Abstract
Pregnancy-associated plasma protein-A (PAPP-A) functions to increase local IGF-I bioactivity. In this study, we used transgenic mice that constitutively express human PAPP-A in arterial smooth muscle to test the hypothesis that overexpression of PAPP-A enhances vascular smooth muscle cell (SMC) response to IGF-I in vivo. PAPP-A transgenic (Tg) and wild-type (WT) mice underwent unilateral carotid ligation, a model of injury-induced SMC hyperplasia and neointimal formation. In both WT and PAPP-A Tg mice, endogenous PAPP-A mRNA expression showed peak elevation 5 days after carotid ligation. However, PAPP-A Tg mice had 70–75% less neointima than WT at 5 and 10 days postligation, with a significant reduction in occlusion of the ligated artery. WT and PAPP-A Tg mice had equivalent increases in medial area and vessel remodeling postligation. There was little change in medial area and no evidence of neointima in the contralateral carotid of WT or PAPP-A Tg mice. Both WT and PAPP-A Tg carotids exhibited signs of dedifferentiation of SMC, which precedes the increase in proliferation and migration that results in neointimal formation. However, the number of proliferating cells in the media and neointima of the ligated PAPP-A Tg artery was reduced by 90% on day 5 postsurgery compared with WT. This decrease was associated with a significant decrease in an in vivo marker of IGF-I bioactivity and reduced IGF-I-stimulated receptor phosphorylation ex vivo. These data suggest differential effects of chronic (transgenic) and transient (endogenous) PAPP-A expression on neointimal formation following vascular injury that may be due in part to the differential impact on IGF-I signaling.
Keywords: insulin-like growth factor I, pregnancy-associated plasma protein-A, vascular smooth muscle cells, hyperplasia, restenosis, carotid ligation
insulin-like growth factor i (IGF-I) has been implicated in physiological and pathological responses to vascular injury or insult (1, 10, 13). IGF-I was found to be elevated in the media and neointima in various animal injury models and in human restenotic vessels following percutaneous revascularization procedures (14, 18, 27), and targeted expression of IGF-I stimulated vascular smooth muscle cell (SMC) proliferation and migration in vivo (32). In vitro studies of SMCs have shown that increases in IGF-I receptor signaling lead to increased proliferation and migration, characteristics of dedifferentiated medial SMCs that infiltrate the intimal layer and contribute to neointimal formation (1, 2, 10, 13). However, the IGF system is complex, and its role in vascular response to injury is yet to be completely understood.
Pregnancy-associated plasma protein-A (PAPP-A) is a protease in the metzincin superfamily that functions to increase local IGF-I bioavailability through cleavage of inhibitory IGF-binding proteins (IGFBPs; reviewed in Ref. 4). PAPP-A is expressed by vascular SMCs in vitro, and marked increases in PAPP-A expression precede neointimal formation in pig coronary arteries following balloon angioplasty and in murine carotid arteries following ligation (3, 27). In the latter study (27), coordinate increases in IGF-I, IGFBP-4, and PAPP-A mRNA expression occurred in response to the injury, suggesting a situation of enhanced local IGF-I bioavailability. Furthermore, deletion of the PAPP-A gene in mice resulted in resistance to the development of neointimal hyperplasia as an acute injury response (27). However, these correlative and loss-of-function studies are not definitive in terms of in vivo mechanisms. Therefore, to determine the role of PAPP-A in arterial remodeling, we evaluated the influence of PAPP-A overexpression in SMCs on vascular response in a murine carotid artery injury model. We hypothesized that the transgenic mice overexpressing PAPP-A would show enhanced neointimal formation in response to injury.
MATERIALS AND METHODS
PAPP-A transgenic mice.
Development of founder lines of PAPP-A transgenic (Tg) mice on an FVB background was reported recently (9). PAPP-A Tg mice designated Tg-4′ by Conover et al. (9) were used for all experiments except for the comparison studies in Table 1, which also included Tg-6′. Briefly, human PAPP-A transgene expression in arterial smooth muscle was driven by a minimal smooth muscle 22α (SM22α) promoter mutated to delete a G/C rich region responsible for repression of transcription in the vascular injury response (25, 29). PAPP-A Tg mice were crossed with FVB mice (The Jackson Laboratory, Bar Harbor, ME) to get PAPP-A Tg and wild-type (WT) mice for experiments. In addition, Tg mice that express a mutant form of PAPP-A (E483A) that is devoid of proteolytic activity were developed (5). These “protease-dead” PAPP-A Tg (TgPD) mice will be detailed in a separate publication (unpublished observations). Genotypes were routinely confirmed at harvest.
Table 1.
Neointimal hyperplasia following unilateral carotid ligation
| % Occlusion |
|||
|---|---|---|---|
| Postligation | WT | PAPP-A Tg′ | PAPP-A TgPD |
| Day 5 | 26 ± 4 (5) | 11 ± 3 (8)* | |
| Day 10 | 45 ± 10 (9) | 22 ± 3 (9)* | 36 ± 12 (9) |
| Day 15 | 72 ± 9 (9) | 38 ± 10 (6)* | |
Results are means ± SE, with nos. of mice in parentheses. WT, wild type; PAPP-A, pregnancy-associated plasma protein-A; Tg, transgenic; PAPP-A Tg′, different PAPP-A Tg founder (PAPP-A Tg6′ in 9 mice); PAPP-A TgPD, “protease-dead” PAPP-A Tg (mutation E483A). Unilateral carotid ligation was performed as described in materials and methods. %Occlusion was calculated as 100 minus [luminal area divided by internal elastic lamina].
P < 0.05 vs. WT.
Carotid ligation.
WT and PAPP-A Tg mice underwent unilateral carotid ligation for the evaluation of vascular remodeling (19, 27). This ligation model induces arterial injury due to cessation of blood flow, resulting in rapid proliferation of medial SMCs and migration, leading to neointimal formation. Mice were anesthetized, a midline incision on the ventral surface of the neck was made, and the right and left common carotid arteries were isolated from surrounding tissues. A suture was passed under the left vessel just proximal to the carotid bifurcation and the artery ligated; the right carotid was treated similarly (minus the ligation) and was considered the contralateral sham control. All procedures were approved by Mayo Clinic's Institutional Animal Care and Use Committee and complied with the standards stated in the National Institutes of Health's Guide for the Care and Use of Laboratory Animals.
Gene expression.
WT and PAPP-A Tg mice were euthanized at various times following ligation for the measurement of mRNA expression of mouse IGF system components [PAPP-A, IGF-I, IGFBP-4, IGFBP-5, IGF-I receptor (IGF-IR)], mouse arterial SMC differentiation markers [αSM actin (αSMA), SM myosin heavy chain (Myhll), SM22α], and reference genes [TATA box-binding protein (TBP), glyceraldehyde-3-phosphate dehydrogenase (GAPDH)] by real-time RT-PCR, as described previously (17, 27). The left (experimental) and right (sham) carotid arteries were immediately isolated, dissected, and lysed with Trizol (Life Technologies, Carlsbad, CA) for total RNA isolation. This RNA was then reverse transcribed and evaluated by quantitative PCR using the iCycler Detection System with SYBR green PCR Master Mix (Bio-Rad, Hercules, CA). Amplification plots were analyzed with iCycler Detection System analysis software version 3.0.6070. The following primer sequences were used for the differentiation markers and reference genes: αSMANM_07392.2 (forward: 5′-ggcatccacgaaaccacctat-3′; reverse: 5′-agccaccgatccagacagagta-3′), MyhllNM_013607.2 (forward: 5′-atgctgggaaggtggactacaa-3; reverse: 5′-gtgcggaacatgcccttttt-3′), SM22αGenBank:U36588.1 (forward: 5′-ctctaatggctttgggcagtttg-3′; reverse: 5′-tgcagttggctgtctgtgaagtc-3′), GAPDHNM_008084.2 (forward: 5′-ttcaccaccatggagaagg-3′; reverse: 5′-ctcgtggttcacacccatc-3′), and TBPNM_013684.3 (forward: 5′-ctcagttacaggtggcagca-3′; reverse: 5′-cagcacagagcaagcaact-3′).
Histomorphometry and immunohistology.
WT and PAPP-A Tg mice were euthanized 5, 10, and 15 days following ligation and the carotid arteries fixed in situ by perfusion with PBS-buffered formalin at physiological pressure. Individual arteries were removed, placed into PBS-buffered formalin, and fixed for 24 h prior to paraffin embedding. Five-micrometer-thick sections were collected 0.5, 1.0, and 1.5 mm distal to a reference point (0.25 mm distal the ligature). At each distance, Verhoff von Giessen-stained (Accustain; Sigma) sections were evaluated using Adobe PhotoShop software version 6.0.1 (Adobe Systems). Neointimal area [area within the internal elastic lamina (IEL) minus luminal area] for each animal was expressed as the mean of the neointimal area measured at the three distinct distances. Medial area was quantified as area defined by the external elastic lamina (EEL) minus that defined by the IEL. Vascular remodeling was calculated as EEL ligated divided by EEL unligated times 100. Percent occlusion was calculated as 100 minus [luminal area divided by IEL].
Ki-67 staining was employed as a nuclear marker of cell proliferation (28). Formalin-fixed paraffin embedded tissue sections were deparaffinized in xylene, dipped in decreasing concentrations of ethyl alcohol, and then rehydrated in distilled water. Antigen retrieval for Ki-67 (TEC3) was performed by placing slides in preheated citrate solution (Dako, Carpinteria, CA) in a water bath at 98°C for 40 min and then allowed to cool for 20 min prior to staining. The staining procedure was carried out in the Dako Autostainer Plus as follows: the tissue sections were treated with Peroxidase Blocking Reagent (Dako, Carpinteria, CA) for 5 min, washed with 1× wash buffer (Dako), and treated with Rodent Block M (Biocare Medical, Concord, CA) for 30 min. The tissue was then incubated with the primary antibody for 60 min at room temperature. After washing in wash buffer, the sections were incubated in a species-specific micropolymer secondary antibody and its paired tertiary, rat probe, and rat horseradish peroxidase for 15 min each (Biocare Medical), with a wash step in between each part of the kit. The slides were washed in 1× wash buffer before the High-Sensitivity Betazoid diaminobenzidine (DAB+) chromogenic substrate system (Biocare Medical, Concord, CA) that is used for colorimetric visualization was applied. Counterstaining with hematoxylin followed by dehydration in increasing concentrations of ethyl alcohol and xylene was performed prior to coverslipping.
IGF-I receptor phosphorylation-ligated carotids were removed from the animals, cleaned of adventia and cleared of blood, opened longitudinally, and placed in serum-free medium (SFM) containing 0.1% bovine serum albumin (BSA) for 60 min at 37°C. Vessels were then washed three times with SFM-BSA and placed in SFM-BSA ± 50 nM recombinant human IGF-I (R & D Systems, Minneapolis, MN) for 10 min. Carotids were transferred to M-PER (Thermo Scientific, Rockford, IL) containing protease and phosphatase inhibitors and snap-frozen in liquid nitrogen prior to homogenization. Protein content was measured by BCA protein assay kit (Thermo), and 20 μg of total protein was resolved on a 4–20% gradient gel (Bio-Rad) and transferred to a polyvinylidene difluoride membrane (Bio-Rad). Membranes were blocked and subsequently probed for phosphorylated IGF-I receptor β-subunit (Thermo) or total IGF-I receptor β-subunit (Novus Biologicals, Littleton, CO) overnight at 4°C. Images were captured using an Odyssey infrared imager (Li-COR Biosciences, Lincoln, NE) and band intensities quantified using Image J analysis software (National Institutes of Health, Bethesda, MD; http://rsbweb.nih.gov/ij/download.html).
Data analysis.
ANOVA and post hoc t-test were used to compare means ± SE. Relative Expression Software Tool 2009 (Qiagen), which automates data analysis using the Pfaffl model, was used for quantitation of gene expression relative to TBP and GAPDH. Significance was set as P < 0.05.
RESULTS
PAPP-A mRNA expression.
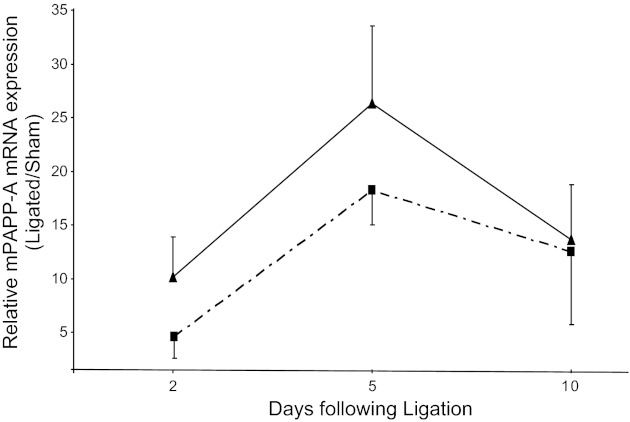
The time course of PAPP-A mRNA expression in the carotid ligation injury model is shown in Fig. 1. In WT mice, endogenous PAPP-A showed peak expression 5 days postinjury. PAPP-A Tg mice showed a time course and magnitude of endogenous PAPP-A expression following injury that was not significantly different from WT. However, these mice also had constitutive human PAPP-A transgene expression (9). It is of note that the time course for endogenous PAPP-A expression is shifted somewhat compared with our earlier study (27), but this is likely due to the different genetic backgrounds. The FVB mouse used in the present study is more susceptible to neointimal formation in response to carotid injury (16).
Fig. 1.
Endogenous pregnancy-associated plasma protein-A (PAPP-A) gene expression in wild-type mice (dashed line) and PAPP-A transgenic (Tg) mice (solid line) following unilateral carotid ligation. Mouse PAPP-A mRNA levels were measured by real-time PCR, and ligated values are expressed relative to the unligated (sham) artery. Results are means ± SE of 4–6 mice. There were no significant differences between WT and PAPP-A Tg mice.
Vascular response.
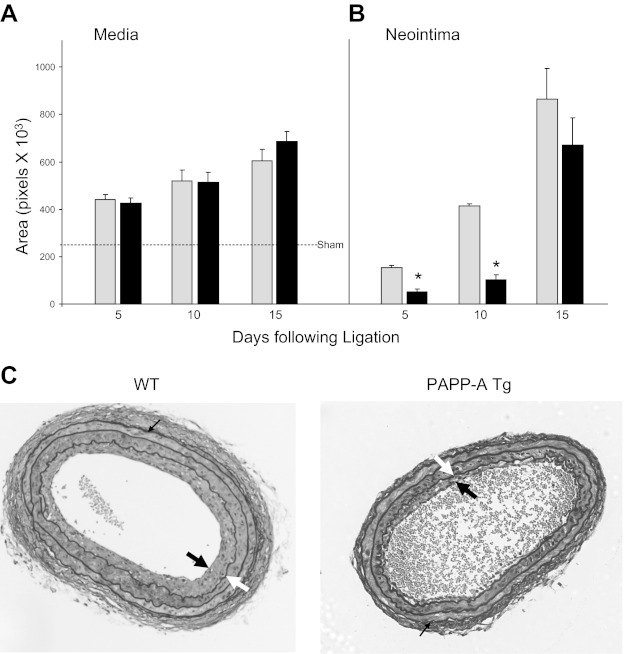
As shown in Fig. 2A, there was a progressive and equivalent increase in medial area in the ligated left artery of WT and PAPP-A Tg mice at 5, 10, and 15 days postinjury. There was little or no change in medial area in the contralateral (sham) artery. Neointima was apparent in WT mice 5 days following carotid ligation (Fig. 2B), progressing to occlusion or near occlusion at 15 days. Somewhat unexpectedly, neointimal formation was significantly suppressed in PAPP-A Tg mice. There was a 70–75% reduction in neointimal area in PAPP-A Tg mice compared with WT mice at 5 and 10 days postinjury (Fig. 2, B and C). On day 15, the difference in neointimal area between groups was not significant, likely due to the variability of absolute neointimal area in totally occluded vessels. If expressed as percent occlusion, then the reduction in neointimal formation was significant at all three time points (data not shown). Similar reductions in neointimal formation were seen with a different founder PAPP-A Tg mouse (Table 1). PAPP-A Tg mice on a mixed C57BL6/129/FVB background had 12 ± 4% occlusion (n = 5) 10 days following carotid ligation compared with 30 ± 6% occlusion (n = 5) in nontransgenic controls. Therefore, these findings were not associated with a specific transgene insertion site or genetic background. Interestingly, PAPP-A TgPD mice expressing “protease-dead” PAPP-A did not show this reduction in neointimal formation or consequent vessel occlusion (Table 1).
Fig. 2.
Medial (A) and neointimal (B) area in wild-type (WT; gray bars) and PAPP-A Tg mice (black bars) following unilateral carotid ligation. Results are means ± SE of 8–12 mice. Dashed line indicates sham values in medial area. *Significant difference between WT and PAPP-A Tg mice at P < 0.0001. C: sections of carotids 10 days following unilateral ligation stained with Verhoff von Giessen. Thick black arrows indicate the neointima, thick white arrows indicate the internal elastic lamina (IEL), and thin black arrows indicate the external elastic lamina (EEL). Neointimal area was defined by IEL minus luminal area. Medial area was defined by EEL minus IEL.
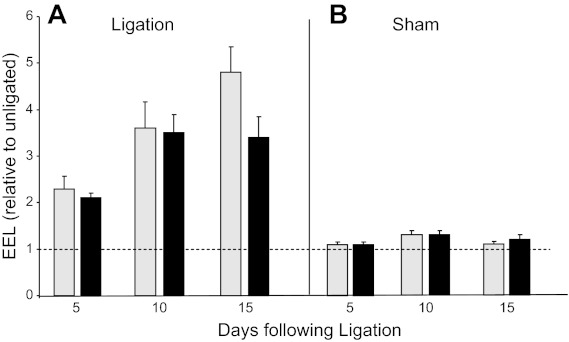
In the employed ligation model (19), vessels can adapt to chronic changes in blood flow by undergoing compensatory adjustment in lumen size. To determine the effect of chronic PAPP-A expression on vascular remodeling, total vessel area of ligated carotid (EEL of left carotid) was determined relative to total area of nonligated carotid (EEL of right carotid). Both WT and PAPP-A Tg mice exhibited equivalent positive remodeling in the ligated vessel (Fig. 3).
Fig. 3.
Vessel remodeling in WT (gray bars) and PAPP-A Tg mice (black bars) following unilateral carotid ligation. Vascular remodeling was calculated as EEL ligated divided by EEL unligated times 100. Results are means ± SE of 8–12 mice.
SMC regulation.
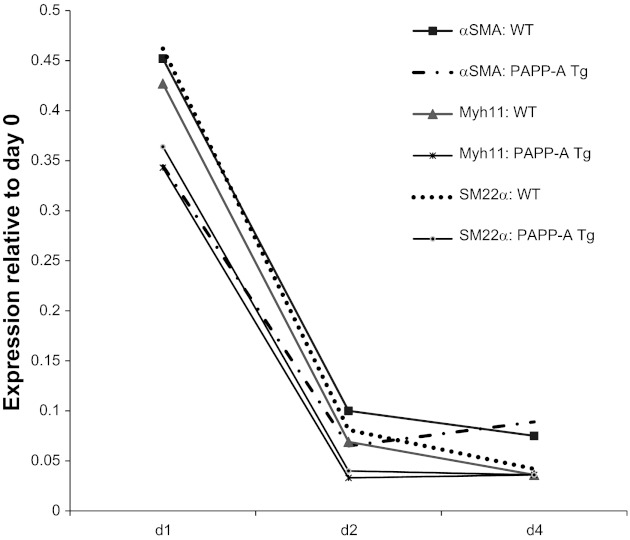
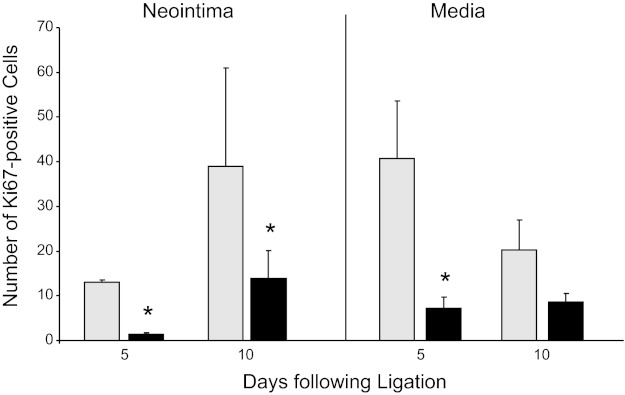
Phenotypic switching of SMCs from a primarily contractile to a more proliferative/synthetic phenotype is a key factor in neointimal formation following vascular injury (25). To test the hypothesis that PAPP-A overexpression in arterial smooth muscle cells impedes this dedifferentiation, differentiation marker expression was measured. As shown in Fig. 4, mRNA levels of murine αSMA, Myhll, and SM22α were decreased dramatically in response to carotid ligation, but there was no significant difference between WT and PAPP-A Tg mice. Following vascular injury, subsequent proliferation was indicated by strong Ki-67 staining of numerous SMCs in the media and neointima of WT carotids 5 and 10 days following injury. However, the number of proliferating cells was markedly reduced in PAPP-A Tg carotids (Fig. 5).
Fig. 4.
Expression of vascular differentiation markers in WT and PAPP-A Tg mice following carotid ligation. mRNA levels were measured by real-time PCR. Results are means (n = 5 or 6 mice) expressed relative to day 0. αSMA, α-smooth muscle actin; Myhll, smooth muscle myosin heavy chain; SM22α, smooth muscle 22α.
Fig. 5.
Ki-67 immunostaining of carotid arteries in WT (gray bars) and PAPP-A Tg mice (black bars) following ligation. Results are means ± SE (n = 4–6 mice). *Significant difference between WT and PAPP-A Tg mice at P < 0.001.
IGF-I responsiveness.
To determine whether chronic PAPP-A transgene expression, as opposed to the wave of endogenous PAPP-A expression, affected the early IGF response to injury, levels of IGF-I, IGF-IR, IGFBP-4, and IGFBP-5 mRNA were measured on day 2. IGFBP-5 is an IGF-I-responsive gene that is used as a marker of in vivo IGF-IR activation in vascular SMCs (9, 11, 21, 27). As shown in Table 2, there was no significant difference in IGF-I, IGF-IR, or IGFBP-4 mRNA expression in carotids from WT and PAPP-A Tg mice. However, IGFBP-5 mRNA levels were significantly decreased in the ligated carotids from PAPP-A Tg vs. WT mice.
Table 2.
IGF system gene expression following unilateral carotid ligation
| mRNA | Relative Expression | P Value |
|---|---|---|
| IGF-I | 0.92 (0.63–1.43) | 0.655 |
| IGFBP-4 | 0.83 (0.56–1.13) | 0.366 |
| IGF-IR | 0.94 (0.82–1.12) | 0.542 |
| IGFBP-5 | 0.60 (0.38–0.91) | 0.048 |
Results are presented as mean expression (with SE range in parentheses) for PAPP-A Tg relative to WT. IGFBP, IGF-binding protein; IGF-IR, IGF-I receptor. Carotids were harvested 2 days following ligation, processed for real-time RT-PCR, and analyzed using the Pfaff1 model for relative expression.
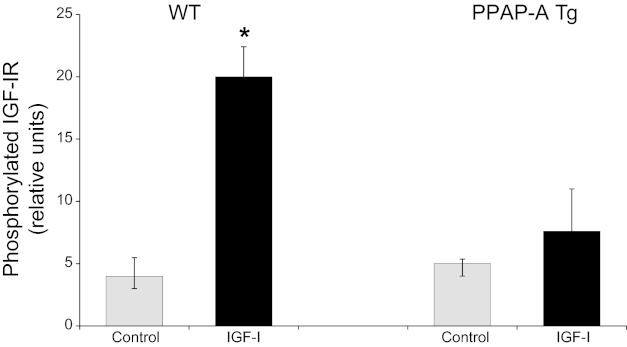
To assess more directly the effect of constitutive PAPP-A expression on IGF-I response to injury, carotid arteries were harvested from WT and PAPP-A Tg mice 2 days postligation and put in media ± 50 nM IGF-I for 10 min. Tissue was then lysed and subjected to Western immunoblot for phosphorylated IGF-IR. As shown in Fig. 6, IGF-I significantly stimulated IGF-IR phosphorylation in previously ligated carotids from WT but not PAPP-A Tg mice. Total IGF-IR was not significantly different in carotids from WT and PAPP-A Tg mice.
Fig. 6.
IGF-I-stimulated IGF-I receptor (IGF-IR) phosphorylation ex vivo. Two days postligation, carotids from WT and PAPP-A Tg mice were harvested and then treated without (gray bars) or with 50 nM IGF-I (black bars) for 10 min. Western immunoblotting for phosphorylated IGF-IR was performed and analyzed as described in materials and methods. Results are means ± SE (n = 5 mice). *Significant IGF-I effect at P < 0.05.
DISCUSSION
In a previous study, mice null for PAPP-A were found to be resistant to injury-induced neointimal hyperplasia at least in part through reduced IGF-I bioavailability (27). Therefore, we hypothesized that overexpression of PAPP-A in arterial smooth muscle, and consequently increased IGF-I bioavailability, would result in neointimal formation exceeding that seen in WT mice. This would fit with studies from other laboratories where targeted overexpression of PAPP-A to osteoblasts in mice resulted in significant increases in bone formation (24) and targeted overexpression PAPP-A to skeletal muscle resulted in increased skeletal muscle mass (26). However, here we demonstrate the exact opposite. PAPP-A Tg mice overexpressing human PAPP-A in arterial smooth muscle had markedly reduced/delayed neointimal formation compared with WT mice. This constitutive human PAPP-A expression is not a general inhibitor of vascular response, since these same Tg mice showed accelerated atherosclerosis in a chronic injury model, i.e., apolipoprotein E deficiency and high-fat diet (9).
Proliferation of medial SMCs, migration to the intima, and proliferation of intimal cells are sequential steps in the formation of neointima after acute arterial injury, and these have all been shown to be stimulated by IGF-I (1, 2, 10, 13). Indeed, previous in vitro studies showed significantly reduced IGF-stimulated proliferation of aortic SMCs from PAPP-A knockout compared with WT mice (27). If PAPP-A expression results in enhanced IGF-I activity, then why would overexpression of PAPP-A in arterial smooth muscle inhibit neointimal formation? We believe the seemingly paradoxical effect of PAPP-A overexpression is in large part due to the particular timing of signaling events in the acute vs. chronic injury response, which are not fully understood. In support of this view, Zhao et al. (31) reported that chronic arterial overexpression of IGF-I was associated with increased vascular contractility, whereas acute IGF-I had the opposite effect. It is also of note that the anabolic effect of PAPP-A overexpression in the aforementioned studies (24, 26) was under basal constitutive conditions and not in response to an acute injury event. We did not observe differences in medial area in unligated control carotids from PAPP-A Tg or WT mice. This may be due to different levels of PAPP-A expression in the various studies.
In the repair response in many tissues, including brain, skeletal muscle, and vascular tissue, there is a wave of IGF-I activity following injury that stimulates cells to proliferate (7, 8, 22). The importance of timing in IGF-I expression/action is highlighted in the brain injury model, where administration of exogenous IGF-I after but not prior to or simultaneous with the insult has a beneficial effect (12). Similarly, pretreatment with IGF-I activated cell cycle arrest following irradiation injury in salivary glands, whereas IGF-I treatment after the injury normalized cell proliferation (15, 20). Indeed, in contrast to acute increases in IGF-I that offered cardiac protection, chronic IGF-I overexpression was associated with reduced functional recovery after ischemic insult (23). Thus, constitutive PAPP-A expression promoting IGF-I action prior to and at the time of injury might impair the normal tissue response. Interestingly, perivascular release of IGF-I at the time of arterial balloon injury reduced neointimal formation (30). Also, insulin implants prior to arterial injury resulted in reduced neointima (6). The investigators interpreted their findings as inhibition of migration. If proliferation of medial SMCs precedes migration to the intima, then our finding of reduced SMC proliferation in the medial compartment of PAPP-A Tg carotids compared with WT 5 days after ligation would suggest an effect prior to migration.
One possibility for reduced proliferation could be that constitutive PAPP-A expression inhibited phenotypic switching of SMCs, resulting in decreases in proliferation and migration. The hallmark of SMC phenotypic switching is the coordinate downregulation of expression of smooth muscle differentiation markers, including αSMA, SM22α, and Myhll. Carotid ligation has been shown to decrease αSMA and SM22α in medial layer 3–7 days after ligation, which is why we used a mutated, constitutively active SM22α promoter to drive PAPP-A transgene expression (9, 25, 29). However, PAPP-A Tg had similar loss of αSMA, Myhll, and SM22α as WT in response to carotid ligation.
Acute and chronic IGF-I activity can have differential effects on injury response (7, 8, 12, 15, 20, 22, 23); therefore, the effects of acute (endogenous) and chronically (transgene) elevated PAPP-A expression on IGF-I activity were examined in the carotid ligation model. There was no difference in IGF-I, IGF-IR, or IGFBP-4 mRNA expression between WT and PAPP-A Tg. However, levels of the IGF-I-responsive gene IGFBP-5 were decreased significantly in PAPP-A Tg relative to WT. This would be consistent with diminished IGF-IR activity early in the injury response that could impact subsequent proliferation and formation of neointima in this model. Diminished IGF-IR activity was supported by ex vivo experiments. PAPP-A Tg carotids harvested 2 days postligation demonstrated significantly reduced IGF-I-stimulated receptor phosphorylation compared with WT carotids. Further support that the “protective” effect of chronically increased PAPP-A levels is mediated through regulation of local IGF-I bioactivity is the finding that the reduction in neointimal formation was not observed in the PAPP-A TgPD mice that are unable to increase IGF bioavailability through IGFBP proteolysis.
Further studies will be necessary to delineate the precise molecular mechanisms underlying the observed effect of chronic increased PAPP-A activity to suppress vascular SMC proliferation and neointimal formation following vascular injury. Nevertheless, these data underscore the complexity of the injury response, in particular the acute injury response, and may have consequences on the development of therapeutic strategies involving PAPP-A (and IGF-I) in vascular response.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-74871 to C. A. Conover
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.K.B., Z.T.R., S.L.H., M.T.O., and C.A.C. performed the experiments; L.K.B., S.L.H., and C.A.C. interpreted the results of the experiments; L.K.B., S.L.H., M.T.O., and C.A.C. edited and revised the manuscript; L.K.B., Z.T.R., S.L.H., M.T.O., and C.A.C. approved the final version of the manuscript; Z.T.R., M.T.O., and C.A.C. did the conception and design of the research; Z.T.R., S.L.H., and C.A.C. analyzed the data; S.L.H. and C.A.C. prepared the figures; C.A.C. drafted the manuscript.
ACKNOWLEDGMENTS
We thank Megan Mason, Jessica Mader, Jacquelyn Grell, Emily Mason, and Catherine Ishitani for their excellent technical assistance.
REFERENCES
- 1. Bayes-Genis A, Conover CA, Schwartz RS. The insulin-like growth factor axis: a review of atherosclerosis and restenosis. Circ Res 86: 125–130, 2000 [DOI] [PubMed] [Google Scholar]
- 2. Bayes-Genis A, Schwartz RS, Bale LK, Conover CA. Effects of insulin-like growth factor-I on cultured human coronary artery smooth muscle cells. Growth Horm IGF Res 13: 246–253, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Bayes-Genis A, Schwartz RS, Lewis DA, Overgaard MT, Christiansen M, Oxvig C, Ashai K, Holmes DR, Jr, Conover CA. Insulin-like growth factor binding protein-4 protease produced by smooth muscle cells increases in the coronary artery after angioplasty. Arterioscler Thromb Vasc Biol 21: 335–341, 2001 [DOI] [PubMed] [Google Scholar]
- 4. Boldt HB, Conover CA. Pregnancy-associated plasma protein-A (PAPP-A): a local regulator of IGF bioavailability through cleavage of IGFBPs. Growth Horm IGF Res 17: 10–18, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Boldt HB, Overgaard MT, Laursen LS, Weyer K, Sottrup-Jensen L, Oxvig C. Mutational analysis of the proteolytic domain of pregnancy-associated plasma protein-A (PAPP-A): classification as a metzincin. Biochem J 358: 359–367, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Breen DM, Chan KK, Dhaliwall JK, Ward MR, Koudsi NA, Lam L, De Souza M, Ghanim H, Dandona P, Stewart DJ, Bendeck MP, Giacca A. Insulin increases reendothelialization and inhibits cell migration and neointimal growth after arterial injury. Arterioscler Thromb Vasc Biol 29: 1060–1066, 2009 [DOI] [PubMed] [Google Scholar]
- 7. Cercek B, Fishbein MC, Forrester JS, Helfant RH, Fagin JA. Induction of insulin-like growth factor I messenger RNA in rat aorta after balloon denudation. Circ Res 66: 1755–1760, 1990 [DOI] [PubMed] [Google Scholar]
- 8. Clemmons DR. Modifying IGF1 activity: an approach to treat endocrine disorders, atherosclerosis and cancer. Nat Rev Drug Discov 6: 821–833, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Conover CA, Mason MA, Bale LK, Harrington SC, Nyegaard M, Oxvig C, Overgaard MT. Transgenic overexpression of pregnancy-associated plasma protein-A in murine arterial smooth muscle accelerates atherosclerotic lesion development. Am J Physiol Heart Circ Physiol 299: H284–H291, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Delafontaine P, Song YH, Li Y. Expression, regulation, and function of IGF-1, IGF-1R, and IGF-1 binding proteins in blood vessels. Arterioscler Thromb Vasc Biol 24: 435–444, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Duan C, Hawes SB, Prevette T, Clemmons DR. Insulin-like growth factor-I (IGF-I) regulates IGF-binding protein-5 synthesis through transcriptional activation of the gene in aortic smooth muscle cells. J Biol Chem 271: 4280–4288, 1996 [DOI] [PubMed] [Google Scholar]
- 12. Endres M, Piriz J, Gertz K, Harms C, Meisel A, Kronenberg G, Torres-Aleman I. Serum insulin-like growth factor I and ischemic brain injury. Brain Res 1185: 328–335, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Frystyk J, Ledet T, Møller N, Flyvbjerg A, Orskov H. Cardiovascular disease and insulin-like growth factor I. Circulation 106: 893–895, 2000 [DOI] [PubMed] [Google Scholar]
- 14. Grant MB, Wargovich TJ, Ellis AE, Tarnuzzer R, Caballero S, Estes K, Rossing M, Spoerri PE, Pepine C. Expression of IGF-I, IGF-I receptor and IGF binding proteins-1, -2, -3, -4 and -5 in human atherectomy specimens. Regul Pept 67: 137–144, 1996 [DOI] [PubMed] [Google Scholar]
- 15. Grundmann O, Fillinger JL, Victory KR, Burd R, Limesand KH. Restoration of radiation therapy-induced salivary gland dysfunction in mice by post therapy IGF-1 administration. BMC Cancer 10: 417–425, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Harmon KJ, Couper LL, Lindner S. Strain-dependent vascular remodeling phenotypes in inbred mice. Am J Pathol 156: 1741–1748, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Harrington SC, Simari RD, Conover CA. Genetic deletion of pregnancy-associated plasma protein-A is associated with resistance to atherosclerotic lesion development in apolipoprotein E-deficient mice challenged with a high-fat diet. Circ Res 100: 1696–1702, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Khorsandi M, Fagin JA, Fishbein MC, Forrester JS, Cercek B. Effects of hypophysectomy on vascular insulin-like growth factor-I gene expression after balloon denudation in rats. Atherosclerosis 93: 115–122, 1992 [DOI] [PubMed] [Google Scholar]
- 19. Kumar A, Lindner V. Remodeling with neointima formation in the mouse carotid artery after cessation of blood flow. Arterioscler Thromb Vasc Biol 17: 2238–2244, 1997 [DOI] [PubMed] [Google Scholar]
- 20. Mitchell G, Fillinger J, Sittadjody S, Avila J, Burd R, Limesand K. IGF1 activates cell cycle arrest following irradiation by reducing binding of ΔNp63 to the p21 promoter. Cell Death Dis 1: e50-–e59., 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nichols TC, du Laney T, Zheng B, Bellinger DA, Nickols GA, Engleman W, Clemmons DR. Reduction in atherosclerotic lesion size in pigs by alphaVbeta3 inhibitors is associated with inhibition of insulin-like growth factor-I-mediated signaling. Circ Res 85: 1040–1045, 1999 [DOI] [PubMed] [Google Scholar]
- 22. O'Donnell SL, Frederick TJ, Krady JK, Vannucci SJ, Wood TL. IGF-I and microglia/macrophage proliferation in the ischemic mouse brain. Glia 38: 85–97, 2002 [DOI] [PubMed] [Google Scholar]
- 23. Prele CM, Reichelt ME, Mutsaers SE, Davies M, Delbridge LM, Headrick JP, Rosenthal N, Bogoyevitch MA, Grounds MD. Insulin-like growth factor-1 overexpression in cardiomyocytes diminishes ex vivo heart functional recovery after acute ischemia. Cardiovasc Pathol 21: 17–27, 2012 [DOI] [PubMed] [Google Scholar]
- 24. Qin X, Wergedal JE, Rehage M, Tran K, Newton J, Lam P, Baylink DJ, Mohan S. Pregnancy-associated plasma protein-A increases osteoblast proliferation in vitro and bone formation in vivo. Endocrinology 147: 5653–5661, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Regan CP, Adam PJ, Madsen CS, Owens GK. Molecular mechanisms of decreased smooth muscle differentiation marker expression after vascular injury. J Clin Invest 106: 1139–1147, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rehage M, Mohan S, Wergedal JE, Bonafede B, Tran K, Hou D, Phang D, Kumar A, Qin X. Transgenic overexpression of pregnancy-associated plasma protein-A increases the somatic growth and skeletal muscle mass in mice. Endocrinology 148: 6176–6185, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Resch ZT, Simari RD, Conover CA. Targeted disruption of the pregnancy-associated plasma protein-A gene is associated with diminished smooth muscle cell response to insulin-like growth factor-I and resistance to neointimal hyperplasia after vascular injury. Endocrinology 147: 5634–5640, 2006 [DOI] [PubMed] [Google Scholar]
- 28. Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol 182: 311–322, 2000 [DOI] [PubMed] [Google Scholar]
- 29. Wamhoff BR, Hoofnagle MH, Burns A, Sinha S, McDonald OG, Owens GK. A G/C element mediates repression of the SM22α promoter within phenotypically modulated smooth muscle cells in experimental atherosclerosis. Circ Res 95: 981–988, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Wong AH, Amabile PG, Yuksel E, Waugh JM, Dake MD. Perivascular release of insulin-like growth factor-1 limits neointima formation in the balloon-injured artery by redirecting smooth muscle cell migration. J Vasc Interv Radiol 12: 347–350, 2001 [DOI] [PubMed] [Google Scholar]
- 31. Zhao G, Sutliff RL, Weber CS, Wang J, Lorenz J, Paul RJ, Fagin JA. Smooth muscle-targeted overexpression of insulin-like growth factor I results in enhanced vascular contractility. Endocrinology 142: 623–632, 2001 [DOI] [PubMed] [Google Scholar]
- 32. Zhu B, Zhao G, Witte DP, Hui DY, Fagin JA. Targeted overexpression of IGF-I in smooth muscle cells of transgenic mice enhances neointimal formation through increased proliferation and cell migration after intraarterial injury. Endocrinology 142: 3598–3606, 2001 [DOI] [PubMed] [Google Scholar]