Abstract
Elevated ghrelin has been shown to be associated with reduced luteinizing hormone (LH) pulsatility in Rhesus monkeys, rats, men, and recently women. We previously reported that 24-h ghrelin concentrations are elevated in women following a 3-mo exercise and diet program leading to weight loss. We investigated whether the elevations in ghrelin following an ∼3-mo exercise and diet program leading to weight loss are associated with a decrease in LH pulsatility. The nonexercising control group (Control, n = 5) consumed a controlled diet that matched energy needs, whereas energy intake in the exercise group (Energy Deficit, n = 16) was reduced from baseline energy requirements and supervised exercise training occurred five times per a week. Significant decreases in body weight (−3.0 ± 0.6 kg), body fat (−2.9 ± 0.4 kg) and 24-h LH pulse frequency (−0.18 ± 0.08 pulses/h), and a significant increase in 24-h mean ghrelin were observed in only the Energy Deficit group. The pre-post change in LH pulse frequency was negatively correlated with the change in mean 24-h ghrelin (R = −0.485, P = 0.030) and the change in peak ghrelin at lunch (R = −0.518, P = 0.019). Interestingly, pre-post change in night LH pulse frequency was negatively correlated with the change in mean day ghrelin (R = −0.704, P = 0.001). Elevated total ghrelin concentrations are associated with the suppression of LH pulsatility in premenopausal women and may play a role in the suppression of reproductive function following weight loss.
Keywords: luteinizing hormone, ghrelin, exercise training, caloric restriction, weight loss
ghrelin is an orexigenic hormone secreted by distinct endocrine cells of the stomach called X/A-like cells or ghrelin cells (11, 24). Peripherally produced ghrelin is able to cross the blood-brain barrier and regulate neuropeptide Y (NPY) and agouti-related protein (AgRP) release from the arcuate nucleus in the hypothalamus (21). Additionally, ghrelin signals are transmitted though the vagus nerve to also stimulate the release of NPY/AgRP (12). Ghrelin activates ghrelin receptors in the arcuate nucleus to upregulate the release of NPY and AgRP and suppresses the activation of the proopiomelanocortin (POMC) and cocaine- and amphetamine-regulated transcript (CART), leading to an increase in hunger and food intake (21, 48). Through these central mechanisms, ghrelin has been proposed to play a role in short-term energy homeostasis (48). Interestingly, ghrelin may also impact reproductive function through its central actions on hypothalamic neurons in the arcuate nucleus, causing indirect alterations on gonadotropin-releasing hormone (GnRH) neurons (5, 19).
Women with anorexia nervosa and exercising women with functional hypothalamic amenorrhea experience suppressed reproductive function and elevated ghrelin concentrations (8, 13, 34, 39, 40). Animal models have demonstrated a direct relationship between elevated ghrelin and reproductive suppression (14, 16, 43). Kluge and colleagues (22, 23) have demonstrated that pulsatile LH secretion is suppressed in men and women following prolonged ghrelin administration, presumably indicating that a chronic elevation in ghrelin is necessary to elicit a suppression of LH pulses.
We previously conducted a 3-mo randomized controlled trial to examine the impact of caloric restriction combined with exercise on menstrual cyclicity and reproductive function in premenopausal, previously untrained women. We found that fasting and 24-h total ghrelin concentrations were elevated in the experimental group that lost weight during the intervention compared with subjects who did not exercise and maintained body weight (29, 30). Although we have documented that our intervention produced menstrual disturbances (46), we had not examined the impact of our intervention on LH pulsatility. To date, there are no studies that have evaluated whether the chronic elevation in ghrelin concentrations following diet- and exercise-associated weight loss is accompanied by and/or associated with a decrease in LH pulsatility.
The purpose of this study was to investigate whether elevations in 24-h circulating ghrelin concentrations following an ∼3-mo exercise and diet program associated with diet- and exercise-induced weight loss are associated with a decrease in LH pulsatility in premenopausal women. We hypothesized that 1) the women who experienced an energy deficiency would demonstrate a significant decrease in LH pulsatility compared with the women in the Control group, who would demonstrate no significant changes in LH pulsatility; and 2) there would be a significant negative correlation between changes in ghrelin concentration and changes in LH pulse frequency.
METHODS
Screening
This study was part of a larger, prospective study designed to assess changes in endocrine and reproductive function in response to a controlled feeding and exercise intervention. The intervention was implemented in sedentary women to emulate exercise and restrictive eating patterns in which many young women engage. Subjects included nonsmoking, healthy, nonexercising (< 1 h/wk purposeful exercise) women ages 18–30 yr, 15–30% body fat, and BMI between 18 and 25 kg/m2. Exclusion criteria included any evidence of disordered eating or history of an eating disorder, loss/gain of a significant amount of weight (±2.3 kg) in the previous year, or use of hormonal contraceptives or medication that might alter metabolic hormones. Each subject signed an informed consent form approved by the Biomedical Institutional Review Board of The Pennsylvania State University.
Subjects provided information regarding demographics, medical history, menstrual history, and physical activity along with eating attitudes questionnaires. A fasting blood sample was obtained for analysis of a complete blood count, basic chemistry panel, and to rule out abnormal pituitary function or metabolic diseases. Psychological stability and the absence of eating disorders or risk of developing an eating disorder were established in an interview under the supervision of a clinical psychologist. Subjects met with a General Clinical Research Center registered dietician to ensure absence of aberrant dietary habits and suitability for a controlled feeding study. Documentation of 2–3 ovulatory menstrual cycles prior to the study was performed with measurements of midluteal phase serum progesterone and the midcycle urinary LH surge (First Response, Tambrands).
Subject Groupings
Following a screening and a baseline monitoring period of 4 wk, subjects completed a 3-mo diet and exercise intervention. Subjects included in this substudy were the subjects who 1) completed the 24-h blood sampling both pre- and post-intervention and 2) had been randomly assigned to either a nonexercising Control group (n = 5), who consumed enough calories to maintain weight, or to the Energy Deficit group (n = 16), who were prescribed and provided reductions in food calories and exercised to achieve a negative energy balance ranging from −30 to −60% compared with baseline energy needs. One subject in the nonexercising Control group was not included, because she lost over 5 kg and was not considered compliant to the protocol. One subject in the Energy Deficit group was not included because of documentation of a luteal phase defect during her control menstrual cycle.
Dietary intake during the intervention.
Dietary intake was controlled throughout the intervention (30). At baseline, each subject's daily energy requirement to maintain a stable body weight was determined through estimates of energy expenditure followed by a week-long “calibration” period allowing for adjustments in the dietary prescription if body weight fluctuated. To estimate total energy expenditure, resting metabolic rate was measured using indirect calorimetry, and this value was added to the total calories expended during a 24-h period as assessed by a triaxial accelerometer (RT3 accelerometer; Stayhealthy, Monrovia, CA) (29, 30).
After the baseline period, the Control group continued to consume the weight maintenance calorie level estimated from the 7-day calibration period. The Energy Deficit group was provided fewer calories (mean ± SE −25 ± 8%) than those required to maintain initial body weight and began supervised exercise training. The diet comprised 55% carbohydrate, 30% fat, and 15% protein (29, 30).
Exercise Training During the Intervention
Exercise was supervised throughout the entire study (29, 30). Whereas the Control group did not perform any exercise, the Energy Deficit group performed aerobic exercise five times per week at 70–80% of maximum heart rate as determined from tests of maximal aerobic capacity. The total amount of calories expended during each exercise session was measured using the OwnCal feature on the Polar S610 heart rate monitor (Polar Electro Oy, Kempele, Finland).
Fitness and Body Composition
Body composition was determined using hydrostatic weighing, and maximal aerobic capacity was determined using indirect calorimetry during the baseline and post-intervention time points according to previously published methods (29, 30).
Twenty-Four-Hour Repeated Blood Sampling
Subjects reported to the General Clinical Research Center at 0730 subsequent to an overnight fast and having abstained from exercise for 24 h. An intravenous catheter was inserted into a forearm vein. Blood samples were obtained every 10 min for 24 h both before and after the 3-mo weight loss intervention. Subjects remained in a supine position with their upper body and head slightly elevated. All postural changes were recorded. Meals were provided at 0900 (breakfast), 1200 (lunch), 1800 (dinner), and 2100 (snack), and subjects consumed these meals within 30 min. Total calories over the day represented 85% of each subject's weight maintenance intake to account for negligible physical activity during the procedure. Each subject consumed a 500-calorie dinner, and the rest of the daily calories were distributed over the remaining meals (43% at breakfast, 49% at lunch, and 4% for the snack). The macronutrient composition of the food over the entire day averaged 55% carbohydrate, 30% fat, and 15% protein and was not significantly different among the three meals (29).
Total Ghrelin
Total ghrelin was measured in duplicate in serum samples from the 24-h repeated blood sampling procedure hourly from 0800 to 1000 every 20 min from 1000 to 2000 and hourly from 2000 to 0800 using the Linco Research (St. Charles, MO) radioimmunoassay kit. Assay sensitivity was 100 pg/ml. The intra-assay and interassay coefficients of variation for the high control were 2.7% and 16.7%, respectively; the intra-assay and interassay coefficients of variation for the low control were 1.2% and 14.7%, respectively. All samples from a given subject were included in the same assay.
LH
LH was measured in serum samples from the 24-h repeated blood sampling procedure every 10 min from 0800 to 0800 using the Siemens (Deerfield, IL) Immulite kit. Assay sensitivity is 0.1 mIU/ml. The intra-assay and interassay coefficients of variation are 5.7% and 12.3%.
Data Analysis
LH pulse analysis.
The time series of the 24-h LH concentrations was analyzed for pulse frequency, peak amplitude, peak height, and 24-h mean LH using the pulse detection algorithm Cluster (CLUSTER 8) (42). A 2 × 1 pulse configuration was used with a t-statistic value of 2.0 for both upstroke and downstroke. Missing data were linearly interpolated between the two adjacent values. The LH variables of interest included mean LH, LH pulse frequency, maximal peak amplitude, mean interval between LH peaks, and LH area under the curve. LH area under the curve was calculated using the computer program Cluster (42) and was defined as the product of the mean peak amplitude and the time of the interval. Additionally, the 12-h day LH concentrations and 12-h night LH concentrations were analyzed for pulse frequency, peak amplitude, peak height, and 12-h day or night mean LH. Day LH concentrations were from 0800 to 1950; night LH concentrations were from 2000 to 0800.
Ghrelin analysis.
Twenty-four-hour mean ghrelin was represented as the average of all ghrelin concentrations (pg/ml) observed in the 24-h analysis. Total ghrelin area under the curve was calculated using the trapezoidal rule. Meal peaks were defined as the highest ghrelin concentration (pg/ml) that occurred prior to the meal administration. Meal response averages were the mean of ghrelin concentrations (pg/ml) from 2 h prior through 2 h after the meal. Additionally, day and night ghrelin concentrations were analyzed separately. Day ghrelin concentrations were from 0800 to 2100; night ghrelin concentrations were from 2200 to 0800.
Statistical Analysis
Data screening prior to analysis involved outlier detection and tests of normality. A paired t-test was used to determine whether significant within-group changes occurred in LH pulse frequency and ghrelin from pre- to post-intervention. Wilcoxon signed rank tests were used when the data were not normally distributed. For comparisons of the changes between groups, independent t-tests on change scores for LH pulse parameters and ghrelin parameters were employed. Mann-Whitney U-Tests were used when the data were not normally distributed. Pearson correlation coefficient analyses were performed to examine relationships between LH and ghrelin when both groups were combined. Stepwise linear regression was used to determine if the change in ghrelin was an independent predictor of change in LH pulse frequency. To explore the predictors of LH pulse frequency, change in body weight, change in mean 24-h ghrelin, change in lunch peak, and change in body fat were entered into the stepwise linear regression model. All analyses were performed using SPSS software (v. 16.0; SPSS, Chicago, IL). All data were reported as means ± SE.
Sample size was based on previously published data (1) demonstrating a significant correlation of serum ghrelin and serum LH in male rats. Although a population correlation of 0.72 was reported in Abou et al. (1), we chose a conservative value of 0.60. Thus, using a bivariate correlation, using two tails, a population correlation of 0.60, an alpha error probability of 0.05, and a power of 0.80, a total sample size of 19 was needed.
RESULTS
Baseline descriptive data for subjects in Control and Energy Deficit groups are shown in Table 1. The Energy Deficit group had a higher initial height, body weight, and fat mass but were similar with respect to age, BMI, fat-free mass, and initial fitness compared with the Control group.
Table 1.
Subject characteristics during pre- and post-intervention in Control and Energy Deficit groups
| Control, n = 5 |
Energy Deficit, n = 16 |
|||||
|---|---|---|---|---|---|---|
| Pre | Post | Change | Pre | Post | Change | |
| Age, yr | 21.2 ± 0.7 | N/A | N/A | 20.3 ± 0.5 | N/A | N/A |
| Height, cm | 159.1 ± 1.1 | N/A | N/A | 164.9 ± 1.3† | N/A | N/A |
| Weight, kg | 52.1 ± 0.9 | 50.9 ± 1.1 | −1.3 ± 0.7 | 58.4 ± 1.1† | 55.3 ± 1.2* | −3.1 ± 0.6† |
| BMI, kg/m2 | 20.6 ± 0.5 | 20.1 ± 0.7 | −0.5 ± 0.3 | 21.5 ± 0.5 | 20.4 ± 0.5* | −1.1 ± 0.2 |
| Fat mass, % | 24.6 ± 2.2 | 23.2 ± 1.8 | −1.4 ± 0.8 | 28.3 ± 1.2 | 24.6 ± 1.2* | −3.7 ± 0.6 |
| Fat mass, kg | 12.8 ± 1.1 | 11.8 ± 0.9 | −1.0 ± 0.6 | 16.6 ± 0.8† | 13.7 ± 0.9* | −2.9 ± 0.4† |
| Fat-free mass, kg | 39.3 ± 1.4 | 39.0 ± 3.0 | −0.3 ± 0.4 | 42.0 ± 0.9 | 41.8 ± 0.8 | −0.2 ± 0.4 |
| Vo2max, ml·kg−1·min−1 | 36.5 ± 3.0 | 38.0 ± 1.3 | 1.8 ± 4.0 | 37.0 ± 1.1 | 43.0 ± 1.5* | 5.9 ± 1.3 |
Values are means ± SE. Independent t-tests were used to compare change in body composition and fitness. Paired t-tests were used to compare body composition and fitness pre- vs. post-study.
Paired t-test, Pre- vs. Post-study, P < 0.05;
independent t-test, Control vs. Energy Deficit, P < 0.05.
Changes in body composition and fitness are shown in Table 1. As expected, the Energy Deficit group had a significant decrease in body weight, BMI, body fat percentage and fat mass, whereas the Control group demonstrated no changes in body weight, BMI, body fat percentage or fat mass. Additionally, fitness levels increased (P = 0.001) in the Energy Deficit group. A one-way ANOVA demonstrated that the only change in body composition that was significantly different between the groups was fat mass, which was greater in the Energy Deficit group than in the Control group (P = 0.038). Body weight changes ranged from +1.1 to −7.55 kg in the Energy Deficit group and +0.5 to −1.25 kg in the Control group.
Prescribed calorie intake and actual calorie intake varied less than 35 calories per week during the intervention, and no differences in this parameter existed among groups. Final macronutrient intake was in accordance with that prescribed, i.e., of 55% carbohydrates, 30% fat, and 15% protein with no differences between groups. With respect to compliance to the exercise training protocol, 15 of the 16 exercising women (94%) consistently reached their prescribed exercise calorie level per week and target exercise intensity level (70–80% of maximal heart rate).
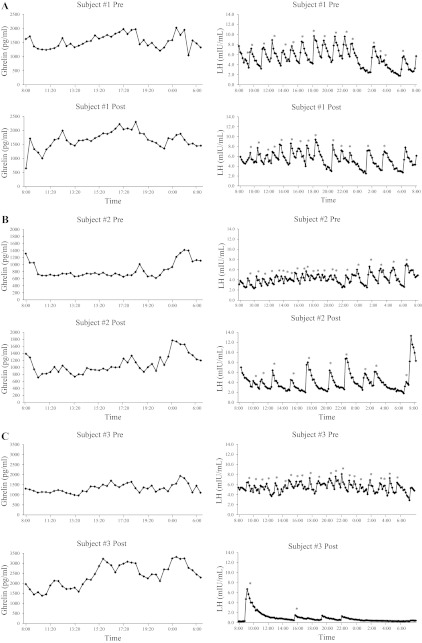
Ghrelin concentrations both pre- and post-intervention are presented in Table 2. Examples of individual mean 24-h ghrelin profiles are presented in Fig. 1. Mean 24-h ghrelin concentrations, 24-h ghrelin area under the curve, breakfast peak, lunch peak, dinner peak, breakfast response average, lunch response average, and dinner response average were all similar (P > 0.05) between the groups before the intervention. Paired t-tests demonstrated that the Energy Deficit group had a significant increase in mean 24-h ghrelin concentrations (P = 0.002), 24-h ghrelin area under the curve (P = 0.005), lunch peak (P < 0.001), dinner peak (P = 0.001), breakfast response average (P = 0.030), lunch response average (P = 0.004), and dinner response average (P = 0.004), whereas the Control group did not demonstrate any changes in ghrelin characteristics. When ghrelin data were analyzed separately for day and night, the change in mean night ghrelin (P = 0.035) and the change in mean day ghrelin (P = 0.006) also increased in the Energy Deficit group.
Table 2.
Comparison of pre- and post-intervention ghrelin concentrations, and meal-related ghrelin concentrations in Control and Energy Deficit groups
| Ghrelin Concentrations | Control, n = 5 |
Energy Deficit, n = 16 |
||||
|---|---|---|---|---|---|---|
| Pre | Post | Change | Pre | Post | Change | |
| Mean 24 h, pg/ml | 1,616 ± 122 | 1,634 ± 178 | 18 ± 74 | 1,217 ± 126 | 1,428 ± 150† | 241 ± 63 |
| Area under the curve | 37,663 ± 2,885 | 37,953 ± 3,752 | 289 ± 1373 | 29,562 ± 2,979 | 35,438 ± 3,588† | 5,876 ± 1731 |
| Meal peaks | ||||||
| Breakfast peak, pg/ml | 1,780 ± 175 | 1,908 ± 303 | 128 ± 230 | 1,475 ± 151 | 1,540 ± 143 | 65 ± 64 |
| Lunch peak, pg/ml | 1,764 ± 170 | 1,774 ± 238 | 11 ± 137 | 1,225 ± 137 | 1,507 ± 159† | 283 ± 60 |
| Dinner peak, pg/ml | 1,929 ± 153 | 1,953 ± 250 | 24 ± 239 | 1,448 ± 157 | 1,794 ± 183† | 346 ± 79 |
| Meal response averages * | ||||||
| Breakfast response average, pg/ml | 1,559 ± 182 | 1,514 ± 238 | −46 ± 164 | 1,241 ± 142 | 1,368 ± 146† | 127 ± 52 |
| Lunch response average, pg/ml | 1,511 ± 173 | 1,514 ± 199 | 3 ± 63 | 1,116 ± 120 | 1,282 ± 133† | 166 ± 48 |
| Dinner response average, pg/ml | 1,655 ± 103 | 1,709 ± 198 | 54 ± 179 | 1,235 ± 141 | 1,499 ± 167† | 264 ± 75 |
Values are expressed as means ± SE. Independent t-tests were used to compare change in lunch peak and breakfast response average. Mann-Whitney U-tests were used to compare the change in mean 24 h, area under the curve, breakfast peak, dinner peak, lunch response average, and dinner response average. Paired t-tests were used to compare all ghrelin concentrations pre- vs. post-study.
Paired t-test, Pre- vs. Post-study, P < 0.05.
Meal response averages were the mean of ghrelin concentrations, pg/ml, from 2 h prior through 2 h after the meal.
Fig. 1.
Examples of individual LH 24-h profiles during pre- and post-intervention. A: example subject 1 was in the Control group, lost 0.1 kg body wt, increased LH pulse frequency by 0.02 pulses/h, and increased mean 24-h ghrelin by 116 pg/ml. B: example subject 2 was in the Energy Deficit group, lost 3.3 kg body wt, decreased LH pulse frequency by 0.75 pulses/h, and increased mean 24-h ghrelin by 239 pg/ml. C: example subject 3 was in the Energy Deficit group, lost 6.3 kg body wt, decreased LH pulse frequency by 0.89 pulses/h, and increased mean 24-h ghrelin by 1,072 pg/ml.
LH characteristics for both pre- and post-intervention 24-h sampling periods are presented in Table 3. Examples of individual 24-h LH profiles are presented in Fig. 1. Mean LH, LH pulse frequency, maximal peak amplitude, mean interval between LH peaks, and LH area under the curve were all similar (P > 0.05) between the groups before the intervention. Paired t-tests demonstrated that LH pulse frequency decreased (P = 0.047) and LH area under the curve increased (P = 0.021) in the Energy Deficit group, whereas no significant change was seen in any LH parameter from pre- to post-intervention in the Control group. When LH pulse characteristics were analyzed separately for day and night, the day LH pulse frequency decreased (P = 0.022) in the Energy Deficit group, whereas no significant change was seen in any day or night parameters in the Control group.
Table 3.
LH characteristics during pre- and post-intervention in Control and Energy Deficit groups
| Control, n = 5 |
Energy Deficit, n = 16 |
|||||
|---|---|---|---|---|---|---|
| Pre | Post | Change | Pre | Post | Change | |
| Mean LH, mIU/ml | 5.2 ± 0.7 | 5.7 ± 1.0 | 0.5 ± 0.5 | 5.1 ± 0.4 | 4.7 ± 0.6 | −0.5 ± 0.5 |
| LH pulse frequency, pulses/h | 0.76 ± 0.11 | 0.75 ± 0.11 | 0.00 ± 0.10 | 0.81 ± 0.06 | 0.64 ± 0.08* | −0.18 ± 0.08 |
| Maximal peak amplitude, mIU/ml | 10.3 ± 0.7 | 10.4 ± 0.9 | 0.1 ± 0.2 | 9.6 ± 0.6 | 10.4 ± 1.0 | 0.9 ± 0.7 |
| Mean interval between LH peaks, min | 85.7 ± 12.3 | 86.3 ± 10.7 | 0.6 ± 13.8 | 86.0 ± 13.1 | 108.7 ± 21.5 | 23.0 ± 30.3 |
| LH area under the curve | 127.7 ± 29.0 | 143.9 ± 28.2 | 16.2 ± 29.2 | 109.9 ± 14.1 | 177.5 ± 28.2* | 56.5 ± 30.4 |
| Cycle day of testing, day | 4.6 ± 1.3 | 6.0 ± 1.3 | 5.3 ± 0.4 | 8.7 ± 2.3 | ||
Values are expressed as means ± SE. Independent t-tests were used to compare change in mean LH, LH pulse frequency, maximal peak amplitude, LH area under the curve, and cycle day of testing. Mann-Whitney U-tests were used to compare the change in mean interval between LH peaks. Paired t-tests were used to compare mean LH, LH pulse frequency, maximal peak amplitude, and LH area under the curve pre- vs. post-study. Wilcoxon signed rank tests were used to compare mean interval between LH peaks pre- vs. post-study.
Paired t-test, Pre- vs. Post-study, P < 0.05.
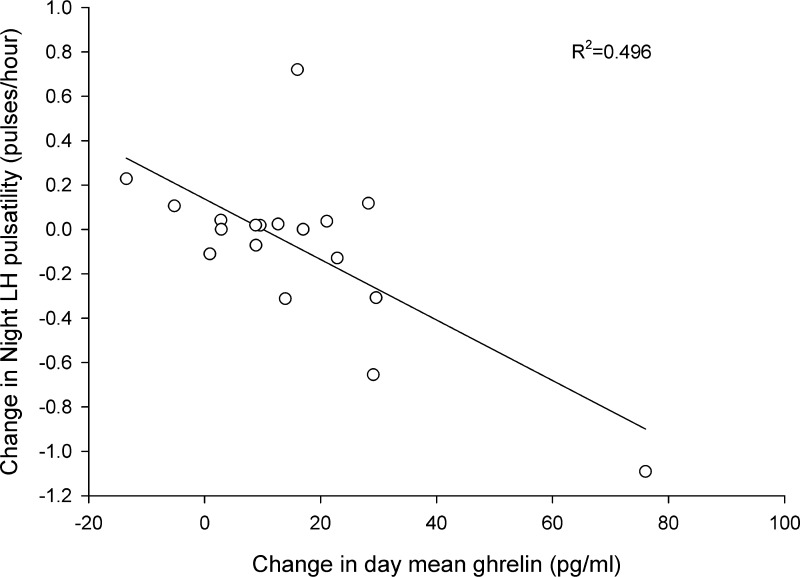
Pearson correlations between change in LH pulse frequency, change in ghrelin concentrations, and changes in body composition in all subjects are presented in Table 4. There were negative correlations between the change in LH pulse frequency and the change in mean 24-h ghrelin, 24-h ghrelin area under the curve, ghrelin lunch peak, ghrelin dinner peak, ghrelin breakfast response average, ghrelin lunch response average, and ghrelin dinner response average. There were no correlations between change in LH pulse frequency and change in BMI, fat mass, or fat-free mass. However, a positive correlation between changes in LH pulse frequency and changes in body weight was demonstrated. Change in night LH pulse frequency was correlated with mean 24-h ghrelin, day mean ghrelin, and night mean ghrelin but not body fat percentage (Table 5; Fig. 2).
Table 4.
Bivariate correlations of change in LH pulse frequency, n = 21
| Change in LH Pulse Frequency |
||
|---|---|---|
| R value | P value | |
| Changes in ghrelin | ||
| Change in mean 24-h ghrelin, pg/ml | −0.485 | 0.030* |
| Change in ghrelin area under the curve | −0.470 | 0.049* |
| Change in ghrelin breakfast peak, pg/ml | 0.034 | 0.888 |
| Change in ghrelin lunch peak, pg/ml | −0.518 | 0.019* |
| Change in ghrelin dinner peak, pg/ml | −0.484 | 0.031* |
| Changes in body composition | ||
| Change in body weight, kg | 0.435 | 0.049* |
| Change in BMI, kg/m2 | 0.426 | 0.054 |
| Change in fat mass, kg | 0.323 | 0.154 |
| Change in fat free mass, kg | 0.282 | 0.229 |
Significant correlations, P < 0.05.
Table 5.
Bivariate correlations of change in night LH pulse frequency, n = 18
| Change in Night LH Pulse Frequency |
||
|---|---|---|
| R value | P value | |
| Changes in ghrelin | ||
| Change in mean 24-h ghrelin, pg/ml | −0.589 | 0.010* |
| Change in mean day ghrelin, pg/ml | −0.704 | 0.001* |
| Change in mean night ghrelin, pg/ml | −0.625 | 0.013* |
Significant correlations, P < 0.05.
Fig. 2.
Relationship between the change in night LH pulsatility and the change in day mean ghrelin, from pre- to post-intervention (n = 18).
To explore the strongest predictors of LH pulse frequency using stepwise linear regression, all variables that were significant in bivariate correlations were entered into the prediction model in addition to variables that had previously been reported to be associated with changes in LH pulse frequency, such as body weight, BMI, fat free mass, mean ghrelin concentration (24-h, day and night), and ghrelin meal peaks. The strongest single predictor of change in 24-h LH pulse frequency was change in day mean ghrelin (R2 = 0.522, adjusted R2 = 0.485, P = 0.002). However, change in percentage body fat significantly increased the variance of change in 24-h LH pulse frequency explained (cumulative model with change in day ghrelin and change in percentage body fat; R2 = 0.714, adjusted R2 = 0.667, P = 0.001).
DISCUSSION
We implemented a well-controlled, intensive exercise and diet intervention in 21 premenopausal, sedentary women and demonstrated that 1) the women in the Energy Deficit group experienced a decrease in LH pulsatility, 2) the women in the Energy Deficit group experienced an increase in mean 24-h total ghrelin concentrations and several meal-related ghrelin parameters, 3) changes in ghrelin were associated with decreases in LH pulsatility, and 4) change in day mean ghrelin was the strongest independent predictor of LH pulse frequency. We speculate that changes in ghrelin may be associated with changes in LH pulse frequency independently of changes in body weight. Perhaps this is beneficial in that ghrelin can change more rapidly than body weight and therefore represents a more sensitive signal of changes in energy balance than changes in body weight (38). Total body weight changes during an intervention employing both calorie restriction and exercise do not always agree with calculations of energy deficit, because exercise training may stimulate increases in muscle mass, plasma volume (9), and body water stored with glycogen (37).
Short-term manipulations of energy intake and exercise expenditure in various combinations have consistently demonstrated that changes in energy availability are related to changes in LH pulse frequency (31, 47). In men (7) and in women (36), 48 h of fasting also resulted in a decrease in LH pulse frequency. Our current study extends these observations from short-term studies by demonstrating that chronic energy deficiency induces a central suppression of reproductive hormone secretion and that a primary predictor of the magnitude of this suppression is circulating total ghrelin. Numerous previous reports have demonstrated a clear association between weight loss and increases in circulating ghrelin (10, 15, 29, 30), but none have demonstrated a significant association between this important metabolic signal and the modulation of the reproductive axis with exercise-induced weight loss. However, although changes in ghrelin were an independent predictor of changes in LH, the correlation was rather modest. This is not unexpected, since numerous other metabolic hormones have been shown to act as metabolic signals that can impact GnRH neurons (6, 45).
Elevated ghrelin concentrations have been associated with decreases in LH in pharmacological studies conducted in men (23, 27) and women (22). Kluge and colleagues demonstrated in men (23) and in women (22) a decrease in LH pulse frequency following four ghrelin injections (50 μg) over a 12-h and a 9-h period, respectively. Similarly, Lanfranco et al. (27) demonstrated that an 8-h administration of acylated ghrelin decreased LH pulsatility. Both of those studies indicate that elevated levels of circulating ghrelin concentrations can inhibit reproductive function by decreasing LH pulse frequency. Future studies need to address more comprehensively what modulates the effects of ghrelin on LH secretion.
The underlying mechanism causing elevated ghrelin to downregulate LH secretion from the anterior pituitary is unknown. Ghrelin may impact LH pulse frequency by altering concentrations of NPY/AgRP and POMC/CART in the arcuate nucleus, either by crossing the blood-brain barrier or by signals transmitted by the vagus nerve to affect appetite and, indirectly, by impacting the hypothalamic-pituitary-ovarian axis (5, 12). Studies in animals suggest that elevated ghrelin causes alterations in GnRH secretion from the GnRH neurons (16). Lebrethon et al. (28) administered ghrelin to male rats and demonstrated a decrease in the GnRH interpulse interval, while Furuta et al. (16) have suggested that, since ghrelin injected in ovariectomized rats decreases only LH pulse frequency and not LH pulse amplitude, ghrelin presumably affects LH secretion at the level of the GnRH pulse generator and not the anterior pituitary. The ghrelin receptor, i.e., the growth hormone secretagogue receptor, has been identified in the hypothalamic neurons including the arcuate nucleus and paraventricular nucleus (17). Elevated ghrelin concentrations can cause alterations of neuropeptides including NPY, POMC, and kisspeptin (19, 32). Alterations in these neuropeptides, NPY, POMC, and KiSS-1, then downregulate GnRH secretion, leading to a downstream decrease in pituitary release of LH (19, 32, 33, 35).
It is important to note that many metabolic signals have been shown to directly or indirectly affect LH pulsatility (3, 6, 44, 45). For example, there is much evidence to suggest that leptin is involved in regulating the hypothalamic-pituitary-ovarian axis in humans (4, 25, 45). Welt et al. (45) administered human recombinant leptin to eight women with FHA and demonstrated an increase in LH pulsatility. Additionally, Ackerman et al. (2) recently demonstrated that elevated ghrelin and suppressed leptin concentrations were associated with suppressed LH secretion in young, exercising women. However, leptin 24-h concentrations were not measured in the current study.
A major limitation to this study is that a cause and effect relationship between ghrelin and the decline in LH pulse frequency cannot be established. However, our data add support to the concept that elevated ghrelin concentrations may cause suppression of LH pulses in exercising women who lose weight. A second limitation may be the sample size. However, the small sample size reflects the difficulties in conducting a study with a high degree of subject burden including 24-h blood draws and a controlled 3-mo intervention that involved both a supervised exercise program and a controlled feeding dietary intervention. A third limitation to this is that total ghrelin (acylated ghrelin + des-acylated ghrelin) was measured as opposed to the biologically active form of ghrelin (acylated ghrelin). However, to date, the majority of weight loss studies have evaluated total ghrelin (acylated ghrelin + des-acylated ghrelin) (10, 15, 18, 26, 29, 30), and the exact endocrine function of des-acylated ghrelin in unknown. Last, while Cluster is a widely used computer software program designed to identify pulses of endocrine hormones with a specificity of ∼99%, it does have a low sensitivity for the detection of pulses (∼80%), indicating that the program may not identify all true secretory bursts. Alternatively, Cluster does have a very low (∼1%) false-positive rate (20). In addition to having low sensitivity, Cluster does not identify secretory bursts by statistically identifying the burst followed by exponential clearance; rather, Cluster identifies hormone pulses defined by probable amplitudes and standard deviations of the expected pulses (20, 41). The detection of a pulse followed by detection of exponential clearance has been evaluated to be a more sensitive (sensitivity ∼96%) method, but has a lower specificity (∼94%) than Cluster.
In summary, our findings suggest that the changes in ghrelin associated with an energy deficiency are also associated with the change in LH pulse frequency in premenopausal women. Understanding the role of ghrelin in regulating the hypothalamic-pituitary-ovarian axis will help to elucidate the mechanism of exercise-associated menstrual disturbances. If ghrelin is directly participating in the downregulation of reproductive function in women with exercise-associated menstrual disturbances, future studies will need to investigate whether improving energy status in women with amenorrhea causes a decrease in ghrelin and if this decrease initiates the reversal of amenorrhea. Moreover, if ghrelin is mechanistically linked to the suppression of the reproductive axis with exercise and/or calorie restriction, research on whether the alteration of the timing and or frequency of meals might minimize the expected increases in ghrelin and thus prevent or reduce subsequent changes in LH pulsatility might prove useful.
GRANTS
National Institutes of Health Grants 1R01 HD-39245-01A1 and M01 RR-10732 (N. I. Williams), Canadian Institute of Health Doctoral Research Award (J. L. Scheid).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.L.S., M.J.D.S., and N.I.W. conception and design of research; J.L.S., B.R.H., and H.J.L. performed experiments; J.L.S., B.R.H., and H.J.L. analyzed data; J.L.S., M.J.D.S., and N.I.W. interpreted results of experiments; J.L.S. prepared figures; J.L.S. drafted manuscript; J.L.S., M.J.D.S., B.R.H., H.J.L., and N.I.W. edited and revised manuscript; J.L.S., M.J.D.S., B.R.H., H.J.L., and N.I.W. approved final version of manuscript.
REFERENCES
- 1. Abou Heif HM, Deif MM, Abdel Aziz HK. Effect of food restriction on ghrelin in adult male rats and its relation to male reproductive hormones. Andrologia 42: 97– 105, 2010 [DOI] [PubMed] [Google Scholar]
- 2. Ackerman KE, Slusarz K, Guereca G, Pierce L, Slattery M, Mendes N, Herzog DB, Misra M. Higher ghrelin and lower leptin secretion are associated with lower LH secretion in young amenorrheic athletes compared with eumenorrheic athletes and controls. Am J Physiol Endocrinol Metab 302: E800– E806, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Adam CL, Findlay PA, Moore AH. Effects of insulin-like growth factor-1 on luteinizing hormone secretion in sheep. Anim Reprod Sci 50: 45– 56, 1998 [DOI] [PubMed] [Google Scholar]
- 4. Audi L, Mantzoros CS, Vidal-Puig A, Vargas D, Gussinye M, Carrascosa A. Leptin in relation to resumption of menses in women with anorexia nervosa. Mol Psychiatry 3: 544– 547, 1998 [DOI] [PubMed] [Google Scholar]
- 5. Budak E, Fernandez Sanchez M, Bellver J, Cervero A, Simon C, Pellicer A. Interactions of the hormones leptin, ghrelin, adiponectin, resistin, and PYY3–36 with the reproductive system. Fertil Steril 85: 1563, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Burcelin R, Thorens B, Glauser M, Gaillard RC, Pralong FP. Gonadotropin-releasing hormone secretion from hypothalamic neurons: stimulation by insulin and potentiation by leptin. Endocrinology 144: 4484– 4491, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Cameron JL, Weltzin TE, McConaha C, Helmreich DL, Kaye WH. Slowing of pulsatile luteinizing hormone secretion in men after forty-eight hours of fasting. J Clin Endocrinol Metab 73: 35– 41, 1991 [DOI] [PubMed] [Google Scholar]
- 8. Christo K, Cord J, Mendes N, Miller KK, Goldstein MA, Klibanski A, Misra M. Acylated ghrelin and leptin in adolescent athletes with amenorrhea, eumenorrheic athletes and controls: a cross-sectional study. Clin Endocrinol (Oxf) 69: 628– 633, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Convertino VA, Brock PJ, Keil LC, Bernauer EM, Greenleaf JE. Exercise training-induced hypervolemia: role of plasma albumin, renin, and vasopressin. J Appl Physiol 48: 665– 669, 1980 [DOI] [PubMed] [Google Scholar]
- 10. Cummings DE, Weigle DS, Frayo RS, Breen PA, Ma MK, Dellinger EP, Purnell JQ. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med 346: 1623, 2002 [DOI] [PubMed] [Google Scholar]
- 11. Date Y, Kojima M, Hosoda H, Sawaguchi A, Mondal MS, Suganuma T, Matsukura S, Kangawa K, Nakazato M. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology 141: 4255, 2000 [DOI] [PubMed] [Google Scholar]
- 12. Date Y, Murakami N, Toshinai K, Matsukura S, Niijima A, Matsuo H, Kangawa K, Nakazato M. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology 123: 1120– 1128, 2002 [DOI] [PubMed] [Google Scholar]
- 13. De Souza MJ, Leidy HJ, O'Donnell E, Lasley B, Williams NI. Fasting ghrelin levels in physically active women: relationship with menstrual disturbances and metabolic hormones. J Clin Endocrinol Metab 89: 3536– 3542, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Fernandez-Fernandez R, Tena-Sempere M, Aguilar E, Pinilla L. Ghrelin effects on gonadotropin secretion in male and female rats. Neurosci Lett 362: 103– 107, 2004 [DOI] [PubMed] [Google Scholar]
- 15. Foster-Schubert KE, McTiernan A, Frayo RS, Schwartz RS, Rajan KB, Yasui Y, Tworoger SS, Cummings DE. Human plasma ghrelin levels increase during a one-year exercise program. J Clin Endocrinol Metab 90: 820, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Furuta M, Funabashi T, Kimura F. Intracerebroventricular administration of ghrelin rapidly suppresses pulsatile luteinizing hormone secretion in ovariectomized rats. Biochem Biophys Res Commun 288: 780, 2001 [DOI] [PubMed] [Google Scholar]
- 17. Guan XM, Yu H, Palyha OC, McKee KK, Feighner SD, Sirinathsinghji DJ, Smith RG, Van der Ploeg LH, Howard AD. Distribution of mRNA encoding the growth hormone secretagogue receptor in brain and peripheral tissues. Brain Res Mol Brain Res 48: 23– 29, 1997 [DOI] [PubMed] [Google Scholar]
- 18. Hansen TK, Dall R, Hosoda H, Kojima M, Kangawa K, Christiansen JS, Jorgensen JO. Weight loss increases circulating levels of ghrelin in human obesity. Clin Endocrinol (Oxf) 56: 203– 206, 2002 [DOI] [PubMed] [Google Scholar]
- 19. Hill JW, Elmquist JK, Elias CF. Hypothalamic pathways linking energy balance and reproduction. Am J Physiol Endocrinol Metab 294: E827– E832, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Johnson ML, Pipes L, Veldhuis PP, Farhy LS, Boyd DG, Evans WS. AutoDecon, a deconvolution algorithm for identification and characterization of luteinizing hormone secretory bursts: description and validation using synthetic data. Anal Biochem 381: 8– 17, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kamegai J, Tamura H, Shimizu T, Ishii S, Sugihara H, Wakabayashi I. Chronic central infusion of ghrelin increases hypothalamic neuropeptide Y and Agouti-related protein mRNA levels and body weight in rats. Diabetes 50: 2438– 2443, 2001 [DOI] [PubMed] [Google Scholar]
- 22. Kluge M, Schussler P, Schmidt D, Uhr M, Steiger A. Ghrelin suppresses secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) in women. J Clin Endocrinol Metab 97: E448– 451, 2012 [DOI] [PubMed] [Google Scholar]
- 23. Kluge M, Schussler P, Uhr M, Yassouridis A, Steiger A. Ghrelin suppresses secretion of luteinizing hormone in humans. J Clin Endocrinol Metab 92: 3202– 3205, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402: 656– 660, 1999 [DOI] [PubMed] [Google Scholar]
- 25. Kopp W, Blum WF, von Prittwitz S, Ziegler A, Lubbert H, Emons G, Herzog W, Herpertz S, Deter HC, Remschmidt H, Hebebrand J. Low leptin levels predict amenorrhea in underweight and eating disordered females. Mol Psychiatry 2: 335– 340, 1997 [DOI] [PubMed] [Google Scholar]
- 26. Kotidis EV, Koliakos GG, Baltzopoulos VG, Ioannidis KN, Yovos JG, Papavramidis ST. Serum ghrelin, leptin and adiponectin levels before and after weight loss: comparison of three methods of treatment–a prospective study. Obes Surg 16: 1425– 1432, 2006 [DOI] [PubMed] [Google Scholar]
- 27. Lanfranco F, Bonelli L, Baldi M, Me E, Broglio F, Ghigo E. Acylated ghrelin inhibits spontaneous luteinizing hormone pulsatility and responsiveness to naloxone but not that to gonadotropin-releasing hormone in young men: evidence for a central inhibitory action of ghrelin on the gonadal axis. J Clin Endocrinol Metab 93: 3633– 3639, 2008 [DOI] [PubMed] [Google Scholar]
- 28. Lebrethon MC, Aganina A, Fournier M, Gerard A, Parent AS, Bourguignon JP. Effects of in vivo and in vitro administration of ghrelin, leptin and neuropeptide mediators on pulsatile gonadotrophin-releasing hormone secretion from male rat hypothalamus before and after puberty. J Neuroendocrinol 19: 181– 188, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Leidy HJ, Dougherty KA, Frye BR, Duke KM, Williams NI. Twenty-four-hour ghrelin is elevated after calorie restriction and exercise training in non-obese women. Obesity 15: 446– 455, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Leidy HJ, Gardner JK, Frye BR, Snook ML, Schuchert MK, Richard EL, Williams NI. Circulating ghrelin is sensitive to changes in body weight during a diet and exercise program in normal-weight young women. J Clin Endocrinol Metab 89: 2659, 2004 [DOI] [PubMed] [Google Scholar]
- 31. Loucks AB, Thuma JR. Luteinizing hormone pulsatility is disrupted at a threshold of energy availability in regularly menstruating women. J Clin Endocrinol Metab 88: 297, 2003 [DOI] [PubMed] [Google Scholar]
- 32. Martini AC, Fernandez-Fernandez R, Tovar S, Navarro VM, Vigo E, Vazquez MJ, Davies JS, Thompson NM, Aguilar E, Pinilla L, Wells T, Dieguez C, Tena-Sempere M. Comparative analysis of the effects of ghrelin and unacylated ghrelin on luteinizing hormone secretion in male rats. Endocrinology 147: 2374– 2382, 2006 [DOI] [PubMed] [Google Scholar]
- 33. Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, Thresher RR, Malinge I, Lomet D, Carlton MB, Colledge WH, Caraty A, Aparicio SA. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci USA 102: 1761– 1766, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nakahara T, Kojima S, Tanaka M, Yasuhara D, Harada T, Sagiyama K, Muranaga T, Nagai N, Nakazato M, Nozoe S, Naruo T, Inui A. Incomplete restoration of the secretion of ghrelin and PYY compared to insulin after food ingestion following weight gain in anorexia nervosa. J Psychiatric Res 41: 814– 820, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Navarro VM, Castellano JM, Fernandez-Fernandez R, Tovar S, Roa J, Mayen A, Nogueiras R, Vazquez MJ, Barreiro ML, Magni P, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. Characterization of the potent luteinizing hormone-releasing activity of KiSS-1 peptide, the natural ligand of GPR54. Endocrinology 146: 156– 163, 2005 [DOI] [PubMed] [Google Scholar]
- 36. Olson BR, Cartledge T, Sebring N, Defensor R, Nieman L. Short-term fasting affects luteinizing hormone secretory dynamics but not reproductive function in normal-weight sedentary women. J Clin Endocrinol Metab 80: 1187– 1193, 1995 [DOI] [PubMed] [Google Scholar]
- 37. Olsson KE, Saltin B. Variation in total body water with muscle glycogen changes in man. Acta Physiol Scand 80: 11– 18, 1970 [DOI] [PubMed] [Google Scholar]
- 38. Scheid JLMJDES, Leidy HJ, Williams NI. Ghrelin but not peptide YY is related to change in body weight and energy availability. Med Sci Sports Exerc 43: 2063– 2071, 2011 [DOI] [PubMed] [Google Scholar]
- 39. Schneider LF, Monaco SE, Warren MP. Elevated ghrelin level in women of normal weight with amenorrhea is related to disordered eating. Fertil Steril 90: 121– 128, 2008 [DOI] [PubMed] [Google Scholar]
- 40. Stock S, Leichner P, Wong AC, Ghatei MA, Kieffer TJ, Bloom SR, Chanoine JP. Ghrelin, peptide YY, glucose-dependent insulinotropic polypeptide, and hunger responses to a mixed meal in anorexic, obese, and control female adolescents. J Clin Endocrinol Metab 90: 2161– 2168, 2005 [DOI] [PubMed] [Google Scholar]
- 41. Veldhuis JD, Carlson ML, Johnson ML. The pituitary gland secretes in bursts: appraising the nature of glandular secretory impulses by simultaneous multiple-parameter deconvolution of plasma hormone concentrations. Proc Natl Acad Sci USA 84: 7686– 7690, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Veldhuis JD, Johnson ML. Cluster analysis: a simple, versatile, and robust algorithm for endocrine pulse detection. Am J Physiol Endocrinol Metab 250: E486– E493, 1986 [DOI] [PubMed] [Google Scholar]
- 43. Vulliemoz NR, Xiao E, Xia-Zhang L, Germond M, Rivier J, Ferin M. Decrease in luteinizing hormone pulse frequency during a five-hour peripheral ghrelin infusion in the ovariectomized rhesus monkey. J Clin Endocrinol Metab 89: 5718– 5723, 2004 [DOI] [PubMed] [Google Scholar]
- 44. Vulliemoz NR, Xiao E, Xia-Zhang L, Rivier J, Ferin M. Astressin B, a nonselective corticotropin-releasing hormone receptor antagonist, prevents the inhibitory effect of ghrelin on luteinizing hormone pulse frequency in the ovariectomized rhesus monkey. Endocrinology 149: 869– 874, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Welt CK, Chan JL, Bullen J, Murphy R, Smith P, DePaoli AM, Karalis A, Mantzoros CS. Recombinant human leptin in women with hypothalamic amenorrhea. N Engl J Med 351: 987, 2004 [DOI] [PubMed] [Google Scholar]
- 46. Williams NI. Predictors of Menstrual Disturbances in Exercising Women. San Diego, CA: Endocrine Society, 2005, p. 684 [Google Scholar]
- 47. Williams NI, Young JC, McArthur JW, Bullen B, Skrinar GS, Turnbull B. Strenuous exercise with caloric restriction: effect on luteinizing hormone secretion. Med Sci Sports Exerc 27: 1390, 1995 [PubMed] [Google Scholar]
- 48. Wren AM, Bloom SR. Gut hormones and appetite control. Gastroenterology 132: 2116– 2130, 2007 [DOI] [PubMed] [Google Scholar]