Abstract
Pancreatic β-cells regulate glucose homeostasis by secreting insulin in response to glucose elevation and G protein-coupled receptor (GPCR) activation. Neuropeptide Y (NPY) and somatostatin (SST) attenuate insulin secretion through Gi activation of Y1 and SSTR1&5 receptors, respectively. The downstream pathways altered by NPY and SST are poorly understood. Thus, we investigated these underlying mechanisms. NPY and SST increase cellular redox potential, suggesting that their inhibitory effect may not be mediated through metabolic inhibition. NPY does not affect intracellular calcium ([Ca2+]i) activity upon glucose stimulation, whereas SST alters this response. Gβγ-subunit inhibition by gallein attenuates insulin secretion but does not alter metabolism or [Ca2+]i. mSIRK-induced Gβγ activation does not modulate glucose metabolism but increases [Ca2+]i activity and potentiates insulin release. Cotreatment with gallein and NPY or SST reduces insulin secretion to levels similar to that of gallein alone. mSIRK and NPY cotreatment potentiates insulin secretion similarly to mSIRK alone, whereas mSIRK and SST treatment decreases insulin release. The data support a model where SST attenuates secretion through Gβγ inhibition of Ca2+ activity, while NPY activates a Ca2+-independent pathway mediated by Gα. GPCR ligands signal through multiple pathways to inhibit insulin secretion, and determining these mechanisms could lead to novel diabetic therapies.
Keywords: G protein-coupled receptor, Ca2+ signaling, Gβγ subunit
glucose homeostasis is a tightly regulated process coordinated by insulin and glucagon secretion. In β-cells, increasing extracellular glucose concentrations raise cellular metabolism and increase the ATP/ADP ratio, inhibiting the ATP-sensitive inward rectifying potassium (KATP) channel. KATP channel inhibition depolarizes the cell and activates voltage-gated Ca2+ channels (VGCC), triggering insulin secretion. This process, termed glucose-stimulated insulin secretion (GSIS), is relatively well understood. In addition to this glucose-dependent pathway, it is widely accepted that several broadly expressed G protein-coupled receptor (GPCR) ligands can regulate insulin and glucagon release by mediating intracellular signaling pathways.
The neurotransmitter neuropeptide Y (NPY) is expressed in sympathetic neurons that innervate the islet (14), and the islet itself, as mRNA of NPY and its receptor, Y1, was detected in isolated mouse islets (22, 23). Steroid induction of NPY expression accompanied by a decrease in insulin release also has been observed in rat β-cells (29) and RINm5F cells (30). However, other studies have suggested that NPY expression is restricted to neonatal β-cells, as expression may be downregulated throughout development (31, 48). NPY inhibits insulin secretion from β-cells by activation of a Gi-coupled receptor, Y1 (28, 35), and may modulate effectors downstream of cAMP generation (43). A hyperinsulinemic response was detected in Y1 receptor knockout mice, suggesting that NPY may tonically inhibit insulin secretion in vivo (22); however, the precise molecular response is yet to be determined.
Somatostatin (SST) is secreted by multiple cell types, including the δ-cells of the pancreatic islet (8, 32). SST receptors (SSTRs) are highly expressed in the β-cell, with SSTR1 and -5 being the predominant receptor subtypes mediating the effect on insulin secretion (38, 44, 47). Similar to Y1, SSTR activation putatively attenuates insulin secretion by coupling through Gi/o proteins to inhibit adenylate cyclase (AC) activity (12, 19, 34). However, it has been previously shown that this mechanism may be more complex, as SST may alter other pathways and targets, such as calcineurin (39).
Although generally the possible pathways activated by NPY and SST have been determined, little is known about the detailed mechanisms modulated by each ligand to alter β-cell insulin secretion. GPCR ligands play a large role in regulating insulin secretion (49) and ∼50% of drugs on the market are targeted against GPCRs (24). Thus, a more thorough understanding of how GPCR ligands impact islet hormone secretion is essential to developing novel diabetic therapies. In this study, we investigated whether NPY and SST modulate insulin secretion through cellular redox potential and intracellular calcium [Ca2+]i activity alteration. We also characterized the metabolic and Ca2+-dependent effects of two Gβγ modulators: an inhibitor of Gβγ-mediated activities, gallein, and mSIRK, a Gβγ activator. By measuring the glucose signaling and secretion responses during treatment with these compounds alone and in combination, we determined whether NPY and SST mediate changes in insulin secretion through a Gβγ-dependent mechanism.
MATERIALS AND METHODS
Islet isolation.
All murine procedures were submitted to, and approved by, the Vanderbilt University Institutional Animal Care and Use Committee. The islet isolation protocol was adapted from previously described methods (41, 46). Pancreata from 8- to 12-wk-old C57BL/6 adult mice (Harlan) were excised and digested in 0.15–0.22% collagenase P (Roche) per milliliter of Hank's balanced salt solution (HBSS, Invitrogen) for 8–12 min under gentle agitation. Samples were centrifuged three times, and the supernatant was replaced with fresh HBSS after each spin. Individual islets were pipetted into fresh media [RPMI 1640 (Invitrogen) supplemented with 10% fetal bovine serum (FBS, Invitrogen), penicillin-streptomycin (Invitrogen), and 11 mM glucose] and incubated at 37°C and 5% CO2 for 24–48 h.
Microfluidic device construction.
Microfluidic devices were constructed using Sylgard 184 silicone elastomer base and curing agent mix (Dow Corning), which was degassed and cured on a master mold for 3 h at 75°C. A well and two access holes for loading and removing the islets were created. The molds were bonded onto 22 × 40 mm cover glass (Corning) following plasma cleaning (Harrick Scientific).
NAD(P)H imaging.
All imaging experiments were conducted in a microfluidic device at 37°C and 5% CO2. The imaging buffer consisted of (in mM) 125 NaCl, 5.7 KCl, 2.5 CaCl2·2H2O, 1.5 MgCl2, 10 HEPES, and 0.1% bovine serum albumin (BSA, Sigma Aldrich) at pH 7.4. NAD(P)H autofluorescence was imaged with an LSM710 microscope (Carl Zeiss) using a Plan-Apochromat 20×/0.8 NA objective and a Coherent Chameleon laser tuned to 710 nm, similar to what was previously described (40). The laser power at the sample was below 3.5 mW to prevent damage to the islet (36). NAD(P)H autofluorescence was measured in intact islets as a function of glucose concentration (2–23 mM) with and without NPY (100 nM; Bachem), SST (1 μM; Sigma Aldrich), NE (1 μM; CalBiochem), gallein (10 μM; CalBiochem), or mSIRK (30 μM; CalBiochem). Addition of increasing glucose concentrations with and without the GPCR ligands/Gβγ modulators occurred at 8-min intervals to allow NAD(P)H levels to plateau, after which Z-stacks were collected.
Imaging of Ca2+ oscillations.
[Ca2+]i oscillation frequency was measured pre- and posttreatment with NPY, SST, gallein, or mSIRK individually and in combination. Intact islets were labeled with Fluo 4-AM (4 μM; Invitrogen) in imaging buffer with 2 mM glucose for 30–45 min prior to data collection. Oscillations in Fluo 4-AM fluorescence over the whole islet area were detected by excitation at 488 nm on an LSM 5Live microscope (Carl Zeiss) with a Plan-Apochromat 20×/0.8 NA lens. Images were collected at one frame every 2 s to measure the fast oscillations in [Ca2+]i generated by changes in ion channel conductances (∼25 s) (6). Cells were imaged at 10 mM glucose for ∼5–10 min to allow sufficient time for synchronous oscillations to appear; the GPCR ligand and/or Gβγ modulator of interest then was added, and oscillations were continuously recorded for another ∼10 min. In the experiment performed at 2 mM glucose, the glucose concentration is below the oscillation threshold. Thus, the Fluo 4-AM fluorescence was collected for 3 min, followed by addition of the GPCR ligand and/or Gβγ modulator of interest. The fluorescence was then monitored for the subsequent 5 min. Data were normalized to the untreated control frequency, amplitude, or intensity for each islet prior to addition of a GPCR ligand and/or Gβγ modulator.
Static incubation insulin secretion assays.
Islets were isolated as described above and allowed to recover overnight. Islets were preincubated for 1 h in KRBH buffer consisting of (in mM) 128.8 NaCl, 4.8 KCl, 1.2 KH2PO4, 1.2 MgSO4·7H2O, 2.5 CaCl2, 20 HEPES, and 5 NaHCO3 (pH7.4) with 2.8 mM glucose. Four islets per sample were incubated in 1 ml of KRBH buffer at low (2.8 mM) or high (16.7 mM) glucose with and without NPY, SST, gallein, mSIRK, mSIRK(L9A; 30 μM, CalBiochem), or nifedipine (10 μM, Sigma) individually or in combination for 45 min at 37°C; each condition was measured in triplicate. The samples were briefly spun at 3,000 rpm (Beckman), and 500 μl of each sample was placed in a new tube. Triton X (500 μl of 2%) was added to each islet sample to lyse the islets prior to storage at −20°C. Insulin content (from the Triton X-containing samples) and secretion were analyzed in duplicate with a Mouse Ultrasensitive Insulin ELISA kit (Alpco) and detected on a Spectra Max M5 spectrometer (Molecular Devices).
Analysis and statistics.
Data were analyzed with Microsoft Excel, ImageJ, MatLab, or GraphPad Prism analysis software. For all imaging data, the background signal was subtracted and the mean ± SE was determined. Student's t-tests and ANOVAs were used where applicable, and P < 0.05 was considered statistically significant unless otherwise noted.
RESULTS
An increase in cellular metabolism is associated with NPY and SST inhibition of insulin release.
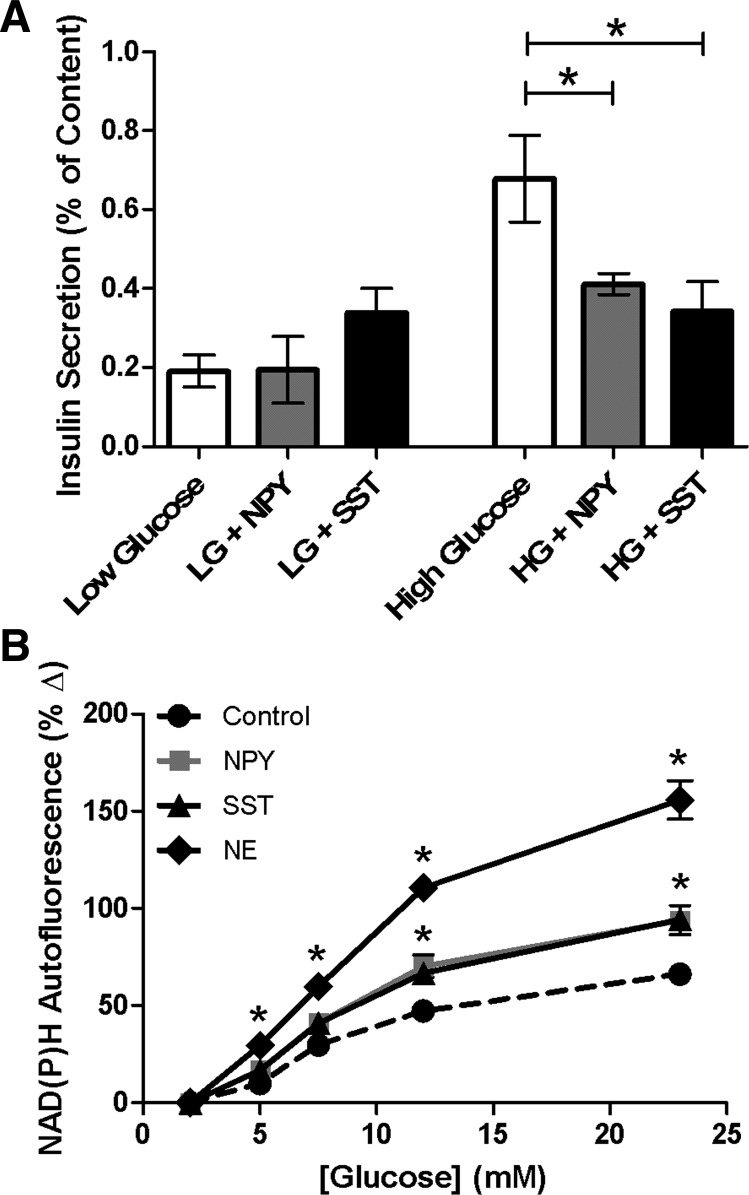
NPY and SST have previously been shown to attenuate insulin release (12, 19, 28, 35). To verify these results and confirm that this inhibition is observed under our conditions, isolated murine islets were incubated at low (2.8 mM) and high (16.7 mM) glucose levels. Treatment with NPY (100 nM) or SST (1 μM) did not alter insulin secretion at low glucose levels compared with untreated control (0.19 ± 0.08 and 0.38 ± 0.08% vs. 0.19 ± 0.04%, respectively, P > 0.05; Fig. 1A). Similar to previously published results (28, 35), insulin secretion decreased upon treatment with NPY at high glucose levels (0.41 ± 0.03%) compared with untreated control (0.68 ± 0.11%, P < 0.05; Fig. 1A). At high glucose concentrations, SST reduced insulin release (0.34 ± 0.08%) compared with untreated control (0.68 ± 0.11%, P < 0.03; Fig. 1A), consistent with prior studies (12, 19, 34, 39).
Fig. 1.
Neuropeptide Y (NPY) and somatostatin (SST) attenuate insulin secretion and increase the cellular redox potential from intact islets. A: percent insulin content secreted from intact islets after static incubation at low (LG, 2.8 mM) or high (HG, 16.7 mM) glucose with and without NPY (100 nM, gray) or SST (1 μM, black). Untreated controls are shown in white. Data are mean ± SE; n = 5–11. *P < 0.05, significance compared with untreated control at high glucose levels. B: glucose dose-response curves of percent change in NAD(P)H from untreated intact islets (black circles) and islets treated with NPY (100 nM, gray squares), SST (1 μM, black triangles), or NE (1 μ M, black diamonds) vs. values at 2 mM glucose. Data are means ± SE; n = 3–11. NPY, SST, and NE treatment at glucose concentrations between 5 and 23 mM is significant, *P < 0.005.
Glucose metabolism and insulin secretion are tightly coupled processes. As glucose is metabolized, β-cells produce the reduced pyridine nucleotides NADH and NADPH [collectively denoted NAD(P)H] through glycolysis and the TCA cycle; autofluorescence of these byproducts serves as an index of the cellular redox state and, by extension, metabolism. Cellular redox potential changes, via alterations in NAD(P)H autofluorescence, can be quantitatively measured by two-photon excitation microscopy (2, 36).
Here, we investigated whether the NPY and SST-induced attenuation of insulin secretion results from changes in the cellular redox state. NAD(P)H autofluorescence from intact islets was measured as a function of glucose with and without NPY or SST. In untreated control islets, increasing glucose concentrations produced a rise in the NAD(P)H autofluorescence and cellular redox potential (Fig. 1B), as expected (2). Islets treated with NPY or SST showed an additional increase in NAD(P)H levels compared with untreated control over a range of glucose concentrations (P < 0.005; Fig. 1B). To determine whether this effect is specific to NPY and SST, NAD(P)H autofluorescence was measured upon treatment with another inhibitor of insulin secretion, norepinephrine (NE, 1 μM) (37). NE additionally increased NAD(P)H levels as a function of glucose compared with untreated control, to an even greater extent than NPY and SST. These three ligands attenuated insulin release; thus, the results suggest their inhibitory effect was due to alteration of pathways downstream or independent of glucose metabolism.
NPY and SST differentially affect Ca2+ activity.
Ca2+ activity is tightly coupled to insulin secretion (20, 50). At glucose concentrations above 7 mM, murine islets display synchronous, fast oscillations in β-cell [Ca2+]i, corresponding to the pulsatile release of insulin (5, 6). Here, we measured the β-cell Ca2+ response in intact islets upon treatment with NPY or SST by detecting changes in the fluorescence intensity of the Ca2+ indicator dye Fluo 4-AM.
Despite studies showing NPY inhibits L-type Ca2+ channels in neuronal (42) and cardiac (10) cells, the putative effects of NPY on β-cell Ca2+ activity have not been determined. Thus, we treated intact islets with NPY and measured changes in [Ca2+]i oscillations at 2 and 10 mM glucose. Upon addition of NPY at 10 mM glucose, neither the [Ca2+]i oscillation frequency nor the amplitude of the oscillating or AC component was significantly altered (P > 0.2; Table 1 and Fig. 2, A and B). At 2 mM glucose, the normalized fluorescence intensity was also unchanged after addition of NPY (Fig. 6A). Although contrary to previous studies in other cell types, these data suggest that NPY does not attenuate insulin secretion by inhibiting islet Ca2+ activity.
Table 1.
Measured [Ca2+]i oscillation parameters, as detected by Fluo 4-AM imaging
| GPCR Ligand/Gβγ Modulator | n | GPCR Ligand/Gβγ Modulator | Frequency, mHz | Amplitude, %Δ |
|---|---|---|---|---|
| Neuropeptide Y | 6 | − | 36.33 ± 1.7 | 9.45 ± 2.0 |
| 6 | + | 40.44 ± 2.2 | 10.04 ± 2.3 | |
| Somatostatin | 7 | − | 34.47 ± 1.9 | 7.05 ± 1.6 |
| 7 | + | 27.16 ± 2.9* | 8.74 ± 1.0 | |
| Gallein | 5 | − | 44.78 ± 7.8 | 4.23 ± 3.9 |
| 5 | + | 41.17 ± 7.9 | 1.32 ± 1.2 | |
| mSIRK | 5 | − | 37.13 ± 7.3 | 16.82 ± 6.1 |
| 5 | + | 57.83 ± 8.2* | 7.10 ± 2.2 | |
| NPY + gallein | 3 | − | 20.54 ± 3.2 | 21.05 ± 3.4 |
| 3 | + | 25.49 ± 9.4 | 17.88 ± 6.1 | |
| NPY + mSIRK | 4 | − | 20.65 ± 2.8 | 13.79 ± 1.7 |
| 4 | + | 32.60 ± 4.8* | 5.01 ± 0.6* | |
| SST + gallein | 3 | − | 18.98 ± 1.9 | 17.49 ± 4.3 |
| 3 | + | 16.58 ± 5.0 | 15.61 ± 2.9 | |
| SST + mSIRK | 4 | − | 30.68 ± 3.1 | 9.76 ± 5.7 |
| 4 | + | 26.57 ± 5.2 | 11.13 ± 7.0 |
Data are means ± SE for frequency and amplitude of [Ca2+]i oscillations measured pre- and posttreatment with the specified G protein-coupled receptor (GPCR) ligand and/or Gβγ modulator. Significance was tested using a two-tailed Student's t-test to compare the frequency or amplitude between untreated and treated islets. Significance P< 0.04.
Fig. 2.
NPY does not affect, whereas SST inhibits, Ca2+ activity. Changes in Fluo 4-AM signal recorded with and without NPY (100 nM) or SST (1 μM) at 10 mM glucose to detect frequency and amplitude of [Ca2+]i oscillations. A and C: representative oscillations in [Ca2+]i recorded from intact islets before (dotted line) and after (solid line) treatment with NPY (A) or SST (C). B and D: normalized frequency of [Ca2+]i oscillations pre- and posttreatment with NPY (B) or SST (D). Data prior to addition of NPY or SST are shown in white; data after addition of the ligand are represented in gray (NPY) or black (SST). Data collected following treatment with the ligand are normalized to the data collected from the islet prior to addition of the ligand. Data are means ± SE; n = 6–7. *P < 0.04.
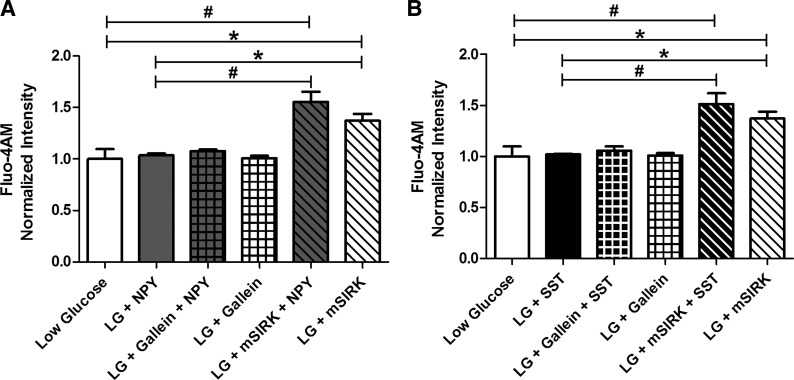
Fig. 6.
NPY, SST, and gallein do not alter [Ca2+]i at low glucose, but mSIRK stimulates [Ca2+]i release. Changes in Fluo 4-AM signal recorded at 2 mM glucose with and without NPY (100 nM), SST (1 μM), gallein (10 μM), mSIRK (30 μM), or a combination. A: Fluo 4-AM fluorescence intensity normalized to untreated control (white) before and after treatment with NPY (gray), gallein (white checkered), NPY and gallein (gray checkered), mSIRK (white striped), or NPY and mSIRK (gray striped). B: normalized Fluo 4-AM fluorescence intensity pre- (white) and posttreatment with SST (black), gallein (black checkered), SST and gallein (black checkered), mSIRK (white striped), or SST and mSIRK (black striped). Data are means ± SE; n = 5. *P < 0.05; #P < 0.005.
To determine whether SST mediates its inhibition of insulin release by modulating Ca2+ activity, we measured changes in [Ca2+]i oscillations from intact islets with and without SST. SST decreased the [Ca2+]i oscillation frequency (P < 0.04; Table 1 and Fig. 2, C and D) but, similarly to NPY, did not affect the oscillation amplitude (Fig. 2C), suggesting that SST's inhibitory effect on insulin release is mediated, at least in part, by alteration of [Ca2+]i oscillation frequency. Similarly, addition of SST did not significantly modulate the Ca2+ response at 2 mM glucose (Fig. 6B).
Gallein and mSIRK differentially alter insulin secretion through a metabolism-independent mechanism.
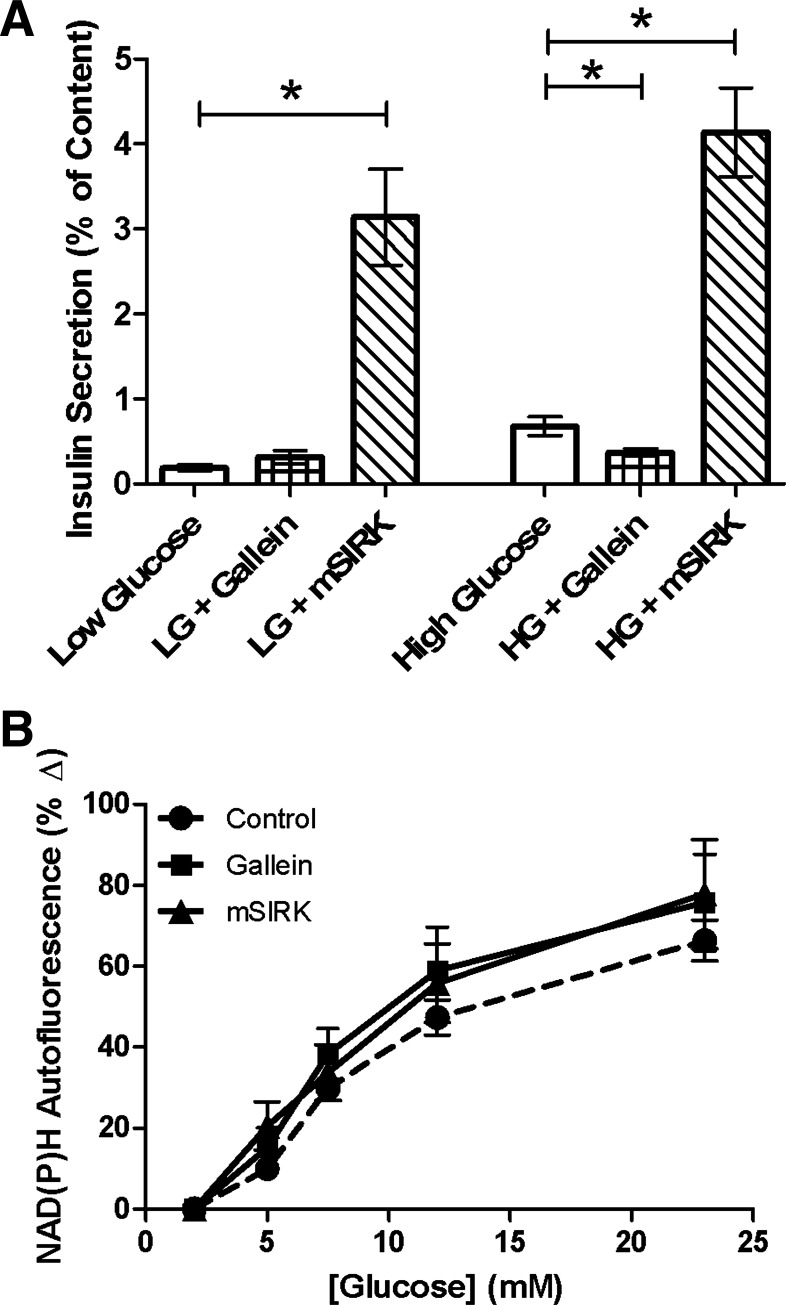
GPCRs are coupled to intracellular G proteins, which are activated upon ligand receptor binding and signal through the Gα or Gβγ subunit (15, 16). To further determine the pathways altered by NPY and SST, two Gβγ modulators, gallein and mSIRK, were studied. Gallein is a cell-permeable compound that blocks Gβγ-dependent activities, while mSIRK is an N-myristoylated Gβγ-binding peptide that binds to and dissociates Gβγ without activating Gα (17). Insulin secretion at high glucose concentrations was attenuated upon incubation with gallein (10 μM, P < 0.02; Fig. 3A), similar to that observed for NPY and SST; no effect of gallein was observed at low glucose. Conversely, mSIRK (30 μM) dramatically increased insulin release at low and high glucose levels compared with untreated controls (P < 0.005; Fig. 3A). Incubation with mSIRK(L9A) (30 μM), the inactive analog of mSIRK, yielded secretion amounts at low and high glucose that were comparable to those of untreated control (P > 0.2; data not shown).
Fig. 3.
Gallein and mSIRK modulate insulin release but do not alter cellular redox potential. A: percent insulin content released from intact islets after static incubation at low (LG, 2.8 mM) or high (HG, 16.7 mM) glucose in the presence and absence of gallein (10 μM, checkered) or mSIRK (30 μM, striped). Untreated control samples are shown in white. Data are means ± SE; n = 5–11. *P < 0.02, significance vs. untreated control. B: percent change in NAD(P)H, as a function of glucose, from untreated intact islets (black circles) and islets treated with gallein (10 μM, black squares) or mSIRK (30 μM, black triangles) vs. values at 2 mM glucose. Data are means ± SE; n = 4–11.
Cellular redox potential changes upon treatment with gallein or mSIRK were measured to determine whether they mediate metabolism to exert their opposing effects on insulin release. NAD(P)H autofluorescence was measured at increasing glucose concentrations with and without gallein or mSIRK. Neither gallein nor mSIRK elicited a significant effect on NAD(P)H levels compared with untreated control (Fig. 3B). Thus, the data suggest that gallein and mSIRK modulate insulin release through a pathway independent of glucose metabolism.
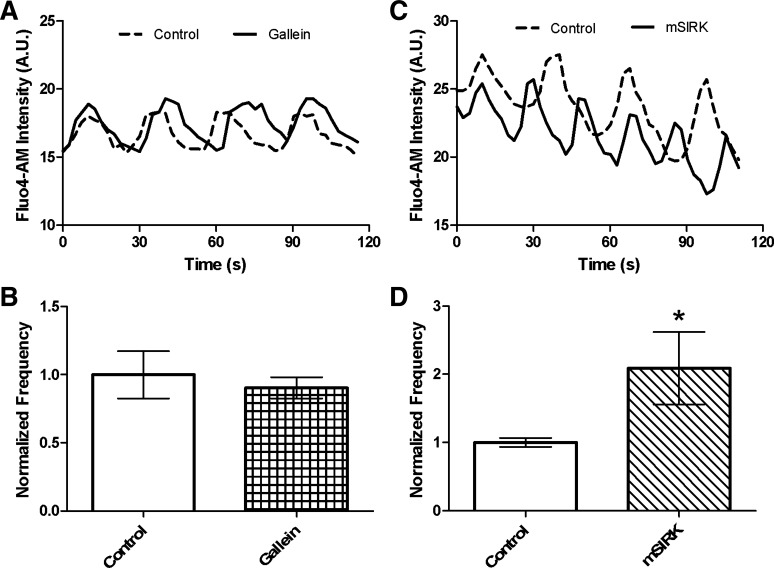
The islet Ca2+response is enhanced by mSIRK but not by gallein.
[Ca2+]i oscillation frequency at 10 mM glucose and the fluorescence intensity at 2 mM glucose were measured in the presence and absence of gallein or mSIRK. Gallein did not affect the [Ca2+]i oscillation frequency or the normalized fluorescence intensity (Figs. 4, A and B, and 6). Alternatively, mSIRK, which was previously shown to stimulate Ca2+ release from intracellular stores (17), increased the frequency of [Ca2+]i oscillations (P < 0.05; Fig. 4, C and D). The amplitude of the oscillations was not significantly affected upon treatment with either drug (Fig. 4, A and C), although there was a trend toward a reduction with mSIRK. At 2 mM glucose, the Fluo 4-AM fluorescence intensity was increased upon addition of mSIRK, consistent with previously published results (17).
Fig. 4.
mSIRK potentiates [Ca2+]i oscillation frequency, but gallein does not alter Ca2+ activity in intact islets. Fluo 4-AM signal recorded at 10 mM glucose in the presence and absence of gallein (10 μM) or mSIRK (30 μM) to detect changes in frequency and amplitude of [Ca2+]i oscillations. A and C: representative [Ca2+]i oscillations from intact islets recorded pre- (dotted line) and posttreatment (solid line) with gallein (A) or mSIRK (C). B and D: normalized [Ca2+]i oscillation frequency measured pre- and posttreatment with gallein (B) or mSIRK (D). Data in the presence of gallein (white checkered) or mSIRK (white striped) are normalized to data collected from the islet prior to Gβγ modulator treatment (white). Data are means ± SE; n = 5. *P < 0.05.
NPY and SST differentially affect Ca2+ activity in the presence of gallein and mSIRK.
To determine whether SST inhibits insulin secretion by altering the islet Ca2+ response via the Gβγ subunit and to verify that NPY does not signal through this pathway, the [Ca2+]i oscillation frequency at 10 mM glucose and the fluorescence intensity at 2 mM glucose were measured with and without combination treatments of SST, NPY, gallein, and mSIRK. Gallein and NPY cotreatment did not affect the [Ca2+]i oscillation frequency (Fig. 5, A and B) or the fluorescence intensity (Fig. 6A). However, treatment with mSIRK and NPY increased the frequency of [Ca2+]i oscillations and the fluorescence intensity at low glucose, similarly to that observed for mSIRK alone (Figs. 5, C and D, and 6A). The amplitude of the oscillating component was decreased upon mSIRK and NPY treatment, not unlike the effect observed for mSIRK alone. Neither cotreatment with gallein and SST nor with mSIRK and SST modulated the [Ca2+]i oscillation frequency (or amplitude; data not shown). Combination treatment with gallein and SST did not alter the fluorescence intensity at low glucose, but cotreatment with mSIRK and SST resulted in elevated fluorescence intensities compared with untreated control. These data suggest that SST reverses the Gβγ-dependent stimulatory effect of mSIRK on Ca2+ activity at high glucose (Fig. 5, E–H).
Fig. 5.
SST abolishes the mSIRK-induced increase in [Ca2+]i oscillation frequency, whereas NPY does not; neither effect [Ca2+]i with gallein. Changes in Fluo 4-AM signal recorded at 10 mM glucose with and without gallein (10 μM) or mSIRK (30 μM) and NPY (100 nM) or SST (1 μM). A and C: representative [Ca2+]i oscillations recorded from intact islets before (dotted line) and after (solid line) treatment with NPY and gallein (A) or mSIRK (C). B and D: normalized frequency of [Ca2+]i oscillations measured before (white) and after treatment with NPY and gallein (B, gray checkered) or mSIRK (D, gray striped). E and G: representative [Ca2+]i oscillations from intact islets recorded pre- (dotted line) and posttreatment (solid line) with SST and gallein (E) or mSIRK (G). F and H: normalized [Ca2+]i oscillation frequency pre- (white) and posttreatment with SST and gallein (F, black checkered) or mSIRK (H, black striped). Data are means ± SE; n = 3–4. *P < 0.05.
NPY inhibits insulin secretion through a Gβγ-independent pathway.
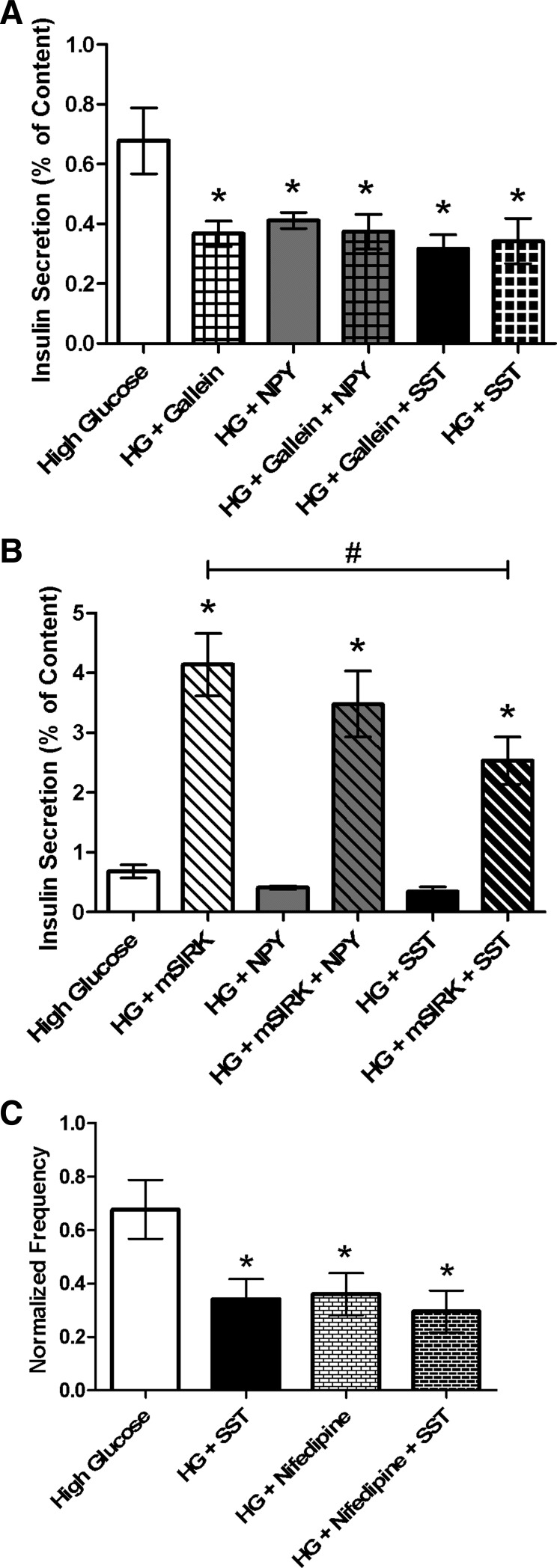
As discussed earlier, NPY attenuates insulin secretion through a Gi-mediated pathway that may modulate effectors downstream of cAMP (28, 35, 43). To determine whether NPY attenuates insulin secretion through a Gβγ-dependent mechanism, isolated islets were incubated at low and high glucose levels with and without NPY and gallein or mSIRK. NPY and gallein combination treatment reduced insulin secretion at stimulatory glucose levels compared with untreated control (0.37 ± 0.06% vs. 0.68 ± 0.11%, respectively); however, NPY and gallein cotreatment did not alter secretion compared with incubation with gallein alone (Fig. 7A). To further examine whether NPY signals through Gβγ, islets were incubated with NPY and mSIRK. No effect of NPY was detected upon activation of Gβγ by mSIRK at either glucose concentration (P > 0.4; Fig. 7B). Together, the data suggest that NPY mediates its inhibitory effect on insulin release through a Gβγ-independent mechanism.
Fig. 7.
NPY likely acts through a Gβγ-independent pathway, but SST modulates a mechanism dependent on Gβγ to attenuate insulin release. Percent insulin content secreted from intact islets after static incubation at high glucose (HG, 16.7 mM) with and without treatment. Untreated control samples are shown in white throughout. A: percent insulin content secreted in the presence and absence of Gβγ inhibitor gallein (10 μM, white checkered), NPY (gray), or SST (black) alone, or combination treatment with gallein and NPY (100 nM, gray checkered) or SST (1 μM, black checkered) at high glucose. B: percent insulin content secreted at high glucose concentrations with and without Gβγ-activating peptide mSIRK (30 μM, white striped), NPY (gray), or SST (black) alone, or combination treatment with mSIRK and NPY (100 nM, gray striped) or SST (1 μM, black striped). C: percent insulin content secreted at high glucose with and without SST (1 μM, black), L-type Ca2+ channel blocker nifedipine (1 μM, white bricked), or combination treatment with nifedipine and SST (black bricked). Data are means ± SE; n = 4–10. *P < 0.04, #P < 0.005: significance vs. untreated control.
Insulin secretion inhibition by SST is mediated through Gβγ.
SST inhibits insulin secretion by SSTR stimulation of G proteins. To determine whether this inhibition is mediated through Gβγ, we measured insulin secretion from intact islets following incubation with gallein and SST. Insulin release at stimulatory glucose levels was decreased upon gallein and SST cotreatment compared with untreated control (P < 0.03; Fig. 7A). However, gallein and SST treatment showed no significant difference at high glucose concentrations compared with SST only (P > 0.6). Additional inhibition of insulin secretion upon combination treatment was not detected; this, in conjunction with our [Ca2+]i data at 10 mM glucose, suggests that SST's effect is mediated primarily through Gβγ. To test this further, insulin release at high glucose was measured following cotreatment with mSIRK and SST, and a decrease in secretion was detected compared with mSIRK only (P < 0.03; Fig. 7B). SSTRs have previously been shown to associate with VGCCs (33). Thus, to determine whether SST inhibits insulin secretion by altering VGCC activity, insulin release was measured following treatment with nifedipine, an L-type Ca2+ channel blocker, alone or in combination with SST. Secretion decreased under both conditions compared with untreated control; however, no additional decrease was noted upon treatment with nifedipine and SST compared with nifedipine alone (Fig. 7C).
DISCUSSION
Although GPCR ligands are known to modulate insulin secretion, little is known about the mechanistic details by which this occurs. Thus, we examined the effect of NPY and SST on cellular metabolism and Ca2+ activity in the β-cells of intact islets and determined whether the Gβγ complex is the primary G protein subunit that mediates the ligand response.
Insulin secretion is inhibited through a metabolism-independent mechanism by NPY and SST.
NPY and SST inhibit insulin secretion through a pertussis toxin-sensitive process, suggesting that they transduce their actions via Gi/o proteins (28, 34, 35). In this paper, we confirm that NPY and SST attenuate insulin release from isolated murine islets (Fig. 1A). Similar to previously published data (47), we also find that insulin secretion is not altered by SST treatment at substimulatory glucose concentrations, suggesting that SST-dependent inhibition requires glucose metabolism; this was also confirmed when insulin secretion was induced by mSIRK treatment at low glucose (data not shown). Comparable results were observed with NPY. NPY is an orexigenic peptide that regulates energy homeostasis and stimulates weight gain (45). In the central nervous system, SST inhibits the release of growth hormone, which modulates protein, lipid, and carbohydrate metabolism (9, 27). However, little is known about NPY's and SST's effects on β-cell metabolism. We determined that both potentiate β-cell redox potential, and thus the metabolism of glucose (Fig. 1B). A previous study found that SST attenuates glucose metabolism by inhibiting the O2 consumption rate in MIN6 cells and isolated mouse islets (13). However, O2 consumption measurements from islets are difficult to interpret. Here, changes in NAD(P)H were measured to determine the cellular redox potential, which correlates to metabolism. Thus, the data are not necessarily contradictory, as SST may inhibit O2 consumption and still increase glucose metabolism.
The central dogma of insulin secretion states that an increase in glucose metabolism will shift the ATP/ADP ratio to favor the production of ATP, inhibiting the KATP channel; this depolarizes the cell and allows for VGCC activation and insulin secretion. Since the NAD(P)H data in the presence of NPY and SST are consistent with a potentiation (not attenuation) of secretion, NPY and SST may alter pathways downstream of glucose metabolism. Similar results were observed for another inhibitor of insulin secretion, NE (Fig. 1B). Secretion is an ATP-dependent process, as granule priming requires ATP hydrolysis (51, 52), so NE, NPY, and SST inhibition of secretion likely increases the amount of available ATP, elevating NAD(P)H levels and leading to the associated rise in metabolic activity detected here. This may not be a general characteristic of all insulin inhibitors, but it is a reasonable explanation for these three, although this postulate remains to be tested. Alternatively, alteration of pathways between NAD(P)H generation and conversion by NE, NPY, and SST cannot be excluded as a possible mechanism. In mouse islets, NPY was shown to promote β-cell replication (11). Consequently, the increase in metabolism we observe with NE, NPY, and SST may be, at least in part, a secondary effect of the increased ATP, resulting in stimulation of β-cell replication.
NPY utilizes a Gβγ-independent pathway to attenuate insulin release.
In β-cells, insulin secretion requires increases in [Ca2+]i (20, 50), where synchronous, coupled oscillations in [Ca2+]i elicit pulsatile insulin release (1, 4, 5). Thus, we measured [Ca2+]i with and without NPY or SST to determine whether these ligands mediate insulin secretion through [Ca2+]i alteration. Prior studies showed that NPY inhibits L-type Ca2+ channels in neuronal (42) and cardiac (10) cells. However, we found no change in the Ca2+ response with NPY treatment in β-cells (Figs. 2, A and B, and 6A), suggesting that cell type differences are important factors in NPY signaling. The data support a model where NPY attenuates insulin release through a Ca2+-independent pathway. This is further corroborated by the lack of effect noted on [Ca2+]i oscillations and Fluo 4-AM fluorescence intensity (at low glucose) from islets treated with mSIRK and NPY compared with mSIRK alone (Figs. 5, C and D, and 6A).
To determine whether NPY signaling occurs through the Gβγ complex, we utilized two Gβγ modulators, gallein and mSIRK. Gallein inhibits Gβγ activity and, as shown in Fig. 3A, attenuates insulin release at stimulatory glucose levels. Although the specificity of gallein to different Gβγ subunits remains unknown, it has been shown that gallein binds to and inhibits effector binding to Gβγ. Gallein is also a competitor of many Gβγ protein-protein interactions (25). mSIRK, a Gβγ-activating peptide, stimulates insulin release (Fig. 3A). These results are contrary to a prior study that found that mSIRK decreases exocytosis in INS832/13 cells (53). However, our data are consistent with mSIRK stimulation of Ca2+ release from intracellular stores (17), which would cause an increase in secretion. Cell type differences between primary cultured islets, as we used, and tissue culture models likely contribute to the variability between the results observed here and previously (53). Furthermore, treatment with the inactive analog of mSIRK, mSIRK(L9A), did not affect secretion at low or high glucose, in support of our assumption that peptide activation of Gβγ is responsible for the increase in insulin release observed with mSIRK incubation. Neither gallein nor mSIRK significantly modulate the NAD(P)H response, so these compounds are unlikely to affect secretion through alteration of metabolism (Fig. 3B). Next, we determined whether gallein and mSIRK modulate the Ca2+ response. Gallein does not alter [Ca2+]i oscillations or fluorescence intensity at low glucose; however, mSIRK significantly increases [Ca2+]i oscillation frequency (Fig. 4) and fluorescence intensity (Fig. 6), consistent with a previous observation that found mSIRK releases Ca2+ from intracellular stores (17). This release of intracellular Ca2+ likely drives the glucose-independent potentiation of insulin secretion observed in Figs. 3A and 7B. The data suggest that, while mSIRK potentiates insulin secretion by upregulating [Ca2+]i, gallein likely inhibits insulin release by altering pathways further downstream, possibly at the level of exocytosis.
We measured insulin release upon incubation at low and high glucose concentrations with NPY and gallein. Cotreatment reduces insulin release at stimulatory glucose levels compared with untreated control, similar to gallein treatment alone (Fig. 7A). If NPY signals through a Gβγ-dependent pathway, we would predict an attenuation of secretion upon cotreatment with NPY and mSIRK. However, no change was observed with NPY and mSIRK combination treatment compared with mSIRK alone (Fig. 7B), similar to the observed results for Ca2+ activity (Fig. 5, D–F). Since mSIRK stimulates the Gβγ subunit without activating Gα, the data support a model where NPY signaling is mediated through the Gα subunit (Fig. 8). Previously, it was shown that, upon receptor activation, both the Gα and Gβγ subunits function to mediate the downstream signaling response via activation of various effectors (15, 16). Furthermore, NPY was shown to modulate effectors downstream of cAMP generation signaling through a Gi-coupled receptor (28, 35, 43). Together, the data suggest that NPY likely inhibits insulin secretion through a Gβγ- and metabolism-independent pathway downstream of cAMP that does not alter Ca2+ (Fig. 8).
Fig. 8.
NPY signals through the Gα subunit, while SST activates the Gβγ complex. Model illustrating the known (A, solid lines) and proposed (B, dashed lines) mechanisms of action for NPY and SST modulation of insulin release. Y1, NPY receptor; SSTR5, SST receptor; VGCC, voltage-gated calcium channel, PLC, phospholipase C; AC, adenylate cyclase; ER, endoplasmic reticulum. Our data suggest that NPY signals through a Gα- and metabolism-dependent pathway that does not modulate the Ca2+ response; SST inhibits insulin release through a Gβγ- and Ca2+-dependent pathway that increases metabolism.
Insulin release is inhibited by SST through a Gβγ-dependent pathway that alters the Ca2+ response.
In this study, we measured β-cell Ca2+ activity prior to and following application of SST. The islet Ca2+ response to increased glucose, which is closely coupled to insulin secretion, was significantly reduced when treated with SST (Fig. 2, C and D). Using gallein and mSIRK, we determined that SST likely exerts this effect on Ca2+ oscillation frequency at elevated glucose levels through a Gβγ-dependent mechanism (Figs. 5, E–H, and 7) acting on downstream effectors (26). Although a prior study in dispersed β-cells found no effect of SST on Ca2+ currents (39), our data are consistent with other published results in HIT and MIN6 cells, which showed that SST decreases the Ca2+ influx through VGCCs (21, 44). These inconsistencies may be accounted for by the differences in tissue culture models and the use of dispersed β-cells, which removes them from the islet microenvironment that is required for normal β-cell function (3). In our work, β-cells in intact islets were utilized to minimize these possible complications. Application of mSIRK and SST returned Ca2+ oscillation frequency to pretreatment levels (Fig. 5, G and H); however, the Fluo 4-AM fluorescence intensity at low glucose and secretion levels upon combination treatment with these drugs remained elevated compared with untreated control. This could be due to mSIRK stimulation of Ca2+-independent pathways that are not altered by SST or by partial SST attenuation of Ca2+ release from intracellular stores. Figure 6B also suggests that, consistent with the secretion data, the inhibition of insulin secretion by SST may be glucose dependent.
SSTR isoforms expressed in the β-cell associated with VGCCs and phospholipase C (PLC) (33). Thus, SST may locally inhibit VGCCs and/or PLC to decrease Ca2+ influx and/or release of Ca2+ from intracellular stores, respectively. As previously mentioned, our mSIRK secretion data suggest that SST may partially attenuate Ca2+ release from intracellular stores (Fig. 5, G and H, and 7B). Moreover, the reduced frequency of [Ca2+]i oscillations is consistent with VGCC modulation (4, 7), which would attenuate β-cell secretion. Treatment with the L-type Ca2+ blocker nifedipine in combination with SST reduces insulin secretion compared with untreated control but not compared with either compound alone (Fig. 7C). If SST's effects are mediated through a mechanism independent of these channels, secretion would be further reduced. Thus, SST may inhibit insulin release at stimulatory glucose through alteration of VGCC activity, specifically L-type Ca2+ channels. However, SST and nifedipine alone may maximally inhibit secretion and thus render no additional effect upon combination treatment; this possibility cannot be eliminated. Alternatively, secretory granules located near L-type Ca2+ channels may be deprimed and calcineurin activated upon SSTR activation, as has been proposed in α-cells (18). SSTRs are also coupled to inward-rectifying K+ channels, including KATP channels (33). It is possible, therefore, that SSTR activation at high glucose stimulates the Gβγ complex and locally activates KATP channels, hyperpolarizing the membrane and decreasing Ca2+ influx through VGCCs. SST action on VGCCs, KATP channels, or a combination of these two effects would explain our data. This effect, however, appears to be limited to stimulatory glucose concentrations, when insulin secretion is elevated.
Summary.
Our data show that, although NPY and SST both inhibit insulin release, they do so through different mechanisms. NPY binding to its receptor likely acts through the Gα subunit to inhibit a Ca2+-independent pathway, further downstream of glucose metabolism. Attenuation of the Ca2+ response at high glucose upon treatment with SST is mediated through Gβγ complex signaling; our data indicate that this occurs downstream of glucose metabolism and may be through direct alteration of electrophysiological activity and/or intracellular signaling. Decreased NPY and/or SST secretion may be a compensatory method by which the body attempts to overcome the insulin-resistant state of type 2 diabetes. Thus, insight into the mechanism(s) by which these two ligands exerts their effects will allow for a more comprehensive understanding of islet function and the pathology of type 2 diabetes. Considering the possible influence of GPCRs on insulin secretion and the abundance of available therapies targeting this receptor class, possible relief of these insulin-inhibiting pathways could lead to novel therapeutic targets for type 2 diabetes.
GRANTS
This work was supported by NIH Grants F32 DK-091181 (to T. A. Schwetz) and T32 DK-007563, R01 DK-053434, and R01 DK-085064 (to D. W. Piston). Some experiments were performed in part through the use of the VUMC Cell Imaging Shared Resource, which is supported by NIH grants CA-68485, DK-20593, DK-58404, HD-15052, DK-59637 and EY-08126.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: T.A.S. and D.W.P. conception and design of research; T.A.S. and A.U. performed experiments; T.A.S. and A.U. analyzed data; T.A.S. and D.W.P. interpreted results of experiments; T.A.S. and A.U. prepared figures; T.A.S. drafted manuscript; T.A.S., A.U., and D.W.P. edited and revised manuscript; T.A.S., A.U., and D.W.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank M. J. Merrins (University of Michigan) for kindly providing us with the MatLab program to analyze [Ca2+]i oscillation amplitude and A.D. Elliott (Vanderbilt University) for discussion.
REFERENCES
- 1.Aslanidi OV, Mornev OA, Skyggebjerg O, Arkhammar P, Thastrup O, Sorensen MP, Christiansen PL, Conradsen K, Scott AC. Excitation wave propagation as a possible mechanism for signal transmission in pancreatic islets of Langerhans. Biophys J 80: 1195–1209, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bennett BD, Jetton TL, Ying GT, Magnuson MA, Piston DW. Quantitative subcellular imaging of glucose metabolism within intact pancreatic islets. J Biol Chem 271: 3647–3651, 1996 [DOI] [PubMed] [Google Scholar]
- 3.Benninger RKP, Head WS, Zhang M, Satin LS, Piston DW. Gap junctions and other mechanisms of cell-cell communication regulate basal insulin secretion in the pancreatic islet. J Physiol 589: 5453–5466, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benninger RKP, Zhang M, Head WS, Satin LS, Piston DW. Gap junction coupling and calcium waves in the pancreatic islet. Biophys J 95: 5048–5061, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergsten P. Glucose-induced pulsatile insulin release from single islets at stable and oscillatory cytoplasmic Ca2+. Am J Physiol Endocrinol Metab 274: E796–E800, 1998 [DOI] [PubMed] [Google Scholar]
- 6.Bergsten P. Slow and Fast oscillations of cytoplasmic Ca2+ in pancreatic-islets correspond to pulsatile insulin release. Am J Physiol Endocrinol Metab 268: E282–E287, 1995 [DOI] [PubMed] [Google Scholar]
- 7.Bertram R, Sherman A, Satin LS. Metabolic and electrical oscillations: partners in controlling pulsatile insulin secretion. Am J Physiol Endocrinol Metab 293: E890–E900, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Brazeau P, Guillemi R. Somatostatin—newcomer from hypothalamus. N Engl J Med 290: 963–964, 1974 [DOI] [PubMed] [Google Scholar]
- 9.Brazeau P, Vale W, Burgus R, Ling N, Butcher M, Rivier J, Guillemi R. Hypothalamic polypeptide that inhibits secretion of immunoreactive pituitary growth-hormone. Science 179: 77–79, 1973 [DOI] [PubMed] [Google Scholar]
- 10.Bryant SM, Hart G. Effects of neuropeptide Y on L-type calcium current in guinea-pig ventricular myocytes. Br J Pharmacol 118: 1455–1460, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cho YR, Kim CW. Neuropeptide Y promotes beta-cell replication via extracellular signal-regulated kinase activation. Biochem Biophys Res Commun 314: 773–780, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Claro A, Grill V, Efendic S, Luft R. Studies on mechanisms of somatostatin action on insulin release. 4. Effect of somatostatin on cyclic-AMP levels and phosphodiesterase activity in isolated rat pancreatic-islets. Acta Endocrinol 85: 379–388, 1977 [PubMed] [Google Scholar]
- 13.Daunt M, Dale O, Smith PA. Somatostatin inhibits oxidative respiration in pancreatic beta-cells. Endocrinology 147: 1527–1535, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Ding WG, Fujimura M, Mori A, Tooyama I, Kimura H. Light and electron-microscopy of neuropeptide-Y containing nerves in human liver, gallbladder, and pancreas. Gastroenterology 101: 1054–1059, 1991 [DOI] [PubMed] [Google Scholar]
- 15.Ford CE, Skiba NP, Bae HS, Daaka YH, Reuveny E, Shekter LR, Rosal R, Weng GZ, Yang CS, Iyengar R, Miller RJ, Jan LY, Lefkowitz RJ, Hamm HE. Molecular basis for interactions of G protein beta gamma subunits with effectors. Science 280: 1271–1274, 1998 [DOI] [PubMed] [Google Scholar]
- 16.Gilman AG. G-Proteins—transducers of receptor-generated signals. Annu Rev Biochem 56: 615–649, 1987 [DOI] [PubMed] [Google Scholar]
- 17.Goubaeva F, Ghosh M, Malik S, Yang J, Hinkle PM, Griendling KK, Neubig RR, Smrcka AV. Stimulation of cellular signaling and G protein subunit dissociation by G protein beta gamma subunit-binding peptides. J Biol Chem 278: 19634–19641, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Gromada J, Hoy M, Buschard K, Salehi A, Rorsman P. Somatostatin inhibits exocytosis in rat pancreatic alpha-cells by G(i2)-dependent activation of calcineurin and depriming of secretory granules. J Physiol 535: 519–532, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hellman B, Lernmark A. Inhibition of in vitro secretion of insulin by an extract of pancreatic alpha1 cells. Endocrinology 84: 1484-&, 1969 [DOI] [PubMed] [Google Scholar]
- 20.Hoenig M, Sharp GWG. Glucose induces insulin release and a rise in cytosolic calcium-concentration in a transplantable rat insulinoma. Endocrinology 119: 2502–2507, 1986 [DOI] [PubMed] [Google Scholar]
- 21.Hsu WH, Xiang HD, Rajan AS, Kunze DL, Boyd AE. Somatostatin inhibits insulin-secretion by a G-protein-mediated decrease in Ca2+ entry through voltage-dependent Ca2+ channels in the beta-cell. J Biol Chem 266: 837–843, 1991 [PubMed] [Google Scholar]
- 22.Imai Y, Patel HR, Hawkins EJ, Doliba NM, Matschinsky FM, Ahima RS. Insulin secretion is increased in pancreatic islets of neuropeptide Y-deficient mice. Endocrinology 148: 5716–5723, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Jamal H, Jones PM, Byrne J, Suda K, Ghatei MA, Kanse SM, Bloom SR. Peptide contents of neuropeptide-Y, vasoactive intestinal polypeptide, and beta-calcitonin gene-related peptide and their messenger ribonucleic-acids after dexamethasone treatment in the isolated rat islets of Langerhans. Endocrinology 129: 3372–3380, 1991 [DOI] [PubMed] [Google Scholar]
- 24.Klabunde T, Hessler G. Drug design strategies for targeting G-protein-coupled receptors. Chem Biochem 3: 929–944, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Lehmann DM, Seneviratne AMPB, Smrcka AV. Small molecule disruption of G protein beta gamma subunit signaling inhibits neutrophil chemotaxis and inflammation. Mol Pharmacol 73: 410–418, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin Y, Smrcka AV. Understanding molecular recognition by G protein betagamma subunits on the path to pharmacological targeting. Mol Pharmacol 80: 551–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moller N, Jorgensen JOL. Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. Endocr Rev 30: 152–177, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Morgan DG, Kulkarni RN, Hurley JD, Wang ZL, Wang RM, Ghatei MA, Karlsen AE, Bloom SR, Smith DM. Inhibition of glucose stimulated insulin secretion by neuropeptide Y is mediated via the Y1 receptor and inhibition of adenylyl cyclase in RIN 5AH rat insulinoma cells. Diabetologia 41: 1482–1491, 1998 [DOI] [PubMed] [Google Scholar]
- 29.Myrsen U, Ahren B, Sundler F. Neuropeptide-Y is expressed in subpopulations of insulin and non-insulin islet cells in the rat after dexamethasone treatment—a combined immunocytochemical and in-situ hybridization study. Diabetes 44: A132–A132, 1995 [DOI] [PubMed] [Google Scholar]
- 30.Myrsen U, Mulder H, Ahren B, Sundler F. Dexamethasone induces NPY expression in RINm5F cells: evidence for constitutive release of NPY and impaired insulin secretion. Diabetes 45: 555–555, 1996 [Google Scholar]
- 31.Myrsen Axcrona U, Ekblad E, Sundler F. Developmental expression of NPY, PYY and PP in the rat pancreas and their coexistence with islet hormones. Regul Pept 68: 165–175, 1997 [DOI] [PubMed] [Google Scholar]
- 32.Orci L, Baetens D, Dubois MP, Rufener C. Evidence for D-cell of pancreas secreting somatostatin. Horm Metab Res 7: 400–402, 1975 [DOI] [PubMed] [Google Scholar]
- 33.Patel YC. Somatostatin and its receptor family. Front Neuroendocrinol 20: 157–198, 1999 [DOI] [PubMed] [Google Scholar]
- 34.Patel YC, Greenwood MT, Warszynska A, Panetta R, Srikant CB. All 5 cloned human somatostatin receptors (Hsstr1–5) are functionally coupled to adenylyl-cyclase. Biochem Biophys Res Commun 198: 605–612, 1994 [DOI] [PubMed] [Google Scholar]
- 35.Pettersson M, Ahren B, Lundquist I, Bottcher G, Sundler F. Neuropeptide-Y—intrapancreatic neuronal localization and effects on insulin-secretion in the mouse. Cell Tissue Res 248: 43–48, 1987 [DOI] [PubMed] [Google Scholar]
- 36.Piston DW, Knobel SM. Real-time analysis of glucose metabolism by microscopy. Trends Endocrinol Metab 10: 413–417, 1999 [DOI] [PubMed] [Google Scholar]
- 37.Porte D, Williams RH. Inhibition of insulin release by norepinephrine in man. Science 152: 1248-&, 1966 [DOI] [PubMed] [Google Scholar]
- 38.Portela-Gomes GM, Stridsberg M, Grimelius L, Oberg K, Janson ET. Expression of the five different somatostatin receptor subtypes in endocrine cells of the pancreas. Appl Immunohistochem Mol Morphol 8: 126–132, 2000 [DOI] [PubMed] [Google Scholar]
- 39.Renstrom E, Ding WG, Bokvist K, Rorsman P. Neurotransmitter-induced inhibition of exocytosis in insulin-secreting beta cells by activation of calcineurin. Neuron 17: 513–522, 1996 [DOI] [PubMed] [Google Scholar]
- 40.Rocheleau JV, Head WS, Piston DW. Quantitative NAD(P)H/flavoprotein autofluorescence imaging reveals metabolic mechanisms of pancreatic islet pyruvate response. J Biol Chem 279: 31780–31787, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Scharp DW, Kemp CB, Knight MJ, Ballinge WF, Lacy PE. Use of Ficoll in preparation of viable islets of Langerhans from rat pancreas. Transplantation 16: 686–689, 1973 [DOI] [PubMed] [Google Scholar]
- 42.Silva AP, Carvalho AP, Carvalho CM, Malva JO. Functional interaction between neuropeptide Y receptors and modulation of calcium channels in the rat hippocampus. Neuropharmacology 44: 282–292, 2003 [DOI] [PubMed] [Google Scholar]
- 43.Skoglund G, Gross R, Ahren B, Loubatieresmariani MM. Different mechanisms are involved in neuropeptide Y-induced pancreatic vasoconstriction and inhibition of insulin-secretion. Eur J Pharmacol 236: 69–74, 1993 [DOI] [PubMed] [Google Scholar]
- 44.Smith PA. N-type Ca(2+)-channels in murine pancreatic beta-cells are inhibited by an exclusive coupling with somatostatin receptor subtype 1. Endocrinology 150: 741–748, 2009 [DOI] [PubMed] [Google Scholar]
- 45.Stanley BG, Anderson KC, Grayson MH, Leibowitz SF. Repeated hypothalamic-stimulation with neuropeptide-Y increases daily carbohydrate and fat intake and body-weight gain in female rats. Physiol Behav 46: 173–177, 1989 [DOI] [PubMed] [Google Scholar]
- 46.Stefan Y, Meda P, Neufeld M, Orci L. Stimulation of insulin-secretion reveals heterogeneity of pancreatic B-cells in vivo. J Clin Invest 80: 175–183, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Strowski MZ, Parmar RM, Blake AD, Schaeffer JM. Somatostatin inhibits insulin and glucagon secretion via two receptor subtypes: an in vitro study of pancreatic islets from somatostatin receptor 2 knockout mice. Endocrinology 141: 111–117, 2000 [DOI] [PubMed] [Google Scholar]
- 48.Whim MD. Pancreatic beta cells synthesize neuropeptide Y and can rapidly release peptide co-transmitters. PLos One 6: 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Winzell MS, Ahren B. G-protein-coupled receptors and islet function—implications for treatment of type 2 diabetes. Pharmacol Therapeut 116: 437–448, 2007 [DOI] [PubMed] [Google Scholar]
- 50.Wollheim CB, Sharp GWG. Regulation of insulin release by calcium. Physiol Rev 61: 914–973, 1981 [DOI] [PubMed] [Google Scholar]
- 51.Xu T, Ashery U, Burgoyne RD, Neher E. Early requirement for alpha-SNAP and NSF in the secretory cascade in chromaffin cells. EMBO J 18: 3293–3304, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu T, Binz T, Niemann H, Neher E. Multiple kinetic components of exocytosis distinguished by neurotoxin sensitivity. Nat Neurosci 1: 192–200, 1998 [DOI] [PubMed] [Google Scholar]
- 53.Zhao Y, Fang QH, Straub SG, Lindau M, Sharp GWG. Noradrenaline inhibits exocytosis via the G protein beta gamma subunit and refilling of the readily releasable granule pool via the alpha(i1/2) subunit. J Physiol 588: 3485–3498, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]