Abstract
Background:
Dedifferentiation of thyroid follicular cells renders radioiodine therapy ineffective in patients of differentiated thyroid cancer (DTC). An alternative therapy to treat the disease or reinduce radioiodine uptake is necessary.
Materials and Methods:
We evaluated the role of retinoic acid therapy in 13 cases of DTC with raised thyroglobulin and/or clinically evident disease. Retinoic acid was given in a dose of 1.5 mg/kg for a period ranging between 1.5 and 18 months.
Results:
Age of the patients was between 18 and 65 years with a median of 49 years. Ten patients had papillary while two had follicular and one patient had mixed papillary and follicular thyroid cancer. Mean radioiodine given before starting retinoic acid was 164 mCi. Mean duration of therapy was 6.4 months. Thyroglobulin decreased in 2 patients and increased in 11 patients at the end of therapy. Radioiodine uptake was demonstrable in six patients, though faintly, while 7 cases showed no uptake. Based on the clinical and biochemical parameters, four patients had progressive disease, eight had stable disease and one patient showed partial response. Of the six patients with reinduction of radioiodine uptake, three had biochemical progression and the other three had stable disease.
Conclusion:
Our findings suggest that retinoic acid therapy may induce radioiodine uptake and reduce serum thyroglobulin levels in some patients with DTC, but whether this results in clinically significant response can only be ascertained on long-term follow-up.
Keywords: Radioiodine, redifferentiation, retinoic acid, thyroglobulin
INTRODUCTION
Carcinoma thyroid, although the most frequent malignancy of the endocrine system, is a rare disease. It accounts for about 1% of all human cancers, with the prevalence in women (5–9 in 100,000) higher as compared to that in men (2–4 of 100,000).[1] Differentiated thyroid cancer (DTC) is a slow-growing and treatable disease with good prognosis. However, about 20–40% of patients with DTC have recurrent disease and about 5% have distant metastases at presentation.[2] Until recently, treatment of recurrent and metastatic disease consisted of surgery (when feasible), thyroid stimulating hormone (TSH) suppressive therapy with levothyroxine (T4), and radioiodine (I-131) treatment when radioiodine (RI) uptake is present in neoplastic foci. During tumor progression, cellular dedifferentiation occurs in up to 30% of cases,[3] and is commonly accompanied by more aggressive growth, metastatic spread and loss of iodine uptake owing to decreased expression of sodium/iodide symporter (NIS). TSH receptor, whose presence indicates a good prognosis, is lost in such dedifferentiated tumors, rendering TSH suppression therapy ineffective.[4] Patients with dedifferentiated thyroid cancer foci continue to have a much worse prognosis than those with I-131 uptake[5,6] and lack adequate therapeutic options. Metastatectomy is useful only in few patients who have surgically approachable and solitary/restricted number of sites.[7] While external radiation therapy can palliate metastatic symptoms, it rarely leads to remission.[8] Conventional chemotherapy, i.e., doxorubicin alone or with cisplatin, is very toxic and provides <20% rate of mostly very transient, partial responses.[9] In recent years, research has focused on targeted approaches addressing the pathological characteristics of RI non-avid thyroid carcinoma. It is known that inability to take up I-131 is associated with poor differentiation and increased tumor grade.[5] However, the molecular basis dedifferentiation is not well known. So far, p53 mutation is the only genetic change clearly shown to correlate with poor differentiation or lacking differentiation. Some recent reports show a significant correlation between the presence of initiating BRAF mutation and poorer outcome of DTC or loss of function of DTC metastases.[10]
The vitamin A (retinol)-derived retinoic acids (RAs) are important regulators of a diverse spectrum of physiological processes, including cell proliferation, differentiation, morphogenesis, angiogenesis, and apoptosis. RA receptors are expressed in human thyroid carcinomas in varying degrees.[11] Differentiation effects of RA have been demonstrated in many types of tumor cells.[12,13] Retinoids inhibit tumor growth and exert several redifferentiating effects: induction of 5’-deiodinase,[14,15] increased expression of NIS mRNA[16] and of the differentiation marker, alkaline phosphatase, or decreased expression of CD97, which is highly expressed in anaplastic thyroid carcinoma,[17] as well as stimulation of intercellular adhesion molecule-1 synthesis.[18] Experimental studies have shown that RAs may increase the expression of NIS, type I 5’-deiodinase, intercellular adhesion molecule-1 (ICAM-1) and thyroglobulin (Tg), which were known to be decreased or even lost in thyroid cancer cells.[15,16,18–20] The results of a few early clinical pilot trials demonstrated that RA may restore RI uptake and decrease the tumor size.[21–24] However, the clinical outcomes of 13-cis RA in subsequent studies were disappointing as <20% of patients showed I-131 uptake after RA pre-treatment.[25]
We wanted to assess the role of all-trans retinoic acid (ATRA) as a rediffrentiating and antineoplastic agent in dedifferentiated thyroid tumor patients in our institute.
MATERIALS AND METHODS
The following inclusion and exclusion criteria were applied.
Inclusion criteria
DTC of follicular cell origin
Serum Tg level ≥10 ng/ml with TSH stimulation
RI whole-body scintigraphy (WBS) showing no or therapeutically insignificant (<0.3%) uptake
Initial therapy must have included total or near-total thyroidectomy and RI ablation therapy
For female patients, the following criteria were additionally applied:
Negative pregnancy test within 1 week of enrollment
Practicing adequate birth control methods
Exclusion criteria
Coexisting second malignancy
Pregnancy
Abnormal liver function tests, renal function tests, and lipid profile
Treatment and follow-up
Thirteen cases of DTC with raised Tg and/or clinically evident disease and negative WBS were treated with RA (Vesanoid, ATRA) in a dose of 1.5 mg/kg/day for a period ranging between 1.5 and 18 months [Tables 1 and 2].
Table 1.
Details of patients and redifferentiation therapy
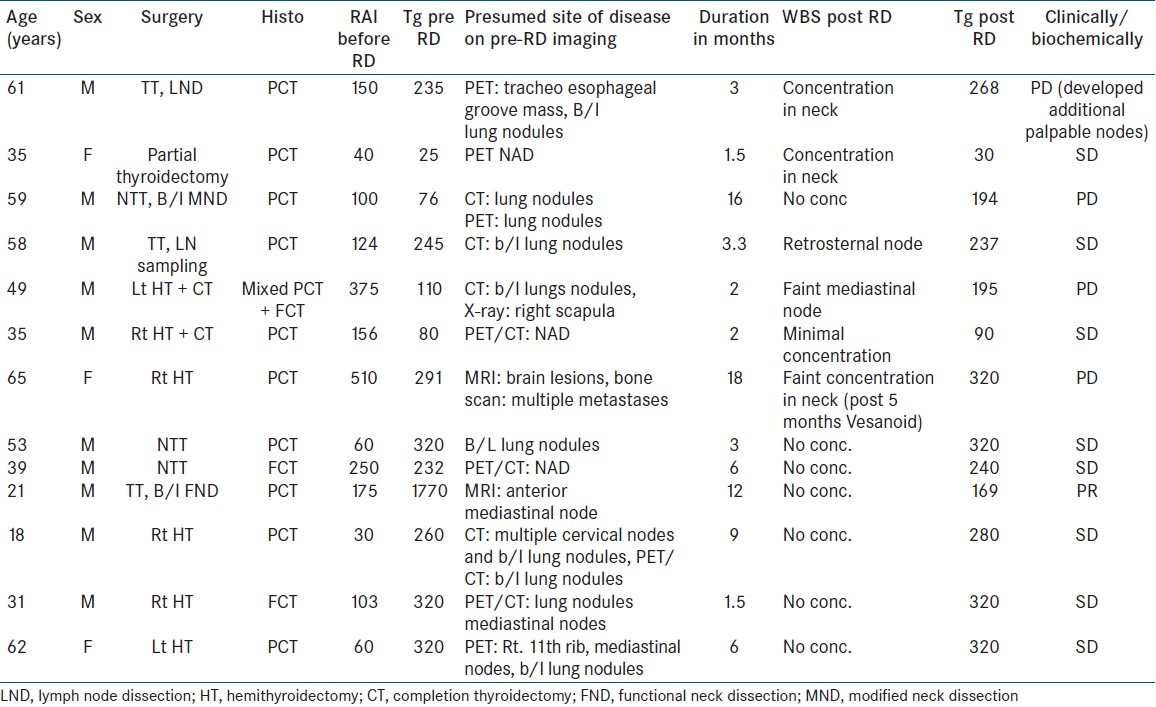
Table 2.
Patient characteristics

A complete response to the drug was defined as a decrease in serum Tg levels to less than 10 ng/ml of thyroid hormone with no evidence of disease on clinical evaluation. A partial response was defined as more than 30% decrease in Tg level with no evidence of progression on clinical evaluation. Stable disease was defined as no / ≤30% change in Tg level with no evidence of progression on clinical evaluation. Progressive disease was defined as an increase in serum Tg level of >30% or progression on clinical evaluation or both. Patients were monitored by physical examination, laboratory tests and imaging studies where feasible, on their follow-up visits.
RESULTS
Age of the patients was between 18 and 65 years with a median of 49 years. Ten patients had papillary while two had follicular and one patient had mixed papillary and follicular thyroid cancer. Mean RI given before starting RA was 164 mCi. Mean duration of therapy was 6.4 months. Tg decreased in 2 patients and increased in 11 patients at the end of therapy. RI uptake was demonstrable in six patients, though faintly, while seven cases showed no uptake [Figures 1 and 2]. Based on clinical and/or biochemical parameters, four patients had progressive disease, eight had stable disease and one patient showed partial response. Of the six patients with RI uptake, three had biochemical progression and the other three had stable disease.
Figure 1.
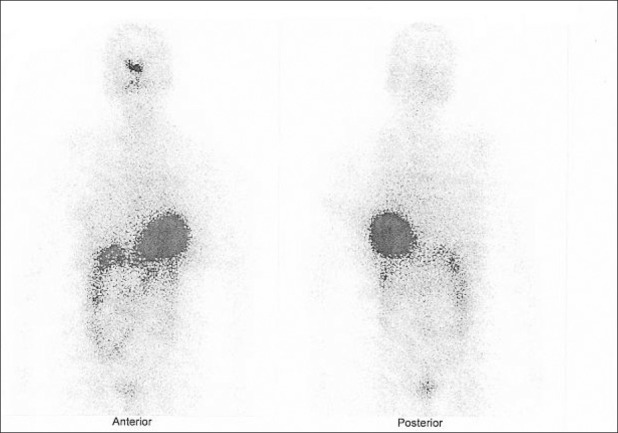
I-131 whole body scan before redifferentiation therapy shows no uptake in the neck
Figure 2.
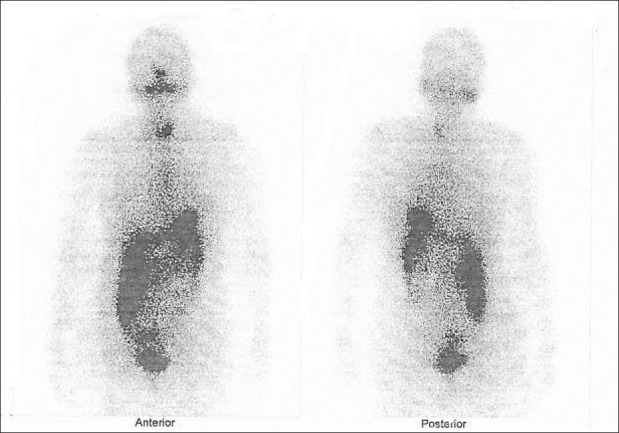
I-131 whole body scan after 3 months of redifferentiation therapy shows tracer uptake in the neck
Adverse reactions
Six out of 13 patients reported minor side effects of nausea and vomiting and 5 patients reported giddiness which eventually subsided. Three patients developed signs of tumor inflammation during the course of therapy, but were asymptomatic for those. No patient reported any major adverse reaction necessitating stopping of the drug.
DISCUSSION
Thyroid cancer is the most common endocrine malignancy. Majority of the thyroid cancers arise from follicular cells. Surgery and RI therapy result in cure of majority of DTC patients; however, some patients with DTC dedifferentiate, which is associated with loss of iodide uptake ability, more aggressive growth, and metastatic spread, making the tumor resistant to the traditional therapeutic modalities including RI. Patients with advanced DTC at presentation often have dedifferentiated cancers on follow-up. Unresectable dedifferentiated thyroid cancer is very difficult to treat. Conventional radiotherapy and chemotherapy have very limited role in the treatment of dedifferentiated thyroid cancer. Aggressive nature of the disease and lack of effective treatment results in dedifferentiated thyroid cancer being responsible for the majority of deaths attributable to thyroid cancer.
Several redifferentiating agents and targeted molecules have been studied in the treatment of dedifferentiated thyroid cancer. Besides inducing RI uptake, redifferentiating agents have shown other effects like promotion of apoptosis, growth inhibition and cell cycle regulation.
The vitamin A (retinol)-derived RAs are important regulators of many physiological processes, including cell proliferation, differentiation, morphogenesis, angiogenesis, and apoptosis.[26] The pleiotropic effects of retinoids are mediated by a nuclear heterodimeric pair of retinoid receptors (RAR/RXR). Retinoid-activated RAR/RXR heterodimers mediate the transcription of specific gene networks by binding to specific DNA response elements and recruiting cofactor complexes which cause the local chromatin structure to alter and engage the basal transcription machinery. RARs and RXRs also integrate a variety of signaling pathways through phosphorylation events and are involved in the control of cell growth, differentiation, and apoptosis.[27] RA has been successfully used for the treatment of hematological malignancies as well as therapy and chemoprevention of solid cancers including thyroid carcinomas.[28] Cell culture experiments in thyroid carcinoma cell lines showed that RA treatment affects thyroid-specific functions, cell–cell or cell–matrix interaction, differentiation markers, growth, and tumorigenicity. RA has an antiproliferative effect on the follicular thyroid carcinoma cell lines FTC-133 and FTC-238. Furthermore, pre-treatment of these cell lines with RA results in decreased in vitro proliferation rates and reduced tumor cell growth of xenotransplants.[29]
A study by Handkiewicz-Junak et al in 2009 showed that RA increase radioactive iodine (RAI) uptake in thyroid tissue in 17% of the 53 epithelial cell thyroid carcinoma patients studied, whose previous post-therapeutic I-131 scans were negative. Forty-one (77%) patients were evaluable for Tg response before and after RA treatment. There was a statistically significant increase in median Tg level (60 vs. 90 ng/ml, P<0.05). There was no difference in Tg increase between scintigraphic responders and non-responders.[30] Zhang et al in 2005 evaluated the effect of ATRA in 11 patients: iodine uptake was increased in 4 and there was a partial response (PR) of target lesions in 5 patients. Two patients had stable disease.[31] Simon et al in 2002 evaluated the response of 50 patients on the basis of reduction in tumor size and Tg levels. Thirteen patients showed a clear increase in RI uptake, while eight showed mild increase. Tg levels were unchanged or decreased in 20 patients. Tumor size was assessable in 37 patients, tumor regression was observed in 6 and there was no change in 22 patients. In total, a response was seen in 19 patients (38%). Response to retinoid therapy did not always correlate with increased RI uptake, so the authors assumed other direct antiproliferative effects.[32] Courbon et al in 2006 treated 11 patients with a progressive disease, with 13-cis-RA (1.5 mg/kg/day) over 8 weeks prior to I-131 irradiation. The redifferentiating effect of RA was evaluated by serum Tg monitoring during RA treatment and qualitative analysis of iodine uptake on the post-therapeutic whole body scan. This study showed iodine uptake was only slightly improved in two patients. The clinical benefits of RA seemed to be very poor. Five patients died of a metastatic disease. Five others presented new clinical evidences of a progressive disease.[33]
Our findings are in agreement with the previously published studies of Zhang et al and Courbon et al.[31,33] RA appears to increase the RI uptake in some patients, but to a mild degree which may not be therapeutically significant. Also, progressive disease was seen in more than 4/13 patients within a short duration. That 8/13 patients showed stable disease and 1/13 showed partial response is a figure which may change on longer follow-up. There are some limitations to our study. Firstly, the response criteria based on RI uptake and serum Tg levels used in previous studies are not standard. Although RI uptake in the disease site is considered to be beneficial, the long-term clinical implication of such uptake is not known. We used serum Tg and not RI uptake as a marker to assess the response. This is also a debatable method as serum Tg levels could have decreased or increased with redifferentiation therapy. It could have increased due to redifferentiation of thyroid cancer that leads to increased Tg expression by the tumor, or it could have decreased due to reduction of tumor volume. Also, the mean follow-up duration was 6.4 months (median 3.3 months). Thyroid cancers are usually indolent and slow growing, so a follow-up period of 6.4 months may not be long enough to detect the clinical events. Of the six patients in whom RI uptake was reinduced in our study, three had biochemical progression and the other three had stable disease. Thus, mere reinduction of I-131 uptake cannot be justifiably included as a response criterion.
CONCLUSION
We conclude that RA therapy may induce RI uptake and reduce serum Tg levels in some patients with DTC, but whether this results in clinically significant response can only be ascertained on long-term follow-up. In our opinion, RA therapy may be beneficial, but definite proof of its efficacy and long-term safety is lacking. Other drugs also need to be evaluated for the treatment of RI negative DTC and clinical or biochemical evidence of disease.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Braverman LE, Utiger RD. Philadelphia: Lippincott-Raven; 1996. The Thyroid: A Fundamental and Clinical Text. [Google Scholar]
- 2.Pacini F, Schlumberger M, Dralle H, Elisei R, Smit JW, Wiersinga W. European consensus for the management of patients with differentiated thyroid carcinoma of the follicular epithelium. Eur J Endocrinol. 2006;154:787–803. doi: 10.1530/eje.1.02158. [DOI] [PubMed] [Google Scholar]
- 3.Goretzki PE, Simon D, Frilling A, Witte J, Reiners C, Grussendorf M, et al. Surgical reintervention for differentiated thyroid cancer. Br J Surg. 1993;80:1009–12. doi: 10.1002/bjs.1800800826. [DOI] [PubMed] [Google Scholar]
- 4.Filetti S, Bidart JM, Arturi F, Caillou B, Russo D, Schlumberger M. Sodium/iodide symporter: a key transport system in thyroid cancer cell metabolism. Eur J Endocrinol. 1999;141:443–57. doi: 10.1530/eje.0.1410443. [DOI] [PubMed] [Google Scholar]
- 5.Mihailovic J, Stefanovic L, Malesevic M. Differentiated thyroid carcinoma with distant metastases: probability of survival and its predicting factors. Cancer Biother Radiopharm. 2007;22:250–5. doi: 10.1089/cbr.2006.313. [DOI] [PubMed] [Google Scholar]
- 6.Durante C, Haddy N, Baudin E, Leboulleux S, Hartl D, Travagli JP, et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab. 2006;91:2892–9. doi: 10.1210/jc.2005-2838. [DOI] [PubMed] [Google Scholar]
- 7.Zettinig G, Fueger BJ, Passler C, Kaserer K, Pirich C, Dudczak R, et al. Long-term follow-up of patients with bone metastases from differentiated thyroid carcinoma – surgery or conventional therapy? Clin Endocrinol (Oxf) 2002;56:377–82. doi: 10.1046/j.1365-2265.2002.01482.x. [DOI] [PubMed] [Google Scholar]
- 8.Arcangeli G, Micheli A, Arcangeli G, Giannarelli D, La Pasta O, Tollis A, et al. The responsiveness of bone metastases to radiotherapy: the effect of site, histology and radiation dose on pain relief. Radiother Oncol. 1989;14:95–101. doi: 10.1016/0167-8140(89)90053-4. [DOI] [PubMed] [Google Scholar]
- 9.Shimaoka K, Schoenfeld DA, DeWys WD, Creech RH, DeConti R. A randomized trial of doxorubicin versus doxorubicin plus cisplatin in patients with advanced thyroid carcinoma. Cancer. 1985;56:2155–60. doi: 10.1002/1097-0142(19851101)56:9<2155::aid-cncr2820560903>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 10.Xing M, Westra WH, Tufano RP, Cohen Y, Rosenbaum E, Rhoden KJ, et al. BRAF mutation predicts a poorer clinical prognosis for papillary thyroid cancer. J Clin Endocrinol Metab. 2005;90:6373–9. doi: 10.1210/jc.2005-0987. [DOI] [PubMed] [Google Scholar]
- 11.Buletic Z, Soprano KJ, Soprano DR. Retinoid targets for the treatment of cancer. Crit Rev Eukaryot Gene Expr. 2006;16:193–210. doi: 10.1615/critreveukargeneexpr.v16.i3.10. [DOI] [PubMed] [Google Scholar]
- 12.Lotan R. Retinoids and their receptors in modulation of differentiation, development, and prevention of head and neck cancers. Anticancer Res. 1996;16:2415–9. [PubMed] [Google Scholar]
- 13.Dragnev KH, Rigas JR, Dmitrovsky E. The retinoids and cancer prevention mechanisms. Oncologist. 2000;5:361–8. doi: 10.1634/theoncologist.5-5-361. [DOI] [PubMed] [Google Scholar]
- 14.Van Herle AJ, Agatep ML, Padua DN, 3rd, Totanes TL, Canlapan DV, Van Herle HM, et al. Effects of 13-cis retinoic acid on growth and differentiation of human follicular carcinoma cells lines (UCLA RO 82 W-1) in vitro. J Clin Endocrinol Metab. 1990;71:755–63. doi: 10.1210/jcem-71-3-755. [DOI] [PubMed] [Google Scholar]
- 15.Schreck R, Schnaiders F, Schmutzler C, Kohrle J. Retinoids stimulate type 1 iodothyronine 5‘-deiodinase activity in human follicular cell lines. J Clin Endocrinol Metab. 1994;79:791–8. doi: 10.1210/jcem.79.3.8077363. [DOI] [PubMed] [Google Scholar]
- 16.Schmutzler C, Winzer R, Meissner-Weigl J, Köhrle J. Retinoic acid increase sodium/iodine symporter mRNA levels in human thyroid cancer cell lines and suppresses expression of functional symporter in non transformed FRTL-5 rat thyroid cells. Biochem Biophys Res Commun. 1997;240:832–8. doi: 10.1006/bbrc.1997.7715. [DOI] [PubMed] [Google Scholar]
- 17.Hoang-Vu C, Bull K, Schwarz I, Krause G, Schmutzler C, Aust G, et al. Regulation of CD97 protein in thyroid carcinoma. J Clin Endocrinol Metab. 1999;84:1104–9. doi: 10.1210/jcem.84.3.5557. [DOI] [PubMed] [Google Scholar]
- 18.Bassi V, Vitale M, Feliciello A, De Riu S, Rossi G, Fenzi G. Retinoic acid induces intercellular adhesion molecule-1 hyperexpression in human thyroid carcinoma cell lines. J Clin Endocrinol Metab. 1995;80:1129–35. doi: 10.1210/jcem.80.4.7714081. [DOI] [PubMed] [Google Scholar]
- 19.Van Herle AJ, Agatep ML, Padua DN, 3rd, Totanes TL, Canlapan DV, Van Herle HM, et al. Effects of 13 cis-retinoic acid on growth and differentiation of human follicular carcinoma cells (UCLA R0 82 W-1) in vitro. J Clin Endocrinol Metab. 1990;71:755–63. doi: 10.1210/jcem-71-3-755. [DOI] [PubMed] [Google Scholar]
- 20.Kurebayashi J, Tanaka K, Otsuki T, Moriya T, Kunisue H, Uno M, et al. All-trans-retinoic acid modulates expression levels of thyroglobulin and cytokines in a new human poorly differentiated papillary thyroid carcinoma cell line, KTC-1. J Clin Endocrinol Metab. 2000;85:2889–96. doi: 10.1210/jcem.85.8.6732. [DOI] [PubMed] [Google Scholar]
- 21.Simon D, Kohrle J, Schmutzler C, Mainz K, Reiners C, Roher HD. Redifferentiation therapy of differentiated thyroid carcinoma with retinoic acid: basics and first clinical results. Exp Clin Endocrinol Diabetes. 1996;104(Suppl 4):13–5. doi: 10.1055/s-0029-1211692. [DOI] [PubMed] [Google Scholar]
- 22.Simon D, Koehrle J, Reiners C, Boerner AR, Schmutzler C, Mainz K, et al. Redifferentiation therapy with retinoids: therapeutic option for advanced follicular and papillary thyroid carcinoma. World J Surg. 1996;22:569–74. doi: 10.1007/s002689900436. [DOI] [PubMed] [Google Scholar]
- 23.Grünwald F, Menzel C, Bender H, Palmedo H, Otte R, Fimmers R, et al. Redifferentiation therapy-induced radioiodine uptake in thyroid cancer. J Nucl Med. 1998;39:1903–6. [PubMed] [Google Scholar]
- 24.Gruning T, Tiepolt C, Zophel K, Bredow J, Kropp J, Franke WG. Retinoic acid for redifferentiation of thyroid cancer-does it hold its promise? Eur J Endocrinol. 2003;148:395–402. doi: 10.1530/eje.0.1480395. [DOI] [PubMed] [Google Scholar]
- 25.Courbon F, Zerdoud S, Bastie D, Archambaud F, Hoff M, Eche N, et al. Defective efficacy of retinoic Acid treatment in patients with metastatic thyroid carcinoma. Thyroid. 2006;16:1025–31. doi: 10.1089/thy.2006.16.1025. [DOI] [PubMed] [Google Scholar]
- 26.Baris O, Savagner F, Nasser V, Loriod B, Granjeaud S, Guyetant S, et al. Transcriptional profiling reveals coordinated up-regulation of oxidative metabolism genes in thyroid oncocytic tumors. J Clin Endocrinol Metab. 2004;89:994–1005. doi: 10.1210/jc.2003-031238. [DOI] [PubMed] [Google Scholar]
- 27.Niles RM. Recent advances in the use of vitamin A (retinoids) in the prevention and treatment of cancer. Nutrition. 2000;16:1084–9. doi: 10.1016/s0899-9007(00)00436-6. [DOI] [PubMed] [Google Scholar]
- 28.Lengfelder E, Saussele S, Weisser A, Buchner T, Hehlmann R. Treatment concepts of acute promyelocytic leukemia. Critical Reviews in Oncology/Hematology. 2005;56:261–74. doi: 10.1016/j.critrevonc.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 29.Schmutzler C, Hoang-Vu C, Rüger B, Köhrle J. Human thyroid carcinoma cell lines show different retinoic acid receptor repertoires and retinoid responses. Eur J Endocrinol. 2004;150:547–56. doi: 10.1530/eje.0.1500547. [DOI] [PubMed] [Google Scholar]
- 30.Handkiewicz-Junak D, Roskosz J, Hasse-Lazar K, Szpak-Ulczok S, Puch Z, Kukulska A, et al. 13-cis-retinoic acid re-differentiation therapy and recombinant human thyrotropin-aided radioiodine treatment of non-Functional metastatic thyroid cancer: a single-center, 53-patient phase 2 study. Thyroid Res. 2009;2:8. doi: 10.1186/1756-6614-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Y, Jia S, Liu Y, Li B, Wang Z, Lu H, et al. A clinical study of all-trans-retinoid-induced differentiation therapy of advanced thyroid cancer. Nucl Med Commun. 2007;28:251–5. doi: 10.1097/MNM.0b013e3280708ebf. [DOI] [PubMed] [Google Scholar]
- 32.Simon D, Körber C, Krausch M, Segering J, Groth P, Görges R, et al. Clinical impact of retinoids in redifferentiation therapy of advanced thyroid cancer: final results of a pilot study. Eur J Nucl Med Mol Imaging. 2002;29:775–82. doi: 10.1007/s00259-001-0737-6. [DOI] [PubMed] [Google Scholar]
- 33.Courbon F, Zerdoud S, Bastie D, Archambaud F, Hoff M, Eche N, et al. Defective efficacy of retinoic acid treatment in patients with metastatic thyroid carcinoma. Thyroid. 2006;16:1025–31. doi: 10.1089/thy.2006.16.1025. [DOI] [PubMed] [Google Scholar]


