Abstract
The aquaporin-2 (AQP2) water channel relocates mainly to the apical plasma membrane of collecting duct principal cells after vasopressin (VP) stimulation. AQP2 transport to this membrane domain is assumed to be a direct route involving recycling of intracellular vesicles. However, basolateral plasma membrane expression of AQP2 is observed in vivo in principal cells. Here, we asked whether there is a transcytotic pathway of AQP2 trafficking between apical and basolateral membranes. We used MDCK cells in which AQP2 normally accumulates apically after VP exposure. In contrast, both site-specific biotinylation and immunofluorescence showed that AQP2 is strongly accumulated in the basolateral membrane, along with the endocytic protein clathrin, after a brief cold shock (4°C). This suggests that AQP2 may be constitutively targeted to basolateral membranes and then retrieved by clathrin-mediated endocytosis at physiological temperatures. Rab11 does not accumulate in basolateral membranes after cold shock, suggesting that the AQP2 in this location is not associated with Rab11-positive vesicles. After rewarming (37°C), basolateral AQP2 staining is diminished and it subsequently accumulates at the apical membrane in the presence of VP/forskolin, suggesting that transcytosis can be followed by apical insertion of AQP2. This process is inhibited by treatment with colchicine. Our data suggest that the cold shock procedure reveals the presence of microtubule-dependent AQP2 transcytosis, which represents an indirect pathway of apical AQP2 delivery in these cells. Furthermore, our data indicate that protein polarity data obtained from biotinylation assays, which require cells to be cooled to 4°C during the labeling procedure, should be interpreted with caution.
Keywords: clathrin, endocytosis, Rab5, Rab11, MDCK cells
the aquaporin-2 (AQP2) water channel located in kidney collecting duct principal cells (4, 12, 22, 35) accumulates in the plasma membrane upon stimulation by vasopressin (VP) (20, 21, 23, 34). During this process, its phosphorylation pattern changes (11, 15, 29, 36, 48), and filamentous actin depolymerization occurs (37, 43, 47, 54). The intracellular routes by which some membrane proteins are transported to their plasma membrane target have been identified (27, 31, 38, 39), but the precise pathway followed by AQP2 during its intracellular transit remains unclear. For polarized AQP2 intracellular trafficking, it has been postulated that AQP2 is simply targeted to the plasma membrane from intracellular sites by a direct route, without transcytosis. However, some previous data are consistent with the existence of an indirect transcytotic pathway of AQP2 membrane insertion (50). A recent proteomics analysis using rat kidney found that AQP2 associates with vesicles that also contain Sec6, Sec8, and RalA, components of the exocyst, which is involved in basolateral membrane targeting (2, 14).
Furthermore, previous work has clearly shown that while AQP2 is mainly located in the apical plasma membrane of principal cells, it is also frequently detectable in basolateral membranes of these cells, as well as in connecting segments (7). The amount of basolateral AQP2 can be modified under different conditions (8, 19, 24, 34), and we have recently shown that basolateral AQP2 is involved in epithelial cell migration and tubule morphogenesis, revealing a unique new rationale for basolateral expression of this aquaporin (6). In addition, AQP2 plasma membrane localization is different among cultured cell lines (4). In MDCK cells, AQP2 is normally located on the apical plasma membrane after VP treatment. However, after long-term exposure to hypertonic conditions, the adenylyl cyclase-stimulating drug forskolin (FK) induced the accumulation of AQP2 in both apical and basolateral membranes of these cells, suggesting the existence of a complex intracellular pathway, possibly involving transcytosis (50).
Among the methodological approaches to address these issues of membrane protein polarity, site-specific cell surface biotinylation and immunofluorescence microscopy (IF) are frequently applied (10, 30, 49, 55). For site-specific biotinylation, living cells are usually incubated at 4°C, but this procedure disrupts microtubules, which are known to be involved in trafficking of many membrane proteins including AQP2 (53) and which are also critical for the basolateral to apical transcytosis of proteins (18). In fact, it has been reported that AQP2 was basolaterally accumulated after 4 h cold treatment of rat kidney slices in vitro (3).
In this study, we applied cold shock (4°C for 15 min) to AQP2-expressing MDCK cells and performed site-specific biotinylation and IF analysis to determine the influence of low temperature on AQP2 plasma membrane distribution before and after rewarming to 37°C. Our results are consistent with constitutive basolateral targeting of AQP2, followed by clathrin-mediated retrieval, and microtubule-dependent transcytosis of AQP2 from the basolateral to the apical membrane.1
MATERIALS AND METHODS
Reagents and antibodies.
Colchicine, cycloheximide (CHX), arginine vasopressin (VP), FK, mouse anti-β-tubulin antibody (T8328), and chlorpromazine hydrochloride were purchased from Sigma Aldrich (St. Louis, MO). Goat anti-AQP2 antibody (sc9882) was purchased from Santa Cruz (Santa Cruz, CA). Mouse anti-clathrin antibody (610499) was purchased from BD Transduction Labs (San Jose, CA). Rabbit anti-Rab5 antibody (2143S) and rabbit anti-EEA1 antibody (2411S) were purchased from Cell Signaling (Danvers, MA). Rabbit anti-Rab11 (71–5300) antibody was purchased from Invitrogen (Carlsbad, CA). Mouse anti-GAPDH antibody (AM4300) was purchased from Ambion (Carlsbad, CA). Rabbit anti-phospho-AQP2 antibodies were kindly provided by Dr. Mark Knepper (NIH, Bethesda, MD). Monoclonal anti-gp135/podocalyxin antibody was kindly provided by Dr. George Ojakian (SUNY Health Center, Brooklyn, NY).
Cell culture.
MDCK cells stably expressing rat AQP2 were generated as described previously (55). Rat AQP2-inner medullary collecting duct (IMCD) cells were kindly provided by Dr. J. Schwartz (Boston University, Boston, MA). AQP2-MDCK cells were grown at 37°C (5% CO2) in 10% FBS-DMEM containing penicillin-streptomycin (PS) and geneticin (G418) (500 μg/ml), and passaged using TrypLE (GIBCO-BRL, Carlsbad, CA). Untransfected (WT) MDCK cells and AQP2-IMCD cells were grown in 10% FBS and PS containing DMEM without geneticin.
Western blotting.
Cells were grown on six-well plates for 3 days incubated with 50 μM indomethacin overnight to reduce endogenous cAMP levels as previously described (9), then subjected to cold shock or VP/FK treatment. Cells were lysed in lysis buffer (150 mM NaCl, 20 mM Tris·HCl, 5 mM EDTA, and 1% Triton X-100) supplemented with a complete mini (CM) protease inhibitor (1 tablet/40 ml). The lysates were rotated for 30 min at 4°C, and centrifuged for 10 min at 6,000 g. The protein concentration was determined using a BCA kit (Pierce, Rockford, IL). Samples were diluted in lysis buffer and 35 μl NuPage SDS sample buffer to a protein concentration of 50 μg protein in 100 μl loading solution. Ten microliter reduced samples (= 5 μg protein) per lane were run on the NuPage 1.0-mm 15-well 4–12% Bis-Tris gel, and transferred onto PVDF membranes (Invitrogen). The transferred PVDF membranes were incubated in 5% skimmed milk in 0.05% Tween 20-PBS (PBST) for blocking, then incubated with primary antibody diluted at 1:10,000 in PBST. PVDF membranes were washed four times for 15 min in PBST, incubated with HRP-conjugated secondary antibody diluted at 1:10,000 in PBST for 30 min, and washed in PBST four times for 15 min. Signals were visualized using Western Lightning ECL and Biomax XAR film. For loading controls, antibodies on PVDF membranes were stripped in stripping buffer (0.2 M glycine, 0.05% Tween 20; pH was adjusted to 2.5 with HCl) for 1 h and blocked in 5% skimmed milk-PBST again, then reprobed with mouse GAPDH antibody. Protein band intensity was analyzed by ImageJ (NIH, Bethesda, MD).
Site-specific cell surface biotinylation.
MDCK cells were plated on TransWell filters (no. 3412, Costar), incubated in a 5% CO2 incubator at 37°C for 4 days, and incubated with 50 μM indomethacin overnight (9). After treatment with or without VP/FK (20 nM VP and 50 μM FK for 20 min) at 37°C, cells were washed two times in ice-cold PBS containing 1 mM CaCl2 and 1 mM MgCl2 (PBS-CM) and were then incubated in biotinylation buffer (10 mM triethanolamine, 2 mM CaCl2, 125 mM NaCl, pH 7.5) containing 1 mg/ml Sulfo-NHS-Biotin (Pierce) at 4°C for 15 min. In experiments to detect apical membrane proteins, 750 μl of the biotinylation buffer was added to apical chambers while 1,500 μl of the same buffer without Sulfo-NHS-Biotin was added to basolateral chambers. In experiments to detect basolateral membrane proteins, 1,500 μl of the biotinylation buffer were added to basolateral chambers and 750 μl of the same buffer without Sulfo-NHS-Biotin were added to apical chambers. Subsequently, monolayers were washed two times with ice-cold PBS-CM and incubated for 5 min with PBS-CM containing 50 mM NH4Cl to quench the remaining biotin, then washed twice in ice-cold PBS-CM. The filters were cut from their plastic supports and incubated in 1,200 μl of lysis buffer (150 mM NaCl, 20 mM Tris·HCl, 5 mM EDTA, 1% Triton X-100) for 30 min at 4°C, and cells were scraped off the filter. After 150 μl of lysate were reserved to quantify total protein amount (original lysate), 900 μl lysate was mixed with 100 μl of neutravidin beads (Thermo Scientific, Rockford, IL) and incubated overnight at 4°C with rotation to collect biotinylated proteins. Samples were centrifuged, supernatant was removed, and the remaining solution was aspirated using a 30-gauge needle, and then 500 μl ice cold lysis buffer was added. This cycle was performed three times. Finally, beads were incubated in 150 μl NuPage sample buffer (1:1 diluted in lysis buffer) at 70°C for 10 min to extract and denature biotinylated proteins. The protein concentration in each original lysate was measured using the BCA kit. The loading volume of biotinylated samples was adjusted based on the protein concentration of the original lysates. A total of 1.2 μg protein in the original lysate and the respective biotinylated protein lysate were subjected to Western blotting as described above.
Immunofluorescence.
MDCK cells, AQP2-MDCK cells, or AQP2-IMCD cells were plated on polyester filters (no. 3460, Costar) in 12-well plastic supports and incubated for 4 days to form polarized confluent monolayers as above. They were then incubated with 50 μM indomethacin overnight (9). Some monolayers were treated with VP/FK (VP 20 nM and FK 50 μM) for 20 min and fixed in 4% PFA at 37°C for 10 min. For cold shock experiments, culture medium was replaced with ice-cold DMEM, and cells were incubated at 4°C for 15 min before fixation in 4% PFA at 4°C for 30 min. Cells were washed three times with PBS, permeabilized with 0.1% Triton X-100-PBS for 10 min, treated with 0.5% SDS-PBS for 5 min, and blocked in 1% BSA-PBS for 30 min at room temperature. For rewarming experiments, culture medium was replaced with DMEM prewarmed to 37°C. Cells were incubated for 15 min at 37°C, then fixed at 37°C for 10 min in 4% PFA and permeabilized as above. For immunostaining, primary antibody was diluted 1:500 (AQP2, β-tubulin, clathrin, Rab5, EEA1), 1:200 (Rab11), or 1:1 (gp135/podocalyxin), added to the apical chambers, and incubated overnight at 4°C. Cells were washed in PBS (10 min, 3 times), incubated with 1:500 diluted secondary antibody for 3 h at room temperature, then washed in PBS (10 min, 3 times), and mounted on glass slides with Vectashield mounting medium (Vector Labs, Burlingame, CA). All images were acquired within the linear range of intensity, and no thresholding was applied to the images.
Biotin staining.
AQP2-MDCK cells were plated on polyester filters (no. 3460, Costar) in 12-well plastic supports and incubated for 4 days to form polarized confluent monolayers as above. Apical or basolateral membranes of MDCK cells were biotinylated as described above. After PBS-CM washes followed by quenching, cells were fixed in 4% PFA for 30 min at 4°C. Cells were permeabilized with 0.1% Triton X-100-PBS for 10 min, treated with 0.5% SDS-PBS for 5 min, and blocked in 1% BSA-PBS for 30 min at room temperature. Cells were incubated in 1 μg/ml FITC-conjugated streptavidin overnight at 4°C. FITC-streptavidin was added to both apical and basolateral chambers. Cells were washed with PBS three times for 10 min and mounted on glass slides with Vectashield mounting medium.
Cycloheximide studies.
AQP2-MDCK cells were plated on six-well plates for Western blotting (32 × 104 cells) or on polyester filters (no. 3460 Costar) for immunofluorescence (8 × 104 cells). After plating, cells were used 2 days (for Western blotting) or 4 days (for immunofluorescence) later. Cells were incubated with 50 μM indomethacin overnight. For Western blotting, cells were treated with or without 40 μM CHX for 2 h, lysed in 300 μl lysis buffer per well. Then 65 μl lysate was mixed with 35 μl NuPage SDS sample buffer and denatured at 70°C for 10 min, and 10 μl of each denatured sample were subjected to Western blotting as described above. For immunofluorescence, cells were treated with or without CHX, then subjected to cold shock experiments and rewarming experiments as described above.
RESULTS
Subcellular distribution of AQP2 is different when assessed by site-specific biotinylation or conventional immunofluorescence.
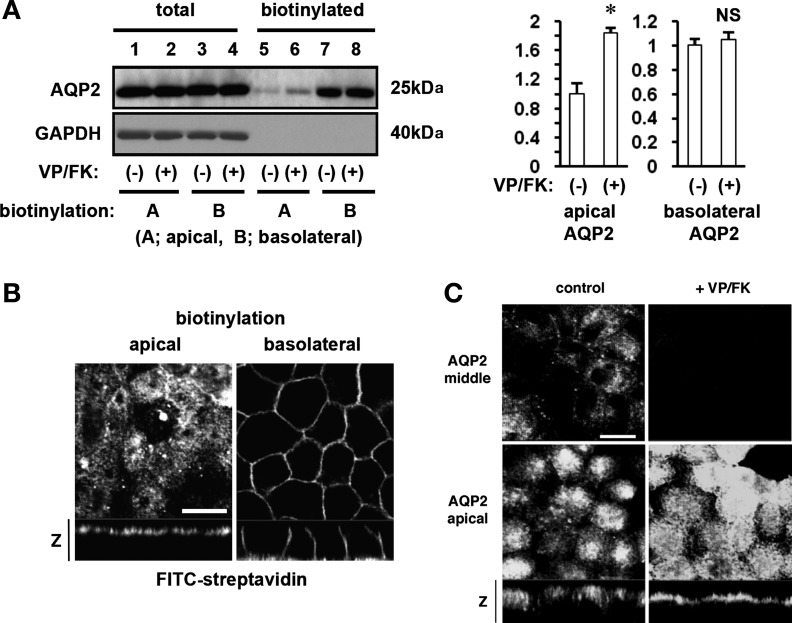
We first determined the subcellular distribution of AQP2 in polarized MDCK cells using site-specific cell surface biotinylation. Apical AQP2 was significantly increased after VP/FK treatment as reported by many groups (Fig. 1A, top, compare lanes 5 and 6) (10, 30, 49, 55). However, as previously reported, a strong basolateral AQP2 signal was also detected under these assay conditions with or without VP/FK treatment (Fig. 1A, top, lanes 7 and 8) (55). These results, which are quantified in Fig. 1A, right, suggest that AQP2 is constitutively targeted to the basolateral membrane of MDCK cells, independently of VP/FK treatment.
Fig. 1.
Subcellular distribution of aquaporin-2 (AQP2) in polarized MDCK cells. A: site-specific biotinylation was performed from the apical (lane 1, 2, 5, 6) or the basolateral (lane 3, 4, 7, 8) side with (lanes 2, 4, 6, 8) or without (lane 1, 3, 5, 7) vasopressin (VP)/forskolin (FK) treatment (VP 20 nM and FK 50 μM for 20 min). As quantified in the histograms to the right of the immunoblot, apical AQP2 was significantly increased after VP/FK treatment (lane 5 and 6, top, and left histogram). Basolateral AQP2 was strongly and equally detected with or without VP/FK treatment (lane 7 and 8, top, and right histogram). Total amounts of AQP2 were not different among these samples (lane 1–4, top). The same PVDF membrane was stripped, and reprobed with GAPDH antibody (bottom). GAPDH was well expressed in MDCK cells (lane 1–4, bottom) but was not detected either in apical or in basolateral membrane fractions (lane 5–8, bottom). *P < 0.05; NS, not significant. B: plasma membrane-bound biotin was visualized using FITC-streptavidin. AQP2-MDCK cells were biotinylated from the apical (left) or basolateral (right) side, and bound biotin was visualized by FITC-streptavidin. FITC-streptavidin was detected only at the side to which biotin was added. The larger panels represent confocal sections through the apical (left) or middle (right) regions of the cells. Bar, 10 μm. The smaller horizontal strips at the bottom are z-sections to show apical and basolateral membranes. C: AQP2-MDCK cells were treated with (right) or without (left) VP/FK (VP 20 nM and FK 50 μM for 20 min), and AQP2 was immunolocalized. The larger panels represent confocal sections through the middle (top) or apical (bottom) regions of the cells as indicated. The smaller horizontal strips at the bottom of the lower (apical) panels are z-sections to show both apical and basolateral regions of the cells. After VP/FK treatment, apical AQP2 was clearly increased and appears as a sharper apical band of staining in the z-section compared to the untreated control on the left. However, basolateral AQP2 accumulation was not detected either with or without VP/FK treatment. The images are representative of three independent experiments. Bar, 10 μm.
To check the accuracy of the biotinylation assay, the same PVDF membrane was reprobed with GAPDH antibody. GAPDH, in which there are several lysine residues that bind to biotin, was not detected in either of the plasma membrane domains (Fig. 1A, bottom, lanes 5-8). This indicates that the detection of AQP2 was specific and not due to experimental problems resulting from, as an example, basolateral biotin binding directly to the polycarbonate filter, detaching from the filter in lysis buffer, and subsequently binding to lysines of all proteins in the lysate.
To further exclude the possibility of technical problems, we visualized Sulfo-NHS-Biotin using FITC-conjugated streptavidin. Biotin was detected only on the side of the cells to which biotin added (Fig. 1B), showing that biotin was not internalized under our assay conditions and was not passing through tight junctions during the experiments. Together, these controls confirm that basolateral AQP2 detection using the biotinylation assay is not due to these technical problems.
We then examined AQP2 distribution in polarized MDCK cells by immunofluorescence (Fig. 1C). MDCK cells were treated with or without VP/FK, then fixed in 4% PFA at 37°C, which avoids the sudden temperature change that is required for the biotinylation assay. Under nonstimulated conditions, AQP2 was found in the cytoplasm and to a lesser extent in apical membranes. AQP2 was barely detectable in basolateral plasma membranes (Fig. 1C, left). After VP/FK treatment, AQP2 was readily detectable in the apical plasma membrane with no basolateral signal (Fig. 1C, right). These results by immunofluorescence staining were, therefore, very different from the results of the site-specific biotinylation assays shown in Fig. 1A. Specifically, the constitutive basolateral AQP2 accumulation suggested by site-specific cell surface biotinylation was not observed in the immunofluorescence studies.
Cold shock-induced basolateral accumulation of AQP2.
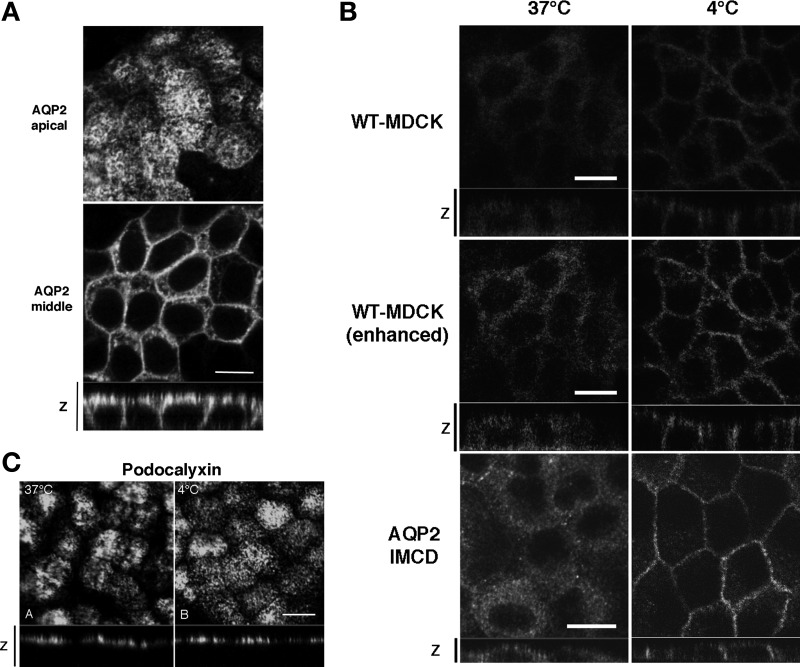
What could explain the difference in results using these two different “polarity” assays? In the cell surface biotinylation assays, MDCK cells were subjected to a sudden temperature change from 37°C to 4°C during the biotin incubation step (13, 42). This cold incubation step is thought to be necessary to reduce or stop exocytosis and endocytosis of membrane proteins. One possibility, therefore, is that AQP2 acutely accumulates in the basolateral membrane during incubation at the cold temperature, possibly due to a more rapid effect of the cold on reducing endocytosis, compared to a slower effect on reducing exocytosis. To examine this further, we incubated polarized MDCK cells in DMEM prechilled to 4°C for 15 min at 4°C (cold shock), and fixed at 4°C for 30 min in 4% PFA to avoid rewarming (Fig. 2A). Surprisingly, AQP2 was now dramatically accumulated in the basolateral plasma membrane (Fig. 2A, bottom). Thus, the rapid and extensive basolateral accumulation of AQP2 was seen also by immunofluorescence after cold treatment. These results were, therefore, different from the results of immunofluorescence performed at 37°C (Fig. 1C), but were similar to data obtained by site-specific biotinylation (Fig. 1A).
Fig. 2.
The effect of cold shock on subcellular distribution of AQP2. A: AQP2-MDCK cells were incubated at 4°C for 15 min (cold shock) and were then stained for AQP2. The larger panels represent confocal sections through the apical (top) or middle (bottom) regions of the cells as indicated. The smaller horizontal strip at the bottom is a z-section to show apical and basolateral membranes. AQP2 staining was abundant in the basolateral membrane after cold shock. Bar, 10 μm. B: WT-MDCK cells that express low amounts of endogenous AQP2 (top) and AQP2-transfected inner medullary collecting duct (IMCD) cells (bottom) were subjected to cold shock (right), in addition to AQP2-MDCK cells (shown in A). The middle set of images are duplicates of the top two images and were digitally enhanced in Photoshop to show more clearly the staining pattern in the low-expressing MDCK cells. In both cell lines, cold shock induced a basolateral accumulation of AQP2. Bar, 10 μm. C: immunostaining for gp135/podocalyxin under control conditions (37°C) and after cold shock for 15 min (4°C). The apical location of podocalyxin was not modified by cold treatment. Images are representative of three independent experiments. Bar, 10 μm.
We also applied cold shock to WT-MDCK (AQP2 untransfected) cells (Fig. 2B, top). As shown previously, a very low level of endogenous AQP2 is expressed in our untransfected MDCK cells (54). Endogenous AQP2 was indeed detectable in the basolateral plasma membrane after cold shock, but not in cells maintained at 37°C before and during fixation (Fig. 2B, top). The low level of endogenous AQP2 was “digitally enhanced” in Fig. 2B, middle, to illustrate more clearly the effect of cold shock on AQP2 in these cells. Cold shock-induced basolateral accumulation of AQP2 was also observed in another cell line, AQP2-transfected rat IMCD cells (Fig. 2B, bottom). These results exclude the possibility that the significant basolateral accumulation after cold shock is due to clonal variation in the MDCK cells, and they confirm that this phenomenon is not restricted to transfected MDCK cells.
We also assessed whether another apical membrane protein found in MDCK cells, gp135/podocalyxin, was redistributed after cold shock. As shown in Fig. 2C, gp135 retained its apical polarity in MDCK cells under all incubation and fixation conditions tested, showing that the cold-induced basolateral redistribution of AQP2 is not due to a nonspecific effect on all membrane proteins.
Cold shock-induced AQP2 basolateral accumulation is not dependent on phosphorylation.
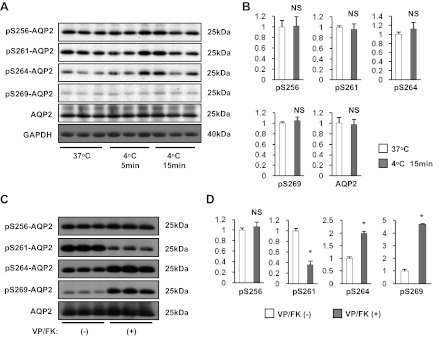
We next investigated whether or not AQP2 phosphorylation patterns were changed during cold shock using antibodies that specifically recognize phosphorylated serine residues S256, S261, S264, and S269, in the COOH terminus of AQP2 (Fig. 3A) (17). After cold shock, the phosphorylation status of these serine residues was not significantly changed (Fig. 3, A and B), demonstrating that the cold shock-induced AQP2 basolateral accumulation was not dependent on detectable changes in its phosphorylation pattern. In addition, AQP2 expression was not significantly changed (Fig. 3A, 5th row from top, and Fig. 3B), That expression levels are not involved in this basolateral targeting process was also shown by the similar results obtained in WT-MDCK cells that express lower levels of AQP2 (Fig. 2B), compared to the transfected cells. The specificity of these antibodies was tested by VP/FK stimulation (VP 20 nM and FK 50 μM for 60 min, Fig. 3, C and D). A significant increase of phosphorylation both at S264 and at S269 and a significant decrease of phosphorylated S261 were detected (Fig. 3, C and D), indicating that these three phospho-AQP2 antibodies are specific. However, the degree of phosphorylation at S256 was not greatly affected by VP/FK treatment in our AQP2-MDCK cells, although a small increase was detectable (Fig. 3, C and D). This result is consistent with a previous report showing that a significant amount of phospho-S256 AQP2 was detected in vasopressin-deficient rats (Brattleboro rats) even under non-dDAVP-stimulated conditions (16). This result does not, therefore, question the specificity of the pS256 antibody.
Fig. 3.
The effect of cold shock on phosphorylation status of AQP2. A: AQP2-MDCK cells were incubated at 37°C or 4°C (5 min or 15 min) and were then subjected to Western blotting. After cold shock, phosphorylation status at residues S256, S261, S264, and S269 was not changed, and the total AQP2 expression level was also not changed. B: protein band intensity was analyzed by ImageJ. Phosphorylated AQP2 signals adjusted to total AQP2 signals, and total AQP2 signals adjusted to the GAPDH signal, were analyzed by a two-tailed Student's t-test (means ± SD, n = 3). C: cells were treated with or without VP/FK (VP 20 nM and FK 50 μM for 60 min) at 37°C, then subjected to Western blotting. Phosphorylated S264 (pS264) and pS269 were significantly increased, and pS261 was significantly decreased after VP/FK treatment. pS256 was much less affected by VP/FK treatment under these conditions. D: protein band intensity was analyzed by ImageJ. Phosphorylated AQP2 signals were adjusted to total AQP2 signals and analyzed by a two-tailed Student's t-test (means ± SD, n = 3, *P < 0.01).
Microtubules are disrupted during cold shock but are not required for AQP2 basolateral accumulation.
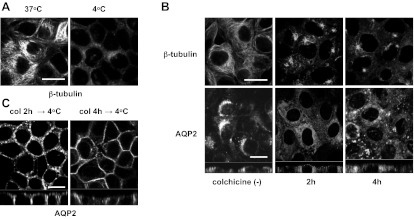
To study the mechanism of cold shock-induced AQP2 basolateral accumulation, we focused on microtubules because it has been reported that cold treatment induces microtubule disruption (3, 52). After staining for β-tubulin, the filamentous structure of microtubules was clearly observed in MDCK cells at 37°C (Fig. 4A, left) but was disrupted after cold treatment (4°C for 15 min, Fig. 4A, right). AQP2 accumulation in the basolateral membrane during cold shock occurred concomitantly with microtubule disruption, suggesting that these two events might be related. Therefore, we examined the polarity of AQP2 expression at 37°C after colchicine-induced microtubule depolymerization. After colchicine treatment (10 μM for 2 h and 4 h), microtubules were clearly depolymerized as expected (Fig. 4B, top). However, intracellular AQP2 appeared uniformly scattered throughout the cytoplasm under these conditions without accumulation in the basolateral plasma membrane (Fig. 4B, bottom). While both cold shock and colchicine treatment induced microtubule disruption, the effect of these two treatments on AQP2 subcellular distribution was, therefore, quite different. We next tested the effect of cold shock on AQP2 distribution after colchicine treatment and found that the dramatic basolateral accumulation of AQP2 also occurred under these conditions (Fig. 4C). Our data suggest, therefore, that microtubule depolymerization is not the dominant mechanism by which cold shock induces AQP2 basolateral accumulation.
Fig. 4.
The effect of colchicine on subcellular distribution of AQP2. A: β-Tubulin in the AQP2-MDCK cells was stained with or without cold shock. The filamentous structure of microtubules was visible at 37°C (left) and was disrupted after cold shock (4°C for 15 min, right). Bar, 10 μm. B: AQP2-MDCK cells were treated with or without 10 μM colchicine for 2 h (middle) and 4 h (right), then β-tubulin (top) and AQP2 (bottom) were stained. After colchicine treatment, microtubules were depolymerized (top), and the usual perinuclear pattern of AQP2 staining (bottom left) was lost and replaced by staining that was scattered throughout the cytoplasm (bottom middle and right). However, basolateral AQP2 accumulation was not observed after colchicine treatment (bottom). The larger panels represent confocal sections through the middle regions of the cells. Bar, 10 μm. C: AQP2-MDCK cells were treated with colchicine (Col) for 2 h and 4 h, then subjected to cold shock (4°C for 15 min). AQP2 was accumulated in the basolateral membrane after cold shock. The larger panels represent confocal sections through the middle regions of the cells. Bar, 10 μm. The images are representative of three independent experiments.
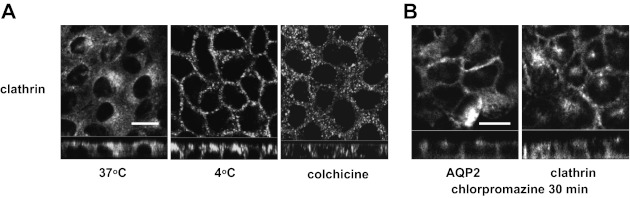
Clathrin accumulates in the basolateral membrane after cold shock.
Next, we focused on the subcellular distribution of clathrin, because this protein mediates the endocytosis of many trans-membrane proteins (28), and its importance for AQP2 endocytosis has been demonstrated in vivo (41) and in vitro (25). We found that the subcellular localization of clathrin was greatly affected by cold shock (Fig. 5A). At 37°C, clathrin was located intracellularly in a perinuclear region, similar to AQP2, with some scattered spots that could represent, at least in part, cell surface-coated pits (Fig. 5A, left). However, after cold shock, clathrin expression at the level of the basolateral plasma membrane was markedly increased (Fig. 5A, middle). After colchicine treatment, the perinuclear appearance of clathrin staining was lost and it became scattered throughout the cytoplasm. Basolateral clathrin accumulation was not observed, however (Fig. 5A, right). Therefore, the subcellular redistribution of clathrin was clearly different between cold shock and colchicine treatment. These results suggest that an acute arrest of clathrin-mediated endocytosis might be a dominant mechanism by which cold shock induces AQP2 basolateral accumulation.
Fig. 5.
The effect of cold shock on subcellular distribution of clathrin. A: at 37°C, clathrin was accumulated in a perinuclear region but not in the basolateral membrane (left). After cold shock (4°C for 15 min), clathrin was greatly accumulated in the basolateral membrane (middle). After colchicine treatment, the perinuclear accumulation of clathrin was also lost, and clathrin-positive vesicles were scattered throughout the cytoplasm (10 μM for 2 h). However, basolateral accumulation of clathrin was not observed (right). The larger panels represent confocal sections through the middle regions of the cells. Bar, 10 μm. B: AQP2-MDCK cells were treated with chlorpromazine (100 μM for 30 min) at 37°C, then AQP2 and clathrin were stained. Both AQP2 (left) and clathrin (right) accumulated in the basolateral membrane after chlorpromazine treatment. The larger panels represent confocal sections through the middle regions of the cells. Bar, 10 μm. The images are representative of three independent experiments.
To further test whether or not AQP2 is internalized from the basolateral membrane by clathrin-mediated endocytosis in polarized MDCK cells at 37°C, we applied chlorpromazine, which inhibits clathrin-mediated endocytosis of Na-K-2Cl cotransporter (NKCC) in MDCK and other cells (32). After chlorpromazine treatment (100 μM for 30 min at 37°C), clathrin clearly accumulated in the basolateral membrane (Fig. 5B, right). Importantly, not only clathrin but also AQP2 was concentrated in the basolateral membrane after chlorpromazine treatment (Fig. 5B, left). These results further support the idea that AQP2 is retrieved from the basolateral membrane by clathrin, and that this retrieval was rapidly inhibited during cold shock.
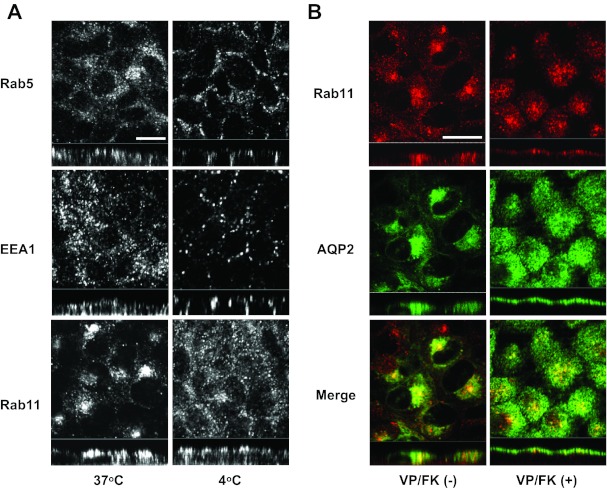
Rab5 and EEA1, but not Rab11, also accumulate in basolateral membranes during cold shock.
It was reported that AQP2 is localized both in Rab5-EEA1-positive vesicles and in Rab11-positive vesicles in vivo and in MDCK cells (2, 45, 46). Furthermore, it has been suggested that AQP2 in Rab11-positive vesicles is regulated by VP or FK stimulation (45, 46, 53). In addition, AQP2 is also constitutively transported independently of VP or FK activation and is retrieved from the plasma membrane via clathrin-coated vesicles (4). Therefore, we examined the effect of cold shock on the localization of these accessory trafficking proteins. Rab5 and EEA1, which are localized on clathrin-coated vesicles (Rab5) and early endosomes (Rab5 and EEA1) (40, 51), are also shifted basolaterally during cold shock (Fig. 6A, top and middle). However, interestingly, while the perinuclear position of Rab11 was disrupted after cold shock, Rab11 did not accumulate in the basolateral membrane (Fig. 6A, bottom). These results suggest that the pool of intracellular AQP2 that is in Rab11-positive vesicles is not directly targeted to the basolateral membrane during cold shock.
Fig. 6.
The effect of cold shock on subcellular localization of Rab5, EEA1, and Rab11. A: at 37°C, Rab5, EEA1, an Rab11 were located intracellularly (left). After cold shock (4°C for 15 min), the subcellular localizations of Rab5 and EEA1 were shifted basolaterally (right top and middle). The perinuclear positioning of Rab11 was lost and vesicles were scattered throughout the cytoplasm. However, Rab11 did not accumulate in the basolateral membrane (right bottom). The larger panels represent confocal sections through the middle regions of the cells. Bar, 10 μm. B: AQP2-MDCK cells were treated with or without VP/FK (VP 20 nM and FK 50 μM for 20 min), then AQP2 and Rab11 were stained. Under nonstimulated conditions, AQP2 and Rab11 were clearly colocalized in the perinuclear region (left). After VP/FK treatment, Rab11 was translocated to the apical membrane together with AQP2 (right). The larger panels represent confocal sections through the middle (left) or apical (right) regions of the cells. Bar, 10 μm. The images are representative of three independent experiments.
To confirm that AQP2 in Rab11-positive vesicles is really regulated by VP/FK in our MDCK cells, we stained Rab11 and AQP2 with or without VP/FK treatment. Rab11 and AQP2 were colocalized under nonstimulated conditions (Fig. 6B, left), and they both accumulated in the apical membrane after stimulation (Fig. 6B, right). These results indicate that AQP2 in Rab11-positive vesicles is a VP/FK-regulated fraction and that this intracellular pool is not sensitive to cold shock-induced basolateral accumulation (Fig. 6A, bottom).
AQP2 is internalized and transported to the apical membrane after rewarming, together with microtubule reorganization.
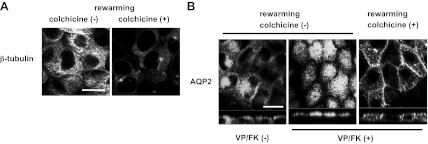
To determine the fate of the basolateral AQP2 that accumulates after cold shock, we rewarmed MDCK cells to 37°C and followed its intracellular fate. Microtubules were clearly reorganized (Fig. 7A, left), and AQP2 was internalized under these conditions and was accumulated in a perinuclear region after rewarming at 37°C for 15 min (Fig. 7B, left). This indicates that the MDCK cells were not irreversibly damaged by cold shock and that AQP2 is internalized from the basolateral membrane and can be transported to a perinuclear region after retrieval from the basolateral membrane. However, after rewarming at 37°C in VP/FK-containing medium, also for 15 min, AQP2 was strongly accumulated in the apical plasma membrane, with no detectable remaining basolateral signal (Fig. 7B, middle). These results demonstrated that AQP2 can be transported from basolateral membranes to apical membranes, indicating the existence of a transcytotic pathway of apical AQP2 insertion. We also attempted to follow directly the endocytosis and transcytosis of basolateral AQP2 to the apical surface after biotinylation of the basolateral cell surface. Under our experimental conditions we were, however, consistently unable to demonstrate any AQP2 internalization from the basolateral plasma membrane after the biotinylation procedure (data not shown). This result suggests an unexplained inhibitory effect of basolateral membrane protein biotinylation on AQP2 endocytosis.
Fig. 7.
Microtubules are required for transcytosis and apical accumulation of AQP2 after VP/FK treatment. AQP2-MDCK cells were treated with or without colchicine (10 μM for 2 h), subjected to cold shock (4°C for 15 min) with or without colchicine, then incubated in DMEM (prewarmed to 37°C) for 15 min at 37°C (rewarming) with or without colchicine. A: after rewarming without colchicine, microtubules were reorganized showing their filamentous structure (left). After rewarming with colchicine, microtubules were maintained in a depolymerized state (right). Bar, 10 μm. B: after rewarming without colchicine, AQP2 was internalized from the basolateral membrane and accumulated in a perinuclear region without VP/FK (left). After rewarming with VP/FK (without colchicine), AQP2 was translocated to the apical membrane with no residual basolateral signal (middle). After rewarming with colchicine and with VP/FK, intracellular AQP2 was increased compared to its location after cold shock alone, but much of the AQP2 still remained in the basolateral membrane (right). The VP/FK-induced apical translocation of AQP2 during rewarming that occurred in the absence of colchicine was not observed in the presence of colchicine (compare right to middle panel). The larger panels represent confocal sections through the middle (left and right) or apical (middle) regions of the cells. Bar, 10 μm. The images are representative of three independent experiments.
AQP2 apical translocation during rewarming with VP/FK is inhibited by colchicine.
While microtubule disruption is apparently not a dominant mechanism of cold shock-induced AQP2 basolateral accumulation (Fig. 4), whether or not microtubules are involved in AQP2 transport from the basolateral membrane during rewarming is unknown. Therefore, we next investigated whether continuous colchicine treatment affects AQP2 internalization and translocation from basolateral to the apical membrane during rewarming. AQP2-MDCK cells were treated with 10 μM colchicine for 2 h, subjected to cold shock in DMEM (4°C, 15 min) containing colchicine (10 μM), then rewarmed for 15 min in 37°C DMEM containing colchicine (10 μM) and VP/FK (VP 20 nM, FK 50 μM). Microtubules were, in this way, maintained in a depolymerized state even after rewarming (Fig. 7A, right, compare to Fig. 7A, left). After rewarming, AQP2 internalization did increase (compare Fig. 7B, right, to Fig. 4C), but AQP2 was still detected in the basolateral plasma membrane and was not strongly accumulated in the apical plasma membrane (compare Fig. 7B, right, with Fig. 7B, middle). This result shows that microtubule organization is necessary for AQP2 transport from the basolateral to the apical pole in these cells.
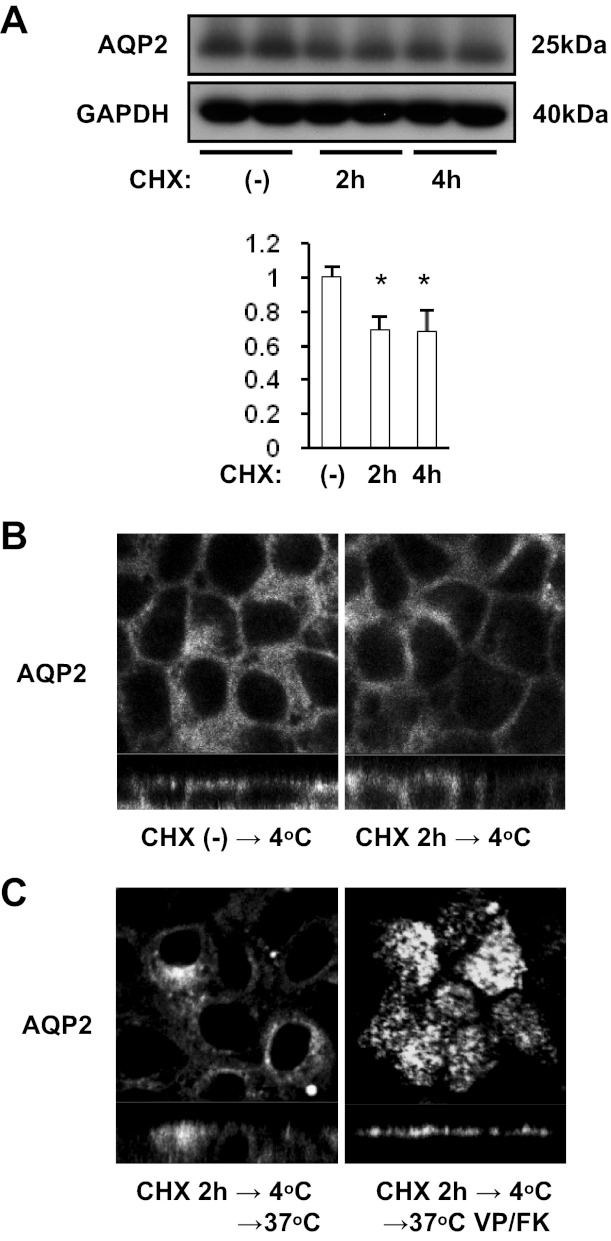
Mature AQP2 follows a transcytotic pathway.
To determine whether or not newly synthesized AQP2 is targeted to basolateral membranes and is then transcytosed, we applied CHX before cold shock to block protein synthesis (Fig. 8) (30). After 2 h and 4 h CHX (40 μM) treatment, AQP2 expression level was significantly decreased (Fig. 8A). After CHX treatment for 2 h, AQP2 also clearly accumulated in basolateral membranes after cold shock (Fig. 8B, top). After rewarming without VP/FK, AQP2 accumulated in a perinuclear region (Fig. 8C, bottom left). After rewarming with VP/FK (VP 20 nM, FK 50 μM), AQP2 markedly accumulated in the apical membrane (Fig. 8C, bottom right). These results demonstrate that mature AQP2 is targeted to the basolateral membrane and then follows a transcytotic pathway to the apical pole of MDCK cells.
Fig. 8.
The effect of cycloheximide (CHX) on cold shock-induced and rewarming-induced AQP2 subcellular localization. A: AQP2-MDCK cells were treated with 40 μM CHX for 2 h or 4 h, then subjected to Western blotting. Protein band intensity was analyzed by ImageJ. After CHX treatment, AQP2 expression was significantly decreased (30%). AQP2 signal intensity was adjusted to the GAPDH signal and analyzed by a two-tailed Student's t-test (means ± SD, n = 3, *P < 0.05). B: after CHX treatment (40 μM for 2 h), AQP2-MDCK cells were subjected to cold shock (4°C for 15 min). The cold shock induced AQP2 basolateral accumulation (left) was reproduced even after CHX treatment (right). C: AQP2-MDCK cells were treated with CHX (40 μM for 2 h), subjected to cold shock (4°C for 15 min), then rewarmed (37°C for 15 min) with (right) or without VP/FK (VP 20 nM and FK 50 μM for 20 min; left). After rewarming without VP/FK, AQP2 was internalized and accumulated in a perinuclear region (left). After rewarming with VP/FK, AQP2 accumulated in the apical membrane (right). The larger panels represent confocal sections through the middle (top in B and left in C) or apical (right in C) regions of the cells. The images are representative of three independent experiments.
DISCUSSION
The work described in this study is based on our initial observation that the subcellular distribution of AQP2 is different when assessed by site-specific biotinylation or by immunofluorescence. We discovered that the cold exposure that is required for the biotinylation technique triggers a dramatic basolateral accumulation of AQP2 in polarized MDCK cells within 15 min, even in the absence of any hormonal stimulation. We believe that this experimental protocol reveals a transcytotic pathway of AQP2 trafficking that involves initial basolateral insertion of AQP2, followed by retrieval and transcytosis into an apical delivery pathway. This indirect apical route from the basolateral membrane is defined as a transcytotic pathway, and it has been described for other proteins such as the polymeric IgA receptor and its ligand in these cells (1, 44). Cold shock is a frequently used protocol for biotinylation assays (13, 42), and the observed basolateral accumulation of AQP2 could possibly represent a cell culture phenomenon. However, AQP2 clearly accumulates on the basolateral plasma membrane of kidney principal cells in vivo, and the degree of basolateral targeting can be modified under different conditions (8, 19, 24, 34). Furthermore, cold-induced basolateral accumulation of AQP2 has been documented in principal cells in rat kidney slices incubated in vitro (3).
Nonetheless, these results imply that the basolateral accumulation of some transmembrane proteins might be overestimated by site-specific biotinylation. The results of such assays should, therefore, be carefully interpreted and combined with immunofluorescence with or without cold shock. Importantly, site-specific biotinylation can be utilized to reveal transient basolateral targeting of some transmembrane proteins as we show here. It is believed that both exocytosis and endocytosis are instantly and simultaneously arrested by exposure to cold temperature (13, 42). While this may vary among different cells and even different proteins, indeed gp135/podocalyxin remained apically located in our cold-treated MDCK cells, our results indicate that endocytosis of AQP2 might be arrested more rapidly and thoroughly than exocytosis in MDCK cells and IMCD cells.
Our results using cold shock and rewarming are best explained by the existence of a constitutive basolateral targeting process, and subsequent transcytosis of AQP2 that is summarized in Fig. 9. In MDCK cells, AQP2 is accumulated in the apical plasma membrane following VP and/or FK treatment, while, in other cell lines (for example, IMCD cells, LLC-PK1 cells, CD8 cells) the polarity of AQP2 expression ranges from mainly basolateral to bipolar (4, 47). This indicates the existence of cell-specific transport pathways. In MDCK cells, we propose that basolateral membrane targeting is normally transient and is usually not detectable by immunofluorescence performed at 37°C. AQP2 is rapidly internalized from this membrane followed by routing into an apical “transcytotic” pathway, possibly via an intermediate step involving a perinuclear compartment (Fig. 9). Importantly, it was reported that AQP2-containing vesicles isolated from rat IMCD interact with Sec6, Sec8, and RalA. Sec6 and Sec8 are components of the exocyst, and RalA is an exocyst-associated small GTPase. The signal complex formed by these AQP2 vesicle-associated proteins is involved in trafficking of proteins to basolateral plasma membranes (2).
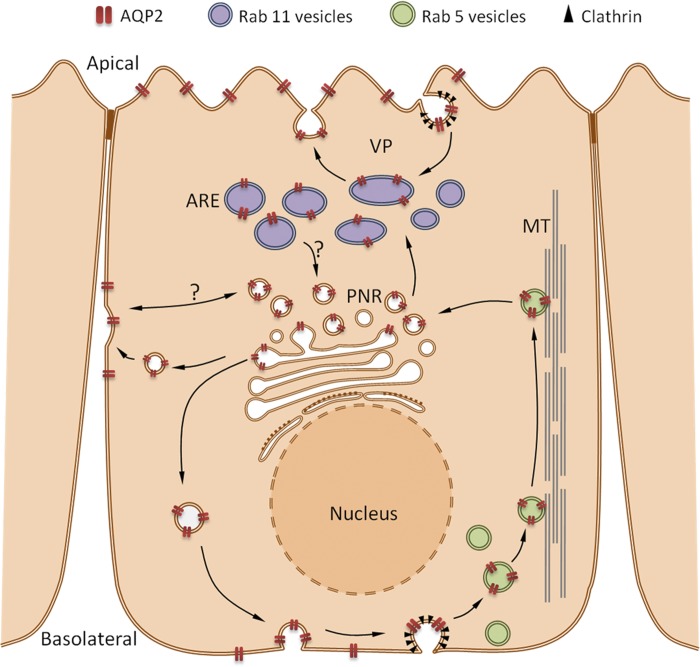
Fig. 9.
Diagram of the proposed pathway by which AQP2 cycles between different intracellular pools of vesicles and different plasma membrane domains. Newly synthesized and possibly some recycled AQP2 moves from the perinuclear region to the basolateral plasma membrane, from where it is retrieved by clathrin-mediated endocytosis into Rab5-positive endosomes (green). These endosomes enter a microtubule (MT)-dependent transcytotic pathway that initially delivers AQP2 to the perinuclear region (PNR), and subsequently to Rab11-positive apical recycling endosomes (ARE, purple). The AQP2 in Rab11-positive vesicles recycles constitutively between the apical plasma membrane and the perinuclear region of the cell (which may include the trans-Golgi network and Rab11-positive vesicles). Vasopressin (VP) increases apical AQP2 expression by increasing exocytosis from the Rab11 compartment, and by inhibiting endocytosis from the apical surface by clathrin-coated pits. After endocytosis, some AQP2 recycles through Rab11 vesicles but we have no evidence that Rab11 vesicles communicate directly with the basolateral plasma membrane in our system. These distinct but convergent pathways may be important to allow simultaneous, constitutive basolateral expression of some AQP2, which we believe is important for epithelial cell migration and collecting duct tubulogenesis (6), as well as vasopressin-induced apical insertion of AQP2 that is critical for regulation of collecting duct water permeability.
A proteomics analysis also revealed that Rab11 and Rab5 are associated with AQP2-containing vesicles (2). Such a complex, multiple vesicular localization of AQP2 was also shown in MDCK cells (45, 46). Our current study shows that Rab5, EEA1, and clathrin are also basolaterally accumulated during cold shock. In contrast, Rab11 (a recycling endosome marker), which clearly accumulates in the apical pole of MDCK cells after VP/FK treatment, did not show basolateral accumulation after cold treatment. Because cold-induced AQP2 basolateral accumulation occurred without VP/FK treatment, these results suggest that the AQP2 fraction that targets to the basolateral membrane is not in Rab11-positive vesicles and is independent of VP/FK stimulation. This basolateral fraction of cellular AQP2 is also continuously retrieved into Rab5-positive vesicles by clathrin-mediated endocytosis at the basolateral cell surface, as shown in Fig. 9. Interestingly, Rab5 interacts with microtubules and is necessary for the motility of vesicles along microtubules (33), consistent with our finding that AQP2 internalization during rewarming after cold shock was blunted by colchicine-induced microtubule disruption. These data demonstrate that AQP2 transcytosis from the basolateral membrane is dependent on microtubules, as previously reported for other transcytosed proteins (18).
In a previous study, we reported that FK-induced AQP2 apical induction was not affected after basolateral membrane treatment with tannic acid, an inhibitor of endocytosis, and we concluded that FK-induced apical transport of AQP2 did not include a transcytotic step (55). Based on our current data, we now conclude that the VP/FK-induced apical translocation of the pool of AQP2 that resides in Rab11-positive vesicles (in a perinuclear region) is not transcytotic, but that AQP2 from the basolateral membrane can be rerouted apically by transcytosis upon VP/FK stimulation, possibly via an intermediate compartment that is Rab11 positive.
Our working hypothesis to explain the current data is shown in Fig. 9. We propose that AQP2 is internalized from the basolateral membrane into clathrin- and Rab5-positive vesicles and then moves to a perinuclear location. The subcellular localization of clathrin, Rab5, and AQP2 under nonstimulated conditions (without VP/FK treatment under 37°C) supports this pathway. After accumulating in the perinuclear region, some AQP2 may be moved into Rab11-positive vesicles that recycle between the apical membrane and the perinuclear region under nonstimulated conditions. Indeed, some AQP2 is targeted to the apical membrane without VP/FK treatment as shown in Fig. 1C. This supports our previous studies showing that apical AQP2 is continually and constitutively recycling (4, 25). Upon VP/FK stimulation, exocytosed AQP2 accumulates in the apical membrane as previously demonstrated, by a combination of inhibition of endocytosis and stimulation of exocytosis (26, 30). Some AQP2 may also recycle from the VP-sensitive Rab11 compartment to other perinuclear compartments, perhaps the trans-Golgi network, from which it can enter the basolateral trafficking pathway.
In summary, we show that AQP2 is constitutively targeted to the basolateral membrane, is retrieved by clathrin-mediated endocytosis into Rab5-positive vesicles, and then undertakes microtubule-dependent transcytosis in polarized MDCK cells. This “hidden” basolateral targeting and transcytosis pathway was revealed using cold shock and rewarming of the cells. Our finding also raises important questions regarding the physiological role of basolateral AQP2 in the kidney. Our recent data highlight a novel role of basolateral AQP2 in cell migration and epithelial morphogenesis that appears to be independent of its water channel function (6). This raises the possibility that mistargeting of AQP2 may lead not only to water balance disorders, but also possibly to structural defects in the connecting and collecting duct system in the kidney.
GRANTS
This work was supported by NIH Grants DK-38452 and DK-96586 (to D. Brown), DK-75940 and a Gottschalk research grant (American Society of Nephrology) to H. A. J. Lu. Additional support for the Program in Membrane Biology Microscopy Core comes from the Boston Area Diabetes and Endocrinology Research Center (DK-57521) and the MGH Center for the Study of Inflammatory Bowel Disease (DK-43351). N. Yui and N. Nomura were also supported by a grant for “Strategic young researcher overseas visits program for accelerating brain circulation” from the Japan Society for the Promotion of Science.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
N.Y., H.A.J.L., Y.C., N.N., R.B., and D.B. conception and design of the research; N.Y., Y.C., and N.N. performed the experiments; N.Y., H.A.J.L., R.B., and D.B. analyzed the data; N.Y., H.A.J.L., Y.C., N.N., R.B., and D.B. interpreted the results of the experiments; N.Y. and N.N. prepared the figures; N.Y. drafted the manuscript; N.Y., H.A.J.L., R.B., and D.B. edited and revised the manuscript; N.Y., H.A.J.L., Y.C., N.N., R.B., and D.B. approved the final version of the manuscript.
ACKNOWLEDGMENTS
We thank Dr. S. Sasaki and Dr. S. Uchida (Tokyo Medical and Dental University) for providing MDCK clones and Dr. M. Knepper (NIH) for providing the phosphorylated AQP2 polyclonal antibodies. Finally, we thank Yechun (Sharon) Ruan for preparing the diagram shown in Fig. 9.
Footnotes
This article is the topic of an Editorial Focus by Curtis T. Okamoto (37a).
REFERENCES
- 1. Apodaca G, Katz LA, Mostov KE. Receptor-mediated transcytosis of IgA in MDCK cells is via apical recycling endosomes. J Cell Biol 125: 67–86, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barile M, Pisitkun T, Yu MJ, Chou CL, Verbalis MJ, Shen RF, Knepper MA. Large scale protein identification in intracellular aquaporin-2 vesicles from renal inner medullary collecting duct. Mol Cell Proteomics 4: 1095–1106, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Breton S, Brown D. Cold-induced microtubule disruption and relocalization of membrane proteins in kidney epithelial cells. J Am Soc Nephrol 9: 155–166, 1998 [DOI] [PubMed] [Google Scholar]
- 4. Brown D. The ins and outs of aquaporin-2 trafficking. Am J Physiol Renal Physiol 284: F893–F901, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Chen Y, Rice W, Gu Z, Li J, Huang J, Brenner MB, Van Hoek A, Xiong J, Gundersen GG, Norman JC, Hsu VW, Fenton RA, Brown D, Lu HA. Aquaporin 2 promotes cell migration and epithelial morphogenesis. J Am Soc Nephrol 23: 1506–1517, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Coleman RA, Wu DC, Liu J, Wade JB. Expression of aquaporins in the renal connecting tubule. Am J Physiol Renal Physiol 279: F874–F883, 2000 [DOI] [PubMed] [Google Scholar]
- 8. De Seigneux S, Nielsen J, Olesen ET, Dimke H, Kwon TH, Frøkiaer J, Nielsen S. Long-term aldosterone treatment induces decreased apical but increased basolateral expression of AQP2 in CCD of rat kidney. Am J Physiol Renal Physiol 293: F87–F99, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Deen PM, Rijss JP, Mulders SM, Errington RJ, van Baal J, van Os CH. Aquaporin-2 transfection of Madin-Darby canine kidney cells reconstitutes vasopressin-regulated transcellular osmotic water transport. J Am Soc Nephrol 8: 1493–1501, 1997 [DOI] [PubMed] [Google Scholar]
- 10. Deen PM, Van Balkom BW, Savelkoul PJ, Kamsteeg EJ, Van Raak M, Jennings ML, Muth TR, Rajendran V, Caplan MJ. Aquaporin-2: COOH terminus is necessary but not sufficient for routing to the apical membrane. Am J Physiol Renal Physiol 282: F330–F340, 2002 [DOI] [PubMed] [Google Scholar]
- 11. Fenton RA, Moeller HB, Hoffert JD, Yu MJ, Nielsen S, Knepper MA. Acute regulation of aquaporin-2 phosphorylation at Ser-264 by vasopressin. Proc Natl Acad Sci USA 105: 3134–3139, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fushimi K, Uchida S, Hara Y, Hirata Y, Marumo F, Sasaki S. Cloning and expression of apical membrane water channel of rat kidney collecting tubule. Nature 361: 549–552, 1993 [DOI] [PubMed] [Google Scholar]
- 13. Gottardi CJ, Dunbar LA, Caplan MJ. Biotinylation and assessment of membrane polarity: caveats and methodological concerns. Am J Physiol Renal Fluid Electrolyte Physiol 268: F285–F295, 1995 [DOI] [PubMed] [Google Scholar]
- 14. Grindstaff KK, Yeaman C, Anandasabapathy N, Hsu SC, Rodriguez-Boulan E, Scheller RH, Nelson WJ. Sec6/8 complex is recruited to cell-cell contacts and specifies transport vesicle delivery to the basal-lateral membrane in epithelial cells. Cell 93: 731–740, 1998 [DOI] [PubMed] [Google Scholar]
- 15. Hoffert JD, Fenton RA, Moeller HB, Simons B, Tchapyjnikov D, McDill BW, Yu MJ, Pisitkun T, Chen F, Knepper MA. Vasopressin-stimulated increase in phosphorylation at Ser269 potentiates plasma membrane retention of aquaporin-2. J Biol Chem 283: 24617–24627, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hoffert JD, Nielsen J, Yu MJ, Pisitkun T, Schleicher SM, Nielsen S, Knepper MA. Dynamics of aquaporin-2 serine-261 phosphorylation in response to short-term vasopressin treatment in collecting duct. Am J Physiol Renal Physiol 292: F691–F700, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Hoffert JD, Pisitkun T, Wang G, Shen RF, Knepper MA. Quantitative phosphoproteomics of vasopressin-sensitive renal cells: regulation of aquaporin-2 phosphorylation at two sites. Proc Natl Acad Sci USA 103: 7159–7164, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hunziker W, Mâle P, Mellman I. Differential microtubule requirements for transcytosis in MDCK cells. EMBO J 9: 3515–3525, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jeon US, Joo KW, Na KY, Kim YS, Lee JS, Kim J, Kim GH, Nielsen S, Knepper MA, Han JS. Oxytocin induces apical and basolateral redistribution of aquaporin-2 in rat kidney. Nephron Exp Nephrol 93: e36–e45, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Katsura T, Gustafson CE, Ausiello DA, Brown D. Protein kinase A phosphorylation is involved in regulated exocytosis of aquaporin-2 in transfected LLC-PK1 cells. Am J Physiol Renal Physiol 272: F817–F822, 1997 [PubMed] [Google Scholar]
- 21. Klussmann E, Maric K, Wiesner B, Beyermann M, Rosenthal W. Protein kinase A anchoring proteins are required for vasopressin-mediated translocation of aquaporin-2 into cell membranes of renal principal cells. J Biol Chem 274: 4934–4938, 1999 [DOI] [PubMed] [Google Scholar]
- 22. Knepper MA, Verbalis JG, Nielsen S. Role of aquaporins in water balance disorders. Curr Opin Nephrol Hypertens 6: 367–371, 1997 [DOI] [PubMed] [Google Scholar]
- 23. Kuwahara M, Fushimi K, Terada Y, Bai L, Marumo F, Sasaki S. cAMP-dependent phosphorylation stimulates water permeability of aquaporin-collecting duct water channel protein expressed in Xenopus oocytes. J Biol Chem 270: 10384–10387, 1995 [DOI] [PubMed] [Google Scholar]
- 24. Li C, Wang W, Summer SN, Westfall TD, Brooks DP, Falk S, Schrier RW. Molecular mechanisms of antidiuretic effect of oxytocin. J Am Soc Nephrol 19: 225–232, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lu H, Sun TX, Bouley R, Blackburn K, McLaughlin M, Brown D. Inhibition of endocytosis causes phosphorylation (S256)-independent plasma membrane accumulation of AQP2. Am J Physiol Renal Physiol 286: F233–F243, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Lu HA, Sun TX, Matsuzaki T, Yi XH, Eswara J, Bouley R, McKee M, Brown D. Heat shock protein 70 interacts with aquaporin-2 and regulates its trafficking. J Biol Chem 282: 28721–28732, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Maxfield FR, McGraw TE. Endocytic recycling. Nat Rev Mol Cell Biol 5: 121–132, 2004 [DOI] [PubMed] [Google Scholar]
- 28. McMahon HT, Boucrot E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat Rev Mol Cell Biol 12: 517–533, 2011 [DOI] [PubMed] [Google Scholar]
- 29. Moeller HB, Knepper MA, Fenton RA. Serine 269 phosphorylated aquaporin-2 is targeted to the apical membrane of collecting duct principal cells. Kidney Int 75: 295–303, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Moeller HB, Praetorius J, Rützler MR, Fenton RA. Phosphorylation of aquaporin-2 regulates its endocytosis and protein-protein interactions. Proc Natl Acad Sci USA 107: 424–429, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mostov KE, Verges M, Altschuler Y. Membrane traffic in polarized epithelial cells. Curr Opin Cell Biol 12: 483–490, 2000 [DOI] [PubMed] [Google Scholar]
- 32. Mykoniatis A, Shen L, Fedor-Chaiken M, Tang J, Tang X, Worrell RT, Delpire E, Turner JR, Matlin KS, Bouyer P, Matthews JB. Phorbol 12-myristate 13-acetate-induced endocytosis of the Na-K-2Cl cotransporter in MDCK cells is associated with a clathrin-dependent pathway. Am J Physiol Cell Physiol 298: C85–C97, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nielsen E, Severin F, Backer JM, Hyman AA, Zerial M. Rab5 regulates motility of early endosomes on microtubules. Nat Cell Biol 1: 376–382, 1999 [DOI] [PubMed] [Google Scholar]
- 34. Nielsen S, Chou CL, Marples D, Christensen EI, Kishore BK, Knepper MA. Vasopressin increases water permeability of kidney collecting duct by inducing translocation of aquaporin-CD water channels to plasma membrane. Proc Natl Acad Sci USA 92: 1013–1017, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nielsen S, Frøkiaer J, Marples D, Kwon TH, Agre P, Knepper MA. Aquaporins in the kidney: from molecules to medicine. Physiol Rev 82: 205–244, 2002 [DOI] [PubMed] [Google Scholar]
- 36. Nishimoto G, Zelenina M, Li D, Yasui M, Aperia A, Nielsen S, Nairn AC. Arginine vasopressin stimulates phosphorylation of aquaporin-2 in rat renal tissue. Am J Physiol Renal Physiol 276: F254–F259, 1999 [DOI] [PubMed] [Google Scholar]
- 37. Noda Y, Horikawa S, Kanda E, Yamashita M, Meng H, Eto K, Li Y, Kuwahara M, Hirai K, Pack C, Kinjo M, Okabe S, Sasaki S. Reciprocal interaction with G-actin and tropomyosin is essential for aquaporin-2 trafficking. J Cell Biol 182: 587–601, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37a. Okamoto CT. Caring about the other 47% of the water channels. Focus on “Basolateral targeting and microtubule-dependent transcytosis of the aquaporin-2 water channel.” Am J Physiol Cell Physiol (November 7, 2012). doi:10.1152/ajpcell.00348.2012 [DOI] [PubMed] [Google Scholar]
- 38. Paladino S, Pocard T, Catino MA, Zurzolo C. GPI-anchored proteins are directly targeted to the apical surface in fully polarized MDCK cells. J Cell Biol 172: 1023–1034, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Polishchuk R, Di Pentima A, Lippincott-Schwartz J. Delivery of raft-associated, GPI-anchored proteins to the apical surface of polarized MDCK cells by a transcytotic pathway. Nat Cell Biol 6: 297–307, 2004 [DOI] [PubMed] [Google Scholar]
- 40. Rubino M, Miaczynska M, Lippe R, Zerial M. Selective membrane recruitment of EEA1 suggests a role in directional transport of clathrin-coated vesicles to early endosomes. J Biol Chem 275: 3745–3748, 2000 [DOI] [PubMed] [Google Scholar]
- 41. Russo LM, McKee M, Brown D. Methyl-β-cyclodextrin induces vasopressin-independent apical accumulation of aquaporin-2 in the isolated, perfused rat kidney. Am J Physiol Renal Physiol 291: F246–F253, 2006 [DOI] [PubMed] [Google Scholar]
- 42. Sargiacomo M, Lisanti M, Graeve L, Le Bivic A, Rodriguez-Boulan E. Integral and peripheral protein composition of the apical and basolateral membrane domains in MDCK cells. J Membr Biol 107: 277–286, 1989 [DOI] [PubMed] [Google Scholar]
- 43. Sasaki S, Noda Y. Aquaporin-2 protein dynamics within the cell. Curr Opin Nephrol Hypertens 16: 348–352, 2007 [DOI] [PubMed] [Google Scholar]
- 44. Su T, Bryant DM, Luton F, Vergés M, Ulrich SM, Hansen KC, Datta A, Eastburn DJ, Burlingame AL, Shokat KM, Mostov KE. A kinase cascade leading to Rab11-FIP5 controls transcytosis of the polymeric immunoglobulin receptor. Nat Cell Biol 12: 1143–1153, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tajika Y, Matsuzaki T, Suzuki T, Ablimit A, Aoki T, Hagiwara H, Kuwahara M, Sasaki S, Takata K. Differential regulation of AQP2 trafficking in endosomes by microtubules and actin filaments. Histochem Cell Biol 124: 1–12, 2005 [DOI] [PubMed] [Google Scholar]
- 46. Takata K, Matsuzaki T, Tajika Y, Ablimit A, Hasegawa T. Localization and trafficking of aquaporin 2 in the kidney. Histochem Cell Biol 130: 197–209, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tamma G, Klussmann E, Oehlke J, Krause E, Rosenthal W, Svelto M, Valenti G. Actin remodeling requires ERM function to facilitate AQP2 apical targeting. J Cell Sci 118: 3623–3630, 2005 [DOI] [PubMed] [Google Scholar]
- 48. Tamma G, Robben JH, Trimpert C, Boone M, Deen PM. Regulation of AQP2 localization by S256 and S261 phosphorylation and ubiquitination. Am J Physiol Cell Physiol 300: C636–C646, 2011 [DOI] [PubMed] [Google Scholar]
- 49. Van Balkom BW, Savelkoul PJ, Markovich D, Hofman E, Nielsen S, van der Sluijs P, Deen PM. The role of putative phosphorylation sites in the targeting and shuttling of the aquaporin-2 water channel. J Biol Chem 277: 41473–41479, 2002 [DOI] [PubMed] [Google Scholar]
- 50. Van Balkom BW, van Raak M, Breton S, Pastor-Soler N, Bouley R, van der Sluijs P, Brown D, Deen PM. Hypertonicity is involved in redirecting the aquaporin-2 water channel into the basolateral, instead of the apical, plasma membrane of renal epithelial cells. J Biol Chem 278: 1101–1107, 2003 [DOI] [PubMed] [Google Scholar]
- 51. Van der Bliek AM. A sixth sense for Rab5. Nat Cell Biol 7: 548–550, 2005 [DOI] [PubMed] [Google Scholar]
- 52. Verrey F, Groscurth P, Bolliger U. Cytoskeletal disruption in A6 kidney cells: impact on endo/exocytosis and NaCl transport regulation by antidiuretic hormone. J Membr Biol 145: 193–204, 1995 [DOI] [PubMed] [Google Scholar]
- 53. Vossenkamper A, Nedvetsky PI, Wiesner B, Furkert J, Rosenthal W, Klussmann E. Microtubules are needed for the perinuclear positioning of aquaporin-2 after its endocytic retrieval in renal principal cells. Am J Physiol Cell Physiol 293: C1129–C1138, 2007 [DOI] [PubMed] [Google Scholar]
- 54. Yui N, Lu HJ, Bouley R, Brown D. AQP2 is necessary for vasopressin- and forskolin-mediated filamentous actin depolymerization in renal epithelial cells. Biol Open 1: 101–108, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yui N, Okutsu R, Sohara E, Rai T, Ohta A, Noda Y, Sasaki S, Uchida S. FAPP2 is required for aquaporin-2 apical sorting at trans-Golgi network in polarized MDCK cells. Am J Physiol Cell Physiol 297: C1389–C1396, 2009 [DOI] [PubMed] [Google Scholar]