Abstract
Objective:
To compare the effectiveness and safety of cefpodoxime and ciprofloxacin for the treatment of mild to moderate cases of acute exacerbation of chronic suppurative otitis media (AECSOM).
Materials and Methods:
Adult patients diagnosed with AECSOM were screened and patients fulfilling the inclusion criteria were randomized to receive either cefpodoxime 200 mg twice daily or ciprofloxacin 500 mg twice daily orally for 7 days. The primary outcome of this randomized, open-labeled, phase IV clinical trial (Registration Number - CTRI/2011/10/002079) was clinical success rate at day 14 visit and the secondary outcome was incidence of adverse events (AEs). Forty-six patients were enrolled: 23 in the cefpodoxime group and 23 in the ciprofloxacin group.
Results:
The clinical success rates were 95.6% in the cefpodoxime group versus 90.9% in the ciprofloxacin group. These rates are comparable, but no statistically significant difference was observed between the groups. Few mild and self-limiting AEs were observed and the tolerability of both the drugs was also good.
Conclusion:
The results of this randomized, open-labeled phase IV clinical trial showed that a 7-day course of cefpodoxime is therapeutically comparable to ciprofloxacin in terms of both clinical effectiveness and safety for the treatment of patients with AECSOM.
Keywords: Cefpodoxime, ciprofloxacin, CSOM
INTRODUCTION
Chronic suppurative otitis media (CSOM) is defined as long-standing chronic suppuration of the middle ear cleft and its mucoperiosteal lining resulting in discharging ear and deafness. Chronic suppuration can occur with (tympano-mastoid type) or without (tubo-tympanic type) cholesteatoma. The tubo-tympanic type is the usually safe variety and follows a benign course. It usually occurs as a complication of acute otitis media, where there is perforation of the tympanic membrane. The perforation is always central and can be of various sizes.[1] Bacterial pathogens in the tubo-tympanic type CSOM vary considerably, and can be a combination of both aerobic and anaerobic bacteria. In CSOM, the bacteria may be aerobic (e.g., Escherichia coli, Staphylococcus aureus, Streptococcus pyogenes, Proteus mirabilis, Klebsiella species, Escherichia species, Haemophilus influenzae, etc.) or anaerobic (e.g., Bacteroides, Peptostreptococcus, Proprionibacterium, etc.).[2–4] Bacteriological cultures may not be needed to establish the diagnosis of CSOM as exhaustive studies have established that 90–100% of chronic draining ears yield two or more isolates consisting of both aerobic and anaerobic bacteria.[4,5] The choice of the antibiotic agent depends greatly on the knowledge of the type of bacteria most frequently implicated in CSOM and their sensitivity to antibiotics.
Since their introduction, systemically acting fluoroquinolones have opened up new therapeutic possibilities for using an orally administered antibiotic in the treatment of CSOM. Ciprofloxacin, in particular, has proven to be very active in vitro against a large number of Gram-negative and Gram-positive organisms. Moreover, ciprofloxacin concentrations within the structures of the middle ear have been shown to exceed the minimum inhibitory concentration (MIC) for the microorganisms responsible for CSOM.[6]
Cefpodoxime, an oral third-generation cephalosporin, has good antimicrobial activity against aerobic gram-positive and gram-negative bacteria. It is also effective against anaerobic organisms. There are few studies that have evaluated the effectiveness of cephalosporins like cephalexin and ceftriaxone in acute exacerbation of chronic suppurative otitis media (AECSOM), and results have shown that they can be considered as an effective agent for the treatment of AECSOM.[7,8] Against this backdrop, the present study was planned to compare the effectiveness and safety of cefpodoxime with ciprofloxacin in AECSOM. The objective of this study was to demonstrate equivalence between cefpodoxime (test drug) and ciprofloxacin (comparator) with respect to their effectiveness in AECSOM. Another objective was to compare their safety and tolerability profile.
MATERIALS AND METHODS
This was a prospective, randomized, open-labeled study. The study was approved by the Institutional Ethics Committee and conducted according to the ICMR guidelines for Biomedical Research on Human Subjects, 2006, and the Declaration of Helsinki. Subjects were recruited in the ENT Outpatient Department of a tertiary care teaching hospital and the study was conducted between June 2011 and October 2011. The study was registered under Clinical Trials Registry - India (Registration Number - CTRI/2011/10/002079).
The objective of the study was to demonstrate equivalence in the effectiveness between the two treatment groups. A difference of 10% in clinical cure rates was assumed to be the largest clinically acceptable effect for which equivalence could be accepted (equivalence limit). Considering the true mean difference between the two treatment groups as zero and the expected standard deviation of 10% in the study population, 90% power and α = 0.05, the number of subjects required in each treatment group was 21. The sample size was calculated by using primer of biostatistics software (version 5.0).
Inclusion criteria
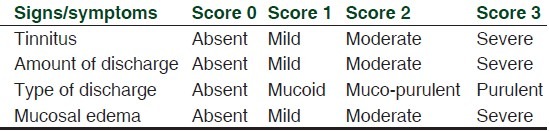
Adults of either sex, aged between 18 and 60, were considered. Clinically documented cases of tubo-tympanic-type CSOM, defined as long-standing chronic suppuration of middle ear cleft and its muco-periosteal lining resulting in discharging ear and deafness presenting with clinical symptoms and signs of acute exacerbation of the disease[1] and baseline otological symptom score[9] [Table 1] of >4 but ≤8 were included in the study.
Table 1.
Otological symptom score[9]
Exclusion criteria
Female patients who are pregnant or lactating were excluded. Severe cases of AECSOM for which hospitalization or parenteral antibiotic treatment is required and patients with otological symptom score of ≤4 and >8 were also excluded from the study. Patients with foul-smelling discharge and those who received antibiotic in the preceding 4 weeks of screening were left out.
Effectiveness parameters
Number of subjects achieving “treatment success” in each treatment group was considered to be the effectiveness parameter. Treatment success was based on changes in the otological symptoms scores at day 14 visit. It was subdivided into two categories: (a) “clinical cure” if the otological symptom score was <3 at day 14 visit or (b) “clinical improvement” if the otological symptom score was between 3 and 5 on day 14. “Treatment failure” was declared if there was no change or increase in the baseline otological symptom score on day 14.
Study visits
Each patient was evaluated for 2 weeks. The first week was the active treatment period. The following week was treatment-free follow-up. Patients were evaluated clinically at baseline (day 0) and at subsequent follow-up visits on Days 3, 7 and 14. Otological symptom score was recorded at every visit.
Grouping
Patients who fulfilled the selection criteria were randomly allocated in both treatment groups. Randomization was done by coin toss. Patients in group A received cefpodoxime (Ranbaxy Laboratories Ltd. Mumbai, India) tablet (200 mg) and group B received ciprofloxacin (Ranbaxy Laboratories Ltd. Mumbai, India) tablet (500 mg). Study medications were dispensed twice during the study period: first during baseline visit for 3 days and next during day 3 visit for the next 4 days. Patients were advised to take each of their respective study medications orally twice daily after food for the first 7 days. Compliance was assessed by the traditional pill count method at each follow-up visit and at the end of the study.
Patients with worsening clinical conditions or treatment failure were withdrawn prematurely from the study. Apart from the study drugs, no concomitant medication was administered to the patients. All patients were advised to stop smoking and consumption of alcohol during the study period. Patients were monitored continuously throughout the study for any adverse event (AE). Safety monitoring was performed continuously throughout the study. All AEs spontaneously reported by the subjects or elicited by the investigators were recorded and causality analysis was done as per the World Health Organization-Uppasala Monitoring Centre (WHO-UMC) criteria.[10]
Statistical analysis
Data were analyzed as per modified intention to treat basis. Subjects reporting for at least one postbaseline follow-up visit were analyzed. All patients who were randomized were considered for safety analysis. Data in ordinal scale were analyzed by Friedman's test for intragroup comparison and by Mann–Whitney U test for inter-group comparison. Categorical data were analyzed by Chi-square test. P-value < 0.05 was considered to be statistically significant.
RESULTS
Of the 55 subjects screened, 46 fulfilled the selection criteria and were randomized - 23 to group A (cefpodoxime) arm and 23 to group B (ciprofloxacin). One subject was lost during follow-up in group B and did not attend the hospital after the first visit. The consort flow chart has been shown in [Figure 1]. The mean age of patients was 35.8 years and 28.1 years in the cefpodoxime and ciprofloxacin groups, respectively. 69.5% were male in the cefpodoxime group and 56.5% were male in the ciprofloxacin group.
Figure 1.
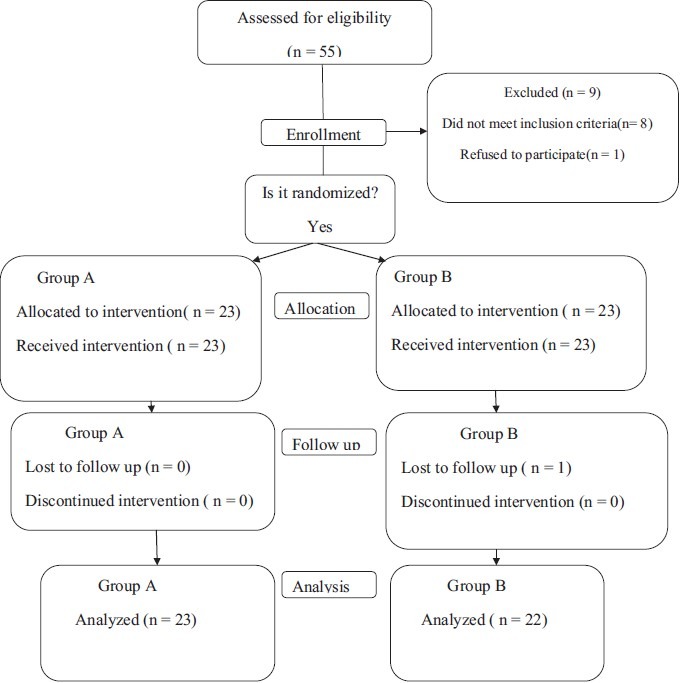
The CONSORT flowchart
There was no statistically significant difference in the baseline demographic profile and baseline otological symptom score. Changes in otological symptom score from baseline have been shown in Figures 2 and 3 and Table 2.
Figure 2.
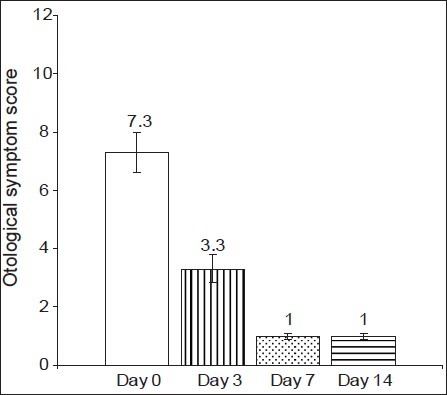
Changes in otological symptom score in the cefpodoxime group (Group A).P< 0.05- Days 3, 7 ,14 vs Day 0
Figure 3.
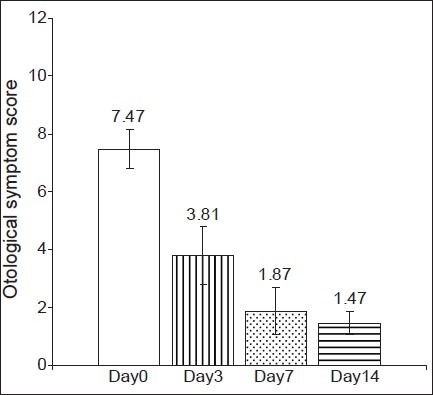
Changes in otological symptom score in the ciprofloxacin group (Group B). P< 0.05- Days 3, 7 ,14 vs Day 0
Table 2.
Intergroup comparison of otological symptom score
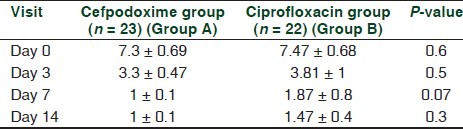
Intragroup analysis of otological symptom score at baseline (day 0) against day 3, day 7 and day 14 scores showed a highly significant decrease in both groups [Figures 2 and 3], and there was a clinically significant improvement in the signs and symptoms of the AECSOM. Thus, it can be suggested that both cefpodoxime and ciprofloxacin are effective in the treatment of AECSOM. An intergroup analysis of the otological symptom scores showed that there was no statistically significant difference in the baseline, day 3 and day 7 and day 14 otological symptom score [Table 2]. Therefore, it can be suggested that both cefpodoxime and ciprofloxacin are equally effective in the treatment of AECSOM.
Table 3 shows the number and the percentage of patients categorized as “treatment success” or “treatment failure” at the day 14 visit. Twenty-two subjects of the 23 enrolled in the cefpodoxime group achieved “treatment success,” i.e. either clinical improvement or clinical cure, and the remaining one subject was categorized as treatment failure. Similarly, in the ciprofloxacin group, 20 subjects of the 22 evaluated showed “treatment success,” i.e. either clinical improvement or clinical cure, and the remaining two were categorized as treatment failure. Intergroup comparison of the percentage of subjects who were categorized as treatment success showed no statistically significant difference (P = 0.25).
Table 3.
Comparison of treatment success rates
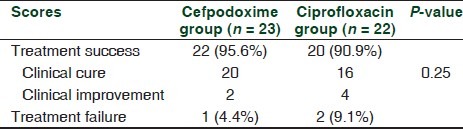
There were one patient in the cefpodoxime group and two patients in the ciprofloxacin group who were categorized as treatment failure and had to be put on other antibiotics after the day 7 evaluation.
Safety analysis was carried out as per the Intention to Treat (ITT) analysis. All patients who were randomized were considered for safety analysis. Only two AEs were noted during the entire study period. Two patients in the cefpodoxime group reported to have mild diarrhea. None of the patients in the ciprofloxacin group reported any AE. These AEs were mild in nature and did not require any dose reduction or withdrawal of the study medications. Causality analysis showed that they were in the “possible” category. Therefore, the safety and tolerability profile of both the study drugs were good without any reported cases of serious AE.
DISCUSSION
Several published studies have shown the effectiveness of ciprofloxacin for the treatment of AECSOM in adult patients.[6,11,12] Few studies have also proved the effectiveness of cephalexin and ceftriaxone in adult patients with AECSOM.[7,8] But, there is no published comparative controlled trial that evaluated ciprofloxacin with an oral third-generation cephalosporin in adult patients with AECSOM. The results of our study showed that cefpodoxime and ciprofloxacin are equally effective in clinically diagnosed cases of AECSOM, both in terms of effectiveness and in terms of safety. After treatment with a 7-day course, the clinical success rates were comparable, i.e. 95.6% in the cefpodoxime group and 90.9% in the ciprofloxacin group. The incidence of AEs was also minimal, i.e. two in the cefpodoxime group. These AEs were nonserious in nature and did not require dose modification or withdrawal of drug therapy. Patient compliance in both the groups was also good.
Baba et al. conducted a clinical trial that showed that ceftriaxone was effective in 65% patients with acute otitis media and 72% patients with AECSOM.[8] Another trial by Baba et al. reported that cephalexin has a 35.5% failure rate in patients with AECSOM.[7] Clinical cure rate with cefpodoxime in our study is significantly higher compared with those two trials with other cephalosporins. Very few studies have evaluated the effectiveness of oral third-generation cephalosporins in patients with otitis media.[13,14] A study reported by Block et al.[13] compared the effectiveness of cefdinir with cefprozil, and the results showed that the overall clinical cure rate with cefdinir was 80% versus 82.5% with cefprozil in the treatment of pediatric acute otitis media. Another study by Kafetzis showed that the overall clinical cure rate with cefixime was 85% in patients with acute otitis media.[14] Our study suggests that cefpodoxime has higher cure rates (95.6%) compared with other oral third-generation cephalosporins.
Some limitations of this study were as follows. A double-blind study could not be conducted due to financial constraints and logistic problems. Secondly, we did not perform bacteriological culture of the cases as exhaustive studies have established that 90–100% of chronic draining ears yield two or more isolates consisting of both aerobic and anaerobic bacteria.[4] Another reason is that, very often, clinicians start antimicrobial therapy at outpatient setting before the bacteriological culture report arrives, which takes about 72 h. Therefore, we conducted this study mainly to provide information to clinicians on the comparative effectiveness of these two antibiotics as initial antibiotics for AECSOM patients based on clinical assessment scores.
CONCLUSION
The results of this study demonstrated that a 7-day course of cefpodoxime is comparable to ciprofloxacin in terms of both clinical effectiveness and safety for the treatment of AECSOM in an outpatient setting. Although cost of drug therapy was higher with cefpodoxime compared with ciprofloxacin, it should not be considered as a drawback. Future trials are warranted to evaluate the bacteriological cure and relapse rates of these two drugs to provide additional supportive scientific evidence.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared
REFERENCES
- 1.Verhoeff M, van der Veen EL, Rovers MM, Sanders EAM, Anne GM, Schilder AGM. Chronic suppurative otitis media: A review. Int J Pediatr Otorhinolaryngol. 2006;70:1–12. doi: 10.1016/j.ijporl.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 2.Brobby GW, Zadik P. Bacteriology of otitis media in Ghana. Trop Doct. 1987;17:91–2. doi: 10.1177/004947558701700216. [DOI] [PubMed] [Google Scholar]
- 3.Fairbanks DN. Antimicrobial therapy for chronic suppurative otitis media. Ann Otol Rhinol Laryngol Suppl. 1981;90:S58–62. doi: 10.1177/00034894810903s214. [DOI] [PubMed] [Google Scholar]
- 4.Chronic suppurative otitis media-Burden of Illness and Management Options. [monograph on the internet] World health organization. 2004. [Last accessed on 2011 Nov 15]. Available from: http://www.who.int/entity/pbd/deafness/activities/hearing…/otitis_media.pdf .
- 5.Bluestone CD, Kenna MA. Chronic suppurative otitits media: Antimicrobial therapy or surgery? Pediatr Ann. 1984;13:417–21. [PubMed] [Google Scholar]
- 6.Gehanno P. Multicenter study of the effectiveness and safety of oral ciprofloxacin in the treatment of chronic suppurative otitis media in adults. The French Study Group. Otolaryngol Head Neck Surg. 1997;117:83–90. doi: 10.1016/S0194-59989770212-2. [DOI] [PubMed] [Google Scholar]
- 7.Baba S, Murai K, Kinoshita H, Kawai T, Koyama K, Tsukiyama M. Double-blind comparative trial of cefroxadine and cephalexin in the treatment of acute suppurative otitis media and acute exacerbation of chronic suppurative otitis media. Jpn J Antibiot. 1989;42:212–47. [PubMed] [Google Scholar]
- 8.Baba S, Mori Y, Suzuki K, Unno T, Kawabori S, Yanai T. Evaluation of the effectiveness of ceftriaxone in acute suppurative otitis media and acute exacerbation of chronic suppurative otitis media. A comparative study with cefotiam as the control. J Med Assoc Thai. 2000;83:61–8. [PubMed] [Google Scholar]
- 9.Al-Abbasi AM. Effectiveness of Povidone Iodine in Treatment of Active Chronic Suppurative Otitis Media. J Indian Med Assoc. 2006;38:118–22. [Google Scholar]
- 10.Edwards R, Aronson JK. Adverse Drug Reactions: Definitions, diagnosis and management. Lancet. 2000;356:1255–9. doi: 10.1016/S0140-6736(00)02799-9. [DOI] [PubMed] [Google Scholar]
- 11.Esposito S, D’Errico G, Montanaro C. Topical and oral treatment of chronic otitis media with ciprofloxacin. A preliminary study. Arch Otolaryngol Head Neck Surg. 1990;116:557–9. doi: 10.1001/archotol.1990.01870050057006. [DOI] [PubMed] [Google Scholar]
- 12.Dohar JE, Alper CM, Rose EA. Treatment of chronic suppurative otitis media with topical ciprofloxacin. Ann Otol Rhinol Laryngol. 1998;107:865–71. doi: 10.1177/000348949810701010. [DOI] [PubMed] [Google Scholar]
- 13.Block SL, Kratzer J, Nemeth MA, Tack KJ. Five-day cefdinir course vs.ten-day cefprozil course for treatment of acute otitis media. Pediatr Infect Dis J. 2000;19:S147–52. doi: 10.1097/00006454-200012001-00003. [DOI] [PubMed] [Google Scholar]
- 14.Kafetzis DA. Review Multi-investigator evaluation of the efficacy and safety of cefprozil, amoxicillin-clavulanate, cefixime and cefaclor in the treatment of acute otitis media. Eur J Clin Microbiol Infect Dis. 1994;13:857–65. doi: 10.1007/BF02111353. [DOI] [PubMed] [Google Scholar]


