Abstract
Acid-sensing ion channels (ASICs) are sodium channels gated by extracellular protons. ASIC1a channels possess intersubunit Cl−-binding sites in the extracellular domain, which are highly conserved between ASIC subunits. We previously found that anions modulate ASIC1a gating via these sites. Here we investigated the effect of anion substitution on native ASICs in rat sensory neurons and heterologously expressed ASIC2a and ASIC3 channels by whole cell patch clamp. Similar to ASIC1a, anions modulated the kinetics of desensitization of other ASIC channels. However, unlike ASIC1a, anions also modulated the pH dependence of activation. Moreover, the order of efficacy of different anions to modulate ASIC2a and -3 was very different from that of ASIC1a. More surprising, mutations of conserved residues that form an intersubunit Cl−-binding site in ASIC1a only partially abrogated the effects of anion modulation of ASIC2a and had no effect on anion modulation of ASIC3. The effects of anions on native ASICs in rat dorsal root ganglion neurons mimicked those in heterologously expressed ASIC1a/3 heteromeric channels. Our data show that anions modulate a variety of ASIC properties and are dependent on the subunit composition, and the mechanism of modulation for ASIC2a and -3 is distinct from that of ASIC1a. We speculate that modulation of ASIC gating by Cl− is a novel mechanism to sense shifts in extracellular fluid composition.
Keywords: ASICs, protons, ion channels
acid-sensing ion channels (ASICs) are cation channels activated by extracellular protons. They are members of the DEG/epithelial sodium channel (ENaC) family of ion channels and include four genes that encode at least six subunits in mammals: ASIC1a, ASIC1b, ASIC2a, ASIC2b, ASIC3, and ASIC4 (24, 34). Functional ASIC channels consist of a complex of three subunits; individual subunits form homotrimeric channels, whereas two or more subunits can assemble to generate heterotrimeric channels. Each subunit composition displays unique biophysical and pharmacological properties (5, 18, 20). ASICs are expressed primarily in neurons and are evolutionarily conserved; they have been identified in neurons of birds, fish, lamprey, and simple chordates (11).
The recently resolved ASIC1a crystal structure has added significantly to our understanding of ASIC channels (20). The overall structure of a single subunit resembles an upheld arm with a loosely clenched fist, the two transmembrane domains akin to the forearm and the fist representing the large extracellular loop (occupying 70% of the total protein). A surprising finding was an intersubunit Cl−-binding site in the extracellular “thumb” domain formed by two residues (Arg310 and Glu314) on an α-helix of one subunit and a residue (Lys212) from an adjacent subunit, residues that are conserved in all H+-gated ASIC isoforms. We recently tested the functional effects of anions on the pH-dependent gating of heterologously expressed ASIC1a, as well as native ASICs in hippocampal neurons, where ASIC1a is the predominant subunit (2). We found that anion substitution altered the kinetics of desensitization and tachyphylaxis, and mutation of the residues that form the anion-binding site in ASIC1a completely abolished the modulatory effects of anions (22). In particular, we found that Cl−, more than other anions, stabilized an open conformation of the channel, resulting in slowing of desensitization, and it facilitated tachyphylaxis (a diminishment of current with repeated exposure to acidic solution). These modulatory effects were mirrored in cultured hippocampal neurons and anion substitution altered ASIC-dependent cell death in neurons exposed to acidosis.
Whereas in the central nervous system ASIC1a homomers and ASIC1a/2 heteromers are the principle channels, in peripheral sensory neurons ASICs primarily consist of heteromeric channels with ASIC3 being a necessary component (5, 35). ASIC3 is activated by small drops in pH and is highly expressed in sensory nerves that innervate tissues with high metabolic capacity such as cardiac and skeletal muscle (17, 26, 31). Both pharmacological and genetic manipulations of ASIC3 support its role as a metabolic or pain sensor during acidic conditions (13, 29, 32). In addition to being activated by protons, ASIC3 is potentiated by physiological concentrations of extracellular lactate, ATP, arachadonic acid, and perhaps other metabolites (1, 6, 19, 25). In combination with ASIC3, ASIC1a, and -2a also contribute to the formation of ASICs in peripheral sensory neurons (5), with different heteromeric combinations being variably expressed in specific subpopulations. For example, we recently found that the ASIC channels in cardiac sensory neurons are composed of ASIC2a/3 heteromeric channels (17), whereas in skeletal muscle sensory neurons ASIC1a, -2, and -3 subunits all contribute (14a).
Since the residues involved in Cl−-binding of ASIC1a are completely conserved between all H+-gated ASIC subunits, here we studied heterologously expressed ASIC2a and ASIC3 channels to see if anion modulation is a common characteristic among ASICs. In addition, we tested the effect of anion substitution on native ASICs in mammalian sensory neurons. We report that anions do modulate all ASICs studied; however, the modulatory effects and the mechanisms were different dependent on the subunit composition of the channels.
MATERIALS AND METHODS
Heterologous expression of cDNA in Chinese hamster ovarian cells.
Mouse ASIC1a, mouse ASIC2a, and rat ASIC3 in pMT3 vectors were cloned as previously described (5, 14). Point mutations were generated by site-directed mutagenesis using the QuikChange kit (Stratagene, La Jolla, CA) and sequenced at the University of Iowa DNA core. Chinese hamster ovarian (CHO) cells plated at ∼10% confluence were transfected with ASICs cDNAs (0.18 μg/1.5 ml for wild-type ASICs or 1.0 μg/1.5 ml for some ASIC3 mutations) using Transfast transfection reagent (Promega, Madison, WI) in 35-mm dishes according to the manufacturer's recommendations. For ASIC subunit coexpression experiments, cDNAs were transfected at equal concentrations. DsRed (Express-C1, Clontech) cDNA (1.82 μg/1.5 ml for wild-type ASICs or 1.0 μg/1.5 ml for some mutations) and GFP (pGreen Lantern; Life Technologies) cDNA (0.33 μg /1.5 ml) was cotransfected to keep cDNA concentrations constant and facilitate detection of expressing cells by epifluorescence. Cells were cultured in F-12 nutrient medium (GIBCO) supplemented with 10% FBS and 1% penicillin-streptomycin at 37°C, 5% CO2 and were studied 48–72 h after transfection.
Culture of rat DRG neurons.
Rat lumbar DRG neurons were cultured as previously described (4). Briefly, rats were sedated and then decapitated, and the DRG neurons (L4–5) were collected, dissociated with papain and collagenase/dispase, plated on poly-d-lysine/laminin-coated 35-mm plastic dishes, and stored at 37°C in F1–2 medium supplemented with nerve growth factor. Cells were studied 18–48 h after plating. Animal care and experiments were approved by the Institutional Animal Care and Use Committee of the University of Iowa.
Electrophysiology.
Whole cell patch-clamp recordings (at −70 mV) from CHO cells and DRG neurons were performed at room temperature with an Axopatch 200B amplifier (Axon Instruments, Foster City, CA) and were acquired and analyzed with PULSE/PULSEFIT 8.70 (HEKA Electronics, Lambrecht, Germany) and IGOR Pro 6.01 (WaveMetrics, Lake Oswego, OR) software or pCLAMP 8.2 (Axon Instruments). Currents were filtered at 5 kHz and sampled at 2 or 0.2 kHz. Micropipettes (2–4 MΩ) were filled with internal solution (mM): 100 KCl, 10 EGTA, 40 HEPES, and 5 MgCl2, pH 7.4 with KOH. For Cl−-free internal solution, KCl and MgCl2 were substituted with K-gluconate and Mg-gluconate2. Standard external solutions contained the following (mM): 120 NaCl, 5 KCl, 1 MgCl2, 2 CaCl2, 10 HEPES, and 10 MES; pH was adjusted with tetramethylammonium hydroxide (TMA-OH), and osmolarity was adjusted with tetramethylammonium chloride (TMA-Cl). The total Cl in each pH solution was as follows (mM): 148 pH 5.0, 143 pH 6.0, 140 pH 6.5, 138 pH 6.8, 137 pH 7.0, 135 pH 7.2, 134 pH 7.4, and 131 pH 8.0. In anion substitution experiments, we substituted NaCH3SO3, NaSCN, or NaBr for NaCl and omitted TMA-Cl for a total Cl− concentration of 11 mM. We also used the following Cl−-free external solutions (mM): 120 Na-gluconate, 5 K-gluconate, 1 Mg-gluconate2, 2 Ca-gluconate2, 10 HEPES, and 10 MES; pH was adjusted with TMA-OH and omitted TMA-Cl. Because gluconate can chelate Ca2+ and Mg2+ (8), we added 6.7 mM Ca-gluconate and 1.7 mM Mg-gluconate to achieve free Ca2+ and Mg2+ concentrations of 2 and 1 mM, respectively (calculated by using CaBuf program ftp.cc.kuleuven.ac.be/pub/droogmans/cabuf.zip; Guy Droogmans, KU Leuven, Belgium). For PO43− and SO42− anion experiments, we “clamped” Ca2+ and Mg2+ at a low concentration by adding HEDTA since PO43− and SO42− either precipitates and/or chelates these divalent cations. These solutions contained the following (mM): 120 NaCl (or NaPO4 or NaSO4), 5 KCl, 1 MgCl2, 1.33 CaCl2, and 20 MES; pH was adjusted with TMA-OH, and osmolarity was adjusted with TMA-Cl.
Extracellular solutions were changed within 20 ms by using a computer-driven solenoid valve system (4). Control solution (pH 8) flowed on cells for 30 s between acidic pH applications to allow for recovery from desensitization. The kinetics of desensitization were fit to single exponential equations, and time constants (τ) are reported. pH-dependent activation and steady-state desensitization curves were fit to the Hill equation with IGOR Pro 6.01. Data are means ± SE. Statistical significance between two groups was assessed using paired or unpaired Student's t-test.
RESULTS
Anions modulate native ASICs in rat DRG neurons.
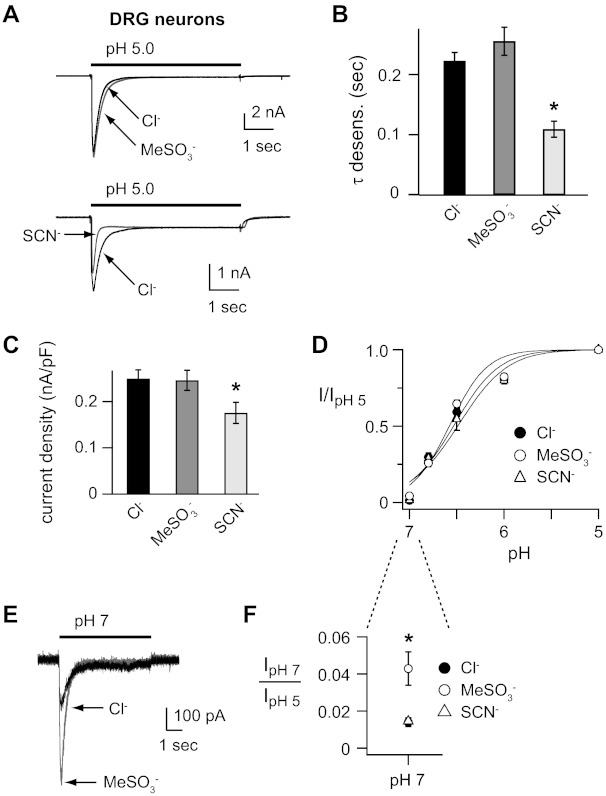
In rodent DRG neurons, ASIC channels are composed of heteromers of multiple ASIC subunits, including ASIC1a, -2a, and -3 (5, 35). We tested if anions modulate the properties of native ASICs in rat DRG neurons. Figure 1A shows that rapid solution change from pH 8 to 5 evoked fast activating and desensitizing currents, which are signatures of ASIC channels. Replacement of Cl− in the extracellular solution to the inert anion methanesulfonate (MeSO3−) did not alter most of the properties of the pH-evoked currents. The rates of desensitization (τ) were not significantly altered (Fig. 1B) nor were the peak current amplitudes (Fig. 1C). Likewise, the pH dose response of activation was not shifted (Fig. 1D). However, at the threshold of activation (pH 7), currents were larger in MeSO3− (10.9 ± 2.6 pA/pF, p = 0.02) compared with Cl− (3.37 ± 0.77 pA/pF), due to a shift in the threshold of the activation curve (Fig. 1E and F). On the other hand, anion substitution to the hydrophobic anion thiocyanate (SCN−) accelerated the rate of desensitization (Fig. 1, A and B) and reduced the peak current amplitude compared with Cl− (Fig. 1, A and C). SCN− did not cause a significant shift in the pH dose response of activation in DRG neurons (Fig. 1, D and F).
Fig. 1.
Anions modulate H+-gated currents in rat dorsal root ganglion (DRG) neurons. A: overlays of representative currents evoked by stepping from pH 8 to pH 5 solutions recorded from rat DRG neurons in either Cl−, MeSO3−, or SCN− solutions. B: mean time constants of desensitization (τ) as measured from single exponential fits to the falling phase of pH 5-evoked currents in Cl−, MeSO3−, or SCN− solutions (n ≥ 19; *P < 0.01 vs. Cl−). C: mean current density of pH 5-evoked currents in Cl−, MeSO3−, or SCN− solutions (n ≥ 22; *P < 0.01 vs. Cl−). D: pH dose-response curves for activation in Cl−, MeSO3−, or SCN− solutions. Data are acquired by stepping from pH 8.0 to the indicated test solutions as in A and are normalized to the peak currents evoked by pH 5.0 (n ≥ 6). Lines are fits of Hill equations. Note the error bars are often smaller than the markers. E: superimposed pH 7-evoked currents recorded from a rat DRG neuron in either Cl− or MeSO3− solutions. F: magnified view of pH 7-evoked current amplitudes, normalized to the pH 5-evoked current amplitude in indicated anion solutions. SCN− data (▵) is superimposed over Cl− data (●; n ≥ 8; *P < 0.01 vs. Cl−).
Some of the effects of anions on DRG neurons were similar to those we described in ASIC1a and hippocampal neurons. For example, SCN− accelerated the rates of desensitization compared with Cl− and anions did not shift the overall pH dose response of activation (22). However, we also found important differences. In DRG neurons, MeSO3− substitution had no effect on the rate of desensitization, whereas MeSO3− caused a significant acceleration in the ASIC1a desensitization rate (22). In addition, MeSO3− shifted the threshold of activation (pH 7) in DRG neurons, whereas it had no effect on pH sensitivity of ASIC1a (22). These results suggest that the modulatory effects of anions are dependent on the ASIC subunit composition and that anions may have different effects on different ASIC subunits.
Extracellular anions modulate the pH-dependent gating of ASIC3.
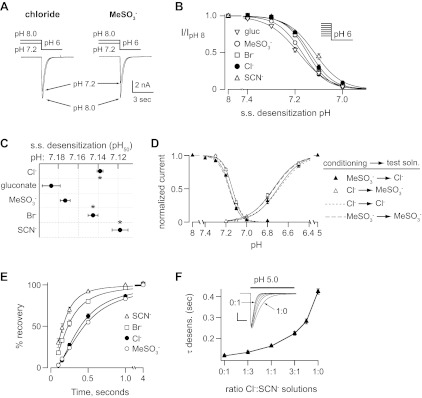
Most ASIC channels in rodent DRG neurons have ASIC3 as a required component (5, 35). In fact, only heteromeric channels consisting of ASIC3 plus another ASIC subunit will generate the fast desensitization kinetics illustrated in Fig. 1, A and B (5, 18). Therefore, we tested if extracellular anions modulate ASIC3 expressed in CHO cells. Given the subtle effects of MeSO3− substitution on ASIC currents in DRG neurons, we also studied ASIC3 properties in Na-gluconate as another “null” anion. Figure 2, A and B, shows that Cl− accelerated the rate of pH 5-evoked current desensitization compared with currents recorded in MeSO3− or gluconate. Moreover, SCN− and Br− caused an even greater acceleration of the desensitization kinetics (Fig. 2, A and B).
Fig. 2.
Extracellular anion modulation of the pH sensitivity and desensitization kinetics of acid-sensing ion channel 3 (ASIC3). A: superimposed pH 5-evoked currents from Chinese hamstar ovarian (CHO) cells transfected with ASIC3 in Cl−, gluconate, or SCN− solutions. B: mean time constants of desensitization of currents evoked by pH 5 solutions containing gluconate (gluc), MeSO3−, Cl−, Br−, or SCN− (n ≥ 13; *P < 0.01 vs. MeSO3−). C: superimposed pH 5- and pH 7-evoked currents recorded in Cl− or MeSO3− solutions. Note the different time scale compared with A. D: mean pH 5-evoked current amplitudes in the indicated anion solutions (n ≥ 16). E: pH dose-response curves for activation in the indicated anion solutions. Data are normalized to the peak currents evoked by pH 5.0 (n ≥ 5). Lines are fits of the Hill equation. F: mean pH50 of fits of Hill equations of the dose responses for activation (n ≥ 5; *P < 0.01 vs. MeSO3−).
Anion substitution had no effect on peak current amplitudes activated by pH 5 (Fig. 2, A, C, left, and D). However, at the threshold of activation pH 7-evoked currents were larger in MeSO3− (127 ± 25 pA/pF; P < 0.02) compared with Cl− (52 ± 13 pA/pF; Fig. 2C, right). These results occurred because anions significantly altered the pH-dependent activation of ASIC3. Compared with the null anions MeSO3− and gluconate, Br−, Cl−, and SCN−caused significant shifts to the right in the pH dose response curve of activation, indicating that they reduced ASIC3 sensitivity to protons (Fig. 2E). Figure 2F plots the mean pH50 as determined by fits of the Hill equation. Next, we tested the effect of Cl− substitution on steady-state desensitization. Figure 3A demonstrates that when the conditioning solution was lowered from pH 8.0 to pH 7.2, the resulting currents evoked by pH 6.0 were smaller in MeSO3− compared with Cl−. Figure 3B shows plots of steady-state desensitization in various anions. Compared with MeSO3− and gluconate, again Br−, Cl−, and SCN− produced shifts to the right indicating they decreased the pH sensitivity of steady-state desensitization (Fig. 3, B and C). The effect of anion substitution on pH dependent gating was fast and reversible: selective substitution of MeSO3− only in the conditioning solution reproduced the shift in the steady-state desensitization dose response but had no effect on activation, whereas substituting MeSO3− only in the activating solution shifted the activation dose response but had no effect on steady-state desensitization (Fig. 3D). We also measured the effect of anion substitution on the rate of recovery from desensitization. After desensitization, ASIC3 channels require exposure to a more alkaline pH for some time before they can then again be activated by acidic pH. Figure 3E shows that SCN− and Br− increased the rate of recovery compared with other anions. Assuming that results obtained in gluconate or MeSO3− represent the absence of a modulating anion, then the greater shift to the right of the pH dose-response curves, as well as the accelerated desensitization and recovery from desensitization observed in SCN− compared with Cl−, suggest that SCN− more effectively modulates ASIC3 gating than Cl−. To further test this possibility, we mixed Cl− and SCN− solutions at varying ratios and measured the desensitization kinetics of ASIC3 in these various solutions. Figure 3F demonstrates that small concentrations of SCN− had marked effects on the rate of desensitization, and the nonlinearity of the curve suggests that SCN− more potently modulates the kinetics of ASIC3 desensitization compared with Cl−.
Fig. 3.
Extracellular anion modulation of steady-state desensitization and recovery of ASIC3. A: representative currents evoked by stepping from conditioning solutions of pH 8.0 or pH 7.2 to a test solution of pH 6.0 in either Cl− or MeSO3− solutions. Note that a greater percentage of the current underwent “steady-state desensitization” in MeSO3− compared with the Cl− at pH 7.2. B: pH dose-response curves for steady-state (s.s.) desensitization in the indicated anion solutions. Data are acquired by varying the conditioning pH and then stepping to pH 6 test solutions as in A and are normalized to the currents evoked by stepping from pH 8 to pH 6 (n ≥ 8). Lines are fits of the Hill equation. C: mean pH50 of fits of Hill equations of the dose responses for steady-state desensitization in the indicated external anion solutions (n ≥ 5; *P < 0.01 vs. MeSO3−). D: pH dose responses for both activation and steady-state desensitization for ASIC3 when the conditioning and test solutions were selectively substituted between Cl− and MeSO3−. ▲: data collected when the conditioning solution was MeSO3−, and the test solution was Cl−. △: data collected when the conditioning solution was Cl−, and the test solution was MeSO3−. Solid lines are fits of the Hill equation. Smaller dashed line is the fit of the Hill equation for data collected in all Cl− solutions, and Larger dashed line is the fit of data collected in all MeSO3− solutions from Figs. 2E and 3B (n ≥ 5). E: recovery from desensitization in indicated solutions. Current was completely desensitized with a 4-s pulse to pH 6. Cells were then exposed to pH 7.4 solution for indicated times before they were stimulated again with pH 6. Recovery is percentage of current evoked by the second pH 6 pulse compared with the first. Lines are fits of single exponentials. Time constants for each fit are (seconds): SCN− 0.19, Br− 0.29, Cl− 0.44, and MeSO3− 0.52 (n ≥ 6). F, Mean time constants of desensitization of currents recorded in the indicated mixtures of Cl− and SCN− solutions (n ≥ 7). Inset: overlay of pH 5-evoked currents in the various Cl−:SCN− solution mixtures (vertical scale bar: 2 nA; horizontal scale bar: 0.5 s).
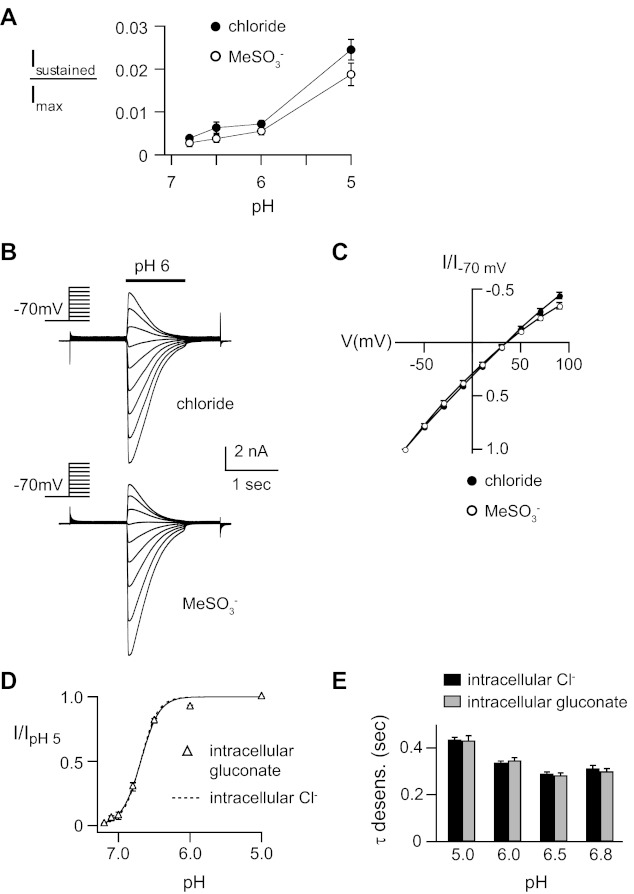
ASIC3 can generate small sustained currents due incomplete desensitization (33). We found that substitution of Cl− with MeSO3− did not significantly alter the amplitude of relative pH-evoked sustained currents (Fig. 4A). Similarly, extracellular anions did not affect the relative selectivity of Na+ permeability, as the reversal potential was not shifted when MeSO3− was substituted for Cl− (Fig. 4, B and C). The effects of anion substitution were specific to the extracellular solution; substitution of the intracellular (pipette) Cl− solution with gluconate had no effect on the desensitization kinetics or on the pH dose response of activation (Fig. 4, D and E).
Fig. 4.
Anions do not effect ASIC3 sustained currents or Na+ selectivity. A: mean sustained current amplitudes normalized to the peak pH 5-evoked current in Cl− and MeSO3− solutions (n ≥ 12). B: overlay of currents evoked by pH 6 in CHO cells transfected with ASIC3 during steps to various membrane potentials (−70 to +90 mV) in extracellular NaCl or NaMeSO3 solutions. Internal solution was KCl. C: current vs. voltage curves for the data in A. Reversal potential for both curves is ∼35 mV, indicating Na+ selective permeability over K+ (n = 12). D: pH dose-response curves for activation of ASIC3 with the pipette solution containing K-gluconate (△) compared with data in KCl pipette solution (dashed line is fit of data from Fig. 2E), normalized to the peak currents evoked by pH 5 (n ≥ 8). E: mean time constants of desensitization of currents evoked by the indicated pH with the pipette solution containing K-gluconate compared with data in KCl pipette solution (n ≥ 9). External solutions are normal Cl− solutions.
In summary, our data show that extracellular anions modulate ASIC3 gating; however, the effects were quite different than what we previously observed with ASIC1a. First, anions effected the pH sensitivity of both activation and steady-state desensitization of ASIC3, whereas anion substitution had no effect on the pH sensitivity of ASIC1a (22). Second, the effect of Cl− on the kinetics of desensitization of ASIC3 was opposite to ASIC1a: whereas Cl− accelerated desensitization of ASIC3 compared with the effect of null anions, Cl− slowed desensitization of ASIC1a almost 10-fold compared with MeSO3− (22). By comparing the results of anion modulation of gating kinetics, the sequence of anion efficacy to modulate ASIC3 gating was: gluconate < MeSO3− < Cl− < Br− < SCN−. The order was the same for anion modulation of pH sensitivity, expect that the efficacy of Cl− and Br− was reversed.
Mutation of putative intersubunit anion-binding site alters ASIC3 gating but does not abrogate anion modulation.
The ASIC1a crystal structure identified specific residues that bind Cl− and are conserved among all H+-gated ASIC isoforms (20). In previous work, we found that mutation of these residues abolished anion modulation of ASIC1a (22). Therefore, we tested if mutation of the equivalent residues in ASIC3 would similarly disrupt anion modulation of channel function (Fig. 5A). We studied channels with mutations of single residues, as well as combined mutations of multiple residues. Of note, currents generated by all of the mutations, except ASIC3K204A, were smaller in amplitude than wild-type ASIC3 (data not shown). In fact, CHO cells transfected with ASIC3E322A, or the double mutations ASIC3K204A/E322A and ASIC3K204A/E322A, did not express consistent transient pH-evoked currents unless we transfected five times our usual cDNA concentration. Even at higher transfection concentrations, transient pH-evoked currents were not evoked from cells transfected with ASIC1aR318A/E322A or the triple mutant channel. Interestingly, the kinetics of desensitization of the various mutant channels did not mimic the effect of MeSO3−or gluconate on wild-type ASIC3. Rather than slowing the rate of desensitization, each of the mutant channels desensitized at a faster rate than wild-type ASIC3 (Fig. 5B). Moreover, each of the mutant channels showed shifts in the pH sensitivity of activation, steady-state desensitization, or both (Fig. 5, C–E). In fact, the double mutant ASIC3K204A/E322A displayed marked shifts in pH sensitivity such that the pH50 for activation was 7.0 and that for steady-state desensitization was 7.4, well beyond the shifts in pH50 caused by gluconate on wild-type ASIC3 (Fig. 5E).
Fig. 5.
Mutations of a conserved putative intersubunit anion-binding site does not alter anion modulation of ASIC3. A: sequence alignment of mouse ASIC1a, mouse ASIC2a, and rat ASIC3. Highlighted residues bind Cl− according to the ASIC1a crystal structure. B: mean time constants of desensitization of pH 5-evoked currents recorded in cells expressing wild-type (WT) or indicated ASIC3 single or double mutations in Cl− solution (n ≥ 4; *P < 0.01 for all mutants, except P = 0.03 for K204A, vs. wild type). pH dose-response curves for activation (C) and steady-state desensitization (D) in cells expressing the indicated ASIC3 mutants compared with fits of data from WT ASIC3 (dashed lines) (n ≥ 6). Solid lines are fits of the Hill equation. E: mean pH50 of fits of Hill equations of pH dose responses of activation and steady-state desensitization in cells expressing the indicated ASIC3 mutants, compared with data obtained from WT ASIC3 in Cl− or gluconate from Figs. 2F and 3C (n ≥ 6; *P < 0.01 vs. WT in Cl−). F: mean time constants of desensitization of pH 5-evoked currents recorded in cells expressing WT or indicated ASIC3 mutants in the indicated anion solutions. Data are normalized to the results in Cl− for each construct to highlight anion selectivity and efficacy (n ≥ 4).
Next, we tested if mutations of the intersubunit anion-binding site residues are required for anion modulation of ASIC3 gating. If so, then mutation of these sites would be expected to either abolish the effect of anions or alter the selectivity of anion efficacy. Surprisingly, the capacity of anions to modulate the desensitization kinetics was relatively preserved in each of the mutants, and the order of anion efficacy was unchanged (Fig. 5F).
These results lead us to two conclusions. First, the three residues that bind Cl− in the related isoform ASIC1a are also important for pH-dependent gating of ASIC3. Mutations of these sites generated channels with markedly altered pH sensitivity of both activation and steady-state desensitization, and in fact, these shifts in pH sensitivity were greater than the effect of replacing extracellular Cl− with “null” anions (MeSO3− or gluconate) on wild-type ASIC3. Second, and unexpectedly, these sites are not required for anion modulation of ASIC3. Mutations of the putative intersubunit anion-binding sites had the opposite effect on the desensitization kinetics compared with null anion substitution on wild-type ASIC3, they did not abolish anion modulation, and they did not disrupt the order of anion efficacy to modulate ASIC3.
Anion modulation of ASIC2a is partially dependent on the putative intersubunit anion-binding site.
ASIC2 subunits are major components of ASIC channels in central nervous system neurons and in cardiac sensory neurons, as well as other populations of sensory neurons (2, 3, 17). We tested if anions might also modulate the properties of ASIC2a. Figure 6A shows normalized pH 3.5-evoked currents demonstrating that anion substitution had marked effects on the kinetics of both activation and desensitization of ASIC2a. Note that ASIC2a required much higher H+ concentrations for activation. Substitution of extracellular Cl− with the “null” anion gluconate reduced the pH sensitivity of activation (Fig. 6B), reduced peak amplitude (Fig. 6C) and slowed the kinetics of desensitization (Fig. 6, A and E). Interestingly, substitution to MeSO3− did not cause significant changes in current properties (Fig. 6, A, B, C, and E). However, given the marked changes in properties with gluconate substitution, we conclude that Cl− potently modulates ASIC2a.
Fig. 6.
Anion modulation of ASIC2a is partially dependent on the putative intersubunit anion-binding site. A: superimposed pH 3.5-evoked currents in SCN−, Cl−, MeSO3−, or gluconate solutions from CHO cells expressing WT ASIC2a, ASIC2aR308A, ASIC2aE312A, or ASIC2aR308A\E312A respectively. Current amplitudes were normalized to demonstrate the differences in kinetics [amplitudes (nA) for ASIC2aWT: 3.0, 3.2, 2.2, and 3.7; ASIC2aR308A: 0.15, 6.9, 7.1, and 2.7; ASIC2aE312A: 1.2, 19.7, 4.3, and 2.1; ASIC2aR308A\E312A: 0.10, 2.7, 2.3, and 2.2 for SCN−, Cl−, MeSO3−, or gluconate, respectively]. B: pH dose-response curves for activation in indicated anion solutions for each of the above ASIC2a constructs, normalized to the peak currents evoked by pH 3.5 (n ≥ 6). Lines of SCN− data are fits of Hill equations. Only very small sustained currents were activated from ASIC2aR308A or ASIC2aR308A\E312A in SCN−; thus a dose response could not be measured. MeSO3− data for WT is nearly superimposed with Cl− data. C: mean pH 3.5 current amplitudes for the indicated ASIC2a constructs in the indicated anion solutions (n ≥ 6; *P < 0.01 vs. currents evoked in Cl−, except P = 0.012 for ASIC2aR308A\E312A data evoked in gluconate). D: mean time constants of desensitization of currents evoked by pH 3.5 in Cl− solution in the indicated ASIC2a constructs (n ≥ 8; *P < 0.01 vs. ASIC2aWT). E: mean time constants of desensitization of WT ASIC2a, ASIC2aR308A, ASIC2aE312A, or ASIC2aR308A\E312A currents evoked by pH 3.5 in the indicated anion solutions normalized to Cl− data (n ≥ 6; *P < 0.05 and **P < 0.01 vs. currents evoked in Cl− in the same construct). ASIC2aR308A currents in gluconate desensitized too slow to measure time constants, thus, are labeled “sustained,” and ASIC2aR308A and ASIC2aR308A\E312A currents in SCN− were too small to measure time constants.
To test if Cl− modulation of ASIC2a is dependent on the putative intersubunit anion-binding site, we studied the effect of anion substitution on ASIC2a channels with mutations of the equivalent residues (see Fig. 5A). First, we compared the properties of mutant channels to wild-type ASIC2a recorded in Cl−. Notable differences include a slight increase in pH sensitivity of the double mutant channel ASIC2aR308A/E312A (Fig. 6B; compare pH 5-evoked data), and all mutant channels had slightly faster desensitization kinetics (Fig. 6D). ASIC2aK211A did not generate acid-evoked currents (data not shown). Next, we tested if these mutations disrupt Cl− modulation. As apparent from Figure 6A, the single mutations ASIC2aR308A and ASIC2aE312A did not alter Cl− modulation of the desensitization kinetics (Fig. 6, A and E) nor did they disrupt its effect on current amplitude (Figs. 6C). However, the difference in pH sensitivity between in Cl− and gluconate seen in the wild-type channel was partially abrogated in ASIC2aR308A (Fig. 6B). The results with the double mutant channel ASIC2aR308A/E312A were more revealing. Figure 6, A and E, demonstrates that anion substitution had minimal effect on the rate of desensitization of ASIC2aR308A/E312A. Moreover, the effect of anions on the pH dose response of ASIC2aR308A/E312A was reversed compared with that on ASIC2aWT (gluconate shifted the dose response to the left making the channels more pH sensitive; Fig. 6B). These data suggest that the modulatory effects of Cl− are at least partially dependent on the intersubunit anion-binding site.
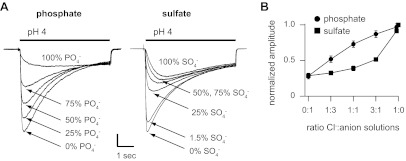
We then tested the effect of SCN− on ASIC2a currents. SCN− caused a marked shift in ASIC2aWT pH sensitivity; whereas pH 5 generated only minimal currents in Cl−, pH 5-evoked currents in SCN− were near maximal (Fig. 6B). SCN− also caused a near 20-fold acceleration in the kinetics of desensitization (Fig. 6, A and E), and similar to the effect of gluconate, currents were significantly smaller in amplitude compared with those in Cl− (Fig. 6C). In summary, the effects of anion modulation were greater on ASIC2a channels than seen with ASIC3; however, the order of anion efficacy, notwithstanding the effects on amplitude, was the same as that for ASIC3: gluconate < MeSO3− < Cl− < SCN−. Next, we tested if ASIC2a modulation by SCN− is dependent on the intersubunit anion-binding site. The effects of SCN− were unchanged in ASIC2aE312A compared with wild type (Fig. 6, A–C, and E). However, pH-evoked currents could not be activated from ASIC2aR308A or ASIC2aR308A/E312A in SCN−, even though robust currents were activated in Cl− from the same cells (Fig. 6A). To test SCN− modulation of these mutants, we performed mixed Cl− and SCN− molar ratio studies. For comparison, we first studied wild-type ASIC2a. Figure 7A demonstrates that small concentrations of SCN− markedly reduced ASIC2aWT acid-evoked current amplitude (Fig. 7B) and accelerated the rate of desensitization (Fig. 7C). Like our results with ASIC3, the nonlinearity of these curves suggests that SCN− more potently modulates ASIC2a than Cl−. We next tested if SCN− can modulate mutant ASIC2a channels. Figure 7A shows that acid-evoked currents were generated as Cl− was added to SCN− solutions for both ASIC2aR308A and ASIC2aR308A/E312A, and similar to results with ASIC2aWT, increasing the concentration of SCN− inhibited current amplitude and accelerated the rate of desensitization (Fig. 7, B and C). Finally, we tested if the more physiological anions PO43− or SO42− can modulate ASIC2a. Mixed molar ratio studies with Cl− demonstrate that both PO43− and SO4− reduced the peak current amplitude of ASIC2a (Fig. 8A). Plotting the current amplitudes at varying ratios reveals that PO43− less potently modulates ASIC2a compared with Cl−; however, SO42− was more potent such that small concentrations have the capacity to modulate ASIC2a (Fig. 8B).
Fig. 7.
SCN− is a potent modulator of ASIC2a, independent of the putative intersubunit anion-binding site. A: superimposed pH 4-evoked currents recorded from a CHO cells expressing WT ASIC2a, ASIC2aR308A, or ASIC2aR308A\E312A in the indicated mixtures of Cl− and SCN− solutions. B: mean pH 4-evoked current amplitudes from cells expressing ASIC2a WT, ASIC2aR308A, or ASIC2aR308A\E312A in the indicated mixtures of Cl− and SCN− solutions, normalized to data in 100% Cl− (n ≥ 7). C: mean time constants of desensitization of pH 4-evoked currents from the same cells with same solutions, normalized to data in 100% Cl− (n ≥ 3).
Fig. 8.
PO43− and SO42− modulate ASIC2a. A: superimposed pH 4-evoked currents recorded from a CHO cells expressing WT ASIC2a in the indicated mixtures of Cl− and PO43− or SO42− solutions (vertical scale bar: 1.0 and 2.1 nA for PO43− and SO42− solutions, respectively). B: mean pH 4-evoked current amplitudes from cells expressing ASIC2a in the indicated mixtures of anion solutions, normalized to data in 100% Cl− (n ≥ 7).
From these data, we conclude that anions are potent modulators of ASIC2a gating. These effects are partially dependent on the intersubunit anion-binding sites since mutating two of the three residues abrogated the effects of Cl− on the kinetics of desensitization rates and the pH dose response compared with gluconate. However, the profound effects of SCN− on ASIC2a gating appear to be independent of the intersubunit anion-binding site: mutations of this site did not disrupt the capacity of SCN− to inhibit current or accelerate desensitization.
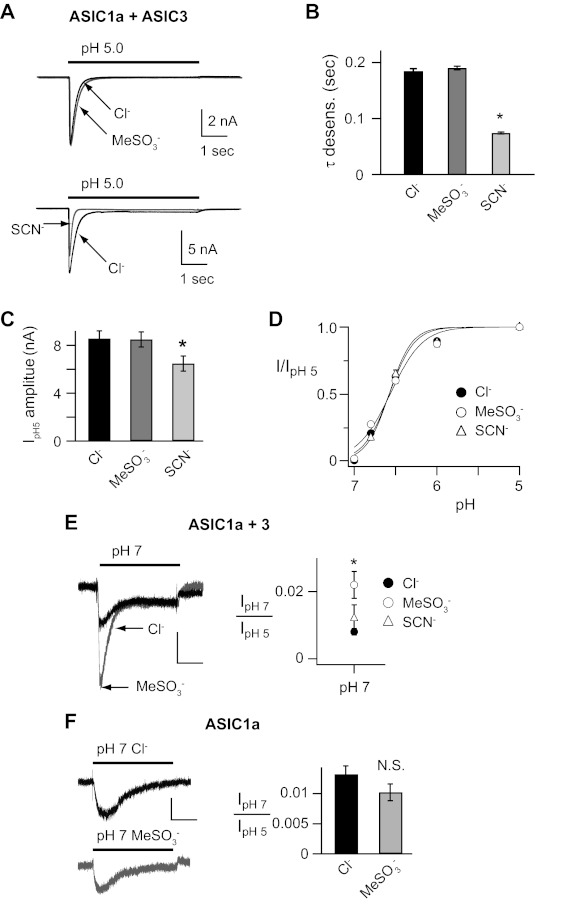
Modulatory effects of anions on rat DRG neurons are mimicked by the effects of anions on ASIC1a/3 heteromeric channels.
We have shown that anions modulate ASIC1a, -2a, and -3 pH-dependent gating properties. However, the effect is different for each of the subunits. We found that anions also modulate native ASICs in DRG neurons; however, the pattern of modulation does not match that of any individual subunit. The shift in the activation threshold in MeSO3− compared with Cl− in DRG neurons (Fig. 1, E and F) matched that seen in heterologously expressed ASIC3 (Fig. 2C). On the other hand, the lack of overall shift in pH dose response of activation (Fig. 1D), and the diminished amplitude in SCN− compared with Cl− or MeSO3− (Fig. 1C), are similar to the effects of anions on ASIC1a (22). For these reasons, and because the majority of ASICs in rodent DRG are heteromeric channels, we tested if coexpression of ASIC1a and ASIC3 might reproduce the effects of anions on native ASIC channels in DRG neurons. Figure 9, A and B, shows that coexpression of ASIC1a and ASIC3 reproduced the fast desensitization kinetics of DRG neurons indicating they formed heteromeric channels (compare to Fig. 1, A and B), and these channels desensitized about twofold faster than those from heterologously expressed ASIC3 (Fig. 2B), which has the fastest desensitization kinetics of the ASIC homomeric channels. Moreover, current amplitudes were smaller in SCN− (Fig. 9C) and anion substitution did not shift the pH dose response of activation (Fig. 9D). However, at the threshold of activation, ASIC1a/3 pH 7-evoked currents were larger in MeSO3− compared with Cl− (Fig. 9E), an effect that was not seen with heterologously expressed ASIC1a homomeric channels (Fig. 9F). Overall, the modulatory effects of anion substitution on ASIC1a/3 heteromeric channels were different than those on ASIC homomeric channels and closely matched those on native ASICs in DRG neurons.
Fig. 9.
Anion modulation of ASIC1a/3 heteromeric channels mimics that in DRG neurons. A: superimposed pH 5-evoked currents recorded from CHO cells coexpressing both ASIC1a and ASIC3 in either Cl−, MeSO3−, or SCN− solutions. B: mean time constants of desensitization of pH 5-evoked currents in Cl−, MeSO3−, or SCN− solutions (n ≥ 26; *P < 0.01 vs. Cl−). C: mean current amplitude of pH 5-evoked currents in Cl−, MeSO3−, or SCN− solutions (n ≥ 32; *P < 0.01 vs. Cl−). D: pH dose-response curves for activation in Cl−, MeSO3−, or SCN− solutions, normalized to the peak currents evoked by pH 5 (n ≥ 6). Lines are fits of Hill equations. E: superimposed pH 7-evoked currents recorded from a CHO expressing both ASIC1a and ASIC3, or F: ASIC1a alone in either Cl− or MeSO3− solutions. At right are the mean pH 7-evoked current amplitudes, normalized to the pH 5-evoked current amplitude in Cl−, MeSO3−, or SCN− solutions (n ≥ 10; *P < 0.01 vs. Cl−).
DISCUSSION
We previously found that extracellular anions modulated the H+-dependent gating properties of ASIC1a. This effect was completely abolished by mutation of an intersubunit Cl−-binding site that was identified in a resolved crystal structure of ASIC1a. Here we tested the effect of anions on native ASICs in DRG neurons and found different modulatory effects. This led us to study the effects of anions on related subunits, ASIC2a and ASIC3, as well as heteromeric ASIC channels. We found that anions also modulate these related channels; however, the effects were unique for each subunit. Remarkably, anion modulation of ASIC2a was only partially dependent on the intersubunit Cl−-binding site, and anion modulation of ASIC3 was independent of this site.
Anion modulation of ASICs depends on the subunit composition.
For ASIC1a, anion substitution altered the kinetics of desensitization, without affecting other gating properties (22). In particular, Cl− potentiated ASIC1a currents by markedly slowing the rate of desensitization. This suggests Cl− either stabilized the open state and/or increased the energy barrier required for the channel to move from the open to the desensitized state. On the other hand, anions had no effect on the apparent proton affinity of ASIC1a or its recovery from desensitization. The effects of Cl− on ASIC1a are remarkably similar to anion modulation of kainate glutamate receptors. With these channels, Cl− has been shown to bind and stabilize the interface between subunit dimers, which slows the rate of desensitization without effecting recovery from desensitization (7, 27). In fact, the order of anion affinity to slow desensitization of kainate receptors (MeSO3− < F− < NO3− < I− < Br− ≈ Cl−) is similar to that for ASIC1a (SCN− < MeSO3− < I− < Br− < Cl−). The ASIC-related channel ENaC is also modulated by extracellular anions with a similar selectivity sequence (SCN− < SO42− < H2PO43− < F− < I− < Cl− < Br−) (9). For ASIC1a, kainate receptors, and ENaC, it appears that Cl− anions are particularly suited, with the appropriate size and charge, to bind and modulate their gating properties.
The effect of anions on ASIC3 and ASIC2a gating was more complex. Anions not only modulated the kinetics of desensitization of ASIC3, but they also altered the apparent proton affinity of both activation and steady-state desensitization, as well as the kinetics of recovery from desensitization. Similarly, anions modulated the proton affinity of activation and the desensitization kinetics of ASIC2a. However, the effect of anions on the apparent proton affinity of ASIC2a was opposite to that for ASIC3. Whereas SCN− slightly reduced the pH sensitivity of ASIC3, it caused a marked increase in pH-dependent activation of ASIC2a. We found that the effect of anions on native ASICs in sensory neurons did not match the effect of anion modulation of any ASIC homomeric channel. Instead, coexpression of ASIC1a and -3 generated heteromeric channels that were modulated by anions in a unique pattern, which mirrored the effects of anions on sensory neurons. Thus, while anion modulation is a common characteristic shared by ASICs, the effect of anions to modulate channel properties varies depending on the subunit composition.
The order of efficacy of different anions to modulate ASIC3 and -2a was also very different than that of ASIC1a. The rank order of anion efficacy for ASIC3 (gluconate < MeSO3− < Cl− < Br− < SCN−) and for ASIC2a (gluconate < MeSO3− < Cl− < SCN−) coincides with the so called “lyotropic” or “chaotropic” series, which postulates that anions that are more readily dehydrated can more effectively bind to proteins than more hydrophilic anions and hence have greater potential to modulate biological activity (12). Gluconate and MeSO3− are hydrophilic and do not compete with Cl− binding or permeation in Cl− channels, and by substituting these “null” anions we learned that Cl− modulates ASIC3 and -2a to a moderate degree. We speculate that the differences in the results obtained in MeSO3− compared with gluconate solutions might indicate a difference in the effects of the two anions. For example, MeSO3− has been shown to alter the gating properties of the chloride channel ClC-1 (28). SCN− is a more hydrophobic anion than Cl−, and it had a greater effect on ASIC3 and -2a properties. In addition, our Cl−:SCN− molar ratio mixing studies support the conclusion that SCN− has a greater apparent affinity for ASIC2a and ASIC3 than Cl−. The effects of lyotropic anions on ion channel function are well documented, as it largely governs anion permeability through chloride channels (28, 30) and anion modulation of the voltage dependence of Na+ channel activation and steady-state inactivation in frog skeletal muscle (12).
Intersubunit anion-binding site is not sufficient for anion modulation of ASIC2a and ASIC3.
With ASIC1a, we found that the intersubunit anion-binding site was necessary for anion modulation. Mutating any one of the three residues defined by the ASIC1a crystal structure as a Cl−-binding site (K211, R309, and E313) completely disrupted anion modulation, and the properties of the mutant channels mimicked the effect of replacing extracellular Cl− with MeSO3−. Given the conservation of these residues in other ASIC subunits, we anticipated that this site would similarly coordinate anion binding in these channels. In fact, in the related channel ENaC mutations of corresponding residues either abolished regulation by Cl− or altered anion selectivity of modulation (9, 10). Surprisingly, we found that mutations of these sites only partially abrogated anion modulation of ASIC2a and had no effect on anion modulation of ASIC3. Thus we conclude that the Cl−-binding site in ASIC1a is not sufficient for anion modulation of ASIC2a and -3.
How do anions modulate ASIC2a and -3? Several pieces of evidence support the possibility that anions bind to an alternative site(s) in the extracellular domain. First, the site must be extracellular; substituting the intracellular solution from Cl− to gluconate had no effect on ASIC3 properties, and the selectivity filter would exclude access of anions to the channel pore (15). Moreover, binding and unbinding of anions must be fast, at least as fast as our extracellular solution changes (∼20 ms), suggesting a direct effect on the channel. Second, the different modulatory effects and the different anion selectivity suggest that anions bind to ASIC2a and -3 at alternative sites compared with ASIC1a. Third, the effects of different anions on ASIC2a suggest more than one anion-binding site. We found that mutating two of the three residues of the putative intersubunit anion-binding site (ASIC2aR308A\E312A) abolished the capacity of Cl− to modulate the pH sensitivity of activation and the desensitization kinetics compared with gluconate. This suggests that this site is required for Cl− modulation of ASIC2a. However, our Cl−:SCN− mixing studies demonstrated that SCN− could still potently modulate ASIC2aR308A\E312A. We interpret these data to suggest that the intersubunit anion-binding site is required for Cl− modulation, but SCN− can bind and modulate ASIC2a at an alternative site. Moreover, our Cl−:SCN− mixing data suggest that within a single channel Cl− and SCN− can simultaneously bind and modulate channel activity. Whereas acid-evoked currents could not be generated from ASIC2aR308A and ASIC2aR308A\E312A in 100% SCN− solutions, mixing Cl− and SCN− solutions allowed for activation of currents, suggesting that the binding of Cl− stabilized the open state of the channel. In addition, at the same time, increasing concentrations of SCN− reduced current amplitude and accelerated the kinetics of desensitization, suggesting that both Cl− and SCN− can simultaneously modulate the channels. The possibility of multiple anion binding sites is also supported by the fact that we found differing anion selectivity for different channel properties for both ASIC2a and -3.
Our data do not rule out the possibility that anions bind to interacting proteins or adjacent lipid membrane moieties. Nevertheless, since anions modulate a variety of gating properties, and had such a profound effect on the pH sensitivity of ASIC2a, we speculate that the anion-binding sites in these channels must be within important gating regions.
Importance of the intersubunit interface in the thumb domain for gating.
Although mutations of the intersubunit Cl−-binding site did not abolish anion modulation of ASIC2a or -3, they caused significant changes in gating that were independent of anions. These mutations generated significant shifts in the apparent proton affinity of the channels and in the kinetics of desensitization. In addition, ASIC2aK210A, as well as some of the combined mutations in ASIC3, did not generate functional acid-activated channels. It is interesting that this same lysine mutation in ASIC3 (ASIC3K204A) generated the most profound shift in pH sensitivity of the single mutations (Fig. 5E). In the ASIC1a crystal structure, this lysine extends from an adjacent subunit into the thumb of an adjoining subunit to coordinate Cl− binding, and our data suggest this intersubunit interface might be necessary for channel assembly or stability. Although the intersubunit Cl−-binding site was not sufficient for anion modulation of ASIC2a and -3, our results do highlight the importance of this region to ASIC gating.
Potential physiological important of anion modulation of ASICs.
What is the physiological significance of our findings? ASICs are highly expressed it tissues that are capable of high metabolic activity, including skeletal and cardiac muscle and the brain. In addition to being gated by protons, ASICs are modulated by several other chemicals released by ischemic or metabolically stressed tissue. Anion modulation might represent another means by which ASICs sense changes in the extracellular compartment. In the brain, ischemia, trauma, and seizures are all associated with profound transmembrane shifts of ions and other molecules. Na+ and Cl− move into cells from the extracellular spaces, and during anoxia or spreading depression the extracellular Cl− concentration in the brain can drop from a normal of ∼140 mM to the 50-to 90-mM range (16, 21). We previously demonstrated that changes in Cl− concentration within this range can modulate ASIC1a (22). Similar shifts of Cl− into skeletal myocytes occur with muscle ischemia (23). Moreover, the mechanism defined here might provide a means for ASICs to be modulated by other biological anions, and here we provide evidence that small concentrations of SO42− have the capacity to modulate ASICs. We hypothesize that anion fluxes associated with ischemia and other hyperexcited states modulate ASICs and represent a novel mechanism to sense changes in the extracellular environment.
GRANTS
This work was supported the National Heart, Lung, and Blood Institute Grant HL-076419 and Veterans Affairs Merit Award to C. J. Benson.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: N.K., P.M.S., and C.J.B. conception and design of research; N.K., M.G., and A.M.S.H. performed experiments; N.K., M.G., A.M.S.H., P.M.S., and C.J.B. analyzed data; N.K., P.M.S., and C.J.B. interpreted results of experiments; N.K., M.G., and C.J.B. prepared figures; N.K. and C.J.B. drafted manuscript; N.K., M.G., A.M.S.H., P.M.S., and C.J.B. approved final version of manuscript; P.M.S. and C.J.B. edited and revised manuscript.
ACKNOWLEDGMENTS
We thank Kathryn Kaufman, Justin Yesis, He Gu, and the DNA Core Facility (National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-25295) for technical assistance.
Present address of N. Kusama: Division of Anesthesiology and Medical Crisis Management, Nagoya City University Hospital, Japan.
REFERENCES
- 1. Allen NJ, Attwell D. Modulation of ASIC channels in rat cerebellar Purkinje neurons by ischaemia-related signals. J Physiol 543: 521–529, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Askwith CC, Wemmie JA, Price MP, Rokhlina T, Welsh MJ. Acid-sensing ion channel 2 (ASIC2) modulates ASIC1 H+-activated currents in hippocampal neurons. J Biol Chem 279: 18296–18305, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Baron A, Waldmann R, Lazdunski M. ASIC-like, proton-activated currents in rat hippocampal neurons. J Physiol 539: 485–494, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Benson CJ, Eckert SP, McCleskey EW. Acid-evoked currents in cardiac sensory neurons: a possible mediator of myocardial ischemic sensation. Circ Res 84: 921–928, 1999 [DOI] [PubMed] [Google Scholar]
- 5. Benson CJ, Xie J, Wemmie JA, Price MP, Henss JM, Welsh MJ, Snyder PM. Heteromultimers of DEG/ENaC subunits form H+-gated channels in mouse sensory neurons. Proc Natl Acad Sci USA 99: 2338–2343, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Birdsong WT, Fierro L, Williams FG, Spelta V, Naves LA, Knowles M, Marsh-Haffner J, Adelman JP, Almers W, Elde RP, McCleskey EW. Sensing muscle ischemia: coincident detection of acid and ATP via interplay of two ion channels. Neuron 68: 739–749, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chaudhry C, Plested AJ, Schuck P, Mayer ML. Energetics of glutamate receptor ligand binding domain dimer assembly are modulated by allosteric ions. Proc Natl Acad Sci USA 106: 12329–12334, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Christoffersen CR, Skibsted LH. Calcium ion activity in physiological salt solutions: influence of anions substituted for chloride. Comp Biochem Physiol A Comp Physiol 52: 317–322, 1975 [DOI] [PubMed] [Google Scholar]
- 9. Collier DM, Snyder PM. Extracellular chloride regulates the epithelial sodium channel. J Biol Chem 284: 29320–29325, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Collier DM, Snyder PM. Identification of epithelial Na+ channel (ENaC) intersubunit Cl- inhibitory residues suggests a trimeric alpha gamma beta channel architecture. J Biol Chem 286: 6027–6032, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Coric T, Passamaneck YJ, Zhang P, Di Gregorio A, Canessa CM. Simple chordates exhibit a proton-independent function of acid-sensing ion channels. FASEB J 22: 1914–1923, 2008 [DOI] [PubMed] [Google Scholar]
- 12. Dani JA, Sanchez JA, Hille B. Lyotropic anions: Na channel gating and Ca electrode response. J Gen Physiol 81: 255–281, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Deval E, Noel J, Lay N, Alloui A, Diochot S, Friend V, Jodar M, Lazdunski M, Lingueglia E. ASIC3, a sensor of acidic and primary inflammatory pain. EMBO J 27: 3047–3055, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Eshcol JO, Harding AM, Hattori T, Costa V, Welsh MJ, Benson CJ. Acid-sensing ion channel 3 (ASIC3) cell surface expression is modulated by PSD-95 within lipid rafts. Am J Physiol Cell Physiol 295: C732–C739, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14a. Gautam M, Benson CJ. Acid-sensing ion channels (ASICs) in mouse skeletal muscle afferents are heteromers composed of ASIC1a, ASIC2, and ASIC3 subunits. FASEB J Epub ahead of print. doi: 10.1096/fj.12-220400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gonzales EB, Kawate T, Gouaux E. Pore architecture and ion sites in acid-sensing ion channels and P2X receptors. Nature 460: 599–604, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hansen AJ, Zeuthen T. Extracellular ion concentrations during spreading depression and ischemia in the rat brain cortex. Acta Physiol Scand 113: 437–445, 1981 [DOI] [PubMed] [Google Scholar]
- 17. Hattori T, Chen J, Harding AM, Price MP, Lu Y, Abboud FM, Benson CJ. ASIC2a and ASIC3 heteromultimerize to form pH-sensitive channels in mouse cardiac dorsal root ganglia neurons. Circ Res 105: 279–286, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hesselager M, Timmermann DB, Ahring PK. pH Dependency and desensitization kinetics of heterologously expressed combinations of acid-sensing ion channel subunits. J Biol Chem 279: 11006–11015, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Immke DC, McCleskey EW. Lactate enhances the acid-sensing Na+ channel on ischemia-sensing neurons. Nat Neurosci 4: 869–870, 2001 [DOI] [PubMed] [Google Scholar]
- 20. Jasti J, Furukawa H, Gonzales EB, Gouaux E. Structure of acid-sensing ion channel 1 at 1.9 A resolution and low pH. Nature 449: 316–323, 2007 [DOI] [PubMed] [Google Scholar]
- 21. Jiang C, Agulian S, Haddad GG. Cl− and Na+ homeostasis during anoxia in rat hypoglossal neurons: intracellular and extracellular in vitro studies. J Physiol 448: 697–708, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kusama N, Harding AM, Benson CJ. Extracellular chloride modulates the desensitization kinetics of acid-sensing ion channel 1a (ASIC1a). J Biol Chem 285: 17425–17431, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lai ZF, Nishi K. Intracellular chloride activity increases in guinea pig ventricular muscle during simulated ischemia. Am J Physiol Heart Circ Physiol 275: H1613–H1619, 1998 [DOI] [PubMed] [Google Scholar]
- 24. Lingueglia E. Acid-sensing ion channels in sensory perception. J Biol Chem 282: 17325–17329, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Mamet J, Baron A, Lazdunski M, Voilley N. Proinflammatory mediators, stimulators of sensory neuron excitability via the expression of acid-sensing ion channels. J Neurosci 22: 10662–10670, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Molliver DC, Immke DC, Fierro L, Pare M, Rice FL, McCleskey EW. ASIC3, an acid-sensing ion channel, is expressed in metaboreceptive sensory neurons. Mol Pain 1: 35, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Plested AJ, Mayer ML. Structure and mechanism of kainate receptor modulation by anions. Neuron 53: 829–841, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Rychkov GY, Pusch M, Roberts ML, Jentsch TJ, Bretag AH. Permeation and block of the skeletal muscle chloride channel, ClC-1, by foreign anions. J Gen Physiol 111: 653–665, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sluka KA, Price MP, Breese NM, Stucky CL, Wemmie JA, Welsh MJ. Chronic hyperalgesia induced by repeated acid injections in muscle is abolished by the loss of ASIC3, but not ASIC1. Pain 106: 229–239, 2003 [DOI] [PubMed] [Google Scholar]
- 30. Smith SS, Steinle ED, Meyerhoff ME, Dawson DC. Cystic fibrosis transmembrane conductance regulator. Physical basis for lyotropic anion selectivity patterns. J Gen Physiol 114: 799–818, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sutherland SP, Benson CJ, Adelman JP, McCleskey EW. Acid-sensing ion channel 3 matches the acid-gated current in cardiac ischemia-sensing neurons. Proc Natl Acad Sci USA 98: 711–716, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ugawa S, Ueda T, Ishida Y, Nishigaki M, Shibata Y, Shimada S. Amiloride-blockable acid-sensing ion channels are leading acid sensors expressed in human nociceptors. J Clin Invest 110: 1185–1190, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Waldmann R, Bassilana F, de Weille J, Champigny G, Heurteaux C, Lazdunski M. Molecular cloning of a noninactivating proton-gated Na+ channel specific for sensory neurons. J Biol Chem 272: 20975–20978, 1997 [DOI] [PubMed] [Google Scholar]
- 34. Wemmie JA, Price MP, Welsh MJ. Acid-sensing ion channels: advances, questions and therapeutic opportunities. Trends Neurosci 29: 578–586, 2006 [DOI] [PubMed] [Google Scholar]
- 35. Xie J, Price MP, Berger AL, Welsh MJ. DRASIC contributes to pH-gated currents in large dorsal root ganglion sensory neurons by forming heteromultimeric channels. J Neurophysiol 87: 2835–2843, 2002 [DOI] [PubMed] [Google Scholar]