Abstract
Glucagon-like peptide-1 (GLP-1) causes vasodilation and increases muscle glucose uptake independent of insulin. Recently, we have shown that GLP-1 recruits muscle microvasculature and increases muscle glucose use via a nitric oxide (NO)-dependent mechanism. Protein kinase A (PKA) is a major signaling intermediate downstream of GLP-1 receptors. To examine whether PKA mediates GLP-1's microvascular action in muscle, GLP-1 was infused to overnight-fasted male rats for 120 min in the presence or absence of H89, a PKA inhibitor. Hindleg muscle microvascular recruitment and glucose use were determined. GLP-1 infusion acutely increased muscle microvascular blood volume within 30 min without altering microvascular blood flow velocity or blood pressure. This effect persisted throughout the 120-min infusion period, leading to a significant increase in muscle microvascular blood flow. These changes were paralleled with an approximately twofold increase in plasma NO levels and hindleg glucose extraction. Systemic infusion of H89 completely blocked GLP-1-mediated muscle microvascular recruitment and increases in NO production and muscle glucose extraction. In cultured endothelial cells, GLP-1 acutely increased PKA activity and stimulated endothelial NO synthase phosphorylation at Ser1177 and NO production. PKA inhibition abolished these effects. In ex vivo studies, perfusion of the distal saphenous artery with GLP-1 induced significant vasorelaxation that was also abolished by pretreatment of the vessels with PKA inhibitor H89. We conclude that GLP-1 recruits muscle microvasculature by expanding microvascular volume and increases glucose extraction in muscle via a PKA/NO-dependent pathway in the vascular endothelium. This may contribute to postprandial glycemic control and complication prevention in diabetes.
Keywords: glucagon-like peptide 1, protein kinase A, microvascular recruitment, nitric oxide
glucagon-like peptide-1 (GLP-1) is a major incretin hormone and acts on its 64-kDa G protein-coupled receptor to increase cAMP production and mediates glucose-induced insulin secretion. Recent evidence suggests that GLP-1 is capable of increasing muscle glucose uptake independent of insulin (2). This was most convincingly demonstrated by an observation of increased myocardial uptake of glucose in Langendorff-perfused rat heart preparation (41). In conscious dogs, both GLP-1 and its active metabolite are able to increase myocardial glucose uptake without altering plasma insulin concentrations (32, 33).
The mechanisms underlying GLP-1-stimulated glucose uptake in muscle are poorly understood. Endothelial cells (ECs) express abundant GLP-1 receptors (35), and GLP-1 has been shown to increase coronary blood flow and myocardial glucose uptake independent of insulin (34, 41). We have reported recently that GLP-1 acutely increases muscle glucose use via increased muscle microvascular recruitment and insulin delivery through a nitric oxide (NO)-dependent mechanism (8). We and others have shown in the past that factors increasing NO production are able to recruit muscle microvasculature, leading to increased endothelial exchange surface area and substrate and hormonal exchanges between plasma and muscle interstitium (4, 5, 12, 24).
Endothelial NO synthase (eNOS) is regulated by signaling pathways involving multiple sites of phosphorylation. The coordinated phosphorylation of eNOS at Ser1177 and dephosphorylation at Thr497 activate whereas Ser1177 dephosphorylation and Thr497 phosphorylation deactivate the enzyme. In our previous report, we have speculated that protein kinase A (PKA), a major signaling intermediate downstream of GLP-1 receptors, may have played an important role in GLP-1-mediated NO production and microvascular recruitment, since PKA has been shown to activate eNOS in response to various stimuli (6, 13, 30, 31), and we have demonstrated that GLP-1 potently increased PKA activity in cultured endothelial cells (8). Because treatment of spontaneously hypertensive rats with sitagliptin for 2 wk improved endothelium-dependent relaxation in renal arteries, restored renal blood flow, and reduced systolic blood pressure, and these effects were prevented by inhibition of either GLP-1 receptor, PKA or NO synthase (27), currently available evidence strongly suggests that GLP-1 exerts its vasodilatory actions via the GLP-1 receptor, PKA, and NO signaling pathways.
In the current study, we examined whether GLP-1-induced PKA activity indeed plays important an role in GLP-1-mediated NO production, microvascular recruitment, and glucose use in muscle. Our results in rats, cultured ECs, and ex vivo arteries indicate that GLP-1 recruits muscle microvasculature by expanding microvascular volume and increases substrate exchange in muscle via a PKA/eNOS-dependent pathway.
MATERIALS AND METHODS
Animal preparations and experimental protocols.
Adult male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) weighing 220–320 g were studied after an overnight fast. Rats were housed at 22 ± 2°C on a 12:12-h light-dark cycle and fed standard laboratory chow and water ad libitum before the study. After being anesthetized with pentobarbital sodium (50 mg/kg ip; Abbott Laboratories, North Chicago, IL), rats were placed in a supine position on a heating pad to ensure euthermia and intubated to maintain a patent airway. Polyethylene cannulae (PE-50; Fisher Scientific, Newark, DE) were inserted into the carotid artery and jugular vein for arterial blood pressure monitoring, arterial blood sampling, and various infusions.
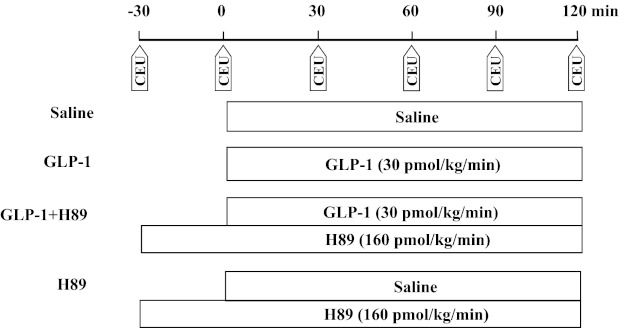
After a 30- to 45-min baseline period to ensure hemodynamic stability and a stable level of anesthesia, rats were randomly studied in one of the following four groups (Fig. 1). Each rat received an intravenous infusion of either saline (control) or GLP-1-(7–36) amide (30 pmol·kg−1·min−1; Bachem Americas, Torrance, CA) for 120 min in the presence or absence of systemic infusion of H89 (160 pmol·kg−1·min−1; Sigma-Aldrich, St. Louis, MO). At the doses selected, GLP-1 has been shown to potently recruit muscle microvasculature (8) and H89 to abolish morphine-mediated attenuation of microvascular hyperpermeability via the PKA-dependent pathway (37). H89 infusion was started 30 min before the commencement of saline or GLP-1 infusion and had no significant effect on muscle microvascular recruitment (Fig. 2). Skeletal muscle microvascular blood volume (MBV), microvascular blood flow velocity (MFV), and microvascular blood flow (MBF) were determined using contrast-enhanced ultrasound, as described previously (8–10, 40). Hindleg glucose extraction, plasma NO levels, and plasma insulin concentrations were measured as described below. Rat gastrocnemius muscles were collected for measurement of Akt1 (Ser473), eNOS (Ser1177), and ERK1/2 (Thr202/Tyr204) phosphorylation using Western blotting and PKA activity. Rat hearts and aortae were collected for measurement of PKA activity.
Fig. 1.
Experimental protocols. CEU, contrast-enhanced ultrasound; GLP-1, glucagon-like peptide 1.
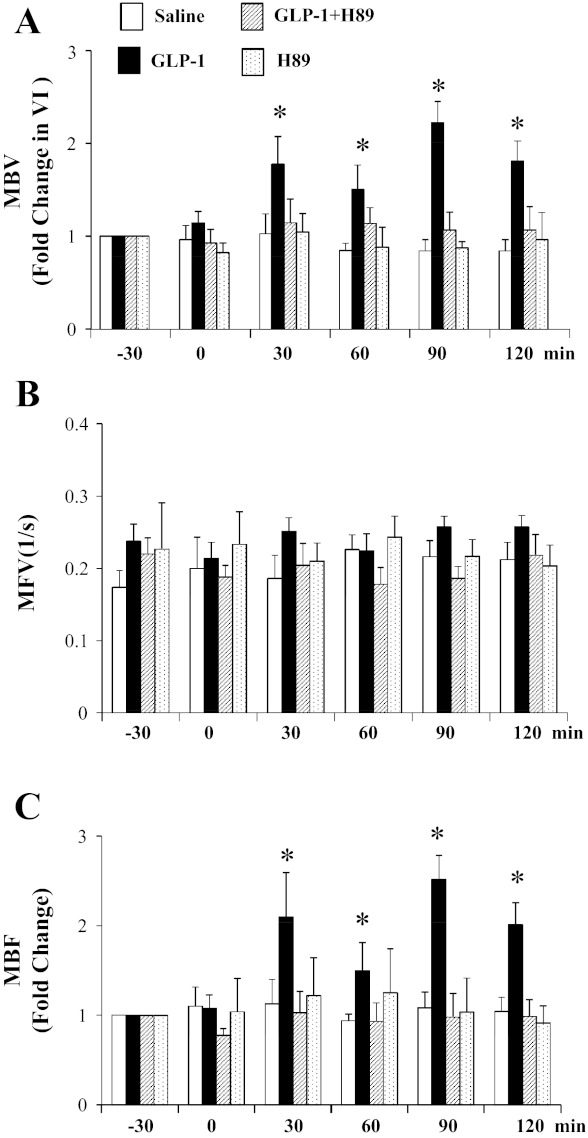
Fig. 2.
Inhibition of protein kinase A (PKA) abolishes GLP-1-mediated muscle microvascular recruitment. GLP-1 was infused continuously at 30 pmol·kg−1·min−1 in the absence or presence of H89. A: changes in microvascular blood volume (MBV). B: changes in microvascular blood flow velocity (MFV). C: changes in microvascular blood flow (MBF). *P < 0.05 compared with −30 or 0 min; n = 5–11.
Mean arterial blood pressure (MAP) was monitored via a sensor connected to the carotid arterial catheter (Harvard Apparatus, Holliston, MA, and ADInstruments, Colorado Springs, CO), and pentobarbital sodium was infused at a variable rate to maintain steady levels of anesthesia and blood pressure throughout the study. The investigation conformed to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (Publication No. 85-23, revised 1996), and the study protocol was approved by the Animal Care and Use Committee of the University of Virginia.
Determination of hindleg muscle glucose extraction.
Carotid arterial and femoral venous blood glucose concentrations were determined using an Accu-Chek Advantage blood glucometer (Roche Diagnostics, Indianapolis, IN). Glucose levels were determined four to six times per time point, and the numbers were averaged. Hindleg glucose uptake (mg/dl) was calculated as the arterial-venous glucose difference.
Culture of bovine aortic ECs.
Bovine aortic ECs (bAECs) in primary culture were purchased from Lonza (Walkersville, MD) and cultured as described previously (25, 26). After serum starvation for 14 h, cells between passages 3 and 8 were exposed to GLP-1-(7–36) amide (1 ng/ml; Bachem Americas, Torrance, CA) for 20 min in the absence or presence of H89 (10 μM; Sigma-Aldrich) and then used for either Western blotting, measurement of NO production, or PKA activity.
Measurement of plasma NO and insulin levels and endothelial NO production.
Plasma NO levels were measured using a 280i Nitric Oxide Analyzer (GE Analytical, Boulder, CO) according to the manufacturer's instructions and as described previously (8, 9). Plasma insulin concentrations were determined using a rat insulin ELISA assay kit (Mercodia, Uppsala, Sweden). For endothelial NO production, bAECs were exposed to GLP-1-(7–36) amide (1 ng/ml) for 20 min in the absence or presence of H89 (10 μM). NO levels in media were measured using the Nitric Oxide Synthase Detection System (Sigma-Aldrich) according to the manufacturer's instructions. Insulin (100 nM) with or without l-NAME (10 mM) was used as positive and negative control for NO production.
Quantification of PKA activity.
PKA activities in tissue and ECs were quantified using a PKA assay kit (Promega, Madison, WI) according to the manufacturer's instructions. Briefly, skeletal muscle, heart, aorta, or ECs (5 × 106) were homogenized in cold PKA extraction buffer, and the lysate was centrifuged for 5 min at 4°C at 14,000 g. For aorta, samples from four rats were pooled together. The supernatant was mixed with assay mixture and incubated for 30 min at room temperature, and the reaction was stopped by heating the mixture to 95°C for 10 min. Samples were then separated on a 0.8% agarose gel at 100 V for 15 min. The densities of both the phosphorylated and nonphosphorylated peptides were quantified using Image J software, and the ratios of phosphospecific to total density were calculated.
Western blotting.
Phosphorylation of eNOS, Akt1, and ERK1/2 in muscle and cultured ECs was determined by Western blotting, as described previously (8, 9). Primary antibody against phospho-Akt1 (Ser473) and Akt1 was obtained from Upstate Biotechnology (Lake Placid, NY), and primary antibody against phospho-eNOS (Ser1177), eNOS, phospho-ERK1/2 (Thr202/Tyr204), and ERK1/2 was obtained from Cell Signaling Technology (Beverly, MA). Both the total and phosphospecific densities were quantified and the ratios of phosphospecific to total density calculated.
Myograph experiments.
Distal saphenous artery was dissected from overnight-fasted rats immediately after the rats were euthanized. After the adhering connective tissue was trimmed off, each artery was cut into three ring segments ∼2 mm in length. Each segment was mounted in a Multi Myograph System (Danish Myo Technology, Aarhus, Denmark). The organ chamber was filled with 6 ml of physiological salt solution buffer (130 mM NaCl, 4.7 mM KCl, 1.6 mM CaCl2, 1.17 mM MgSO4, 1.18 mM KH2PO4, 14.9 mM NaHCO3, 0.026 mM EDTA, and 5.5 mM glucose, pH 7.4), which was constantly bubbled with 95% O2-5% CO2 and maintained at 37°C. Each ring was stretched initially to 5 mN, an optimal tension, and then allowed to stabilize at baseline tone. Relaxant responses to phenylephrine (300 nM) or acetylcholine (1 μM) were performed to test for vessel viability and integrity of endothelium. After precontraction with phenylephrine, GLP-1 (1 ng/ml) was added to the incubation bath with or without H89 (1 μM) pretreatment for 1 h. The relaxant response was expressed as percentage of the contraction induced by phenylephrine.
Statistical analysis.
All data are presented as means ± SE. Statistical analyses were performed with SigmaStat 3.1.1 software (Systat Software, Chicago, IL), using either Student's t-test or analysis of variance (ANOVA) with post hoc analysis as appropriate. A P value of <0.05 was considered statistically significant.
RESULTS
PKA inhibition abolishes GLP-1-induced increase in muscle microvascular perfusion.
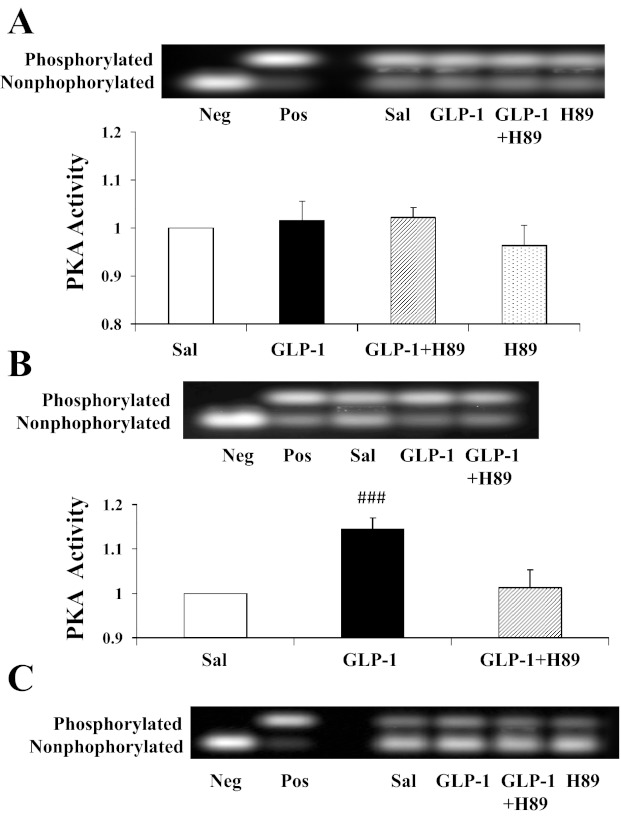
We have demonstrated previously that GLP-1 recruits muscle microvasculature and increases glucose use via a NO-dependent mechanism and that incubation of bAECs with GLP-1 increases endothelial PKA activity significantly (8). To examine the potential role of PKA in GLP-1's microvascular action in muscle, we infused rats with the selective PKA inhibitor H89 prior to beginning GLP-1 infusion. Control rats received saline or H89 infusion only. Similarly to our previous report (8), GLP-1 potently recruited muscle microvasculature by increasing muscle MBV (∼2-fold, P < 0.001, ANOVA) without affecting MFV (Fig. 2). This led to an approximately twofold increase in muscle MBF (P < 0.001, ANOVA). This was associated with a significant increase in cardiac muscle and aorta PKA activity (Fig. 3). Skeletal muscle PKA activity did not change significantly across any of the groups. Inhibition of PKA activity by coinfusion of H89 completely prevented a GLP-1-induced increase in PKA activity (Fig. 3) and abolished GLP-1-induced microvascular recruitment (Fig. 2). H89 infusion alone did not alter muscle microvascular parameters despite a brief decrease in MAP either alone or with GLP-1 (Table 1).
Fig. 3.
Effects of H89 on GLP-1-mediated changes in PKA activity. GLP-1 was infused continuously (30 pmol·kg−1·min−1) for 120 min in the absence or presence of H89 (160 pmol·kg−1·min−1). A: skeletal muscle. B: heart. C: aorta (samples from 4 rats were pooled together). Neg, negative control; Pos, positive control; Sal, saline. ###P = 0.002 compared with saline; n = 4–5.
Table 1.
Changes in MAP and plasma insulin concentrations
| Time (min) | −30 | 0 | 30 | 60 | 90 | 120 |
|---|---|---|---|---|---|---|
| MAP, mmHg | ||||||
| Saline | 106 ± 4 | 98 ± 6 | 94 ± 6# | 96 ± 4# | 103 ± 3 | |
| GLP-1 | 100 ± 3 | 105 ± 2 | 108 ± 2 | 104 ± 3 | 103 ± 2 | |
| H89 + GLP-1 | 104 ± 3 | 94 ± 4* | 98 ± 3* | 101 ± 3 | 104 ± 2 | 106 ± 2 |
| H89 | 107 ± 2 | 107 ± 2 | 99 ± 2 | 94 ± 4 | 90 ± 5* | 103 ± 4 |
| Insulin concentration, pM | ||||||
| Saline | 75 ± 13 | 70 ± 11 | 76 ± 13 | 92 ± 17 | 83 ± 12 | |
| GLP-1 | 103 ± 28 | 101 ± 30 | 99 ± 36 | 129 ± 43 | 157 ± 42 | |
| H89 + GLP-1 | 73 ± 17 | 64 ± 16 | 61 ± 16 | 42 ± 3 | 58 ± 10 | 64 ± 5 |
| H89 | 121 ± 27 | 111 ± 20 | 106 ± 23 | 101 ± 27 | 120 ± 27 | 134 ± 37 |
Values are means ± SE.
MAP, mean arterial blood pressure; GLP-1, glucagon-like peptide 1.
P < 0.05 compared with −30 min;
P < 0.05 compared with 0 min.
PKA inhibition blocks GLP-1-induced NO production and muscle glucose extraction.
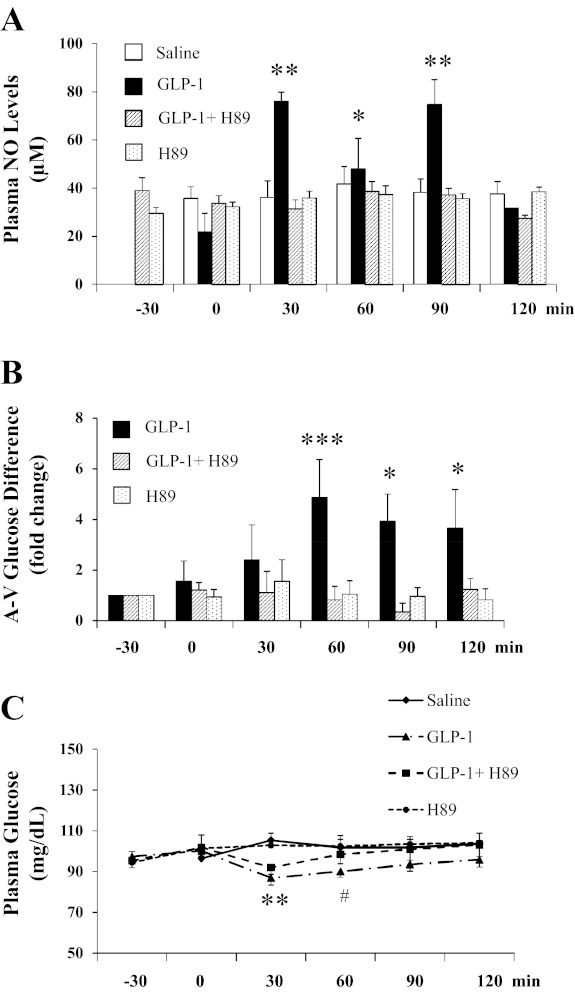
Because microvascular endothelial surface area determines substrate exchange between plasma and muscle interstitium and we have shown previously that GLP-1 increases muscle microvascular recruitment and glucose extraction via NO-dependent mechanism (8), we next assessed whether inhibition of PKA also prevented GLP-1-induced NO production and muscle glucose extraction. As shown in Fig. 4A, GLP-1 infusion increased plasma NO levels approximately threefold within 30 min, which remained elevated for 90 min (P = 0.001, ANOVA). This was associated with a two- to threefold increase in muscle glucose extraction (P < 0.04; Fig. 4B), as reflected by femoral arterial-venous glucose difference and a significant decrease in arterial plasma glucose levels at 30 and 60 min (P < 0.001 and P < 0.02, respectively; Fig. 4C). Concurrent H89 infusion completely abolished GLP-1-stimulated increase in both NO production and muscle glucose utilization and prevented a GLP-1-induced decrease in arterial plasma glucose levels (P = 0.169, ANOVA). Plasma insulin concentrations remained stable during the experiments for all groups (Table 1).
Fig. 4.
PKA inhibition blunts GLP-1-mediated nitric oxide (NO) production and muscle glucose extraction. GLP-1 was infused continuously at 30 pmol·kg−1·min−1 in the absence or presence of H89. A: changes in arterial plasma NO levels. B: changes in muscle glucose extraction. C: changes in plasma glucose levels. **P < 0.001, *P < 0.05, ***P = 0.005, and #P < 0.02 compared with −30 or 0 min; n = 4–8.
GLP-1 effects on Akt1, eNOS, and ERK1/2 phosphorylation in skeletal muscle in vivo.
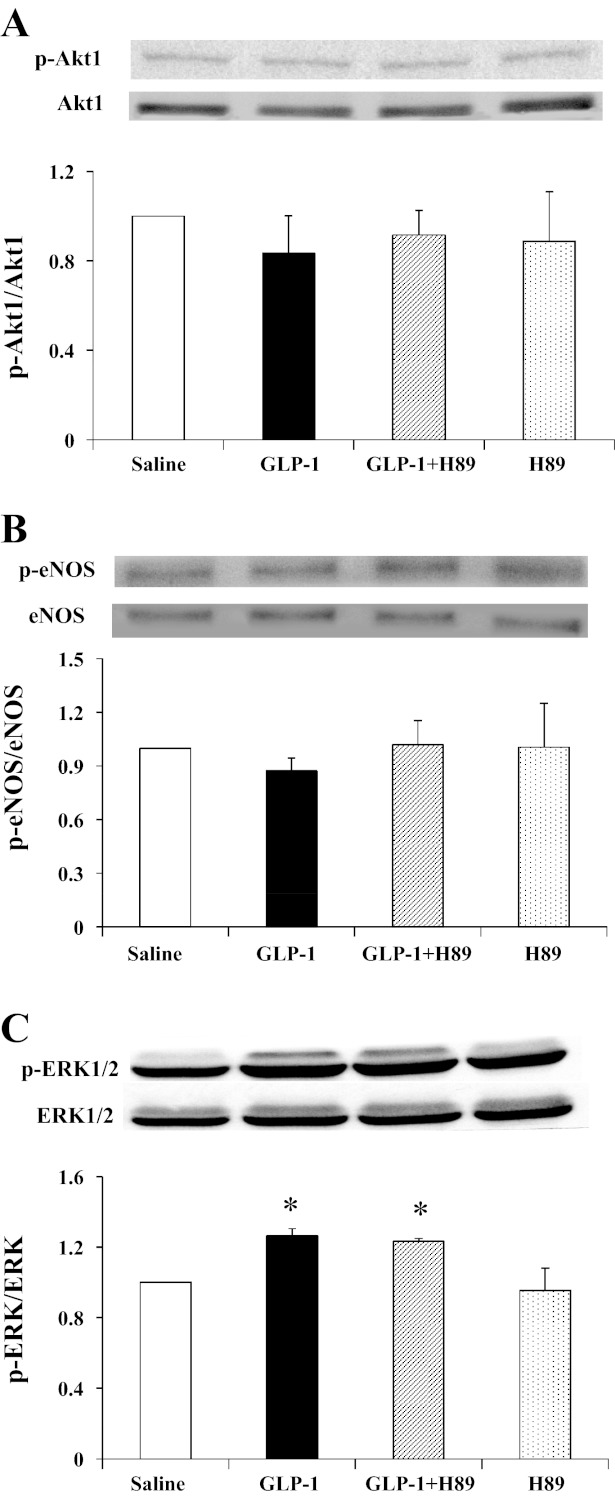
Because we have shown that GLP-1 increased the phosphorylation of Akt1 and eNOS in cultured endothelial cells, we further examined whether GLP-1 also stimulates phosphorylation of Akt1, eNOS, and ERK1/2 in skeletal muscle. As shown in Fig. 5, GLP-1 and H89 had no effect on either Akt1 or eNOS phosphorylation in the skeletal muscle. On the contrary, muscle ERK1/2 phosphorylation increased significantly during GLP-1 infusion (P = 0.027, ANOVA), and pretreatment with H89 did not block this effect (P = 0.762, ANOVA).
Fig. 5.
Effects of PKA inhibition on GLP-1-mediated muscle Akt1 (A), endothelial NO synthase (eNOS; B), and ERK1/2 (C) phosphorylation. A: changes in Akt1 phosphorylation. B: changes in eNOS phosphorylation. C: changes in ERK1/2 phosphorylation. *P < 0.05 compared with saline; n = 3–5.
PKA mediates GLP-1-induced NO production in cultured bAECs.
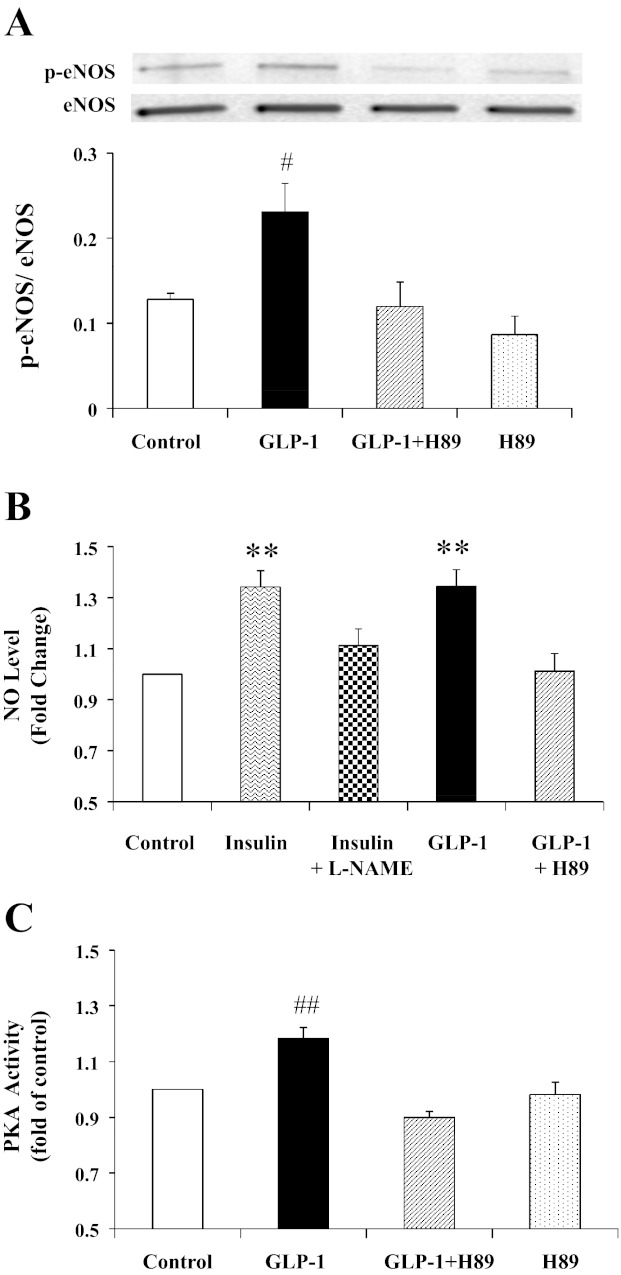
Because plasma NO increased but muscle eNOS phosphorylation did not increase, the increase in plasma NO concentrations were most likely secondary to endothelial production of NO. Figure 6 shows that incubation of bAECs with GLP-1 for 20 min potently increased endothelial eNOS phosphorylation at Ser1177 (P = 0.019, ANOVA) and NO production (by ∼30%, P < 0.001), which was associated with a significant increase in PKA activity (P = 0.001, ANOVA). Incubation of cells with H89 completely abolished GLP-1-induced increases in PKA activity, eNOS phosphorylation, and NO production, confirming that GLP-1-mediated endothelial NO production is PKA-dependent.
Fig. 6.
GLP-1 increases NO production in cultured endothelial cells via PKA-dependent pathway. Cells were incubated with or without l-NAME (10 mM), GLP-1 (1 ng/ml), and/or H89 (10 μM). A: changes in eNOS phosphorylation. B: changes in endothelial NO production. Insulin (100 nM) was used as positive control. C: changes in PKA activity. #P < 0.02, **P < 0.001, and ##P = 0.001 compared with control; n = 3–9.
PKA mediates GLP-1-induced vasodilation in isolated saphenous artery communicating branch.
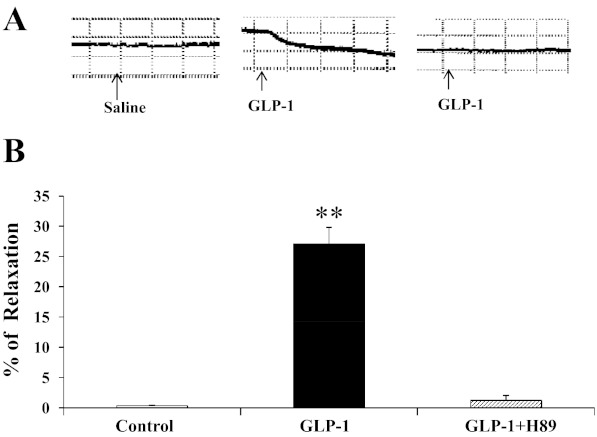
Because we observed increased PKA activity in heart and aorta but not in the skeletal muscle, we finally used an ex vivo approach to examine the role of PKA activation in isolated rat saphenous (Fig. 7). Incubation of the artery rings with GLP-1 potently increased vasorelaxation (∼30%, P < 0.001, ANOVA), and this effect was once again prevented by pretreatment of the vessels with H89. Thus, GLP-1-mediated PKA activity within the vessel wall is indeed responsible for GLP-1-induced vasorelaxation.
Fig. 7.
PKA mediates GLP-1-induced arterial vasodilation ex vivo. Rat saphenous artery was isolated, preconstricted with phenylnephrine, and incubated with GLP-1 (1 ng/ml) for 30 min with or without H89 (1 μM) pretreatment for 1 h. **P < 0.001 compared with control; n = 3.
DISCUSSION
The current study demonstrates that GLP-1-induced PKA activity plays a pivotal role in GLP-1-mediated microvascular recruitment and glucose use in muscle. Whereas systemic infusion of GLP-1 increased tissue PKA activity in vivo and incubation of ECs with GLP-1 potently increased PKA activity in vitro, administration of H89, a selective inhibitor of PKA, completely abolished GLP-1-induced NO production, microvascular recruitment, and glucose use in muscle and ex vivo vasodilation. Thus, our data strongly suggest that PKA signaling is required in GLP-1's microvascular actions and the associated metabolic consequences.
Although ex vivo studies using rat arterial rings or pulmonary arteries have shown that vascular endothelium and NO production are essential in the vasorelaxant effect of GLP-1 (16, 38), and incubation of ECs with GLP-1 analog liraglutide dose and time dependently increases NO production (19), our recent report demonstrated a direct stimulatory effect of GLP-1 on eNOS phosphorylation and that endothelial production of NO is crucial in GLP-1-induced microvascular recruitment and glucose use in muscle, as inhibition of NO production completely abolished the microvascular effects of GLP-1 (8). The current study further extends our previous findings by demonstrating that these effects are mediated via the PKA signaling pathway. This is consistent with the fact that activation of the GLP-1 receptor triggers the generation of second-messenger cAMP via a direct action on the adenylate cyclase (18) and leads to the activation of PKA (14) and that eNOS is colocalized with the catalytic subunit of PKA in EC junctions (20).
The role of PKA in GLP-1-mediated glucose-stimulated insulin secretion is well characterized in pancreatic β-cells (3, 14). Binding of GLP-1 to its receptors activates adenylate cyclase and increases intracellular cAMP levels, leading to activation of PKA (21). Our observations that PKA inhibition completely prevented GLP-1-induced NO production, microvascular recruitment, and muscle extraction of glucose confirm that PKA signaling plays a key role in GLP-1's microvascular actions. That administration of PKA inhibitor H89 completely blocked GLP-1-induced PKA activity in the aorta and abolished GLP-1-induced vasodilatation ex vivo in the rat saphenous artery strongly suggests that PKA mediates GLP-1 actions in conduit artery and resistance arterioles as well.
In the current study, we observed that GLP-1 infusion increased the phosphorylation of ERK1/2 but not Akt1 in muscle. Although the increased phosphorylation of ERK1/2 was consistent with a previous report (1), the same study showed that incubation of isolated rat soleus muscles with GLP-1 for 3 min potently increased phosphatidylinositol 3-kinase (PI 3-kinase) activity and Akt phosphorylation. This discrepancy may be explained by differences in GLP-1 exposure time (2 h vs. 3 min), variations in Akt isoforms (the antibody we used did not cross-react with other Akt isoforms), and study setting (in vivo vs. in vitro). Indeed, in isolated rat hearts GLP-1 markedly increased cardiac muscle glucose uptake without altering Akt1 phosphorylation (41).
Inasmuch as both PKA and the PI 3-kinase are able to phosphorylate eNOS and increase NO production and GLP-1 has been shown to activate the PI 3-kinase pathway in muscle (1) and β-cells (7), our study findings suggest that the PI 3-kinase pathway is likely uninvolved in GLP-1-induced NO production and microvascular recruitment since inhibition of PKA alone with H89 completely blocked GLP-1-induced NO production and vascular responses both in vivo and ex vivo. Our findings are in agreement with prior reports in cultured ECs that PKA but not PI 3-kinase mediates GLP-1-induced attenuation on ROS-induced senescence (36).
H89 is a potent and selective inhibitor of PKA (11, 23) and has been widely used to inhibit PKA in biochemical studies. It blocks PKA actions through competitive inhibition of the adenosine triphosphate site on the PKA catalytic subunit. A limitation of the current study is that H89 at high concentrations also inhibits several other kinases, such as p70S6K, MSK1, ROCK11, and MAPKAP-K1b (28, 29). However, it is less likely that these kinases contributed to GLP-1-mediated NO production since inhibition of PKA activity with another specific inhibitor, Rp-cAMPS, also abolished exendin-4-induced activation of eNOS and cell proliferation (15). Rp-cAMPS is a cell-permeable cAMP analog that specifically inhibits PKA by interacting with cAMP-binding sites on the regulatory subunits (39).
It seems contradictory that we observed that GLP-1 increased muscle microvascular perfusion via the PKA-dependent pathway but not PKA activity in the skeletal muscle. However, PKA activity did increase significantly in both heart and aorta after GLP-1 infusion. This is not surprising since skeletal muscle per se expresses much lower GLP-1 receptors than heart (8-fold less) and aorta (4-fold less) (17). Together with our findings in cultured cells and ex vivo saphenous artery study, our findings are entirely consistent with a direct action of GLP-1 on its receptors to activate PKA in the vasculature.
In conclusion, GLP-1 recruits muscle microvasculature, which leads to expansion of the microvascular blood volume and increased glucose use in muscle through a process that involves PKA activation and NO production. Given that in the resting state only ∼30% of muscle capillaries are perfused (22) and an increase in muscle endothelial surface area could significantly increase insulin delivery and action and substrate exchange in muscle (4, 12), GLP-1's microvascular actions in muscle may contribute significantly to postprandial glycemic control and diabetes complication prevention in diabetes.
GRANTS
This work was supported by American Diabetes Association Grants 1-11-CR-30 and 9-09-NOVO-11 (to Z. Liu) and National Heart, Lung, and Blood Institute Grant R01-HL-094722 (to Z. Liu).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Z.D., W. Chai, L.Z., and Z.F. performed the experiments; Z.D., W. Chai, W.W., L.Z., Z.F., and Z.L. analyzed the data; Z.D. edited and revised the manuscript; Z.D., W. Chai, W.W., L.Z., Z.F., W. Cao, and Z.L. approved the final version of the manuscript; W.W., W. Cao, and Z.L. interpreted the results of the experiments; Z.L. did the conception and design of the research; Z.L. prepared the figures; Z.L. drafted the manuscript.
REFERENCES
- 1.Acitores A, González N, Sancho V, Valverde I, Villanueva-Peñacarrillo ML. Cell signalling of glucagon-like peptide-1 action in rat skeletal muscle. J Endocrinol 180: 389–398, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Ayala JE, Bracy DP, James FD, Julien BM, Wasserman DH, Drucker DJ. The glucagon-like peptide-1 receptor regulates endogenous glucose production and muscle glucose uptake independent of its incretin action. Endocrinology 150: 1155–1164, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology 132: 2131–2157, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Barrett E, Eggleston E, Inyard A, Wang H, Li G, Chai W, Liu Z. The vascular actions of insulin control its delivery to muscle and regulate the rate-limiting step in skeletal muscle insulin action. Diabetologia 52: 752–764, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barrett EJ, Wang H, Upchurch CT, Liu Z. Insulin regulates its own delivery to skeletal muscle by feed-forward actions on the vasculature. Am J Physiol Endocrinol Metab 301: E252–E263, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boo YC, Hwang J, Sykes M, Michell BJ, Kemp BE, Lum H, Jo H. Shear stress stimulates phosphorylation of eNOS at Ser635 by a protein kinase A-dependent mechanism. Am J Physiol Heart Circ Physiol 283: H1819–H1828, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Buteau J, Spatz ML, Accili D. Transcription factor FoxO1 mediates glucagon-like peptide-1 effects on pancreatic b-cell mass. Diabetes 55: 1190–1196, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Chai W, Dong Z, Wang N, Wang W, Tao L, Cao W, Liu Z. Glucagon-like peptide 1 recruits microvasculature and increases glucose use in muscle via a nitric oxide-dependent mechanism. Diabetes 61: 888–896, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chai W, Wang W, Dong Z, Cao W, Liu Z. Angiotensin II receptors modulate muscle microvascular and metabolic responses to insulin in vivo. Diabetes 60: 2939–2946, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chai W, Wang W, Liu J, Barrett EJ, Carey RM, Cao W, Liu Z. Angiotensin II type 1 and type 2 receptors regulate basal skeletal muscle microvascular volume and glucose use. Hypertension 55: 523–530, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chijiwa T, Mishima A, Hagiwara M, Sano M, Hayashi K, Inoue T, Naito K, Toshioka T, Hidaka H. Inhibition of forskolin-induced neurite outgrowth and protein phosphorylation by a newly synthesized selective inhibitor of cyclic AMP-dependent protein kinase, N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide (H-89), of PC12D pheochromocytoma cells. J Biol Chem 265: 5267–5272, 1990 [PubMed] [Google Scholar]
- 12.Clark MG. Impaired microvascular perfusion: a consequence of vascular dysfunction and a potential cause of insulin resistance in muscle. Am J Physiol Endocrinol Metab 295: E732–E750, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dixit M, Loot AE, Mohamed A, Fisslthaler B, Boulanger CM, Ceacareanu B, Hassid A, Busse R, Fleming I. Gab1, SHP2, and protein kinase A are crucial for the activation of the endothelial NO synthase by fluid shear stress. Circ Res 97: 1236–1244, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Drucker DJ. The biology of incretin hormones. Cell Metab 3: 153–165, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Erdogdu Ö, Nathanson D, Sjöholm Å, Nyström T, Zhang Q. Exendin-4 stimulates proliferation of human coronary artery endothelial cells through eNOS-, PKA- and PI3K/Akt-dependent pathways and requires GLP-1 receptor. Mol Cell Endocrinol 325: 26–35, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Golpon HA, Puechner A, Welte T, Wichert PV, Feddersen CO. Vasorelaxant effect of glucagon-like peptide-(7–36)amide and amylin on the pulmonary circulation of the rat. Regul Pept 102: 81–86, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Green BD, Hand KV, Dougan JE, McDonnell BM, Cassidy RS, Grieve DJ. GLP-1 and related peptides cause concentration-dependent relaxation of rat aorta through a pathway involving KATP and cAMP. Arch Biochem Biophys 478: 136–142, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Hallbrink M, Holmqvist T, Olsson M, Ostenson CG, Efendic S, Langel U. Different domains in the third intracellular loop of the GLP-1 receptor are responsible for Gas and Gai/Gao activation. Biochim Biophys Acta 1546: 79–86, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Hattori Y, Jojima T, Tomizawa A, Satoh H, Hattori S, Kasai K, Hayashi T. A glucagon-like peptide-1 (GLP-1) analogue, liraglutide, upregulates nitric oxide production and exerts anti-inflammatory action in endothelial cells. Diabetologia 53: 2256–2263, 2010 [DOI] [PubMed] [Google Scholar]
- 20.Heijnen HF, Waaijenborg S, Crapo JD, Bowler RP, Akkerman JW, Slot JW. Colocalization of eNOS and the catalytic subunit of PKA in endothelial cell junctions: A clue for regulated NO production. J Histochem Cytochem 52: 1277–1285, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev 87: 1409–1439, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Honig CR, Odoroff CL, Frierson JL. Active and passive capillary control in red muscle at rest and in exercise. Am J Physiol Heart Circ Physiol 243: H196–H206, 1982 [DOI] [PubMed] [Google Scholar]
- 23.Johannes FJ, Prestle J, Dieterich S, Oberhagemann P, Link G, Pfizenmaier K. Characterization of activators and inhibitors of protein kinase Cμ. Eur J Biochem 227: 303–307, 1995 [DOI] [PubMed] [Google Scholar]
- 24.Ko SH, Cao W, Liu Z. Hypertension management and microvascular insulin resistance in diabetes. Curr Hypertens Rep 12: 243–251, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li G, Barrett EJ, Ko SH, Cao W, Liu Z. Insulin and insulin-like growth factor-I receptors differentially mediate insulin-stimulated adhesion molecule production by endothelial cells. Endocrinology 150: 3475–3482, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li G, Barrett EJ, Wang H, Chai W, Liu Z. Insulin at physiological concentrations selectively activates insulin but not insulin-like growth factor I (IGF-I) or insulin/IGF-I hybrid receptors in endothelial cells. Endocrinology 146: 4690–4696, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Liu L, Liu J, Wong WT, Tian XY, Lau CW, Wang YX, Xu G, Pu Y, Zhu Z, Xu A, Lam KS, Chen ZY, Ng CF, Yao X, Huang Y. Dipeptidyl peptidase 4 inhibitor sitagliptin protects endothelial function in hypertension through a glucagon-like peptide 1-dependent mechanism. Hypertension 60: 833–841, 2012 [DOI] [PubMed] [Google Scholar]
- 28.Lochner A, Moolman JA. The many faces of H89: a review. Cardiovasc Drug Rev 24: 261–274, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Marunaka Y, Niisato N. H89, an inhibitor of protein kinase A (PKA), stimulates Na+ transport by translocating an epithelial Na+ channel (ENaC) in fetal rat alveolar type II epithelium. Biochem Pharmacol 66: 1083–1089, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Michell BJ, Chen Zp, Tiganis T, Stapleton D, Katsis F, Power DA, Sim AT, Kemp BE. Coordinated control of endothelial nitric-oxide synthase phosphorylation by protein kinase C and the cAMP-dependent protein kinase. J Biol Chem 276: 17625–17628, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Michell BJ, Harris MB, Chen ZP, Ju H, Venema VJ, Blackstone MA, Huang W, Venema RC, Kemp BE. Identification of regulatory sites of phosphorylation of the bovine endothelial nitric-oxide synthase at serine 617 and serine 635. J Biol Chem 277: 42344–42351, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Nikolaidis LA, Elahi D, Hentosz T, Doverspike A, Huerbin R, Zourelias L, Stolarski C, Shen YT, Shannon RP. Recombinant glucagon-like peptide-1 increases myocardial glucose uptake and improves left ventricular performance in conscious dogs with pacing-induced dilated cardiomyopathy. Circulation 110: 955–961, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Nikolaidis LA, Elahi D, Shen YT, Shannon RP. Active metabolite of GLP-1 mediates myocardial glucose uptake and improves left ventricular performance in conscious dogs with dilated cardiomyopathy. Am J Physiol Heart Circ Physiol 289: H2401–H2408, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Nystrom T. The potential beneficial role of glucagon-like peptide-1 in endothelial dysfunction and heart failure associated with insulin resistance. Horm Metab Res 40: 593–606, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Nyström T, Gutniak MK, Zhang Q, Zhang F, Holst JJ, Ahrén B, Sjöholm A. Effects of glucagon-like peptide-1 on endothelial function in type 2 diabetes patients with stable coronary artery disease. Am J Physiol Endocrinol Metab 287: E1209–E1215, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Oeseburg H, de Boer RA, Buikema H, van der Harst P, van Gilst WH, Silljé HH. Glucagon-like peptide 1 prevents reactive oxygen species-induced endothelial cell senescence through the activation of protein kinase A. Arterioscler Thromb Vasc Biol 30: 1407–1414, 2010 [DOI] [PubMed] [Google Scholar]
- 37.Puana R, McAllister RK, Hunter FA, Warden J, Childs EW. Morphine attenuates microvascular hyperpermeability via a protein kinase A-dependent pathway. Anesth Analg 106: 480–485, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Richter G, Feddersen O, Wagner U, Barth P, Göke R, Göke B. GLP-1 stimulates secretion of macromolecules from airways and relaxes pulmonary artery. Am J Physiol Lung Cell Mol Physiol 265: L374–L381, 1993 [DOI] [PubMed] [Google Scholar]
- 39.Schaap P, van Ments-Cohen M, Soede RD, Brandt R, Firtel RA, Dostmann W, Genieser HG, Jastorff B, van Haastert PJ. Cell-permeable non-hydrolyzable cAMP derivatives as tools for analysis of signaling pathways controlling gene regulation in Dictyostelium. J Biol Chem 268: 6323–6331, 1993 [PubMed] [Google Scholar]
- 40.Wang N, Ko SH, Chai W, Li G, Barrett EJ, Tao L, Cao W, Liu Z. Resveratrol recruits rat muscle microvasculature via a nitric oxide-dependent mechanism that is blocked by TNFα. Am J Physiol Endocrinol Metab 300: E195–E201, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao T, Parikh P, Bhashyam S, Bolukoglu H, Poornima I, Shen YT, Shannon RP. Direct effects of glucagon-like peptide-1 on myocardial contractility and glucose uptake in normal and postischemic isolated rat hearts. J Pharmacol Exp Ther 317: 1106–1113, 2006 [DOI] [PubMed] [Google Scholar]