Abstract
A major side effect of insulin treatment of diabetes is weight gain, which limits patient compliance and may pose additional health risks. Although the mechanisms responsible for this weight gain are poorly understood, it has been suggested that there may be a link to the incidence of recurrent episodes of hypoglycemia. Here we present a rodent model of marked weight gain associated with weekly insulin-induced hypoglycemic episodes in the absence of diabetes. Insulin treatment caused a significant increase in both body weight and fat mass, accompanied by reduced motor activity, lowered thermogenesis in response to a cold challenge, and reduced brown fat uncoupling protein mRNA. However, there was no effect of insulin treatment on total food intake nor on hypothalamic neuropeptide Y or proopiomelanocortin mRNA expression, and insulin-treated animals did not become insulin-resistant. Our results suggest that repeated iatrogenic hypoglycemia leads to weight gain, and that such weight gain is associated with a multifaceted deficit in metabolic regulation rather than to a chronic increase in caloric intake.
Keywords: obesity, diabetes, insulin, hypoglycemia
intensive insulin therapy (IIT) is now well-established as the optimal approach to control diabetic hyperglycemia in both type 1 and, increasingly, type 2 diabetes mellitus. However, two major side effects limit acceptance of, and compliance with, such therapy: an increased incidence of hypoglycemia and frequent weight gain that continues over several years. Weight gain as a result of insulin therapy is a consistent finding of major trials (11, 16) and is often greater than that seen with other treatment regimes. Such weight gain may not only act as a barrier to patient compliance but may to some extent also oppose the beneficial health consequences of improved glycemic control.
Several explanations for the mechanism(s) by which IIT causes weight gain have been suggested, including hyperphagia following hypoglycemic stimuli, alteration of physical activity level, the anabolic and/or lipogenic actions of insulin, and/or decreased glycosuria (1, 5, 17, 19, 40). However, it has proven difficult to establish the mechanisms underlying weight gain in human studies due to the complex nature of potential mechanisms, difficulty in achieving optimal experimental glycemic control and detection of hypoglycemia, difficulty in accurately recording food intake and motor activity, and the ethical issues in constructing control groups with potentially suboptimal treatment regimes; the overall conclusion has been that the cause(s) of weight gain associated with IIT remain unclear (17). Weight gain appears to be associated with the incidence of repeated insulin-induced hypoglycemia (RH; see Refs. 12 and 35); however, this has not been directly studied. Moreover, the link between insulin therapy and increased weight appears somewhat paradoxical, given the well-established anorectic action of insulin on hypothalamically mediated feeding responses.
We previously reported (30) that an animal model of long-term, clinically relevant RH produced significant alteration of subsequent neural function. Consistent with the human literature, this model also produced markedly impaired neuroendocrine responses to further hypoglycemic episodes, which is the defining characteristic of hypoglycemia-associated autonomic failure seen in human diabetic patients following RH. It became clear that our RH protocol was causing significant weight gain and hence might also serve as an animal model for insulin-induced weight gain seen in human patients. Here, we present the first characterization of such a model, which is a further report on the same animals whose neural function and counterregulatory hormonal responses we previously described (30).
METHODS
Subjects.
Ninety eight male Sprague-Dawley rats (Charles River, Wilmington, MA) were studied, starting at 1 mo of age. Rats were individually housed, with food and water available ad libitum, on a 12:12-h light-dark schedule (lights on 7:00 A.M.). A standard laboratory chow was provided to all animals throughout, and room/cage temperature was maintained at 70°F. All procedures were approved by the Yale University Institutional Animal Care and Use Committee. After a 1-wk acclimatization period, animals received one injection per week, intraperitoneally, of either sterile saline (0.5 ml, control animals) or human insulin (Humulin, RH animals; Eli Lilly). The insulin dose was initially 10 U/kg, given in 0.5 ml saline, which in our hands lowers tail vein blood glucose to 30–40 mg/dl (e.g., see Refs. 13 and 29); insulin was given between 10:00 and 11:00 A.M. on the same day each week. RH animals were closely observed after induction of hypoglycemia, and food was withheld for 3 h. During a random subset of the weekly hypoglycemic episodes, blood glucose levels were monitored to confirm that hypoglycemia was consistently achieved. There were no instances of blood glucose levels below 30 mg/dl. Rats who became nonresponsive to mild tail pinch received 50% dextrose, intraperitoneally, to achieve target hypoglycemia; this occurred fewer than 10 times in total. No animal experienced coma or seizure. Insulin doses (but not volume) were gradually reduced to maintain this level of induced hypoglycemia; the RH rats required progressively less insulin to achieve target hypoglycemia. This most likely resulted in large part from loss of the counterregulatory response to induction of hypoglycemia (7). By the end of the experiment, the dose of insulin required to achieve and maintain rats at 30–40 mg/dl tail vein blood glucose for 3 h was only 0.5 U/kg.
Studies were carried out on animals at 4, 8, and 12 mo of age. The initial aim of this protocol was to examine the impact of weekly bouts of hypoglycemia on subsequent cognitive performance (30); hence, measurements of feeding and detailed weight gain were not performed between 1 and 4 mo. Therefore, an additional group of animals (n = 16) were used for a repeat of the weekly saline or insulin intraperitoneal injection protocol, with measurement of daily food intake, motor activity, and core temperature starting at 1 mo of age.
Surgery.
Some animals underwent one of two types of surgery for the placement of either 1) indwelling vascular catheters, as described previously (30), or 2) intraperitoneal minitransmitters (for acquisition of motor activity and core temperature data; MiniMitter). Animals received atropine sulfate (0.2 ml of 540 mg/ml solution, ip) followed by anesthesia with a ketamine-xylazine mix and were allowed to recover for 1 wk following surgery, before any testing or measurement, during which time they were handled extensively. Surgeries were not performed on the day of insulin administration nor on the following day.
Euthanasia.
Animals were killed with an overdose of pentobarbital sodium. Immediately following death, random subsets of animals were analyzed for body fat by differential X-ray absorption (DEXA), had their fat pads dissected and weighed, had their interscapular brown adipose tissue (BAT) removed for uncoupling protein (UCP) 1 mRNA analysis, or had their brains removed for later study. No animal was used for more than one of these measures to avoid confounds, minimize any impact of outlying individuals, and maximize confidence in the different measures. Animals were killed either 3 or 4 days after the day of RH, between 12:00 and 3:00 P.M.
Intravenous glucose tolerance test studies.
An intravenous glucose bolus (500 mg/kg) was administered through an indwelling catheter, followed by sampling of plasma glucose and insulin at 5, 10, 30, and 60 min. These measures were taken either 3 or 4 days after the day of RH, during the light cycle.
Arcuate mRNA measurements.
These measurements were taken at 12 mo of age, to maximize any long-term neural changes caused by RH. Immediately following pentobarbital overdose, brains were removed into chilled isopentane (−30°C) and then transferred to storage at −80°C until analyzed. Frozen brains were cut on a cryostat at −12°C in 300-μm sections. Cut sections were placed in RNA Later (Ambion) until micropunched using modifications (24, 25) of the method of Palkovits (33). Micropunched brain nuclei were sonicated in a guanadinium thiocyanate solution and purified using magnetic beads (MagMax-96; Ambion). Quantitation of mRNA was carried out by quantitative real-time reverse transcription-polymerase chain reaction as previously described (24). Briefly, genomic DNA was removed with DNase, and mRNA was reverse-transcribed with random hexamer priming using Superscript-3 (Invitrogen) and treated with RNaseH (Ambion). Primer sets for proopiomelanocortin (POMC) and neuropeptide Y (NPY) were designed by reference to published sequences, and their specificity was verified using Genebank: NPY forward TCGTGTGTTTGGGCATTCTG, reverse GCGGAGTAGTATCTGGCCATGT, probe ACAATCCGGGCGAGGA; POMC forward GGCGTGCGGAGGAAGAG, reverse GCCCTCCCGTGGACTTG, probe TGGCCGTCCGGAGC; and cyclophilin forward AATGGCACTGGTGGCAAGTC, reverse GCCAGGACCTGTATGCTTCAG, probe TCTACGGAGAGAAATT. Primers and their sequence-specific probes prepared by Applied Biosystems were sequenced and then quantified with an Applied Biosystems 7700 real-time PCR system set for 40 PCR cycles. Standard curves were generated from serially diluted pooled samples for each probe and for constitutively expressed mRNA (cyclophilin) to control for differences in amplification efficiency. Results were calculated from the standard curve relative to cyclophilin.
Fat pad/BAT measurements.
Immediately following pentobarbital overdose, the abdominal cavity was opened, and four fat pads were removed for immediate weighing: the mesenteric, perirenal, retroperitoneal, and epididymal pads; the perirenal and retroperitoneal pads were combined for ease of measurement. In separate animals, the interscapular fat pad was removed, and the central BAT section was dissected out on chilled glass plates and then immediately placed in chilled isopentane before being stored at −80°C until analysis.
DEXA body fat measurement.
Animals were studied at 8 mo old; at 12 mo, the larger RH animals were too large to fit into the scanner. Body composition was measured by DEXA (PiximusII; Lunar, Madison, WI) as a torso scan (the torso filled the scan field), separated into the body compartments fat mass, lean mass, and bone.
Thyroid hormone.
Total thyroxine (T4) was measured in 5 ml of rat serum as previously described (15). Briefly, each assay tube contained 100 ml barbital buffer (0.11 M sodium barbital, 0.1% wt/vol 8 anilino-1-napthalen-sulfonic acid sodium salt, 15% wt/vol bovine γ-globulin, Cohn fraction II, and 0.1% wt/vol gelatin, pH 8.6), 100 ml anti-T4 (rabbit; Sigma) diluted to a final concentration of 1:30,000, and 100 ml 125I-labeled T4 (12,000–15,000 counts/min; Perkin Elmer/NEN). Standards were prepared from T4 (Sigma) measured using a Cahn electrobalance; standards were run in triplicate, whereas samples were run in duplicate. Standards were calibrated to measure serum T4 levels from 0.4 to 25.6 mg/dl. Tubes were incubated at 37°C for 1 h and then chilled on wet ice for 30 min. Bound counts were precipitated by adding 300 ml ice-cold polyethylene glycol 6000 (20% wt/vol; Sigma). Tubes were centrifuged at 1,800 g for 20 min at 4°, the supernatant was aspirated, and the pellet was counted in a gamma counter (Packard Cobra II). The assay was run at 40–50% binding; nonspecific binding was generally below 8%.
BAT UCP1 mRNA measurement.
Total RNA was extracted from BAT according to the TRI Reagent protocol with minor modifications (6). Tissue was homogenized with Trizol reagent (GIBCO-BRL, Paisley, UK) (10 μl Trizol/mg tissue), and total RNA was precipitated with isopropanol after phase separation with chloroform (200 μl/ml Trizol). The aqueous phase was removed, and total RNA was precipitated with isopropanol. The subsequent RNA pellet was washed with 75% ethanol and then stored at −80°C in 100% ethanol until quantification.
Aliquots of RNA, reconstituted in 10 mM Tris·HCl and 0.1 mM EDTA, were denatured and read on a spectrophotometer (DU-640; Beckman Coulter, Fullerton, CA) at 230, 260, 270, 280, and 320 nm. Samples were fixed to nylon membranes according to the slot-blot method (31). After the membranes were soaked in six times standard sodium citrate (6× SSC), 2 μg of total RNA from each sample was dissolved in 7.4% formaldehyde and SSC and applied to a nylon membrane through a microfiltration apparatus. Membranes were shadowed with ultraviolet (UV) light to verify that the samples were evenly loaded and then UV cross-linked to fix the RNA to the membrane (39).
A random primer labeling kit (Stratagene, Cedar Creek, TX) was used to quantify UCP1 and β-actin mRNA on the nylon membranes from the slot blot. Membranes were prehybridized for 30 min at 68°C in QuikHybe Hybridization Solution (Stratagene, La Jolla, CA). For UCP1 hybridization, cDNA probes, graciously supplied by Dr. Daniel Riquier (Meudon, France), specific for rodent UCP1, were used. A purchased β-actin probe (ONCOR, Gaithersburg, MD) was used for β-actin hybridization. Membranes were hybridized for 1 h at 68°C in a medium containing QuikHybe Hybridization Solution, random oligonucleotide primers, 5× buffer, [32P]2-deoxycytidine 5′-triphosphate, Klenow polymerase, salmon testes DNA, and purified probes. After hybridization, the membranes were washed two times in 2× SSC, 0.1% sodium dodecyl sulfate (SDS) at room temperature and then washed again in 0.1× SSC, 0.1% SDS at 60°C. The membranes were placed in a cassette with a phosphor screen for 2 days. After exposure, the phosphor screens were scanned with a densitometer (Storm 860-PC Scanner; Molecular Dynamics, Sunnyvale, CA), and mRNA was quantified using ImageQuaNT version 5.0 software (Molecular Dynamics). Gene expression levels are expressed in optical density units. The membranes were then stripped of the UCP1 probe and radioactive label and washed with 10 mM Na2HPO4 for 1 h at 60°C followed by a second wash with 25 mM Na2HPO4 for 20 min at room temperature. After verifying by phosphor screen exposure that the radioactivity was completely removed from the membranes, the membranes were then hybridized with the β-actin probe, placed in a cassette with a phosphor screen, and quantified as described above. The data are represented as a ratio of UCP1 mRNA and β-actin mRNA to account for nonspecific changes in gene expression and potential variability during loading of the samples during the slot-blot procedure.
Food intake.
In animals whose food intake was measured, the standard chow was replaced with a powdered chow of identical composition that was given in a fixed glass bowl designed to prevent spillage and attached to the cage base with adhesive; pilot tests with additional (untreated) animals in cages without bedding, to allow observation of any spillage, suggested that spillage was minimal at most (below 1%), a conclusion supported by the consistent consumption measurements across days. Food was weighed each morning and replenished. Food intake was not measured at 12 mo, as no group differences were seen at 4 or 8 mo. On the rare occasion where spillage was observed (n = 2), the data from that cage for that day were discarded.
Motor activity/core body temperature monitoring.
To allow for objective monitoring of animals' motor activity and core body temperature, implanted intraperitoneal minitransmitters (MiniMitter) were used. After a 1-wk postsurgery recovery period, data were collected continuously via a platform mounted underneath each cage (the animals remaining in their home cages throughout), with measures of activity and temperature collected every minute. Following baseline measurements, animals were placed in a chilled room (ambient temperature held at 4°C) to allow measurement of core temperature response to a cold challenge.
Statistical analysis.
Analysis was done using Excel and/or PRISM, with an a priori alpha level set at 0.05. Weight gain data, insulin sensitivity data, motor activity data, and core temperature data were analyzed using repeated-measures two-way ANOVA. UCP mRNA comparison used a single t-test, and comparison of fat pat weights at 8 mo used single t-tests Bonferroni-corrected for multiple comparisons. Food-intake data were analyzed between groups by week using a repeated-measure two-way ANOVA and by day within the early weeks of RH treatment using a simple t-test comparing day of treatment against all other days combined. Follow-up comparisons between groups, where done, used Bonferroni-corrected t-tests.
RESULTS
Body weight.
Animals in the RH group became significantly heavier than did control animals, with significant differences observed as early as 4 wk after the start of treatment. Average body weights at 4, 8, and 12 mo of age are shown in Fig. 1A. At all ages, there was a complete separation of animal weights between the two groups, with all animals in the RH groups being heavier than every animal in the saline control group. A median-weight member of each group, at 8 mo, is shown in Fig. 1B. Control animals' weight plateaued at 8 mo, but that of RH animals continued to increase between 8 and 12 mo.
Fig. 1.
A: mean weight at 4, 8, and 12 mo of age; error bars show SE. Weights of animals at 4 wk (before treatment) are shown as solid black bars below the shaded weight gain bars and did not differ. At all ages, there was a complete separation of weight gain between groups, so that all repeated insulin-induced hypoglycemia (RH) animals were heavier than all control animals. There were significant effects of both age and treatment on weight gain, as well as an interaction between treatment and age: control animals' mean weight was significantly lower than that of RH animals at all ages (all P < 0.0001). B: median-weight animals from the control (top left) and RH (bottom right) groups are pictured at 8 mo to illustrate differences in body size.
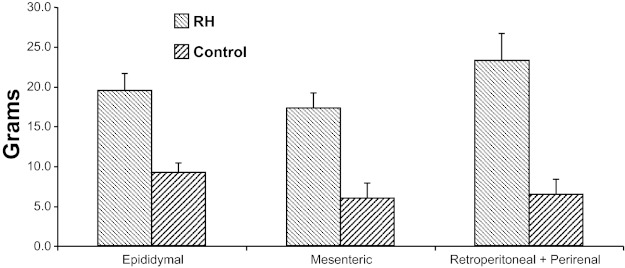
Fat mass.
RH animals' greater weight was reflected in significantly greater fat pad mass. Data at 8 mo are shown in Fig. 2; RH animals showed increases in the size of all pads, with their combined weight averaging 280% that of controls; by 12 mo, the RH animals' fat pad weights averaged 420% of control values (both 8- and 12-mo group differences, P < 0.0001). The group differences in fat mass were confirmed by DEXA measurements at 8 mo: control animals had an average percentage fat of 14 ± 1%, whereas RH animals had 25 ± 2% body fat (P < 0.0001).
Fig. 2.
Fat pad weights from control and RH animals at 8 mo old (data at 12 mo were similar; see text). RH animals had more fat than control animals in all fat pad locations; P < 0.0001 for all comparisons.
Insulin sensitivity.
As noted above, the dose of insulin needed to produce target hypoglycemia decreased across the study, from 10 U/kg to an average of 0.5 U/kg at 12 mo, suggesting that the weight gain associated with the RH protocol might not be associated with insulin resistance. This issue was evaluated at 8 mo using an intravenous glucose tolerance test (IVGTT). No group differences were seen in the IVGTT, with average plasma glucose returning to baseline by 30 min after glucose bolus administration in both groups and no difference in plasma glucose or insulin profiles (Fig. 3). As previously reported (30), fasting plasma glucose and insulin did not differ between groups at any age. The failure to observe any group difference either in the IVGTT or using the homeostasis model assessment (HOMA) model of insulin resistance (26) suggests that there was no group difference in systemic insulin sensitivity, although it is possible that any difference was below the sensitivity of these measures.
Fig. 3.
Plasma glucose and insulin levels in 8-mo-old animals in response to an iv glucose bolus given at 0 min. Repeated-measures ANOVA showed no effect of group on either glucose or insulin data.
We confirmed that the reduction in insulin dose required to produce the weekly hypoglycemic bouts was not the result of any conditioning to the injection per se by administering a saline injection to 12-mo-old RH animals at the time when they would have expected to receive their weekly insulin injection: this had no effect on plasma glucose (−5 ± 4 mg/dl), comparable to the lack of effect in control animals [2 ± 3 mg/dl, P = not significant (NS)].
Counterregulatory hormone response to hypoglycemia.
These data have been previously reported (30), along with characterization of the impact of RH on cognitive and hippocampal function, and are hence not described in detail here: RH led to the expected marked reduction in hormone release to subsequent hypoglycemia.
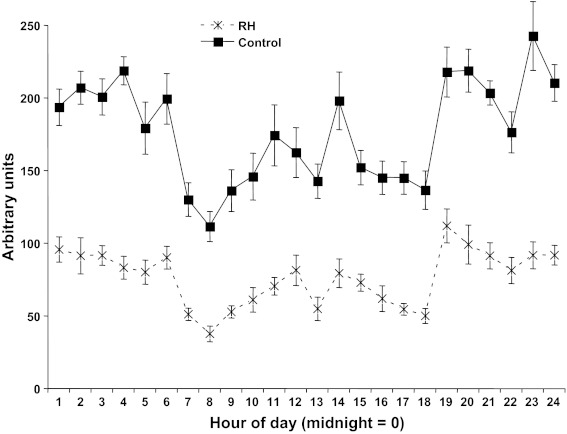
Motor activity.
At 4 mo, motor activity level was extremely variable, both inter- and intraanimal, even when averaged across several days, and no group effects were observed. However, in the 12-mo animals, variance was greatly reduced, and several clear effects emerged (Fig. 4). Both RH and control animals showed an increase in activity during the hours of darkness (1–6 and 18–24), but control animals were significantly more active than RH animals at all times, with control rats' overall average activity increase across the 24-h cycle being 232% that of RH animals.
Fig. 4.
Motor activity, in arbitrary units of distance traveled, at 12 mo of age. RH animals were significantly less active than control animals throughout the diurnal cycle (effect of group, P < 0.0001), with both groups showing reduced activity during the light portion of the cycle (hours 7–18; P < 0.0001 for effect of time on activity but no significant interaction between group and time).
Thyroid hormone.
Measurements of serum T4 at 12 mo showed no significant group difference (controls 36.2 ± 2.5 ng/ml, RH 32.9 ± 2.7 ng/ml, P = NS).
Core temperature.
At baseline, there were no group differences in core body temperature in either 4- or 12-mo animals (all means between 37.0 and 37.3°C). At 4 mo, there was similarly no group difference in response to being placed in an ambient temperature of 4°C. However, in 12-mo animals, a small but significant group difference was seen (Fig. 5A): with prolonged exposure to cold, control animals raised their core temperature while the core temperature of RH animals did not change (P < 0.0001).
Fig. 5.
A: core body temperature of 12-mo-old rats after being placed in a 4°C chamber at time 0. Control animals raised their core temperature significantly more than did RH animals (P < 0.001 for interaction between group and time). B: uncoupling protein 1 (UCP1) mRNA in the brown adipose tissue (BAT) of 12-mo-old rats, standardized to β-actin expression. UCP1 mRNA was significantly higher in BAT from control animals than from RH animals, P < 0.01.
BAT UCP1 mRNA.
UCP1, within the BAT, is a major contributor to thermogenesis (and hence energy use) in the rat. Hence, we quantified UCP1 mRNA in BAT from 12-mo-old RH and control animals. BAT from RH animals showed a significant reduction in expression of UCP1 mRNA relative to that of β-actin, corrected for sample weight, with a relative mRNA abundance of 44 ± 5 in controls vs. 23 ± 4 in RH animals (P < 0.01, Fig. 5B). Importantly, this group difference was also significant when calculated as total UCP1 mRNA per pad, which avoids any confound due to a difference in white adipose infiltration (arbitrary units: 6.7 ± 0.5 for controls vs. 4.5 ± 0.4 for RH, P < 0.01).
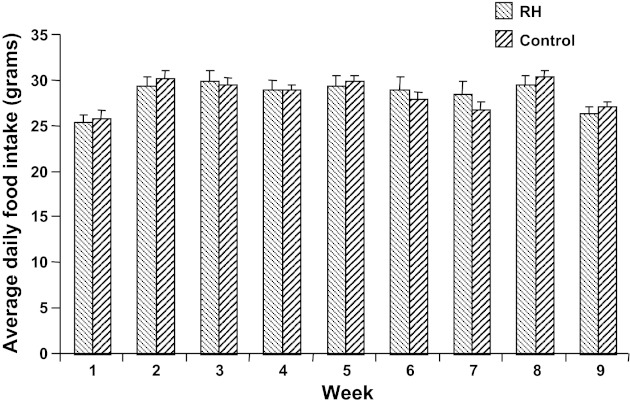
Food intake.
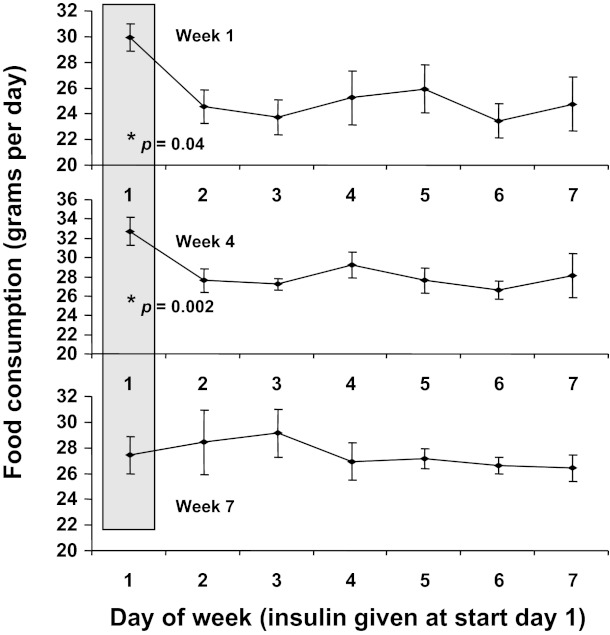
No group differences were seen in total food intake, neither during the early stages of treatment (Fig. 6) nor at 8 mo (RH average intake 30.4 ± 1.1 g/day, controls average intake 30.7 ± 1.2 g/day, P = NS). Initially, RH animals showed a hyperphagic response during at least a portion of the 24 h following insulin treatment but compensated for this by eating less than the control animals on other days; this pattern persisted for the first 4 wk of treatment, but the hyperphagic response to hypoglycemia then attenuated (consistent with the observed reduction in other autonomic response to repeated hypoglycemia) until by week 7, as shown in Fig. 7, there was no difference in food consumption in the 24-h posthypoglycemia from that on other days. The absence of posthypoglycemic hyperphagia persisted thereafter.
Fig. 6.
Average daily food intake, by week, starting at 1 mo of age. No group effect was observed at any time point, nor was there an effect of time (effects of week and of group, both P > 0.05).
Fig. 7.
Daily food intake of RH animals during weeks 1, 4, and 7 of treatment (week 1 being at 1 mo of age). Shaded column indicates the day immediately following insulin-induced hypoglycemia. Intake on this day was significantly higher than on the remaining days during weeks 1 through 4 (P < 0.05, simple t-test for day of treatment vs. pooled other days) but not during week 7 or thereafter.
Hypothalamic mRNA.
NPY activity within the hypothalamic arcuate nucleus has a well-established orexigenic role and is increased during glucoprivic feeding; hence, we measured arcuate NPY as well as POMC mRNA in 12-mo-old RH and control animals. Consistent with the absence of group differences in feeding, there were no group effects on abundance of either mRNA: standardized to cyclophilin, NPY had a relative abundance of 0.80 ± 0.04 (RH) vs. 0.84 ± 0.11 (controls), whereas POMC had relative abundances of 0.86 ± 0.15 (RH) and 0.86 ± 0.09 (controls; all P = NS).
DISCUSSION
The data presented here characterize a novel animal model of the weight gain associated with insulin-induced hypoglycemia. Such weight gain is seen, in human diabetic patients, across many months or years, and this is matched by the chronic weight gain of the RH animals in the present experiment. Our protocol was designed to mimic the RH seen in such patients, with a frequency of hypoglycemia comparable to or less than that reported in clinical studies (e.g., see Refs. 5 and 19). After 11 mo of RH, the insulin-treated animals had a mean body mass of 951 vs. 543 g for their control counterparts, an increase of 75%. The amounts of insulin given to the RH animals during the later stages of treatment were small, but weight gain continued. One possibility is that the early period during which animals responded to hypoglycemia with bouts of hyperphagia (shown in Fig. 7) may act to prime adipocytes and lead to long-term fat accumulation, even in the absence of altered overall food consumption (Fig. 6). There are some data to suggest that body composition is altered in rats who feed during the light phase and/or in single bouts (which would be the case for hypoglycemia-induced binge feeding in the present experiment) rather than the more normal dark-phase feeding, including possible increase in adipocyte proliferation (4, 14, 38), although the impact on long-term obesity is uncertain (34). Such an effect would be expected to cause obesity to persist even after cessation of RH, a possibility that we did not address. Our data are also consistent with the possibility that hypoglycemia per se plays a role in at least the maintenance of weight gain: this is true for, for example, the loss of some counterregulatory responses after non-insulin-induced hypoglycemia (27). Animal models of obesity commonly exhibit systemic insulin resistance (e.g., see Ref. 23), which was not evident in the present model with RH and control animals showing similar levels of insulin sensitivity; this suggests that weekly, insulin-induced hypoglycemic episodes may provide a more appropriate model for weight gain seen with IIT, as human studies suggest that responses to insulin administration are maintained or improved with such therapy (2). We have previously reported that the RH animals examined here had markedly impaired neuroendocrine responses to hypoglycemia compared with control animals, again consistent with the sequelae of IIT in human diabetic patients (30). Specifically, the RH animals in this study had markedly reduced counterregulatory release of epinephrine to further hypoglycemia (a reduction of roughly 75% compared with control animals), which we have reported previously in the context of cognitive studies (30). This model has the further advantage of not requiring long-term management of diabetic animals; our previous work on RH and cognition suggests that the impact of long-term RH is similar in diabetic and control animals (29), but it is possible that the effect of RH on weight gain might differ in the context of underlying diabetes.
Our findings suggest that RH produces both profound weight gain and a significant increase in body fat percentage; moreover, the data suggest that the weight gain observed is a consequence of reduced energy expenditure and/or altered adipose storage rather than any increase in food intake. RH animals showed a complete attenuation of hypoglycemia-induced hyperphagia by week 7 of treatment and compensated for the initial treatment-associated hyperphagia with reduced food consumption at other times during the week between hypoglycemic episodes, so that average food consumption was identical in RH and saline-treated animals at all times measured, although the pattern of food consumption likely varied in the first few weeks of treatment, which may have primed future adipose storage (14, 34, 38). This finding is consistent with the extensive literature on impaired responses to hypoglycemia after RH, and the relatively long time course of the attenuation in hyperphagia (several weeks) is consistent with the time course of reduced response to hypoglycemia in human diabetic patients (e.g., see Ref. 32). Our data also are consistent with several clinical studies that have reported a loss of hunger feelings during hypoglycemia after RH and others in which an increase in weight accompanied a decrease in food intake (1, 17, 19). In addition, the failure to see any group difference in hypothalamic mRNA expression for the feeding-related peptides NPY and POMC supports the conclusion that the observed weight gain in RH animals is not as a result of altered food intake. Our data are in contrast to a recent report that feeding responses are maintained after RH; in that study, however, the model of RH used was only a 2-day treatment with daily 6- to 8-h hypoglycemic periods (37). Given that RH produces marked alteration of hippocampal function (28–30), it is interesting and consistent with the present findings that several recent studies have suggested that the hippocampus may play a significant role in regulation of metabolic control (8–10, 22, 36).
In contrast to the feeding data, RH animals showed significant reductions in several estimates of energy expenditure. Twelve-month-old RH animals showed a large reduction in spontaneous motor activity during all phases of the day-night cycle. Importantly, this difference was not due to any incapacity in the RH animals: we showed that the two groups of animals have identical motor activity, at all ages studied, when placed in a novel (cognitive testing) environment (30). Thus, the reduced spontaneous activity of RH animals is unlikely to be due to any physical impairment but is rather due to altered internal drive. Animals were also closely monitored and handled, with no sign of impaired mobility observed in any animal. Although the RH animals became larger, hence requiring additional energy expenditure for a given amount of motor activity, the reduction in activity appears to be greater than could be explained by this variable alone: at 12 mo, RH animals were 69.5% heavier on average than control animals, but control animals' activity was 132% higher than that of the RH animals. Intriguingly, the motor activity data are consistent with data in humans, suggesting that insulin therapy may increase weight initially primarily via increased fat deposition, with a shift to acting via a decrease in energy expenditure after roughly 6 mo (19); other investigators have also found that reduction in energy expenditure is a primary mechanism for weight gain (5). Reduced motor activity would tend to lower lean body mass, hence potentially lowering resting metabolic rate and further promoting weight gain in the relatively inactive RH animals. Notwithstanding the higher fat mass, RH animals also had greater lean mass than their control counterparts, so that relatively lower energy maintenance requirement of adipose tissue would not, alone, explain continued weight gain in the RH animals in the absence of hyperphagia; however, insulin-induced lipogenesis may also affect subsequent metabolic efficiency (19, 20). A further contributing factor to initial fat gain in the RH animals, and perhaps to ongoing lowered energy usage, might have been altered metabolic rate caused by early lipogenesis from repeated insulin administration (19, 20). One possible explanation for altered energy expenditure might have been an alteration in thyroid function, and it has been recently suggested that thyroid hormone may regulate both insulin signaling and hippocampal function (21). However, we found no difference in serum thyroid (T4) between the two groups, although it is conceivable that differences between groups in T4 to triiodothyronine conversion might exist. The fact that the dose of insulin required to induce hypoglycemia decreased markedly across the study appears to reflect loss of counterregulation rather than increased insulin sensitivity, given that no group difference in IVGTT or HOMA was observed; of interest, the loss of hypoglycemia-induced hyperphagia shown in Fig. 7 occurred by 7 wk of treatment, whereas the reduction in insulin dosage occurred fairly gradually over the 12 mo of study.
In addition to the reduction in spontaneous motor activity, RH animals showed a reduced level of UCP mRNA in the interscapular BAT and had lower core body temperature (at 12 mo) in response to a cold challenge. RH animals may have had reduced central thermal stress because of additional insulation from their fat mass, but that same insulation would presumably act to retain more heat once produced. The difference in core temperature was small, however, and neither group showed a decrease in temperature below baseline in response to the cold challenge, so impairment in thermogenic regulation in the RH animals is unlikely to be the major determinant of obesity in these animals. However, both metabolic and motor activity-related energy expenditure appear to be reduced following RH. Recent work suggests that the difference in BAT may, perhaps, be at least in part a consequence of the reduced activity level in the RH animals (3). Future studies, involving long-term indirect calorimetry, may allow for more precise measurements of the exact causes of the observed RH-induced obesity.
The multifaceted pattern of deficits seen in response to this model of RH, with impaired neuroendocrine (30), behavioral (feeding), motor, and thermogenic responses, suggests that the impact of RH on homeostasis may be a general phenomenon with multiple physiological bases, possibly associated with reduced sympathetic tone (18) that would itself be a potential contributor to weight gain, rather than simply impairing counterregulatory responses to subsequent hypoglycemic events. While there are important differences between rodents and humans with regard to energy balance (notably, the reduction of BAT in adult humans), the present protocol of chronic RH appears to offer many useful features as an experimental model of the weight gain associated with insulin therapy.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-077106 (to E. C. McNay) and DK-20495 and P30 DK-45735 (to R. S. Sherwin).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: E.C.M., R.J.M., and R.S.S. conception and design of research; E.C.M., J.A.T., C.M.K., A.D.-M., and R.J.M. performed experiments; E.C.M., J.A.T., C.M.K., A.D.-M., B.E.L., R.J.M., and R.S.S. analyzed data; E.C.M., J.A.T., C.M.K., A.D.-M., B.E.L., R.J.M., and R.S.S. interpreted results of experiments; E.C.M. and J.A.T. prepared figures; E.C.M. and R.S.S. drafted manuscript; E.C.M., J.A.T., C.M.K., A.D.-M., B.E.L., R.J.M., and R.S.S. edited and revised manuscript; E.C.M., J.A.T., C.M.K., A.D.-M., B.E.L., R.J.M., and R.S.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Yuyan Ding and WanLing Zhu for expert surgical preparation and assistance with long-term animal care and Amin Danai and Dr. R. Thomas Zoeller for conducting the thyroid measurements.
REFERENCES
- 1.Bagg W, Plank L, Gamble G, Drury P, Sharpe N, Braatvedt G. The effects of intensive glycaemic control on body composition in patients with type 2 diabetes. Diabetes Obesity Metab 3: 410–416, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Beck-Nielsen H, Richelsen B, HAsling C, Nielsen O, Heding L, Sorensen N. Improved in vivo insulin effect during subcutaneous insulin infusion in patients with IDDM. Diabetes 33: 832–837, 1984 [DOI] [PubMed] [Google Scholar]
- 3.Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Bostrom EA, Choi JH, Long JZ, Kajimura S, Zingaretti MC, Vind BF, Tu H, Cinti S, Hojlund K, Gygi SP, Spiegelman BM. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 481: 463–468, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braun T, Kazdova L, Fabry P, Lojda Z, Hromadkova V. Meal eating and refeeding after a single fast as a stimulus for increasing the number of fat cells in abdominal adipose tissue of rats. Metabolism 17: 825–832, 1968 [DOI] [PubMed] [Google Scholar]
- 5.Carlson M, Campbell P. Intensive insulin therapy and weight gain in IDDM. Diabetes 42: 1700–1707, 1993 [DOI] [PubMed] [Google Scholar]
- 6.Chomczynski P. A reagent for the single-step simultaneous isolation of RNA, DNA and proteins from cell and tissue sample. Biotechniques 15: 532–537, 1993 [PubMed] [Google Scholar]
- 7.Cryer PE. Hypoglycaemia-associated autonomic failure. In: Hypoglycaemia and Diabetes: Clinical and Physiological Aspects, edited by Frier BM, Fisher BM. London, UK: Arnold, 1993, p. 275– 283 [Google Scholar]
- 8.Davidson TL, Chan K, Jarrard LE, Kanoski SE, Clegg DJ, Benoit SC. Contributions of the hippocampus and medial prefrontal cortex to energy and body weight regulation. Hippocampus 19: 235–252, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davidson TL, Kanoski SE, Chan K, Clegg DJ, Benoit SC, Jarrard LE. Hippocampal lesions impair retention of discriminative responding based on energy state cues. Behav Neurosci 124: 97–105, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davidson TL, Kanoski SE, Schier LA, Clegg DJ, Benoit SC. A potential role for the hippocampus in energy intake and body weight regulation. Curr Opin Pharmacol 7: 613–616, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diabetes Care and Complications Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in diabetes mellitus. N Engl J Med 329: 977–986, 1993 [DOI] [PubMed] [Google Scholar]
- 12.Diabetes Care and Complications Group Hypoglycemia in the Diabetes Control and Complications Trial. Diabetes 46: 271–286, 1997 [PubMed] [Google Scholar]
- 13.Flanagan DE, Keshavarz T, Evans ML, Flanagan S, Fan X, Jacob RJ, Sherwin RS. Role of corticotrophin-releasing hormone in the impairment of counterregulatory responses to hypoglycemia. Diabetes 52: 605–613, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Fuller RW, Diller ER. Diurnal variation of liver glycogen and plasma free fatty acids in rats fed ad libitum or single daily meal. Metabolism 19: 226–229, 1970 [DOI] [PubMed] [Google Scholar]
- 15.Gauger KJ, Kato Y, Haraguchi K, Lehmler HJ, Robertson LW, Bansal R, Zoeller RT. Polychlorinated biphenyls (PCBs) exert thyroid hormone-like effects in the fetal rat brain but do not bind to thyroid hormone receptors. Environ Health Perspect 112: 516–523, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Group UKPDS UKPDS. Diabetologia 34: 877–890, 1991 [PubMed] [Google Scholar]
- 17.Heller S. Weight gain during insulin therapy in patients with type 2 diabetes mellitus. Diabetes Res Clin Pract 65: S23–S27, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Herlein J, Morgan D, Phillips B, Haynes W, Sivitz W. Antecedent hypoglycemia, catecholamine depletion, and subsequent neural responses. Endocrinology 147: 2781–2788, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Jacob A, Salinas K, Adams-Huet B, Raskin P. Potential causes of weight gain in type 1 diabetes mellitus. Diabetes Obesity Metab 8: 404–411, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Jacob AN, Salinas K, Adams-Huet B, Raskin P. Weight gain in type 2 diabetes mellitus. Diabetes Obes Metab 9: 386–393, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Jahagirdar V, McNay EC. Thyroid hormone's role in regulating brain glucose metabolism and potentially modulating hippocampal cognitive processes. Metab Brain Dis 27: 101–111, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanoski SE. Cognitive and neuronal systems underlying obesity. Physiol Behav 106: 337–344, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levin BE, Dunn-Meynell AA, Balkan B, Keesey R. Selective breeding for diet-induced obesity and insulin resistance in Sprague-Dawley rats. Am J Physiol Regul Integr Comp Physiol 273: R725–R730, 1997 [DOI] [PubMed] [Google Scholar]
- 24.Levin BE, Dunn-Meynell AA, Banks WA. Obesity-prone rats have normal blood-brain barrier transport but defective central leptin signaling prior to obesity onset. Am J Physiol Regul Integr Comp Physiol 286: R143–R150, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Levin BE, Sullivan AC. Catecholamine levels in discrete brain nuclei of seven month old genetically obese rats. Pharmacol Biochem Behav 11: 77–82, 1979 [DOI] [PubMed] [Google Scholar]
- 26.Matthews D, Hosker J, Rudenski A, Naylor B, Treacher D, Turner R. Homeostasis model asessment: insulin resistance and beta-cell function from fasting glucose and insulin concentrations in man. Diabetologia 28: 412–419, 1985 [DOI] [PubMed] [Google Scholar]
- 27.McCrimmon RJ, Evans ML, Jacob RJ, Fan X, Zhu Y, Shulman GI, Sherwin RS. AICAR and phlorizin reverse the hypoglycemia-specific defect in glucagon secretion in diabetic BB rats. Am J Physiol Endocrinol Metab 283: E1076–E1083, 2002 [DOI] [PubMed] [Google Scholar]
- 28.McNay E, Cotero V. Mini-review: impact of recurrent hypoglycemia on cognitive and brain function. Physiol Behav 100: 234–238, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McNay EC, Sherwin RS. Effect of recurrent hypoglycemia on spatial cognition and cognitive metabolism in normal and diabetic rats. Diabetes 53: 418–425, 2004 [DOI] [PubMed] [Google Scholar]
- 30.McNay EC, Williamson A, McCrimmon RJ, Sherwin RS. Cognitive and neural hippocampal effects of long-term moderate recurrent hypoglycemia. Diabetes 55: 1088–1095, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Milner R, Trayhurn P. Rapid quantitation of uncoupling protein in brown adipose tissue mitochondria by a dot immunobinding (“dot blot”) procedure: application to the measurement of uncoupling protein in Richardson's ground squirrel, rats, and mice. Biochem Cell Biol 68: 973–979, 1990 [DOI] [PubMed] [Google Scholar]
- 32.Mokan M, Mitrakou A, Veneman T, Ryan C, Korytkowski M, Cryer P, Gerich J. Hypoglycemia unawareness in IDDM. Diabetes Care 17: 1397–1403, 1994 [DOI] [PubMed] [Google Scholar]
- 33.Palkovits M. Isolated removal of hypothalamic or other brain nuclei of the rat. Brain Res 59: 449–450, 1973 [DOI] [PubMed] [Google Scholar]
- 34.Philippens KM, von Mayersbach H, Scheving LE. Effects of the scheduling of meal-feeding at different phases of the circadian system in rats. J Nutr 107: 176–193, 1977 [DOI] [PubMed] [Google Scholar]
- 35.Purnell J, Hokanson J, Marcovina S, Steffes M, Clearly P, Brunzell J. Effect of excessive weight gain with intensive therapy of type 1 diabetes on lipid levels and blood pressure: results from the DCCT. J Am Med Assoc 280: 140–146, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruiz N, Pacheco LF, Farrell B, Cox CB, Ermolinsky BS, Garrido-Sanabria ER, Nair S. Metabolic gene expression changes in the hippocampus of obese epileptic male rats in the pilocarpine model of temporal lobe epilepsy. Brain Res 1426: 86–95, 2011 [DOI] [PubMed] [Google Scholar]
- 37.Sanders N, Figlewicz D, Taborsky G, Wilkinson C, Daumen W, Levin B. Feeding and neuroendocrine responses after recurrent insulin-induced hypoglycemia. Physiol Behav 87: 700–706, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Sitren HS, Stevenson NR. The effects of meal-feeding at different times of the day on daily changes in serum insulin, gastrin and liver enzymes in the rat. J Nutr 108: 1393–1401, 1978 [DOI] [PubMed] [Google Scholar]
- 39.Thurston S, Saffer J. Ultraviolet shadowing nucleic acids on nylon membranes. Anal Biochem 178: 41–42, 1989 [DOI] [PubMed] [Google Scholar]
- 40.Torbay N, Bracco E, Geliebter A, Stewart I, Hashim S. Insulin increases body fat despite control of food intake and physical activity. Am J Physiol Regul Integr Comp Physiol 248: R120–R124, 1985 [DOI] [PubMed] [Google Scholar]