Abstract
During spermatogenesis, extensive restructuring takes place at the cell-cell interface since developing germ cells migrate progressively from the basal to the adluminal compartment of the seminiferous epithelium. Since germ cells per se are not motile cells, their movement relies almost exclusively on the Sertoli cell. Nonetheless, extensive exchanges in signaling take place between these cells in the seminiferous epithelium. c-Yes, a nonreceptor protein tyrosine kinase belonging to the Src family kinases (SFKs) and a crucial signaling protein, was recently shown to be upregulated at the Sertoli cell-cell interface at the blood-testis barrier (BTB) at stages VIII–IX of the seminiferous epithelial cycle of spermatogenesis. It was also highly expressed at the Sertoli cell-spermatid interface known as apical ectoplasmic specialization (apical ES) at stage V to early stage VIII of the epithelial cycle during spermiogenesis. Herein, it was shown that the knockdown of c-Yes by RNAi in vitro and in vivo affected both Sertoli cell adhesion at the BTB and spermatid adhesion at the apical ES, causing a disruption of the Sertoli cell tight junction-permeability barrier function, germ cell loss from the seminiferous epithelium, and also a loss of spermatid polarity. These effects were shown to be mediated by changes in distribution and/or localization of adhesion proteins at the BTB (e.g., occludin, N-cadherin) and at the apical ES (e.g., nectin-3) and possibly the result of changes in the underlying actin filaments at the BTB and the apical ES. These findings implicate that c-Yes is a likely target of male contraceptive research.
Keywords: testis, c-Yes, nonreceptor protein tyrosine kinase, Sertoli cell, spermatogenesis, seminiferous epithelial cycle, ectoplasmic specialization
during spermatogenesis, extensive restructuring and communication take place at the Sertoli cell-cell and Sertoli-germ cell interface across the seminiferous epithelium to accommodate germ cell movement and to coordinate cellular events that occur simultaneously at different stages of the seminiferous epithelial cycle in the mammalian testis (12, 23, 48). For instance, at stage VIII of the epithelial cycle, preleptotene spermatocytes, differentiated from type B spermatogonia, residing in the basal compartment traverse the blood-testis barrier (BTB) to enter the adluminal compartment while transforming to leptotene and zygote spermatocytes to prepare for meiosis I/II (23, 48). However, fully developed spermatids that become spermatozoa at the adluminal edge of the tubule lumen undergo spermiation; the release of sperm also takes place at stage VIII of the cycle, involving degeneration of the apical ectoplasmic specialization (apical ES) at the Sertoli-spermatid interface in rat testes (11, 13, 50). Recent studies have shown that these events, such as those that occur at stage VIII of the epithelial cycle, are coordinated by a local functional axis known as the apical ES-BTB-hemidesmosome in the seminiferous epithelium (77). In short, biologically active fragments released from laminin-β3 or -γ3 chains at the apical ES at spermiation during degeneration of the apical ES perturb the BTB function, inducing its restructuring, which in turn facilitates the transit of preleptotene spermatocytes across the BTB (13). However, the signaling molecule(s) that regulates these events in the apical ES-BTB axis remains unknown. Herein, we demonstrate the critical role of c-Yes in regulating BTB dynamics as well as cell adhesion at the apical ES, illustrating c-Yes is an important signaling molecule in this axis.
c-Yes (or Yes 1, Yamaguchi sarcoma viral oncogene homolog 1), a nonreceptor protein tyrosine kinase and a member of the Src family kinases (SFKs) and also a proto-oncogene whose expression is upregulated in various cancers, including melanoma (29), colon cancer (58), and brain cancer (53), is also known to be involved in regulating cell cycle and cytokinesis (28). In fact, members of the SFKs are targets of chemotherapy (1, 67). Moreover, members of SFKs, including c-Yes, are found ubiquitously in mammalian cells, including Sertoli and germ cells, and are known to be involved in normal cellular function (11, 15, 68). For instance, it has been shown that c-Yes is involved in regulating junction dynamics at the Sertoli cell BTB, such as protein endocytosis (76). Unlike c-Src, which shuttles between plasma membrane and late endosome/lysosomes, c-Yes is exocytosed to plasma membrane via the Golgi pool of caveolin, which is a protein transcytosis markers in tissues, including testis (59, 60), illustrating that c-Yes is likely involved in endocytic vesicle-mediated protein trafficking. In polarized epithelial cells, c-Yes is required for the transcytosis of polymeric immunoglobulin A, and interstingly, the deficiency of c-Yes cannot be substituted by c-Src (44, 66), even though both c-Yes and c-Src are members of the SFKs. Expression pattern of c-Yes in the seminiferous epithelium during the epithelial cycle is also distinctly different from that of c-Src at the BTB and the apical ES in adult rat testes. For instance, the expression of c-Yes was at its lowest at the apical ES but highest at the BTB at stages VIII and IX (76), coinciding with the timing of apical ES disruption and BTB restructuring, respectively. Indeed, c-Yes interacts physically with BTB proteins, such as the tight junction (TJ) protein occludin and the basal ES protein N-cadherin, as well as with apical ES proteins, such as β1-integrin (76). Inhibition of c-Yes activity in cultured primary Sertoli cells with an established functional TJ barrier by either a selective c-Yes inhibitor or its downregulation by TGFβ3, a regulator of BTB dynamics (43), was found to induce an increase in internalization of occludin and N-cadherin, thereby redistributing these proteins from the cell-cell interface into the cell cytosol, destabilizing the Sertoli cell BTB, leading to TJ barrier disruption (76). Herein, we report that the use of siRNA to knockdown c-Yes was found to perturb the Sertoli cell TJ barrier integrity, and it also affected apical ES adhesion, illustrating that c-Yes is an important signaling molecule in the apical ES-BTB functional axis that coordinates cellular events across the epithelium during the epithelial cycle. More importantly, it showed that c-Yes mediated its effects via changes in the localization of junction proteins (e.g., occludin, nectin-3), polarity proteins [e.g., partitioning-defective protein 6 (PAR6)], and signaling proteins [e.g., focal adhesion kinase (FAK)], illustrating that c-Yes plays a crucial role in coordinating other regulatory proteins at the BTB and apical ES to regulate cell adhesion function.
MATERIALS AND METHODS
Animals.
Male Sprague-Dawley rats of 20 and 60–70 days of age (at ∼250–300 g body wt) were purchased from Charles River Laboratories (Kingston, NY). Pups were used for preparation of Sertoli cell cultures. Adult rats at 60–70 days of age were used for in vivo c-Yes knockdown experiments. The use of animals was approved by The Rockefeller University Institutional Animal Care and Use Committee (with protocol nos. 09-016 and 12-506).
Primary Sertoli cell cultures.
Sertoli cells isolated from testes of 20-day old rats were cultured in serum-free DMEM-F-12 medium (Sigma-Aldrich, St. Louis, MO) supplemented with different growth factors at 35°C with 95% air-5% CO2 (vol/vol) in a humidified atmosphere as described (47). Freshly isolated Sertoli cells were seeded at a density of 1) 0.5 × 106 cells/cm2 in 12-well dishes for lysate preparation, 2) 1.2 × 106 cells/cm2 on Millicell cell culture inserts (surface area, 0.6-cm2; EMD Millipore, Billerica, MA) for transepithelial electrical resistance (TER) measurements to assess the TJ-permeability barrier function, or 3) 0.05 × 106 cells/cm2 on 18-mm-diameter circle cover glasses (Thomas Scientific, Swedesboro, NJ) for epifluorescence microscopy at time 0 and cultured in DMEM-F-12 as described (47). About 36 h thereafter, cultures were subjected to brief hypotonic treatment, using 20 mM Tris, pH 7.4, at 22°C for 2.5 min as described (19) to lyse residual germ cells, and Sertoli cells were rinsed twice with DMEM-F-12 to remove Tris buffer and lysed cellular debris. These cultures were >98% pure, with negligible contaminations of either Leydig cells, peritubular myoid cells, or germ cells using specific markers for these cell types by either immunoblotting or RT-PCR, using the corresponding specific antibodies or primers, as detailed elsewhere (30, 31), and based on microscopic analysis. All dishes, bicameral inserts, or cover glasses were coated with BD Matrigel Basement Membrane Matrix (BD Biosciences, San Jose, CA) at 1:7 as described (47). When Sertoli cells were plated at time 0, a functional TJ-permeability barrier was established in ∼2 days (76), and ultrastructures of the TJ, basal ES, gap junction, and desmosome were detected by electron microscopy (64), which mimicked the Sertoli cell BTB in vivo.
Knockdown of c-Yes in Sertoli cells in vitro by RNAi.
Sertoli cells cultured for 2 days, with a functional TJ barrier established, were used for c-Yes knockdown by RNAi. On day 2, Sertoli cells plated at different cell densities for lysate preparation (for immunoblotting), for TJ barrier assessment, and for fluorescence microscopy were transfected with 100, 200, and 50 nM nontargeting control siRNA duplexes, respectively (catalog no. D-001810-10), or ON-TARGETplus siRNA duplexes for rat c-Yes (GAUCAAUUGCUACCGGAAA, catalog no. J-085484-11; Thermo Fisher Scientific, Lafayette, CO) with RiboJuice siRNA Transfection Reagent (EMD Millipore Bioscience, Madison, WI), following the manufacturer's protocols. The selected concentrations of siRNA duplexes were based on pilot experiments. About 24 h after transfection (i.e., day 3), cells were washed twice and incubated in fresh media for another 1–3 days before termination, as specified in various experiments.
Immunoblotting and coimmunoprecipitation.
Immunoblotting and coimmunoprecipitation (Co-IP) were carried out as described previously (76). To obtain lysates for Co-IP, Sertoli cells were harvested 2 days after transfection in IP lysis buffer [10 mM Tris, 0.15 M NaCl, 1% NP-40 (vol/vol), and 10% glycerol (vol/vol), pH 7.4, at 22°C] freshly supplemented with protease and phosphatase inhibitor cocktails (Sigma-Aldrich) at a 1:100 dilution (vol/vol). Protein concentration was estimated using DC (detergent compatible) Protein Assay kits (Bio-Rad) with BSA as a standard. About 500 μg of protein derived from Sertoli cell lysate was used for Co-IP in each reaction tube and incubated with 2 μg of anti-c-Yes monoclonal antibody (see Table 1) as described (76). Immunoblots were visualized by enhanced chemiluminescence, using a kit prepared in our laboratory as described (46). Images were obtained using a Fujifilm LAS-4000 Mini imaging system and analyzed in Multi Gauge software (Version 3.1; Fujifilm), which was to be followed by Image J software (http://rsbweb.nih.gov/ij/download.html; version 1.46). Antibodies used for immunoblotting experiments are listed in Table 1.
Table 1.
Antibodies used for different experiments in this report
| Antibody | Host Species | Vendor | Catalog No. | Application(s)/Dilution(s) |
|---|---|---|---|---|
| c-Yes | Mouse | BD Transduction laboratories | 610375 | IB (1:1,000), IF (1:100) |
| c-Yes | Mouse | Santa Cruz Biotechnology | sc-8403 | IP (2 μg) |
| Actin | Goat | Santa Cruz Biotechnology | sc-1616 | IB (1:200) |
| c-Src | Mouse | Santa Cruz Biotechnology | sc-8056 | IB (1:200) |
| p-Src-Y530 | Rabbit | Abcam | ab4817 | IB (1:1,000) |
| Hck | Rabbit | Abcam | ab32860 | IB (1:500) |
| Fyn | Rabbit | Santa Cruz Biotechnology | sc-16 | IB (1:150) |
| FAK | Rabbit | Millipore | 06-543 | IB (1:1,000) |
| p-FAK-Y397 | Rabbit | Invitrogen | 44-625G | IB (1:1,000) |
| p-FAK-Y576 | Rabbit | Millipore | DAM1394797 | IB (1:1,000) |
| p-FAK-Y407 | Rabbit | Invitrogen | 44-650G | IB (1:1,000), IF (1:100) |
| Pyk2 | Rabbit | Santa Cruz Biotechnology | sc-9019 | IB (1:200) |
| p-Pyk2-Y402 | Rabbit | Invitrogen | 44-618G | IB (1:250) |
| Occludin | Rabbit | Invitrogen | 71-1500 | IB (1:250), IF (1:100) |
| ZO-1 | Rabbit | Invitrogen | 61-7300 | IB (1:250), IF (1:100) |
| CAR | Rabbit | Santa Cruz Biotechnology | sc-15405 | IB (1:200), IF (1:100) |
| JAM-A | Rabbit | Invitrogen | 36-1700 | IB (1:300) |
| N-Cadherin | Rabbit | Santa Cruz Biotechnology | sc-7939 | IB (1:200), IF (1:100) |
| β-Catenin | Rabbit | Invitrogen | 71-2700 | IB (1:250), IF (1:100) |
| Arp3 | Mouse | Sigma-Aldrich | A5979 | IB (1:3,000) |
| N-WASP | Rabbit | Santa Cruz Biotechnology | sc-20770 | IB (1:200) |
| Drebrin | Rabbit | Abcam | ab11068-50 | IB (1:1,000) |
| Eps8 | Mouse | BD Transduction Laboratories | 610143 | IB (1:5,000), IF (1:100) |
| Par6 | Rabbit | Abcam | ab45394 | IF (1:100) |
| Nectin-3 | Goat | Santa Cruz Biotechnology | sc-14806 | IF (1:30) |
IB, immunoblotting; IF, immunofluorescence microscopy; IP, immunoprecipitation; FAK, focal adhesion kinase; CAR, coxsackievirus and adenovirus receptor; JAM-A, junctional adhesion molecule-A; Par6, partitioning-defetive protein 6. Each antibody used herein cross-reacted with its corresponding protein in the rat.
Assessment of Sertoli cell TJ-permeability barrier in vitro after c-Yes knockdown.
The Sertoli cell TJ-permeability barrier function was quantified by measuring TER across the Sertoli cell epithelium using a Millicell electrical resistance system (Millipore, Bedford, MA) as described (47). Cells were maintained for ≤6 days (i.e., 4 days after transfection) on Matrigel-coated bicameral units.
Actin polymerization assay.
Effects of c-Yes knockdown on actin polymerization were assessed through the initial rate of fluorescence increase that occurs during pyrene-conjugated G-actin conversion into F-actin using Actin Polymerization Biochem Kits obtained from Cytoskeleton (Denver, CO), as described earlier (40). In brief, on day 4 (i.e., 2 days after transfection), cells were lysed in 20 mM Tris (pH 7.5 at 22°C) containing 20 mM NaCl and 0.5% Triton X-100 (vol/vol) freshly supplemented with protease and phosphatase inhibitor cocktails (Sigma-Aldrich) at a 1:100 dilution (vol/vol). Cellular debris was removed by two successive centrifugations at 20,000 g at 4°C for 1 and 1.5 h, respectively. The supernatant containing the cleared lysate was immediately subjected to actin polymerization assay according to the manufacturer's instructions. Cell lysates (∼30 μl) from control and c-Yes RNAi-treated groups with equal amounts of protein were added to the final reaction mix (∼100 μl) containing 60 μl of G-actin stock and 10 μl of 10× actin polymerization buffer. The kinetics of fluorescence enhancement were monitored in Corning 96-well solid black flat bottom polysene microplate (via top reading) using a FilterMax F5 Multi-Mode Microplate Reader and the Multi-Mode Analysis Software (Molecular Devices, Sunnyvale, CA), with an excitation filter at 360 nm and an emission filter at 430 nm and 50 μs integration time. The initial rate of filament growth (5–7 min) was measured as described (17), and the linear regression analysis was performed using Microsoft Excel. This experiment was repeated three times, excluding pilot experiments that yielded similar results.
Immunofluorescence analysis by epifluorescence.
Epifluorescence analysis was performed as described (76) using antibodies shown in Table 1. Sertoli cells cultured for 2 days on round cover glasses with a cell density of 0.05 × 106 cells/cm2 after transfection were fixed either in methanol at −20°C for 5 min or in 4% paraformaldehyde (PFA) (wt/vol) in PBS (10 mM NaH2PO4 and 0.15 M NaCl, pH 7.4) at room temperature (22°C) for 10 min. PFA-fixed cells were permeabilized in 0.1% Triton X-100 (vol/vol) in PBS prior to blocking in 1% BSA (wt/vol) for 30 min. After overnight incubation with primary antibodies (Table 1), secondary antibodies conjugated with Alexa Fluor dye (Invitrogen, Carlsbad, CA) were used for protein visualization. Nuclei were stained with DAPI (4′,6-diamidino-2-phenylindole; Invitrogen, Eugene, OR). For in vivo studies, frozen cross-sections of testes at 7 μm thick in a cryostat at −22°C were collected on poly-l-lysine-coated microscope slides (Polysciences, Warrington, PA) and fixed in Bouin's fixative or 4% PFA and were treated similarly to Sertoli cells, as described above. For F-actin staining, cells or cross-sections of testes were fixed in 4% PFA (wt/vol) in PBS at room temperature for 10 min, to be followed by an incubation with FITC-conjugated phalloidin (diluted 1:50 in 1% BSA-PBS; Sigma-Aldrich) for cells or rhodamine phalloidin (Invitrogen) for testis sections for 30 min (40, 76). Images were captured by a Nikon Eclipse 90i microscope using NIS (Nikon Imaging Software)-Elements AR (Advanced Research) software (version 3.2; Nikon, Melville, NY) in JPEG format. Images were subsequently analyzed and merged using Adobe Photoshop in Adobe Creative Suite (version 3.0). For semiquantitative data analysis, such as fluorescence intensity and mislocalization of proteins at the BTB (e.g., distance of proteins that moved away from the BTB site near the basement membrane), Image J software (version 1.46, National Institutes of Health, Bethesda, MD) was used. To avoid interexperimental variations, all cross-sections of testes or Sertoli cell epithelium in treatment vs. control groups were processed simultaneously.
Confocal microscopy.
Confocal microscopy was performed at the Bio-Imaging Resource Center, The Rockefeller University, as detailed elsewhere (39, 65). In brief, Sertoli cells were cultured at 0.75–1 × 106 cells/cm2 for 2 days on Matrigel-coated Transwell Permeable Supports (Costar polyester membrane inserts, 24-mm diameter, 0.4-μm pore size; Corning, Lowell, MA). Each insert was placed in the well of a six-well dish so that a functional TJ-permeability barrier was formed in ∼48 h. Thereafter, Sertoli cells were transfected with the c-Yes siRNA duplexes vs. nontargeting control duplexes to knock down c-Yes by ∼75%. Two days later, Sertoli cells on polyester membranes were fixed using 4% PFA (wt/vol) in PBS for 10 min, permeabilized with 0.1% Triton X-100 (vol/vol) in PBS for 10 min, and blocked with 1% BSA (wt/vol) in PBS for 30 min. Cells were stained with an anti-c-Yes or an anti-Eps8 antibody (see Table 1) at room temperature overnight, to be followed by incubation with Alexa Fluor 488-conjugated goat-anti-mouse secondary antibody (Invitrogen) at a 1:200 dilution in PBS containing 1% BSA (wt/vol) at 22°C for 30 min. Images were acquired using an inverted Zeiss LSM510 laser-scanning confocal microscope (Carl Zeiss MicroImaging, Thornwood, NY) equipped with multiple laser lines (Diode: 405 nm; Argon/2: 458, 477, 488, and 514 nm; HeNe1: 543 nm; HeNe2: 633 nm) and Zeiss LSM 510 software. Optical slices <0.8 μm were collected at 0.41-μm intervals along the z-axis to obtain a series of images (i.e., z-stack). Image deconvolution of z-stacks was performed using 3D Huygens Deconvolution Software (Scientific Volume Imaging, Hilvesum, The Netherlands) to increase the signal-to-noise ratio. z-Stacks were adjusted for brightness and contrast, and optical sections were reconstructed with Image J software, as described (39, 65).
Semiquantitative analysis of fluorescence images.
For the fluorescence images of Sertoli cells obtained by confocal microscopy in the c-Yes silenced cells vs. control cells by RNAi to illustrate the knockdown of c-Yes, the intensity of fluorescence signals was quantified using Image J (version 1.46). Intensity of the corresponding fluorescence signals from control siRNA-transfected Sertoli cells was arbitrarily set at 1 for comparison. To assess the effects of c-Yes knockdown on the localization of Eps8, the width of Eps8 fluorescence at the x-z view in c-Yes-silenced cells was measured and compared with control cells. At least 40 images and an x-z view from two independent experiments were scored.
In vivo knockdown of c-Yes in adult rat testes.
For transfection with c-Yes siRNA duplexes to testicular cells in vivo for c-Yes knockdown, adult male rats (∼275–300 g body wt at 60–70 days of age) received an intratesticular injection of a transfection mixture on days 1 and 3 (i.e., a total of 2 doses). Each transfection mixture had a volume of ∼200 μl and consisted of 100 nM siRNA duplexes and 7.5 μl of RiboJuice siRNA Transfection Reagent in ∼190 μl Opti-MEM Reduced Serum Medium (Invitrogen) via a 28-gauge needle attached to a 1-ml syringe (BD, Franklin Lakes, NJ) as described (37). The volume of each testis was estimated to be 1.6 ml. Nontargeting control siRNA duplexes serving as control were applied to one testis of a rat, and c-Yes siRNA duplexes were administered to the other testis of the same rat. Rats were euthanized 2 days after the last intratesticular injection, and testes were removed immediately for subsequent analysis. Each time point had n = 5 rats. Pilot experiments were conducted to establish the regimen reported herein to assess changes in phenotypes after in vivo knockdown of c-Yes in the testis since rats euthanized on day 3 after the last transfection displayed similar phenotypes as those rats that were euthanized on day 2 to day 2.5, and rats euthanized on day 4 did not display more severe phenotypic changes. However, we did not examine changes beyond day 4.
BTB integrity assay.
The BTB integrity assay was performed as described (33), which assessed the ability of an intact BTB to block the diffusion of a fluorescence tag (inulin-FITC, Mr 4.6 kDa) across the barrier from the basal to the adluminal compartment in the seminiferous epithelium. In brief, rats (∼300 g body wt) were under anesthesia with ketamine-HCl (60 mg/kg body wt ip) and xylazine (an analgesic, 15 mg/kg body wt im). Thereafter, a small incision was made over the jugular vein, and ∼1.5 mg of inulin-FITC (Sigma-Aldrich) suspended in 300 μl of PBS was administered to the jugular vein (iv) using a 28-gauge needle. About 30–45 min later, rats were euthanized by CO2 asphyxiation, and testes were removed and snap-frozen in liquid nitrogen. Cryostat sections were obtained and examined by fluorescence microscopy. Distance traveled by inulin-FITC (green fluorescence) from the basement membrane (DFITC) was measured vs. the radius of a seminiferous tubule (DR) in both the treatment and control groups and randomly scored from ∼80 tubules from n = 3 rats. Rats treated with CdCl2 at 3 mg/kg body wt ip for 2 days, which was shown to irreversibly disrupt BTB integrity, served as positive controls (n = 4 rats).
Histological analysis to assess changes in the status of spermatogenesis following c-Yes knockdown.
For histological analysis, testes were fixed in Bouin's fixative and embedded in paraffin, and sections (5-μm thickness) were obtained with a microtome. Sections were deparaffinized and rehydrated through successive incubation of xylene and ethanol and tap and deionized water as described (37). Cross-sections of testes were mounted on microscopic slides and placed in Hematoxylin 7211 (Richard-Allan Scientific, Richland, MI) to stain nuclei, to be followed by an incubation in Clarifier and Scott's solution, and finally in Eosin-Y to stain cytoplasm. Sections were dehydrated in absolute ethanol, cleared in 100% xylene, and sealed in PolyMount (Polysciences, Warrington, PA) and examined microscopically. Damages to spermatogenesis, such as defects in spermiation, loss of spermatid polarity, and loss of germ cell adhesion that led to premature germ cell loss, were assessed by examination of at least 50 randomly selected seminiferous tubules from cross-sections of each testis with n = 3 rats. A seminiferous tubule was scored as defective if it met any of the following criteria: 1) loss of spermatid polarity (it was defined by the presence of at least 15 elongated spermatids per cross-section of a seminiferous tubule in which the heads of these spermatids were not pointing toward the basement membrane but at least 90° from the intended orientation), 2) premature germ cell loss [it was defined by the presence of at least 10 germ cells of mixed cell types (e.g., elongating/elongated spermatids, round spermatids, and spermatocytes) in the tubule lumen], and 3) defects in spermiation (it was defined by the presence of at least 10 elongating/elongated spermatids that were embedded in the seminiferous epithelium after spermiation in a late stage VIII or IX tubule).
Statistical analysis.
Statistical comparisons between treatment and corresponding control groups were performed by one-way ANOVA and post hoc Dunnett's test; comparisons involving only two correlated groups were performed by Student's t-test. GB-STAT (Version 7.0; Dynamic Microsystems) was used for all statistical analyses. In vitro experiments were repeated three to five times using different batches of Sertoli cells, and each point contained triplicate dishes, bicameral units, or cover glasses. For in vivo experiments, each time point had at least n = 3–5 rats.
RESULTS
Knockdown of c-Yes in Sertoli cells by RNAi leads to a disruption of the TJ barrier function mediated by mislocalization of proteins at the cell-cell interface and reorganization of actin filaments.
Sertoli cells cultured for 2 days with an established TJ-permeability barrier and also with the ultrastructures of TJ, basal ES, gap junction, and desmosome that mimicked the Sertoli cell BTB in vivo (11) were used herein to study the role of c-Yes on BTB function. It is noted that this in vitro system has been widely used by investigators to study BTB regulation (7, 20, 26, 27, 52). Furthermore, Sertoli cells isolated from 20-day-old rat testes ceased to divide, differentiated and mitotically inactive (54), and they mimicked adult Sertoli cells morphologically and functionally on the basis of several comparative studies (32, 41). Furthermore, the purity of adult Sertoli cells was ∼85 vs. ∼95–98% of that isolated from 20-day-old rat testes (32, 41). Compared with Sertoli cells transfected with nontargeting control siRNA duplexes, the efficacy of the c-Yes knockdown using c-Yes-specific siRNA duplexes was found to be >50% by day 1 and ∼75% by day 2, which persisted through day 3 (Fig. 1, A and B). More importantly, the knockdown of c-Yes did not elicit any off-target effects, as shown in Fig. 1C, when a number of BTB-associated structural and regulatory proteins were assessed by immunoblotting. For instance, by day 2 after transfection when c-Yes was knockdown by ∼75%, no unintended knockdown was detected for either selected members of SFKs and FAKs, including FAK and Pyk2, or their corresponding phosphorylated forms, as well as TJ and basal ES proteins (Fig. 1C). However, the knockdown of c-Yes by ∼75% in these cells significantly impeded the Sertoli cell TJ barrier function (Fig. 1D). In pilot experiments, it was noted that when Sertoli cells were transfected on day 3 or 4, when the TJ barrier was firmly established, as illustrated by a stable TER across the cell epithelium (Fig. 1D), a knockdown of c-Yes by RNAi was not as effective as when cells were transfected on day 2 to perturb the TJ barrier function, illustrating that c-Yes was critically involved in the assembly of the TJ barrier, but it was less important for its maintenance. Consistent with findings shown in Fig. 1, A and B, a considerable loss of c-Yes was indeed detected at the Sertoli cell-cell interface by immunofluorescence analysis following c-Yes knockdown vs. nontargeting control (Fig. 1E), confirming its silencing by RNAi. Although c-Yes knockdown failed to downregulate any of the BTB proteins that were examined by immunoblotting, including regulatory/signaling and structural proteins at the BTB (Fig. 1C), considerable changes in the localization of TJ (e.g., occludin, ZO-1) and basal ES (e.g., N-cadherin, β-catenin) were detected. These proteins were found to become mislocalized, and instead of being localized to the Sertoli cell-cell interface, they were found to be internalized (Fig. 1E). Moreover, the organization of actin filaments in Sertoli cells was found to be perturbed following c-Yes knockdown, in which actin filaments appeared to be truncated and defragmented, and actin filaments were at the cell periphery but somewhat retracted from the cell-cell interface. This phenotype was consistent with an earlier report in which an inhibitor was used to block the c-Yes function (76). This pattern of actin reorganization (Fig. 1E) thus contributed to the instability of the TJ-permeability barrier, as shown in Fig. 1D.
Fig. 1.
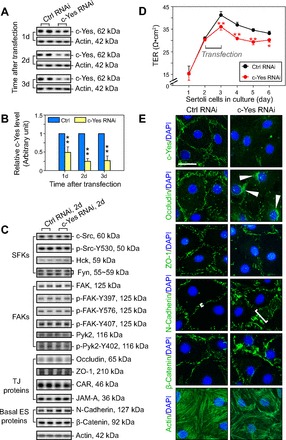
Knockdown of c-Yes by RNAi perturbs the Sertoli cell tight junction (TJ)-permeability barrier in vitro via changes in the localization and distribution of proteins at the Sertoli cell-cell interface. A: an assessment by immunoblotting on the changes in the steady-state protein level of c-Yes after its knockdown in Sertoli cells by RNAi. Sertoli cells were cultured alone at 0.5 × 106 cells/cm2 for 2 days, forming a functional TJ-permeability barrier with ultrastructures of TJ, basal ectoplasmic specialization (ES), gap junction, and desmosome under electron microscopy as described (35, 38, 64). Thereafter, cells were transfected with 100 nM of rat c-Yes-specific siRNA duplexes (c-Yes RNAi) vs. nontargeting control (Ctrl RNAi) and incubated for 1 day (1d). Thereafter, cells were washed twice and terminated immediately (1d; i.e., 24 h after transfection), to be followed by 2 (2d) and 3 days (3d), respectively, with up to ∼75% knockdown noted by 2d to 3d, as summarized in B. B: each data point was normalized against actin. Protein level in Ctrl RNAi was arbitrarily set at 1. Each bar = mean ± SD of n = 3–4 independent experiments. **P < 0.01. C: immunoblotting to examine changes in selected proteins at the blood-testis barrier (BTB) after c-Yes silencing 2 days after transfection on 1) members of the Src family kinases (SFKs), 2) members of focal adhesion kinases (FAKs), 3) TJ proteins, and 4) basal ES proteins, illustrating that there was no off-target effect of c-Yes silencing. D: effects of c-Yes knockdown on the Sertoli cell TJ barrier function were assessed by quantifying transepithelial electrical resistance across the Sertoli cell epithelium, with 1.2 × 106 cells/cm2 cultured on Matrigel-coated Millicell bicameral units. Within 24 h after transfection with 200 nM siRNA duplexes, a significant decline on the TJ-permeability barrier was noted. Each data point is the mean ± SD of triplicates of a representative experiment, and this experiment was repeated 3 times using different batches of Sertoli cells and yielded similar results. *P < 0.05; **P < 0.01. E: changes in the distribution and/or localization of TJ (e.g., occludin, ZO-1) and basal ES (e.g., N-cadherin, β-catenin) proteins and F-actin in Sertoli cells cultured at 0.05 × 106 cells/cm2 on Matrigel-coated coverslips ∼2d after transfection with 50 nM c-Yes siRNA duplexes vs. nontargeting control siRNA duplexes. Following the knockdown of c-Yes, both TJ and basal ES proteins were found to become mislocalized, moving from the Sertoli cell-cell interface into the cell cytosol, such as occludin, in which it was accumulated in cell cytosol (see white arrowheads), likely mediated by an increase in protein endocytosis, thereby destabilizing the Sertoli TJ-permeability barrier, as noted in D. For instance, N-cadherin was no longer restricted to the cell-cell interface; instead, it became diffusely localized at the Sertoli cell-cell interface (see white brackets). The actin filament network in Sertoli cells was also perturbed following c-Yes knockdown, with actin filaments appearing to be “retracted” from the Sertoli cell-cell interface, possibly being used to facilitate internalization of integral membrane proteins at the BTB. Bar = 40 μm, which applies to all micrographs.
Knockdown of c-Yes in Sertoli cells by RNAi accelerates actin polymerization mediated by mislocalization of actin-binding protein Eps8 at the cell-cell interface.
Knockdown of c-Yes by ∼75% did not affect the expression of several actin regulatory proteins, including 1) Arp3 and N-WASP, which are known to induce branched actin polymerization (11, 22, 49), 2) actin-binding protein drebrin E, which is known to recruit Arp3 to the BTB to elicit its restructuring at stage VIII of the epithelial cycle (4, 34), and 3) Eps8, an actin barbed-end capping and bundling protein in the testis (Fig. 2A) (2, 37). In this context, it was of interest to note that c-Yes structurally interacted with Eps8 but not Arp3 or drebrin E, illustrating that it could activate Eps8 to elicit changes in actin organization via its effects on actin barbed-end capping and bundling activity during the epithelial cycle of spermatogenesis (Fig. 2B). Indeed, the knockdown of c-Yes in the Sertoli cell epithelium led to an increase in actin polymerization (Fig. 2, C and D), providing the biochemical basis that supports the findings that there was an extensive reorganization of F-actin in c-Yes knockdown Sertoli cells that led to a disruption of the Sertoli cell TJ-permeability barrier and mislocalization of multiple proteins at the cell-cell interface (Fig. 1, D and E). We next used confocal microscopy to examine any changes in the localization of Eps8, a binding partner of c-Yes, as shown in Fig. 2B, following c-Yes knockdown (Fig. 2E). A considerable loss of c-Yes was detected at the Sertoli cell-cell interface after c-Yes knockdown, as shown in Fig. 2E, images a–d (see also the histogram in Fig. 2E, top, that illustrates a loss of c-Yes fluorescence by ∼70% in c-Yes knockdown cells vs. control cells). More importantly, it is of interest to note that Eps8 was found to become mislocalized and no longer tightly localized at the Sertoli cell-cell interface. Instead, it became “diffusively” localized (see Fig. 2E, images g and h vs. images e and f), moving away from the cell-cell contact site (see also the histogram in Fig. 2E, bottom, that depicts changes in the localization of Eps8 by quantifying the “width” of Eps8 fluorescence at the x-z view). Thus, actin filaments were no longer properly organized in Sertoli cells near the cell surface following c-Yes knockdown to confer the TJ-permeability barrier, supporting the findings shown in Fig. 1E, regarding an alteration of F-actin organization that led to barrier disruption.
Fig. 2.
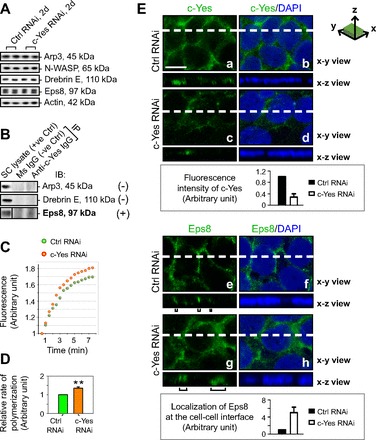
A study to assess changes in the actin regulatory and actin-binding proteins in Sertoli cells, their interaction with c-Yes, and kinetics of actin polymerization following c-Yes knockdown. Sertoli cells were cultured alone at 0.5 × 106 cells/cm2 (A–D) or at 0.75–1 × 106 cells/cm2 for confocal microscopy (E) for 2 days to form an intact cell epithelium with an established TJ-permeability barrier (see Fig. 1D); thereafter, cells were transfected with c-Yes-specific siRNA duplexes (c-Yes RNAi) vs. nontargeting control siRNA duplexes (Ctrl RNAi) for 24 h, and cells were terminated 1d thereafter (i.e., 2d after transfection) for immunoblotting (A), coimmunoprecipitation (Co-IP; B), actin polymerization assay (C and D), and confocal microscopy (E). A: consistent with findings shown in Fig. 1C that there were no off-target effects following c-Yes knockdown, the steady-state levels of proteins that were shown to regulate branched actin polymerization (e.g., Arp3, N-WASP), actin binding (e.g., drebrin E), and actin-bundling and barbed-end capping (e.g., Eps8) were unaffected after c-Yes knockdown. B: it was also noted that Eps8 that promotes actin filament bundles, but not Arp3 and drebrin E, structurally interacted with c-Yes, as demonstrated by Co-IP. C and D: an increase in actin polymerization was detected following c-Yes knockdown vs. control. E: 2 days after transfection to knockdown c-Yes by ∼75% (see Fig. 1, A and B), z-stacks of the Sertoli cell epithelium stained for c-Yes or Eps8 (green fluorescence) were obtained by confocal microscopy. Each column in E shows an optical slice from the x-y plane (i.e., parallel to the plane of Sertoli cell attachment on the culture dish; see the x-y section illustrated at top to depict orientation), with the left column for either c-Yes (images a and c) or Eps8 (images e and g) alone and the right column for either c-Yes and DAPI (images b and d) or Eps8 and DAPI (images f and h). Micrographs at bottom are vertical views of the Sertoli cell epithelium, which are reconstructed optical slices from the x-z plane (i.e., perpendicular to the plane of Sertoli cell attachment on the culture dish; see the x-z section illustrated at top to depict orientation) corresponding to the sliced positions on the x-y plane marked by white dashed lines. The findings that illustrate a loss of c-Yes staining at the Sertoli cell-cell interface by confocal microscopy (images c and d vs. images a and b), supported by the histogram at bottom, in which the intensity of c-Yes in the c-Yes knockdown cells was compared with control cells, are consistent with immunoblotting data shown in Fig. 1, A, B, and E. Black brackets underneath image g for Eps8 illustrate that following c-Yes knockdown by RNAi there was a mislocalization of Eps8 in the Sertoli cell epithelium, since more Eps8 was found to move away from the cell-cell contact site and failed to confer actin filament bundles to maintain the TJ-permeability barrier at the Sertoli cell-cell interface, as supported by data shown in Fig. 1, D and E. Histogram shown at the bottom also supported the mislocalization of Eps8 since the knockdown of c-Yes caused Eps8 to be localized less tightly to the cell-cell contact site by as much as ∼4-fold vs. control cells. These findings are representative results of 3 independent experiments. Bar in E, image a = 15 μm, which applies to E, images b–h.
Knockdown of c-Yes in the testis in vivo perturbs BTB integrity.
To further extend the findings in vitro and their physiological relevancy in vivo, we next examined changes in the BTB integrity following c-Yes knowndown by RNAi in vivo. Using a functional assay to assess the BTB integrity (33) following transfection of testes with c-Yes siRNA duplexes vs. nontargeting control siRNA duplexes via intratesticular injection (2 doses of siRNA duplexes with 1 dose on days 1 and 3, respectively, and rats subjected to BTB integrity assay on day 5), it was shown that c-Yes knockdown in vivo had a disruptive effect on the BTB integrity (Fig. 3, A and B), consistent with the findings in vitro shown in Fig. 1. Rats treated for 2 days with CdCl2 (3 mg/kg body wt ip), which is known to irreversibly disrupt BTB integrity (25, 61, 72), served as positive controls (Fig. 2, A and B).
Fig. 3.
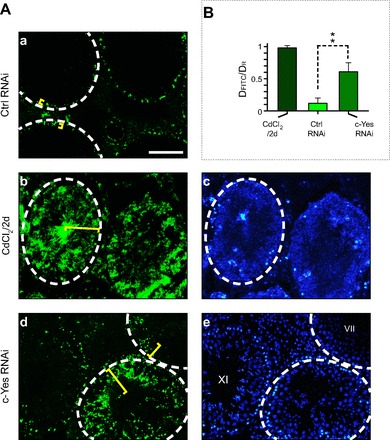
An in vivo assay to assess the effects of c-Yes knockdown vs. control in adult rat testes on the BTB integrity following intratesticular administration of siRNA duplexes. A: 2 days after the last intratesticular injection of siRNA duplexes using adult rats at 60–70 days of age (∼250–300 g body wt) to knock down c-Yes via transfection, rats received ∼1.5 mg inulin-FITC through the jugular vein. About 30 min thereafter, rats were euthanized, and the testes were snap-frozen in liquid nitrogen. Frozen sections (10 μm) were obtained in a cryostat, and sections on poly-l-lysine-coated slides were mounted in ProLong Gold Antifade reagent with DAPI (blue, to visualize Sertoli cell nuclei; Invitrogen), and the diffusion of inulin-FITC (green) from the basement membrane (annotated by the white dashed circle, illustrating the relative location of the BTB) into the seminiferous epithelium (see yellow brackets) was examined by fluorescence microscopy. In image a, this is the control rat testis subjected to nontargeting control knockdown (Ctrl RNAi). Positive control (images b and c) consisted of rats (n = 4) treated with 3 mg/kg body wt CdCl2 (ip) and used for BTB integrity assays 2 days thereafter. Images d and e are rat testes with c-Yes knockdown illustrating that the BTB was damaged (images d and e vs. image a). Images c and e are the corresponding images of b and d stained with DAPI. B: histogram showing semiquantitative analysis of the BTB integrity assay shown in A. Results were the ratio of the distance traveled by inulin-FITC (DFITC) from the BTB (white dashed line near the basement membrane) toward the center of the tubule lumen to the radius of the seminiferous tubule (DR). For tubules that were obliquely sectioned, the radius is the mean of the longest and the shortest distance from the basement membrane to the center of the tubule. Each data point is the mean ± SD of ∼80 randomly selected seminiferous tubules from 3 different rats. **P < 0.01. Bar in a = 130 μm, which applies to images b–e.
Disruption of BTB integrity after c-Yes knockdown in the testis is mediated via mislocalization of proteins and reorganization of F-actin network at the BTB.
To further delineate the underlying mechanism(s) by which a knockdown of c-Yes impedes BTB integrity, changes in the 1) localization and/or distribution of BTB constituent proteins, such as the TJ protein occludin and basal ES protein N-cadherin, 2) F-actin organization, and 3) distribution of actin regulatory protein Eps8, which is known to induce actin barbed-end capping and bundling (2, 14, 70, 78) at the BTB, were assessed and analyzed (Fig. 4, A–F). Consistent with findings in vitro shown in Fig. 1, a knockdown of c-Yes in vivo (Fig. 4, A and B) led to mislocalization of occludin at the BTB, where it was relocalized and moved away from the Sertoli cell-cell interface (Fig. 4C, images a and b). As such, ≥50% of occludin was no longer confined to the Sertoli cell BTB site (Fig. 4C). For N-cadherin, it was also redistributed, moving (or diffusing) away from the Sertoli cell-cell interface at the BTB, with as much as an approximately threefold increase in the distance where N-cadherin moved away from the BTB site (Fig. 4, C and D). F-actin and the actin-bundling protein Eps8 were also redistributed and mislocalized (Fig. 4, E and F). Thus, the actin filaments were not capable of conferring the tightly bundled ultrastructure at the basal ES that coexisted with TJ to constitute the BTB, and these changes (e.g., F-actin and Eps8 moved away from the BTB), shown in Fig. 4, E and F, coupled with mislocalization of integral membrane proteins occludin and N-cadherin at the site (Fig. 4, C and D), thus destabilized BTB, leading to its disruption (see Fig. 3).
Fig. 4.
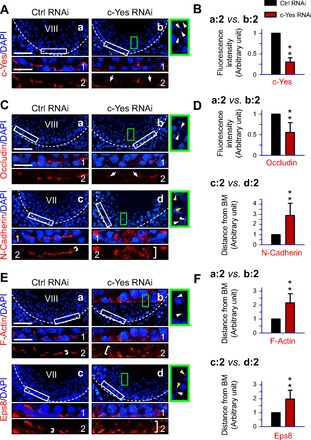
Changes in the distribution of BTB integral membrane proteins and actin regulatory protein Eps8 and F-actin organization following in vivo c-Yes knockdown in adult rat testes. Frozen sections of rat testes obtained 2 days after administration of the last dose of siRNA duplexes for c-Yes knockdown in vivo in adult rats at 60–70 days of age (∼250–300 g body wt) vs. nontargeting control siRNA duplexes were stained for c-Yes (A and B) and for BTB integral membrane proteins occludin and N-cadherin (C and D) as well as F-actin and the actin-bundling and barbed-end capping protein Eps8 (E and F). Cell nuclei were visualized with DAPI (blue). Areas boxed by white rectangles in A, images a and b, and C and E, images a–d, were magnified to better illustrate changes in protein localization at the BTB and shown as 1 and 2 in A, C, and E, which were analyzed for either changes in fluorescence intensity or mislocalization by diffusing away from the basement membrane (BM), as shown in B, D, and F. Dashed lines mark the relative location of the BTB near the basement membrane in images a–d. Areas boxed by green rectangles in images b and d from the c-Yes RNAi group were enlarged and are shown to the right of images b or d with white arrowheads to indicate that the elongating/elongated spermatids there were embedded in the seminiferous epithelium vs. control tubules of the same stages, such as in stage VIII of the epithelial cycle, showing defects in spermiation, some of which were misoriented/misaligned, losing their polarity with the heads no longer pointing toward the basement membrane (see yellow arrowheads). The silencing of c-Yes was also confirmed in these tubules in which c-Yes immunostaining was diminished considerably (A, image b2 vs. image a2), and the c-Yes staining intensity was diminished by ∼75% (B), consistent with findings shown in Fig. 1, A and B. The distribution of occludin was also found to be altered and mislocalized, and it was no longer tightly restricted to the BTB (as denoted by 2 white arrows in C, image b2 vs. image a2), consistent with findings shown in Fig. 1. N-cadherin (C, image d2 vs. image c2, and D), F-actin (E, image b2 vs. image a2, and F), and Eps8 (E, image d2 vs. image c2) were also displayed a considerably diffused pattern of distribution at the BTB (see white brackets). Semiquantitative analysis of densitometric data, such as changes in staining intensity (e.g., c-Yes and occludin) and protein mislocalization, where a target protein (e.g., N-cadherin, F-actin, Eps8) that moved away from the BM was summarized in B, D, and F with data in the nontargeting control group arbitrarily set at 1. Each bar is the mean ± SD of ∼50 seminiferous tubules from 3 different rats (**P < 0.01). Scale bar in image a = 100 μm, which applies to images b–d. Scale bar in 1 = 30 μm, which applies to 2 and also micrographs in green boxes.
Knockdown of c-Yes in the testis in vivo perturbs spermiation and disrupts spermatid polarity and adhesion in the seminiferous epithelium.
Changes in the status of spermatogenesis in the testis following in vivo knockdown of c-Yes were assessed using paraffin sections stained by hematoxylin and eosin (Fig. 5, images a–f). It was noted that step 19 elongated spermatids (and/or spermatozoa) were found to be embedded in the epithelium due to defects in spermiation in stage VIII tubules, and many elongated spermatids were also misaligned due to loss of cell polarity, and these changes were found mostly in stage VIII and IX tubules (Fig. 5, images c–f) vs. controls in which rat testes were transfected with nontargeting control siRNA duplexes (Fig. 5, images a and b). For instance, some tubules appeared as mixed stages in which most of the elongated spermatids had already moved to the adluminal edge of the tubule lumen, undergoing spermiation, which is analogous of a stage VIII tubule, but many elongated spermatids were also embedded deep inside the epithelium, similar to a stage VI–VII tubule [Fig. 5, image c (i, ii, and iii)]. Stages of some tubules were difficult to assign when extensive germ cell loss occurred, and spermatids remained embedded in the epithelium, which appeared to be a stage VIII–IX tubule (Fig. 5, image d). In Fig. 5, images e and f, both appear to be stage IX tubules, but elongated spermatids were found embedded in the seminiferous epithelium due to defects in spermiation. In Fig. 6, the percentage of tubules that displayed defects in spermatogenesis following c-Yes knockdown (2 days after the last administration of siRNA duplexes for transfection) and typical morphological abnormalities were also shown, illustrating that the knockdown of c-Yes in vivo affected ∼25% of the tubules that displayed defects in spermatid polarity, premature germ cell loss, and defects in spermiation (e.g., elongated spermatids embedded in the epithelium that failed to be released). It was noted that these changes were confined mostly to stage VIII–IX tubules, perhaps including some late stage VII tubules in which spermatids were still “trapped” inside the epithelium instead of lying at the adluminal edge of the tubule, making it difficult to distinguish late stage VII from early stage VIII tubules. Thus, this percentage of damaged tubules at ∼25% (see Fig. 6) matched well with the frequency of late stage VII–IX tubules in rats since in Sprague-Dawley rats the percentages of stages VII, VIII, and IX were estimated at 20.9, 7.6, and 3%, respectively (24).
Fig. 5.
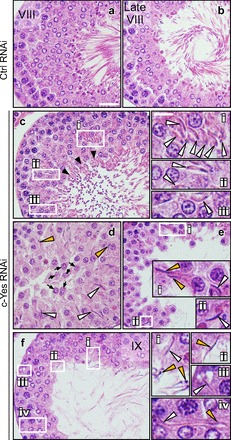
c-Yes knockdown in vivo by RNAi causes defects in spermiation. Rat testes from adult rats at 60–70 days of age (∼250–300 g body wt) were transfected with nontargeting control siRNA duplexes (Ctrl RNAi; images a and b) vs. c-Yes-specific siRNA (c-Yes RNAi; images c–f). Rats were euthanized 2 days after the administration of the last dose of siRNA duplexes, fixed in Bouin's fixative, dehydrated, and embedded in paraffin, and the paraffin sections were stained by hematoxylin and eosin. The knockdown of c-Yes was found to cause defects in spermiation, as noted in stage VIII to late stage VIII/early IX tubules. In control testes, a stage VIII and a late stage VIII tubule was shown in images a and b, respectively. Cross-sections of rat testes from the c-Yes RNAi group were shown in images c-f. Boxed areas in images c, e, and f annotated by Roman numerals i, ii, and iii were magnified. Black arrowheads in image c indicate that most of the fully developed elongated spermatids (i.e., spermatozoa) were released from the epithelium into the tubule lumen at spermiation. Black arrows in image d indicate that some round spermatids were found in the lumen following c-Yes knockdown. Many elongating/elongated spermatids were also embedded in the seminiferous epithelium, as indicated by white arrowheads shown in i, ii, iii, and iv. Yellow arrowheads annotated misaligned spermatids with their heads pointed away from the BM. Bar = 40 μm in image a, which applies to images b–f. Bar = 12 μm in i, which also applies to ii–iv.
Fig. 6.
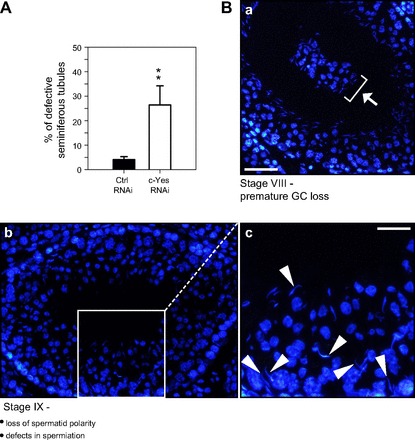
Evaluation of the effects of c-Yes knockdown on germ cell adhesion and spermiation. A: histogram summarizing semiquantitative analysis of defective tubules after c-Yes knockdown in vivo [2 days after the last administration of siRNA duplexes to testes of adult rats at 60–70 days of age (∼250–300 g body wt) for transfection, i.e., on day 5 after the initial administration of siRNA duplexes]. Each bar is the mean ± SD of ∼80 seminiferous tubules from 3 different rats (**P < 0.01) that had defects in spermatogenesis. B: the most observed defective phenotypes in the seminiferous epithelium following c-Yes knockdown by RNAi were as follows. Image a: premature germ cell loss, such as the presence of groups of round spermatids and spermatocytes in tubule lumen at stage VIII of the epithelial cycle, as annotated by white arrow and white bracket. Image b: in stage IX tubules after spermiation had just occurred, elongated spermatids were still embedded in the seminiferous epithelium, which were misoriented, losing polarity since the heads of these elongated spermatids no longer pointed toward the BM (annotated by white arrowheads in image c, which is the magnified view of the boxed area in image b). Scale bar in image a = 60 μm, which also applies to image b. Scale bar in image c = 30 μm.
Knockdown of c-Yes in the testis in vivo disrupts distribution and localization of PAR6, p-FAK-Tyr407, and nectin-3 at the apical ES.
PAR6 is a PAR-based polarity protein shown recently to confer cell adhesion and polarity at the BTB and apical ES in the testis (74). p-FAK-Tyr407, however, is an apical ES and BTB regulatory signaling protein that affects F-actin organization at these sites (40). Nectin-3 is an integral membrane protein at the apical ES restricted to elongated spermatids and functions as a cell adhesion protein (55). Nectins are known to activate Cdc42 (a small Rho GTPase known to regulate protein endocytosis in the testis), and nectins also colocalize with PAR6, a polarity protein that is known to confer elongating spermatid polarity in the seminiferous epithelium (73), suggesting their involvement in spermatid polarity. c-Yes was also found to participate in Cdc42-mediated signaling function in human breast epithelial cells (16). Thus we examined changes in nectin-3 distribution at the apical ES following local transfection of testis using c-Yes-specific siRNA duplexes for its knockdown (Fig. 7). In control testes transfected with nontargeting control siRNA duplexes, the localization of PAR6 (Fig. 7A, image a) and p-FAK-Tyr407 (Fig. 7B, image a) at the apical ES in stage VIII tubules was near the tip of the head of elongated spermatids and at the concave side of the elongated spermatid head, respectively, as described earlier (40, 74). However, following the knockdown of c-Yes, PAR6 (Fig. 7A, image b) and p-FAK-Tyr407 (Fig. 7B, image b) were found to be mislocalized and no longer tightly packed at the corresponding site at the apical ES as in control testes; instead, they “diffused” away from the apical ES. In normal rat testes, nectin-3 was localized to the apical ES and restricted to the front end of elongating spermatid heads in stage VI–VII tubules (Fig. 7C, image a), but it was considerably diminished to an almost nondetectable level at stage VIII to prepare spermatids for spermiation (Fig. 7C, image b), and nectin-3 reappeared in step 9 spermatids at the apical ES in stage IX tubules, which was seen prominently over the acrosomal region (Fig. 7C, image c). In c-Yes knockdown tubules, nectin-3 was noticeably mislocalized in that it was no longer restricted to the front end of elongating spermatid heads. Instead, it surrounded the entire spermatid head (Fig. 7C, image d) and persisted in stage VIII tubules (Fig. 7C, image e vs. image b) and also in elongating spermatids embedded in the epithelium of a stage IX tubule (Fig. 7C, image f). It is likely that, due to these defects in PAR6, p-FAK-Tyr407, and nectin-3 localization, there were changes in spermatid adhesion in the epithelium, causing defects in spermiation, spermatid adhesion, and polarity, as shown in Figs. 5 and 6.
Fig. 7.
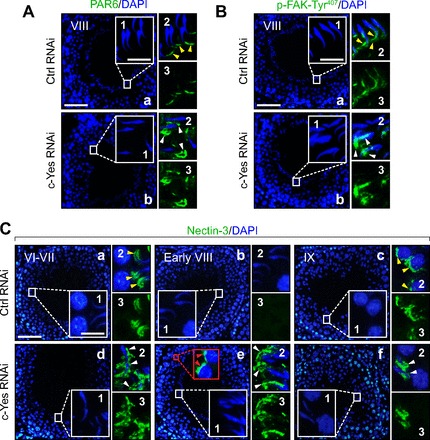
Changes in cell adhesive function at the apical ES in the seminiferous epithelium following in vivo c-Yes knockdown in testes in which defects in spermiation are mediated via an upregulation and/or mislocalization of partitioning-defective protein 6 (PAR6), p-FAK-Tyr407, and nectin-3 at the apical ES. Micrographs shown herein are rat testes from adult rats at 60–70 days of age (∼250–300 g body wt) that were transfected with either nontargeting control siRNA duplexes, as shown in A and B, image a, and C, images a–c, or c-Yes-specific siRNA duplexes for c-Yes knockdown shown in A and B, image b, and C, images d–f, and stained for PAR6 (A), p-FAK-Tyr407 (B), or nectin-3 (C). Insets 1-3 are the corresponding magnified views of the boxed areas in A and B, images a and b, and C, images a–f, and were stained with either DAPI (blue; inset 1), DAPI + either PAR6, p-FAK-Tyr407, or nectin-3 (green; inset 2), or target protein (i.e., PAR6, p-FAK-Tyr407 or nectin-3; green) alone (image 3). In control testes transfected with nontargeting control siRNA duplexes, PAR6 or p-FAK-Tyr407 was localized at the apical ES either near the tip of the head of elongated spermatids (see yellow arrowheads) or at the concave side of the elongated spermatid head (see yellow arrowheads), respectively, in stage VIII tubules, as described earlier (40, 74). However, following the knockdown of c-Yes, PAR6 and p-FAK-Tyr407 were found to be mislocalized and no longer tightly packed at the corresponding site at the apical ES as that in control testes; instead they “diffused” away from the apical ES, as annotated by white arrowheads in A and B. In rat testes that received nontargeting Ctrl RNAi duplexes in C, images a–c, the apical ES adhesion protein nectin-3 was localized at the apical ES in stage VI-VII tubules (image a), as indicated by yellow arrowheads in image a, inset 2. Nectin-3 staining was diminished considerably at the apical ES in stage VIII tubules just prior to spermiation to a level that was virtually undetectable (image b) until it reappeared again at the apical ES in stage IX tubules (image c) surrounding step 9 spermatids, as annotated by yellow arrowheads, as shown in image c, inset 2. Following c-Yes knockdown in rat testes, nectin-3 was found to be mislocalized (see white arrowheads in image d, insets 2 and 3, vs. image a, insets 2 and 3), and nectin-3 was found to be detected persistently at the apical ES even in stage VIII tubules, when its disappearance appeared to be needed to allow spermiation in a stage VIII tubule in control testes (image e vs. image b). Nectin-3 was also detected at the apical ES in step 8 spermatids; see red arrowheads in the red boxed area with enlarged view shown immediately at right. In short, nectin-3 staining was persistently found at the apical ES in c-Yes knockdown rat testes. This thus perturbed spermatid movement and adhesion, causing defects in spermiation since a transient loss of nectin-3 was necessary for spermatid movement and spermiation; see images a–c in controls. Thus, in a stage IX tubule shown in image f after spermiation had occurred in this c-Yes knockdown testis, elongated spermatids remained embedded in the seminiferous epithelium, and nectin-3 staining was also retained between Sertoli cells and the elongated spermatids, perturbing their release from the epithelium. White arrowheads in image d, inset 2, image e, inset 2, and image f, inset 2) illustrate “unwanted” and “mislocalized” nectin-3 at the apical ES. Scale bar in image a = 100 μm, which applies to image b or images b–f. Scale bar in inset 1 = 15 μm, which also applies to A–C, insets 2 and 3.
DISCUSSION
c-Yes, similar to other members of the SFKs (e.g., c-Src), is a (proto)oncogene, with its expression intimately related to carcinogenesis (11, 29, 53, 57, 58). For instance, overexpression of c-Yes in human colorectal carcinoma cells promotes metastatsis (3), but recent studies have shown that it also plays critical roles in different cellular physiological function, such as growth factor activation, endocytic vesicle-mediated protein trafficking, and cell signaling (11, 15, 68, 69). c-Yes, similarly to other members of SFKs, possesses SH (Src homology) domains such as SH1, SH2, SH3, and SH4, which, besides modulating intrinsic kinase activity and other functions, are being used for protein-protein interactions (18, 36, 56). As such, c-Yes can recruit a large number of binding partners to a specific cellular site or domain, altering the phosphorylation status of these interacting protein partners and changing the molecular composition of a protein complex, such as at the BTB (e.g., occludin-ZO-1, N-cadherin/β-catenin) or apical ES (e.g., α6β1-integrin-laminin-α3β3γ3), thus eliciting changes in molecular function of the protein complex in response to changes in environment, growth, pathogenesis, and most notably spermatogenesis. For instance, it was reported earlier that treatment of Sertoli cells with an established TJ permeability in vitro that mimicked the BTB in vivo with SU-6656, an inhibitor of c-Yes, caused mislocalization of N-cadherin at the Sertoli cell-cell interface, with N-cadherin moved from the cell surface into the cytosol, destabilizing Sertoli cell adhesive function that led to a disruption of Sertoli cell TJ-permeability barrier (76). These findings are consistent with earlier studies that have shown N-cadherin to be an important adhesion molecule in the testis (8, 45), in particular at the Sertoli cell-cell interface at the BTB (31).
In this context, it is of interest to note that c-Yes can be monopalmitoylated at its SH4 domain near its NH2 terminus, which facilitates its involvement in the transport of the Golgi pool of caveolin [a marker of transcytosis (21)], regulating endocytic vesicle-mediated protein trafficking, most notably transcytosis, and yet c-Src is nonpalmitoylated but can shuttle between the plasma membrane and late endosome/lysosome, thereby involving in protein endocytosis and protein degradation (60). Thus, although both c-Yes and c-Src are involved in intracellular protein trafficking, they play distinctive roles in the endocytic vesicle-mediated trafficking events (60). In a recent report, we demonstrated that c-Yes was upregulated at the BTB in the seminiferous epithelium during the epithelial cycle, which is most notably associated with the BTB at stages VIII–IX of the epithelial cycle during BTB restructuring to facilitate the transit of preleptotene spermatocytes at the site (76). Furthermore, c-Yes was also shown to be an integrated component of the apical ES, which was tightly associated with β1-integrin and laminin-β3/γ3 chains at stage IV to early stage VIII but diminished considerably at late stage VIII of the epithelial cycle (76). c-Yes was also shown to be involved in regulating protein trafficking function at the Sertoli cell BTB, affecting protein localization/distribution at the Sertoli cell-cell interface (76). However, these studies relied on the use of SU-6656, which is a selective c-Yes inhibitor, and thus the specificity of these findings was not certain. For instance, in this earlier study, SU-6656 was used at 20 nM, which is the IC50 for c-Yes vs. the IC50 of SU-6656 for c-Src, Fyn, and Lyn at 280, 170, and 130 nM, respectively (5, 6). In short, at 20 nM only c-Yes was selectively inhibited, but other nonreceptor Src family kinase members can also be “weakly” inhibited. Furthermore, these studies were in vitro in nature (76), making it likely that the in vivo response could be different. As such, the physiological relevance of c-Yes in vivo in regulating cell adhesion at the BTB and the apical ES remains largely unknown. Herein, we have demonstrated unequivocally that the knockdown of c-Yes by RNAi can perturb the Sertoli cell BTB integrity both in vitro and in vivo without any detectable off-target effects. More importantly, it was shown that the disruptive effects of c-Yes on the BTB integrity following its knockdown perturbed protein localization and/or distribution at the Sertoli cell-cell interface both in vitro and in vivo. Additionally, a knockdown of c-Yes was also found to perturb spermatid adhesion and polarity in vivo, and the disruptive effects associated with a knockdown of c-Yes at the apical ES appeared to be mediated via changes in protein localization/distribution of necin-3 and also PAR6 and p-FAK-Tyr407 at the site. These findings are significant since PAR6 was shown to confer spermatid adhesion and polarity during spermiogenesis (74), and p-FAK-Tyr407 was demonstrated recently to be critically involved in regulating cell adhesion at the apical ES and the BTB via its effects on actin dynamics (40). These findings thus illustrate that c-Yes forms a regulatory complex with the PAR-based regulatory module (e.g., PAR6) and FAK [which is an integrated component of the BTB and apical ES (63)]. Thus, c-Yes, together with FAK, can fine-tune the phosphorylation status of multiple protein complexes at the apical ES (e.g., nectin-3-afadin) and/or BTB (e.g., occludin-ZO-1, N-cadherin-β-catenin) as well as actin-regulating proteins (e.g., Eps8), thereby modulating the distribution and/or localization of these proteins at the site via changes in the actin filament organization in Sertoli cells, altering the adhesion function of the apical ES and BTB during the epithelial cycle of spermatogenesis. However, it is equally possible that a loss of c-Yes signaling may affect other yet-to-be identified signaling pathway(s) in the seminiferous epithelium, contributing to the phenotypic changes detected in this report regarding changes in spermatid polarity, spermatid adhesion, and defects in spermiation. This possibility must be carefully evaluated in future studies.
It is also noted that in our earlier study that examined the role of c-Yes on the Sertoli cell BTB function, the disruptive effects of the c-Yes SU-6656 on the TJ-permeability barrier can be blocked by the use of testosterone (76), illustrating that steroids are involved in the c-Yes-mediated physiological responses in the seminiferous epithelium. Other studies have shown that testosterone and 17β-estradiol secreted by testicular cells are crucial to maintain spermatogenesis (9, 10, 51, 62, 71). Thus, future studies should include an assessment if the use of steroids, in particular 17β-estradiol and testosterone, can modify the phenotypic changes following the knockdown of c-Yes as reported herein.
A recent report has shown that Cdc42, a small GTPase at the BTB and an integrated component of the PAR-based polarity complex (73), regulates endocytic vesicle-mediated protein trafficking at the Sertoli cell BTB, such as by modulating TGF-β3-mediated BTB disruption (75). Interestingly, c-Yes was shown to be responsible for Cdc42 activation during Wnt-5a-induced signaling in human mammary epithelial cells (16). Additionally, Cdc42 was also shown to regulate spermatid adhesion at the apical ES via its interactions with IQ motif-containing GTPase-activating protein (an actin-binding protein) and the N-cadherin/β-catenin adhesion protein complex (42). Thus, future studies should include a careful evaluation on the involvement of Cdc42 activation by c-Yes during spermatogenesis by which c-Yes regulates actin-filament bundles at the apical ES and the BTB. Additionally, since the knockdown of c-Yes also perturbs spermatid polarity in the seminiferous epithelium, it is likely that c-Yes is also a component of the PAR-based protein complex that modulates protein phosphorylation of PAR proteins (e.g., PAR3, PAR6) at the apical ES. Furthermore, c-Yes mediates these changes via its effects on the actin filament network at the apical ES, possibly via changes in the phosphorylation status of the actin regulatory proteins.
In summary, we have shown that c-Yes, a nonreceptor protein tyrosine kinase, is an important regulatory protein involved in the maintenance of BTB integrity, spermatid polarity, adhesion, and spermiation in vivo. These effects appear to be stage specifically limited to stage VIII–IX tubules and possibly mediated via its effects on protein recruitment and localization at the BTB and the apical ES mediated by PAR6, p-FAK-Tyr407, and nectins as well as other yet-to-be identified signaling pathway(s).
GRANTS
This work was supported by grants from the National Institute of Child Health and Human Development (U54-HD-029990 Project 5 to C. Y. Cheng; R01-HD-056034 to C. Y. Cheng).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
X.X. and C.Y.C. performed the experiments; X.X., D.D.M., and C.Y.C. analyzed the data; X.X. and C.Y.C. prepared the figures; X.X. and C.Y.C. drafted the manuscript; X.X., D.D.M., and C.Y.C. approved the final version of the manuscript; D.D.M. and C.Y.C. interpreted the results of the experiments; C.Y.C. did the conception and design of the research; C.Y.C. edited and revised the manuscript.
REFERENCES
- 1. Ahluwalia MS, De Groot J, Liu WM, Gladson CL. Targeting SRC in glioblastoma tumors and brain metastases: rationale and preclinical studies. Cancer Lett 298: 139–149, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ahmed S, Goh WI, Bu W. I-BAR domains, IRSp53 and filopodium formation. Semin Cell Dev Biol 21: 350–356, 2010 [DOI] [PubMed] [Google Scholar]
- 3. Barraclough J, Hodgkinson C, Hogg A, Dive C, Welman A. Increases in c-Yes expression level and activity promote motility but not proliferation of human colorectal carcinoma cells. Neoplasia 9: 745–754, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bazellières E, Massey-Harroche D, Barthélémy-Requin M, Richard F, Arsanto JP, Le Bivic A. Apico-basal elongation requires a drebrin-E-EB3 complex in columnar human epithelial cells. J Cell Sci 125: 919–931, 2012 [DOI] [PubMed] [Google Scholar]
- 5. Blake RA, Broome MA, Liu X, Wu J, Gishizky M, Sun L, Courtneidge SA. SU6656, a selective src family kinase inhibitor, used to probe growth factor signaling. Mol Cell Biol 20: 9018–9027, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bowman T, Broome MA, Sinibaldi D, Wharton W, Pledger WJ, Sedivy JM, Irby R, Yeatman T, Courtneidge SA, Jove R. Stat3-mediated Myc expression is required for Src transformation and PDFG-induced mitogenesis. Proc Natl Acad Sci USA 98: 7319–7324, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Byers S, Hadley MA, Djakiew D, Dym M. Growth and characterization of epididymal epithelial cells and Sertoli cells in dual environment culture chambers. J Androl 7: 59–68, 1986 [DOI] [PubMed] [Google Scholar]
- 8. Byers SW, Sujarit S, Jegou B, Butz S, Hoschutzky H, Herrenknecht K, MacCalman C, Blaschuk OW. Cadherins and cadherin-associated molecules in the developing and maturing rat testis. Endocrinology 134: 630–639, 1994 [DOI] [PubMed] [Google Scholar]
- 9. Carreau S, Hess RA. Oestrogens and spermatogenesis. Philos Trans R Soc Lond B Biol Sci 365: 1517–1535, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Carreau S, Wolczynski S, Galeraud-Denis I. Aromatase, oestrogens and human male reproduction. Philos Trans R Soc Lond B Biol Sci 365: 1571–1579, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cheng CY, Mruk DD. The blood-testis barrier and its implications for male contraception. Pharmacol Rev 64: 16–64, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cheng CY, Mruk DD. An intracellular trafficking pathway in the seminiferous epithelium regulating spermatogenesis: a biochemical and molecular perspective. Crit Rev Biochem Mol Biol 44: 245–263, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cheng CY, Mruk DD. A local autocrine axis in the testes that regulates spermatogenesis. Nat Rev Endocrinol 6: 380–395, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cheng CY, Mruk DD. Regulation of spermiogenesis, spermiation and blood-testis barrier dynamics: novel insights from studies on Eps8 and Arp3. Biochem J 435: 553–562, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Clump DA, Qazi IH, Sudol M, Flynn DC. c-Yes response to growth factor activation. Growth Factors 23: 263–272, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Dejmek J, Safholm A, Kamp Nielsen C, Andersson T, Leandersson K. Wnt-5a/Ca2+-induced NFAT activity is counteracted by Wnt-5a/Yes-Cdc42-casein kinase 1alpha signaling in human mammary epithelial cells. Mol Cell Biol 26: 6024–6036, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Disanza A, Carlier MF, Stradal TE, Didry D, Frittoli E, Confalonieri S, Croce A, Wehland J, Di Fiore PP, Scita G. Eps8 controls actin-based motility by capping the barbed ends of actin filaments. Nat Cell Biol 6: 1180–1188, 2004 [DOI] [PubMed] [Google Scholar]
- 18. Filippakopoulos P, Muller S, Knapp S. SH2 domains: modulators of nonreceptor tyrosine kinase activity. Curr Opin Struct Biol 19: 643–649, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Galdieri M, Ziparo E, Palombi F, Russo MA, Stefanini M. Pure Sertoli cell cultures: a new model for the study of somatic-germ cell interactions. J Androl 2: 249–254, 1981 [Google Scholar]
- 20. Grima J, Pineau C, Bardin CW, Cheng CY. Rat Sertoli cell clusterin, alpha 2-macroglobulin, and testins: biosynthesis and differential regulation by germ cells. Mol Cell Endocrinol 89: 127–140, 1992 [DOI] [PubMed] [Google Scholar]
- 21. Hansen CG, Nichols BJ. Exploring the caves: cavins, caveolins and caveolae. Trends Cell Biol 20: 177–186, 2010 [DOI] [PubMed] [Google Scholar]
- 22. Hertzog M, Milanesi F, Hazelwood L, Disanza A, Liu H, Perlade E, Malabarba MG, Pasqualato S, Maiolica A, Confalonieri S, Le Clainche C, Offenhauser N, Block J, Rottner K, Di Fiore PP, Carlier MF, Volkmann N, Hanein D, Scita G. Molecular basis for the dual function of Eps8 on actin dynamics: bundling and capping. PLoS Biol 8: e1000387, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hess RA, de Franca LR. Spermatogenesis and cycle of the seminiferous epithelium. Adv Exp Med Biol 636: 1–15, 2008 [DOI] [PubMed] [Google Scholar]
- 24. Hess RA, Schaeffer DJ, Eroschenko VP, Keen JE. Frequency of the stages of the cycle of the seminiferous epithelium in the rat. Biol Reprod 43: 517–524, 1990 [DOI] [PubMed] [Google Scholar]
- 25. Hew KW, Heath GL, Jiwa AH, Welsh MJ. Cadmium in vivo causes disruption of tight junction-associated microfilaments in rat Sertoli cells. Biol Reprod 49: 840–849, 1993 [DOI] [PubMed] [Google Scholar]
- 26. Janecki A, Jakubowiak A, Steinberger A. Effect of cadmium chloride on transepithelial electrical resistance of Sertoli cell monolayers in two-compartment cultures—a new model for toxicological investigations of the “blood-testis” barrier in vitro. Toxicol Appl Pharmacol 112: 51–57, 1992 [DOI] [PubMed] [Google Scholar]
- 27. Janecki A, Jakubowiak A, Steinberger A. Regulation of transepithelial electrical resistance in two-compartment Sertoli cell cultures: in vitro model of the blood-testis barrier. Endocrinology 129: 1489–1496, 1991 [DOI] [PubMed] [Google Scholar]
- 28. Jun J, Lee MK, Jin Y, Fu SB, Rosales JL, Lee KY. Clues for c-Yes involvement in the cell cycle and cytokinesis. Cell Cycle 10: 1502–1503, 2011 [DOI] [PubMed] [Google Scholar]
- 29. Lee JH, Pyon JK, Kim DW, Lee SH, Nam HS, Kim CH, Kang SG, Lee YJ, Park MY, Jeong DJ, Cho MK. Elevated c-Src and c-Yes expression in malignant skin cancers. J Exp Clin Cancer Res 29: 116, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lee NP, Mruk DD, Conway AM, Cheng CY. Zyxin, axin, and Wiskott-Aldrich syndrome protein are adaptors that link the cadherin/catenin protein complex to the cytoskeleton at adherens junctions in the seminiferous epithelium of the rat testis. J Androl 25: 200–215, 2004 [DOI] [PubMed] [Google Scholar]
- 31. Lee NP, Mruk D, Lee WM, Cheng CY. Is the cadherin/catenin complex a functional unit of cell-cell actin-based adherens junctions in the rat testis? Biol Reprod 68: 489–508, 2003 [DOI] [PubMed] [Google Scholar]
- 32. Li JC, Lee TW, Mruk TD, Cheng CY. Regulation of Sertoli cell myotubularin (rMTM) expression by germ cells in vitro. J Androl 22: 266–277, 2001 [PubMed] [Google Scholar]
- 33. Li MW, Xia W, Mruk DD, Wang CQ, Yan HH, Siu MK, Lui WY, Lee WM, Cheng CY. Tumor necrosis factor {alpha} reversibly disrupts the blood-testis barrier and impairs Sertoli-germ cell adhesion in the seminiferous epithelium of adult rat testes. J Endocrinol 190: 313–329, 2006 [DOI] [PubMed] [Google Scholar]
- 34. Li MW, Xiao X, Mruk DD, Lam YL, Lee WM, Lui WY, Bonanomi M, Silvestrini B, Cheng CY. Actin-binding protein drebrin E is involved in junction dynamics during spermatogenesis. Spermatogenesis 1: 1–14, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li MW, Mruk DD, Lee WM, Cheng CY. Disruption of the blood-testis barrier integrity by bisphenol A in vitro: is this a suitable model for studying blood-testis barrier dynamics? Int J Biochem Cell Biol 41: 2302–2314, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li SS, Wu CC. Using peptide array to identify binding motifs and interaction networks for modulator domains. Methods Mol Biol 570: 67–76, 2009 [DOI] [PubMed] [Google Scholar]
- 37. Lie PP, Mruk DD, Lee WM, Cheng CY. Epidermal growth factor receptor pathway substrate 8 (Eps8) is a novel regulator of cell adhesion and the blood-testis barrier integrity in the seminiferous epithelium. FASEB J 23: 2555–2567, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lie PP, Cheng CY, Mruk DD. Crosstalk between desmoglein-2/desmocollin-2/Src kinase and coxsackie and adenovirus receptor/ZO-1 protein complexes, regulates blood-testis barrier dynamics. Int J Biochem Cell Biol 42: 975–986, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lie PP, Cheng CY, Mruk DD. Interleukin-1alpha is a regulator of the blood-testis barrier. FASEB J 25: 1244–1253, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lie PP, Mruk DD, Mok KW, Su L, Lee WM, Cheng CY. Focal adhesion kinase-Tyr407 and -Tyr397 exhibit antagonistic effects on blood-testis barrier dynamics in the rat. Proc Natl Acad Sci USA 109: 12562–12567, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lui WY, Lee WM, Cheng CY. Transforming growth factor beta3 regulates the dynamics of Sertoli cell tight junctions via the p38 mitogen-activated protein kinase pathway. Biol Reprod 68: 1597–1612, 2003 [DOI] [PubMed] [Google Scholar]
- 42. Lui WY, Mruk DD, Cheng CY. Interactions among IQGAP1, Cdc42, and the cadherin/catenin protein complex regulate Sertoli-germ cell adherens junction dynamics in the testis. J Cell Physiol 202: 49–66, 2005 [DOI] [PubMed] [Google Scholar]
- 43. Lui WY, Wong CH, Mruk DD, Cheng CY. TGF-beta3 regulates the blood-testis barrier dynamics via the p38 mitogen activated protein (MAP) kinase pathway: an in vivo study. Endocrinology 144: 1139–1142, 2003 [DOI] [PubMed] [Google Scholar]
- 44. Luton F, Vergés M, Vaerman JP, Sudol M, Mostov KE. The SRC family protein tyrosine kinase p62yes controls polymeric IgA transcytosis in vivo. Mol Cell 4: 627–632, 1999 [DOI] [PubMed] [Google Scholar]
- 45. MacCalman C, O'Brien D, Byers S, Blaschuk O. N-Cadherin expression in the seminiferous epithelium of the mouse testis. Endocr J 1: 519–525, 1993 [Google Scholar]
- 46. Mruk DD, Cheng CY. Enhanced chemiluminescence (ECL) for routine immunoblotting: An inexpensive alternative to commercially available kits. Spermatogenesis 1: 121–122, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mruk DD, Cheng CY. An in vitro system to study Sertoli cell blood-testis barrier dynamics. Methods Mol Biol 763: 237–252, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mruk DD, Silvestrini B, Cheng CY. Anchoring junctions as drug targets: role in contraceptive development. Pharmacol Rev 60: 146–180, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nurnberg A, Kitzing T, Grosse R. Nucleating actin for invasion. Nat Rev Cancer 11: 177–187, 2011 [DOI] [PubMed] [Google Scholar]
- 50. O'Donnell L, Nicholls PK, O'Bryan MK, McLachlan RI, Stanton PG. Spermiation: The process of sperm release. Spermatogenesis 1: 14–35, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. O'Donnell L, Robertson KM, Jones ME, Simpson ER. Estrogen and spermatogenesis. Endocr Rev 22: 289–318, 2001 [DOI] [PubMed] [Google Scholar]
- 52. Okanlawon A, Dym M. Effect of chloroquine on the formation of tight junctions in cultured immature rat Sertoli cells. J Androl 17: 249–255, 1996 [PubMed] [Google Scholar]
- 53. Orr BA, Bai H, Odia Y, Jain D, Anders RA, Eberhart CG. Yes-associated protein 1 is widely expressed in human brain tumors and promotes gioblastoma growth. J Neuropathol Exp Neurol 70: 568–577, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Orth JM. Proliferation of Sertoli cells in fetal and postnatal rats: a quantitative autoradiographic study. Anat Rec 203: 485–492, 1982 [DOI] [PubMed] [Google Scholar]
- 55. Ozaki-Kuroda K, Nakanishi H, Ohta H, Tanaka H, Kurihara H, Mueller S, Irie K, Ikeda W, Sakai T, Wimmer E, Nishimune Y, Takai Y. Nectin couples cell-cell adhesion and the actin scaffold at heterotypic testicular junctions. Curr Biol 12: 1145–1150, 2002 [DOI] [PubMed] [Google Scholar]
- 56. Reebye V, Frilling A, Hajitou A, Nicholls JP, Habib NA, Mintz PJ. A perspective on non-catalytic Src homology (SH) adaptor signalling proteins. Cell Signal 24: 388–392, 2012 [DOI] [PubMed] [Google Scholar]
- 57. Rho O, Kim DJ, Kiguchi K, Digiovanni J. Growth factor signaling pathways as targets for prevention of epithelial carcinogenesis. Mol Carcinog 50: 264–279, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sancier F, Dumont A, Sirvent A, Paquay de Plater L, Edmonds T, David G, Jan M, de Montrion C, Cogé F, Léonce S, Burbridge M, Bruno A, Boutin JA, Lockhart B, Roche S, Cruzalegui F. Specific oncogenic activity of the Src-family tyrosine kinase c-Yes in colon carcinoma cells. PLos One 6: e17237, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sandilands E, Brunton VG, Frame MC. The membrane targeting and spatial activation of Src, Yes and Fyn is influenced by palmitoylation and distinct RhoB/RhoD endosome requirements. J Cell Sci 120: 2555–2564, 2007 [DOI] [PubMed] [Google Scholar]
- 60. Sato I, Obata Y, Kasahara K, Nakayama Y, Fukumoto Y, Yamasaki T, Yokoyama KK, Saito T, Yamaguchi N. Differential trafficking of Src, Lyn, Yes and Fyn is specified by the state of palmitoylation in the SH4 domain. J Cell Sci 122: 965–975, 2009 [DOI] [PubMed] [Google Scholar]
- 61. Setchell BP, Waites GMH. Changes in the permeability of the testicular capillaries and of the “blood-testis barrier” after injection of cadmium chloride in the rat. J Endocrinol 47: 81–86, 1970 [DOI] [PubMed] [Google Scholar]
- 62. Sharpe RM. Regulation of spermatogenesis. In: The Physiology of Reproduction, edited by Knobil E, Neill JD. New York: Raven, 1994, p. 1363–1434 [Google Scholar]
- 63. Siu ER, Wong EW, Mruk DD, Porto CS, Cheng CY. Focal adhesion kinase is a blood-testis barrier regulator. Proc Natl Acad Sci USA 106: 9298–9303, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Siu MK, Wong CH, Lee WM, Cheng CY. Sertoli-germ cell anchoring junction dynamics in the testis are regulated by an interplay of lipid and protein kinases. J Biol Chem 280: 25029–25047, 2005 [DOI] [PubMed] [Google Scholar]
- 65. Su L, Mruk DD, Lui WY, Lee WM, Cheng CY. P-glycoprotein regulates blood-testis barrier dynamics via its effects on the occludin/zonula occludens 1 (ZO-1) protein complex mediated by focal adhesion kinase (FAK). Proc Natl Acad Sci USA 108: 19623–19628, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Su T, Bryant DM, Luton F, Verges M, Ulrich SM, Hansen KC, Datta A, Eastburn DJ, Burlingame AL, Shokat KM, Mostov KE. A kinase cascade leading to Rab11-FIP5 controls transcytosis of the polymeric immunoglobulin receptor. Nat Cell Biol 12: 1143–1153, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sudol M. From Rous sarcoma virus to plasminogen activator, src oncogene and cancer management. Oncogene 30: 3003–3010, 2011 [DOI] [PubMed] [Google Scholar]
- 68. Summy JM, Sudol M, Eck MJ, Monteiro AN, Gatesman A, Flynn DC. Specificity in signaling by c-Yes. Front Biosci 8: 185–205, 2003 [DOI] [PubMed] [Google Scholar]
- 69. Tatárová Z, Brábek J, Rösel D, Novotný M. SH3 domain tyrosine phosphorylation—sites, role and evolution. PLoS One 7: e36310, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Vaggi F, Disanza A, Milanesi F, Di Fiore PP, Menna E, Matteoli M, Gov NS, Scita G, Ciliberto A. The Eps8/IRSp53/VASP network differentially controls actin capping and bundling in filopodia formation. PLoS Comput Biol 7: e1002088, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wang RS, Yeh S, Tzeng CR, Chang C. Androgen receptor roles in spermatogenesis and fertility: lessons from testicular cell-specific androgen receptor knockout mice. Endocr Rev 30: 119–132, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Wong CH, Mruk DD, Lui WY, Cheng CY. Regulation of blood-testis barrier dynamics: an in vivo study. J Cell Sci 117: 783–798, 2004 [DOI] [PubMed] [Google Scholar]
- 73. Wong EW, Cheng CY. Polarity proteins and cell-cell interactions in the testis. Int Rev Cell Mol Biol 278: 309–353, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wong EW, Mruk DD, Lee WM, Cheng CY. Par3/Par6 polarity complex coordinates apical ectoplasmic specialization and blood-testis barrier restructuring during spermatogenesis. Proc Natl Acad Sci USA 105: 9657–9662, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wong EW, Mruk DD, Lee WM, Cheng CY. Regulation of blood-testis barrier dynamics by TGF-beta3 is a Cdc42-dependent protein trafficking event. Proc Natl Acad Sci USA 107: 11399–11404, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Xiao X, Mruk DD, Lee WM, Cheng CY. c-Yes regulates cell adhesion at the blood-testis barrier and the apical ectoplasmic specialization in the seminiferous epithelium of rat testes. Int J Biochem Cell Biol 43: 651–665, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Yan HH, Mruk DD, Wong EW, Lee WM, Cheng CY. An autocrine axis in the testis that coordinates spermiation and blood-testis barrier restructuring during spermatogenesis. Proc Natl Acad Sci USA 105: 8950–8955, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zwaenepoel I, Naba A, Da Cunha MM, Del Maestro L, Formstecher E, Louvard D, Arpin M. Ezrin regulates microvillus morphogenesis by promoting distinct activities of Eps8 proteins. Mol Biol Cell 23: 1080–1094, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]


