Abstract
Topiramate, presumably through antagonism of excitatory glutaminergic pathways and facilitation of inhibitory gamma-aminobutyric acid neurons in the cortico-mesolimbic system, might reduce cocaine’s abuse liability. We tested whether topiramate (100 mg twice daily) would reduce the euphoria, subjective mood, craving, and preference for cocaine over money induced by low and high doses (0.325 and 0.650 mg/kg i.v., respectively) of experimentally administered cocaine in 24 male and female, cocaine-dependent, non-treatment-seeking research volunteers in a university inpatient laboratory. We utilized a randomized, double-blind, placebo-controlled, within-subject, Latin-square crossover design in which 3 experimental challenge doses of low-dose cocaine, high-dose cocaine, and placebo were administered in counterbalanced order after 5 days of topiramate or matching placebo pretreatments separated by a 1-week washout period (2006–2009). After placebo pretreatments, cocaine produced dose-related increases in euphoria, stimulant effects, craving for more cocaine, and monetary value of cocaine in a behavioral preference test of cocaine vs. money choice. Topiramate pretreatment reduced the cocaine-related craving and monetary value of high-dose cocaine while increasing the monetary value, euphoria, and stimulant effects of low-dose cocaine. Validated and standardized human experimental methods evaluating the potential for topiramate to alter cocaine’s abuse liability suggest that topiramate may reduce the reinforcing effects and craving induced by higher cocaine doses. Low-dose cocaine might appear to have some enhancement of its stimulant properties in the presence of topiramate’s prominent sedative effects.
Keywords: Cocaine, Craving, Mood, Preference, Reward, Topiramate
INTRODUCTION
Medications that alter the effects of excitatory amino acids (EAAs) such as glutamate or facilitate the actions of gamma-aminobutyric acid (GABA) neurons in the brain may reduce cocaine reinforcement in animals (Kaddis, Uretsky & Wallace 1995; Koob 1992; Rockhold 1998; Wolf 1998). An intact GABA efferent system from the nucleus accumbens, corpus striatum, and ventral pallidum to cortical structures (Kalivas, Churchill & Klitenick 1993) is critical for the expression of cocaine reinforcement (Hemby, Johnson & Dworkin 1997). Additionally, inhibition of corticofugal glutaminergic (GLUergic) pathways or activation of GABA pathways can decrease the extracellular release of dopamine (Dewey et al. 1997; Karler et al. 1998), the neurotransmitter central to the mediation of cocaine reinforcement. EAAs, including glutamate, also play a role in the acquisition of preference conditioning and other rewarding effects of cocaine (Cervo & Samanin 1995; David, Durkin & Cazala 1998; de Vries et al. 1998), and chronic cocaine administration results in reductions in midbrain GABA function (Cameron & Williams 1994; Peris 1996). These findings support the idea that medications that restore the GABA/glutamate balance could reduce cocaine use.
Furthermore, the action of EAAs at alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA)/kainate receptors is important in the development and expression of context-conditioned behavioral sensitization to cocaine (Bell & Kalivas 1996; Li et al. 1997; Zhang et al. 1997). Consistent with this neuropharmacological framework, AMPA antagonists have been shown to block cocaine-induced behavioral sensitization (Kaddis et al. 1995) and decrease the extracellular release of dopamine in the brain (Moghaddam & Bolinao 1994; Pap & Bradberry 1995). The locomotor effects of cocaine and other psychostimulants also are attenuated by AMPA antagonists (Schiffer et al. 2001; Witkin 1993). Thus, topiramate may have a promising profile as a potential treatment for cocaine dependence because it both facilitates GABAergic function (Petroff et al. 1999, 2001; White et al. 1997), presumably because it activates a novel site on the GABAA-receptor complex (Czuczwar & Patsalos 2001), and antagonizes the action of EAAs at AMPA/kainate receptors in the cortico-mesolimbic system (Gibbs et al. 2000; Skradski & White 2000)—effects that may synergistically contribute to a suppression of cortico-mesolimbic dopamine function.
Consistent with this proposal, in a pilot study in humans (N=40), Kampman et al. (2004) showed that cocaine-dependent individuals who received topiramate, compared with placebo recipients, were significantly more likely to abstain from using cocaine for 3 continuous weeks. Hence, initial clinical data support topiramate’s efficacy in treating cocaine dependence. Notably, however, the mechanism by which topiramate might have exerted its anti-cocaine-taking effects in humans has not been characterized. The present human laboratory study is the first direct test in humans to determine whether topiramate (100 mg twice daily) can reduce significantly low-dose (0.325 mg/kg i.v.) and high-dose (0.65 mg/kg i.v.) cocaine-induced subjective mood changes and preference for money over drug taking, both of which can be associated with its abuse liability. Successful demonstration that topiramate reduces cocaine-induced subjective measures that can be associated with abuse liability in humans may enable us to substantiate the idea that neuromodulation of certain critical neuromodulatory pathways in the cortico-mesolimbic system could be one method by which an efficacious medicine can treat cocaine dependence.
MATERIALS AND METHODS
Subjects
We studied 24 DSM-IV (American Psychiatric Association 1994)-diagnosed, male and female cocaine-dependent individuals between the ages of 18 and 55 years, with experience of having injected either cocaine or other psychoactive substances. Subjects were research volunteers who were not seeking treatment and who were recruited by local newspaper, radio, and television advertisements. These subjects did not meet diagnostic criteria for any other axis 1 psychiatric disorder except nicotine dependence. See Table 1 for additional demographic information. The Human Subjects Committee at the University of Virginia provided ethical approval for this study, which was in accordance with the 1964 Declaration of Helsinki. All subjects gave written informed consent before being included in the study.
Table 1.
Demographics and Drug Use of 24 Non-Treatment-Seeking, Cocaine-Dependent Subjects at Intake
| Age, yr * | 34 (7.19) |
| Sex † | |
| Male | 19 (79) |
| Female | 5 (21) |
| Ethnicity † | |
| White | 7 (29) |
| Black | 15 (63) |
| Other | 2 (8) |
| Marital status † | |
| Married | 1 (4) |
| Separated | 4 (17) |
| Divorced | 5 (21) |
| Never married | 14 (58) |
| Education years * | 12 (1.30) |
| Hollingshead occupational category (Hollingshead & Redlich 1958) † | |
| Professional/skilled worker (1–3) | 1 (4) |
| Semi-skilled/clerical (4–6) | 15 (63) |
| Unskilled/unemployed (7–9) | 8 (33) |
| Days of cocaine use in last 30 days * | 13.04 (8.34) |
| Days of alcohol use in last 30 days * | 12.13 (9.81) |
| Days of alcohol intoxication in last 30 days * | 4.30 (7.09) |
Values are expressed as mean (SD).
Values are expressed as number (%).
Experimental Design
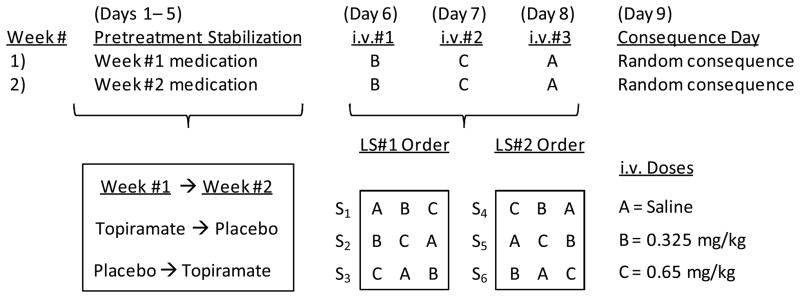
As illustrated in Figure 1, the study was a double-blind, within-subject, Latin-square, crossover design in which all subjects received each of three dose levels (0, 0.325, and 0.65 mg/kg) of cocaine intravenously following repeated oral dose pretreatments with both placebo and topiramate (100 mg twice daily). Topiramate or matching placebo doses were administered on a twice-daily regimen for 5 days to achieve a steady state (Schneiderman 1998) before commencing with 3 experimental days of cocaine administration. The sequence of topiramate and placebo (i.e. which comes first) was counterbalanced across subjects in two 8-day sequences. On each of the 3 experimental days (Days 6, 7, and 8), subjects received a single intravenous infusion of one of the three cocaine doses (0, 0.325, or 0.65 mg/kg) as a cocaine challenge. The sequence of the three cocaine doses was completely counterbalanced with two separate digram-balanced 3 × 3 Latin squares (Maxwell & Delaney 1990) so that both ordinal position and sequence were perfectly balanced across 6 subjects. Twelve subjects received topiramate pretreatment first and placebo second, and the other 12 received pretreatments in the opposite order. Within each set of 12 subjects, 6 received the same sequence of cocaine doses under both the topiramate and placebo pretreatment conditions, whereas an alternative sequence of intravenous challenges was given following the second pretreatment period for the other 6 subjects. The overall Latin-square balancing of these dose sequences yielded a cohort of 24 individuals. To establish the Multiple Choice Questionnaire (MCQ) as a measure of cocaine reinforcement (Griffiths, Rush & Puhala 1996), study Day 9 was a “consequence day” on which subjects received one of their randomly selected MCQ responses (see MCQ methods below). Subjects resided in an inpatient environment at the Clinical Pharmacological Research Unit (CPRU) for two separate 9-day blocks interspersed by a 1-week washout period. All doses (oral and i.v.) were administered under double-blind experimental conditions.
Figure 1.
Study Design Illustration of Within-Subject Crossover Pretreatments with Digram-Balanced Latin-Square (LS) Counterbalanced Sequences of Cocaine Dosing After 5 Days of Pretreatment Stabilization.
Topiramate (100 mg twice daily) and placebo were given as crossover pretreatments for 1 week each. After 5 days of stabilization, three intravenous (i.v.) doses of saline or cocaine (0.325 or 0.65 mg/kg) were administered over 3 days (Days 6–8) in an order completely counterbalanced across a group of 6 subjects. Day 9 was the day of delivery of a money-versus-cocaine choice from the Multiple Choice Questionnaire.
Choice of Medication Dose and Its Preparation
The topiramate dose (100 mg twice daily, orally) that we chose was that found to be efficacious for treating cocaine-dependent humans in a pilot study (Kampman et al. 2004). Topiramate tablets (100 mg) were purchased from Ortho-McNeil Pharmaceutical, Inc. (Raritan, NJ, USA) and over-encapsulated in royal blue size 0 capsules (Shionogi Qualicaps, Inc., Whitsett, NC, USA) filled with cornstarch. Placebo topiramate capsules were identical in both color and size and contained only cornstarch.
The low and high cocaine doses that we chose (0.325 and 0.65 mg/kg i.v., respectively) were those found previously to produce significant effects on brain blood flow and subjective mood (Johnson et al. 1998a,b). These doses were similar to the low and high doses of 25 mg and 50 mg tested intravenously in classic studies by Foltin and Fischman (1992) characterizing the pharmacobehavioral effects of cocaine. The intravenous cocaine dose procedures were in accordance with National Institute on Drug Abuse guidelines for cocaine administration. Cocaine HCl ampoules suitable for human intravenous use were supplied by the National Institute on Drug Abuse, mixed with sterile 0.9% w/v normal saline, and measured to obtain the correct dose administered to subjects by body weight. The 0 mg/kg cocaine dose was 0.9% w/v sterile saline administered as the placebo cocaine.
Laboratory Conditions
Study procedures were conducted on an inpatient research unit, the CPRU. After insertion of a venous catheter into a non-dominant arm or hand vein, subjects remained seated in a comfortable chair for the 90-minute period beginning 30 minutes before through 60 minutes after cocaine or placebo was infused by an automated syringe pump. Cardiovascular function was monitored continuously for medical safety, and subjective assessments of mood, euphoria, craving, and drug preference were monitored repeatedly before and after the intravenous infusion.
Experimental Measures
The study employed standard measures of abuse liability assessment (Fischman & Foltin 1991; Jasinski, Johnson & Henningfield 1984; Roache 2010; Roache & Griffiths 1989) to assess how topiramate or placebo might alter the abuse liability of cocaine. Abuse liability studies typically assess euphoria and drug reinforcement as well as other measures of general mood state and specific expected target symptoms to establish an overall profile of drug effects (Foltin & Fischman 1991; Roache 2010). The specific experimental assessments are described below.
Visual Analog Scale of Cocaine Effects (VAS-C)
For the VAS-C, subjects used a pencil to place a perpendicular mark on a 100-mm line labeled (left to right) “not at all” to “extremely” so as to rate their current feelings on 14 adjectival scales that have been used previously in human abuse liability assessments for cocaine (Johnson et al. 2004; Roache et al. 2005) and methamphetamine (Johnson et al. 1999, 2007a). The 14 items were: 1) measures of euphoria (“high”, “like”, “rush or thrill”, “feel good or elated”); 2) general measures of stimulant effect/side effect (“aroused or stimulated”, “mind-racing”, “slow or lethargic”, “nervous”, “shaky or jittery”, “nauseous”), and 3) measures of craving (“crave”, “desire”, “want cocaine”, and “could refuse cocaine”). These 14 items are clustered into three factors (euphoria, stimulant, and craving) and used to calculate mean factor scores, based upon previous factor analyses demonstrating reliable factor structure (Johnson et al. 2004). The VAS-C was completed 60 minutes before the morning dose of topiramate or placebo topiramate, just before the cocaine or placebo cocaine infusion (i.e. “baseline”), and at 2, 4, 6, 8, 10, 20, 40, 60, and 120 minutes after the infusion.
Multiple Choice Questionnaire (MCQ)
The MCQ is a paper-and-pencil task measuring preference for drug over monetary reward. We used it in previous studies demonstrating reliable dose-related cocaine effects amenable to alteration by putative treatment agents (Johnson et al. 2004, 2007a; Roache et al. 2005). The MCQ is a modification of the task originally developed by Griffiths et al. (1993). On the form, subjects were asked to indicate their preference between “today’s cocaine dose” and a designated amount of money. Over a series of 72 items, the amount of the monetary alternative was sequentially increased from $0.25 to $25. Typically, cocaine users prefer to receive drug over money at low monetary values but switch to a preference for money over drug at high monetary values. The switch is called the “crossover value”, or “MCQ-Price”, and is a quantitative measure of preference for the drug over money. As reported by Griffiths et al. (1993, 1996), the MCQ is considered a measure of drug reinforcement because at the end of the study, subjects are given the commodity (i.e. drug or money) that they preferred on one of their randomly selected choices. In this study, a training and familiarization session was used to reinforce MCQ choices and establish an expectation of receiving consequences. Subsequently, and during the experiment, MCQ responses were completed after subjects received an experimental dose of cocaine; the questions were framed about receiving this same dose again. Then, on the last day of the experiment (Day 9), subjects received, as a direct consequence of their previous MCQ choices, either the money or the cocaine injection that they chose on one of the “lottery” choices, drawn at random from all of the MCQ responses on the previous six cocaine challenge days.
Addiction Research Center Inventory (ARCI)
The ARCI is a 49-item true/false questionnaire (Haertzen, Hill & Belleville 1963) that is used routinely in abuse liability assessment (Fischman & Foltin 1991). In its original description, five factor scales were empirically derived based on responses to drugs from different pharmacological classes. The morphine/benzedrine group (MBG) items evaluate opioid and stimulant-like euphoria. The benzedrine group (BG) and amphetamine (A) factor scales assess general stimulant effects. The lysergic acid diethylamide (LSD) scale assesses hallucinogen effects that generally are considered to be dysphoric. The pentobarbital-chlorpromazine-alcohol group (PCAG) scale measures sedative-like intoxicating effects. The ARCI was completed 60 minutes before the morning dose of topiramate or placebo topiramate and again 20, 60, and 120 minutes after the cocaine or placebo cocaine infusion.
Global Rating of Stimulation (GRS)
The GRS assesses the overall level of general stimulation that is prototypic of stimulant administration (Johnson et al. 1996; Silverstone et al. 1992) with a five-point scale: 0—normal; 1—slightly light-headed, restless, or speeded-up; 2—moderately light-headed, restless, or speeded-up; 3—very much light-headed, restless, or speeded-up, and 4—extremely light-headed, restless, or speeded-up. Subjects were asked to circle one answer. The GRS was completed at the same time points as the VAS-C measures.
Profile of Mood States (POMS)
The POMS is a 65-item standardized five-point interval rating scale composed of adjectives describing different moods or feeling states (McNair, Lorr & Droppleman 1971). The 65 items are uniquely distributed into six validated mood factors measuring the “Friendliness”, “Tension-Anxiety”, “Depression-Dejection”, “Vigor”, “Fatigue”, and “Confusion-Bewilderment” mood dimensions, and composite scores for “Arousal” and “Total Mood Disturbance” are determined by combinations of the other six factors. In the present experiment, as in other studies (Fischman & Foltin 1991), the POMS was used as a general assessment of drug-related mood changes. It was completed at the same time points as the ARCI.
General Procedures
All subjects were caffeine abstinent while residing on the CPRU. Cigarette smokers were limited to smoke breaks several times throughout the day but never within 1 hour of the cocaine infusion, resulting in the smoking of only 5 to 10 cigarettes/day. On a separate experimental day before double-blind testing, all subjects were trained on the proper use of the rating scales and a single-blind dose of cocaine (0.325 mg/kg i.v.) was administered to ensure that subjects could tolerate cocaine infusion.
As in previous studies of this type (Johnson et al. 2004, 2007a; Roache et al. 2005), female subjects who were not already taking oral contraceptives were stabilized on contraceptive pills to prevent pregnancy and to control for the fluctuation in brain dopamine levels that occurs during different parts of the menstrual cycle (Di Paolo 1994; King, Steger & Morgan 1986). After a medical and gynecological examination, oral contraceptive medication was started in the month preceding testing and terminated the month afterwards. Medical consultation ensured that female subjects were aware of the risks of taking oral contraception and cigarette smoking.
Test Day Procedures
Beginning in the evening before the first day and continuing each day thereafter, topiramate or matching placebo doses were administered at 1900 h, and subjects retired to bed at 2300 h. On the morning of each subsequent inpatient hospital day (i.e. Days 2–9), subjects received an oral dose of topiramate or matching placebo at 0700 h. Each subject provided a urine sample at 0600 h before receiving a standard hospital breakfast. OnTrak TesTcup® (Varian Inc., Palo Alto, CA, USA) was used to verify that urine samples were free from the presence of opiates, amphetamines, benzodiazepines, or barbiturates on every day and were free from cocaine on Days 1–5 before the cocaine challenge doses began. Unbeknownst to subjects, cocaine was not actually tested on Days 6–8 to maintain the blinding of the staff to the challenge dose conditions. Also, breath alcohol concentrations were measured daily, and both urine drug screens and breath alcohol concentration had to be negative for further testing to proceed. On each of the intravenous challenge infusion days, a vein suitable for venipuncture was cleaned and numbed with EMLA® cream, and a 20-G intravenous catheter was inserted. The vein was kept patent by an infusion of 0.9% w/v saline at a rate of 8 ml/hour. Recordings of vital signs (e.g. heart rate and blood pressure) and 12-lead EKG were monitored continuously through a Spacelabs Ultraview® 1050, Module 90496 cardiac monitor (Spacelabs Medical Inc., Issaquah, WA, USA). At 0800 h, subjects completed baseline responses on the ARCI, POMS, and GRS. At 0900 h on each of the infusion days, cocaine or saline (placebo cocaine) was administered intravenously as a 10-ml infusion over 1 minute by an automated infusion pump (Baxter International Inc., Deerfield, IL, USA). Repeated measurements of ARCI, POMS, VAS-C, GRS, and assessments on the MCQ were done at scheduled intervals as described above. The experiment ended at 1200 h, and lunch was provided at 1230 h.
Statistical Analysis
For all measures, mixed-effects analysis of variance models (SAS® PROC MIXED; SAS Institute Inc., Cary, NC, USA) were used to study the main effects of treatment (topiramate vs. placebo) and cocaine dose level (0, 0.325 mg/kg, and 0.65 mg/kg) and their interaction. Some analyses also included “time” in the model to measure the onset and offset of cocaine effects, but since cocaine effects were short-lived (i.e. dissipating after 30 minutes), clinically meaningful interpretation of treatment effects eliminated the time effect by examining peak and mean cocaine effects observed over the first 30 minutes after infusion. Although only significant covariates were retained in the final model, we tested between-subject factors such as age, gender, race, and years of cocaine use as possible covariates, as well as the daily “baseline” assessment taken before cocaine injection. Generally, after adjustment for the daily baseline assessment, other covariate factors were neither significant nor retained in the final model. For the MCQ, there was an additional covariate of self-reported money spent on cocaine in the past 30 days. Each of the primary outcome measures was analyzed by a separate univariate mixed-model analysis of variance. Kenward-Roger approximation was used to compute the appropriate number of degrees of freedom for hypothesis testing in the mixed-effects models due to the small sample size. The significance level of all tests was set at 0.05 without correction for multiple dependent variables, but uncorrected P values are reported to allow readers to gauge the experiment-wise error rate for themselves. A priori contrasts between topiramate and placebo at each level of cocaine dose were done only if there was a significant treatment × dose interaction from the mixed models. All tests were two-sided.
RESULTS
Most subjects were male (19 out of 24), with a mean age of 34 (see Table 1). The majority were unmarried (96%) African-Americans (63%) of an unskilled (33%) or semi-skilled (63%) occupational category on the Hollingshead classification (Hollingshead & Redlich 1958) who used cocaine on an average of 13 days in the past month.
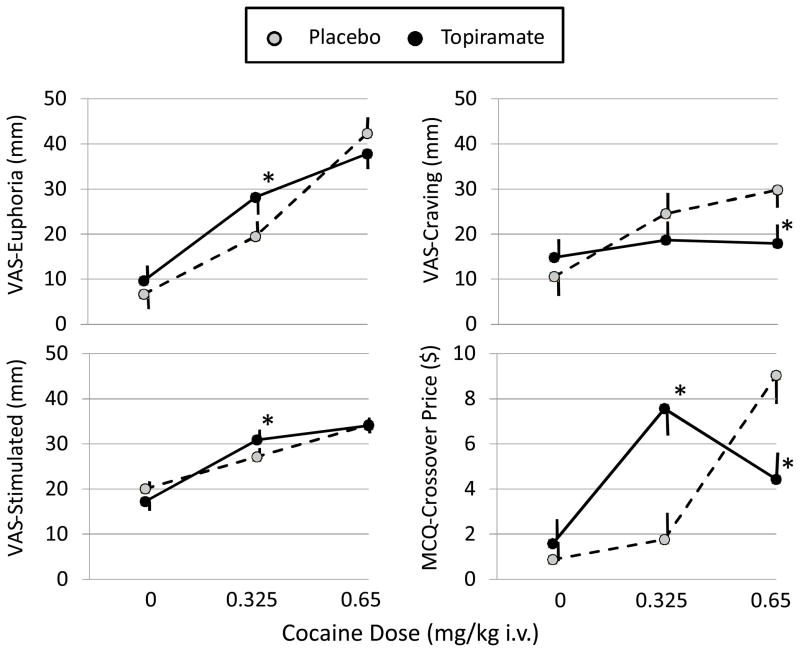
Figure 2 shows the effects of cocaine and topiramate on the primary experimental measures of cocaine abuse potential. Cocaine produced significant main effects indicating large dose-related increases on the VAS-Euphoria (F=43.94, P<0.0001) and Stimulated (F=43.75, P<0.0001) subscales, and smaller effects to increase VAS-Craving (F=6.51, P=0.0034) and the MCQ-Price (F=6.39, P=0.0064) measure of reinforcement. Topiramate main effects were not significant for any of these measures (all F<3.0, P>0.1), but topiramate produced significant interactions with cocaine dose on VAS-Euphoria (F=7.03, P=0.004), VAS-Stimulated (F=7.53, P=0.0042), VAS-Craving (F=7.55, P=0.0026), and MCQ-Price (F=32.80, P<0.0001). Post-hoc tests of this interaction revealed a bidirectional effect of topiramate wherein the effects of the highest cocaine dose (0.65 mg/kg) on VAS-Craving and MCQ-Price were decreased by topiramate, while the effects of the low cocaine dose (0.325 mg/kg) were enhanced for VAS-Euphoria, VAS-Stimulated, and MCQ-Price.
Figure 2.
Effects of Topiramate on Cocaine Dose-Related Activation of Euphoria, Stimulation, Craving, and Behavioral Preference for Additional Cocaine.
Shown are the mean ± SEM Euphoria, Stimulated, and Craving factor score ratings from the Visual Analog Scale (VAS) and the Crossover Price from the Multiple Choice Questionnaire (MCQ). Data are means ± SEM from the N=24 subjects given cocaine doses (mg/kg) intravenously (i.v.) after oral pretreatment with placebo or topiramate (100 mg twice daily). *Topiramate was significantly different from placebo at the same cocaine dose.
The effects of cocaine and topiramate on the ARCI and POMS subjective mood measures (Table 2) roughly mirrored the effects observed with the VAS subscales and the MCQ. Significant main effects of cocaine in the absence of topiramate’s effects indicate that cocaine reduced anger-hostility (POMS-AH) and total mood disturbance (POMS-TMD) and produced mild stimulating effects measured by the GRS, ARCI-BG, and tension-anxiety (POMS-TA) scales. An overall main effect of topiramate to reduce vigor (POMS-VIG) indicates a general sedative effect of topiramate, as does its interaction with cocaine to reduce POMS-AROUSAL and increase fatigue (POMS-FAT) at the 0.65 mg/kg dose of cocaine. Cocaine tended—although these were not large effects—to increase ratings on the ARCI-LSD scale, indicating dysphoria, and the ARCI-PCAG scale, indicating sedation; however, topiramate counteracted the effects of the high (0.65 mg/kg) cocaine dose. Finally, a cocaine main effect on the confusion-bewilderment (POMS-CB) scale indicates a central nervous system confusional state from cocaine alone (i.e. with placebo topiramate), but the interaction with topiramate was due to the finding that topiramate alone (with 0 mg/kg cocaine) elevated this scale. The only truly bidirectional effect of topiramate seen in the subjective mood ratings was the topiramate-related increase in the ARCI-MBG euphoric effects of the low (0.325 mg/kg) dose of cocaine, which was not seen at the high (0.65 mg/kg) dose of cocaine.
Table 2.
Cocaine and Topiramate Effects on Subjective Mood
| Least Squares Means (95% Confidence Intervals) | ANOVA Table | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo Topiramate | 100 mg Topiramate | Main Effect of Cocaine | Main Effect of Topiramate | Interaction of Cocaine and Topiramate | ||||||||
| Subjective Mood Subscale | 0 mg/kg Cocaine | 0.325 mg/kg Cocaine | 0.65 mg/kg Cocaine | 0 mg/kg Cocaine | 0.325 mg/kg Cocaine | 0.65 mg/kg Cocaine | F | P | F | P | F | P |
| ARCI | ||||||||||||
| MBG (euphoria) | 6.1 (5.3, 7.0) | 5.6 (4.8, 6.5) | 7.1 (6.3, 8.0) | 7.1 (6.2, 7.9) | 7.7* (6.8, 8.5) | 6.6 (5.7, 7.4) | 0.23 | 0.80 | 7.27 | 0.014 | 4.21 | 0.023 |
| BG (benzedrine) | 4.5 (3.9, 5.1) | 4.9 (4.3, 5.5) | 5.0 (4.4, 5.6) | 4.4 (3.8, 5.0) | 5.3 (4.7, 5.9) | 5.2 (4.6, 5.8) | 4.10 | 0.025 | 0.34 | 0.57 | 0.49 | 0.62 |
| A (amphetamine) | 4.2 (3.5, 4.8) | 4.3 (3.7, 5.0) | 4.5 (3.9, 5.2) | 4.1 (3.5, 4.7) | 5.0 (4.3, 5.7) | 4.2 (3.5, 4.8) | 1.36 | 0.27 | 0.13 | 0.73 | 1.40 | 0.26 |
| LSD (dysphoria) | 2.8 (2.1, 3.6) | 3.6 (2.8, 4.3) | 4.3 (3.6, 5.0) | 2.9 (2.1, 3.6) | 3.7 (3.0, 4.4) | 3.4* (2.7, 4.1) | 12.77 | 0.0001 | 1.21 | 0.29 | 5.47 | 0.011 |
| PCAG (sedation) | 2.4 (2.0, 2.8) | 3.5 (3.2, 3.9) | 3.9 (3.5, 4.2) | 2.4 (2.0, 2.7) | 3.3 (2.9, 3.6) | 3.2* (2.8, 3.5) | 18.57 | 0.0001 | 9.05 | 0.008 | 4.97 | 0.018 |
| POMS | ||||||||||||
| FRD (friendliness) | 16.5 (15.2, 17.8) | 15.38 (14.0, 16.7) | 15.1 (13.9, 16.4) | 15.7 (14.4, 16.9) | 15.4 (14.1, 16.6) | 14.8 (13.6, 16.1) | 1.66 | 0.20 | 0.48 | 0.49 | 0.26 | 0.77 |
| TA (tension-anxiety) | 3.0 (2.0, 4.0) | 4.3 (3.3, 5.3) | 5.7 (4.7, 6.7) | 3.1 (2.2, 4.1) | 4.4 (3.4, 5.4) | 4.6 (3.6, 5.6) | 11.36 | 0.0001 | 0.61 | 0.45 | 1.52 | 0.23 |
| AH (anger-hostility) | 3.2 (2.6, 3.9) | 3.0 (2.3, 3.7) | 2.4 (1.7, 3.1) | 2.8 (2.2, 3.5) | 2.8 (2.1, 3.4) | 2.7 (2.1, 3.4) | 4.78 | 0.01 | 0.15 | 0.70 | 2.73 | 0.08 |
| FAT (fatigue) | 0.7 (0.2, 1.2) | 0.8 (0.3, 1.3) | 0.6 (0.1, 1.1) | 1.0 (0.5, 1.5) | 0.4 (0, 0.9) | 1.4* (0.9, 1.9) | 1.95 | 0.15 | 2.06 | 0.15 | 4.82 | 0.01 |
| VIG (vigor) | 14.9 (13.5, 16.3) | 14.1 (12.7, 15.5) | 15.7 (14.3, 17.1) | 14.7 (13.3, 16.1) | 14.8 (13.4, 16.1) | 13.2 (11.9, 14.6) | 0.19 | 0.82 | 1.43 | 0.24 | 2.86 | 0.06 |
| CB (confusion-bewilderment) | 2.6 (2.0, 3.3) | 3.0 (2.4, 3.7) | 3.6 (3.0, 4.3) | 3.7* (3.1, 4.3) | 3.2 (2.6, 3.8) | 3.7 (3.1, 4.3) | 5.89 | 0.006 | 2.65 | 0.13 | 4.47 | 0.020 |
| DD (depression-dejection) | 1.4 (0.7, 2.2) | 1.4 (0.7, 2.2) | 1.7 (1.0, 2.5) | 1.3 (0.6, 2.0) | 1.5 (0.8, 2.2) | 1.2 (0.5, 2.0) | 0.12 | 0.89 | 0.34 | 0.57 | 0.87 | 0.44 |
| AROUSAL (arousal) | 15.7 (13.5, 17.9) | 15.1 (12.9, 17.4) | 17.1 (15.0, 19.3) | 13.3 (11.1, 15.5) | 15.2 (13.0, 17.4) | 12.4* (10.2, 14.5) | 0.31 | 0.73 | 11.26 | 0.001 | 4.25 | 0.018 |
| TMD (total mood disturbance) | −5.1 (−8.8, −1.4) | −1.6 (−5.3, 2.2) | −1.4 (−5.1, 2.3) | −2.7 (−6.4, 1.0) | −2.8 (−6.5, 0.8) | 0.4 (−3.3, 4.0) | 3.96 | 0.02 | 0.87 | 0.35 | 1.38 | 0.26 |
| GRS | 0.1 (−0.1, 0.4) | 0.7 (0.5, 1.0) | 1.0 (0.7, 1.2) | 0.1 (−0.2, 0.3) | 0.7 (0.5, 0.9) | 0.9 (0.7, 1.2) | 47.88 | 0.0000 | 0.12 | 0.73 | 0.02 | 0.98 |
Abbreviations: ANOVA, analysis of variance; ARCI, Addiction Research Center Inventory; POMS, Profile of Mood States; GRS, Global Rating of Stimulation.
Topiramate was significantly different from placebo at the same cocaine dose.
For the total POMS score, there was a significant treatment main effect, and those pretreated with topiramate compared with placebo had a reduction in general mood (mean difference= −3.79, 95% CI: −6.73 to −0.86, P=0.015); however, there was no effect of cocaine. On the POMS subscales, when subjects received cocaine, compared with placebo, there was a decrease in total mood disturbance but no main effect of treatment and no interaction between cocaine dose level and treatment. Whilst topiramate decreased vigor, there was no main effect of cocaine dose level and no interaction between cocaine and treatment. During cocaine administration, there was a significant increase in tension-anxiety but no significant effect of treatment and no interaction between cocaine and treatment. There was a significant cocaine dose level effect to increase confusion-bewilderment and an interaction between cocaine and treatment; however, there was no effect of treatment, and none of the post-hoc within-dose contrasts were significant (see Table 2).
Adverse Events
There were no serious adverse events in any subject throughout the experiment, and all adverse events resolved spontaneously, with no need for medication intervention. Generally, topiramate was well tolerated, with the relative frequencies of the five most common adverse events between topiramate and placebo being: a) headache (26% vs. 26%, respectively); b) paresthesia (24% vs. 12%, respectively); c) fatigue (26% vs. 18%, respectively); d) dizziness (20% vs. 12%, respectively), and e) taste alteration (14% vs. 10%, respectively). None of these adverse events were considered to be of substantial clinical importance, and none occurred at a significantly greater frequency than placebo (i.e. all P>0.05). No clinically important cardiovascular event occurred following cocaine administration with or without topiramate pretreatment.
DISCUSSION
In a group of cocaine-dependent, i.v.-experienced, research volunteers given cocaine under experimental conditions, we found that cocaine produced dose-related increases in euphoria, stimulating mood effects, craving for more cocaine, and willingness of subjects to pay money to obtain more cocaine. The study utilized standardized and validated experimental human laboratory approaches (Fischman & Foltin 1991; Jasinski et al. 1984; Roache 2010) to determine whether topiramate has the potential, as a putative treatment for cocaine dependence, to reduce the abuse liability of cocaine. Importantly, we also observed that oral doses of topiramate (100 mg twice daily) decreased the craving for cocaine and the amount of money that subjects were willing to pay to receive additional high (0.65 mg/kg i.v.) doses of cocaine. Decreases in craving and a willingness to pay for additional doses of cocaine indicate that the reinforcing effects of the high dose of cocaine were reduced by topiramate. Curiously, we also found evidence that certain effects of cocaine can be enhanced by topiramate. Specifically, the euphoria, stimulating effects, and MCQ-Price measure of reinforcement for the low dose (0.325 mg/kg) of cocaine were increased by topiramate compared with placebo in this experimental study. These results suggest a potential for topiramate to enhance the positive effects of low cocaine doses without enhancing the cocaine-induced craving for more cocaine. The mechanism for this effect is unknown but could be related to the subacute, short-term topiramate dosing conditions of the experiment. Although 5 days of dosing with topiramate would achieve steady-state levels of topiramate (Johnson & Ait-Daoud 2010; Schneiderman 1998), that time period is not long enough to increase gradually topiramate doses—a procedure known to reduce adverse central nervous system side effects with topiramate. There was a tendency for topiramate to produce more frequent reports of fatigue, dizziness, and paresthesia as adverse events although none of these differences achieved statistical significance. In contrast, significant effects of topiramate were detected: decreased vigor and arousal and increased confusion ratings on the POMS mood scales. With these kinds of subjective mood changes after topiramate pretreatment, it is likely that cocaine-dependent research volunteers expecting to receive cocaine would find even low doses of cocaine to be more desirable than usual. Although higher doses of cocaine normally produce greater euphoria and reinforcing effect than low cocaine doses, and did so under placebo pretreatment conditions in the present experiment, this clearly did not occur following topiramate pretreatment where these effects were attenuated.
Bidirectional effects of topiramate to enhance the effects of the low cocaine dose while reducing the effects of the high cocaine dose also could be related to topiramate’s known mood-stabilizing effects (Correll, Sheridan & DelBello 2010). We had partial support for this hypothesis wherein topiramate enhanced low cocaine dose stimulation and euphoria while also normalizing both positive and negative mood effects measured on the POMS and ARCI following the high cocaine dose. Although we did not measure any signs of cocaine abstinence, mood stabilization could ameliorate the unpleasant effects of abstinence (Zullino et al. 2004), and cocaine-withdrawal-related irritability has been shown to enhance the euphoric effects of a low cocaine challenge dose (Newton et al. 2003). Additionally, cocaine administration is known to produce a craving for more cocaine by acting as a “priming cue” that triggers an expectation of more cocaine (Highfield et al. 2002). When this is not followed by further cocaine taking, it can be unpleasant due to the uncoupling of the salience of reward (Wheeler et al. 2011).
Bidirectional effects of topiramate might also suggest a possible leftward shift in the cocaine dose-response curve, suggesting sensitization. Because dose-response curves are inverted U-shaped, a leftward shift could increase the effects of a low dose while decreasing the effects of a high dose. However, an argument against this interpretation is that even with U-shaped dose-response curves, higher doses are uniformly determined to be more reinforcing even when fewer of the high doses are self-administered (Roache & Meisch 1995). Thus, sensitization would not result in decreases in the MCQ measure of reinforcement. Clearly, these complex effects of topiramate on cocaine-induced stimulation, euphoria, and reinforcement need further exploration as they might provide additional clues as to other mechanisms that could be useful in reducing cocaine-taking behavior. This would, however, require additional focused research in both animals and humans, perhaps with different and multiple doses of topiramate and cocaine under alternate schedules of administration, to disentangle the various discrete mechanisms by which topiramate might alter the stimulant and reinforcing effects of cocaine in addicted individuals.
Regardless of the mechanism for the observed bidirectional effects of topiramate, the seminal finding of the present study is the treatment-related reduction in cocaine-induced craving and the amount of money that subjects were willing to spend to receive a future cocaine dose following the injection of the high cocaine dose. This finding suggests that topiramate may have some efficacy to reduce the potential for the use and abuse of cocaine. Such a conclusion would be consistent with and may explain the behavioral mechanism behind a previous observation that the same dose of topiramate reduced cocaine use by cocaine-dependent outpatients in a preliminary treatment trial (Kampman et al. 2004). Reis et al. (2008) also found, albeit in an open-label trial, that 25% of cocaine-dependent outpatients treated with a range of topiramate doses up to 300 mg reported a significant reduction in craving.
Our study employed standard and accepted methods of human laboratory cocaine abuse liability assessment in non-treatment seekers (Fischman & Foltin 1991; Jasinski et al. 1984; Roache & Griffiths 1989) to evaluate the potential of topiramate as a putative treatment agent (Roache 2010). Because human laboratory studies have reliably found a positive and monotonically increasing relationship between drug dose and abuse potential (Fischman & Foltin 1991; Jasinski et al. 1984; Roache & Griffiths 1989), which is true for MCQ preference and cocaine craving as well (Griffiths et al. 1993, 1996; Johnson et al. 2004, 2007a; Mumford, Rush & Griffiths 1995; Roache et al. 2005), the current finding of a topiramate-related reduction at the high cocaine dose is all the more compelling.
The use of other measures of subjective mood change in abuse liability evaluation is based upon the presumption that drugs are abused by humans because of their positive subjective effects (Chutuape & de Wit 1994; Fischman & Foltin 1991; Griffiths & Balster 1979; Preston, Walsh & Sannerud 1997). However, this issue is controversial (Hughes, Alison & Terry 1996; Woods, Katz & Winger 1987) because drug use is determined by variables other than subjective mood (Roache 2010). Thus, the finding that topiramate did not significantly reduce cocaine-induced euphoria or stimulation does not negate other possible benefits of topiramate.
Similarly, the relationship between craving and drug taking has been questioned because craving reductions are not always associated with a decrease in drug taking (Haney & Spealman 2008; Sofuoglu et al. 2009; Weiss, Griffin & Hufford 1995), and craving is not always an essential trigger for drug taking (de Wit 2000). Nevertheless, there is a reasonably good correspondence between craving and drug use in daily life (Preston et al. 2009) or drug preference or drug liking in drug abusers (Fischman & Foltin 1991; Jasinski et al. 1984; Roache 2010; Roache & Griffiths 1989). Thus, the finding that topiramate’s anti-craving effect was coupled with a reduction in the amount of money that subjects were willing to pay for additional cocaine doses, but was not associated with a reduction in cocaine-induced euphoria, suggests that a possible mechanism to explain these findings could be reduced motivation for high doses or for more cocaine, rather than diminished subjective euphoria.
Clearly, to appreciate fully its importance, it is necessary to determine the ability to replicate both the bidirectional effect of topiramate as well as its suppressant effect at high cocaine doses. Limitations of our study include the needs to have a larger sample, to determine whether there are gender differences in response, and to repeat this experiment in a paradigm where cocaine is administered repeatedly or self-administered to determine the extent to which these effects are sustained. We also don’t know the optimal dose of topiramate. Although both this study and the outpatient treatment trial employed 200 mg/day, Kampman et al. (2004) suggested the need for testing higher doses. This is in contrast to Johnson et al. (2007b), who, in their alcoholism treatment study, suggested testing <300 mg/day to avoid adverse effects. Resolution of these issues may require parametric drug interaction studies of various combinations of topiramate and cocaine doses.
In sum, our study provides limited evidence from the inpatient human laboratory that topiramate may exhibit anti-craving effects coupled with a lowering of the amount of money that subjects are willing to pay to receive additional cocaine, although these effects may be limited to alteration of the motivational properties of high-dose cocaine. We suggest possible mechanisms for our finding but argue that it is evidence for topiramate’s effects to decrease cocaine’s abuse liability and the propensity of cocaine-dependent individuals to seek and use cocaine. We suggest that topiramate should be examined further as a possible pharmacological treatment for cocaine dependence.
Acknowledgments
This research was supported by National Institute on Drug Abuse grant 5 R01 DA012191-09 to B. A. Johnson. We thank the staff of the Clinical Pharmacological Research Unit at the University of Virginia. We also thank Robert H. Cormier, Jr., for his skilled technical assistance with preparation of the manuscript. The experiment described herein is in compliance with the current laws of Virginia and the United States of America.
Footnotes
AUTHORS’ CONTRIBUTION
All authors have critically reviewed the content of this manuscript and approved the final version for publication.
DISCLOSURES
B. A. Johnson has served as a consultant to Johnson & Johnson (Ortho-McNeil Janssen Scientific Affairs, LLC), Transcept Pharmaceuticals, Inc., D&A Pharma, Organon, ADial Pharmaceuticals, LLC (with which he also serves as Chairman), Psychological Education Publishing Company (PEPCo), LLC, and Eli Lilly and Company. The other authors have no disclosures to report.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Bell K, Kalivas PW. Context-specific cross-sensitization between systemic cocaine and intra-accumbens AMPA infusion in the rat. Psychopharmacology (Berl) 1996;127:377–383. doi: 10.1007/s002130050101. [DOI] [PubMed] [Google Scholar]
- Cameron DL, Williams JT. Cocaine inhibits GABA release in the VTA through endogenous 5-HT. J Neurosci. 1994;14:6763–6767. doi: 10.1523/JNEUROSCI.14-11-06763.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervo L, Samanin R. Effects of dopaminergic and glutamatergic receptor antagonists on the acquisition and expression of cocaine conditioning place preference. Brain Res. 1995;673:242–250. doi: 10.1016/0006-8993(94)01420-m. [DOI] [PubMed] [Google Scholar]
- Chutuape MA, de Wit H. Relationship between subjective effects and drug preferences: ethanol and diazepam. Drug Alcohol Depend. 1994;34:243–251. doi: 10.1016/0376-8716(94)90163-5. [DOI] [PubMed] [Google Scholar]
- Correll CU, Sheridan EM, DelBello MP. Antipsychotic and mood stabilizer efficacy and tolerability in pediatric and adult patients with bipolar I mania: a comparative analysis of acute, randomized, placebo-controlled trials. Bipolar Disord. 2010;12:116–141. doi: 10.1111/j.1399-5618.2010.00798.x. [DOI] [PubMed] [Google Scholar]
- Czuczwar SJ, Patsalos PN. The new generation of GABA enhancers: potential in the treatment of epilepsy. CNS Drugs. 2001;15:339–350. doi: 10.2165/00023210-200115050-00001. [DOI] [PubMed] [Google Scholar]
- David V, Durkin TP, Cazala P. Rewarding effects elicited by microinjection of either AMPA or NMDA glutaminergic antagonists into the ventral tegmental area revealed by an intracranial self-administration paradigm in mice. Eur J Neurosci. 1998;10:1392–1402. doi: 10.1046/j.1460-9568.1998.00150.x. [DOI] [PubMed] [Google Scholar]
- de Vries TJ, Schoffelmeer AN, Binnekade R, Mulder AH, Vanderschuren LJ. MK-801 reinstates drug-seeking behaviour in cocaine-trained rats. Neuroreport. 1998;9:637–640. doi: 10.1097/00001756-199803090-00014. [DOI] [PubMed] [Google Scholar]
- de Wit H. Laboratory-based assessment of alcohol craving in social drinkers. Addiction. 2000;95:S165–S169. doi: 10.1080/09652140050111735. [DOI] [PubMed] [Google Scholar]
- Dewey SL, Chaurasia CS, Chen CE, Volkow ND, Clarkson FA, Porter SP. GABAergic attenuation of cocaine-induced dopamine release and locomotor activity. Synapse. 1997;25:393–398. doi: 10.1002/(SICI)1098-2396(199704)25:4<393::AID-SYN11>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Di Paolo T. Modulation of brain dopamine transmission by sex steroids. Rev Neurosci. 1994;5:27–41. doi: 10.1515/revneuro.1994.5.1.27. [DOI] [PubMed] [Google Scholar]
- Fischman MW, Foltin RW. Utility of subjective-effects measurements in assessing abuse liability of drugs in humans. Br J Addict. 1991;86:1563–1570. doi: 10.1111/j.1360-0443.1991.tb01749.x. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Fischman MW. Smoked and intravenous cocaine in humans: acute tolerance, cardiovascular and subjective effects. J Pharmacol Exp Ther. 1991;257:247–261. [PubMed] [Google Scholar]
- Foltin RW, Fischman MW. Self-administration of cocaine by humans: choice between smoked and intravenous cocaine. J Pharmacol Exp Ther. 1992;261:841–849. [PubMed] [Google Scholar]
- Gibbs JW, Sombati S, DeLorenzo RJ, Coulter DA. Cellular actions of topiramate: blockade of kainate-evoked inward currents in cultured hippocampal neurons. Epilepsia. 2000;41(suppl 1):S10–S16. doi: 10.1111/j.1528-1157.2000.tb02164.x. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Balster RL. Opioids: similarity between evaluations of subjective effects and animal self-administration results. Clin Pharmacol Ther. 1979;25:611–617. doi: 10.1002/cpt1979255part1611. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Rush CR, Puhala KA. Validation of the multiple-choice procedure for investigating drug reinforcement in humans. Exp Clin Psychopharmacol. 1996;4:97–106. [Google Scholar]
- Griffiths RR, Troisi JR, Silverman K, Mumford GK. Multiple-choice procedure: an efficient approach for investigating drug reinforcement in humans. Behav Pharmacol. 1993;4:3–13. [PubMed] [Google Scholar]
- Haertzen CA, Hill HE, Belleville RE. Development of the Addiction Research Center Inventory (ARCI): selection of items that are sensitive to the effects of various drugs. Psychopharmacologia. 1963;4:155–166. doi: 10.1007/BF02584088. [DOI] [PubMed] [Google Scholar]
- Haney M, Spealman R. Controversies in translational research: drug self-administration. Psychopharmacology (Berl) 2008;199:403–419. doi: 10.1007/s00213-008-1079-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemby SE, Johnson BA, Dworkin SI. Neurobiological basis of drug reinforcement. In: Johnson BA, Roache JD, editors. Drug Addiction and its Treatment: Nexus of Neuroscience and Behavior. Philadelphia, PA: Lippincott-Raven; 1997. pp. 137–169. [Google Scholar]
- Highfield DA, Mead AN, Grimm JW, Rocha BA, Shaham Y. Reinstatement of cocaine seeking in 129X1/SvJ mice: effects of cocaine priming, cocaine cues and food deprivation. Psychopharmacology (Berl) 2002;161:417–424. doi: 10.1007/s00213-002-1047-9. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB, Redlich FC. Social Class and Mental Illness: A Community Study. New York: Wiley; 1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR, Alison HO, Terry SY. Saying versus doing and other methodological issues in the study of drug self-administration. Exp Clin Psychopharmacol. 1996;4:234–238. [Google Scholar]
- Jasinski DR, Johnson RE, Henningfield JE. Abuse liability assessment in human subjects. Trends Pharmacol Sci. 1984;5:196–200. [Google Scholar]
- Johnson B, Barron B, Fang B, Lamki L, Wagner L, Wells L, Kenny P, Overton D, Dhother S, Abramson D, Chen R, Kramer L. Isradipine prevents global and regional cocaine-induced changes in brain blood flow: a preliminary study. Psychopharmacology (Berl) 1998a;136:335–341. doi: 10.1007/s002130050575. [DOI] [PubMed] [Google Scholar]
- Johnson B, Lamki L, Fang B, Barron B, Wagner L, Wells L, Kenny P, Overton D, Dhother S, Abramson D, Chen R, Kramer L. Demonstration of dose-dependent global and regional cocaine-induced reductions in brain blood flow using a novel approach to quantitative single photon emission computerized tomography. Neuropsychopharmacology. 1998b;18:377–384. doi: 10.1016/S0893-133X(97)00168-1. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Ait-Daoud N. Topiramate in the new generation of drugs: efficacy in the treatment of alcoholic patients. Curr Pharm Des. 2010;16:2103–2112. doi: 10.2174/138161210791516404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA, Oldman D, Goodall EM, Chen YR, Cowen PJ. Effects of GR 68755 on d-amphetamine-induced changes in mood, cognitive performance, appetite, food preference, and caloric and macronutrient intake in humans. Behav Pharmacol. 1996;7:216–227. [PubMed] [Google Scholar]
- Johnson BA, Roache JD, Ait-Daoud N, Wells LT, Mauldin JB. Effects of isradipine on cocaine-induced subjective mood. J Clin Psychopharmacol. 2004;24:180–191. doi: 10.1097/01.jcp.0000115662.45074.c3. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Roache JD, Ait-Daoud N, Wells LT, Wallace CL, Dawes MA, Liu L, Wang X-Q. Effects of acute topiramate dosing on methamphetamine-induced subjective mood. Int J Neuropsychopharmacol. 2007a;10:85–98. doi: 10.1017/S1461145705006401. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Roache JD, Bordnick PS, Ait-Daoud N. Isradipine, a dihydropyridine-class calcium channel antagonist, attenuates some of d-methamphetamine’s positive subjective effects: a preliminary study. Psychopharmacology (Berl) 1999;144:295–300. doi: 10.1007/s002130051007. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Rosenthal N, Capece JA, Wiegand F, Mao L, Beyers K, McKay A, Ait-Daoud N, Anton RF, Ciraulo DA, Kranzler HR, Mann K, O’Malley SS, Swift RM Topiramate for Alcoholism Advisory Board, Topiramate for Alcoholism Study Group. Topiramate for treating alcohol dependence: a randomized controlled trial. JAMA. 2007b;298:1641–1651. doi: 10.1001/jama.298.14.1641. [DOI] [PubMed] [Google Scholar]
- Kaddis FG, Uretsky NJ, Wallace LJ. DNQX in the nucleus accumbens inhibits cocaine-induced conditioned place preference. Brain Res. 1995;697:76–82. doi: 10.1016/0006-8993(95)00786-p. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Churchill L, Klitenick MA. GABA and enkephalin projection from the nucleus accumbens and ventral pallidum to the ventral tegmental area. Neuroscience. 1993;57:1047–1060. doi: 10.1016/0306-4522(93)90048-k. [DOI] [PubMed] [Google Scholar]
- Kampman KM, Pettinati H, Lynch KG, Dackis C, Sparkman T, Weigley C, O’Brien CP. A pilot trial of topiramate for the treatment of cocaine dependence. Drug Alcohol Depend. 2004;75:233–240. doi: 10.1016/j.drugalcdep.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Karler R, Calder LD, Thai DK, Bedingfield JB. The role of dopamine and GABA in the frontal cortex of mice in modulating a motor stimulant effect of amphetamine and cocaine. Pharmacol Biochem Behav. 1998;60:237–244. doi: 10.1016/s0091-3057(97)00581-9. [DOI] [PubMed] [Google Scholar]
- King TS, Steger RW, Morgan WW. Effect of ovarian steroids to stimulate region-specific hypothalamic 5-hydroxytryptamine synthesis in ovariectomized rats. Neuroendocrinology. 1986;42:344–350. doi: 10.1159/000124461. [DOI] [PubMed] [Google Scholar]
- Koob GF. Drugs of abuse: anatomy, pharmacology and function of reward pathways. Trends Pharmacol Sci. 1992;13:177–184. doi: 10.1016/0165-6147(92)90060-j. [DOI] [PubMed] [Google Scholar]
- Li Y, Vartanian AJ, White FJ, Xue CJ, Wolf ME. Effects of the AMPA receptor antagonist NBQX on the development and expression of behavioral sensitization to cocaine and amphetamine. Psychopharmacology (Berl) 1997;134:266–276. doi: 10.1007/s002130050449. [DOI] [PubMed] [Google Scholar]
- Maxwell SE, Delaney HD. Designing Experiments and Analyzing Data: A Model Comparison Perspective. Pacific Grove, CA: Brooks/Cole; 1990. [Google Scholar]
- McNair DM, Lorr M, Droppleman LF. Manual of the Profile of Mood States. San Diego, CA: Educational and Industrial Testing Service; 1971. [Google Scholar]
- Moghaddam B, Bolinao ML. Glutamatergic antagonists attenuate ability of dopamine uptake blockers to increase extracellular levels of dopamine: implications for tonic influence of glutamate on dopamine release. Synapse. 1994;18:337–342. doi: 10.1002/syn.890180409. [DOI] [PubMed] [Google Scholar]
- Mumford GK, Rush CR, Griffiths RR. Abecarnil and alprazolam in humans: behavioral, subjective and reinforcing effects. J Pharmacol Exp Ther. 1995;272:570–580. [PubMed] [Google Scholar]
- Newton TF, Kalechstein AD, Tervo KE, Ling W. Irritability following abstinence from cocaine predicts euphoric effects of cocaine administration. Addict Behav. 2003;28:817–821. doi: 10.1016/s0306-4603(01)00273-8. [DOI] [PubMed] [Google Scholar]
- Pap A, Bradberry CW. Excitatory amino acid antagonists attenuate the effects of cocaine on extracellular dopamine in the nucleus accumbens. J Pharmacol Exp Ther. 1995;274:127–133. [PubMed] [Google Scholar]
- Peris J. Repeated cocaine injections decrease the function of striatal gamma-aminobutyric acid(A) receptors. J Pharmacol Exp Ther. 1996;276:1002–1008. [PubMed] [Google Scholar]
- Petroff OA, Hyder F, Mattson RH, Rothman DL. Topiramate increases brain GABA, homocarnosine, and pyrrolidinone in patients with epilepsy. Neurology. 1999;52:473–478. doi: 10.1212/wnl.52.3.473. [DOI] [PubMed] [Google Scholar]
- Petroff OA, Hyder F, Rothman DL, Mattson RH. Topiramate rapidly raises brain GABA in epilepsy patients. Epilepsia. 2001;42:543–548. doi: 10.1046/j.1528-1157.2001.18800.x. [DOI] [PubMed] [Google Scholar]
- Preston KL, Vahabzadeh M, Schmittner J, Lin JL, Gorelick DA, Epstein DH. Cocaine craving and use during daily life. Psychopharmacology (Berl) 2009;207:291–301. doi: 10.1007/s00213-009-1655-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston KL, Walsh SL, Sannerud CA. Measures of interoceptive stimulus effects: relationship to drug reinforcement. In: Johnson BA, Roache JD, editors. Drug Addiction and its Treatment: Nexus of Neuroscience and Behavior. Philadelphia, PA: Lippincott-Raven; 1997. pp. 91–114. [Google Scholar]
- Reis AD, Castro LA, Faria R, Laranjeira R. Craving decrease with topiramate in outpatient treatment for cocaine dependence: an open label trial. Rev Bras Psiquiatr. 2008;30:132–135. doi: 10.1590/s1516-44462008005000012. [DOI] [PubMed] [Google Scholar]
- Roache JD. Role of the human laboratory in the development of medications for alcohol and drug dependence. In: Johnson BA, editor. Addiction Medicine: Science and Practice. New York: Springer Science + Business Media, LLC; 2010. pp. 129–157. [Google Scholar]
- Roache JD, Griffiths RR. Diazepam and triazolam self-administration in sedative abusers: concordance of subject ratings, performance and drug self-administration. Psychopharmacology (Berl) 1989;99:309–315. doi: 10.1007/BF00445549. [DOI] [PubMed] [Google Scholar]
- Roache JD, Johnson BA, Ait-Daoud N, Mauldin JB, Thornton JE, Wells LT, Murff WL. Effects of repeated-dose isradipine on the abuse liability of cocaine. Exp Clin Psychopharmacol. 2005;13:319–326. doi: 10.1037/1064-1297.13.4.319. [DOI] [PubMed] [Google Scholar]
- Roache JD, Meisch RA. Findings from self-administration research on the addiction potential of benzodiazepines. Psychiatr Ann. 1995;25:153–157. [Google Scholar]
- Rockhold RW. Glutamatergic involvement in psychomotor stimulant action. Prog Drug Res. 1998;50:155–192. doi: 10.1007/978-3-0348-8833-2_4. [DOI] [PubMed] [Google Scholar]
- Schiffer WK, Gerasimov MR, Marsteller DA, Geiger J, Barnett C, Alexoff DL, Dewey SL. Topiramate selectively attenuates nicotine-induced increases in monoamine release. Synapse. 2001;42:196–198. doi: 10.1002/syn.10000. [DOI] [PubMed] [Google Scholar]
- Schneiderman JH. Topiramate: pharmacokinetics and pharmacodynamics. Can J Neurol Sci. 1998;25:S3–S5. doi: 10.1017/s031716710003482x. [DOI] [PubMed] [Google Scholar]
- Silverstone PH, Oldman D, Johnson B, Cowen PJ. Ondansetron, a 5-HT3 receptor antagonist, partially attenuates the effects of amphetamine: a pilot study in healthy volunteers. Int Clin Psychopharmacol. 1992;7:37–43. doi: 10.1097/00004850-199200710-00005. [DOI] [PubMed] [Google Scholar]
- Skradski S, White HS. Topiramate blocks kainate-evoked cobalt influx into cultured neurons. Epilepsia. 2000;41(suppl 1):S45–S47. doi: 10.1111/j.1528-1157.2000.tb02171.x. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Waters AJ, Mooney M, O’Malley SS. Minocycline reduced craving for cigarettes but did not affect smoking or intravenous nicotine responses in humans. Pharmacol Biochem Behav. 2009;92:135–140. doi: 10.1016/j.pbb.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss RD, Griffin ML, Hufford C. Craving in hospitalized cocaine abusers as a predictor of outcome. Am J Drug Alcohol Abuse. 1995;21:289–301. doi: 10.3109/00952999509002698. [DOI] [PubMed] [Google Scholar]
- Wheeler RA, Aragona BJ, Fuhrmann KA, Jones JL, Day JJ, Cacciapaglia F, Wightman RM, Carelli RM. Cocaine cues drive opposing context-dependent shifts in reward processing and emotional state. Biol Psychiatry. 2011;69:1067–1074. doi: 10.1016/j.biopsych.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White HS, Brown SD, Woodhead JH, Skeen GA, Wolf HH. Topiramate enhances GABA-mediated chloride flux and GABA-evoked chloride currents in murine brain neurons and increases seizure threshold. Epilepsy Res. 1997;28:167–179. doi: 10.1016/s0920-1211(97)00045-4. [DOI] [PubMed] [Google Scholar]
- Witkin JM. Blockade of the locomotor stimulant effects of cocaine and methamphetamine by glutamate antagonists. Life Sci. 1993;53:L405–L410. doi: 10.1016/0024-3205(93)90496-p. [DOI] [PubMed] [Google Scholar]
- Wolf ME. The role of excitatory amino acids in behavioral sensitization to psychomotor stimulants. Prog Neurobiol. 1998;54:679–720. doi: 10.1016/s0301-0082(97)00090-7. [DOI] [PubMed] [Google Scholar]
- Woods JH, Katz JL, Winger G. Abuse liability of benzodiazepines. Pharmacol Rev. 1987;39:251–413. [PubMed] [Google Scholar]
- Zhang XF, Hu XT, White FJ, Wolf ME. Increased responsiveness of ventral tegmental area dopamine neurons to glutamate after repeated administration of cocaine or amphetamine is transient and selectively involves AMPA receptors. J Pharmacol Exp Ther. 1997;281:699–706. [PubMed] [Google Scholar]
- Zullino DF, Khazaal Y, Hättenschwiler J, Borgeat F, Besson J. Anticonvulsant drugs in the treatment of substance withdrawal. Drugs Today. 2004;40:603–619. doi: 10.1358/dot.2004.40.7.850478. [DOI] [PubMed] [Google Scholar]