Abstract
Objective:
To detect incidence of adverse drug reactions (ADRs) in hospitalized patients and to assess their causality, seriousness, preventability, and the possible economic impact.
Materials and Methods:
This was a prospective study carried out in two medical units at a tertiary care, teaching hospital, for about 18 months. All the admitted patients who developed an ADR after admission (group A) or who were admitted primarily for the treatment of an ADR (group B) were included. Descriptive statistics with 95% CI, χ2, χ2 for the trend and kappa test were used.
Results:
Out of 6601 patients, 140 patients developed 154 ADRs with an incidence of 2.12%. Causality of the majority of the ADRs in group A was ‘possible’ while those in group B was ‘probable’. Among 109 ADRs (34 serious) in group A, 38 were preventable. On the other hand, out of 45 serious ADRs in group B, 19 were preventable. The total cost of 154 ADRs in 140 patients was Rs. 1,49,803 with an average of Rs. 1070 per patient. The preventable cost for 57/154 ADR was Rs. 96,310.
Conclusion:
Around 2% of the hospital patients develop ADRs. A large number of these ADRs were preventable. A substantial saving can be made if adequate caution is exerted.
Keywords: Adverse drug reaction, economic impact, intensive monitoring, preventability
INTRODUCTION
In some countries, the adverse drug reactions (ADRs) rank among the top 10 leading causes of death.[1] Additionally, it is estimated that 5–8% of all hospitalized patients experience serious ADRs and approximately 10% of the hospital costs are related to ADRs.[2–4] These data, however, have been reported from different settings in the US or Europe and may not reflect the actual situation in India.
ADR monitoring methods have their own merits and demerits. Spontaneous reporting depends solely on clinicians to report the details of suspected adverse reaction in a patient. In addition, we cannot detect the incidence and the spontaneity in prescribers which is always lacking, captures only a small fraction of the adverse events.[5,6] Intensive hospital-based monitoring consists of routine prospective recording of demographic and clinical information on hospitalized patients. One could detect adverse reactions, whether or not physicians suspected any associations between drugs and events and incidence of ADRS.[7] In India, very few intensive monitoring studies are published.[8] Hence, this study was planned to detect the incidence of the ADRs in hospitalized patients and to assess their causality, seriousness, preventability, and the possible economic impact.
MATERIALS AND METHODS
This prospective, observational, and longitudinal single centre study was conducted after Ethics Committee permission in indoor patients of two units of the Department of General Medicine, a tertiary care teaching hospital, over a period of 18 months from December 2007 to June 2009. There are a total number of 100 beds in these two units and usually the occupancy is 100%. All patients enrolled during this period were followed up until discharge. All admitted patients who developed a clinically suspected ADR after admission or who were admitted primarily because of an ADR, were included.
The investigator visited the units daily and studied every patient from admission to discharge. The attending doctors and the nursing staff were appraised about the study objectives and were requested to inform the investigator about any ADR. The detection of the ADRs was therefore done both by the investigator himself as well as by the attending medical and paramedical personnel. For all the ADR-related patients, the necessary data were obtained and recorded on a pre-designed case record form (CRF). The data obtained included the demographic details, past history, findings on general and systemic examination, laboratory investigation reports, diagnosis, and treatment.
Suspected medications, treatment given, and the outcome were documented in a slightly modified Central Drug Standard Control Organization (CDSCO) form. A causality analysis of all the observed ADRs was undertaken as per the WHO-UMC (1972) and Naranjo probability score.[9,10] The preventability of an ADR was estimated by using the criteria of Schumock and Thornton, and the severity was evaluated by Hartwig's criteria.[11,12] The patients were divided into two groups for analysis: (A) those developing ADRs during hospitalization for other ailments and (B) included those admitted to the hospital primarily for the treatment of ADRs only.
Statistics
No formal sample size was calculated. Descriptive statistics with 95% CI was used. The other tests of significance used were χ2/Fisher exact test, χ2 for the trend and Cohen's weighted kappa test for agreement. P < 0.05 was considered significant.
RESULTS
Of 6601 admitted patients, 140 patients were observed to have an ADR. Amongst these, 96 (1.45%) developed 109 ADRs during hospitalization (group A) and 44 (0.66%) patients were admitted primarily for the treatment of 45 ADRs that had developed outside the hospital (group B). Thus, 154 ADRs showed up in the two study units. The majority of the patients (42/140) with ADRs were in the age group of 46–60 years. However, it is noteworthy that about 28/140 patients belonged to the geriatric group (age > 60 years). The incidence of the ADRs was equal in both genders (2%).
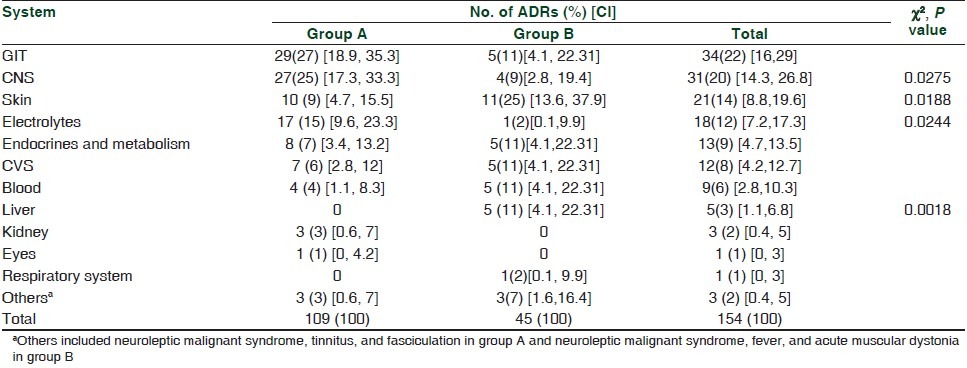
Gastrointestinal ADRs developed most commonly during hospitalization (29, group A) while cutaneous ADRs (11) were the most common cause of hospitalization (group B) [Table 1]. Significant differences were observed between the two groups with regard to CNS, skin, electrolyte, and liver-related ADRs (P < 0.05).
Table 1.
System wise distribution of adverse drug reactions
Out of the 233 suspected drugs, most of these drugs were antimicrobial agents (85) followed by those used in cardiovascular diseases (66) and acting on the central nervous system (25). A significant difference existed, in number of ADRs, between groups A and B in the case of antiretrovirals [A (2), B (11), P = 0.0001], diuretics [A (17), B (1), P = 0.02], and nitrates [A (14), B (0), P = 0.01]. Cardiovascular agents were associated with significantly (P = 0.005) larger number of ADRs in group A as compared to those in group B [Figure 1]. It was observed that furosemide, nitroglycerin, insulin, and enalapril were most frequently suspected drugs in group A while isoniazid, rifampicin, efavirenz, and digoxin were the common causes of ADRs requiring hospitalization (group B).
Figure 1.
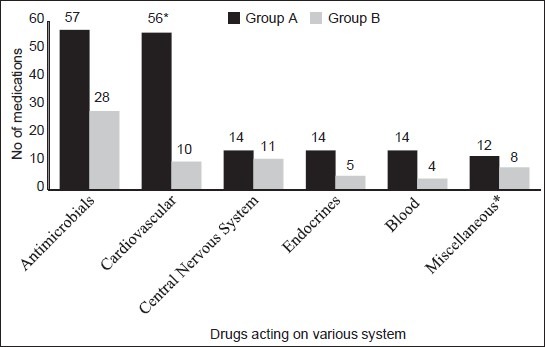
Medications suspected to cause the adverse drug reactions. *P = 0.0058 (χ2 – test). Miscellaneous: Vitamins, NSAIDs, iron, salbutamol + ipratropium bromide, lactulose, herbal agents, lactulose
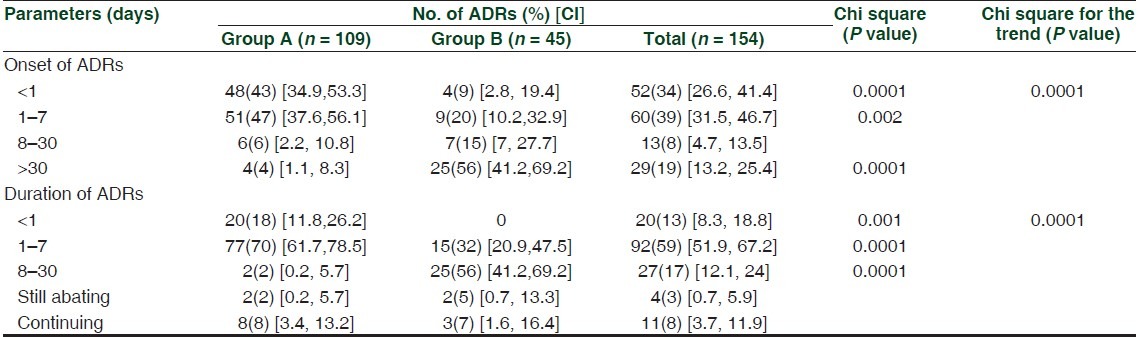
As shown in Table 2 the onset of the ADRs was early (within a week) in group A (99, 90%, P < 0.0001) and late (>30 days) in group B (25, 56%, P < 0.0001). Most of the ADRs (97, 88%) resolved within a week in group A and within a month in group B (25, 56%). ADRs (82, 75%) in group A were treated immediately (patients already hospitalized) while it took about 1–7 days to begin the treatment in group B (26, 58%). Type A and B ADRs were seen significantly (P = 0.006) more in group A (type A: 101) and B (type B: 11), respectively. Suspected medications were administered by the oral route (156/233) followed by intravenous (58/233). In the case of 99 ADRs, dechallenge was done and response was positive. In rest of them, either it was not attempted (due to therapeutic reasons) or could not be assessed (loss of follow-up). An accidental rechallenge occurred in 6/154 cases, and it was found to be positive in three.
Table 2.
Onset and duration of adverse drug reactions
By WHO-UMC criteria majority of the ADRs in group A were ‘possible’ (60, P = 0.008) in nature while those in group B were ‘probable’ (28, P = 0.03). However, in the Naranjo scale this difference between groups A and B was much smaller as well as statistically insignificant [Table 3]. The assessment of the agreement–disagreement between the WHO-UMC criteria and the Naranjo scale was 133 or 86% (k = 0.5).
Table 3.
Causality assessment of adverse drug reactions

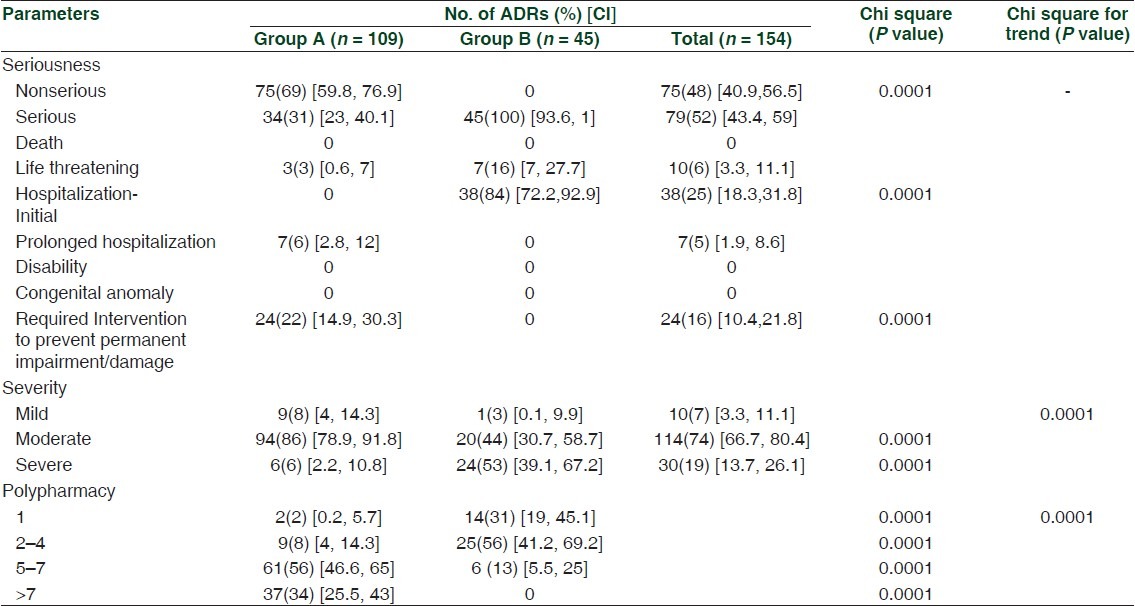
Out of 154 reports, 79 ADRs were serious and 75 ADRs non-serious. Among serious ADRs, 45 were seen in group B and 34 in group A [Table 4]. All non-serious ADRs (75) belonged to group A (P < 0.0001). No nonserious ADRs were seen in group B. Fifty-seven (37%) of the total and 47 (60%) of serious ADRs were preventable. Thus, the majority of serious ADRs were preventable. Amongst serious ADRs, 28 (35%) were definitely preventable and 19 (25%) were probably preventable. Of the total of 57 preventable ADRs, 38 belonged to group A and 19 to group B. Out of a total number of 45 ADRs that caused the hospitalization in patients of group B, 19 (or 45%) were preventable. It was observed that the majority of ADRs (114 or 74%) were moderate in severity. Moderate ADRs were significantly (P < 0.0001) more in group A while severe ADRs were more in group B (P < 0.0001). As shown in Table 4, the number of ADRs was directly proportional to the number of drugs prescribed. Thus, 104 (68%) ADRs were developed when five or more drugs were administered. We observed that chances for the development of an ADR were significantly higher in patients receiving five or more drugs (group A: 98, group B: 6, P < 0.0001) as compared to those given less number of drugs (group A: 11, group B: 39).
Table 4.
Characteristics of adverse drug reactions
ADRs leading to hospitalization or prolonging hospital stay were 52/154 in number, and they caused 321 days of extended hospital stay. Out of these 52 ADRs, 45 ADRs were in group B (causing hospitalization) and only 7 in group A (prolonging hospital stay). ADRs in group A prolonged hospitalization by a total number of 14 days and those in group B caused 307 days of hospitalization. The total cost of 154 ADRs in 140 patients was Rs. 1,49,803 with an average of Rs. 1070 per patient. The total cost of 57/154 preventable ADRs was Rs. 96,310. In group A, there were 109 ADRs. The median direct cost was Rs. 50 (range, Rs. 1–9714) and median indirect cost was Rs. 179 (range, Rs. 57–1110). The total cost of 38 preventable ADRs was Rs. 16,554. Serious ADRs in this group (34) amounted to a total of Rs. 7706. In group B, there were 45 ADRs. The median direct cost was Rs. 473 (range, Rs. 120–40124) and the median indirect cost was Rs. 1025 (range, Rs. 0–5500). The total cost of 19 preventable ADRs alone was Rs. 79,756. Both the direct as well as indirect costs were significantly higher in group B (P < 0.0001).
DISCUSSION
This prospective study was conducted in two medical units of a tertiary care, teaching hospital for about 18 months. The incidence of ADRs was 2.12% (group A, 1.45%, group B, 0.66%). A total of 79 (52%) ADRs were serious in nature and 47 (60%) of them were preventable.
The incidence of the ADRs in in-patients was 4.4% and 3% in other studies.[12,13] It may be because of anticancer and antitubercular medications that were also included in these studies but not in ours. Out of 140 patients, 42 patients belonged to the age group of 42-60 years and 28 belonged to the geriatric age group (>60 years). This is an agreement with other studies.[13–16] We have observed no gender difference in the incidence of ADRs in this study as has also been recorded by others.[15] Most of the ADRs in group A related to GIT, CNS, and skin in that order. Group B ADRs related to skin and GIT. Since most of the suspected medications were administered by the oral route, it is possible that GIT falls an easy prey to all such medications. At the same time, adverse reactions on skin are also noticed and reported quickly by the patients. Chemotherapeutic agents are a frequent cause of ADRs followed by drugs acting on CVS, CNS, and endocrines in that order. Antimicrobial drugs are among the most frequently prescribed drugs in the hospital and therefore, they are likely to be a common causative group. Our finding is in line with other studies.[14,15,17,18]
Most of the patients in group A developed the ADRs within a week of initiation of drug therapy while in group B developed the ADRs quite late. In fact, 25 (56%) patients of group B came up with the ADRs after a month of the initiation of drug therapy. Group A ADRs were quickly observed and thus treated early. Similar observations have been made before.[15] Group B patients reported the ADRs quite late till it became moderate or severe and thus resulted in longer time (8–30 days) to resolve.
Group A ADRs were ‘possible’ (P = 0.008) while group B were ‘probable’ (P = 0.0329). In the Naranjo scale such differences between groups A and B were much smaller as well as statistically insignificant. Multiple medications and lack of dechallenge are some of the reasons for most of the ADRs in group A being termed as ‘possible’ by WHO-UMC. In group B, few cases of multiple medications and dechallenge being done in all cases make a category of ‘probable’ to be more common in group B when Naranjo scale was used. Our findings are in agreement with others studies.[17,19,20] We have found both methods to be in agreement with each other in the majority of the cases (86%). We have experienced that the WHO-UMC method is simple and less time consuming while the Naranjo scale is more accurate.
Most of the ADRs in group B were serious in nature. Seventy nine (52%) of the 154 ADRs were serious. In other studies, it is about 3–30%.[18,19,21] It may be because we included the ADRs that developed during hospitalization and were a cause for the hospitalization. Nearly all ADRs in group B were of a serious nature. Fifty seven (37%) of the total and 47 (60%) of the serious ADRs were preventable. The 35% of serious ADRs were definitely preventable. In a related study, 10% of the ADRs are definitely avoidable, 50% of the ADRs are possibly preventable and about 40% of the ADRs are unavoidable.[22] Ninety-four (86%) ADRs in group A were moderate, while in group B 24 (53%) were serious. Most of the ADRs developed in the hospital may receive a prompt attention and thus they may not be allowed to become severe. Out-patients may not report to hospital until ADR is truly severe. A total of 104 (68%) ADRs were seen in patients receiving five or more drugs. It appears that polypharmacy may be common in hospitalized patients (Group A). The total cost of 154 ADRs in 140 patients was Rs. 1,49,803 with an average of Rs. 1070 per patient. The total cost of 57 preventable ADRs was Rs. 96,310. We thus opined that around 64% of the total cost of the ADRs was contributed by the preventable cases. This has also been an observation of other studies.[5,6,9] Substantial saving can be made if adequate caution is exerted toward preventable ADRs.
Our study has few limitations such as we studied only medical units and we did not follow up patients after discharge. Some of these patients may have suffered from late ADRs. We, however, feel that the duration of the study and sample size was adequate as it was able to cover all the seasons in a year.
In conclusion, around 2% of the hospital patients develop ADRs and a significant number of these ADRs were preventable (a lot can be saved in terms of financial resources and human suffering). Our study has generated a useful data particularly in the Indian context. In any case, our study is one more effort in making the drug use much more rational and safe.
ACKNOWLEDGMENTS
We would like to acknowledge Dr. Asha Shah and Dr. Bipin Amin for their help to conduct this study.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared
REFERENCES
- 1.World Health Organization (WHO) Safety Monitoring on Medicinal Products. Geneva (Switzerland): Office of Publications, World Health Organization; 2002. The Importance on Pharmacovigilance. [Google Scholar]
- 2.Einarson TR. Drug-related hospital admissions. Ann Pharmacother. 1993;27:832–8. doi: 10.1177/106002809302700702. [DOI] [PubMed] [Google Scholar]
- 3.Lazarous J, Pomeranz BH, Corey PN. Incidence of Adverse drug reactions in hospitalized patients. JAMA. 1998;279:1200–5. doi: 10.1001/jama.279.15.1200. [DOI] [PubMed] [Google Scholar]
- 4.Moore N, Lecointre D, Noblet C, Mabille M. Frequency and cost of serious adverse drug reactions in a department of general medicine. Br J Clin Pharmacol. 1998;45:301–8. doi: 10.1046/j.1365-2125.1998.00667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dikshit RK, Desai CK, Desai MK. Pleasures and pains of running a pharmacovigilance center. Indian J Pharmacol. 2008;40(Suppl 1):s31–3. [PMC free article] [PubMed] [Google Scholar]
- 6.Pirmohamed M, Breckenridge AM, Kitteringham NR, Park BK. Adverse drug reactions. BMJ. 1998;316:1295–8. doi: 10.1136/bmj.316.7140.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Storm BL, Kimmel SE. Text book of pharmacoepidemiology. 4th ed. England: John Willey and Sons, Ltd; 2007. pp. 432–8. [Google Scholar]
- 8.Incidence of adverse drug reactions, India. [Last cited on 2011 Sep 26]. Available from: http://pharmacovigilance.co.in/resources/Incidence%20of%20ADR%20india%20R1.htm .
- 9.World health organisation. Sweden. [Last cited on 2010 Nov 12]. Available from: http://www.who-umc.org .
- 10.Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239–45. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- 11.Lau PM, Stewart K, Dooley MJ. Comment: Hospital admissions resulting from preventable adverse drug reactions. Ann Pharmacother. 2003;37:303–5. doi: 10.1177/106002800303700229. [DOI] [PubMed] [Google Scholar]
- 12.Hartwig S, Seigel J, Schneider PJ. Preventability and severity assessment in reporting adverse drug reactions. Am J Hosp Pharm. 1992;49:2229–32. [PubMed] [Google Scholar]
- 13.Ramesh M, Pandit J, Parthasarathi G. Adverse drug reactions in a south Indian hospital - their severity and cost involved. Pharmacoepidemiol Drug Saf. 2003;12:687–92. doi: 10.1002/pds.871. [DOI] [PubMed] [Google Scholar]
- 14.Fattinger K, Roos M, Vergeres P, Holenstein C, Kind B, Masche U, et al. Epidemiology of drug exposure and adverse drug reactions in two swiss departments of internal medicine. Br J Clin Pharmacol. 2000;49:158–67. doi: 10.1046/j.1365-2125.2000.00132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lagnaoui N, Moore N, Fach J, Longy-Boursier M, Begaud B. Adverse drug reactions in a department of systemic diseases-oriented internal medicine: Prevalence, incidence, direct costs and avoidability. Eur J Clin Pharmacol. 2000;55:181–6. doi: 10.1007/s002280050738. [DOI] [PubMed] [Google Scholar]
- 16.Jose J, Rao PG. Pattern of adverse drug reactions notified by spontaneous reporting in an Indian tertiary care teaching hospital. Pharmacol Res. 2006;54:226–33. doi: 10.1016/j.phrs.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 17.Gor AP, Desai SV. Adverse Drug Reactions (ADR) in the inpatients of Medicine Department of a Rural Tertiary Care Teaching Hospital and Influence of Pharmacovigilance in Reporting ADR. Indian J Pharmacol. 2008;40:37–40. doi: 10.4103/0253-7613.40488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hurwitz N, Wade OL. Intensive hospital monitoring of adverse reactions to drugs. BMJ. 1969;1:531–6. doi: 10.1136/bmj.1.5643.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bates DW, Cullen DJ, Laird N, Petersen LA, Small SD, Servi D, et al. Incidence of adverse drug events and potential adverse drug events: Implications for prevention. JAMA. 1995;274:29–34. [PubMed] [Google Scholar]
- 20.Dormann H, Neubert A, Criegee-Rieck M, Egger T, Radespiel-TröGer M, Azaz-Livshits T, et al. Readmissions and adverse drug reactions in internal medicine: The economic impact. J Intern Med. 2004;255:653–63. doi: 10.1111/j.1365-2796.2004.01326.x. [DOI] [PubMed] [Google Scholar]
- 21.Faich GA. US Adverse Drug Reaction Surveillance 1989-1994. Pharmacoepidemiol Drug Saf. 1996;5:393–8. doi: 10.1002/(SICI)1099-1557(199611)5:6<393::AID-PDS235>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 22.Patel KJ, Kedia MS, Bajpai D, Mehta SS, Kshirsagar NA, Gogtay NJ. Evaluation of the prevalence and economic burden of adverse drug reactions presenting to the medical emergency department of a tertiary referral centre: A prospective study. BMC Clin Pharmacol. 2007;7:8. doi: 10.1186/1472-6904-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


