been implicated in memory encoding and retrieval (Eichenbaum, Yonelinas, & Ranganath, 2007; Viskontas, Knowlton, Steinmetz, & Fried, 2006) and therefore may support improved memory function during development.
The MPFC is involved in self-regulation, mentalizing, and reward valuation (C. D. Frith & Frith, 1999; Van Overwalle & Baetens, 2009; Walter, Abler, Ciaramidaro, & Erk, 2005). This area is activated during a range of social cognition and mentalizing tasks (Amodio & Frith, 2006; Blakemore, 2008; Gilbert, et al., 2006). A number of studies show that the long-range connections between the posterior cingulate cortex (PCC) and MPFC develop over the course of adolescence and serve to integrate the anterior and posterior portions of the DMN (Supekar, et al., 2010; Uddin, et al., 2011). The current results corroborate these previous findings and confirm that the MPFC becomes more integrated with the DMN during adolescent and early adult development. The current study found that MPFC connections were increased with all TN seeds, suggesting that the development of these connections occurred across the DMN and was not specific to PCC-MPFC connections. Developmental differences occurred mainly within the dorsal MPFC, with some extension into the more ventral part of the MPFC, including orbitofrontal cortex, suggesting that they support improved social cognition and may also contribute to reduced impulsivity and risk-taking in adulthood.
Reduced connectivity between these two regions has also been implicated in neurodevelopmental disorders such as attention deficit hyperactivity disorder (ADHD) and autism (Castellanos, et al., 2008; Uddin, 2011; Uddin, et al., 2008). In ADHD, it has been proposed that pathology arises from delayed development of these systems. Fair et al. (2010) found reduced connectivity within the DMN in young adults with ADHD and the pattern of altered connections were consistent with that of younger children. In autism, it has been proposed that abnormal function within the MPFC contributes to impairments in social cognition (Uddin, 2011). Autism is characterized by abnormal social responsivity and introspective thought (U. Frith, 2001; Pelphrey & Carter, 2008). Atypical development of TN correlations may, therefore, contribute to impulsivity, social cognition and reward processing problems observed in these disorders.
4.3 Developmental Changes in (Anti)Correlations Between TP and TN Networks
A series of TP regions showed stronger anticorrelation in the TN maps of adults than those of children. These regions form distributed sections of the TP network and spatially correspond with several previously-identified sub-units within the TP network (Power, et al., 2011). The areas showing overlap with TP network subunits include: the right anterior insula and bilateral IPL/supramarginal gyrus with the cingulo-opercular network (CON), the bilateral SPL/precuneus with the dorsal attention network (DAN), and the right anterior insula/IFC and right IPL with the ventral attention network (VAN). This correspondence with previously-defined functional “sub-networks” suggests that the development of stronger anticorrelations between late childhood and early adulthood may be associated with the maturation of cognitive control functions related to these specific “sub-networks”.
Subunits of the TP network have been identified using both resting state functional connectivity and task-based methods (Dosenbach, Fair, Cohen, Schlaggar, & Petersen, 2008; Dosenbach, et al., 2007; Fair, et al., 2007; Fox, Corbetta, Snyder, Vincent, & Raichle, 2006; Fox, Snyder, Barch, Gusnard, & Raichle, 2005; Power, et al., 2011). The CON is a network that maintains activity over the course of task blocks for a range of tasks and these regions have been implicated in the instantiation and maintenance of task information (Dosenbach, et al., 2008; Dosenbach, et al., 2007; Fox, Snyder, Barch, et al., 2005). The current results suggest that the relationship between this set of regions and the DMN becomes more competitive in adulthood. This competition may contribute to more focused goal-directed behavior during attentionally-demanding tasks, and may reduce distraction from self-reflective thoughts.
Although the current set of regions showed correspondence with a number of CON regions, the right anterior insula/IFC region also overlapped with the ventral attention network (VAN). The peak coordinate of the right IFC seed identified as part of the VAN (Fox, et al., 2006) showed close correspondence with the region identified in the current study. The VAN has been identified across a number of task activation studies during the identification of task-relevant, infrequent stimuli and is thought to be involved in alerting attention during the detection of rare events (Corbetta, Patel, & Shulman, 2008; Corbetta & Shulman, 2002). This network includes both the right IFC and the right temporo-parietal junction (TPJ) and resting state studies have found that these regions also form a right-lateralized network at rest (Fox, et al., 2006; Power, et al., 2011). The current set of regions show strong overlap with the right anterior insula/IFC; however, they do not overlap with the peak TPJ coordinates previously identified as part of the VAN. Instead, a slightly more anterior region of the IPL is activated in the current study. Although this region does not overlap with the peak TPJ coordinate of the VAN, there may still be some overlap with the extent of TPJ connectivity during resting state as well as the extent of activation during visual attention studies. Some evidence that TPJ may be functionally-related to the anterior IPL comes from a study in which resting-state functional connectivity was used to functionally-parcellate the TPJ (Mars, et al., 2011). This study found that the anterior portion of the TPJ was strongly connected with both the anterior portion of the IPL as well as the anterior insula.
In addition to its role in detecting rare events, the right IFC has also been implicated in response inhibition and retrieval of task relevant information (Levy & Wagner, 2011; Petrides & Pandya, 2009). Both the right IFC and the right IPL identified in the current study overlap with regions recently identified during a response inhibition task. For this reason, brain-behavior relationships were examined to determine whether (anti)correlations between these regions and parts of the DMN were related to inhibitory control function. First, the relationship with inhibitory control performance was examined between the set of regions showing developmental changes within the TN network (i.e. the MPFC and parahippocampal gyrus, which had increased connectivity with TN regions in adults, and the entire set of regions that showed greater anticorrelation with TN regions in adults). Across both groups, inhibitory control performance was related to the strength of (anti)correlation between these two sets of regions, although this relationship was stronger within the adult group.
In addition to establishing that TP-TN (anti)correlations within developing regions are associated with inhibitory control function, we were also interested in determining whether (anti)correlation across a set of regions, which are commonly implicated in inhibitory control, is likewise important for inhibitory control function. These regions included pre-SMA, right anterior insula/IFC and right anterior IPL, which are involved in basic response inhibition, as well as the right DLPFC, which is recruited during conditions when working memory is necessary to guide response inhibition (Mostofsky, et al., 2003; Simmonds, et al., 2008). To determine whether (anti)correlations in this set of regions were consistently related to response inhibition, MANCOVA analyses were performed to relate the strength of (anti)correlation between these regions with inhibitory control behavior. The current findings show that connectivity between this set of regions and both the anterior and posterior nodes of the DMN are related to inhibitory control performance on two Go/No-go tasks. Further, connectivity with the right DLPFC was specifically related to response inhibition on the Complex version of the Go/No-go task, which recruited working memory in addition to inhibitory control functions.
The current results suggest that TP-TN (anti)correlations among these regions are important for inhibitory control. In a previous study, it was found that anticorrelation between the TP and TN networks was not uniform across all regions, and was stronger between particular TP and TN regions (Anderson, et al., 2011). The authors noted that the anterior insula was one region that exhibited some of the strongest anti-correlations with both the anterior and posterior nodes of the DMN. Given that this region also had displayed robust developmental changes in the current study, it may be that the establishment of anti-correlation between this region and the DMN is particularly important for mature cognitive control.
Task activation and resting state connectivity studies have found altered TP-TN connectivity in a number of studies including patient populations. There is less suppression of the DMN during task performance and less TP-TN anticorrelation during rest in autistic patients (Kennedy & Courchesne, 2008; Kennedy, Redcay, & Courchesne, 2006). Castellanos et al. (2008) found that adults with ADHD have reduced anticorrelation between TP and TN regions. In addition, reduced deactivation of DMN regions has been found during inhibitory control in children with ADHD (Liddle, et al., 2011). Response inhibition problems are a common finding in ADHD patients (Suskauer, et al., 2008) and may be related to the altered development of these TP-TN connections.
4.4 Potential Limitations
Recent examination has found that participant motion over the course of the scan session produces non-uniform changes in resting state correlations across the brain (Power, et al., 2012; Power, et al., 2011; Satterthwaite, et al., 2012; Van Dijk, et al., 2012). These changes may reduce precision in localizing network boundaries (Power, et al., 2011), and may also lead to spurious group differences between clinical or developmental populations with differing amounts of motion (Power, et al., 2012; Satterthwaite, et al., 2012; Van Dijk, et al., 2012).
In the current study, four motion scores (mean and max absolute motion and mean and max differential motion) were significantly greater in the children than in the adults. Therefore, group differences in motion may have contributed to the observed developmental differences in connectivity. Although motion may have influenced the current connectivity differences between the two groups, there is evidence that these differences are due to actual changes in functional coupling rather than just motion-induced artifact. First, developmental increases in long-range connectivity between the MPFC and PCC have been replicated in a number of studies and these developmental changes coincide with developmental increases in white matter tracts between these two regions (Supekar, et al., 2010; Uddin, et al., 2011). In addition, Van Dijk et al. (2012) found that the strength of connectivity between these two DMN regions is related to age, even after accounting for subject motion. Second, for all pairwise connections that were examined in the current study, there were no significant relationships between strength of connectivity and participant motion. Third, previous studies found that greater movement may result in stronger short-range connectivity and disrupted long-range connectivity, thereby obscuring true developmental changes (Satterthwaite, et al., 2012). The conjunction analyses performed in the current study show that there is strong overlap in the group differences found for each of the individual TN seed maps, and therefore these connectivity differences are not due to connection distance. Third, the separate examination of developmental group differences with a low-movement group of children and a high-movement group of children found good correspondence in the regions identified, suggesting that group differences are not merely an artifact of motion.
The brain-behavior associations in the current study may be limited by the large group differences in the mean and variability of the behavioral measures. However, there are several reasons to believe that these associations are valid. First, ANCoVA models, which included a group effect, were used. This allowed for testing of brain-behavior relationships that were not just driven by group differences in performance. Second, these relationships were examined in the two groups separately and a consistent brain-behavior relationship was found in the adult group between commission error rate and connectivity between the two sets of developing regions. This establishes that connectivity in these regions is related to response inhibition abilities in adults. Third, there is close anatomical correspondence between the regions showing greater anticorrelation with the TN network in adults and regions that are active during response inhibition tasks. Fourth, the brain-behavior relationships were specifically associated with commission error rate and did not generalize to intra-subject variability, which also showed group differences.
Another potential limitation for the current study is the interpretation of anticorrelations at rest. While TP and TN networks might have an inherently antagonistic relationship; it may also be the case that the use of global signal regression artificially causes this opposing relationship. Murphy et al. (2009) found that examination of the full-brain connectivity with a PCC seed shifts the correlation distribution so that the correlations are no longer mostly positive and are instead centered around zero. Although this commonly used method does cause a shift in the distribution of correlations, there are several reasons to believe that TP-TN anticorrelations are biologically plausible. First, the TP and TN seeds selected by Fox and colleagues (2005) were chosen based on their activity patterns during task fMRI. These two sets of regions show opposing activity during task (i.e. task positive regions were activated by attentionally demanding and working memory tasks while task negative regions were deactivated by the same tasks). This opposing task-related activity was found in the absence of any global signal regression, suggesting that it is behaviorally relevant. In addition, this activity has been found to be parametrically modulated based on task demands (i.e. TP regions were more active during more demanding conditions and TN regions were more deactive during demanding conditions). Fox et al. (2005) seeded these task-derived regions in resting state (using global signal regression) and found that these regions also form anti-correlated networks at rest. In the current study, the sets of regions that were found to become more anti-correlated in adulthood, were also related to response inhibition abilities, suggesting that these anti-correlations are behaviorally-relevant and not just artifactual. In addition, a previous study (Fox, Zhang, Snyder, & Raichle, 2009) specifically examined the effect of global signal regression on the anticorrelations between TP and TN regions and found that it is unlikely that the relationship is artifactual. In the present study, follow-up analyses showed that the TN+-correlations were reduced in adults even when the analyses were re-run without global signal regression. These findings confirm that the developmental differences were not the result of global signal regression and that many of these between-network connections were still anticorrelated in adults even without global signal regression.
4.5 Future Directions
As hypothesized, we found developmental changes in specific resting state connections within and between TP and TN networks. Using a cross sectional approach that included separate child and adult cohorts, we identified consistent changes in connection strength. The current study identified a number of developmental changes in connections that have previously been implicated in psychopathology. Further examination of brain-behavior relationships may help to establish whether the atypical development of these connections is related to behavioral deficits, such as inhibitory control in ADHD and social cognition in autism. Better understanding of the developmental trajectories and behavioral outcomes that arise from abnormal development of connectivity may lead to more targeted interventions for these disorders.
The current cross sectional approach does limit the interpretability of the findings. Future research on network development may include intermediate age ranges to characterize non-linear connectivity changes that occur during adolescence. Studies using age as a continuous variable or a longitudinal design, will provide a better characterization of the trajectory of development. This type of approach may be useful in answering a number of questions such as whether early maturation may account for improved behavioral performance in children, whether early maturation results in better performance in adulthood, and whether there are certain developmental windows that are important for typical development of specific behavioral abilities.
Table 1a.
Regions that showed stronger positive correlations in the TN maps (i.e. within network connectivity) of Adults than Children. Comparison of 30 children with the highest movement versus adults on the left. Comparison of 30 children with the lowest movement versus adults on the right.
| Adults - High Movement Children | Adults - Low Movement Children | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||||
| region | side | BA | significance | size | peak Z | x | y | z | region | side | BA | significance | size | peak Z | x | y | z |
| Medial Prefrontal Cortex/Superior Frontal Gyrus/Anterior Cingulate | B | 10/8/9/32/11 | 0.000 | 3722 | 5.57 | −16 | 42 | 50 | Medial Prefrontal Cortex | B | 9/10 | 0.001 | 184 | 4.77 | 8 | 50 | 28 |
| 5.35 | 24 | 32 | 52 | 3.68 | 2 | 52 | 42 | ||||||||||
| 5.35 | 4 | 56 | 38 | Superior Frontal Gyrus | L | 10 | 0.014 | 121 | 4.59 | −24 | 60 | 12 | |||||
|
| |||||||||||||||||
| Middle Temporal Gyrus/Inferior Temporal Gyrus/Middle Temporal Pole | L | 21/20/38 | 0.000 | 322 | 4.95 | −56 | −2 | −26 | |||||||||
| 3.93 | −44 | 6 | −34 | ||||||||||||||
| 3.8 | −50 | 10 | −26 | ||||||||||||||
|
|
|||||||||||||||||
| Inferior Temporal Gyrus/Middle Temporal Gyrus | R | 21/20 | 0.000 | 358 | 4.78 | 52 | −10 | −20 | |||||||||
| 4.57 | 60 | −14 | −24 | ||||||||||||||
| 3.89 | 44 | −16 | −30 | ||||||||||||||
|
|
|||||||||||||||||
| Parahippocampal Gyrus | R | 35/28 | 0.034 | 107 | 4.37 | 22 | −24 | −22 | |||||||||
| 3.63 | 18 | −8 | −24 | ||||||||||||||
| Hippocampus/Parahippocampal Gyrus | L | 28/34 | 0.005 | 155 | 3.92 | −26 | −18 | −22 | |||||||||
| 3.86 | −28 | −24 | −16 | ||||||||||||||
| 3.69 | −16 | −14 | −20 | ||||||||||||||
|
|
|||||||||||||||||
| PCC/Precuneus/Lingual Gyrus/Mid Cingulate | B | 31/30/23/29 | 0.000 | 461 | 4.24 | 10 | −52 | 6 | |||||||||
| 3.74 | −4 | −62 | 24 | ||||||||||||||
| 3.72 | −6 | −46 | 34 | ||||||||||||||
Table 1b.
Regions that showed stronger anticorrelations in the TN maps of Adults than Children. Comparison of 30 children with the highest movement versus adults on the left. Comparison of 30 children with the lowest movement versus adults on the right.
| High Movement Children - Adults | Low Movement Children – Adults | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||||
| region | side | BA | significance | size | peak Z | x | y | z | region | side | BA | significance | size | peak Z | x | y | z |
| Inferior Frontal Operculum/Anterior Insula/Inferior Frontal Rolandic/Precentral Gyrus/Temporal Pole/Inferior Frontal Trigeminal | R | 13/44/22/9/47/45 | 0.000 | 1434 | 5.84 | 38 | 6 | 4 | |||||||||
| 4.8 | 54 | 10 | 20 | ||||||||||||||
| 4.69 | 40 | 16 | 2 | ||||||||||||||
| Anterior Insula/Inferior Frontal Rolandic/Inferior Frontal Operculum/Superior Temporal | L | 22/13/6/44 | 0.000 | 451 | 4.91 | −56 | 2 | 6 | |||||||||
| 4.26 | −46 | 0 | 6 | ||||||||||||||
| 4.11 | −28 | 2 | 6 | ||||||||||||||
|
| |||||||||||||||||
| Supramarginal Gyrus/Inferior Parietal/Superior Temporal/Superior Parietal/Postcentral/Precuneus | R | 40/7/2/3/22/42/5 | 0.000 | 2172 | 5.71 | 62 | −32 | 38 | Inferior Parietal Cortex | R | 40 | 0.019 | 115 | 4.28 | 30 | −42 | 40 |
| 5.41 | 64 | −24 | 32 | 3.75 | 36 | −44 | 48 | ||||||||||
| 4.95 | 62 | −38 | 14 | Superior Parietal/Postcentral Gyrus/Precuneus | R | 7/5 | 0.010 | 129 | 4.24 | 16 | −54 | 68 | |||||
| 4.03 | 24 | −50 | 66 | ||||||||||||||
| 3.85 | 34 | −52 | 62 | ||||||||||||||
| Supramarginal Gyrus/Inferior Parietal/Superior Temporal/Postcentral Gyrus | L | 40/2/42/1/3/22 | 0.000 | 1498 | 5.28 | −62 | −28 | 24 | |||||||||
| 4.74 | −42 | −46 | 54 | ||||||||||||||
| 4.58 | −64 | −32 | 36 | ||||||||||||||
|
| |||||||||||||||||
| Anterior Cingulate Cortex/Supplementary Motor Area | B | 24/32 | 0.000 | 288 | 4.83 | 10 | 8 | 38 | |||||||||
| 4.23 | −6 | −4 | 42 | ||||||||||||||
| 4.06 | 6 | 2 | 44 | ||||||||||||||
| Superior Frontal Gyrus/pre-SMA | R | 6 | 0.004 | 165 | 4.29 | 20 | −8 | 70 | |||||||||
| 3.91 | 14 | −16 | 64 | ||||||||||||||
| 3.3 | 28 | −6 | 64 | ||||||||||||||
|
| |||||||||||||||||
| Precuneus/Superior Parietal/Mid Cingulate Gyrus | L | 7/31/5/40 | 0.000 | 519 | 4.73 | −12 | −48 | 58 | |||||||||
| 4.37 | −22 | −48 | 58 | ||||||||||||||
| 4.36 | −14 | −56 | 62 | ||||||||||||||
| Cuneus/Precuneus | R | 7/19 | 0.001 | 215 | 4.36 | 14 | −80 | 38 | Precuneus/Cuneus | R | 7/19 | 0.002 | 168 | 3.94 | 10 | −74 | 40 |
| 3.4 | 12 | −66 | 44 | 0.013 | 123 | 3.94 | 62 | −32 | 36 | ||||||||
| 3.35 | 52 | −22 | 38 | ||||||||||||||
| Mid Cingulate Cortex/Paracentral Lobule | R | 31/5 | 0.005 | 159 | 4.52 | 12 | −32 | 42 | Mid Cingulate Cortex | R | 31 | 0.014 | 121 | 4.51 | 16 | −34 | 42 |
| 4.08 | 10 | −28 | 38 | ||||||||||||||
|
| |||||||||||||||||
| Inferior Frontal Trigeminal/Middle Frontal Gyrus | R | 46/10 | 0.000 | 270 | 4.69 | 46 | 38 | 10 | |||||||||
| 3.8 | 38 | 48 | 24 | ||||||||||||||
| Inferior Frontal Trigeminal/Middle Frontal Gyrus | L | 46/10 | 0.000 | 318 | 4.42 | −48 | 36 | 16 | |||||||||
| 4.4 | −34 | 42 | 28 | ||||||||||||||
| 4.19 | −44 | 42 | 24 | ||||||||||||||
Highlights.
Network development leads to specific changes between children and adults
Adults have stronger within-network connectivity to circumscribed regions
Adults have more anticorrelated between-network connectivity to circumscribed regions
Inhibitory control is related to the strength of anticorrelation in developing areas
Acknowledgments
The Autism Speaks Foundation and NIH (R01 NS048527, R01MH078160 and R01MH085328), Johns Hopkins General Clinical Research Center (M01 RR00052), the National Center for Resource (P41 RR15241), and the Intellectual and Developmental Disabilities Research Center (HD-24061).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Anderson JS, Ferguson MA, Lopez-Larson M, Yurgelun-Todd D. Connectivity Gradients Between the Default Mode and Attention Control Networks. Brain Connect. 2011;1:147–157. doi: 10.1089/brain.2011.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Braver TS, Nystrom LE, Forman SD, Noll DC, Cohen JD. Dissociating working memory from task difficulty in human prefrontal cortex. Neuropsychologia. 1997;35:1373–1380. doi: 10.1016/s0028-3932(97)00072-9. [DOI] [PubMed] [Google Scholar]
- Bedard AC, Nichols S, Barbosa JA, Schachar R, Logan GD, Tannock R. The development of selective inhibitory control across the life span. Dev Neuropsychol. 2002;21:93–111. doi: 10.1207/S15326942DN2101_5. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ. The social brain in adolescence. Nat Rev Neurosci. 2008;9:267–277. doi: 10.1038/nrn2353. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, den Ouden H, Choudhury S, Frith C. Adolescent development of the neural circuitry for thinking about intentions. Soc Cogn Affect Neurosci. 2007;2:130–139. doi: 10.1093/scan/nsm009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE, Noll DC. A parametric study of prefrontal cortex involvement in human working memory. Neuroimage. 1997;5:49–62. doi: 10.1006/nimg.1996.0247. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Getz S, Galvan A. The adolescent brain. Dev Rev. 2008;28:62–77. doi: 10.1016/j.dr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Jones RM, Hare TA. The adolescent brain. Ann N Y Acad Sci. 2008;1124:111–126. doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos FX, Margulies DS, Kelly C, Uddin LQ, Ghaffari M, Kirsch A, Shaw D, Shehzad Z, Di Martino A, Biswal B, Sonuga-Barke EJ, Rotrosen J, Adler LA, Milham MP. Cingulate-precuneus interactions: a new locus of dysfunction in adult attention-deficit/hyperactivity disorder. Biol Psychiatry. 2008;63:332–337. doi: 10.1016/j.biopsych.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JD, Perlstein WM, Braver TS, Nystrom LE, Noll DC, Jonides J, Smith EE. Temporal dynamics of brain activation during a working memory task. Nature. 1997;386:604–608. doi: 10.1038/386604a0. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Courtney SM, Ungerleider LG, Keil K, Haxby JV. Transient and sustained activity in a distributed neural system for human working memory. Nature. 1997;386:608–611. doi: 10.1038/386608a0. [DOI] [PubMed] [Google Scholar]
- Crone EA, Donohue SE, Honomichl R, Wendelken C, Bunge SA. Brain regions mediating flexible rule use during development. J Neurosci. 2006;26:11239–11247. doi: 10.1523/JNEUROSCI.2165-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone EA, Wendelken C, Donohue S, van Leijenhorst L, Bunge SA. Neurocognitive development of the ability to manipulate information in working memory. Proc Natl Acad Sci U S A. 2006;103:9315–9320. doi: 10.1073/pnas.0510088103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Esposito M. From cognitive to neural models of working memory. Philos Trans R Soc Lond B Biol Sci. 2007;362:761–772. doi: 10.1098/rstb.2007.2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. Trends Cogn Sci. 2008;12:99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, Fox MD, Snyder AZ, Vincent JL, Raichle ME, Schlaggar BL, Petersen SE. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci U S A. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annu Rev Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Dosenbach NU, Church JA, Miezin FM, Barch DM, Raichle ME, Petersen SE, Schlaggar BL. The maturing architecture of the brain’s default network. Proc Natl Acad Sci U S A. 2008;105:4028–4032. doi: 10.1073/pnas.0800376105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Power JD, Dosenbach NU, Church JA, Miezin FM, Schlaggar BL, Petersen SE. Functional brain networks develop from a “local to distributed” organization. PLoS Comput Biol. 2009;5:e1000381. doi: 10.1371/journal.pcbi.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Dosenbach NU, Church JA, Cohen AL, Brahmbhatt S, Miezin FM, Barch DM, Raichle ME, Petersen SE, Schlaggar BL. Development of distinct control networks through segregation and integration. Proc Natl Acad Sci U S A. 2007;104:13507–13512. doi: 10.1073/pnas.0705843104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Posner J, Nagel BJ, Bathula D, Dias TG, Mills KL, Blythe MS, Giwa A, Schmitt CF, Nigg JT. Atypical default network connectivity in youth with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2010;68:1084–1091. doi: 10.1016/j.biopsych.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci U S A. 2006;103:10046–10051. doi: 10.1073/pnas.0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Barch DM, Gusnard DA, Raichle ME. Transient BOLD responses at block transitions. Neuroimage. 2005;28:956–966. doi: 10.1016/j.neuroimage.2005.06.025. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Zhang D, Snyder AZ, Raichle ME. The global signal and observed anticorrelated resting state brain networks. J Neurophysiol. 2009;101:3270–3283. doi: 10.1152/jn.90777.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P, Aden U, Blennow M, Lagercrantz H. The functional architecture of the infant brain as revealed by resting-state fMRI. Cereb Cortex. 2011;21:145–154. doi: 10.1093/cercor/bhq071. [DOI] [PubMed] [Google Scholar]
- Fransson P, Skiold B, Engstrom M, Hallberg B, Mosskin M, Aden U, Lagercrantz H, Blennow M. Spontaneous brain activity in the newborn brain during natural sleep--an fMRI study in infants born at full term. Pediatr Res. 2009;66:301–305. doi: 10.1203/PDR.0b013e3181b1bd84. [DOI] [PubMed] [Google Scholar]
- Fransson P, Skiold B, Horsch S, Nordell A, Blennow M, Lagercrantz H, Aden U. Resting-state networks in the infant brain. Proc Natl Acad Sci U S A. 2007;104:15531–15536. doi: 10.1073/pnas.0704380104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith CD, Frith U. Interacting minds--a biological basis. Science. 1999;286:1692–1695. doi: 10.1126/science.286.5445.1692. [DOI] [PubMed] [Google Scholar]
- Frith U. Mind blindness and the brain in autism. Neuron. 2001;32:969–979. doi: 10.1016/s0896-6273(01)00552-9. [DOI] [PubMed] [Google Scholar]
- Geier CF, Garver K, Terwilliger R, Luna B. Development of working memory maintenance. J Neurophysiol. 2009;101:84–99. doi: 10.1152/jn.90562.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier CF, Terwilliger R, Teslovich T, Velanova K, Luna B. Immaturities in reward processing and its influence on inhibitory control in adolescence. Cereb Cortex. 2010;20:1613–1629. doi: 10.1093/cercor/bhp225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert SJ, Spengler S, Simons JS, Steele JD, Lawrie SM, Frith CD, Burgess PW. Functional specialization within rostral prefrontal cortex (area 10): a meta-analysis. J Cogn Neurosci. 2006;18:932–948. doi: 10.1162/jocn.2006.18.6.932. [DOI] [PubMed] [Google Scholar]
- Hampson M, Driesen NR, Skudlarski P, Gore JC, Constable RT. Brain connectivity related to working memory performance. J Neurosci. 2006;26:13338–13343. doi: 10.1523/JNEUROSCI.3408-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazbec S, Hardin MG, Schroth E, McClure E, Pine DS, Ernst M. Age-related influence of contingencies on a saccade task. Exp Brain Res. 2006;174:754–762. doi: 10.1007/s00221-006-0520-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AM, Di Martino A, Uddin LQ, Shehzad Z, Gee DG, Reiss PT, Margulies DS, Castellanos FX, Milham MP. Development of anterior cingulate functional connectivity from late childhood to early adulthood. Cereb Cortex. 2009;19:640–657. doi: 10.1093/cercor/bhn117. [DOI] [PubMed] [Google Scholar]
- Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Competition between functional brain networks mediates behavioral variability. Neuroimage. 2008;39:527–537. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Kennedy DP, Courchesne E. Functional abnormalities of the default network during self- and other-reflection in autism. Soc Cogn Affect Neurosci. 2008;3:177–190. doi: 10.1093/scan/nsn011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy DP, Redcay E, Courchesne E. Failing to deactivate: resting functional abnormalities in autism. Proc Natl Acad Sci U S A. 2006;103:8275–8280. doi: 10.1073/pnas.0600674103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiebel SJ, Poline JB, Friston KJ, Holmes AP, Worsley KJ. Robust smoothness estimation in statistical parametric maps using standardized residuals from the general linear model. Neuroimage. 1999;10:756–766. doi: 10.1006/nimg.1999.0508. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Tordesillas-Gutierrez D, Martinez M, Salinas F, Evans A, Zilles K, Mazziotta JC, Fox PT. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Hum Brain Mapp. 2007;28:1194–1205. doi: 10.1002/hbm.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy BJ, Wagner AD. Cognitive control and right ventrolateral prefrontal cortex: reflexive reorienting, motor inhibition, and action updating. Ann N Y Acad Sci. 2011;1224:40–62. doi: 10.1111/j.1749-6632.2011.05958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddle EB, Hollis C, Batty MJ, Groom MJ, Totman JJ, Liotti M, Scerif G, Liddle PF. Task-related default mode network modulation and inhibitory control in ADHD: effects of motivation and methylphenidate. J Child Psychol Psychiatry. 2011;52:761–771. doi: 10.1111/j.1469-7610.2010.02333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mars RB, Sallet J, Schuffelgen U, Jbabdi S, Toni I, Rushworth MF. Connectivity-Based Subdivisions of the Human Right “Temporoparietal Junction Area”: Evidence for Different Areas Participating in Different Cortical Networks. Cereb Cortex. 2011 doi: 10.1093/cercor/bhr268. [DOI] [PubMed] [Google Scholar]
- Mennes M, Kelly C, Zuo XN, Di Martino A, Biswal BB, Castellanos FX, Milham MP. Inter-individual differences in resting-state functional connectivity predict task-induced BOLD activity. Neuroimage. 2010;50:1690–1701. doi: 10.1016/j.neuroimage.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennes M, Zuo XN, Kelly C, Di Martino A, Zang YF, Biswal B, Castellanos FX, Milham MP. Linking inter-individual differences in neural activation and behavior to intrinsic brain dynamics. Neuroimage. 2011;54:2950–2959. doi: 10.1016/j.neuroimage.2010.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Erickson CA, Desimone R. Neural mechanisms of visual working memory in prefrontal cortex of the macaque. J Neurosci. 1996;16:5154–5167. doi: 10.1523/JNEUROSCI.16-16-05154.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostofsky SH, Schafer JG, Abrams MT, Goldberg MC, Flower AA, Boyce A, Courtney SM, Calhoun VD, Kraut MA, Denckla MB, Pekar JJ. fMRI evidence that the neural basis of response inhibition is task-dependent. Brain Res Cogn Brain Res. 2003;17:419–430. doi: 10.1016/s0926-6410(03)00144-7. [DOI] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage. 2009;44:893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelphrey KA, Carter EJ. Brain mechanisms for social perception: lessons from autism and typical development. Ann N Y Acad Sci. 2008;1145:283–299. doi: 10.1196/annals.1416.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Distinct parietal and temporal pathways to the homologues of Broca’s area in the monkey. PLoS Biol. 2009;7:e1000170. doi: 10.1371/journal.pbio.1000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, Vogel AC, Laumann TO, Miezin FM, Schlaggar BL, Petersen SE. Functional network organization of the human brain. Neuron. 2011;72:665–678. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueda MR, Fan J, McCandliss BD, Halparin JD, Gruber DB, Lercari LP, Posner MI. Development of attentional networks in childhood. Neuropsychologia. 2004;42:1029–1040. doi: 10.1016/j.neuropsychologia.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Sala R, Granero R, Ezpeleta L. Dimensional analysis of a categorical diagnostic interview: the DICA-IV. Psicothema. 2006;18:123–129. [PubMed] [Google Scholar]
- Santos NS, Kuzmanovic B, David N, Rotarska-Jagiela A, Eickhoff SB, Shah JN, Fink GR, Bente G, Vogeley K. Animated brain: a functional neuroimaging study on animacy experience. Neuroimage. 2010;53:291–302. doi: 10.1016/j.neuroimage.2010.05.080. [DOI] [PubMed] [Google Scholar]
- Satterthwaite TD, Wolf DH, Loughead J, Ruparel K, Elliott MA, Hakonarson H, Gur RC, Gur RE. Impact of in-scanner head motion on multiple measures of functional connectivity: relevance for studies of neurodevelopment in youth. Neuroimage. 2012;60:623–632. doi: 10.1016/j.neuroimage.2011.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds DJ, Pekar JJ, Mostofsky SH. Meta-analysis of Go/No-go tasks demonstrating that fMRI activation associated with response inhibition is task-dependent. Neuropsychologia. 2008;46:224–232. doi: 10.1016/j.neuropsychologia.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Hare T, Casey BJ. Frontostriatal maturation predicts cognitive control failure to appetitive cues in adolescents. J Cogn Neurosci. 2011;23:2123–2134. doi: 10.1162/jocn.2010.21572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Stevens WD, Chamberlain JP, Gilmore AW, Schacter DL. Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. Neuroimage. 2010;53:303–317. doi: 10.1016/j.neuroimage.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekar K, Uddin LQ, Prater K, Amin H, Greicius MD, Menon V. Development of functional and structural connectivity within the default mode network in young children. Neuroimage. 2010;52:290–301. doi: 10.1016/j.neuroimage.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suskauer SJ, Simmonds DJ, Fotedar S, Blankner JG, Pekar JJ, Denckla MB, Mostofsky SH. Functional magnetic resonance imaging evidence for abnormalities in response selection in attention deficit hyperactivity disorder: differences in activation associated with response inhibition but not habitual motor response. J Cogn Neurosci. 2008;20:478–493. doi: 10.1162/jocn.2008.20032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ. The self in autism: an emerging view from neuroimaging. Neurocase. 2011;17:201–208. doi: 10.1080/13554794.2010.509320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Kelly AM, Biswal BB, Margulies DS, Shehzad Z, Shaw D, Ghaffari M, Rotrosen J, Adler LA, Castellanos FX, Milham MP. Network homogeneity reveals decreased integrity of default-mode network in ADHD. J Neurosci Methods. 2008;169:249–254. doi: 10.1016/j.jneumeth.2007.11.031. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Supekar KS, Ryali S, Menon V. Dynamic reconfiguration of structural and functional connectivity across core neurocognitive brain networks with development. J Neurosci. 2011;31:18578–18589. doi: 10.1523/JNEUROSCI.4465-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk KR, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59:431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Overwalle F, Baetens K. Understanding others’ actions and goals by mirror and mentalizing systems: a meta-analysis. Neuroimage. 2009;48:564–584. doi: 10.1016/j.neuroimage.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol. 2008;100:3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viskontas IV, Knowlton BJ, Steinmetz PN, Fried I. Differences in mnemonic processing by neurons in the human hippocampus and parahippocampal regions. J Cogn Neurosci. 2006;18:1654–1662. doi: 10.1162/jocn.2006.18.10.1654. [DOI] [PubMed] [Google Scholar]
- Vogel AC, Power JD, Petersen SE, Schlaggar BL. Development of the brain’s functional network architecture. Neuropsychol Rev. 2010;20:362–375. doi: 10.1007/s11065-010-9145-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter H, Abler B, Ciaramidaro A, Erk S. Motivating forces of human actions. Neuroimaging reward and social interaction. Brain Res Bull. 2005;67:368–381. doi: 10.1016/j.brainresbull.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Williams BR, Ponesse JS, Schachar RJ, Logan GD, Tannock R. Development of inhibitory control across the life span. Dev Psychol. 1999;35:205–213. doi: 10.1037//0012-1649.35.1.205. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Liang M, Jiang T, Tian L, Liu Y, Liu Z, Liu H, Kuang F. Functional dysconnectivity of the dorsolateral prefrontal cortex in first-episode schizophrenia using resting-state fMRI. Neurosci Lett. 2007;417:297–302. doi: 10.1016/j.neulet.2007.02.081. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Liang M, Tian L, Wang K, Hao Y, Liu H, Liu Z, Jiang T. Functional disintegration in paranoid schizophrenia using resting-state fMRI. Schizophr Res. 2007;97:194–205. doi: 10.1016/j.schres.2007.05.029. [DOI] [PubMed] [Google Scholar]