Abstract
Oxytocin (OT) is a neurohypophyseal peptide traditionally associated with female reproductive functioning, and more recently with prosocial behavior. OT and its receptor are also expressed in the heart and vascular tissue and play a role in cardiovascular homeostasis. In vitro, it has been demonstrated that OT decreases NADPH-dependent superoxide production and pro-inflammatory cytokine release from vascular endothelial cells and macrophages, suggesting that OT may attenuate pathophysiological processes involved with atherosclerotic lesion formation. The present study sought to determine the effect of chronic exogenous OT administration on inflammation and atherosclerosis in an animal model of dyslipidemia and atherosclerosis, the Watanabe Heritable Hyperlipidemic (WHHL) rabbit. Twenty-two, 3-month-old WHHLs were surgically implanted with osmotic mini-pumps containing OT (n=11) or vehicle (n=11), and then were individually housed for the entire study. Blood and 24-hour urine samples were taken at baseline and after 8 (midpoint) and 16 (endpoint) weeks of treatment. At endpoint, the aortas and visceral fat samples were dissected and stored for analyses. There were no group differences in body weight, serum lipids, plasma/urinary measures of oxidative stress, plasma cortisol or urinary catecholamines over the 16-week treatment. OT-treated animals exhibited significantly lower plasma C-reactive protein levels at midpoint and endpoint and developed significantly less atherosclerosis in the thoracic aorta relative to vehicle control animals at endpoint (p<0.05). Cytokine gene expression from visceral adipose tissue samples suggested that there was a decrease in adipose tissue inflammation in the OT-treated group compared to the vehicle control group, however these differences were not statistically significant. These results suggest that chronic peripheral OT administration can inhibit inflammation and atherosclerotic lesion development.
Keywords: Oxytocin, atherosclerosis, inflammation, WHHL rabbit
Introduction
It is well established that the neuropeptide, oxytocin (OT), acts centrally to facilitate a variety of prosocial behaviors (Carter et al., 2008; Ross and Young, 2009). In addition, affiliative social behaviors and warm contact stimuli are associated with elevations in plasma OT (Grewen et al., 2005; Light et al., 2005; Holt-Lunstad et al., 2008). It has been suggested that OT may serve as an impetus for the organism to seek out social support and prosocial behavior (Taylor et al., 2006; Taylor et al., 2010). In a separate line of research, it has also been demonstrated that OT works centrally to attenuate peripheral stress responses (Petersson et al., 1999; Neumann et al., 2000; Petersson et al., 2005). These observations are also consistent with the notion that OT may play a role in the beneficial effects of affiliative social interactions on stressful behavior and stress-related disease (Knox and Uvnas-Moberg, 1998; Uvnäs-Moberg, 1998; Smith and Wang, 2012).
In addition to its CNS effects, OT and its receptor (OTR) are expressed in a variety of peripheral tissues, including the heart and blood vessels (Jankowski et al., 2000; Gimpl and Fahrenholz, 2001; Kiss and Mikkelsen, 2005). It has been suggested that this cardiovascular OT system is involved in the maintenance of normal homeostatic functions (Gutkowska et al., 1997; Jankowski et al., 1998; Jankowski et al., 2000), however, it has also been proposed that OT may work directly on vascular cells to slow the progression of pathophysiological processes involved in disease (Paredes et al., 2006; Szeto et al., 2008; Nation et al., 2010). In our laboratory, we demonstrated that OT may also have antiatherogenic properties by inhibiting oxidative stress and inflammation in cultured vascular endothelial and smooth muscle cells, monocytes, and macrophages (Szeto et al., 2008). In vivo, chronic OT infusions in an animal model of hyperlipidemia and atherosclerosis, the ApoE −/− knockout mouse, attenuated aortic atherosclerosis in a site-specific manner and inhibited the ex vivo secretion of IL-6 from visceral adipose tissue (Nation et al., 2010).
A major pathophysiological process underlying atherosclerosis and coronary artery disease (CAD) is chronic inflammation ((Ross, 1999; Mullenix et al., 2005). Systemic and local inflammatory events, driven largely by pro-inflammatory cytokines and oxidative stress, mediate all phases of atherogenesis. The pro-inflammatory cytokines involved in the development and progression of atherosclerosis include interleukin (IL)-1β, tumor necrosis factor (TNF)-α, and IL-6 among others (Ait-Oufella et al., 2011). Of notable importance in CAD is IL-6, which has been shown to up regulate the production of C-reactive protein (CRP), and affect lipid metabolism and the acute phase response (Yudkin et al., 2000). CRP, a molecule found in plasma and produced by hepatocytes, is elevated in inflammatory disorders and is used as a systemic marker of inflammation (Agrawal et al., 2010).
An additional source of circulating proinflammatory cytokines (Yudkin, 2003) is adipose tissue, and increased adiposity has been shown to be a risk factor for a variety of inflammatory disorders, including CAD (Allende-Vigo, 2010; Reaven, 2011). Increased adiposity results in the modification of adipocytes leading to physiological dysfunction, characterized by changes in production and secretion of adipokines (adipose tissue derived cytokines). Altered adipokine concentration mediate functions that are associated with CAD such as fibrinolysis, coagulation, blood pressure, inflammation, insulin resistance, and the development of atherosclerosis (DeClercq et al., 2008; Hajer et al., 2008). Interestingly, it has been demonstrated that the major cellular components of adipose tissue, adipocytes and macrophages, both express OTRs (Bonne and Cohen, 1975; Szeto et al., 2008).
In view of the potential role of OT in the attenuation of inflammatory processes and disease, the current study examined the potential anti-atherogenic effects of in vivo chronic OT infusion in the Watanabe Heritable Hyperlipidemic (WHHL) rabbit. Due to a spontaneous genetic defect in cholesterol clearance, WHHLs are extremely dyslipidemic from birth and develop severe atherosclerosis in a compressed time frame (Buja et al., 1983). It has been demonstrated that WHHL rabbits exposed to a stable social environment, characterized by increased affiliative social behavior, exhibited 50% less aortic atherosclerotic lesions than WHHLs subjected to an unstable social environment or in a social isolation (McCabe et al., 2002). It was also demonstrated that social environment influences inflammatory cytokines and vascular oxidative stress in WHHLs (Nation et al., 2008). Although these studies suggest that social environment and behavior play an important role in disease progression (even in animals with strong genetic determinants), the mechanisms by which social/emotional behaviors influence the course of disease are not clear. The current study further examines the hypothesis that OT, which is linked to social affiliation and other prosocial behaviors, attenuates disease. In addition to the extent of aortic atherosclerosis, we also assessed the effect of OT on metabolic, stress and inflammatory biomarkers, as well as the expression of adipose tissue derived adipokines.
Methods
Experimental Animals
Twenty-two male WHHL rabbits (2.5 months old, 2.9–3.5 kg) were obtained from Brown Family Enterprises (Odenville, AL). Previously, we have shown that social environment can influence the progression of atherosclerosis in WHHL rabbits (McCabe et al., 2002). In order to assess the role of OT independent of differences in social environment, all animals in the current study were maintained in individually-caged conditions. Animals were housed in individual cages (6 sq ft) exposed to 12-hour light and dark conditions (lights on at 0700h) and fed standard rabbit chow (Purina; 2.5% total fat, 0% cholesterol) and water ad libitum. Rabbits were acclimated for seven days before initiation of experiments. The rabbits were weighed weekly. All procedures were approved by the Animal Care and Use Committee of the University of Miami.
Blood Draws and Urine Collection
Rabbits were fasted overnight at treatment baseline (collected 1 day prior to implantation of osmotic mini-pumps), midpoint (week 8), and endpoint (week 16) for measurement of serum cholesterol, triglyceride, insulin, glucose, plasma cortisol, C-reactive protein (CRP) and oxidized LDL. Samples for plasma oxytocin measurement were taken at various times during the study (indicated in Figure 1). Using loose restraint, blood was sampled from the marginal ear vein. Serum or plasma was obtained by centrifugation at 1000 × g for 15 minutes at 4°C and stored in aliquots at −80°C until assay.
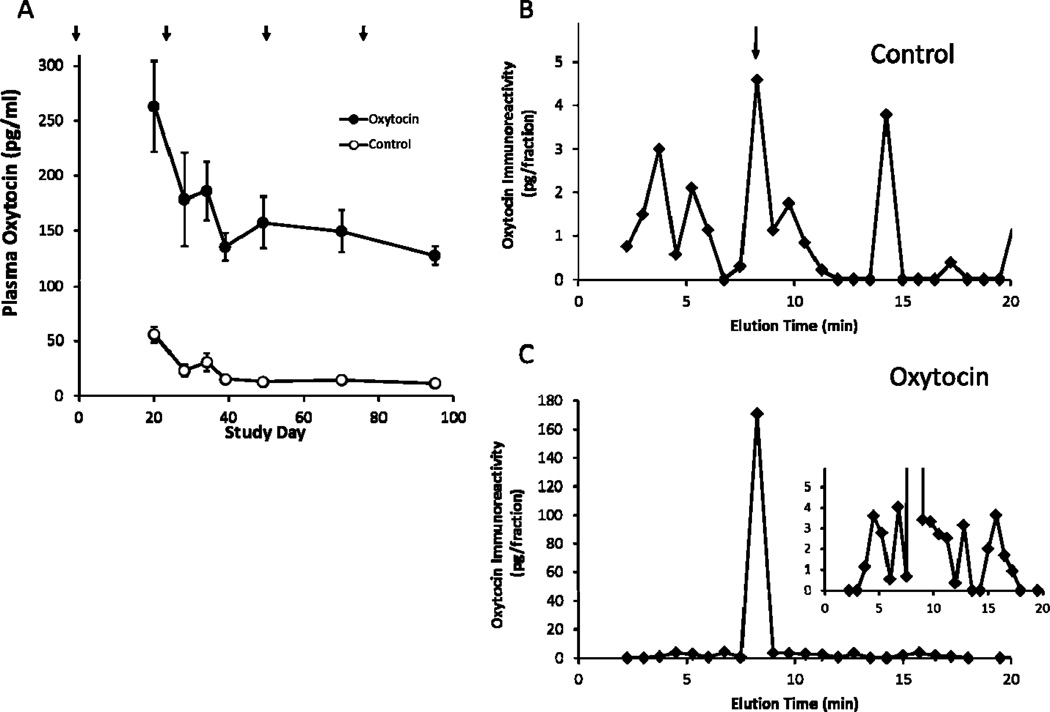
Figure 1.
Plasma OT Levels in control and OT-treated WHHL rabbits and the HPLC profile of oxytocin immunoreactivity of extracted plasmas. Panel A. Plasma OT levels in vehicle (Control) or OT-Treated WHHL animals during the study period. Arrows indicate times that osmotic pumps were implanted or replaced. p <0.05 between groups at all time points. Panels B and C. Plasma from three animals in the Control (B) or Oxytocin-treated (C) group were pooled and extracted as described under Methods and the extract from 3 ml plasma subjected to HPLC separation and the collected fractions were used to measure oxytocin immunoreactivity. Arrow in panel B indicates the elution time of authentic oxytocin standard. Inset in panel C has a reduced scale in order to visualize the minor peaks that were present. Plasma levels of oxytocin in the pooled samples were 7.8 and 70 pg/ml for the control and OT-treated rabbits, respectively.
Animals were placed in metabolic cages for 24-hour urine collection at baseline, midpoint and endpoint for the measurement of epinephrine and norepinephrine. Urine samples were collected on ice and acidified to 5.0 mmol/l HCl, aliquotted prior to storage at −80°C.
Osmotic Minipumps and Surgeries
Osmotic minipumps (Alzet model 2ML4, infusion rates of 2.5 µl/hr, DURECT Corporation, Cupertino, CA) were filled following manufacturer's guidelines with 2 ml of 300 µg/ml OT for an infusion rate of 250ng/kg/hr (Bachem, Torrance, CA) or vehicle (50 mM sodium citrate, pH 4.0) solutions. Osmotic minipumps were used to deliver the peptide in order to obtain a chronic, constant elevation of OT over the entire study period. OT has a half-life of less than 10 minutes, suggesting that single injections would have only short, acute effects. The infusion rate was established in dose response pilot studies and the dose chosen gave on average an 8-fold increase in plasma OT steady state levels compared to vehicle infusions. The pumps were then primed in a sterile saline bath at 37°C for 6 hours. Rabbits were anesthetized with isofluorane (1–3% in 100% oxygen) and scrubbed with 70% isopropanol and betadine solutions before surgery. A small subcutaneous incision was made in the midscapular region, the pumps were inserted and the wound was sutured. Animals were allowed to recover in a 37°C incubator before returning to their home cages. Pump-exchange surgeries were performed every 4 weeks over 4 months.
Tissue Collection
After 16 weeks of treatment, rabbits were euthanized and the entire aorta and heart were removed via a midline thoracic and abdominal incision, and the tissue was placed in a 10% solution of buffered formalin for later staining and quantification of atherosclerotic disease. The epididymal fat were weighed and a portion stored in RNA Qiazol lysis buffer (Qiagen).
Biochemical Assays
Serum cholesterol, triglyceride, insulin, glucose, plasma cortisol and urinary creatinine were measured by automated analyzer (Roche Diagnostics, Indianapolis, IN). Plasma oxidized LDL (Mercodia, Winston Salem, NC), urinary isoprostane (Neogen Corp., Lexington, KY) and urinary catecholamines (Alpco Diagnostics, Salem, NH) were measured by commercially available reagents and following the manufacturer’s instructions. For CRP assay, samples were diluted 1:400 in assay buffer prior to analysis with a commercially available high sensitivity ELISA kit (Immunology Consultants Laboratory, Inc., Newburg, OR). Oyxtocin was measured after extraction of 1 ml of plasma as previously described in detail (Szeto et al., 2011) and quantified using a commercially available OT kit (ADI-900-153; Enzo Life Sciences, Plymouth Meeting, PA). For HPLC analysis plasma was pooled from three animals in each group in order to have sufficient material for analysis and extracted under identical conditions as used for assay. HPLC separation was performed using equipment and conditions as described previously (Szeto et al., 2011).
Quantitation of Atherosclerosis
All histomorphometric procedures were performed in a blind fashion. The method for preparation of mice aortas and quantification of disease was performed as described previously (Karra et al. 2005) by oil-red-O staining of atherosclerotic lesions with digital data analysis. Percent lesion area was calculated from the proportional area of pixels stained with oil-red-O for a given aortic section.
RT-PCR analysis
Total RNA (optical density ratio of 260/280 nm, >1.8) was isolated from approximately 50 mg of adipose tissue using the RNAeasy kit (Qiagen, Valencia, CA) and treated with DNAse I (Qiagen, Valencia, CA). cDNA was prepared using the reverse transcriptase reaction (Applied Biosystems by Life Technologies, Carlsbad, CA) following manufacturer’s protocol. Inventoried rabbit primers for polymerase chain reactions (PCR) were from Applied Biosystems; IL-6 (Cat #: Oc03822686_s1), MCP-1 (Cat #: Oc03823307_s1), adiponectin (Cat #: Oc03823307_s1), IL-10 (Cat #: Oc03396942_m1), TNF-α (Cat #: Oc03397715_m1), and 18S (Cat #: Hs03928990_g1).
Quantitative gene expression of the primers with the use of real-time PCR was performed with the TaqMan gene expression assay (Applied Biosystems by Life Technologies). Twenty micrograms of cDNA were amplified with TaqMan Universal PCR Master Mix and reactions run using universal cycling conditions on an Applied Biosystems Step-One Plus RT-PCR System. Samples were analyzed in triplicate and were normalized to the housekeeping gene, 18S. To analyze relative quantitation (RQ), the Comparative Ct method ΔΔCT (threshold cycle, (Pfaffl, 2001)) was used.
Statistical Analyses
Analysis of variance with repeated measures was used to examine treatment effects (OT group, Vehicle group) on dependent measures across three time points (baseline, midpoint, endpoint). Student's t-tests were used to compare groups on percent lesion area and epididymal fat mRNA expression. A significance level of p < 0.05 was required for all tests.
Results
Validation of OT infusion
To ensure OT was being delivered and elevating peripheral concentrations in the treated group, plasma levels were measured in all rabbits over the course of the study (Figure 1A). In vehicle treated animals we observed that plasma OT values were initially elevated and then reached a steady state level for the remainder of the study (F (1, 20) = 4.86, p=0.004). OT levels in animals receiving OT infusions were increased by an average of 135 pg/ml relative to the vehicle group, which represented an 8-fold increase on average. This elevation in OT was sustained throughout the entire study (p < 0.0001). To confirm that the increase in OT measured in the ELISA was due to intact OT and not other immunoreactive products (Szeto et al., 2011), samples from the final blood collection were subjected to HPLC separation and fractions collected to assess the distribution of oxytocin immunoreactivity (Figure 1B and C). In the sample from the control group, we noted that in addition to a peak of immunoreactivity coincident with authentic OT (eluting at ~8 min), several other peaks of immunoreactivity were observed that account for the majority (60%)of the immunoreactivity in this sample, similar to what we described previously for human plasma samples (Szeto et al., 2011). When the sample from the OT-treated group were similarly analyzed, the increase in immunoreactivity was accounted for almost entirely by intact OT, however, other peaks of immunoreactivity similar to those seen in the control group were still observed, but apparently not altered by OT-infusion suggesting that they represent endogenous OT-immunoreactive substances. These data show that the use of osmotic pumps for delivery of OT results in sustained high levels of intact OT in the peripheral circulation.
Atherosclerosis
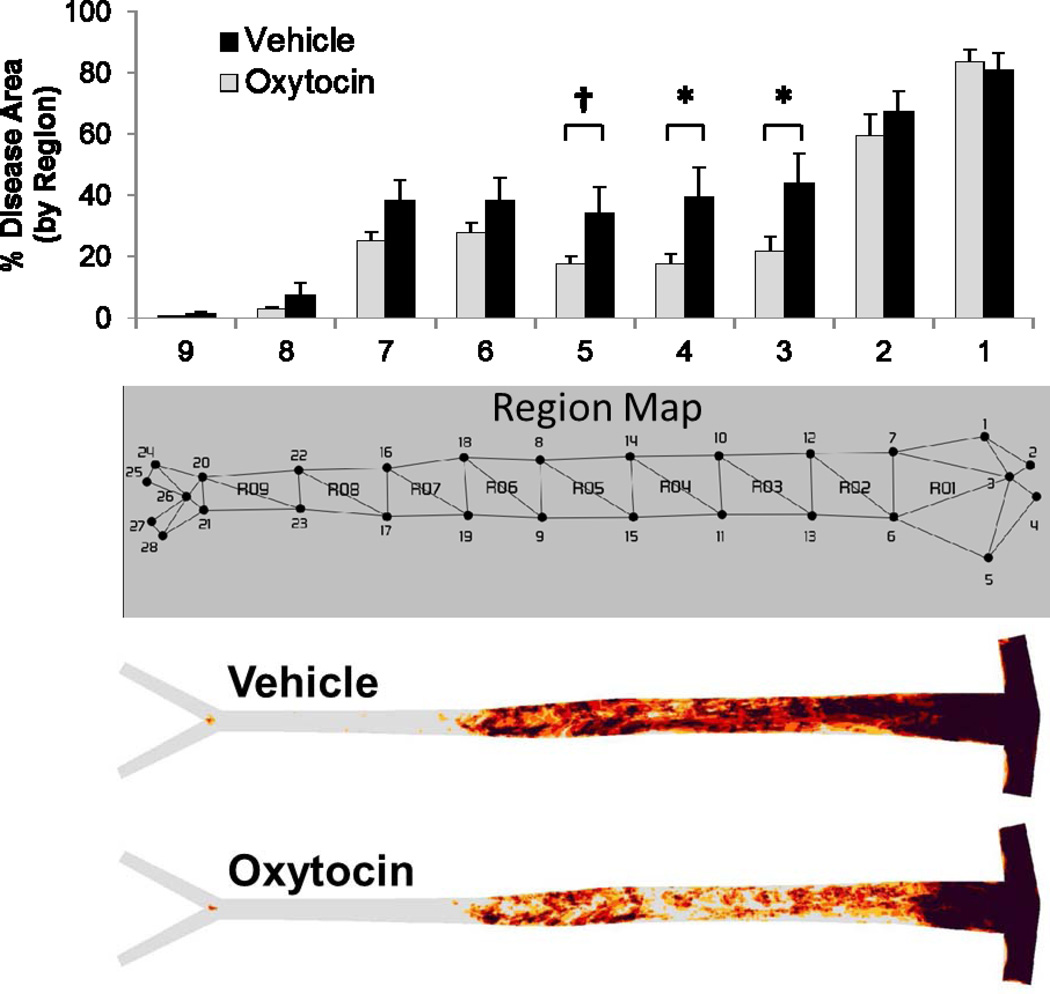
Atherosclerosis was evaluated in all animals at endpoint by en face quantification of Oil Red O stained atherosclerotic lesions. All animals exhibited evidence of atherosclerosis, with the greatest extent of disease at the aortic arch and far less disease in the abdominal aorta (Figure 2). Although there were no significant group differences in disease in the aortic arch (segments 1 & 2), OT-treated animals exhibited less atherosclerosis in the thoracic aorta (segments 3, 4 and 5) compared to vehicle control animals (p < 0.05 for segments 3 & 4, p = 0.088 for segment 5). Although mean values for disease were also less in the abdominal aorta (segments 6–9) for the OT group, these differences were not statistically significant. Therefore, these data suggest that chronic OT infusion decreases aortic atherosclerosis in a site-specific manner.
Figure 2.
Aortic atherosclerosis lesion prevalence map and bar graph showing quantitative lesional area within assigned aortic regions in WHHL rabbits chronically infused with OT or saline for 16 weeks. There was a significant decrease in atherosclerosis in the thoracic aorta identified as regions 3, 4 and 5 and trends toward lower extent of disease in the upper abdominal aorta (regions 6 and 7). No differences were observed in the aortic arch (regions 1 and 2). *p ≤ 0.05 between Groups, † p = 0.088.
Metabolic, Inflammatory, and Stress Biomarkers
Prior to implantation of the pumps, baseline measure of metabolic, inflammatory and stress biomarkers were comparable between the two groups (Tables 1 and 2). OT infusion had no significant influence on body weight or plasma lipids at midpoint or endpoint (Table 1). Fasting glucose levels were also similar between the groups at each time point, however OT infusion increased fasting insulin levels at midpoint (t (20) = −2.20, p=0.038; Table 1). This finding is consistent with prior research (Bobbioni-Harsch et al., 1995) that suggests OT powerfully stimulates insulin secretion in vitro.
Table 1.
Mean Values of Body Weight, Serum Lipids, Insulin, and Glucose Across Time
| Vehicle (n=11) | OT (n=11) | |
|---|---|---|
| Weight (kg) | ||
| Baseline | 3.02 ± 0.09 | 2.87 ± 0.10 |
| Midpoint | 3.26 ± 0.07 | 3.16 ± 0.09 |
| Endpoint | 3.54 ± 0.07 | 3.45 ± 0.12 |
| Cholesterol (mg/dl) | ||
| Baseline | 610.5 ± 55.1 | 596.7 ± 39.1 |
| Midpoint | 601.3 ± 43.2 | 621.2 ± 36.7 |
| Endpoint | 541.5 ± 44.3 | 535.8 ± 36.0 |
| Triglyceride (mg/dl) | ||
| Baseline | 316.5 ± 28.7 | 313.8 ± 38.3 |
| Midpoint | 253.7 ± 44.2 | 211.7 ± 35.6 |
| Endpoint | 227.8 ± 36.5 | 195.8 ± 35.7 |
| Non-Esterified Fatty Acids (µM) | ||
| Baseline | 488.2 ± 23.9 | 545.8 ± 39.7 |
| Midpoint | 593.4 ± 27.6 | 565.4 ± 48.7 |
| Endpoint | 893.6 ± 56.8 | 785.6 ± 20.4 |
| Glucose (mg/dl) | ||
| Baseline | 118.7 ± 1.9 | 120.1 ± 2.7 |
| Midpoint | 121.0 ± 1.7 | 115.5 ± 2.5 |
| Endpoint | 112.9 ± 3.4 | 109.3 ± 1.7 |
| Insulin (ng/ml) | ||
| Baseline | 0.88 ± 0.16 | 0.73 ± 0.15 |
| Midpoint | 0.59 ± 0.11 | 1.15 ± 0.23* |
| Endpoint | 1.08 ± 0.32 | 2.43 ± 0.63 |
Values are mean ± standard error of the mean. Baseline is pretreatment. Midpoint is after 8 weeks of treatment. Endpoint is after 16 weeks of treatment. Bold numbers represent significance differences between Groups.
p <0.05 between Groups
Table 2.
Mean Values of Biomarkers Across Time
| Vehicle (n=11) | OT (n=11) | |
|---|---|---|
| Cortisol (µg/dl) | ||
| Baseline | 0.16 ± 0.11 | 0.16 ± 0.05 |
| Midpoint | 0.11 ± 0.03 | 0.36 ± 0.17 |
| Endpoint | 0.20 ± 0.04 | 0.18 ± 0.05 |
| Urinary Epinephrine (ng/mg creatinine) | ||
| Baseline | 2.0 ± 1.0 | 1.3 ± 0.8 |
| Midpoint | 1.0 ± 0.1 | 1.0 ± 0.2 |
| Endpoint | 1.3 ± 0.1 | 1.5 ± 0.2 |
| Urinary Norepinephrine (ng/mg creatinine) | ||
| Baseline | 25.3 ± 4.6 | 24.5 ± 11.7 |
| Midpoint | 17.7 ± 2.6 | 16.4 ± 2.0 |
| Endpoint | 26.7 ± 3.4 | 23.4 ± 3.9 |
| Oxidized LDL (U/l) | ||
| Baseline | 33.3 ± 1.4 | 32.4 ± 1.3 |
| Midpoint | 33.3 ± 1.8 | 30.3 ± 1.8 |
| Endpoint | 27.2 ± 1.1 | 27.2 ± 1.4 |
| Urinary Isoprostane (ng/mg creatinine) | ||
| Baseline | 16.4 ± 9.3 | 16.0 ± 9.4 |
| Midpoint | 6.9 ± 0.7 | 7.9 ± 1.2 |
| Endpoint | 12.2 ± 0.8 | 12.8 ± 1.4 |
| C-Reactive Protein (CRP; µg/ml) | ||
| Baseline | 35.2 ± 6.6 | 57.6 ± 18.0 |
| Midpoint | 25.8 ± 3.4 | 16.6 ± 2.7* |
| Endpoint | 44.9 ± 6.8 | 28.5 ± 4.2* |
Values are mean ± standard error of the mean. Baseline is pretreatment. Midpoint is after 8 weeks of treatment. End point is after 16 weeks of treatment. Bold numbers represent significance differences between Groups.
p = 0.05 between Groups
Serum cortisol and urinary epinephrine and norepinephrine were not statistically different between groups at midpoint or endpoint, suggesting that OT infusion did not affect these stress biomarkers (Table 2). Oxidative stress was assessed by measuring levels of plasma oxidized LDL and urinary isoprostane. Again, no differences in these measures were observed between the two groups at midpoint or endpoint (Table 2). OT-treated rabbits had significantly exhibited lower levels of systemic inflammation as measured by plasma CRP at midpoint and endpoint compared to vehicle controls (p < 0.05; Table 2).
Adipose Tissue Adipokine Expression
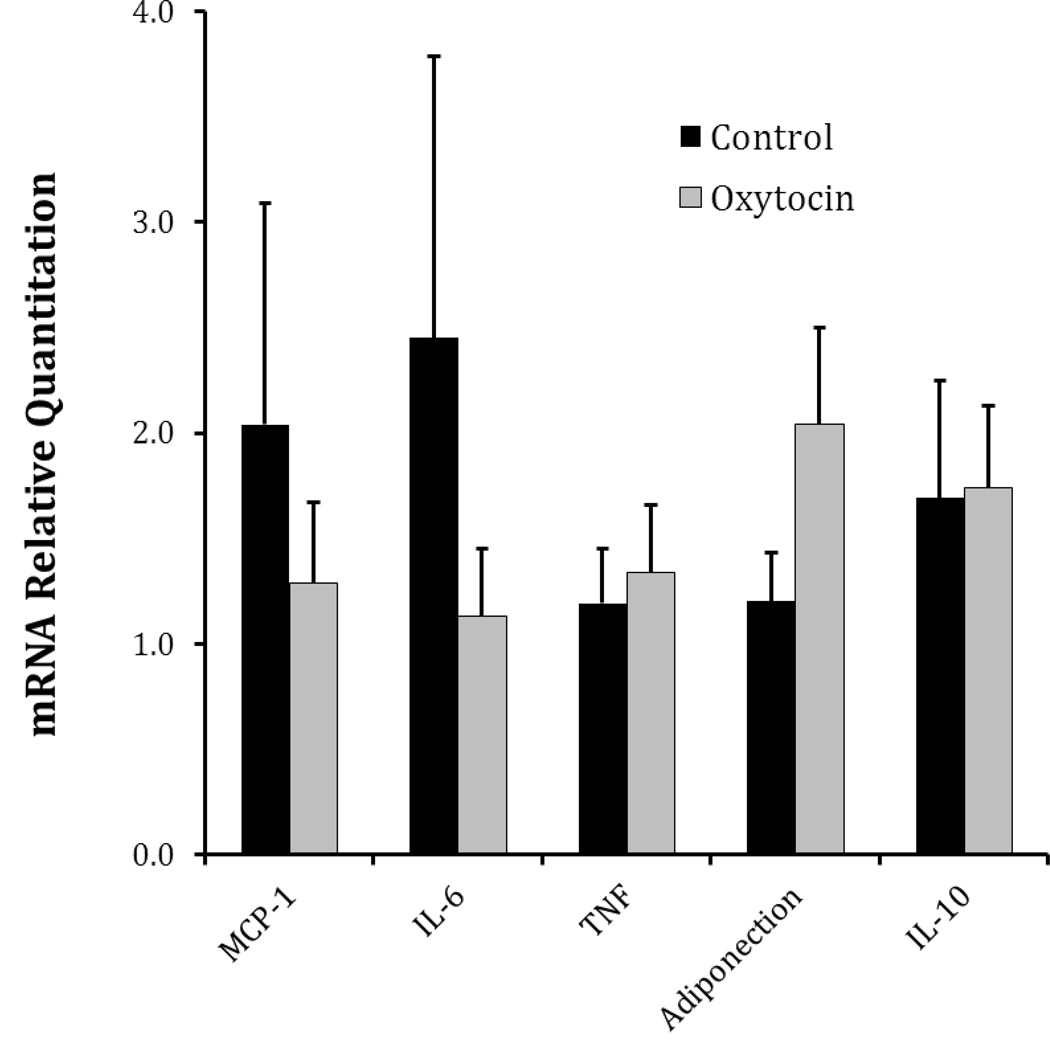
We evaluated the effect of OT infusion on adipokine mRNA expression in epididymal fat, a visceral fat depot (Figure 3). Although there were no significant group differences in adipokine expression, there were several changes suggestive of a decrease in adipose tissue inflammation. For example, the OT-treated group exhibited lower mean expression for the proinflammatory adipokines, MCP-1 and IL-6, and an increase in the anti-inflammatory adipokine, adiponectin. While these changes are in the predicted direction, this study was not sufficiently powered to detect differences in mRNA expression levels in this tissue.
Figure 3.
mRNA expression levels of adipose tissue adipokines in control and OT treated WHHL rabbits. Adipose tissue mRNA of the indicated genes was measured by quantitative rt-PCR. Data represent mean ± S.E.M. Although there were no statistically significant differences between groups, OT treated animals exhibit an adipokine expression pattern consistent with reduced inflammation.
Discussion
The major finding of this study is that chronic OT treatment slows the progression of atherosclerosis in an animal model of disease with strong genetic determinants. Prior work from our laboratory demonstrated that OT inhibits inflammation and oxidative stress in cultured vascular cells and macrophages (Szeto et al., 2008). In the present study, in vivo infusion of OT significantly reduced atherosclerosis and was associated with decreased systemic inflammation, as reflected by circulating CRP. This is consistent with a recent study (Ahmed & Elosaily, 2011) in which OT injections in a methionine-fed rat model of atherosclerosis resulted in a decrease in systemic inflammation and disease. Although not statistically significant, expression of adipokines from visceral fat following chronic exposure to OT was indicative of decreased adipose tissue inflammation. In previous work from our lab, chronic OT infusion significantly reduced the secretion of IL-6 from epididymal fat ex vivo in ApoE (−/−) mice (Nation et al., 2010). It has been demonstrated that transplantation of epididymal fat pads into ApoE (−/−) mice increased inflammation and accelerated the development of atherosclerosis relative to sham operated ApoE (−/−) mice (Ohman et al., 2008). Taken together, these findings suggest that peripheral OT may work directly on vascular tissue, as well as visceral fat, to reduce inflammation and the pathophysiological sequelae leading to disease.
The OT-related attenuation of disease occurred in the aorta below the level of the aortic arch. These findings in the WHHL rabbit are consistent with a prior study from our laboratory that evaluated the effects of chronic OT administration on atherosclerosis in the ApoE−/− knockout mouse, an animal model with a phenotype similar to the WHHL rabbit (Nation el al., 2010). Regional differences in aortic atherosclerosis development over time and in response to treatment have been described (Nakashima et al., 1994; VanderLaan et al., 2004). Lesions develop first in the aortic arch, then the thoracic region, and later in the renal and iliac bifurcations. This is consistent with the current study in which we found progressively more disease from the proximal aorta to the distal aorta. It has also been shown that differential gene expression profiles exist throughout the aorta and may account for the regional differences in disease susceptibility (Karra et al., 2005). Site-specific differences in hemodynamics can also be responsible for differential disease severity at various locations within the aorta (VanderLaan et al., 2004). OT has a partial affinity for the vasopressin V1 receptor at high concentrations (Gimpl and Fahrenholz, 2001) raising the possibility that OT infusion could increase blood pressure by activating the V1 receptor. However, studies have shown that acute subcutaneous administration of OT can transiently reduce blood pressure (Petersson et al., 1996; Maier et al., 1998). Pilot studies from our lab using New Zealand White rabbits infused with OT at a higher (500ng/kg/hr) and lower (125 ng/kg/hr) dose of OT than used in the current study (250 ng/kg/hr) showed no differences in blood pressure or heart rate compared to vehicle controls over a two week period. This suggests that alteration of blood pressure and heart rate did not modulate the OT effect on disease in the current study.
It is interesting that chronic OT infusion slowed the progression of disease without affecting other traditional risks factors for disease or stress hormones. OT administration did not differentially affect body weight, plasma glucose, serum cholesterol, triglycerides, free fatty acids, or plasma and urinary measures of oxidative stress. Similarly, plasma cortisol and urinary catecholamine values were similar between the OT-treated and vehicle control groups, and as mentioned above, at the dose used in this study OT did not significantly alter blood pressure in pilot work. Since OT does not appear to influence these risk factors but does reduce inflammation, these data support the argument that the OT-related attenuation of disease may be through the suppression of local vascular and systemic inflammation. Also, at physiological concentrations such as those in the current study, OT does not cross the blood-brain-barrier (Churchland and Winkielman, 2012), and therefore the results in the current study are due to OT’s peripheral effects.
A relatively unexpected finding was the significant increase of plasma insulin levels at midpoint in the OT-treated rabbits. In fact, previous studies have shown that OT increases glucose uptake in rat cardiomyocytes (Florian et al., 2010) and increases glucose oxidation resembling insulin-like activity in rat epididymal fat pads (Pittman et al., 1961). OT also has been shown to stimulate insulin release from beta cells in the rat pancreas independent of glycemic control (Bobbioni-Harsch et al., 1995; Lee et al., 1995). Therefore, OT has potent insulinogenic properties, whether the increased circulating insulin levels are associated with impaired glucose tolerance or insulin resistance is not known and warrants further study.
Although the measurement of adipokine gene expression from visceral fat was in the predicted direction, none of the differences were statistically significant. Adipokine gene expression is stimulated in the presence of chronic low-grade inflammation due to obesity (Fantuzzi, 2005; Ohman et al., 2008; Zhou et al., 2011). In the current study, although the WHHLs developed atherosclerosis, they were not obese or overweight, and therefore, adipokine expression may not have been elevated, making it more difficult to observe an OT-related attenuation of expression. Another consideration is that in the current study, epididymal fat samples were taken from random regions of this visceral fat depot. A study published subsequently revealed that epididymal adipose tissue may not have uniform metabolic activity across its proximal and distal (relative to the testes) segments (Altintas et al., 2011), which may explain the variability of gene expression in our samples. Given these concerns, it is likely that the present study was not sufficiently powered to detect significant differences in adipokine gene expression.
It is well established that stressful behavior and social isolation are associated with negative disease outcomes (Kaplan et al., 1982; Clarkson et al., 1987; Manuck et al., 1995; Berkman and Orth-Gomer, 1996; Schneiderman and Skyler, 1996; Syme, 1996; Kaplan et al., 2009). Conversely, there is also accumulating evidence that prosocial behavior is associated with the attenuation of disease, and that social affiliation may impart some degree of protection from pathophysiological processes (Knox and Uvnas-Moberg, 1998; Uvnäs-Moberg, 1998; Paredes et al., 2006; Tom and Assinder, 2010; Fekete et al., 2011; McCall and Singer, 2012). Several studies have shown that hyperlipidemic animals exposed to prosocial environments develop less atherosclerotic lesions than animals housed in socially stressful or isolated environments (Shively et al., 1989; McCabe et al., 2002; Paredes et al., 2006; Bernberg et al., 2008). Positive social interactions have also been shown to increase peripheral OT (Uvnas-Moberg, 1997; Uvnäs-Moberg, 1998; Grewen et al., 2005; Light et al., 2005; Holt-Lunstad et al., 2008). Taken together with the findings from the current study, these studies suggest that the beneficial effects of a prosocial environment on disease progression may be, in part, due to elevated levels of peripheral OT associated with positive social behavior. The fact that OT influences both macrophages and adipose tissue, which are not specific to the cardiovascular system, suggests that peripheral OT may have widespread effects on inflammation and disease in other tissues. For example, OT infusion in models of myocardial infarction improves function in the injured heart through reduction of inflammation (Jankowski et al., 2010; Kobayashi et al., 2009). There is also a growing literature examining the influence of OT on cancer cells and neoplastic processes (Cassoni et al., 2004; Strunecka et al., 2009). This being the case, social environment and peripheral OT could represent important targets for therapeutic intervention in disease.
Acknowledgements
We would like to thank Lindsay Conner and Cole Brown for their technical help. This study was supported, in part, by Grants HL-36588 and HL-04726 from the National Heart, Lung, and Blood Institute and the National Institutes of Health.
Role of Funding Source
This study was supported, in part, by Grants HL-36588 and HL-04726 from the National Heart, Lung, and Blood Institute and the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
Angela Szeto took primary responsibility for the conduct of the study, and was involved in the analysis of all biomarkers, and writing of the study.
Maria Rossetti assisted Dr. Szeto with the conduct of the study.
Armando Mendez oversaw the hormonal, lipid, inflammatory and other biomarker measurements, and was involved in the design, analysis and writing of the study.
Crystal Noller assisted Dr. Szeto with the conduct of the study.
Edward Herderick carried out the histological examination of the vascular tissue and the quantification of atherosclerosis.
Julie Gonzales oversaw the animal husbandry and care, and assisted with the conduct of the study.
Neil Schneiderman participated in the design, analysis and writing of the study.
Philip McCabe oversaw all aspects of the study, including design, conduct, analysis and writing.
Conflict of Interest Statement
None of the authors have any conflict of interest that could inappropriately influence or be perceived to influence this work.
References
- Agrawal A, Hammond DJ, Jr, Singh SK. Atherosclerosis-related functions of C-reactive protein. Cardiovascular & hematological disorders drug targets. 2010;10:235–240. doi: 10.2174/187152910793743841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ait-Oufella H, Taleb S, Mallat Z, Tedgui A. Recent advances on the role of cytokines in atherosclerosis. Arterioscler Thromb Vasc Biol. 2011;31:969–979. doi: 10.1161/ATVBAHA.110.207415. [DOI] [PubMed] [Google Scholar]
- Allende-Vigo MZ. Pathophysiologic mechanisms linking adipose tissue and cardiometabolic risk. Endocrine practice : official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 2010;16:692–698. doi: 10.4158/EP09340.RA. [DOI] [PubMed] [Google Scholar]
- Altintas MM, Rossetti MA, Nayer B, Puig A, Zagallo P, Ortega LM, Johnson KB, McNamara G, Reiser J, Mendez AJ, Nayer A. Apoptosis, mastocytosis, and diminished adipocytokine gene expression accompany reduced epididymal fat mass in long-standing diet-induced obese mice. Lipids in health and disease. 2011;10:198. doi: 10.1186/1476-511X-10-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkman LF, Orth-Gomer K. Prevention of cardiovascular morbidity and mortality: Role of social relations. In: Orth-Gomer K, Schneiderman N, editors. Behavioral Medicine Approaches to Cardiovascular Disease Prevention. Erlbaum; Mahwah, NJ: 1996. pp. 51–67. [Google Scholar]
- Bernberg E, Andersson IJ, Gan LM, Naylor AS, Johansson ME, Bergstrom G. Effects of social isolation and environmental enrichment on atherosclerosis in ApoE−/− mice. Stress. 2008;11:381–389. doi: 10.1080/10253890701824051. [DOI] [PubMed] [Google Scholar]
- Bobbioni-Harsch E, Frutiger S, Hughes G, Panico M, Etienne A, Zappacosta F, Morris HR, Jeanrenaud B. Physiological concentrations of oxytocin powerfully stimulate insulin secretionin vitro. Endocrine. 1995;3:55–59. doi: 10.1007/BF02917449. [DOI] [PubMed] [Google Scholar]
- Bonne D, Cohen P. Characterization of oxytocin receptors on isolated rat fat cells. Eur J Biochem. 1975;56:295–303. doi: 10.1111/j.1432-1033.1975.tb02233.x. [DOI] [PubMed] [Google Scholar]
- Buja LM, Kita T, Goldstein JL, Watanabe Y, Brown MS. Cellular pathology of progressive atherosclerosis in the WHHL rabbit. An animal model of familial hypercholesterolemia. Arteriosclerosis. 1983;3:87–101. doi: 10.1161/01.atv.3.1.87. [DOI] [PubMed] [Google Scholar]
- Carter CS, Grippo AJ, Pournajafi-Nazarloo H, Ruscio MG, Porges SW. Oxytocin, vasopressin and sociality. Prog Brain Res. 2008;170:331–336. doi: 10.1016/S0079-6123(08)00427-5. [DOI] [PubMed] [Google Scholar]
- Cassoni P, Sapino A, Marrocco T, Chini B, Bussolati G. Oxytocin and oxytocin receptors in cancer cells and proliferation. J Neuroendocrinol. 2004;16:362–364. doi: 10.1111/j.0953-8194.2004.01165.x. [DOI] [PubMed] [Google Scholar]
- Churchland PS, Winkielman P. Modulating social behavior with oxytocin: how does it work? What does it mean? Horm Behav. 2012;61:392–399. doi: 10.1016/j.yhbeh.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson TB, Kaplan JR, Adams MR, Manuck SB. Psychosocial influences on the pathogenesis of atherosclerosis among nonhuman primates. Circulation. 1987;76:I29–I40. [PubMed] [Google Scholar]
- DeClercq V, Taylor C, Zahradka P. Adipose tissue: the link between obesity and cardiovascular disease. Cardiovascular & hematological disorders drug targets. 2008;8:228–237. doi: 10.2174/187152908785849080. [DOI] [PubMed] [Google Scholar]
- Fantuzzi G. Adipose tissue, adipokines, and inflammation. The Journal of allergy and clinical immunology. 2005;115:911–919. doi: 10.1016/j.jaci.2005.02.023. quiz 920. [DOI] [PubMed] [Google Scholar]
- Fekete EM, Antoni MH, Lopez C, Mendez AJ, Szeto A, Fletcher MA, Klimas N, Kumar M, Schneiderman N. Stress buffering effects of oxytocin on HIV status in low-income ethnic minority women. Psychoneuroendocrinology. 2011;36:881–890. doi: 10.1016/j.psyneuen.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florian M, Jankowski M, Gutkowska J. Oxytocin increases glucose uptake in neonatal rat cardiomyocytes. Endocrinology. 2010;151:482–491. doi: 10.1210/en.2009-0624. [DOI] [PubMed] [Google Scholar]
- Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev. 2001;81:629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- Grewen KM, Girdler SS, Amico J, Light KC. Effects of partner support on resting oxytocin, cortisol, norepinephrine, and blood pressure before and after warm partner contact. Psychosom Med. 2005;67:531–538. doi: 10.1097/01.psy.0000170341.88395.47. [DOI] [PubMed] [Google Scholar]
- Gutkowska J, Jankowski M, Lambert C, Mukaddam-Daher S, Zingg HH, McCann SM. Oxytocin releases atrial natriuretic peptide by combining with oxytocin receptors in the heart. Proc Natl Acad Sci USA. 1997;94:11704–11709. doi: 10.1073/pnas.94.21.11704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajer GR, van Haeften TW, Visseren FL. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. European heart journal. 2008;29:2959–2971. doi: 10.1093/eurheartj/ehn387. [DOI] [PubMed] [Google Scholar]
- Holt-Lunstad J, Birmingham WA, Light KC. Influence of a "warm touch" support enhancement intervention among married couples on ambulatory blood pressure, oxytocin, alpha amylase, and cortisol. Psychosom Med. 2008;70:976–985. doi: 10.1097/PSY.0b013e318187aef7. [DOI] [PubMed] [Google Scholar]
- Jankowski M, Hajjar F, Kawas SA, Mukaddam-Daher S, Hoffman G, McCann SM, Gutkowska J. Rat heart: A site of oxytocin production and action. Proc Natl Acad Sci USA. 1998;95:14558–14563. doi: 10.1073/pnas.95.24.14558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski M, Wang D, Hajjar F, Mukaddam-Daher S, McCann SM, Gutkowska J. Oxytocin and its receptors are synthesized in the rat vasculature. Proc Natl Acad Sci USA. 2000;97:6207–6211. doi: 10.1073/pnas.110137497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan JR, Chen H, Manuck SB. The relationship between social status and atherosclerosis in male and female monkeys as revealed by meta-analysis. American journal of primatology. 2009;71:732–741. doi: 10.1002/ajp.20707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan JR, Manuck SB, Clarkson TB, Lusso FM, Taub DM. Social status, environment, and atherosclerosis in cynomolgus monkeys. Arteriosclerosis. 1982;2:359–368. doi: 10.1161/01.atv.2.5.359. [DOI] [PubMed] [Google Scholar]
- Karra R, Vemullapalli S, Dong C, Herderick EE, Song X, Slosek K, Nevins JR, West M, Goldschmidt-Clermont PJ, Seo D. Molecular evidence for arterial repair in atherosclerosis. Proc Natl Acad Sci U S A. 2005;102:16789–16794. doi: 10.1073/pnas.0507718102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss A, Mikkelsen JD. Oxytocin--anatomy and functional assignments: a minireview. Endocrine regulations. 2005;39:97–105. [PubMed] [Google Scholar]
- Knox SS, Uvnas-Moberg K. Social isolation and cardiovascular disease: an atherosclerotic pathway? Psychoneuroendocrinology. 1998;23:877–890. doi: 10.1016/s0306-4530(98)00061-4. [DOI] [PubMed] [Google Scholar]
- Lee B, Yang C, Chen TH, al-Azawi N, Hsu WH. Effect of AVP and oxytocin on insulin release: involvement of V1b receptors. Am J Physiol. 1995;269:E1095–E1100. doi: 10.1152/ajpendo.1995.269.6.E1095. [DOI] [PubMed] [Google Scholar]
- Light KC, Grewen KM, Amico JA. More frequent partner hugs and higher oxytocin levels are linked to lower blood pressure and heart rate in premenopausal women. Biological Psychology. 2005;69:5–21. doi: 10.1016/j.biopsycho.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Maier T, Dai WJ, Csikos T, Jirikowski GF, Unger T, Culman J. Oxytocin pathways mediate the cardiovascular and behavioral responses to substance P in the rat brain. Hypertension. 1998;31:480–486. doi: 10.1161/01.hyp.31.1.480. [DOI] [PubMed] [Google Scholar]
- Manuck SB, Marsland AL, Kaplan JR, Williams JK. The pathogenicity of behavior and its neuroendocrine mediation: an example from coronary artery disease. Psychosom Med. 1995;57:275–283. doi: 10.1097/00006842-199505000-00009. [DOI] [PubMed] [Google Scholar]
- McCabe PM, Gonzales JA, Zaias J, Szeto A, Kumar M, Herron AJ, Schneiderman N. Social Environment Influences the Progression of Atherosclerosis in the Watanabe Heritable Hyperlipidemic Rabbit. Circulation. 2002;105:354–359. doi: 10.1161/hc0302.102144. [DOI] [PubMed] [Google Scholar]
- McCall C, Singer T. The animal and human neuroendocrinology of social cognition, motivation and behavior. Nature neuroscience. 2012;15:681–688. doi: 10.1038/nn.3084. [DOI] [PubMed] [Google Scholar]
- Mullenix PS, Andersen CA, Starnes BW. Atherosclerosis as inflammation. Annals of vascular surgery. 2005;19:130–138. doi: 10.1007/s10016-004-0153-z. [DOI] [PubMed] [Google Scholar]
- Nakashima Y, Plump AS, Raines EW, Breslow JL, Ross R. ApoE-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arteriosclerosis and thrombosis : a journal of vascular biology / American Heart Association. 1994;14:133–140. doi: 10.1161/01.atv.14.1.133. [DOI] [PubMed] [Google Scholar]
- Nation DA, Gonzales JA, Mendez AJ, Zaias J, Szeto A, Brooks LG, Paredes J, D'Angola A, Schneiderman N, McCabe PM. The effect of social environment on markers of vascular oxidative stress and inflammation in the Watanabe heritable hyperlipidemic rabbit. Psychosom Med. 2008;70:269–275. doi: 10.1097/PSY.0b013e3181646753. [DOI] [PubMed] [Google Scholar]
- Nation DA, Szeto A, Mendez AJ, Brooks LG, Zaias J, Herderick EE, Gonzales J, Noller CM, Schneiderman N, McCabe PM. Oxytocin attenuates atherosclerosis and adipose tissue inflammation in socially isolated ApoE−/− mice. Psychosom Med. 2010;72:376–382. doi: 10.1097/PSY.0b013e3181d74c48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann ID, Kromer SA, Toschi N, Ebner K. Brain oxytocin inhibits the (re)activity of the hypothalamo-pituitary-adrenal axis in male rats: involvement of hypothalamic and limbic brain regions. Regulatory Peptides. 2000;96:31–38. doi: 10.1016/s0167-0115(00)00197-x. [DOI] [PubMed] [Google Scholar]
- Ohman MK, Shen Y, Obimba CI, Wright AP, Warnock M, Lawrence DA, Eitzman DT. Visceral adipose tissue inflammation accelerates atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2008;117:798–805. doi: 10.1161/CIRCULATIONAHA.107.717595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredes J, Szeto A, Levine JE, Zaias J, Gonzales JA, Mendez AJ, Llabre MM, Schneiderman N, McCabe PM. Social experience influences hypothalamic oxytocin in the WHHL rabbit. Psychoneuroendocrinology. 2006;31:1062–1075. doi: 10.1016/j.psyneuen.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Petersson M, Alster P, Lundeberg T, Uvnas-Moberg K. Oxytocin causes a long-term decrease of blood pressure in female and male rats. Physiol Behav. 1996;60:1311–1315. doi: 10.1016/s0031-9384(96)00261-2. [DOI] [PubMed] [Google Scholar]
- Petersson M, Eklund M, Uvnas-Moberg K. Oxytocin decreases corticosterone and nociception and increases motor activity in OVX rats. Maturitas. 2005;51:426–433. doi: 10.1016/j.maturitas.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Petersson M, Hulting A-L, Uvnas-Moberg K. Oxytocin causes a sustained decrease in plasma levels of corticosterone in rats. Neuroscience Letters. 1999;264:41–44. doi: 10.1016/s0304-3940(99)00159-7. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic acids research. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittman JA, Boshell BR, Williams BH, Hamner D, Hill P. Insulin-like activity of vasopressin and oxytocin. Biochem Biophys Res Commun. 1961;6:29–32. doi: 10.1016/0006-291x(61)90179-6. [DOI] [PubMed] [Google Scholar]
- Reaven GM. Insulin Resistance: the Link Between Obesity and Cardiovascular Disease. The Medical clinics of North America. 2011;95:875–892. doi: 10.1016/j.mcna.2011.06.002. [DOI] [PubMed] [Google Scholar]
- Ross HE, Young LJ. Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Front Neuroendocrinol. 2009;30:534–547. doi: 10.1016/j.yfrne.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R. Atherosclerosis -- An Inflammatory Disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Schneiderman N, Skyler JS. Insulin metabolism, sympathetic nervous system regulation, and coronary heart disease prevention. In: Orth-Gomer K, Schneiderman N, editors. Behavioral Medicine Approaches to Cardiovascular Disease Prevention. Mahwah, NJ: Erlbaum; 1996. pp. 105–133. [Google Scholar]
- Shively CA, Clarkson TB, Kaplan JR. Social deprivation and coronary artery atherosclerosis in female cynomolgus monkeys. Atherosclerosis. 1989;77:69–76. doi: 10.1016/0021-9150(89)90011-7. [DOI] [PubMed] [Google Scholar]
- Smith AS, Wang Z. Salubrious effects of oxytocin on social stress-induced deficits. Hormones and Behavior. 2012;61:320–330. doi: 10.1016/j.yhbeh.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strunecka A, Hynie S, Klenerova V. Role of oxytocin/oxytocin receptor system in regulation of cell growth and neoplastic processes. Folia biologica. 2009;55:159–165. [PubMed] [Google Scholar]
- Syme SL. Social class and cardiovascular disease. In: Orth-Gomer K, Schneiderman N, editors. Behavioral Medicine Approaches to Cardiovascular Disease Prevention. Mahwah, NJ: Erlbaum; 1996. pp. 43–50. [Google Scholar]
- Szeto A, McCabe PM, Nation DA, Tabak BA, Rossetti MA, McCullough ME, Schneiderman N, Mendez AJ. Evaluation of enzyme immunoassay and radioimmunoassay methods for the measurement of plasma oxytocin. Psychosom Med. 2011;73:393–400. doi: 10.1097/PSY.0b013e31821df0c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeto A, Nation DA, Mendez AJ, Dominguez-Bendala J, Brooks LG, Schneiderman N, McCabe PM. Oxytocin attenuates NADPH-dependent superoxide activity and IL-6 secretion in macrophages and vascular cells. Am J Physiol Endocrinol Metab. 2008;295:E1495–E1501. doi: 10.1152/ajpendo.90718.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE, Gonzaga GC, Klein LC, Hu P, Greendale GA, Seeman TE. Relation of oxytocin to psychological stress responses and hypothalamic-pituitary-adrenocortical axis activity in older women. Psychosom Med. 2006;68:238–245. doi: 10.1097/01.psy.0000203242.95990.74. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Saphire-Bernstein S, Seeman TE. Are plasma oxytocin in women and plasma vasopressin in men biomarkers of distressed pair-bond relationships? Psychol Sci. 2010;21:3–7. doi: 10.1177/0956797609356507. [DOI] [PubMed] [Google Scholar]
- Tom N, Assinder SJ. Oxytocin in health and disease. The International Journal of Biochemistry & Cell Biology. 2010;42:202–205. doi: 10.1016/j.biocel.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Uvnas-Moberg K. Physiological and endocrine effects of social contact. Ann N Y Acad Sci. 1997;807:146–163. doi: 10.1111/j.1749-6632.1997.tb51917.x. [DOI] [PubMed] [Google Scholar]
- Uvnäs-Moberg K. Oxytocin may mediate the benefits of positive social interaction and emotions. Psychoneuroendocrinology. 1998;23:819–835. doi: 10.1016/s0306-4530(98)00056-0. [DOI] [PubMed] [Google Scholar]
- VanderLaan PA, Reardon CA, Getz GS. Site specificity of atherosclerosis: site-selective responses to atherosclerotic modulators. Arterioscler Thromb Vasc Biol. 2004;24:12–22. doi: 10.1161/01.ATV.0000105054.43931.f0. [DOI] [PubMed] [Google Scholar]
- Yudkin JS. Adipose tissue, insulin action and vascular disease: inflammatory signals. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 2003;27(Suppl 3):S25–S28. doi: 10.1038/sj.ijo.0802496. [DOI] [PubMed] [Google Scholar]
- Yudkin JS, Kumari M, Humphries SE, Mohamed-Ali V. Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link? Atherosclerosis. 2000;148:209–214. doi: 10.1016/s0021-9150(99)00463-3. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Wei Y, Wang L, Wang X, Du X, Sun Z, Dong N, Chen X. Decreased adiponectin and increased inflammation expression in epicardial adipose tissue in coronary artery disease. Cardiovascular diabetology. 2011;10:2. doi: 10.1186/1475-2840-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]