Abstract
Objectives:
To explore the effect of vitamin D supplementation in prevention of respiratory tract infections on the basis of published clinical trials.
Materials and Methods:
Clinical trials were searched from various electronic databases. Five clinical trials were suitable for inclusion. Outcome was events of respiratory tract infections in vitamin D group and placebo group. Data was reported as odds ratio with 95% confidence interval. Both random and fixed model was used for analysis. Analysis was done with the help of Comprehensive meta-analysis software 2.
Results:
Events of respiratory tract infections were significantly lower in vitamin D group as compared to control group [Odds ratio = 0.582 (0.417 – 0.812) P = 0.001] according to random model. Results were similar in fixed model. On separate analysis of clinical trials dealing with groups of children and adults, beneficial effect of vitamin D was observed in both, according to fixed model [Odds ratio = 0.579 (0.416 – 0.805), P = 0.001 and Odd ratio = 0.653 (0.472 – 0.9040, P = 0.010 respectively]. On using random model beneficial effect persisted in children's group but became nonsignificant in adults group [Odds ratio = 0.579 (0.416 – 0.805), P = 0.001 and Odd ratio = 0.544 (0.278 – 1.063) P = 0.075 respectively].
Conclusion:
Vitamin D supplementation decreases the events related to respiratory tract infections. There is need of more well conducted clinical trials to reach to a certain conclusion.
Keywords: Influenza, pneumonia, prevention, respiratory tract infections, vitamin D
INTRODUCTION
Acute respiratory infection (ARI) is widespread not only in the pediatric age group but also in adults. Respiratory tract infections are the major cause of mortality and morbidity.[1]
There are limited options available for treatment and prevention of ARI. Antibiotics are the mainstay of treatment for pneumonia. In the developing countries, zinc and vitamin A supplementation has been studied for the prevention and treatment of ARI. It has been shown that supplements of these micronutrients reduce the ARI rates though evidences are conflicting.[2–4] Vitamin C (ascorbic acid) has also been used for the prevention and treatment of ARI despite the lack of convincing evidence of benefit.[5]
Recent research suggested that vitamin D also has a potential role in the prevention of ARI by increasing immunity. In some observational studies, it was observed that low vitamin D level in blood is associated with the increased incidence of respiratory tract infections.[6] There is a need to refine our knowledge about the functions of vitamin D in the prevention of respiratory tract infections in the light of clinical trials which were done to explore this phenomenon. Hence, we conducted this systematic review and meta-analysis to asses the role of vitamin D supplementation in prevention of ARI.
MATERIALS AND METHODS
Search strategy of clinical trials
This systematic review and meta-analysis was done according to the instructions given in “Cochrane Handbook for Systematic Reviews of Interventions”.[7] Clinical trials were collected from the electronic databases like PubMed, Cochrane Clinical Trial Register, and Google Scholar. References mentioned in full text articles and reviews were also explored to collect the relevant clinical trials. The clinical trials which are not available as free full text were collected from the corresponding authors of these trials through email. In PubMed, various MeSH terms like “Vitamin D”, “Respiratory Tract Infections”, “Influenza”, “Pneumonia”, “Common Cold”, etc., were used in various combinations to retrieve the clinical trials. For Pubmed, clinical trials published from 1966 to present were considered. Only those clinical trials which are published in English language were collected. Summary of various words used for searching the literature is given in appendix 1. Last search was done on 23 November 2011. Both first and second authors [J.K and J.P.G] did the search together from databases. Potential clinical trials were downloaded in hard copies. The clinical trials which were not available online in full text were collected from the corresponding author through email. These clinical trials were assessed on the basis of inclusion and exclusion criteria mentioned in the pretested proforma independently by both authors. Wherever there was discrepancy, the decision of the third author was considered final.
Inclusion and exclusion criteria for clinical trials
Only randomized placebo controlled clinical trials exploring the effect of vitamin D supplementation on prevention of respiratory tract infections like influenza, pneumonia, common cold, etc., were included in the analysis. Only those clinical trials where results were given in categorical variables were included. Non randomized, clinical trials without controls and clinical trials showing results in continuous variables were not included. Clinical trials in which vitamin D was used in combination with other intervention/s were not included in the final analysis. Process of selection of various RCTs for this study is given in the form of preferred reporting items for systematic review and meta - analysis (PRISMA) chart according to PRISMA statement [Figure 1].[8]
Figure 1.
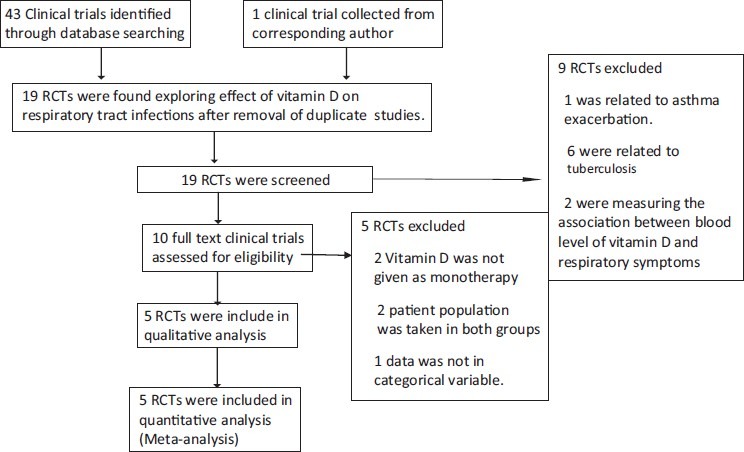
PRISMA Chart
Outcome
Outcome of interest was the event of respiratory tract infection in the case and control groups. In all clinical trials placebo was used in the control group. The software used in this analysis (Comprehensive meta-analysis software 2) converted these events into odds ratio and calculated 95% confidence interval around odds ratio.
Statistics
The statistical analysis was performed by CMA (Comprehensive meta-analysis) Version 2 software and the effect was estimated by calculating the odds ratio (OR) and the 95% confidence interval (CI). No data transformation was done. Events in each group were combined for meta-analysis. Results were given in both random as well as fixed model. Forest plot values were given according to random model and test for heterogeneity (t^2) was done.
RESULTS
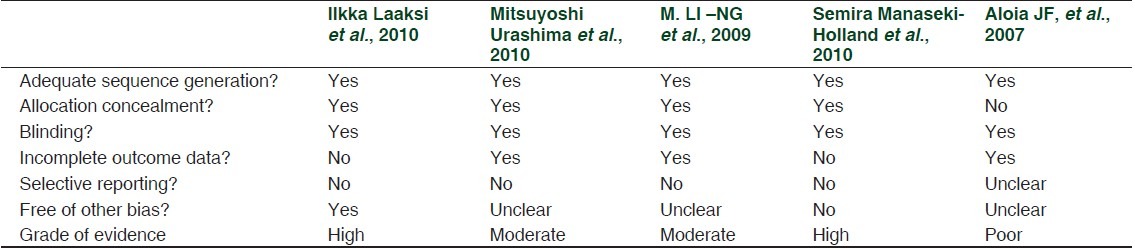
On the basis of inclusion and exclusion criteria, we found 5 clinical trials.[9–14] Dose of vitamin D used in these clinical trials were ranged from 400 IU/day to 2000 IU/day. In one clinical trial single parenteral dose of vitamin D was given (100000 IU). [Table 1] In four clinical trials, events were recorded in the form of number of participants suffered from respiratory tract infections during the trial period. In one clinical trial events were recorded in the form of total number of times participants reported respiratory tract infection in the questionnaire given to them (four times) in trial period. Levels of evidence of these clinical trials were high or moderate. Level of evidence was assessed according to “The Grades of Recommendation, Assessment, Development and Evaluation Working Group” (GRADE Working group).[15,16] Information related to the quality of evidence and biases in these clinical trials are mentioned in Table 2.
Table 1.
Characteristics of clinical trials included in the meta-analysis
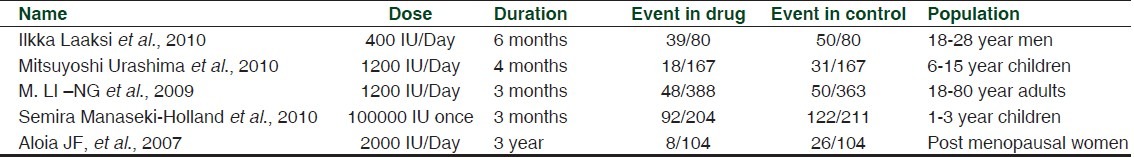
Table 2.
Levels of evidence and methodological bias in clinical trials included in the meta-analysis
Overall events of respiratory tract infection in vitamin D group were 21.7% (205/943) whereas in placebo group it was 30.1% (279/925). There was significant reduction in incidence of respiratory tract infection in vitamin D group as compared to placebo group [Odds ratio = 0.582 (0.417 – 0.812) Z = - 3.185, P value = 0.001, t^2 = 0.064] according to the random model [Figure 2]. The values were similar when fixed model was used [Odds ratio = 0.615 (0.488 – 0.776) Z = - 4. 108, P value = 0.000].
Figure 2.
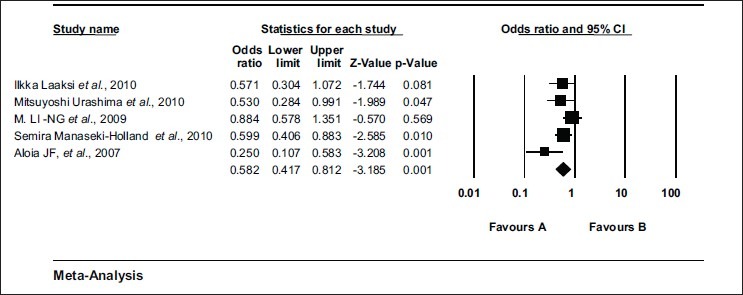
Meta-analysis of trials showing effect of vitamin D supplementation in prevention of respiratory tract infections. Effect of vitamin D supplementation in prevention of respiratory tract infections. Random effect model (DerSimonian laird); Odds ratio = 0.582 (0.417 – 0.812) Z = - 3.185, P value = 0.001, test for heterogeneity t^2= 0.064
There were two clinical trials which were dealing with pediatric age group. These clinical trials were analyzed separately and it was observed that in this age group also vitamin D supplementation reduces the rate of respiratory tract infections significantly [Odds ratio = 0.579 (0.416 – 0.805) Z = – 3.246, P value = 0.001 test for heterogeneity t^2 = 0.000] by random model [Figure 3]. After using the fixed model, values were same [Odds ratio = 0.579 (0.416 – 0.805) Z = - 3.246, P value = 0.001].
Figure 3.
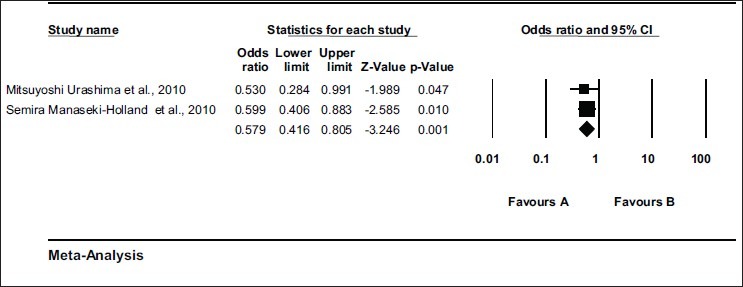
Meta-analysis of trials showing effect of vitamin D supplementation in prevention of respiratory tract infections in pediatric population Effect of vitamin D supplementation in prevention of respiratory tract infections in pediatric population. Random effect model (DerSimonian laird); Odds ratio = 0.579 (0.416 – 0.805) Z = - 3.246 P Value = 0.001 Test for heterogeneity t^2= 0.000
Three clinical trails were dealing with adult population. In these clinical trials, odd ratio was in favor of vitamin D group but it was not significant. [Odd ratio = 0.544 (0.278 – 1.063) Z = - 1.783, P value = 0.075, test for heterogeneity t^2 = 0.247] according to random model. Figure 4 After analyzing on the basis of fixed model, values were significantly in favor of vitamin D [Odd ratio = 0.653 (0.472 – 0.904) Z = - 2. 569, P value = 0.010].
Figure 4.
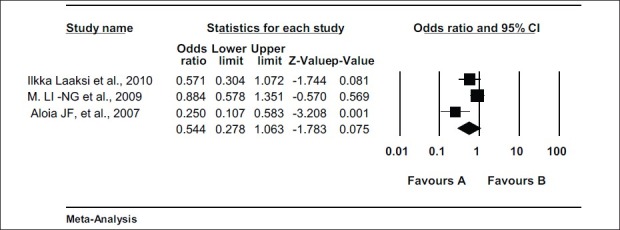
Meta-analysis of trials showing effect of vitamin D supplementation in prevention of respiratory tract infections in adult population Effect of vitamin D supplementation in prevention of respiratory tract infections in adult population. Random effect model (DerSimonian laird); Odds ratio = 0.544 (0.278 – 1.063) Z = - 1.783 P value = 0.075 test for heterogeneity t^2 = 0.247
Publication bias (through funnel plot) was not checked because of small numbers of clinical trials.[17]
DISCUSSION
This systematic review and meta-analysis was done to explore the role of vitamin D supplementation in prevention of respiratory tract infections. According to this systematic review and meta-analysis vitamin D significantly reduces the respiratory tract infection related events as compared to placebo. Beneficial effect of vitamin D was observed in children as well as adults according to fixed model. Beneficial effect of vitamin D was observed only in children according to random model. This assessment is based on only five clinical trials as less clinical trials are done for the exploration of the same.
Various reasons are given for the protective action of vitamin D in prevention of respiratory tract infections. It is believed that vitamin D increases the production of natural antibodies.[18] Vitamin D is also known to strengthen the immunity by inducing monocyte differentiation and inhibiting lymphocyte proliferation.[19] It also postulated that vitamin D enhances the phagocytic activity of macrophages.[18]
This systematic review and meta-analysis has some limitations. In clinical trials dealing with children there was significant protective effect of vitamin D in our analysis but there was significant heterogeneity [Test for heterogeneity t^2 = 0.000]. But same odds ratio was observed in random as well as fixed model. This heterogeneity may be there because of some publication bias, very less numbers of clinical trials, different doses of vitamin D and heterogeneity of participants of clinical trials. We could not check publication bias because interpretation of funnel plot is not possible when clinical trials are less than 10.[17] Because of insufficient reporting we could not conduct the analysis of adverse effects. We could not compare our results with other meta-analyses, as not a single meta-analysis exploring this phenomenon is existed in electronic databases.
On the basis of this study, we can conclude that vitamin D is useful in prevention of respiratory tract infections. But looking at the availability of only five clinical trials there is need of conduction of more clinical trials so that more valid conclusion can be reached.
Appendix 1
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared
REFERENCES
- 1.World Health Organization: The global burden of disease: 2004 update. [Last accessed on 2011 Nov 18]. Available from: http://www.who.int/healthinfo/global_burden_disease/GBD_report_2004update_full.pdf .
- 2.National Family Health Survey(NFHS-3), 2005-06: India. Mumbai: IIPS; 2008. International Institute for Population Sciences (IIPS) and Macro International. [Google Scholar]
- 3.Acharya D, Prasanna KS, Nair S, Rao RS. Acute respiratory infections in children: A community based longitudinal study in south India. Indian J Public Health. 2003;47:7–13. [PubMed] [Google Scholar]
- 4.Yamey G. Zinc supplementation prevents diarrhoea and pneumonia. BMJ. 1999;319:1521–2. doi: 10.1136/bmj.319.7224.1521a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhutta ZA, Black RE, Brown KH, Gardner JM, Gore S, Hidayat A, et al. Zinc Investigators’ Collaborative Group. Prevention of diarrhea and pneumonia by zinc supplementation in developingcountries: Pooled analysis of randomizedcontrolled trials. J Pediatr. 1999;135:689–97. doi: 10.1016/s0022-3476(99)70086-7. [DOI] [PubMed] [Google Scholar]
- 6.Wayse V, Yousafzai A, Mogale K, Filteau S. Association of subclinical vitamin D deficiency with severe acute lower respiratory infection in Indian children under 5 years. Eur J Clin Nutr. 2004;58:563–7. doi: 10.1038/sj.ejcn.1601845. [DOI] [PubMed] [Google Scholar]
- 7.Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Chichester (UK): John Wiley and Sons; 2008. [Google Scholar]
- 8.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009;6:e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laaksi I, Ruohola JP, Mattila V, Auvinen A, Ylikomi T, Pihlajamaki H. Vitamin D Supplementation for the Prevention of Acute Respiratory Tract Infection: A Randomized, Double-Blinded Trial among Young Finnish Men. J Infect Dis. 2010;202:809–14. doi: 10.1086/654881. [DOI] [PubMed] [Google Scholar]
- 10.Urashima M, Segawa T, Okazaki M, Kurihara M, Wada Y, Ida H. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in schoolchildren. Am J Clin Nutr. 2010;91:1255–60. doi: 10.3945/ajcn.2009.29094. [DOI] [PubMed] [Google Scholar]
- 11.Holland SM, Qader G, Masher MI, Bruce J, Mughal MZ, Chandramohan D, et al. Effects of vitamin D supplementation to children diagnosed with neumonia in Kabul: A randomised controlled trial. Trop Med Int Health. 2010;15:1148–55. doi: 10.1111/j.1365-3156.2010.02578.x. [DOI] [PubMed] [Google Scholar]
- 12.Li-Ng M, Aloia JF, Pollack S, Cunha BA, Mikhail M, Yeh J, et al. A randomized controlled trial of vitamin D3 supplementation for the prevention of symptomatic upper respiratory tract infections. Epidemiol Infect. 2009;137:1396–404. doi: 10.1017/S0950268809002404. [DOI] [PubMed] [Google Scholar]
- 13.Aloia JF, Talwar SA, Pollack S, Yeh J. A randomized controlled trial of vitamin D3 supplementation in African American women. Arch Intern Med. 2005;165:1618–23. doi: 10.1001/archinte.165.14.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aloia J, Li-Ng M. Re: Epidemic influenza and vitamin D. Epidemiol Infect. 2007;135:1095–6. doi: 10.1017/S0950268807008308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ. 2004;328:1490–4. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schunemann HJ, Oxman AD, Vist GE, Higgins JP, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Chichester (UK): John Wiley and Sons; 2008. [Google Scholar]
- 17.Sterne JAC, Egger M, Moher D. Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Chichester (UK): John Wiley and Sons; 2008. [Google Scholar]
- 18.Rallof J. The antibiotic vitamin.Deficiency in vitamin D may predispose people to infection. Science. 2006;170:312. [Google Scholar]
- 19.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–3. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]


