Abstract
We previously reported a subset of γδ T cells in mice which preferentially responds following intradermal immunization with collagen in Complete Freund’s Adjuvant (CFA). These cells express a nearly invariant “canonical” Vγ4Vδ4+ TCR. They are potent producers of IL-17A, and promote the development of collagen-induced arthritis. In this study, we report that CFA emulsified with PBS alone (without collagen) is sufficient to induce a strong response of Vγ4Vδ4+ cells in the draining lymph nodes of DBA/1 and C57BL/6 mice, and that the TCRs of the elicited Vγ4Vδ4+ cells in both strains heavily favor the canonical sequence. However, although both CFA and Incomplete Freund’s Adjuvant (IFA, which lacks the killed mycobacteria present in CFA) induced Vγ4Vδ4+ γδ T cell to expand, only CFA stimulated them to express IL-17A. The route of immunization was also critical, since intraperitoneal CFA induced only a weak response by these cells, whereas intradermal or subcutaneous CFA strongly stimulated them, suggesting that the canonical CFA-elicited Vγ4Vδ4+ cells are recruited from Vγ4+ γδ T cells normally found in the dermis. Their IL-17A response requires the toll-like receptor adapter protein MyD88, and their activation is enhanced by IFNγ, although αβ T cells need not be present. The CFA-elicited Vγ4Vδ4+ γδ T cells show a cytokine profile different from that of other previously described IL-17-producing γδ T cells. Finally, the Vγ4Vδ4+ subset appears to promote the Th17 αβ T cell response, suggesting its importance in mounting an effective immune response against certain pathogens.
Keywords: gamma/delta T cells, Th17 response, IL-17, dermis
Introduction
The γδ T cells are often regarded as distinct subsets, based on the TCRs they express, for two reasons: first, because γδ T cells that reside in particular tissues differ with regard to the TCRs they express; and second, because those expressing the same V-genes have been found to carry out similar functions [1]. This observation has led many to speculate that γδ T cells in tissues such as the skin and the intestinal epithelium act as sentinels that help prevent microbial infection, and that their specificity is tied to this role. Despite this, the ligands recognized by the TCRs of γδ T cells responding in various experimental models remain for the most part uncharacterized. γδ T cells have been implicated as regulatory cells in various models [reviewed in [2]]. Conversely, numerous studies have now also shown that γδ T cells, particularly certain subsets, can instead produce IL-17, a cytokine critical for neutrophil recruitment, and thereby exacerbate autoimmunity [reviewed in [3]].
We previously discovered a subset of γδ T cells that preferentially expands in the draining lymph nodes of DBA/1 mice following intradermal injection of complete Freund’s adjuvant (CFA). These cells virtually all express a Vγ4Vδ4+ TCR, whose junctions are limited such that for each chain, the majority represent a particular sequence motif. This “canonical” subset is heavily biased to secrete IL-17, a property that probably explains why DBA/1 male mice, which can be induced to develop collagen-induced arthritis when immunized by intradermal injection of collagen emulsified in CFA, show a reduced disease incidence and severity if the response of Vγ4+ cells is blocked [4]. This canonical Vγ4Vδ4+ subset has many properties in common with a predominant γδ TCR+ subset normally found in the dermis, as recently reported in three different publications [5–7]. The dermal γδ T cells were shown to differ from the fetal thymus-derived γδ TCR+ dendritic epidermal T cells (DETCs) subset, which represents most of the T cells normally present in the epidermis of mice, in terms of the type of TCR they express (since DETCs are Vγ5Vδ1+ but the dermis-associated γδ T cells are not), and also in having an overall lower TCR level and higher CCR6 level (which is nearly absent on DETCs) [5]. The dermis-associated γδ T cells predominantly express IL-17 when stimulated with PMA/ionomycin [5–7], and about half express a Vγ4+ TCR [6, 7]. In one study, mice injected intradermally with BCG were found to undergo a rapid induction of IL-17 secretion among their dermal γδ (but not αβ) T cells, a process also found to be important in subsequent neutrophil recruitment [7]. In another study [6], IL-17-producing γδ T cells were shown to promote psoriasis in a mouse model plus in psoriasis patients, dermal γδ T cells were similarly discovered to be increased. The psoriasis-associated human γδ T cells also produced IL-17 when stimulated in culture, strongly implying that dermal γδ T cell subsets in mice and humans are functional analogues. In this study, we show that Vγ4Vδ4+ IL-17-producing γδ T cells are also preferentially induced by intradermal CFA injection in C57BL/6-background mice. For most of these induced cells, we discovered that their TCRs have junctions that are “canonical,” i.e. that they represent a well-defined sequence motif. We also investigated what is needed to bring about their response, in terms of type of adjuvant, injection route, and host inflammatory signals. Finally, we present evidence that the response of the canonical Vγ4Vδ4+ γδ T cell subset promotes the concomitant development of Th17 CD4+ αβ T cells.
Materials and Methods
Mice
DBA/1LacJ, C57BL/6, B6.TCRβ−/−, B6.TCRδ−/−, and B6.MyD88−/− mice were originally purchased from Jackson Laboratories (Bar Harbor, ME) and thereafter bred in our facility. The B6.TCRβ−/− IFNγ−/− strain was generated by breeding B6.IFNγ−/− (from Jackson Laboratories) with B6.TCRβ−/− mice to create F2 mice, and then establishing a new strain by selecting as breeders the progeny homozygous for both inactivated genes. B6.Vγ4/6−/− mice were bred in our facility, as previously described [8, 9]. Both male and female mice were used unless otherwise designated in the figure legends, at ages ranging from 8–16 weeks. Mice were injected with 100 μl of complete Freund’s adjuvant (CFA) containing 4 mg/ml of killed Mycobacerium tuberculosis H37 RA (Difco; Fisher) emulsified with an equal volume of PBS, either intradermally at the base of the tail, subcutaneously in the scruff of the neck, or intraperitoneally. Mice were also stimulated with 100 μl of incomplete Freund’s adjuvant (IFA) emulsified in an equal volume of PBS (Difco; Fisher), or with 100 μl of 2.25 mg alum (aluminum hydroxide; AlumImuject; Pierce) emulsified in PBS. Mice were boosted as indicated with a second identical injection on day 14–21, and sacrificed on day 26, or on the day indicated in the figure legends. Axillary and inguinal lymph nodes, and popliteal lymph nodes as well in some experiments, were taken for analysis. For naïve controls in many experiments, because of low cell numbers, T cells from 2–4 mice were pooled to allow for the analyses.
TCR Vγ4 Sequences
This analysis was carried out as previously described [4]. Briefly, RNA was isolated from nylon wool purified lymph node cells and amplified with primers specific for Cγ1 and Vγ4. The cDNA products were then TA cloned using the pCR2.1 vector (InVitrogen), and individually sequenced to determine the amino acid sequence encoded in the junctional region.
Flow Cytometry
Single cell suspensions from lymph nodes or from peritoneal lavage were passed over nylon wool to enrich for T cells, and then stained for flow cytometry as previously described [9]. Biotinylated and FITC-labeled anti-Vγ1 (2.11 [10]), anti-Vγ4 (UC3 [11]), and anti-Vδ4 (GL2 [12]) monoclonal antibodies were prepared in our laboratory, and used to stain cells together with streptavidin-APC (eBioscience) or streptavidin–Cychrome (BD Biosciences), and PE-conjugated anti-CD44 monoclonal antibody (BD Biosciences). In some experiments, T cells were instead or also stained using anti-Cβ-FITC (H57-597 [13]) monoclonal antibody, or with anti-CD8β-APC (eBioscience) and anti-CD4-FITC (GK1 [14]). Intracellular cytokine staining was carried out as previously described [4]; briefly, cells were first activated in vitro by culturing them for 4–6 hours with PMA/ionomycin. After surface staining and fixation, cells were permeabilized with saponin-containing buffer, and intracellularly stained with PE-labeled antibodies specific for IL-17F (eBioscience); IL-17A, IL-2, IFNγ, TNFα (BD Biosciences); IL-22 (R&D Systems); and in some experiments also with IL-17A-APC (eBioscience). Stained samples were analyzed on a FACSCalibur or FACScan flow cytometer (BD Biosciences), and the data were processed using FlowJo software (Tree Star). Note that the nomenclature for mouse Vγ genes used in this study is that of Heilig and Tonegawa [15]. The WHO-IUIS equivalent designations are: Vγ1 (GV5S1), Vγ4 (GV3S1), Vδ4 (DV104S1) [16].
Luminex Cytokine Assay
Cells were passed over nylon wool to enrich for T cells, then negatively selected by staining them with biotinylated antibodies against TCRβ and Vγ4 (for Vγ1 enriched cells), or TCRβ and Vγ1 (for Vγ4 enriched cells), followed by incubation with streptavidin-MACS beads and passage over LD magnetic columns to remove the positive cells (Miltenyi Biotec). Cells from the lymph nodes of 3 mice were pooled for each group. After purification, the cells were cultured for 40 hours at 2 x105/well in 96-well plates coated with 10 μg/ml pan-specific anti-TCRδ antibody (GL3 [12]). The culture supernatants were then analyzed using a 20-plex cytokine assay (InVitrogen), and the Luminex 100 system. Values obtained for cytokines in ng/ml were determined from standards analyzed at the same time.
Vγ4+ T cell depletion/inactivation
Mice were immunized by intradermal injection of CFA emulsified in PBS as described above on day 0 and day 21. At day -4, mice were also injected intravenously in the tail vein with 200μg of purified anti-Vγ4 monoclonal antibody, and this treatment was repeated at day 17. Mice were sacrificed on day 26.
Statistical Analysis
Differences between 2 groups were analyzed using a two-tailed Student’s t-test; a p-value of 0.05 or less was considered to be significant. All experiments were performed at least twice, using 3 or more mice per group, unless otherwise noted. For scatter plots, each symbol shows the result obtained from an individual mouse, except for naïve controls in which in most cases cells from 2–4 mice were pooled and the value for a single mouse then calculated; longer lines superimposed over the symbols show the mean, and shorter ones show the s.e.m. unless otherwise indicated. Bar graphs indicate the average obtained, and the errors bars indicate the s.e.m. unless otherwise indicated in the figure legend.
Results
CFA alone preferentially induces Vγ4Vδ4+ cells in the draining lymph nodes in both DBA/1 and C57BL/6 mice
We previously reported that DBA/1 mice with collagen-induced arthritis show preferential expansion of a particular γδ T cell subset, which expresses a Vγ4Vδ4+ TCR, is biased to produce IL-17A, has an activated phenotype based on several different activation markers, and carries TCR chains whose junctional amino acid sequences, particularly in the case of the γ chain, are identical or nearly so. In the same study, we also observed that CFA alone (without collagen), when injected using a similar protocol, was sufficient to induce the preferential expansion of Vγ4Vδ4+ cells biased to produce IL-17A [4]. However, whether or not the TCR junctions of these cells also contained the previously noted canonical conserved amino acid sequence motif was not determined in that study. We therefore examined the junctions of Vγ4 transcripts from T cells isolated from the draining lymph nodes of DBA/1 mice injected intradermally with CFA, and found indeed that most (31/45; about 69%) encode this motif (Fig. 1A). In comparison, only ~25% of naïve DBA/1 Vγ4 transcripts contained the YG(X)LYS junction, whereas it was present in 88% of the Vγ4 transcripts isolated from collagen/CFA treated DBA/1 mice [4]. This indicates that intradermal injection of PBS-emulsified CFA alone, without any collagen, is sufficient to induce the preferential response of the canonical Vγ4Vδ4+ subset in DBA/1 mice.
Fig. 1. DBA/1LacJ mice treated with CFA, but not IFA or alum, show preferential expansion of the canonical Vγ4Vδ4+ γδ T cell subset.
DBA/1LacJ were mice immunized intradermally with collagen/CFA (denoted CIA), or with CFA, IFA, or alum only emulsified in PBS, and the draining lymph nodes harvested on the day indicated. Untreated controls (naïve) are shown for comparison A. Vγ4 transcripts from the lymph nodes of four CFA-treated males, immunized twice on d. 0 and 21 and sacrificed on day 26 (d. 26), were amplified by PCR, and the cDNA transcripts then cloned and sequenced, separately for each mouse. The predicted amino acid sequence encoded by each unique junction is shown (which includes the C-terminal portion of Vγ4, N-encoded amino acids, and N-terminal Jγ1 amino acids), and the number of clones obtained represented by each sequence is indicated; the conserved motif (top line) was predominant. B. The number of Vγ4Vδ4+ cells obtained from DBA/1LacJ mice treated as indicated; some mice were immunized only once (1X) and the lymph node cells harvested as indicated on day 9 or day 11, whereas others were injected twice (2X) on days 0 and 21 and the lymph node cells harvested on day 26. Each group contained 3–9 mice; each symbol within a group represents the result from an individual mouse; error bars show the s.d. C. As for B, except the average percent of Vγ4+ cells co-expressing Vδ4 is indicated for each treatment group.
We therefore went on to examine whether CFA was needed to stimulate the expansion of these Vγ4Vδ4+ cells, or whether incomplete Freund’s adjuvant (IFA) or alum might also suffice. When following the same injection protocol used to induce CIA (denoted d. 26 in Fig. 1), IFA was able to induce Vγ4Vδ4+ cell numbers to expand and the proportion of Vγ4+ cells that co-expressed Vδ4 to increase (Fig. 1B and C), although it was less effective in this regard than CFA/collagen or CFA alone. Injection of alum, in contrast, did not induce any measurable Vγ4Vδ4 expansion. We also examined whether a single injection of CFA might be sufficient to induce the response of this subset. Indeed, among lymphocytes present in lymph nodes 9 days after a single CFA injection, Vγ4Vδ4+ cells were slightly increased in both numbers and proportion, compared to those in the lymph nodes of naïve controls (d. 9 in Fig. 1B and C). A single injection of IFA as well was also able to induce a small but measurable increase of these cells at day 9, though not in their proportion. Not surprisingly, no expansion of this subset after a single dose of alum (tested at day 11) was seen (Fig. 1B).
The TCRs of Vγ4Vδ4+ cells that expand in the lymph nodes of C57BL/6 mice are also canonical
Although C57BL/6 mice, unlike DBA/1 mice, rarely develop CIA when immunized with collagen/CFA, we suspected that collagen/CFA or CFA only might expand Vγ4Vδ4+ cells in this strain as well. We found that both collagen/CFA (not shown) and CFA alone (Fig. 2A) are indeed able to induce a robust expansion of Vγ4Vδ4+ cells and an increase in their proportion (Fig. 2B) following two intradermal injections, when examined on day 26 after the initial injection. At an earlier timepoint (day 19), the expansion of this subset and its proportionate increase were still clearly evident, but substantially weaker. As seen with the DBA/1 strain, a single injection of CFA was also sufficient to induce a weak, though measurable, Vγ4Vδ4+ cell expansion 9 days later in C57BL/6 mice.
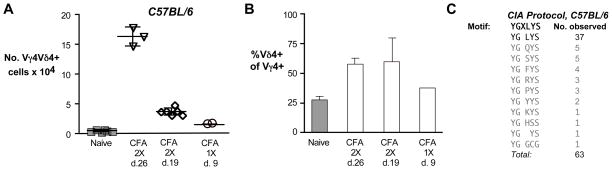
Fig. 2. C57BL/6 mice immunized with CFA also causes preferential expansion of the canonical Vγ4Vδ4+ subset in the draining lymph nodes.
A. The number of Vγ4Vδ4+ cells obtained from the lymph nodes of individual C57BL/6 mice immunized with intradermal CFA on days 0 and 21 and sacrificed on day 26, or immunized on days 0 and 14 and sacrificed on day 19, or immunized on day 0 and sacrificed on day 9, are shown. B. As for A, except the average percent of Vγ4+ cells co-expressing Vδ4 is indicated for each treatment group. C. Four C57BL/6 male mice were treated as for Fig. 1A, except that mice were immunized with CFA/collagen (the CIA protocol) instead of CFA only, and cloned Vγ4 transcripts from each individual were sequenced.
To verify whether, in experiments using C57BL/6 mice, the TCRs of the observed expanded Vγ4Vδ4 population also predominantly contained the conserved junctional motifs, we sequenced cDNA from Vγ4 transcripts derived from the draining lymph nodes of C57BL/6 mice treated using the same protocol we used for DBA/1 mice to elicit CIA. As shown in Fig. 2C, although only 21% of the in-frame Vγ4 transcripts from C57BL/6 naïve controls carried the canonical sequence (4/19; data not shown), here we also found that the junctions of the majority (37/63, or 59%) of the in-frame transcripts from the immunized mice expressed the conserved sequence. Although this was somewhat lower than the frequency observed for DBA/1 mice treated with collagen/CFA (which was 88% [4]) or with CFA only (69%; see Fig. 1A), it is possible that the frequency actually is essentially the same between the two strains, because one of the 4 C57BL/6 mice we tested apparently responded very poorly and had only 30% canonical Vγ4 transcripts (3/10), compared to 64% (8/13), 45% (13/29) and 100% (11/11) for the other 3 mice. Thus, intradermal CFA immunization in C57BL/6 mice also resulted in a clear preferential expansion of Vγ4Vδ4+ γδ T cells having the same conserved Vγ4 sequence, indicating that the induction of this subset does not depend upon the DBA/1 background.
Both CFA and IFA can induce the expansion of Vγ4Vδ4+ γδ T cells, but CFA is needed to stimulate them to express IL-17A
We next tested whether in C57BL/6 mice, the Vγ4Vδ4+ cells induced by CFA also show an induction of activation markers and a bias to produce IL-17A, as we found in DBA/1 mice [4]. As shown in Fig. 3A, both of these characteristics were evident in CFA-immunized C57BL/6 mice, even when the cells were examined only 9 days after a single intradermal CFA injection, which is before the increase in the proportion of Vγ4+ cells co-expressing Vδ4 is evident (see Fig. 2B, above). Because IFA appeared to be less effective in inducing expansion of Vγ4Vδ4+ cells than CFA in DBA/1 mice (see Fig. 1B, above), we wondered whether IFA-induced Vγ4Vδ4+ cells would, like those induced by CFA, show an increased bias to produce IL-17A. As shown in Fig. 3B (left panel), at 9 days following immunization, IL-17A expression in the C57BL/6-derived Vγ4Vδ4+ cells did not differ from that of naïve controls, whereas in the cells from CFA-treated mice, IL-17A had already been induced at this timepoint. Because the expansion of Vγ4Vδ4+ cells at day 9 is barely detectable, we also examined CFA vs. IFA-treated mice given two immunizations, at day 26 after the initial inoculation, to test whether IL-17A induction might be demonstrable after a stronger stimulation. Although a slightly higher percentage was obtained in IFA-treated mice, it was not significantly different from the percent of IL-17A producing Vγ4Vδ4+ cells normally present in naïve B6 mice (Fig. 3B, right panel). Thus, IFA injected intradermally appears to be inadequate for inducing IL-17A production in this subset. IFA did induce some upregulation of CD44 expression on the Vγ4Vδ4+ population, but this tended to be lower than that induced by CFA (data not shown).
Fig. 3. C57BL/6-derived Vγ4Vδ4+ cells induced by intradermal CFA also upregulate CD44 expression and are biased to produce IL-17A.
A. Results of typical experiments showing the percentage of C57BL/6-derived Vγ4Vδ4+ cells with high levels of CD44, and the percent of this subset that was positive for IL-17A after PMA/ionomycin stimulation and intracellular cytokine staining. Mice were treated by intradermal CFA immunization as described in Fig. 2A, and lymph nodes harvested on the day shown. Some points in this graph represent results from only two animals, but the findings were confirmed in other experiments (not shown) using similar but not identical conditions. B. As for A, except the IL-17A+ percentage of Vγ4Vδ4+ cells is compared for day 9 vs. day 26 for mice treated with either CFA or IFA. The result for mice treated with CFA on day 9 represents the average obtained from only 2 animals, but is included for comparison. The IFA and CFA results were confirmed in other experiments under similar but not identical conditions.
Intraperitoneal CFA immunization induces a comparatively weak response by Vγ4Vδ4+ cells
Because intradermal CFA was sufficient for the induction of the canonical Vγ4Vδ4+ cells, we also examined whether this particular route of injection is needed. When CFA was injected twice intraperitoneally into C57BL/6 mice, an increase in Vγ4Vδ4+ cell numbers was evident on day 26 (Fig. 4A), along with a corresponding and significant increase in the proportion of Vγ4+ cells co-expressing Vδ4 (Fig. 4B, left panel). Although the induced peritoneal Vγ4Vδ4+ cells expressed high levels of CD44 (Fig. 4B, right panel), the percentage was not significantly higher than that found on these cells in naïve mice, though both were considerably above that seen for naïve Vγ4Vδ4+ cells in lymph nodes (see Fig. 3A above). We were thus unable to use this characteristic to assess their activation. The increase in peritoneal Vγ4Vδ4+ cell numbers induced by CFA, though obvious, was of a much smaller degree than was found in peripheral lymph nodes following intradermal CFA injection (Fig. 4C). As we previously noted following intradermal treatment with CFA/collagen [4], no increase of Vγ4Vδ4+ cell numbers or proportion was detectable in the spleens of mice injected intraperitoneally with CFA (data not shown). Thus, intraperitoneal injection of CFA elicits a comparatively weak local response by Vγ4Vδ4+ cells.
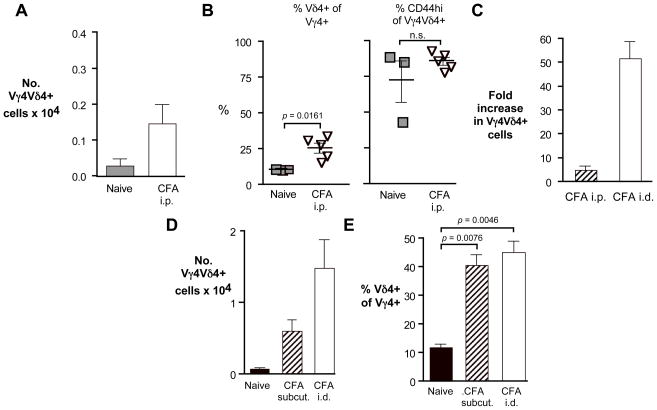
Fig. 4. The route of immunization influences the strength of the response of the Vγ4Vδ4+ subset to CFA.
A–C. C57BL/6 mice were either uninfected (naïve controls), or given an intraperitoneal (i.p.) injection of CFA emulsified in PBS on days 0 and 21. Cells were stained for flow cytometric analysis 26 days after the first injection. A. Peritoneal Vγ4Vδ4+ cell numbers obtained. B. Percent of peritoneal Vγ4+ cells co-expressing Vδ4 (left) and percent of peritoneal CD44-high Vγ4Vδ4+ cells (right). C. The fold-increase in intraperitoneally (i.p) or intradermally (i.d.) immunized mice was calculated by dividing the number of Vγ4Vδ4+ cells obtained in each CFA stimulated mouse by the average obtained per mouse in naïve controls. D–E. C57BL/6 mice were injected twice as for intradermal CFA immunization, but subcutaneously at the scruff of the neck, and cells from the axillary and inguinal lymph nodes were combined and analyzed on day 26. Lymph nodes were pooled for the naïve controls, whereas subcutaneously or intradermally injected mice show the average obtained from 4–5 animals. D. Vγ4Vδ4+ cell numbers obtained from lymph nodes of mice immunized by subcutaneous injection of CFA. E. The percent of Vγ4+ cells co-expressing Vδ4 from the same samples shown in D.
Vγ4Vδ4+ γδ T cells are also strongly and preferentially induced by subcutaneous injection of emulsified CFA
Vγ4+ cells normally found in the dermis appear to have many of the same properties as the canonical Vγ4Vδ4+ cells that preferentially respond following CFA immunization [17, 18, 5–7]. Delivering an inflammatory stimulus to the dermis via subcutaneous injection of CFA might therefore be expected to generate a strong response of Vγ4Vδ4+ cells, comparable to that elicited by intradermal CFA immunization. We tested this and found that subcutaneous injection indeed induced a substantial increase in Vγ4Vδ4+ cell numbers (Fig. 4D), as well as an increase in the proportion of Vγ4+ cells that co-expressed Vδ4 (Fig. 4E), indicating a preferential response by this subset. Based on cell numbers obtained, the response induced by subcutaneous injection may be somewhat weaker than that induced by intradermal injection, however. Because the Vγ4Vδ4+ cells respond efficiently to CFA introduced either into the skin or just beneath it, and but only weakly to CFA introduced intraperitoneally, the dermis is a likely reservoir for this subset.
Induction of IL-17-producing Vγ4Vδ4+ cells does not require the presence of αβ T cells
In a previous study, the in vitro stimulated IL-17 secretion by γδ T cells was found to be greatly enhanced when αβ T cells were present in the same culture [19]. We therefore wondered whether in mice lacking αβ T cells, the Vγ4Vδ4+ subset would respond. When B6.TCRβ−/− mice, which have no αβ T cells, were treated with intradermal CFA, we found that the Vγ4Vδ4+ cells nonetheless expanded vigorously (Fig. 5A). The fold-increase of the Vγ4Vδ4+ subset was considerably less than in wildtype C57BL/6 mice (only about 7-fold compared to more than 50-fold, as shown in Fig. 2E above); this may be a consequence of the already-expanded state of the γδ T cells in the B6.TCRβ−/− strain, the probable result of enhanced homeostatic expansion due to the absence of αβ T cells [20]. Indeed, the Vγ4Vδ4+ cells in B6.TCRβ−/−mice also appeared to be activated because, as in wildtype mice, there was a clear increase in the proportion of Vγ4+ cells co-expressing Vδ4, an induction of IL-17A in these cells, and an upregulation of CD44 expression (Fig. 5B). Therefore, αβ T cells are not necessary for the in vivo expansion and activation of the Vγ4Vδ4+ subset.
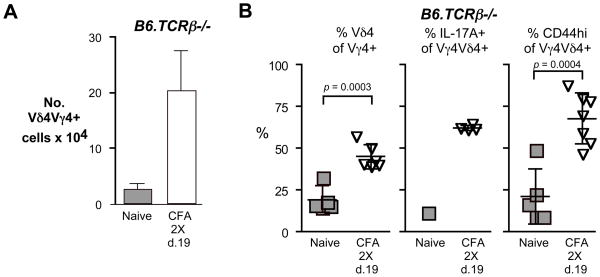
Fig. 5. The response of the Vγ4Vδ4+ cells to intradermal immunization with CFA does not require the presence of αβ T cells.
A. Vγ4Vδ4+ cell numbers obtained from lymph nodes of B6.TCRβ−/− mice immunized by intradermal injection of CFA on days 0 and 14, and sacrificed on day 19. Error bars show s.d. B. B6.TCRβ−/− mice were treated as in A, and lymph nodes cells analyzed by flow cytometry. The percent of Vγ4+ cells co-expressing Vδ4 (left), the percent of IL-17A+ cells among Vγ4Vδ4+ cells (center), and the percent of CD44-high cells among Vγ4Vδ4+ cells (right), are shown. For each condition, 5–7 mice were tested, except for IL-17A expression by naïve Vγ4Vδ4+ cells, which is shown for comparison and represents lymph nodes from two mice that were pooled.
Activation of the Vγ4Vδ4+ cells requires stimulation via a PRR and is enhanced by IFNγ
Induction of IL-17A expression in Vγ4Vδ4+ cells in this system required immunization with CFA, which contains killed mycobacteria, as opposed to intradermal IFA, which does not. IL-17A induction may thus require an additional signal, likely triggered by molecules in the bacterial component of CFA engaging with a pattern recognition receptor (PRR), such a toll-like receptor (TLR). We therefore tested whether in MyD88−/− mice, which are deficient in most TLR signaling due to the absence of a critical signal transduction adaptor protein, the Vγ4Vδ4 cells respond following immunization with intradermal CFA. Compared to wildtype C57BL/6 controls, we found that expansion of this subset was markedly reduced in MyD88−/− mice (Fig. 6A). Although the proportion of Vγ4+ cells that co-expressed Vδ4 was nonetheless clearly elevated (Fig. 6B), CFA immunization was not able to induce IL-17A production in the B6.MyD88−/− derived Vγ4Vδ4+ cells (Fig. 6C). Because IFNγ enhances the macrophage response to TLR2 and TLR4 ligands [21], we next examined whether IFNγ is necessary for induction of the Vγ4Vδ4+ cells. As shown in Fig. 6D (left panel), expansion of the Vγ4Vδ4+ subset was reduced in B6.TCRβ−/− IFNγ−/− mice, compared to B6.TCRβ−/− mice. This may reflect the more general reduction in inflammatory cells that are recruited to the draining lymph nodes in mice that cannot produce IFNγ ((not shown), especially since Vδ4+ cells that instead co-express Vγ1 showed a similarly reduced expansion in this strain (Fig. 6D, right panel; note that like αβ T cells, Vγ1+ cells also normally increase in number in the lymph nodes following intradermal immunization with CFA [4]). Nonetheless, the activation of the Vγ4Vδ4+ cells was impaired in the TCRβ−/− IFNγ−/− mice, because the percent of Vγ4+ cells co-expressing Vδ4 in these mice increased only marginally compared to those in TCRβ−/− controls (Fig. 6E, 1st panel), and was not significant. Despite this overall weaker response, high-level expression of CD44 was still clearly induced on the B6.TCRβ−/− IFNγ−/− derived Vγ4Vδ4+ cells, albeit to a lesser degree than on those from B6.TCRβ−/− mice (Fig. 6E, 3rd panel). In both B6.TCRβ−/− and B6.TCRβ−/− IFNγ−/− mice, as in wildtype mice [4], the percent increase in cells co-expressing Vδ4, and their CD44 upregulation, was specific to Vγ4+ cells; γδ T cells co-expressing Vδ4 instead in conjunction with Vγ1 did not change in proportion in either the TCRβ−/− or TCRβ−/− IFNγ−/− mice (Fig. 6E, 2nd panel), and they did not acquire CD44 expression (Fig. 6E, 4th panel), as we had noted previously in wildtype mice [4]. Given the general decrease in the inflammatory response in the TCRβ−/− IFNγ−/− strain, whether or not the activation of Vγ4Vδ4+ cells is normally enhanced by IFNγ could not be determined from this experiment, but clearly at least some steps towards the activation of this subset are possible without it.
Fig. 6. Neither a TLR signal nor IFNγ is critical for stimulating expansion of the Vγ4Vδ4 subset, but a TLR signal appears to be required for inducing them to produce IL-17A.
A–C. Mice were immunized by intradermal injection of CFA on days 0 and 21, and their lymph nodes were harvested on day 26. For each group, 3–6 mice were analyzed except as noted below. A. The Vγ4Vδ4+ cell numbers obtained from C57BL/6 vs. B6.MyD88−/− mice. Cells from the lymph nodes of 2 mice were pooled for the MyD88 naïve control. B. The percent of Vγ4+ cells co-expressing Vδ4. Cells from the lymph nodes of 3 mice were pooled for the MyD88 naïve control. C. The percent of IL-17A+ cells among Vγ4Vδ4+ cells. Only 2 mice were analyzed for the B6.MyD88−/− CFA treated group, and cells from the lymph nodes of 3 mice were pooled for the MyD88 naïve control. D–E. Mice were immunized as for A except immunizations were on day 0 and 14, and lymph nodes were harvested on day 20. D. The Vγ4Vδ4+ and Vγ1Vδ4+ cell numbers obtained from B6.TCRβ−/− vs. B6.TCRβ−/−IFNγ−/− mice. Error bars show s.d.; n.s.= not significant (p > 0.05). E. The percent of Vγ4+ or Vγ1+ cells co-expressing Vδ4 (left), and the percent of CD44-high Vγ4Vδ4+ or Vγ1Vδ4+ cells (right).
CFA-elicited Vγ4Vδ4+ cell show a distinct cytokine profile
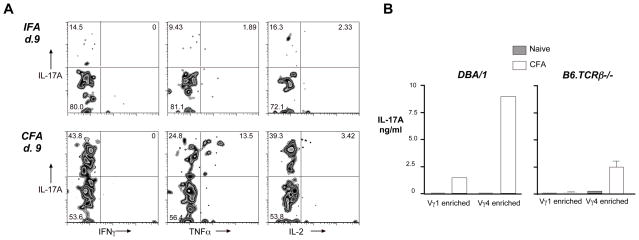
To further characterize the Vγ4Vδ4+ cells induced by intradermal CFA, we examined them for their ability to produce several other cytokines. As shown in Fig. 7A, whereas CFA-induced Vγ4Vδ4+ cells from C57BL/6 mice frequently express IL-17A, they do not produce IL-17F or IL-22, other IL-17 family members. In this way, they differ from IL-17-producing γδ T cells that were previously described in several other studies [22, 6, 5]. Compared to CFA-induced Vγ4Vδ4+ cells, in which nearly half of the cells under the conditions used for this experiment were induced to express IL-17A (Fig. 7, left panels, center), both naïve Vγ4Vδ4+ cells and Vγ4+ cells from CFA-immunized mice that do not co-express Vδ4 showed a less pronounced IL-17A bias (Fig. 7, left two panels, top and bottom).
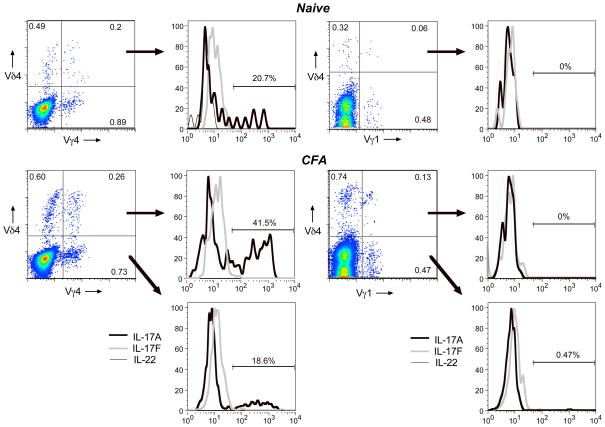
Fig. 7. Activation of the canonical Vγ4Vδ4 subset induces it to produce IL-17A, but not IL-17F or IL-22.
C57BL/6 mice were immunized by intradermal injection of CFA on day 0 and day 14, and the lymph nodes harvested on day 26. A typical example of flow cytometry profiles of cells from an individual mouse is shown (CFA, bottom), along with a typical example from an untreated control mouse (naïve, top). Histograms show the staining for 3 different intracellular cytokines within cells gated by the quadrants indicated.
In contrast, CFA-induced cells instead expressing Vγ1, even if they co-expressed Vδ4, produced almost no intracellular IL-17A (Fig. 7. right two panels). IL-17A induction in Vγ4Vδ4+ cells could be detected as early as day 9 following a single intradermal CFA injection, but not IFA (Fig. 8A). Although a small fraction of the IL-17A+ cells co-expressed TNFα, none expressed IFNγ either at this timepoint or at day 26 (not shown), and only a very few expressed IL-2. We went on to confirm that Vγ4+ cells from the draining lymph nodes of mice immunized with intradermal CFA actually secrete substantially more IL-17A protein than do Vγ1+ cells, by testing supernatants from the cultures of purified cells activated in vitro, using a Luminex Multiplex cytokine assay (Fig. 8B).
Fig. 8. Vγ4Vδ4+ cells induced to express IL-17 do not co-express IFNγ or IL-2, although some co-express TNFα.
A. C57BL/6 mice were immunized by intradermal injection of CFA or IFA, and lymph nodes harvested on day 9. The panels show a typical example of a flow cytometry panel from an individual mouse. B. Draining lymph nodes were taken on day 20 from DBA/1LacJ or B6.TCRβ−/− mice that were either left untreated, or that received intradermal CFA injections on days 0 and 14. Cells from 3 mice were pooled, passed over nylon wool to enrich for T cells, and negatively selected using MACS beads to enrich for either Vγ1+ or Vγ4+ cells. Cells were cultured on wells coated with anti-Cδ monoclonal antibody, and the presence of various cytokines measured using a multi-plex cytokine bead assay with known standards. Cells from DBA/1 mice show the results from single cultures; error bars for cells from B6.TCRβ−/− mice show the range of values obtained from duplicate wells tested separately.
The Vγ4Vδ4+ subset promotes a Th17 αβ T cell response
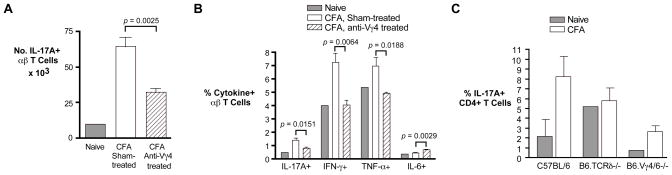
Because the Vγ4Vδ4+ cells provide a relatively early source of IL-17A during inflammation, they could promote the development of αβ TCR+ IL-17-producing cells, including Th17 cells, as we suggested in our previous study [4]. We examined this by pre-treating mice, prior to each intradermal injection of CFA, by intravenous injection of anti-Vγ4 specific monoclonal antibody, which inactivates and/or depletes most of the Vγ4+ cells [23, 9]. The numbers of IL-17-producing αβ T cells present in the draining lymph nodes of mice so treated were found to be substantially reduced compared to those obtained from sham-treated controls (Fig. 9A). Moreover, the proportion of IL-17A-producing αβ T cells was reduced by more than half in mice treated with anti-Vγ4 antibody as compared to untreated controls (Fig. 8B). Somewhat surprisingly, the percentage of IFNγ producing αβ T cells was reduced to a similar degree, and that of αβ T cells producing TNF-α also significantly decreased. Conversely, although the overall percentages were quite low, we observed a highly significant enhancement of IL-6+ αβ T cells in mice treated with anti-Vγ4+ antibody vs. untreated controls; this is interesting because IL-6 is important in promoting the differentiation of Th17 αβ T cells [24] but not of IL-17-producing γδ T cells [25]. In another experiment, we examined IL-17A expression specifically in CD4+ T cells and obtained similar results: the percentage of IL-17A CD4+ cells obtained from mice given intradermal CFA was substantially reduced in TCRδ−/− and Vγ4/6−/− mice (which cannot produce either Vγ4+ or Vγ6+ cells but have near-normal numbers of other γδ T cells) [8, 9], as compared to wildtype mice (Fig. 9C). Because most of the Vγ4+ cells in lymph nodes following treatment with intradermal CFA are canonical Vγ4Vδ4+ cells, this subset appears to be needed for a full-fledged Th17 response.
Fig. 9. The αβ T cell response to intradermal CFA is altered when Vγ4+ cells are inactivated.
C57BL/6 mice were immunized intradermally with CFA on days 0 and 21, with intravenous injections of anti-Vγ4 antibody preceding each immunization by 4 days; lymph nodes were harvested on day 26. A–B: Each group represents the average obtained from 4 mice whose cells were analyzed individually, except the naïve control, included for comparison, for which cells from the lymph nodes of 4 mice were pooled together before the analysis. A. Numbers of αβ T cells obtained expressing IL-17A intracellularly after 4 hours of culture with PMA/ionomycin. B. As for A, except the percent of αβ T cells expressing the indicated cytokine is shown. C. The percent of IL-17A+ cells present in CD4+ cells was determined. Here, mice were immunized by intradermal injection of CFA on day 0 and day 14, and the lymph nodes harvested on d. 19. Error bars show the range obtained from samples from duplicate mice analyzed separately; for naïve TCRδ−/− and naive Vγ4/6−/− mice, shown for comparison, the result was determined from cells obtained from a single mouse only.
Discussion
Several recent reports describe γδ T cells normally present in the dermis that often express Vγ4 and are biased to produce IL-17A [5–7]. These dermal γδ T cells, which are highly motile and appear to be pre-activated to some degree, have not yet been characterized with regard to the Vδ genes they co-express, or the sequences of their Vγ4 TCR junctions. The canonical Vγ4Vδ4+ cells we have studied here that expand in the draining lymph nodes following intradermal CFA injection may be recruited from this dermal population, because we found that Vγ4Vδ4+ cells also preferentially responded in the draining lymph nodes following subcutaneous immunization with CFA. Moreover, in previous studies, subcutaneous injection of CFA-emulsified with a uveitis-inducing retinal peptide, which promotes the subsequent development of uveitis, also led to the preferential activation of a Vγ4Vδ4+ subset [19, 26]. Like the dermal γδ T cells, the CFA-elicited Vγ4Vδ4+ cells to some extent appear to be preactivated: although the CD44 level on this subset increases to a higher level following CFA immunization, it is already unusually high on most of these cells even in naïve mice [4, 7], compared to Vγ1+ cells [4]. According to several reports, Vγ4+ γδ T cells from naïve mice can be induced by culture with cytokines alone (lL-23 with or without added IL-1β) to produce IL-17 [27], and also to proliferate [6], and although the stimulation can be augmented by the presence of pathogen products, these were not required [6]. This suggests that in our system, cytokines elicited by CFA, together with molecules derived from the mycobacteria present in CFA, are likely sufficient to induce proliferation and promote the migration of the Vγ4Vδ4+ cells into lymph nodes. It also implies that, despite their highly conserved TCRs, the Vγ4Vδ4+ γδ T cell response does not require stimulation via the TCR for these steps. This may be a general property of T lymphocytes that differentiate into IL-17-biased cells, because certain cytokine combinations in vitro were recently shown to be sufficient to stimulate IL-17 secretion by both γδ T cells and naïve CD4+ αβ T cells, without any need for a TCR signal [28]. Because killed Mycobacterium tuberculosis was recently shown to stimulate IL-1β and IL-18 production by dendritic cells, and this cytokine combination was able to induce IL-17 production by both αβ and γδ T cells [28], it is possible that TCR stimulation of the Vγ4Vδ4+ cells in our system also is not needed to induce them to secrete IL-17.
Canonical γδ TCRs, with invariant or nearly invariant junctions, are characteristic of three previously described γδ T cell subsets: the Vγ5Vδ1+ “DETC” cells found almost exclusively in the epidermis [29]; the Vγ6Vδ1+ cells that respond in many different types of inflammation and are resident in the tongue, nasal epithelium, and female reproductive tract and may also be resident in the peritoneum [30, 31, 3]; and a Vγ1Vδ6.3 subset that in many ways resembles iNKT αβ T cells [32]. All of these are derived from precursors that develop only in the fetal thymus. Interestingly, Gray et al. presented evidence that at least some of the dermal γδ T cells are also fetal thymus-derived [5]. Thus, if the cells we have investigated in this study are indeed dermis-derived, their canonical TCR may be fetal thymus-derived as well.
Some of the characteristics of the Vγ4Vδ4+ IL-17-producing γδ T cells may be typical of all IL-17 producing γδ T cells; these have been variously reported as expression of high levels of CD44+, CD25, TLR2, and CCR6; and production of IL-22 and IL-17F instead or as well. Among the Vγ6Vδ1+ canonical subset that expands in the lung following repeated intranasal instillation of live Bacillus subtilis, a large fraction secreted IL-17A and produced or co-produced IL-22 or IL-17F, although they did not express IFNγ [22]. Moreover, IL-17 and IL-22 co-expression was common among γδ T cells elicited in the peritoneum following injection of heat-killed mycobacteria with a synthetic aryl hydrocarbon receptor ligand [33]. However, γδ T cells elicited by infection with Listeria monocytogenes, which largely express the canonical Vγ6Vδ1 TCR, often expressed both IL-17A and IFNγ [3, 34]. Thus, even within the Vγ6Vδ1 canonical subset whose members bear virtually identical TCRs, differences in the way the cells are elicited appear to impact their cytokine profile. For the canonical Vγ4Vδ4+ cells studied here, like the Vγ4+ cells of the dermis [6], production of IFNγ was not found, even though Vγ4Vδ4+ cells that produce IFNγ have been previously described following coxsackievirus B3 infection [35]. Whether this discrepancy can be explained by differences in the agents eliciting the response, or reflects a subtle difference in the responding γδ T cell subset, is not clear at this time. Furthermore, dermal Vγ4+ cells were shown in two recently published studies to produce IL-17F and/or IL-22 as well as IL-17A [5, 6], whereas we found in this study that a high percentage of the CFA-elicited lymph node Vγ4Vδ4+ cells express IL-17A, but not IL-22 or IL-17F. If the dermal Vγ4+ cells indeed represent the canonical Vγ4Vδ4 cells that were recruited to skin-draining lymph nodes following intradermal or subcutaneous CFA immunization, as we speculate here, this could indicate that their inherent cytokine bias changes during immunization, or instead that the canonical Vγ4Vδ4+ dermal cells differ from other Vγ4+ dermal cells in this regard.
We were unable in this study to detect TLR2 or TLR4 by flow cytometry on the Vγ4Vδ4+ CFA-responsive cells in lymph nodes (data not shown), although TLR2 expression has been previously noted on IL-17-producing γδ T cells [36]. This was somewhat surprising, since TLR2 is directly involved in the induction of IL-17 expression on CD4+ T cells, IL-23 has been shown to induce TLR2 and TLR4 mRNA expression on splenic γδ T cells [37], and TLR ligands and other PRRs have been shown to enhance IL-23 stimulated expression of IL-17 by γδ T cells [33, 6]. Scart1 and/or Scart2, which are scavenger receptors expressed by Vγ4+ γδ T cells in the dermis and in skin-draining lymph nodes [17, 18], may play a similar role in inducing IL-17 on these cells as do TLRs on other γδ T cells and CD4 αβ T cells, if they are able to bind to pathogen products derived from the mycobacteria present in CFA. The IL-17A response by canonical Vγ4Vδ4+ cells appears to require stimulation not only via cytokine receptors but also TLRs, since IL-17A was not induced on these cells in CFA-immunized MyD88−/− mice (see Fig. 6C above). Consistently, a recent publication showed that IFA containing TLR 2 and 4 ligands can be an effective substitute for the mycobacteria in CFA in inducing IL-17+ γδ T cells and Th17 cells, although it did not show whether the γδ T cells themselves express TLR2 or TLR4, whereas IFA containing instead TLR3 and TLR7 ligands was not found to be very effective [38].
In our experiments, removing certain components from the system - mycobacteria, MyD88, or IFNγ - also decreased the overall strength of the inflammatory response, so that an increase in Vγ4Vδ4+ cells could not be used to assess a response by the Vγ4Vδ4+ cells. However, as noted in mice that received a single dose of CFA and were analyzed 9 days later, even though the increase in numbers of Vγ4Vδ4+ cells was quite small at that timepoint, their response was clear based on the proportionate increase of γδ T cells expressing this TCR, their increase in CD44 expression, and the induction of IL-17A expression within them. Since the Vγ4Vδ4+ cells express neither TLR2 nor TLR4 and do not produce IFNγ when stimulated, presumably their response is instead triggered by the stimulation of other cells that express these molecules, such as dendritic cells. Whether these particular molecules are required for the Vγ4Vδ4 response, or instead others with related functions can substitute for them, remains to be determined. It is possible that the mycobacteria, MyD88, and IFNγ act in this system via a common mechanism: a TLR molecule (likely to be TLR2 or TLR4 since mycobacteria carry ligands for these) whose signaling involves MyD88, which leads to secretion of IFNγ in the responding mice. More likely, the process is more complex, however, because we saw a different outcome depending upon which of these signals was disrupted. Specifically, a defect in MyD88 hindered the induction of IL-17A in Vγ4Vδ4+ cells but did not alter their proportionate increase, whereas a defect in IFNγ reduced the increase in the proportion of Vγ4Vδ4+ cells, but did not prevent them from being induced to express high levels of CD44 (see Fig. 6 above). Delineating the signals that are needed to fully activate the Vγ4Vδ4+ cells in this system will require further study.
We presented evidence in this study that the Vγ4Vδ4 subset promotes a Th17-type immune response (Fig. 8 above). Consistently, Sumaria et al. [7] similarly demonstrated that lack of dermal γδ T cells can reduce the subsequent antigen-specific CD4 response to intradermally-injected mycobacteria, and others [19, 27, 38] also showed that the γδ T cells in other experimental systems that likely represent the canonical Vγ4Vδ4+ subset enhance IL-17 production by αβ T cells, including CD4+ Th17 cells. So, although these cells clearly can promote autoimmunity [4, 19, 27, 18], they are probably also critical in immune defense, for the mounting of an effective Th17 response. The concomitant reduction we noted of the Th1-associated cytokines IFNγ and TNFα when these cells are not present could indicate that the Vγ4Vδ4+ subset might also be important in promoting a subsequent strong Th1 response, particularly since IL-17A induces dendritic cells to produce IL-12, and thereby helps to bring about a Th1 response [39], and in a study concerning the pulmonary CD4 T cell response to vaccination against mycobacteria, IL-23 and IL-17 were found to be critical to eliciting the accelerated response of IFNγ-producing CD4+ cells [40]. Interestingly, we also noted in our study a marked increase in IL-6-producing αβ T cells following treatment of the mice with anti-Vγ4 antibody (Fig. 8B above). This may reflect a lag in the differentiation of CD4 αβ T cells into Th17 cells, since IL-6 is important in this process [41]. Our findings suggest that the IL-17 response of canonical Vγ4Vδ4+ cells is likely to be important in promoting host resistance to certain infectious agents. Because Cai et al. [6] recently showed that psoriasis patients have elevated levels of dermal IL-17-producing γδ T cells, if the canonical Vγ4Vδ4+ cells in mice are indeed derived from γδ T cells normally found in the dermis, similar γδ T cells are likely to play an important role in humans as well, in promoting effective Th17 and perhaps Th1 responses.
References
- 1.O’Brien RL, Roark CL, Jin N, Aydintug MK, French JD, Chain JL, et al. γδ T cell receptors - Functional correlations. Immunol Rev. 2007;215:77–88. doi: 10.1111/j.1600-065X.2006.00477.x. [DOI] [PubMed] [Google Scholar]
- 2.Hayday AC. γδ T cells: A right time and a right place for a conserved third way of protection. Ann Rev Immunol. 2000;18:975–1026. doi: 10.1146/annurev.immunol.18.1.975. [DOI] [PubMed] [Google Scholar]
- 3.O’Brien RL, Roark CL, Born WK. IL-17-producing γδ T cells. Eur J Immunol. 2009;39:662–6. doi: 10.1002/eji.200839120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roark CL, French JD, Taylor MA, Bendele AM, Born WK, O’Brien RL. Exacerbation of collagen-induced arthritis by oligoclonal, IL-17-producing γδ T cells. J Immunol. 2007;179:5576–83. doi: 10.4049/jimmunol.179.8.5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gray EE, Suzuki K, Cyster JG. Cutting edge: Identification of a motile IL-17-producing γδ T cell population in the dermis. J Immunol. 2011;186(11):6091–5. doi: 10.4049/jimmunol.1100427 [doi].. jimmunol.1100427 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cai Y, Shen X, Ding C, Qi C, Li K, Li X, et al. Pivotal role of dermal IL-17-producing γδ T cells in skin inflammation. Immunity. 2011;35(4):596–610. doi: 10.1016/j.immuni.2011.08.001 [doi].. S1074-7613(11)00306-2 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sumaria N, Roediger B, Ng LG, Qin J, Pinto R, Cavanagh LL, et al. Cutaneous immunosurveillance by self-renewing dermal γδ T cells. J Exp Med. 2011;208(3):505–18. doi: 10.1084/jem.20101824 [doi]. jem.20101824 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sunaga S, Maki K, Komagata Y, Miyazaki J-I, Ikuta K. Developmentally ordered V-J recombination in mouse T cell receptor γ locus is not perturbed by targeted deletion of the Vγ4 gene. J Immunol. 1997;158:4223–8. [PubMed] [Google Scholar]
- 9.O’Brien RL, Yin X, Huber SA, Ikuta K, Born WK. Depletion of a γδ T cell subset can increase host resistance to a bacterial infection. J Immunol. 2000;165:6472–9. doi: 10.4049/jimmunol.165.11.6472. [DOI] [PubMed] [Google Scholar]
- 10.Pereira P, Gerber D, Huang SY, Tonegawa S. Ontogenic development and tissue distribution of Vγ1-expressing γ/δ T lymphocytes in normal mice. J Exp Med. 1995;182:1921–30. doi: 10.1084/jem.182.6.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dent AL, Matis LA, Hooshmand F, Widacki SM, Bluestone JA, Hedrick SM. Self-reactive γδ T cells are eliminated in the thymus. Nature. 1990;343:714–9. doi: 10.1038/343714a0. [DOI] [PubMed] [Google Scholar]
- 12.Goodman T, LeCorre R, Lefrancois L. A T-cell receptor γδ-specific monoclonal antibody detects a Vγ5 region polymorphism. Immunogen. 1992;35:65–8. doi: 10.1007/BF00216631. [DOI] [PubMed] [Google Scholar]
- 13.Kubo RT, Born W, Kappler JW, Marrack P, Pigeon M. Characterization of a monoclonal antibody which detects all murine αβ T cell receptors. J Immunol. 1989;142:2736–42. [PubMed] [Google Scholar]
- 14.Dialynas DP, Quan ZS, Wall KA, Pierres A, Quintans J, Loken MR, et al. Characterization of the murine T cell surface molecule, designated L3T4, identified by monoclonal antibody GK1.5: similarity of L3T4 to the human Leu-3/T4 molecule. J Immunol. 1983;131:2445–51. [PubMed] [Google Scholar]
- 15.Heilig JS, Tonegawa S. T-cell γ gene is allelically but not isotypically excluded and is not required in known functional T-cell subsets. Proc Natl Acad Sci USA. 1987;84:8070–4. doi: 10.1073/pnas.84.22.8070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arden B, Clark SP, Kabelitz D, Mak TW. Mouse T-cell receptor variable gene segment families. Immunogen. 1995;42:501–30. doi: 10.1007/BF00172177. [DOI] [PubMed] [Google Scholar]
- 17.Kisielow J, Kopf M, Karjalainen K. SCART scavenger receptors identify a novel subset of adult γδ T cells. J Immunol. 2008;181(3):1710–06. doi: 10.4049/jimmunol.181.3.1710. [DOI] [PubMed] [Google Scholar]
- 18.Fink DR, Holm D, Schlosser A, Nielsen O, Latta M, Lozano F, et al. Elevated numbers of SCART1+ γδ T cells in skin inflammation and inflammatory bowel disease. Mol Immunol. 2010;47(9):1710–8. doi: 10.1016/j.molimm.2010.03.002 [doi].. S0161-5890(10)00081-7 [pii] [DOI] [PubMed] [Google Scholar]
- 19.Cui Y, Shao H, Lan C, Nian H, O’Brien RL, Born WK, et al. Major role of γδ T cells in the generation of IL-17 uveitogenic T cells. J Immunol. 2009;183:560–7. doi: 10.4049/jimmunol.0900241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.French JD, Roark CL, Born WK, O’Brien RL. γδ T cell homeostasis is established in competition with αβ T cells and NK cells. Proc Natl Acad Sci USA. 2005;102:14741–6. doi: 10.1073/pnas.0507520102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-γ: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75(2):163–89. doi: 10.1189/jlb.0603252 [doi]jlb.0603252 [pii].. [DOI] [PubMed] [Google Scholar]
- 22.Simonian PL, Wehmann F, Roark CL, Born WK, O’Brien RL, Fontenot AP. γδ T cells protect against lung fibrosis via IL-22. J Exp Med. 2010;207:2239–53. doi: 10.1084/jem.20100061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peterman GM, Spencer C, Sperling AI, Bluestone JA. Role of γδ T cells in murine collagen-induced arthritis. J Immunol. 1993;151:6546–58. [PubMed] [Google Scholar]
- 24.Stockinger B, Veldhoen M. Differentiation and function of Th17 cells. Curr Opin Immunol. 2007;19:281–6. doi: 10.1016/j.coi.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 25.Shibata K, Yamada H, Hara H, Kishihara K, Yoshikai Y. Resident Vδ1+ γδ T cells control early infiltration of neutrophils after Escherichia coli infection via IL-17 production. J Immunol. 2007;178(7):4466–72. doi: 10.4049/jimmunol.178.7.4466. 178/7/4466 [pii] [DOI] [PubMed] [Google Scholar]
- 26.Nian H, Shao H, O’Brien RL, Born WK, Kaplan HJ, Sun D. Activated γδ T cells promote the activation of uveitogenic T cells and exacerbate EAU development. Invest Ophthalmol Vis Sci. 2011 doi: 10.1167/iovs.10-6758 [doi].. in press. iovs.10-6758 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Ladeville EC, Mills KHG. Interleukin-1 and IL-23 induce innate IL-17 production from γδ T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–41. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 28.Lalor SJ, Dungan LS, Sutton CE, Basdeo SA, Fletcher JM, Mills KH. Caspase-1-processed cytokines IL-1beta and IL-18 promote IL-17 production by γδ and CD4 T cells that mediate autoimmunity. J Immunol. 2011;186(10):5738–48. doi: 10.4049/jimmunol.1003597 [doi].. jimmunol.1003597 [pii] [DOI] [PubMed] [Google Scholar]
- 29.Asarnow DM, Kuziel WA, Bonyhadi M, Tigelaar RE, Tucker PW, Allison JP. Limited diversity of γδ antigen receptor genes of Thy-1+ dendritic epidermal cells. Cell. 1988;55:837–47. doi: 10.1016/0092-8674(88)90139-0. [DOI] [PubMed] [Google Scholar]
- 30.Itohara S, Farr AG, Lafaille JJ, Bonneville M, Takagaki Y, Haas W, et al. Homing of a γδ thymocyte subset with homogeneous T-cell receptors to mucosal epithelia. Nature. 1990;343:754–7. doi: 10.1038/343754a0. [DOI] [PubMed] [Google Scholar]
- 31.Kim CH, Witherden DA, Havran WL. Characterization and TCR variable region gene use of mouse resident nasal γδ T lymphocytes. J Leuk Biol. 2008;84:1259–63. doi: 10.1189/jlb.0108050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grigoriadou K, Boucontet L, Pereira P. Most IL-4-producing γδ thymocytes of adult mice originate from fetal precursors. J Immunol. 2003;171:2413–20. doi: 10.4049/jimmunol.171.5.2413. [DOI] [PubMed] [Google Scholar]
- 33.Martin B, Hirota K, Cua DJ, Stockinger B, Veldhoen M. Interleukin-17-producing γδ T cells selectively expand in response to pathogen products and environmental signals. Immunity. 2009;131:321–30. doi: 10.1016/j.immuni.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 34.Hamada S, Umemura M, Shiono T, Hara H, Kishihara K, Tanaka K, et al. Importance of murine Vδ1 γδ T cells expressing IFNγ and IL-17A in innate protection against Listeria monocytogenes infection. Immunol. 2008;125:170–7. doi: 10.1111/j.1365-2567.2008.02841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huber SA, Graveline DA, Born WK, O’Brien RL. Cytokine production by Vγ+ T cell subsets is an important factor determining CD4 Th-cell phenotype and susceptibility of BALB/c mice to coxsackievirus B3-induced myocarditis. J Virol. 2001;75:5860–9. doi: 10.1128/JVI.75.13.5860-5869.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mokuno Y, Matsuguchi T, Takano M, Nishimura H, Washizu J, Ogawa T, et al. Expression of toll-like receptor 2 on γδ T cells bearing invariant Vγ6/Vδ1 induced by Escherichia coli infection in mice. J Immunol. 2000;165(2):931–40. doi: 10.4049/jimmunol.165.2.931. ji_v165n2p931 [pii] [DOI] [PubMed] [Google Scholar]
- 37.Reynolds JM, Pappu BP, Peng J, Martinez GJ, Zhang Y, Chung Y, et al. Toll-like receptor 2 signaling in CD4(+) T lymphocytes promotes T helper 17 responses and regulates the pathogenesis of autoimmune disease. Immunity. 2010;32(5):692–702. doi: 10.1016/j.immuni.2010.04.010 [doi]. S1074-7613(10)00159-7 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zuo A, Liang D, Shao H, Born WK, Kaplan HJ, Sun D. In vivo priming of IL-17(+) uveitogenic T cells is enhanced by Toll ligand receptor (TLR)2 and TLR4 agonists via γδ T cell activation. Mol Immunol. 2012;50(3):125–33. doi: 10.1016/j.molimm.2011.12.013 [doi].. S0161-5890(11)00838-8 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin Y, Ritchea S, Logar A, Slight S, Messmer M, Rangel-Moreno J, et al. Interleukin-17 is required for T helper 1 cell immunity and host resistance to the intracellular pathogen Francisella tularensis. Immunity. 2009;31(5):799–810. doi: 10.1016/j.immuni.2009.08.025 [doi].. S1074-7613(09)00448-8 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khader SA, Bell GK, Pearl JE, Fountain JJ, Rangel-Moreno J, Cilley GE, et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol. 2007;8(4):369–77. doi: 10.1038/ni1449 [doi].. ni1449 [pii] [DOI] [PubMed] [Google Scholar]
- 41.Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of T(H)17 cells. Nature. 2008;453(7198):1051–7. doi: 10.1038/nature07036. [DOI] [PMC free article] [PubMed] [Google Scholar]