Abstract
Background:
Fine needle aspiration cytology (FNAC) is extensively used in the diagnosis of various clinically palpable lesions of breast and salivary glands. Much interest has been gained in mucosubstances produced in tumors arising from these organs.
Aims:
To evaluate the utility of Periodic acid Schiff with diastase (PAS-D) and Alcian blue (AB) staining pattern on fine needle aspirates of breast and salivary gland neoplasms.
Materials and Methods:
Seventy-five cases of different neoplasm of breast and salivary gland were studied. The staining pattern of PAS-D and AB stains on smears of these neoplasm were observed.
Results:
Among cases of neoplasms of breast, intracytoplasmic PAS-D positive globules were restricted to carcinoma except in one case where PAS-D-positive globules were seen in fibroadenoma. The background substance of both mucinous carcinoma and fibroadenoma with myxoid change stained positive with PAS-D, but the pattern was different. The cases of pleomorphic adenoma and mucoepidermoid carcinoma of salivary gland showed intracytoplasmic PAS-D-positive globules. The cases of pleomorphic adenoma showed stromal positivity which was not seen in basal cell adenoma on smears.
Conclusion:
Intracytoplasmic PAS-D-positive globules may be useful in differentiating benign and malignant lesions of breast. The presence of PAS-D positive granules are useful in differentiating various lesions of salivary glands. AB staining of stromal fragments in pleomorphic adenoma is useful in differentiating it from basal cell adenoma.
Keywords: Alcian blue, breast neoplasms, FNAC, PAS-D, salivary gland neoplasms
Introduction
Breast and salivary glands are two common organs for routine fine needle aspiration cytology (FNAC). Both have the same basic histological architecture and secretory functions.[1] These similarities in the two organs and difficulty in diagnosing different lesions on aspirates has prompted us to undertake this study. We aim to evaluate the utility of Periodic acid Schiff with diastase (PAS-D) and Alcian blue (AB) staining on FNAC of breast and salivary gland neoplasms and ascertain if these stains can be used as an adjunct to routine cytological procedures in aiding the differential diagnosis.
Materials and Methods
This was a 2-year prospective study of FNAC of breast and salivary gland lesions. Seventy eight cases were diagnosed as tumors on FNAC. Three cases were excluded as the histopathology correlation was not available. Following detailed clinical history and examination, FNAC was performed using 22-G needle attached to 10-mL syringe. Leishman and Pap stains were performed using standard procedures. Two slides were fixed immediately in alcohol and preserved for PAS-D and Alcian blue staining. PAS-D staining was done by the method proposed by Johnson and Wadhera[2] and Alcian blue staining was performed by method proposed by Bancroft at a pH of 2.5.[3] The smears were assessed for extracellular and intracellular positivity. The intracellular staining pattern was in the form of intracytoplasmic globules, granules or uniform / patchy cytoplasmic positivity. The PAS-D positivity on smears of carcinoma of breast was graded as per system proposed by Johnson and Wadhera.[2] The smears of breast carcinoma were graded as per Robinson's Criteria[4][Table 1]. We selected grade I and grade II smears to look for PAS-D and AB positivity, which may help in differentiating these lesions from benign lesions with atypia.
Table 1.
Cytological grade of smears of the smears of breast carcinoma as per Robinson Criteria
For the tumors of salivary gland, both the stains were assessed as positive or negative as no specific grading system have been obtained in the literature.
Results
Out of the 50 cases of breast lump, 29 (58%) were fibroadenoma, 19 (36%) were carcinoma and one case was (3%) of benign and malignant phylloides tumor each.
Out of 25 cases of salivary gland tumors, 16 (64%) were pleomorphic adenoma, four (16%) were metastatic deposits of squamous cell carcinoma, two (8%) were mucoepidermoid carcinoma, one (4%) was acinic cell carcinoma, basal cell adenoma and salivary duct carcinoma each. The PAS-D and AB positivity of all lesions along with grading of breast carcinoma is shown in Table 2.
Table 2.
PAS-D and AB positivity of all lesions and grading of carcinoma breast lesions
Most (28/29 or 96%) FNA smears of fibroadenoma were negative for PAS-D and AB. Only one case showed PAS-D-positive intracytoplasmic globules [Figure 1a]. One case showed PAS-D positivity in the form of acellular clumps of acidophilic material in the background [Figure 1b]. The histopathology of this revealed fibroadenoma with myxoid change. In both the cases, AB positivity was not seen. Among the smears of breast carcinoma, 14/19 (70%) showed intracytoplasmic PAS-D-positive globules [Figure 1c]. One case of mucinous carcinoma showed abundant extracellular PAS-D-positive material [Figure 1d], in addition to intracytoplasmic PAS-D-positive globules. On tissue sections, all these cases showed presence of PAS-D-positive globules in the cytoplasm of malignant cells. Some cases also showed PAS-D positivity on the luminal surface of glands.
Figure 1.
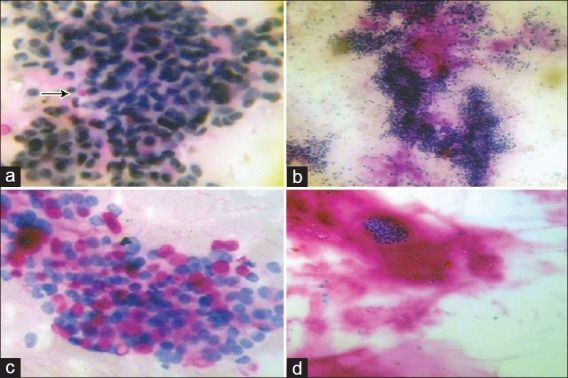
(a) Fibroadenoma showing intracytoplasmic globules (arrow) (PAS-D, ×400), (b) Fibroadenoma showing PAS-D positive clumps of background substance (PAS-D, ×100), (c) Carcinoma breast showing intracytoplasmic globules (PAS-D, ×400), (d) Mucinous carcinoma with abundant background PAS-D positive material (PAS-D, ×100)
Nine out of 16 (56%) cases of pleomorphic adenoma showed intracytoplasmic PAS-D positive globules [Figure 2a] which were usually single and occasionally causing nuclear indentation. Two cases showed PAS-D positive stromal fragments [Figure 2b].
Figure 2.
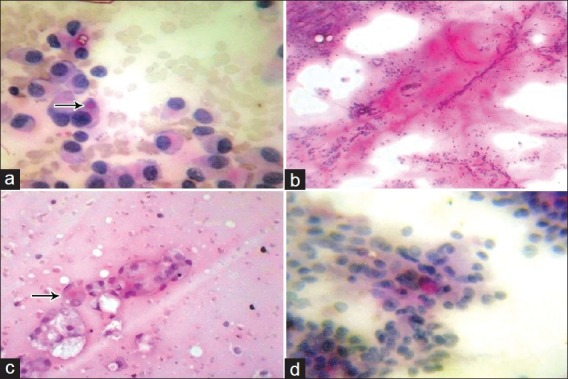
(a) Intracytoplasmic PAS-D positive globules in pleomorphic adenoma (arrow) (PAS-D, ×400), (b) PAS-D positive stromal fragments in pleomorphic adenoma (PAS-D, ×100), (c) Intracytoplasmic PAS-D positive globules (arrow) in mucoepidermoid carcinoma (PAS-D, ×400), (d) Fine intracytoplasmic PAS-D positive granules in acinic cell carcinoma (PAS-D, ×400)
Histopathology of these cases in addition showed positive luminal secretions and all the cases showed AB-positive stromal fragments. The four cases of metastatic deposits of squamous cell carcinoma did not show positivity with PAS-D or AB on smears and sections. The two cases of mucoepidermoid carcinoma showed abundant intracytoplasmic PAS-D-positive globules in glandular and signet cells on smears [Figure 2c]. AB positivity was not seen in these cases. One case of acinic cell carcinoma showed fine PAS-D-positive granules in cytoplasm on smears [Figure 2d]. Histopathology of the same case showed abundant intracytoplasmic PAS-D-positive globules. The single case of basal cell adenoma showed no reactivity with either of the stain on smears and sections.
Discussion
Cellular smears with bimodal benign pattern, numerous single bipolar stromal cell nuclei and presence of fibromyxoid stroma are the essential diagnostic features on aspiration smears from fibroadenoma of breast.[1] The acidophilic mucopolysaccharide stromal fragments is expected to stain positive with AB. However, such staining stroma was not demonstrable in our FNA smears of fibroadenoma. The single case where myxoid stroma was present, also did not stain with AB but stained positive with PAS-D. It is suggested that this fibroadenoma with myxoid change has neutral mucopolysaccharide instead of acid mucopolysaccharide.
Certain cases of fibroadenomas and fibrocystic disease with epithelial hyperplasia may yield cellular smears with suspicious discohesion and atypia of cells suggesting low grade duct carcinoma. Intracytoplasmic PAS-D positivity though seen, is rare in fibroadenomas (only 1 out of 29 cases in our study). They were also not strickingly globular material as seen in carcinomas, but just fulfilling the criteria proposed by Johnson and Wadhera.[2] The maximum grade that could be assigned to this weak positivity was + (the lowest). Strict criteria for recognising PAS-D-positive cytoplasmic globules need to be adhered to.
We found PAS-D-positive intracytoplasmic globules in 14/19 (74%) of our duct carcinomas. We have deliberately included more lower grade carcinomas in our study because equivocal smears with high cellularity variable but subtle discohesion and mild atypia are more likely to be confused with these in cytological smears from fibroadenomas and other benign proliferations. However, the few cases of grade II tumors included in this study showed slightly higher percentage of positivity (4/5 or 80%) as compared to 10/14 (70%) of grade I tumors. This could be the reason for slightly lower positivity (74%) in our study as compared to 90% positivity reported by Nijhawan et al.[5]
The single mucinous carcinoma encountered by us showed intracytoplasmic PAS-D-positive globules in addition to PAS-D-positive lakes of mucinous substance in the background. The quality of staining was different from that seen in the case of fibroadenoma with mucinous change, in that it was more homogenous and appeared fluid-like. Fibroadenoma also do not show intracytoplasmic globules. These differences may aid in the distinction of these two entities when fibroadenoma with mucinous change and few atypical cells are causing worry.
The PAS-D stained intracytoplasmic globules in lobular carcinoma is reported to have a distinct morphology having a dark staining central core and lighter periphery.[6] However, we could not contribute to this observation as we had no cases of this type of carcinoma included in this study.
In pleomorphic adenoma of salivary gland, two types of mucins are demonstrable on histological sections.[7] Our findings of PAS-D-positive material and AB-positive material in FNA smears is consistent with this observation. Luna and Pilch[8] have also reported presence of eosinophillic colloid-like PAS-D positive material in duct lumina. Since myoepitheliomas do not yield stainable material in smears, these stains may help to identify them. Mucoepidermoid carcinoma and adenoid cystic carcinoma also needs to be distinguished from pleomorphic adenoma. Kawahara et al.[9] have found that the extracellular material in adenoid cystic carcinoma stains positive with AB. Hence, this stain is unlikely to be of any help in differential diagnosis. However, use of PAS-D may be useful as we have found that stromal fragments seen in some of our cases of pleomorphic adenoma stain positive with it. This feature has not been noticed in adenoid cystic carcinoma. In addition, adenoid cystic carcinoma do not show intracytoplasmic PAS-D-positive globules.
PAS-D-positive globules seen in mucoepidermoid carcinoma are of help in distinguishing it from deposits of squamous cell carcinoma. Luna et al.[8] emphasized this point by stating that mucin production is invariably present in mucoepidermoid carcinoma and in the absence of this, the tumor should be labelled as epidermoid carcinoma.Aspirates from Warthin tumor may yield mucin-producing cells and metaplastic squamous cells to suggest mucoepidermoid carcinoma. In this situation, mucin detection may not be of help in distinguishing these as observed by Goonewardene and Nasuti.[10]
The presence of PAS-D-positive globules in FNA smears of acinic cell carcinoma have been reported.[11] Our finding of similar globules in the single case of acinic cell carcinoma included in this study confirms this. On the smears, intracytoplasmic PAS-D positivity may be restricted to only a few cells due to the fragility and dispersal of the cytoplasm. Granular cytoplasmic PAS-D positivity is exclusive to acinic cell carcinoma. This feature can be useful in distinguishing it from clear cell tumor and oncocytic tumors.
FNA smears from basal cell adenoma showed numerous basaloid cells in clusters with some metachromatic stromal fragments in the background and hence, could be confused with pleomorphic adenoma. The stromal elements in basal cell adenoma are collagenous in origin and has no mucin.[12] Hence, it is not expected to give positive reaction with PAS-D or AB. This is confirmed by us on sections. The PAS-D or AB-positive stromal fragments consistently present in pleomorphic adenoma help their identification when confused with monomorphic adenoma. In this scenario, the AB positivity of stroma and cytoplasmic PAS-D positive globules seen in adenoid cystic carcinoma can be reassuring in excluding basal cell adenoma.
The smears of salivary duct carcinoma had striking resemblance to those of breast ductal carcinoma. However, the cells failed to stain with either PAS-D or AB in smears or sections in this study. This is in contrast to the observations of Genester et al.[13] who reported presence of apocrine type PAS-D-positive cytoplasmic vacuoles in salivary duct carcinoma.This suggests the possibility of existence of mucinous variant of salivary duct carcinoma as has been reported by Simson et al.[14] The main differential diagnosis here is with high grade mucoepidermoid carcinoma. Since, mucin secretion is the rule in mucoepidermoid carcinoma and reports of mucin production in salivary duct carcinoma are variable, this field needs to be reported further before drawing any conclusions regarding the utility of these stains.
Conclusions
Intracytoplasmic PAS-D-positive globules may be useful in differentiating between benign and malignant lesions of the breast. Here, a higher grade of PAS-D positivity correlates well with malignancy. The intracytoplasmic PAS-D-positive globules in the cells of pleomorphic adenoma may be useful in differentiating it from adenoid cystic carcinoma and mucoepidermoid carcinoma from squamous cell carcinoma. The intracytoplasmic PAS-D-positive granules are exclusive to acinic cell carcinoma and is useful in differentiating it from oncocytic and clear cell tumors. Thus, to conclude, mucin stains like PAS-D and AB could act as an adjunct in differentiating various salivary gland and breast neoplasms. However, a careful cytological interpretation is more important.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Lindholm K. Breast. In: Orell S, Sterett G, Whitaker D, editors. Fine needle aspiration cytology. 4th ed. India: Elsevier; 2005. pp. 165–266. [Google Scholar]
- 2.Johnson S, Wadhera V. The importance of intractoplasmic DPAS positivity in fine needle aspirates of breast lesions. J Clin Pathol. 2001;54:146–51. doi: 10.1136/jcp.54.2.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cook H. Theory and practice of histochemical techniques. 4th ed. New York: Churchill Livingstone; 1996. Carbohydrates; pp. 173–212. [Google Scholar]
- 4.Robinson IA, McKee G, Nicholson A, D’Arcy J, Jackson PA, Cook MG, et al. Prognostic value of cytological grading of fine-needle aspirates from breast carcinomas. Lancet. 1994;343:947–9. doi: 10.1016/s0140-6736(94)90066-3. [DOI] [PubMed] [Google Scholar]
- 5.Nijhawan R, Rajwanshi A, Gautam U, Gupta SK. Cytoplasmic vacuolation, intracytoplasmic lumina and DPAS staining in ductal carcinoma of breast. Diagn Cytopathol. 2003;28:291–4. doi: 10.1002/dc.10272. [DOI] [PubMed] [Google Scholar]
- 6.Crasta J, Makhija P, Kumar KR, Sheriff S. Cytologic features of lobular carcinoma of breast: how important are the intracytoplasmic lumina. Indian J Pathol Microbiol. 2005;48:170–2. [PubMed] [Google Scholar]
- 7.Rosai J. Major and minor salivary glands. In: Rosai J, Ackerman L, editors. Rosai and Ackerman's Surgical Pathology. 9th ed. India: Elsevier; 2004. pp. 873–916. [Google Scholar]
- 8.Luna M. Salivary glands. In: Pilch B, editor. Head and neck surgical pathology. Philadelphia: Lippincot, Williams and Wilkins; 2001. pp. 284–349. [Google Scholar]
- 9.Kawahara A, Harada H, Kage M, Yokoyama T, Kojiro M. Extracellular material in adenoid cystic carcinoma of salivary gland: a comparative cytological study with other salivary myoepithelial tumors. Diagn Cytopathol. 2004;31:14–8. doi: 10.1002/dc.20094. [DOI] [PubMed] [Google Scholar]
- 10.Goonewardene SA, Nastily JF. Value of mucin detection in distinguishing muco-epidermoid carcinoma from Warthin's tumor on fine needle aspiration. Acta Cytol. 2002;46:704–8. [PubMed] [Google Scholar]
- 11.Palma O, Tori AM, de Cristofaro JA, Fiaccavento S. Fine needle aspiration cytology in two cases of well differentiated acinic-cell carcinoma of parotid gland discussion of diagnostic criteria. Acta Cytol. 1985;29:516–21. [PubMed] [Google Scholar]
- 12.Auclair P, Ellis G. Major Salivary glands. In: Silverberg S, Dellis R, Frable W, editors. Principles and practice of surgical pathology and cytopathology. 3rd ed. New York: Churchill Livingstone; 1997. pp. 575–674. [Google Scholar]
- 13.Brandwein-Gensler M, Hille J, Wang BY, Urken M, Gordor R, Wang LJ, et al. Low-grade salivary duct carcinoma: description of 16 cases. Am J Surg Pathol. 2004;28:1040–4. doi: 10.1097/01.pas.0000128662.66321.be. [DOI] [PubMed] [Google Scholar]
- 14.Simpson RH, Prasad AR, Lewis JE, Skalova A, David L. Mucin rich variant of salivary duct carcinoma: A clinicopathologic and immunohistochemical study of four cases. Am J Surg Pathol. 2003;27:1070–9. doi: 10.1097/00000478-200308000-00004. [DOI] [PubMed] [Google Scholar]


