Abstract
This study was aimed at establishing whether specific activation of angiotensin II (ANG II) type 2 receptor (AT2R) modulates adipocyte differentiation and function. In primary cultures of subcutaneous (SC) and retroperitoneal (RET) preadipocytes, both AT2R and AT1R were expressed at the mRNA and protein level. Cells were stimulated with ANG II or the AT2R agonist C21/M24, alone or in the presence of the AT1R antagonist losartan or the AT2R antagonist PD123,319. During differentiation, C21/M24 increased PPARγ expression in both RET and SC preadipocytes while the number of small lipid droplets and lipid accumulation solely increased in SC preadipocytes. In mature adipocytes, C21/M24 decreased the mean size of large lipid droplets. Upon abolishment of AT2R expression using AT2R-targeted shRNAs, expressions of AT2R, aP2, and PPARγ remained very low, and cells were unable to differentiate. In Wistar rats fed a 6-wk high-fat/high-fructose (HFHF) diet, a significant shift toward larger adipocytes was observed in RET and SC adipose tissue depots. C21/M24 treatments for 6 wk restored normal adipocyte size distribution in both these tissue depots. Moreover, C21/M24 and losartan decreased hyperinsulinemia and improved insulin sensitivity impaired by HFHF diet. A strong correlation between adipocyte size area and glucose infusion rate during euglycemic-hyperinsulinemic clamp was observed. These results indicate that AT2R is involved in early adipocyte differentiation, while in mature adipocytes and in a model of insulin resistance AT2R activation restores normal adipocyte morphology and improves insulin sensitivity.
Keywords: angiotensin type 2 receptor, adipocyte, differentiation, PPARγ, high-fat/high-fructose diet
several recent reviews clearly implicate the renin-angiotensin system (RAS) in various aspects of adipose tissue physiology and dysfunction, linking RAS abnormalities with the development of insulin resistance and type 2 diabetes (7, 25, 36, 50). Classically, the hormone angiotensin II (ANG II) mediates its action via two major receptors, namely the ANG II type 1 receptor (AT1R) and the type 2 receptor (AT2R). AT1R is closely associated with the regulation of blood pressure and hydro-mineral balance, with long-term ANG II-induced AT1R activation playing a well-documented role in hypertension, type 2 diabetes, and cardiovascular diseases (7, 25, 36, 50). In contrast, AT2R is highly expressed in fetal life, while in the adult its expression is much more variable, according to tissues and pathophysiological situations (for recent reviews see Refs. 25, 45, 46, 51, 56). In conditions of insulin resistance, several studies, although not all (38), suggest that AT1R blockade provides end-organ protection independently of its antihypertensive effect by decreasing the size of adipocytes in visceral adipose tissue and by reversing the profile of adipokine secretion associated with obesity and type 2 diabetes (13, 17, 34, 48, 61) (for review see Refs. 20, 25). Therapeutic responses to angiotensin receptor blockers (ARBs) may result from enhanced AT2R activation in addition to inhibition of AT1R. Indeed, when AT1R is blocked, circulating ANG II binds to and activates AT2R only (45). However, most of the effects reported for AT2R have been inferred from studies using AT1R inhibition with ARBs (13, 49) or in AT2R-deficient mice (24, 57). Even in these conditions, it has proved difficult to clearly establish whether AT2R may play a role in adipocyte differentiation and adipose tissue physiology. Indeed, in the study of Iwai et al. (24) using atherosclerotic apoE-KO mice with an AT2R deficiency (AT2R/apoE double-knockout mice), the lack of AT2R decreased the expression of adipocyte differentiation factors, whereas adipocyte size, plasma cholesterol, and free fatty acid levels were increased. Conversely, Yvan-Charvet et al. (57) documented an increased number of small adipocytes in AT2R-deficient mice, and those mice were protected against high-fat diet-induced obesity.
In humans, it is well accepted that dysfunctional visceral adipose tissue is associated with an increased risk of insulin resistance and cardiovascular diseases (8, 15, 53). In contrast, increasing lipid storage in subcutaneous (SC) adipose tissue may be protective (1, 2, 15, 23, 27, 52, 53). This safe storage of lipids in adipose tissue is key to preventing lipotoxicity in nonadipose tissues (7, 16, 36). Therefore, an understanding of the specific properties and regulation of visceral (dysfunctional TG storage) as opposed to SC (functional TG storage) adipose tissues is of paramount importance (8, 15, 23).
Until recently, it was difficult to directly address the role of AT2R due to the absence of high affinity and selective ligands. Indeed, in addition to CGP42112A, only two AT2R agonists were available (35, 43), but all are peptides and not readily used in in vivo studies. In this context, we developed a stable, nonpeptide, and highly selective AT2R agonist, C21, now renamed M24 (14, 54). Using this compound, recent publications have argued in favor of a protective role of AT2R in various pathological situations, such as myocardial infarction and remodeling, stroke, subcutaneous injury and cognitive impairment (for recent reviews, see Refs. 44, 46). C21/M24 is the most selective AT2R agonist available to date and represents a unique tool to delineate the specific roles of AT2R on morphological and functional properties of adipocytes.
In light of the above as well as current knowledge on AT2R, the purpose of the present study was to clarify the paradigm regarding the role of AT2R in adipocyte differentiation and in an in vivo situation of insulin resistance. To achieve this goal, we investigated the effect of selective activation of AT2R on cultured rat preadipocytes and in rats submitted to a 6-wk high-fat/high-fructose (HFHF) diet.
RESEARCH DESIGN AND METHODS
Cell cultures and treatments.
All protocols were approved by the Animal Care and Ethics Committee of the Faculté de médecine et des sciences de la santé de l'Université de Sherbrooke. Upon arrival, 8-wk-old male Wistar rats were acclimated to 12-h daylight cycle at constant temperature (22°C). Retroperitoneal (RET) and subcutaneous (SC) preadipocytes derived from stromal vascular cells were obtained by collagenase digestion, as described by Haussman et al. (18). The isolated cells were centrifuged to separate floating adipocytes from the pellet of stromal vascular cells. After filtration and centrifugation, pelleted stromal vascular cells were resuspended in DMEM-F12 + 10% FBS (Invitrogen Life Technologies, Burlington, ON, Canada). Since our aim was to study the process of morphological differentiation, cells were cultured at a density of 1.5 × 104 cells/cm2 to reach near 75% confluence at the end of the experimental period. After 48 h, preadipocytes were allowed to differentiate in serum-free differentiation DMEM-F12 medium containing 2 nM triiodothyronine (T3); 872 nM insulin; 65 μM transferrin, and 5 ng/ml sodium selenite (BD Biosciences, Mississauga, ON, Canada). Cells were cultured with the differentiation medium until day 12 in the absence or presence of ANG II or the AT2R agonist C21/M24, alone or in the presence of the AT1R antagonist losartan (Sigma-Aldrich, Oakville, ON, Canada) or the AT2R antagonist PD123,319 (Tocris, Ellisville, MI), with medium changes every 2 days. Cells were examined daily, and phase-contrast images were acquired using a phase-contrast microscope (Leica, Deerfield, IL).
Gene expression analyses.
After 12 days in culture, cells were harvested, washed, and pelleted. RNA was isolated and processed for reverse transcriptase and quantification by PCR. Rat primers (Invitrogen Life Technologies, Carlsbad, CA) for AT2R and AT1AR, the only AT1R isoform expressed in adipose tissue (3), were designed with the Beacon Designer software (PREMIER Biosoft International, Palo Alto, CA) (Table 1). PCR was performed with an iCycler iQ Detection System (Bio-Rad Laboratories) according to the manufacturer's instructions. PCR amplification was initiated with 1 cycle at 94°C for 3 min for denaturation of cDNA, followed by 40 three-segment cycles for amplification (94°C for 30 s, 61.8°C for 30 s, and 72°C for 30 s) and a final extension at 72°C for 5 min. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and β-actin were chosen as endogenous control (housekeeping genes).
Table 1.
Primer sequences used for PCR
| Genes, Rats | Primer Sequences Forward, 5′-3′, Reverse, 5′-3′ | Amplicon Length, bp |
|---|---|---|
| ATR2 | (F GGTCTGCTGGGATTGCCTTAATG | 142 |
| (R ACTTGGTCACGGGTAATTCTGTTC | ||
| ATR1A | (F CCCTACCCTCTACAGCATCATC | 121 |
| (R GGCGAGATTGAGAAGAAAGACG | ||
| ACTB | Primer pair from Qiagen | 145 |
| GAPDH | (F TGGTGCCAAAAGGGTCATC | 176 |
| (R CTTCCACGATGCCAAAGTTG |
ATR2, angiotensin II receptor, type 2; ATR1A, angiotensin II receptor, type 1A; ACTB, β-actin, GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Western blotting analyses.
Cells were lysed in RIPA (radioimmunoprecipitation assays) buffer, and proteins (20 μg) were separated on 10% SDS-polyacrylamide gels. For rat adipose tissues, total protein extraction was performed on frozen adipose tissues. SC and RET tissues were homogenized using a Teflon potter in RIPA buffer with a ratio of 2.5 ml/g tissue followed by centrifugation at 13,000 g at 4°C for 20 min. Samples from an equivalent amount of protein (between 25 and 30 μg) were separated on 10% SDS polyacrylamide gels. After transfer onto PVDF, membranes were blocked with 5% milk in TBS buffer + 0.1% Tween 20, pH 7.5, and subsequently incubated either 2 h or overnight with primary antibodies at the following dilutions in 5% BSA: anti-AT2R (C-18, 1:500), anti-AT1R (1:500), anti-peroxisome proliferator-activated receptor-γ (PPARγ; SC-7273, 1:1,000) and anti-adipose-specific fatty-acid-binding protein (aP2/FABP4; SC-18661 antibodies, 1:2,000), anti-hormone-sensitive lipase (HSL; Abcam, ab45422, 1:1,000), anti-fatty acid synthase (FAS; New England Biolabs, C20G5, 1:1,000), anti-perilipin A (Abcam, ab3526, 1:2,000), anti-GAPDH (1:1,000), and anti-β-actin antibody (1:8,000). Unless otherwise stated, all antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Membranes were then washed and incubated with the appropriate secondary antibody conjugated to horseradish peroxidase (Cell Signaling Technology, Boston, MA) in 5% milk and TBS-Tween 20 (0.1%) for 1 h. After subsequent washing, detection was performed by chemiluminescence with an ECL system on ECL Hyperfilms.
Classification of cultured cells according to the size of lipid droplets (LD) was performed with a custom-designed software program written using MATLAB (v. R2010b; The MathWorks, Natick, MA). For each treatment, two to three Petri dishes were prepared. For each Petri dish, six images were acquired, thus 18 images for each treatment per experiment. Two to three different experiments were performed. The total number of cells analyzed per experiment varied from 100 to 200 according to the experimental conditions. All procedures were performed in a double-blinded manner. Cells were classified into three categories, large, medium, and small, according to the size of their LDs, which were identified with a colored symbol tag. The ranges of average droplet size differed between SC and RET preadipocytes: type 1, large LD (19–25 μm); type 2, medium LD (13–15 μm), and type 3, small LD (7–13 μm).
Quantification of lipid content by AdipoRed assay.
After 12 days of treatment, lipid content was measured using a commercially available kit according to the manufacturer's instructions (AdipoRed Assay Reagent; Cambrex Bio Science Walkersville, Walkersville, MD). After a 10-min incubation, the plates were placed in a Tecan F200 fluorometer (Tecan, Durham, NC), and fluorescence was measured with an excitation wavelength of 485 nm and an emission wavelength of 572 nm.
Inactivation of AT2 receptor expression by short hairpin RNA.
Stable short hairpin RNA (shRNA)-mediated knockdown of AT2R expression in primary cultures of preadipocytes was achieved using lentiviral constructs. Two AT2R shRNA sequences (shRNA A: TRCN0000027316 NM_007429.2–956s1c1), (shRNA B: TRCN0000027390 NM_007429.2–1229s1c1) subcloned into the pLKO.1-Puro vector and one control shRNA (empty vector) obtained from Sigma-Aldrich were used, as described in Table 2. The shRNA A was more potent in abolishing AT2R expression and was thus selected for subsequent experiments.
Table 2.
Sequence of synthesized oligonucleotides of shAT2R
| Name | Sequence |
|---|---|
| shAT2R, A | 5′-CCGGGCATTCATCATTTGCTGGCTTCTCGAGAAGCCAGCAAATGATGAATGCTTTTT-3′ |
| shAT2R, B | 5′-CCGGCTTAGAGAAATGGACACCTTTCTCGAGAAAGGTGTCCATTTCTCTAAGTTTTT-3′ |
shRNA sequences used to downregulate mRNA level of AT2R. Boldface letters correspond to the loop of the shRNA sequences.
In vivo experiments.
For in vivo experiments, Wistar rats (8 wk old) were randomly distributed in control or treated groups. Experimental animals received a high-fat/high-fructose (HFHF) diet [consisting of Teklad rat chows containing 46.5% fructose and 25.7% lard (TD no. 05482 rat chow), Teklad, Harlan Laboratories, Mississauga, ON, Canada] for 6 wk, whereas the control groups were fed a standard laboratory rodent diet (Rodent laboratory chow 5001 from Purina), as previously described (32). During the experiments, rats had free access to food and water. Weight, energy intake, and water consumption were measured three times a week. Both diet groups were subsequently subdivided into treatment groups: the standard chow diet-fed control group was treated with saline or C21/M24 (0.3 mg·kg−1·day−1), while the HFHF group was treated with saline, C21/M24 (0.3 mg·kg−1·day−1) or losartan (1 mg·kg−1·day−1). Treatments were administered intraperitoneally by constant infusion via Alzet osmotic minipumps (model 2006, Durect, Cupertino, CA). After 6 wk of diet and treatments, rats were fasted overnight and submitted to 2 h of euglycemic-hyperinsulinemic clamps to determine their insulin sensitivity, as previously described (32). At the end of experimentation, rats were euthanized by decapitation. Blood samples were collected for metabolic measurements [glucose, triglycerides (TG), nonesterified-fatty acids (NEFA)] as well as insulin assay. Adipose tissues (SC, RET), liver, heart, and muscles (gastrocnecmius, soleus) were also collected. Adipose tissues were excised, washed with isotonic saline solution, and subsequently fixed in phosphate-buffered 4% formaldehyde and embedded in paraffin blocks; 5 μM paraffin sections were prepared, mounted on slides, and stained with hematoxylin-eosin (H&E). Images were acquired with a Nikon Eclipse 300 microscope (Mississauga, ON, Canada) equipped with a CoolSnap fx digital camera (Roper Scientific, Tucson, AZ). Images were acquired using a ×20 objective. Measurement of adipocyte size (as area in μm2) was performed as described above with a custom-designed software program written using MATLAB.
Laboratory assays.
Blood glucose levels were measured with an Accu-Chek Aviva nanoglucometer (Roche, Mississauga, ON, Canada). Plasma insulin was determined by radioimmunoassay (Linco Research, St. Charles, MO). Triglycerides (TG) and nonesterified fatty acids (NEFA) were measured by enzymatic methods according to the manufacturer's instructions (Wako Chemicals, Hayward, CA). Liver lipids were extracted with chloroform-methanol 2:1 as described by Folch et al. (10). The concentration of hepatic TG was measured with enzymatic reagent kits (Roche, Indianapolis, IN).
Data analysis.
The data are presented as means ± SE of the number of experiments indicated in the text. Statistical analyses of the data were performed using one-way ANOVA followed by the Newman-Keuls post hoc test or two-way ANOVA followed by Bonferroni multiple comparisons. Differences were considered significant for P ≤ 0.05.
RESULTS
SC and RET preadipocytes exhibit different differentiation profiles.
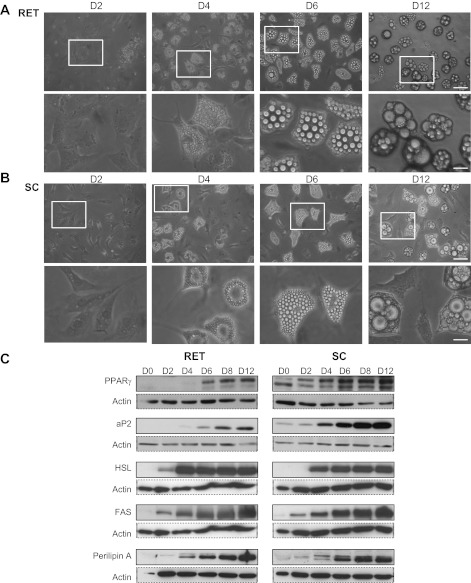
Preadipocytes were cultured in the presence of a well-established cocktail of differentiating factors. Both were characterized by a progressive accumulation of lipid droplets, which began after 3–4 days and progressed until day 12 onward. At that time, RET adipocytes contained two to five large lipid droplets (Fig. 1A), whereas SC adipocytes were more heterogeneous, with cells exhibiting a few large lipid droplets, often surrounded by small lipid droplets (Fig. 1B). These morphological changes were accompanied by a time-dependent increase in the expression of selective markers of differentiation (PPARγ and aP2/FAPB4) and of adipogenesis (HSL, FAS, and perilipin A; Fig. 1C).
Fig. 1.
Time-course of preadipocyte differentiation from rat retroperitoneal (RET) and subcutaneous (SC) adipose tissue in primary culture. Preadipocytes from RET (A) and SC (B) adipose tissues were plated in 35-mm Petri dishes at a density of 1.5 × 104/cm2. To initiate the process of differentiation, cells were cultured in DMEM-F12 medium-enriched differentiation cocktail as described in research design and methods. A and B: phase-contrast microscopy of SC and RET preadipocytes. Images were acquired at days 2, 4, 6, and 12 using a Leica microscope equipped with a ×20 objective. Squares in top represent areas selected for magnification shown in bottom. Undifferentiated cells, elongated fibroblast-like cells or more polygonal cells, still persisted in SC cultures. Scale bars, 60 μm for full-size images, 20 μm for magnifications. C: proteins were extracted before differentiation (day 0, D0) and after 2, 4, 6, 8, and 12 days in differentiation medium and processed for Western blotting using appropriate primary antibodies against PPARγ (1:1,000), aP2/FABP4 (1:2,000), hormone-sensitive lipase (HSL; 1:2,000), fatty acid synthase (FAS; 1:2,000), perilipin A, and β-actin antibody (1:8,000, 1:2,000). Data shown are representative results from 3 independent experiments.
SC and RET adipocytes express AT1R and AT2R.
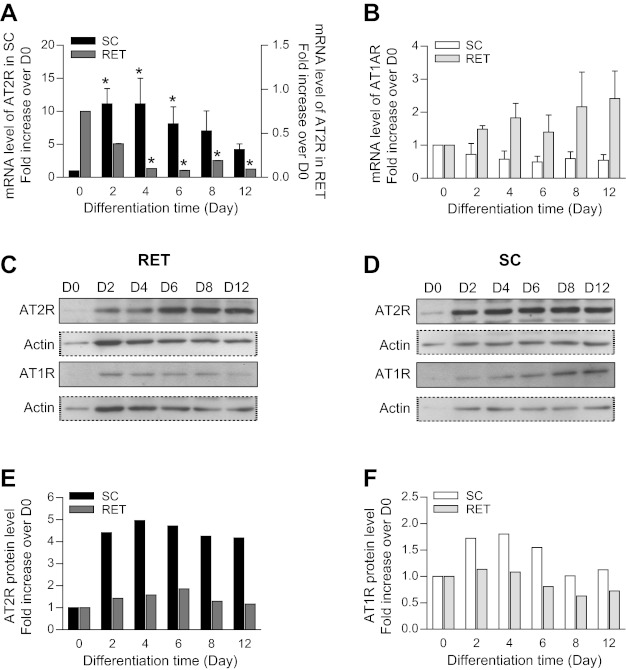
Time course analyses indicated that in SC preadipocytes the level of AT2R mRNA increased at the onset of the differentiation process, remained elevated throughout differentiation, and decreased after day 8. In contrast, in RET cells, AT2R mRNA was already present at the beginning of differentiation and decreased rapidly after 4 days of differentiation (Fig. 2A). AT1R mRNA levels, on the other hand, did not change significantly throughout the time course of differentiation in either SC or RET cultures (Fig. 2B). Western blotting confirmed the presence of the receptors in both cell types. Although there was a marked increase in AT2R expression at day 2 of differentiation, AT1R and AT2R expression levels remained thereafter relatively stable throughout the experimental period (Fig. 2, C–F).
Fig. 2.
ANG II receptor 1A (AT1AR) and AT2R mRNA (A and B) and protein expression (C–F) throughout preadipocyte differentiation. Preadipocytes from RET and SC adipose tissues were plated in 35-mm Petri dishes at a density of 1.5 × 104/cm2. To initiate the process of differentiation, cells were cultured in DMEM-F12 medium-enriched differentiation cocktail as described in research design and methods. Densitometric analysis of mRNA levels of AT2R (A) and AT1AR (B) during differentiation of SC and RET preadipocytes. mRNA of target genes was normalized to that of β-actin (n = 3–4). Statistical analyses were performed using one-way ANOVA followed by Newman-Keuls post hoc test. Statistical significance vs. day 0 of differentiation: *P < 0.02. C–F: cell lysates containing equal concentrations of protein (20 μg) were processed for Western blotting using anti-AT2R antibody (1:500; C) and anti-AT1R antibody (1:500; D). Amount of loaded protein was compared by reprobing the membrane with an anti-β-actin antibody (1:8,000). E and F: corresponding densitometric analysis of Western blotting shown in C and D.
Selective pharmacological activation of AT2R has opposite effects on cell morphology in SC adipocytes.
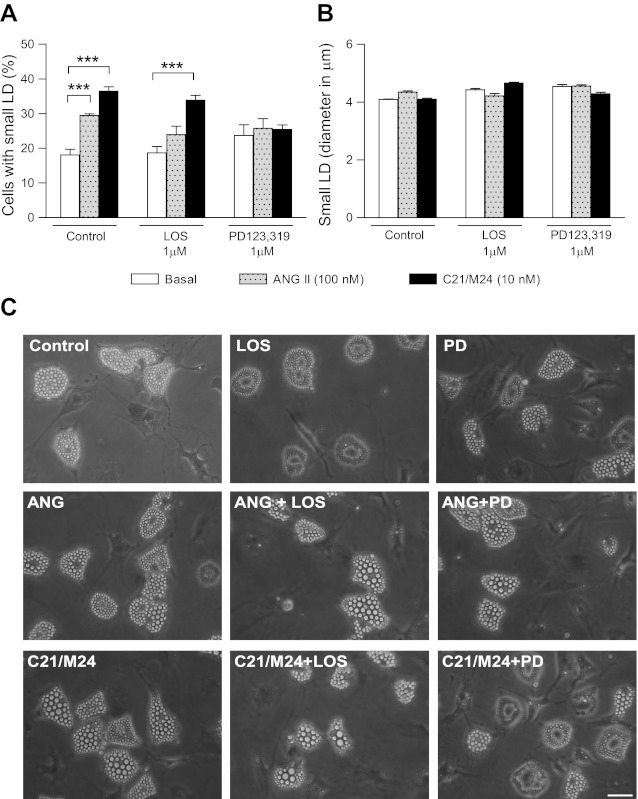
SC and RET preadipocytes were cultured in the absence or presence of ANG II (100 nM) or the AT2R agonist C21/M24 (10 nM), alone or in the presence of the respective AT1R (losartan, 1 μM) or AT2R (PD123,319, 1 μM) antagonists. Although it was difficult to assess changes in the size of adipocytes themselves, selective effects were clearly observed on both the number and size of lipid droplets. During the process of differentiation (between days 2 and 7 of culture), C21/M24 treatment increased the proportion of cells exhibiting small lipid droplets (≤5.5 μm), an effect not modified by cotreatment with losartan, but abolished with PD123,319 (Fig. 3, A and C). ANG II alone also increased the proportion of cells accumulating lipid droplets. However, the various treatments did not affect the size of these small LD (Fig. 3, B and C). In contrast, such effects were not observed in RET preadipocytes (not shown).
Fig. 3.
Effect of selective ligands of type 1 and type 2 receptors on morphology of preadipocytes from SC rat adipose tissue in primary culture. Preadipocytes were plated in 35-mm Petri dishes at a density of 1.5 × 104/cm2 and cultured for 7 days as described in research design and methods in the absence (control) or presence of ANG II (100 nM) or C21/M24 (10 nM) alone or in combination with AT1R antagonist losartan (LOS; 1 μM) or AT2R antagonist PD123,319 (PD; 1 μM). A: histograms illustrating percentage of cell types exhibiting small lipid droplets (LD). B: analyses of mean diameter of small LD. Values were obtained from data analyses (histograms and fitting curves) constructed as described in research design and methods and in Supplemental Data. Data are means ± SE of LD from 36 images taken from 3 different Petri dishes from 2 different experiments. Results include mean values obtained at day 7 in culture, thus n = 24. Statistical analyses were performed using two-way ANOVA followed by Bonferroni multiple comparisons. Statistical significance vs. respective control: ***P < 0.001. C: preadipocytes were plated in 35-mm Petri dishes at a density of 1.5 × 104/cm2 and cultured for 7 days in the absence (control) or presence of ANG II (100 nM), or C21/M24 (10 nM) alone or in combination with losartan (1 μM) or PD123,319 (1 μM). Scale bars, 40 μm.
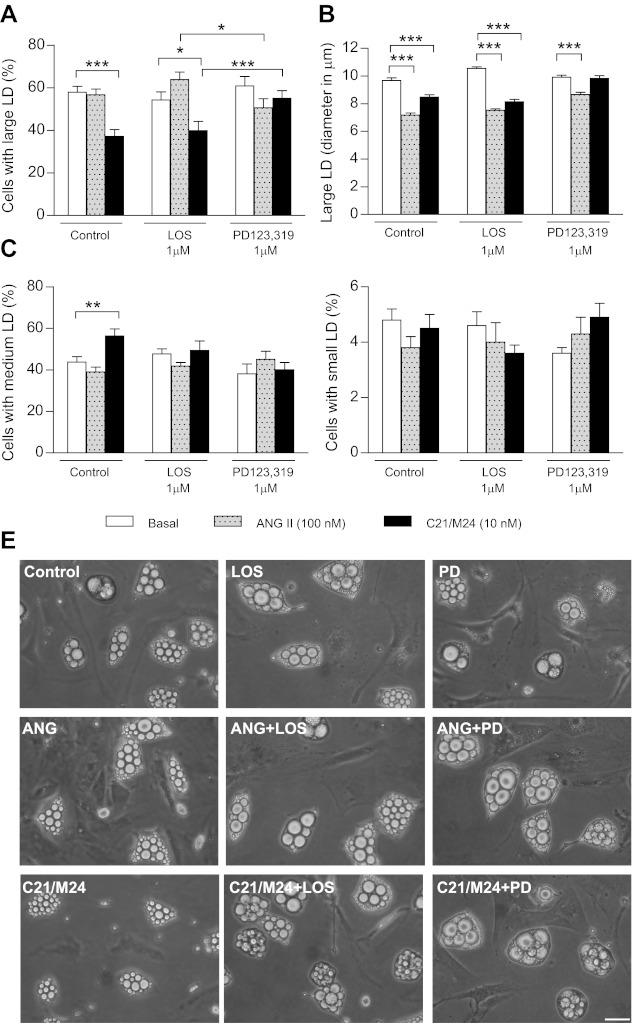
After 11 days of culture and onward, stimulation of SC adipocytes with C21/M24 induced a significant reduction in the number (Fig. 4A) and size (Fig. 4B) of large lipid droplets (19–25 μm) while increasing the proportion of cells exhibiting medium LD (13–15 μm; Fig. 4C), without altering that of small LD (≤5.5 μm; Fig. 4D). These effects were not modified by treatment with losartan, whereas incubation with PD123,319 abolished the effect of C21/M24 and significantly blunted the effect of ANG II on the size of large LD (Fig. 4B). Of note, the percentage of cells exhibiting medium and small LD was not modified by ANG II (Fig. 4, C and D). Representative images are illustrated in Fig. 4E. These same treatments, on the other hand, did not affect the appearance of RET adipocyte morphology, nor their size, which were all very large, even in control conditions (data not shown).
Fig. 4.
Effect of selective ligands of type 1 and type 2 receptors on morphology of adipocytes from SC rat adipose tissue in primary culture. Preadipocytes were plated in 35-mm Petri dishes at a density of 1.5 × 104/cm2 and cultured until day 13 as described in research design and methods in the absence (control) or presence of ANG II (100 nM) or C21/M24 (10 nM) alone or in combination with losartan (1 μM) or PD123,319 (1 μM). A and B: histograms illustrating percentage of cell types exhibiting large LD (A) and their mean diameter (B). C and D: histograms illustrating percentage of cells types exhibiting medium LD (C) and small LD (D). Values were obtained from data analyses (histograms and fitting curves) constructed as described in research design and methods and in Supplemental Data. Data are means ± SE of LD from 54 images taken from 3 different Petri dishes from 3 different experiments. Statistical analyses were performed using two-way ANOVA followed by Bonferroni multiple comparisons. Statistical significance vs. respective control: *P < 0.05, **P < 0.01, and ***P < 0.001. E: adipocytes cultured for 13 days in the absence (control) or presence of ANG II (100 nM) or C21/M24 (10 nM) alone or in combination with losartan (1 μM) or PD123,319 (1 μM). Scale bars, 40 μm.
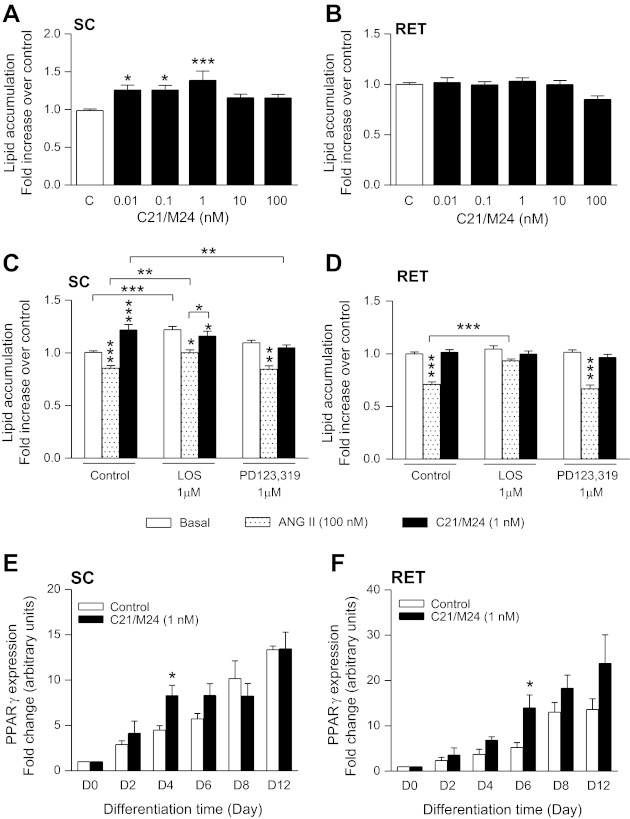
Selective AT2R stimulation increases lipid accumulation and PPARγ during adipogenesis in SC adipocytes.
Stimulation of cells for 11 days with various doses of C21/M24 significantly increased lipid accumulation in SC but not in RET preadipocytes (Fig. 5, A and B). When stimulated with C21/M24 (1 nM), this increase was mainly due to AT2R activation, since this effect was abolished by PD123,319 while not being affected by losartan (Fig. 5C). In contrast, adipocytes stimulated with ANG II showed less lipid accumulation both in SC and RET preadipocytes, an effect decreased by losartan in SC and abolished in RET (Fig. 5, C and D). Furthermore, losartan alone stimulated lipid accumulation in SC adipocytes (Fig. 5C). During the time course of differentiation, C21/M24 increased PPARγ expression in both SC and RET preadipocytes, with significant differences at days 4 and 6, respectively (Fig. 5, E and F).
Fig. 5.
Effect of AT2R activation on lipid accumulation (A–D) and on on PPARγ expression (E and F) in rat adipocytes from SC and RET adipose tissue. SC and RET preadipocytes were plated in 35-mm Petri dishes at a density of 1.5 × 104/cm2 and cultured for 12 days as described in research design and methods. Treatments were initiated at day 0 and continued every 2 days. SC (A) and RET (B) preadipocytes were treated with C21/M24 from 0.01 to 100 nM. Preadipocytes from SC (C) and RET (D) adipose tissues were treated with ANG II (100 nM) or C21/M24 (1 nM) alone or in combination with AT1R antagonist losartan (1 μM), or AT2R antagonist PD123,319 (PD; 1 μM). Lipid accumulation was assessed with an AdipoRed kit on day 11. Results are expressed as means ± SE of ≥6 independent experiments, each condition conducted in quadruplicate. Results were corrected for protein content. Statistical analyses were performed using one-way ANOVA followed by Newman-Keuls post hoc test. Statistical significance: * P < 0.05, ***P < 0.001; vertical asterisks represent comparison with respective controls; horizontal lines indicate comparison between groups. SC (E) and RET (F) preadipocytes were plated in 35-mm Petri dishes at a density of 1.5 × 104/cm2 and cultured until day 12 as described in research design and methods. Proteins were extracted on days 0, 2, 4, 6, 8, and 12, and cell lysates containing equal amounts of protein (20 μg) were processed for Western blotting using a primary antibody against PPARγ (1:1,000). Expression levels of PPARγ were normalized to that of actin content with an anti-β-actin antibody (1:8,000). Statistical analyses were performed using one-way ANOVA followed by Tukey's multiple comparison test. Statistical significance vs. respective control: *P < 0.05 (n = 4–5).
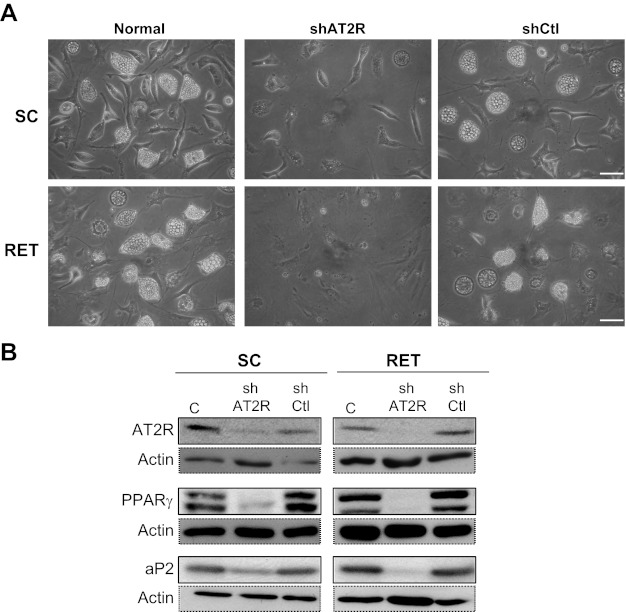
Finally, primary adipocytes, infected with lentivirus expressing shRNA-mediated knockdown of AT2R (shAT2R) exhibited a spindle-like or polygonal morphology, without any lipid droplets, whereas cells infected with control shRNA (shCtl) differentiated normally (Fig. 6A). In shAT2R-infected cells, AT2R protein expression was abolished, and expression of the differentiation markers PPARγ and aP2 remained very low or absent (Fig. 6B). These results clearly indicate that AT2R is critical for initiating preadipocyte differentiation and lipid accumulation.
Fig. 6.
Effect of inactivation of AT2R expression on adipocyte differentiation. SC and RET preadipocytes were noninfected (normal) or infected with control (shCtl) or shAT2R lentivirus, followed by initiation of differentiation by adding a standard differentiation medium for 8 days. A: illustrations of SC and RET differentiated preadipocytes untreated (without shRNA), with control shRNA (shCtl), and with AT2R shRNA (shAT2R) at day 8. Scale bars, 60 μm. B: cell lysates from conditions in A (20 μg) were analyzed by immunoblotting with antibodies against AT2R (1:500), PPARγ (1:1,000), or aP2/FABP4 (1:2,000) and normalized to anti-β-actin antibody (1:8,000) for protein content. Data shown are representative results from 3 independent experiments.
Six-week treatment with C21/M24 restores adipocyte morphology and improves insulin resistance in vivo.
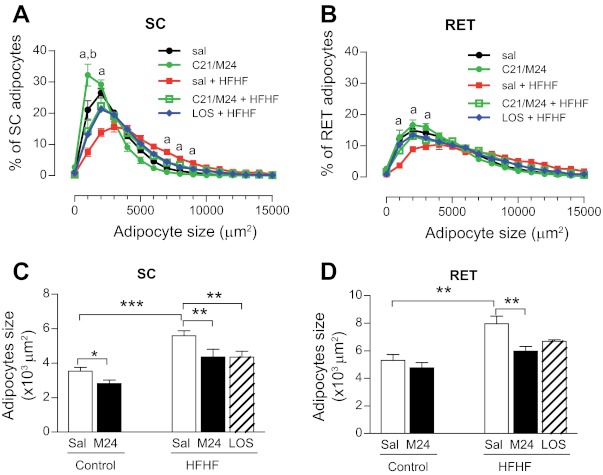
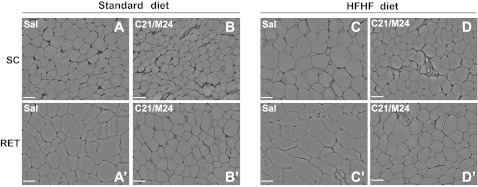
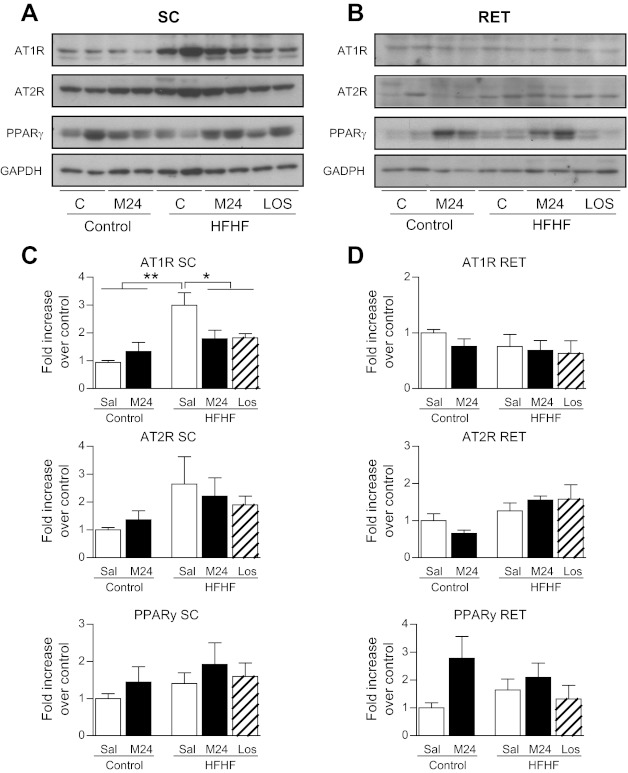
In an attempt to correlate the above in vitro observations with the in vivo situation, rats were fed an HFHF diet for 6 wk to induce insulin resistance. Analyses of adipocyte size distribution in several adipose tissue depots revealed that the HFHF diet resulted in a significant shift toward larger adipocytes, particularly in SC and RET adipose depots (Fig. 7, A–D, and Fig. 8C, C′ vs. A, A′). Treatment of HFHF-fed rats with C21/M24 or with the AT1R antagonist losartan prevented this shift and restored the normal distribution profile of adipocyte size (Fig. 7, A and B, and Fig. 8D, D′ vs. A, A′), and the overall mean diameter of LD decreased both in SC and RET adipocytes (Fig. 7, C and D). These results indicate that both blockade of AT1R and stimulation of AT2R induced the same effect on adipocyte size distribution in HFHF-fed rats. In addition, C21/M24 treatment decreased SC adipocyte size in the control group but not in RET adipose tissue (Fig. 7, A and C, and Fig. 8B, B′ vs. A, A′). Western blot analyses indicated that, in SC adipose tissues, HFHF diet for 6 wk increased AT1R expression compared with control groups and that this increase was prevented by both C21/M24 and losartan. No significant differences between groups were observed among other measured protein expression levels, except for a tendency for C21/M24 to increase PPARγ in RET adipose tissues in control groups (Fig. 9, A–D).
Fig. 7.
Effect of selective stimulation of AT2R as well as AT1R blockade on adipocyte size distribution in control and high-fat/high-fructose (HFHF)-fed rats. Rats were fed for 6 wk with standard laboratory rodent diet or HFHF diet. Control groups were treated with saline or C21/M24 (0.3 mg·kg−1·day−1), the HFHF group with saline, C21/M24 (M24; 0.3 mg·kg−1·day−1) or losartan (1 mg·kg−1·day−1), as described in research design and methods. Adipose tissues were prepared for histology. Paraffin sections (5 μM) were stained with hematoxylin and eosin (H&E), and images were acquired and analyzed as described in research design and methods. A and B: adipocyte size distribution from SC (A) and RET (B) adipose tissue from control and HFHF rats. Statistical significance vs. respective control: aP < 0.01 between saline, C21/M24 in control chow diet with saline, and C21/M24 in HFHF groups, and between Los in HFHF vs. saline in HFHF groups; bP < 0.001, C21/M24 vs. saline in standard chow diet-fed control groups. Adipocyte size areas from SC (C) and RET (D) adipose tissue were measured as described in research design and methods. Data are presented as means ± SE (n = 8–10). Statistical analyses were performed using one-way ANOVA followed by Newman-Keuls post hoc test. Statistical significance: *P < 0.05, **P < 0.01, and ***P < 0.001.
Fig. 8.
Adipocyte size in adipose tissue from rats fed HFHF diet and treated with C21/M24. Histology of SC and RET adipose tissue from Wistar rats fed control or HFHF diet. Saline (Sal; A, A′), C21/M24 (B, B′), Sal in HFHF (C, C′), and C21/M24 in HFHF (D, D′) groups are shown. Sections (5 μm) of SC and RET fat were stained with H&E. Ten images per histological section were used for analysis. Images were acquired using a Leica microscope equipped with a ×10 objective. Scale bar, 40 μm.
Fig. 9.
PPARγ, AT1R, and AT2R protein expression in rats fed standard (control) and HFHF diets for 6 wk. Control groups were treated with saline or C21/M24 (0.3 mg·kg−1·day−1), the HFHF group with saline (Sal), C21/M24 (0.3 mg·kg−1·day−1), or losartan (1 mg·kg−1·day−1), as described in research design and methods. Adipose tissues were prepared for Western blotting. A and B: cell lysates from SC (A) and RET (B) adipose tissue depots containing equal concentrations of protein (20 μg) were processed for Western blotting using primary antibodies against PPARγ (1:1,000), AT2R (1:500), and AT1R (1:500). The amount of loaded protein was compared by reprobing the membrane with an anti-GAPDH antibody (bottom; 1:1,000). B and C: corresponding densitometric analysis of Western blotting shown in A and B. *P < 0.05, **P < 0.01 vs. saline-treated rats in HFHF conditions.
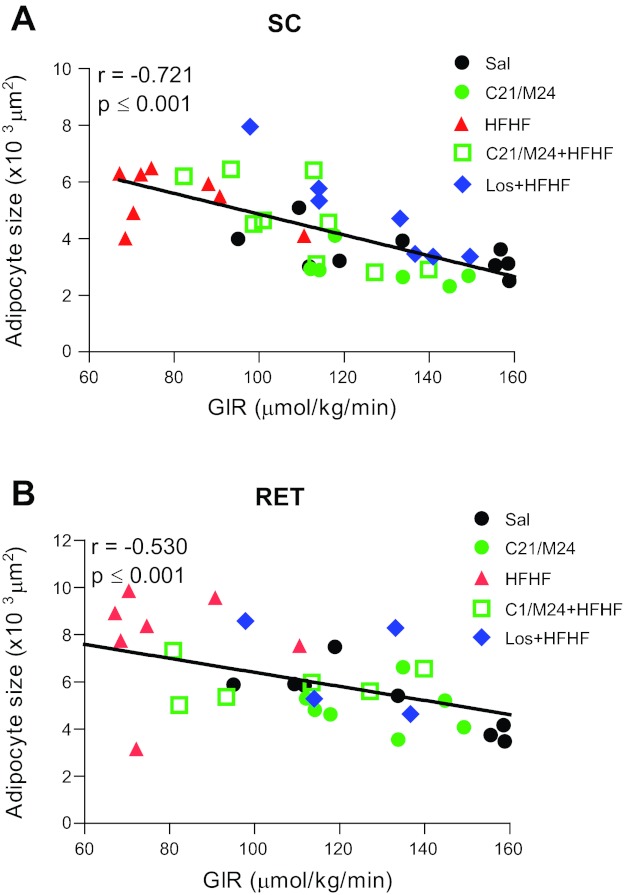
Six weeks of HFHF diet had only a small, nonsignificant impact on caloric intake and body weight but led to an increase in total adipose tissue mass without change in lean body mass (Table 3). Rats under HFHF diet developed hyperinsulinemia and insulin resistance, as shown by the decreased glucose infusion rate (GIR) during euglycemic-hyperinsulinemic clamp. C21/M24 and losartan treatment decreased hyperinsulinemia and improved diet-induced insulin resistance, as indicated by the significant increase in GIR (Table 4). Under fasting conditions, C21/M24 treatment also reduced insulin and TG levels in the control diet, but had no significant effect on NEFA and TG in the HFHF groups. In addition, we observed that only losartan was able to reverse TG content increased by HFHF diet in liver (Table 4). Of note, although there was an absence of significant changes in fasting NEFA and TG in the HFHF groups, C21/M24 improved the insulin-mediated lowering of blood NEFA and TG concentrations during the clamp studies compared with saline treatment in the HFHF group, an effect not observed with losartan treatment. Results also indicated a correlation between larger adipocytes with lower GIR in both SC and RET adipose tissues (Fig. 10, A and B).
Table 3.
Tissue weights after 6-wk treatment with C21/M24 or losartan in control- and HFHF-fed rats
| Diet |
Control |
HFHF |
|||
|---|---|---|---|---|---|
| Treatment | Sal | C21/M24 | Sal | C21/M24 | LOS |
| SC adipose tissue | 3.60 ± 0.37 | 3.49 ± 0.33 | 7.36 ± 1.07** | 8.16 ± 1.03** | 6.92 ± 0.57* |
| RET adipose tissue | 5.0 ± 0.8 | 3.8 ± 0.4 | 10.8 ± 1.2* | 11.3 ± 2.4* | 11.2 ± 1.9* |
| Gastronecmius | 5.36 ± 0.17 | 5.52 ± 0.12 | 5.43 ± 0.12 | 5.14 ± 0.24 | 5.25 ± 0.11 |
| Soleus | 0.47 ± 0.01 | 0.48 ± 0.01 | 0.52 ± 0.03 | 0.49 ± 0.02 | 0.51 ± 0.03 |
| Heart | 1.27 ± 0.03 | 1.22 ± 0.05 | 1.25 ± 0.06 | 1.23 ± 0.06 | 1.27 ± 0.03 |
| Liver | 12.7 ± 0.4 | 12.6 ± 1.1 | 12.0 ± 0.6 | 12.2 ± 1.1 | 12.8 ± 0.6 |
Values are means ± SE, in g, from 9-10 animals per group. Control, standard diet-fed rats; HFHF, high-fat/high-fructose-fed rats; SC, subcutaneous; RET, retroperitoneal; Sal, saline treatment; LOS, losartan treatment; Statistical analysis was performed using one-way ANOVA with Newman-Keuls post hoc test;
P < 0.05,
P < 0.01, difference between HFHF diet vs. saline and C21/M24 in control diet.
Table 4.
Metabolic parameters after 6-wk treatment with C21/M24 or losartan in control- and HFHF-fed rats
| Diet |
Control |
HFHF |
|||
|---|---|---|---|---|---|
| Treatment | Sal | C21/M24 | Sal | C21/M24 | LOS |
| Total caloric intake, kcal/6 wk | 100.8 ± 2.3 | 99.9 ± 2.6 | 102.3 ± 2.4 | 100.6 ± 3.8 | 94.8 ± 2.4 |
| Water intake, ml/day | 33.76 ± 1 | 39.22 ± 0.9 | 28.33 ± 2 | 33.95 ± 2.6 | 31.38 ± 3 |
| Fasting glucose, mmol/l | 8.4 ± 0.5 | 8.5 ± 0.4 | 10.0 ± 0.5 | 9.0 ± 0.6 | 9.6 ± 0.5 |
| Fasting insulin, pmol/l | 129.4 ± 60 | 77.4 ± 20b | 404.6 ± 125a | 207.4 ± 39c | 168.5 ± 33c |
| Fasting NEFA, μmol/l | 454 ± 50 | 414 ± 33 | 514.1 ± 41 | 466 ± 37 | 491.3 ± 27 |
| Fasting TG, μmol/l | 504.1 ± 52 | 369 ± 35b | 662 ± 91 | 553.4 ± 61 | 750 ± 97 |
| Liver TG content, mg/g liver | 2.15 ± 0.15f | 1.76 ± 0.20f | 3.07 ± 0.24 | 3.13 ± 0.56 | 2.08 ± 0.16f |
| Clamp NEFA, μmol/l | 0.041 ± 0.003 | 0.036 ± 0.003e | 0.051 ± 0.003 | 0.037 ± 0.003c | 0.053 ± 0.005g |
| Clamp TG, μmol/l | 0.222 ± 0.016 | 0.159 ± 0.020e | 0.264 ± 0.028 | 0.165 ± 0.013c | 0.221 ± 0.031 |
| GIR, μmol/kg/min | 133.2 ± 8.3 | 138.8 ± 7.5 | 77 ± 5.7d | 107.8 ± 5.5c | 124.7 ± 6.3e |
Values are means ± SE from 9-10 animals per group. NEFA, nonesterified fatty acids; TG, triglycerides; GIR, glucose infusion rate. Statistical analysis was performed using one way ANOVA followed by Newman-Keuls test.
P < 0.05,
P < 0.001, difference between saline in HFHF diet vs. saline and C21/M24 in control diet;
P < 0.05, difference between C21/M24 vs. saline in control diet;
P < 0.05, difference between C21/M24 vs. saline in HFHF diet and between losartan vs. saline in HFHF diet;
P < 0.05 difference between C21/M24 in control diet vs. saline in HFHF diet;
P < 0.05, difference between losartan vs. saline in HFHF diet;
P < 0.05, difference between losartan vs. C21/M24 in HFHF diet.
Fig. 10.
Correlation of large adipocytes with insulin resistance. Rats were fed standard laboratory rodent diet or HFHF diet for 6 wk. Control groups were treated with saline or C21/M24 (0.3 mg·kg−1·day−1), the HFHF group with saline (Sal), C21/M24 (M24) (0.3 mg·kg−1·day−1), or losartan (1 mg·kg−1·day−1), as described in research design and methods. A and B: SC (A) and RET (B) adipocyte size was correlated with glucose-infusion rate (GIR) measured by hyperinsulinemic-euglycemic clamp (from data of Table 4).
DISCUSSION
The role of ANG II receptors in adipocytes and in insulin resistance has generated considerable interest in recent decades (59). The present data are the first to address the direct effects of AT2R stimulation in adipocytes from SC and visceral adipose tissues. Results show that selective AT2R activation acts preferentially on SC preadipocytes and exhibits two opposite effects according to the differentiation status of the cells. In primary cultures of preadipocytes, we demonstrate that AT2R is involved in the initial steps of differentiation by favoring PPARγ expression and early lipid accumulation. However, once adipocytes reach their full capacity to store lipids, AT2R stimulation decreases the size of large lipid droplets but increases lipid accumulation. On the other hand, in a model of diet-induced insulin resistance (HFHF rats), treatment with the AT2R agonist C21/M24 or the AT1R antagonist losartan for 6 wk is sufficient to restore normal adipocyte size distribution and reduce HFHF diet-induced insulin resistance.
We found that both AT1R and AT2R were present in primary cell cultures of preadipocytes from rat SC and RET adipose tissue, thus corroborating previous results in primary cultures of human SC preadipocytes (40, 55). However, AT2R protein expression differs from its mRNA expression in RET preadipocytes. One hypothesis may be that the protein turnover of AT2R could be very slow compared with its mRNA level. Indeed, the mechanism of AT2R degradation is still unknown and may vary according to experimental model (19, 33). Thus the protein turnover of AT2R could be very slow compared with its mRNA level.
Direct activation of AT2R with C21/M24 increased the expression of PPARγ, whereas AT2R downregulation by shRNA infection blunted PPARγ expression and prevented preadipocyte differentiation in both SC and RET preadipocytes. These results thus demonstrate that AT2R is involved in the first steps of preadipocyte differentiation and suggest that PPARγ activation could represent one mechanism by which AT2R initiates differentiation, as previously shown in neuronal cells (60). However, C21/M24 accelerated the appearance of LD and selectively increased lipid accumulation only in SC preadipocytes. These discrepancies between SC and RET preadipocytes are of particular interest, since knockdown of AT2R with shRNA had the same effect in both types of adipocytes, indicating that expression of AT2R is essential, but not necessarily its activation. Such a hypothesis may be explained by the constitutive activity of the receptor prior to differentiation (12). In that condition, AT2R action may thus occur through interaction with some of its recently identified partner proteins such as promyelocytic leukemia zinc finger (PLZF) and AT2R-interacting protein (ATIP) (for recent review see Ref. 21).However, the role of these interactions is not yet fully understood.
In contrast to the C21/M24 results in which lipid storage was increased, cells stimulated solely with ANG II exhibited a decrease in lipid accumulation, suggesting that ANG II, through the AT1R, was unable to favor TG accumulation in both SC and RET adipocytes. Accordingly, Cabassi et al. (4) have shown that ANG II stimulates lipolysis in both SC and RET adipose tissue by AT1R activation in Sprague-Dawley rats. In addition, ANG II-treated rats exhibited lower adipose tissue mass and lower TG content in adipose tissue depots. These effects were inhibited by losartan. Therefore, ANG II-stimulated lipolysis by AT1R may explain the decrease in lipid accumulation observed in our in vitro model.
As largely documented (30, 31, 37, 42, 52), we observed enlarged adipose tissue in both visceral and SC adipose tissue depots in HFHF-treated rats. In this situation, C21/M24 and losartan decreased adipocyte size in SC and RET adipose tissue depots, restoring adipocytes of smaller size, even though these treatments did not change total adipose tissue mass in HFHF-fed rats. Decreasing the size of adipocytes under lipid overload may limit the damages associated with large adipocytes. Such a strategy may also increase the ability of adipocytes to further store TG. Indeed, a new hypothesis proposes that increased adipose tissue storage capacity, enabled by increasing the storage capacity of individual adipocytes as well as stimulating the differentiation of new preadipocytes, may limit adipocyte hypertrophy and improve adipose tissue function (47, 52). The inverse relationship between adipocyte size and insulin sensitivity observed herein has also been described previously (1). In various mouse and rat models of type 2 diabetes, ARB administration increased the number of differentiated adipocytes, PPARγ expression, and improved insulin sensitivity (5, 9, 13, 29, 41, 49, 61). Supporting a link between AT2R-increased PPARγ expression and AT2R-induced differentiation, PPARγ-activating drugs (such as thiazolidinediones) have been described as the prototypical example of the potent antidiabetic effects of PPARγ-mediated adipose tissue expansion and improvement in metabolic functions (28). The present study also demonstrates that direct and selective AT2R stimulation can reproduce the effects of AT1R blockers and are in agreement with studies conducted in preadipocyte cell lines (6) and in AT2R-deficient mice (24, 49), thus suggesting a role of AT2R in adipogenesis. However, certain results obtained herein are in contrast with whole body AT2R-deficient mice studies from Yvan-Charvet et al. (57). The major difference is that we show that AT2R activation improves insulin sensitivity by decreasing the size of large adipocytes and by increasing new, small adipocytes without inducing obesity, whereas Yvan-Charvet and colleagues (57, 58) showed that AT2R deletion improved insulin sensitivity and prevented diet-induced obesity. In both models, adipocyte physiology was restored. However, adipose tissue from AT2R-deficient mice appears to be resistant to normal expansion upon physiological insult such as a high-fat diet. It is known that adipose tissue is an important endocrine tissue and a sink for lipids to prevent lipotoxicity (47, 52). Thus, stimulating AT2R may be better than blocking it to restore normal adipocyte physiology. Our results are nevertheless in agreement with their studies on adipose tissue since we show that AT2R expression is essential for adipogenesis in vitro, an effect similar to a reduction in adipogenesis in AT2R-deficient mice. Moreover, our study also confirms that stimulating AT2R not only does not induce obesity itself, an effect that might be expected from the results from AT2R-deficient mice, but also improves insulin sensitivity.
We also observed some discrepancies between the effects of C21/M24 in vitro vs. in vivo. Indeed, C21/M24 improved adipocyte size in both SC and RET adipose tissue, but in vitro C21/M24 acted only in SC preadipocytes. Besides the fact that C21/M24 could act on other tissues or cells that could improve adipocyte size, lower AT1R expression in SC adipose tissue of C21/M24- and losartan-treated animals cannot explain the improvement of adipocyte size, since in RET adipose tissue AT1R expression is similar among all groups. Moreover, some distinctions should be made about the projection of our pharmacological in vitro results to in vivo, since we worked on differentiated preadipocytes (multilocular) and not fully mature (unilocular) adipocytes as in adipose tissue. Indeed, our in vitro model aims more at the differentiation process that may also affect adipocyte size in vivo than the effect of AT2R and AT1R on adipocyte size from mature adipocyte. On the basis of our results and others' (24, 57), we think that the effect of C21/M24 and losartan on adipocytes size is more due to an increase in number of new adipocytes by increasing adipogenesis, which increases the lipid storage capacity of the adipose tissue rather than by a direct effect on reduction of adipocyte size.
In conditions of insulin resistance and after hyperinsulinemic-euglycemic clamp, Hsieh et al. (22) showed that AT1R blockade increased whole body glucose uptake, in part due to an unmasked AT2R activation by AT1R blockade. In our model of HFHF diet, both C21/M24 and losartan were able to improve insulin resistance. To date, our study is the first to document that selective AT2R activation can also improve insulin resistance as previously depicted with AT1R antagonists. The fact that we did not observe a significant improvement in fasting glucose, TG, or NEFA with C21/M24 or losartan may be due to our experimental approach, designed to induce a moderate state of insulin resistance without any excessive disturbances in AT1R-mediating effects. Indeed, losartan is well known to attenuate several of the deleterious effects associated with well-established insulin resistance, as observed in longer treatment durations in various diet-induced obesity or genetic models of obesity (11, 39, 61). Hence, we selected a moderate duration of HFHF diet to target adipose tissue storage capacity without inducing hypertension- or obesity-associated damage such as inflammation or oxidative stress.
Our results did identify certain differences between AT2R activation and AT1R blockade, especially on TG levels. C21/M24 decreased fasting TG levels in control diet and also had a tendency to reduce fasting TG levels in HFHF-fed animals. Whereas no changes in TG level were observed with losartan in fasting state, we did show a reduction in TG content in liver compared with the HFHF and C21/M24+HFHF groups. Similar results have also been reported by Ran et al. (39), which observed less TG content in liver of obese Zucker rats treated for 4 wk with olmesartan, albeit with no significant changes in fasting TG level. In addition, we observed an increase in insulin-mediated NEFA and TG suppression during clamp studies with C21/M24 but not with losartan. Thus, from these results it is our hypothesis that losartan is less potent than C21/M24 specifically with regard to increasing insulin sensitivity for systemic lipid metabolism, although this hypothesis remains to be explored. Nonetheless, even with high TG levels, losartan-treated rats were more insulin sensitive for glucose metabolism than C21/M24-treated rats. These results may be explained by a different regulation of insulin signaling, lipolysis, lipoprotein lipase activity, and NEFA uptake proteins by AT2R activation vs. AT1R blockade (26).
In conclusion, the present study demonstrates that stimulation of AT2R promotes functional differentiation of SC and RET preadipocytes and improves lipid storage in SC adipocytes but not in visceral adipocytes. Moreover, in vivo AT2R activation is able to restore the adipocyte cell size profile and to reduce the insulin resistance induced by HFHF diet. Therefore, since AT2R is expressed in a limited number of tissues compared with AT1R, C21/M24, in association with ARBs, could represent a new and attractive therapeutic tool for prevention and management of patients presenting a potential risk of developing metabolic complications of obesity.
GRANTS
This work was supported by grants from the Canadian Diabetes Association to N. Gallo-Payet, A. C. Carpentier, M.-F. Langlois, and J.-P. Baillargeon and by the Canada Research Chairs Program to N. Gallo-Payet. J.-P. Baillargeon and M.-F. Langlois are recipients of a Chercheur-boursier senior scholarship from the Fonds de la Recherche en Santé du Québec. N. Gallo-Payet is a recipient of a Canada Research Chair in Endocrinology of the Adrenal Gland. A. C. Carpentier is the recipient of the CIHR-GSK Chair in Diabetes. All are members of the FRSQ-funded Centre de recherche clinique Étienne-Le Bel.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.S., J.-P.B., M.-F.L., A.H., A.C.C., and N.G.-P. conception and design of research; M.S., S.P., M.-O.G., S.M.L., C.R., M.A., and C.W. performed experiments; M.S., S.P., M.-O.G., S.M.L., C.R., J.-P.B., M.-F.L., A.C.C., and N.G.-P. analyzed data; M.S., J.-P.B., M.-F.L., A.C.C., and N.G.-P. interpreted results of experiments; M.S., S.P., M.-O.G., S.M.L., and N.G.-P. prepared figures; M.S., C.R., and N.G.-P. drafted manuscript; M.S., M.-O.G., S.M.L., J.-P.B., M.-F.L., A.H., A.C.C., and N.G.-P. edited and revised manuscript; M.S., S.P., M.-O.G., S.M.L., C.R., J.-P.B., M.-F.L., M.A., C.W., A.H., A.C.C., and N.G.-P. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank our technicians Lucie Chouinard and Lucie Bouffard (both from Division of Endocrinology, Department of Medicine, Faculté de médecine et des sciences de la santé, Université de Sherbrooke, Sherbrooke, QC, Canada) for experimental assistance with cell cultures and metabolic measurements. We are also grateful to Dr. Marcel D. Payet (Department of Physiology and Biophysics, Faculté de médecine et des sciences de la santé, Université de Sherbrooke) for developing morphometric software and assistance with data analyses and to Pierre Pothier for critical reading of the manuscript and editorial assistance (Les Services PM-SYS Enr., Sherbrooke). We also thank Audrey Emond and Thomas Grenier-Larouche (students from our group) for stimulating discussions. Dr. Nicole Gallo-Payet is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
REFERENCES
- 1. Arner E, Westermark PO, Spalding KL, Britton T, Ryden M, Frisen J, Bernard S, Arner P. Adipocyte turnover: relevance to human adipose tissue morphology. Diabetes 59: 105– 109, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arner P, Arner E, Hammarstedt A, Smith U. Genetic predisposition for Type 2 diabetes, but not for overweight/obesity, is associated with a restricted adipogenesis. PLoS One 6: e18284, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Burson JM, Aguilera G, Gross KW, Sigmund CD. Differential expression of angiotensin receptor 1A and 1B in mouse. Am J Physiol Endocrinol Metab 267: E260– E267, 1994 [DOI] [PubMed] [Google Scholar]
- 4. Cabassi A, Coghi P, Govoni P, Barouhiel E, Speroni E, Cavazzini S, Cantoni AM, Scandroglio R, Fiaccadori E. Sympathetic modulation by carvedilol and losartan reduces angiotensin II-mediated lipolysis in subcutaneous and visceral fat. J Clin Endocrinol Metab 90: 2888– 2897, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Clasen R, Schupp M, Foryst-Ludwig A, Sprang C, Clemenz M, Krikov M, Thone-Reineke C, Unger T, Kintscher U. PPARgamma-activating angiotensin type-1 receptor blockers induce adiponectin. Hypertension 46: 137– 143, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Darimont C, Vassaux G, Ailhaud G, Negrel R. Differentiation of preadipose cells: paracrine role of prostacyclin upon stimulation of adipose cells by angiotensin-II. Endocrinology 135: 2030– 2036, 1994 [DOI] [PubMed] [Google Scholar]
- 7. de Kloet AD, Krause EG, Woods SC. The renin angiotensin system and the metabolic syndrome. Physiol Behav 100: 525– 534, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature 444: 881– 887, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Di Filippo C, Lampa E, Tufariello E, Petronella P, Freda F, Capuano A, D'Amico M. Effects of irbesartan on the growth and differentiation of adipocytes in obese zucker rats. Obes Res 13: 1909– 1914, 2005 [DOI] [PubMed] [Google Scholar]
- 10. Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226: 497– 509, 1957 [PubMed] [Google Scholar]
- 11. Foryst-Ludwig A, Hartge M, Clemenz M, Sprang C, Hess K, Marx N, Unger T, Kintscher U. PPARgamma activation attenuates T-lymphocyte-dependent inflammation of adipose tissue and development of insulin resistance in obese mice. Cardiovasc Diabetol 9: 64, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Funke-Kaiser H, Reinemund J, Steckelings UM, Unger T. Adapter proteins and promoter regulation of the angiotensin II type 2 receptor—implications for cardiac pathophysiology. J Renin Angiotensin Aldosterone Syst 11: 7– 17, 2010 [DOI] [PubMed] [Google Scholar]
- 13. Furuhashi M, Ura N, Takizawa H, Yoshida D, Moniwa N, Murakami H, Higashiura K, Shimamoto K. Blockade of the renin-angiotensin system decreases adipocyte size with improvement in insulin sensitivity. J Hypertens 22: 1977– 1982, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Georgsson J, Skold C, Botros M, Lindeberg G, Nyberg F, Karlen A, Hallberg A, Larhed M. Synthesis of a new class of druglike angiotensin II C-terminal mimics with affinity for the AT2 receptor. J Med Chem 50: 1711– 1715, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Golan R, Shelef I, Rudich A, Gepner Y, Shemesh E, Chassidim Y, Harman-Boehm I, Henkin Y, Schwarzfuchs D, Ben Avraham S, Witkow S, Liberty IF, Tangi-Rosental O, Sarusi B, Stampfer MJ, Shai I. Abdominal superficial subcutaneous fat: a putative distinct protective fat subdepot in type 2 diabetes. Diabetes Care 35: 640– 647, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol 9: 367– 377, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hasegawa G, Fukui M, Hosoda H, Asano M, Harusato I, Tanaka M, Shiraishi E, Senmaru T, Sakabe K, Yamasaki M, Kitawaki J, Fujinami A, Ohta M, Obayashi H, Nakamura N. Telmisartan, an angiotensin II type 1 receptor blocker, prevents the development of diabetes in male Spontaneously Diabetic Torii rats. Eur J Pharmacol 605: 164– 169, 2009 [DOI] [PubMed] [Google Scholar]
- 18. Hausman D, Park H, Hausman G. Isolation and culture of preadipocytes from rodent white adipose tissue. In: Adipose Tissue Protocols, edited by Yang K. New York: Humana, 2008, p. 201–219 [DOI] [PubMed] [Google Scholar]
- 19. Hein L, Meinel L, Pratt RE, Dzau VJ, Kobilka BK. Intracellular trafficking of angiotensin II and its AT1 and AT2 receptors: evidence for selective sorting of receptor and ligand. Mol Endocrinol 11: 1266– 1277, 1997 [DOI] [PubMed] [Google Scholar]
- 20. Henriksen EJ. Improvement of insulin sensitivity by antagonism of the renin-angiotensin system. Am J Physiol Regul Integr Comp Physiol 293: R974– R980, 2007 [DOI] [PubMed] [Google Scholar]
- 21. Horiuchi M, Iwanami J, Mogi M. Regulation of angiotensin II receptors beyond the classical pathway. Clin Sci (Lond) 123: 193– 203, 2012 [DOI] [PubMed] [Google Scholar]
- 22. Hsieh PS, Tai YH, Loh CH, Shih KC, Cheng WT, Chu CH. Functional interaction of AT1 and AT2 receptors in fructose-induced insulin resistance and hypertension in rats. Metabolism 54: 157– 164, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Ibrahim MM. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev 11: 11– 18, 2010 [DOI] [PubMed] [Google Scholar]
- 24. Iwai M, Tomono Y, Inaba S, Kanno H, Senba I, Mogi M, Horiuchi M. AT2 receptor deficiency attenuates adipocyte differentiation and decreases adipocyte number in atherosclerotic mice. Am J Hypertens 22: 784– 791, 2009 [DOI] [PubMed] [Google Scholar]
- 25. Jing F, Mogi M, Horiuchi M. Role of renin-angiotensin-aldosterone system in adipose tissue dysfunction. Mol Cell Endocrinol 2012. March 23 [Epub ahead of print] PMID: 22465098 [DOI] [PubMed] [Google Scholar]
- 26. Jones BH, Standridge MK, Moustaid N. Angiotensin II increases lipogenesis in 3T3-L1 and human adipose cells. Endocrinology 138: 1512– 1519, 1997 [DOI] [PubMed] [Google Scholar]
- 27. Kim JY, van de Wall E, Laplante M, Azzara A, Trujillo ME, Hofmann SM, Schraw T, Durand JL, Li H, Li G, Jelicks LA, Mehler MF, Hui DY, Deshaies Y, Shulman GI, Schwartz GJ, Scherer PE. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest 117: 2621– 2637, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Laplante M, Festuccia WT, Soucy G, Gelinas Y, Lalonde J, Berger JP, Deshaies Y. Mechanisms of the depot specificity of peroxisome proliferator-activated receptor gamma action on adipose tissue metabolism. Diabetes 55: 2771– 2778, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Lee MH, Song HK, Ko GJ, Kang YS, Han SY, Han KH, Kim HK, Han JY, Cha DR. Angiotensin receptor blockers improve insulin resistance in type 2 diabetic rats by modulating adipose tissue. Kidney Int 74: 890– 900, 2008 [DOI] [PubMed] [Google Scholar]
- 30. Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 117: 175– 184, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lundgren M, Svensson M, Lindmark S, Renstrom F, Ruge T, Eriksson JW. Fat cell enlargement is an independent marker of insulin resistance and “hyperleptinaemia”. Diabetologia 50: 625– 633, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Menard SL, Croteau E, Sarrhini O, Gelinas R, Brassard P, Ouellet R, Bentourkia M, van Lier JE, Des Rosiers C, Lecomte R, Carpentier AC. Abnormal in vivo myocardial energy substrate uptake in diet-induced type 2 diabetic cardiomyopathy in rats. Am J Physiol Endocrinol Metab 298: E1049– E1057, 2010 [DOI] [PubMed] [Google Scholar]
- 33. Mogi M, Iwai M, Horiuchi M. Emerging concepts of regulation of angiotensin II receptors: new players and targets for traditional receptors. Arterioscler Thromb Vasc Biol 27: 2532– 2539, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Munoz MC, Giani JF, Dominici FP, Turyn D, Toblli JE. Long-term treatment with an angiotensin II receptor blocker decreases adipocyte size and improves insulin signaling in obese Zucker rats. J Hypertens 27: 2409– 2420, 2009 [DOI] [PubMed] [Google Scholar]
- 35. Ohinata K, Fujiwara Y, Fukumoto S, Iwai M, Horiuchi M, Yoshikawa M. Angiotensin II and III suppress food intake via angiotensin AT(2) receptor and prostaglandin EP(4) receptor in mice. FEBS Lett 582: 773– 777, 2008 [DOI] [PubMed] [Google Scholar]
- 36. Olivares-Reyes JA, Arellano-Plancarte A, Castillo-Hernandez JR. Angiotensin II and the development of insulin resistance: implications for diabetes. Mol Cell Endocrinol 302: 128– 139, 2009 [DOI] [PubMed] [Google Scholar]
- 37. Pausova Z. From big fat cells to high blood pressure: a pathway to obesity-associated hypertension. Curr Opin Nephrol Hypertens 15: 173– 178, 2006 [DOI] [PubMed] [Google Scholar]
- 38. Perlstein TS, Henry RR, Mather KJ, Rickels MR, Abate NI, Grundy SM, Mai Y, Albu JB, Marks JB, Pool JL, Creager MA. Effect of angiotensin receptor blockade on insulin sensitivity and endothelial function in abdominally obese hypertensive patients with impaired fasting glucose. Clin Sci (Lond) 122: 193– 202, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ran J, Hirano T, Adachi M. Angiotensin II type 1 receptor blocker ameliorates overproduction and accumulation of triglyceride in the liver of Zucker fatty rats. Am J Physiol Endocrinol Metab 287: E227– E232, 2004 [DOI] [PubMed] [Google Scholar]
- 40. Schling P. Expression of angiotensin II receptors type 1 and type 2 in human preadipose cells during differentiation. Horm Metab Res 34: 709– 715, 2002 [DOI] [PubMed] [Google Scholar]
- 41. Shiuchi T, Iwai M, Li HS, Wu L, Min LJ, Li JM, Okumura M, Cui TX, Horiuchi M. Angiotensin II type-1 receptor blocker valsartan enhances insulin sensitivity in skeletal muscles of diabetic mice. Hypertension 43: 1003– 1010, 2004 [DOI] [PubMed] [Google Scholar]
- 42. Skurk T, Alberti-Huber C, Herder C, Hauner H. Relationship between adipocyte size and adipokine expression and secretion. J Clin Endocrinol Metab 92: 1023– 1033, 2007 [DOI] [PubMed] [Google Scholar]
- 43. Speth R, Kim K. Discrimination of two angiotensin II receptor subtypes with a selective agonist analogue of angiotensin II, p-aminophenylalanine6 angiotensin II. Biochem Biophys Res Commun 169: 997– 1006, 1990 [DOI] [PubMed] [Google Scholar]
- 44. Steckelings UM, Larhed M, Hallberg A, Widdop RE, Jones ES, Wallinder C, Namsolleck P, Dahlof B, Unger T. Non-peptide AT2-receptor agonists. Curr Opin Pharmacol 11: 1– 6, 2011 [DOI] [PubMed] [Google Scholar]
- 45. Steckelings UM, Rompe F, Kaschina E, Namsolleck P, Grzesiak A, Funke-Kaiser H, Bader M, Unger T. The past, present and future of angiotensin II type 2 receptor stimulation. J Renin Angiotensin Aldosterone Syst 11: 67– 73, 2010 [DOI] [PubMed] [Google Scholar]
- 46. Steckelings UM, Unger T. Angiotensin II type 2 receptor agonists—where should they be applied? Expert Opin Investig Drugs 21: 763– 766, 2012 [DOI] [PubMed] [Google Scholar]
- 47. Tan CY, Vidal-Puig A. Adipose tissue expandability: the metabolic problems of obesity may arise from the inability to become more obese. Biochem Soc Trans 36: 935– 940, 2008 [DOI] [PubMed] [Google Scholar]
- 48. Tian F, Luo R, Zhao Z, Wu Y, Ban D. Blockade of the RAS increases plasma adiponectin in subjects with metabolic syndrome and enhances differentiation and adiponectin expression of human preadipocytes. Exp Clin Endocrinol Diabetes 118: 258– 265, 2009 [DOI] [PubMed] [Google Scholar]
- 49. Tomono Y, Iwai M, Inaba S, Mogi M, Horiuchi M. Blockade of AT1 receptor improves adipocyte differentiation in atherosclerotic and diabetic models. Am J Hypertens 21: 206– 212, 2008 [DOI] [PubMed] [Google Scholar]
- 50. van der Zijl NJ, Moors CC, Goossens GH, Blaak EE, Diamant M. Does interference with the renin-angiotensin system protect against diabetes? Evidence and mechanisms. Diabetes Obes Metab 14: 586– 595, 2012 [DOI] [PubMed] [Google Scholar]
- 51. Verdonk K, Danser AH, van Esch JH. Angiotensin II type 2 receptor agonists: where should they be applied? Expert Opin Investig Drugs 21: 501– 513, 2012 [DOI] [PubMed] [Google Scholar]
- 52. Virtue S, Vidal-Puig A. Adipose tissue expandability, lipotoxicity and the Metabolic Syndrome—an allostatic perspective. Biochim Biophys Acta 1801: 338– 349, 2010 [DOI] [PubMed] [Google Scholar]
- 53. Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev 21: 697– 738, 2000 [DOI] [PubMed] [Google Scholar]
- 54. Wan Y, Wallinder C, Plouffe B, Beaudry H, Mahalingam AK, Wu X, Johansson B, Holm M, Botoros M, Karlen A, Pettersson A, Nyberg F, Fandriks L, Gallo-Payet N, Hallberg A, Alterman M. Design, synthesis, and biological evaluation of the first selective nonpeptide AT2 receptor agonist. J Med Chem 47: 5995– 6008, 2004 [DOI] [PubMed] [Google Scholar]
- 55. Ye Z, Wu X, Jiang J. Expression changes of angiotensin II pathways and bioactive mediators during human preadipocytes-visceral differentiation. Metabolism Clin and Exp 58: 1288– 1296, 2009 [DOI] [PubMed] [Google Scholar]
- 56. Yu L, Zheng M, Wang W, Rozanski GJ, Zucker IH, Gao L. Developmental changes in AT1 and AT2 receptor-protein expression in rats. J Renin Angiotensin Aldosterone Syst 11: 214– 221, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yvan-Charvet L, Even P, Bloch-Faure M, Guerre-Millo M, Moustaid-Moussa N, Ferre P, Quignard-Boulange A. Deletion of the angiotensin type 2 receptor (AT2R) reduces adipose cell size and protects from diet-induced obesity and insulin resistance. Diabetes 54: 991– 999, 2005 [DOI] [PubMed] [Google Scholar]
- 58. Yvan-Charvet L, Massiera F, Lamande N, Ailhaud G, Teboul M, Moustaid-Moussa N, Gasc JM, Quignard-Boulange A. Deficiency of angiotensin type 2 receptor rescues obesity but not hypertension induced by overexpression of angiotensinogen in adipose tissue. Endocrinology 150: 1421– 1428, 2009 [DOI] [PubMed] [Google Scholar]
- 59. Yvan-Charvet L, Quignard-Boulange A. Role of adipose tissue renin-angiotensin system in metabolic and inflammatory diseases associated with obesity. Kidney Int 79: 162– 168, 2011 [DOI] [PubMed] [Google Scholar]
- 60. Zhao Y, Foryst-Ludwig A, Bruemmer D, Culman J, Bader M, Unger T, Kintscher U. Angiotensin II induces peroxisome proliferator-activated receptor gamma in PC12W cells via angiotensin type 2 receptor activation. J Neurochem 94: 1395– 1401, 2005 [DOI] [PubMed] [Google Scholar]
- 61. Zorad S, Dou JT, Benicky J, Hutanu D, Tybitanclova K, Zhou J, Saavedra JM. Long-term angiotensin II AT1 receptor inhibition produces adipose tissue hypotrophy accompanied by increased expression of adiponectin and PPARgamma. Eur J Pharmacol 552: 112– 122, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]