Abstract
Background:
Actinomyces species are part of mucosal surfaces of oral cavity, gastrointestinal and genital tracts. When these mucosal surfaces disrupt, Actinomyces become pathogen and cause infection. Eosinophil leucocytes participate in host defense against helminthic infestation and they generally play a role in asthma and allergy. However, the role of eosinophil leucocytes in host defense against bacteria is conflicting.
Aim:
To determine whether there is a relationship between Actinomyces-like organisms (ALOs) and eosinophil leucocytes at light microscopic level.
Materials and Methods:
Cervicovaginal samples obtained from 200 patients were examined by both Pap smear microscopy and anaerobic culturing. Since the results obtained by these methods were not concordant for diagnosis of genital Actinomyces, 6 of 200 patients (3%) diagnosed with ALOs by Pap smear microscopy became the study group. Patients without any infectious agents (n=134) were the control group. Statistical analyses were conducted with χ2 test using SPSS program.
Results:
The study and control groups were compared statistically in view of the presence of eosinophil leucocytes and it was found that there was a significant correlation between the presence of ALOs and eosinophil leucocytes (P<0.05). Abundant polymorphonuclear leucocytes (PMNLs) and macrophages were also detected in the study group.
Conclusion:
This study implies that eosinophil leucocytes might have a role in host defense against Actinomyces in addition to PMNLs and macrophages.
Keywords: Actinomyces-like organisms, eosinophil leucocytes, cervicovaginal smears
Introduction
Actinomyces species are part of mucosal surfaces of oral cavity, gastrointestinal and genital tracts. They do not penetrate this intact mucosa under normal conditions. However, a foreign body, neoplasia and diabetes mellitus can be predisposing factors for crossing the normal mucosa. The mucosa becomes disrupted and damaged; hence these opportunistic organisms can be pathogenic and cause infection.[1,2] In host defense against Actinomyces infection, polymorphonuclear leucocytes (PMNLs) and mononuclear cells are the first cells migrating from the blood into tissue sites where they participate in the early inflammatory response.[3] However, the roles of eosinophil leucocytes in host defense against bacteria such as Actinomyces are not fully settled.
Eosinophil leucocytes are bone marrow-derived granulocytes and are predominantly found in tissues that have an interface with the external environment such as gastrointestinal and respiratory tracts.[4] They are believed to participate in host defense against parasitic infestation and are also generally involved in diseases with allergic inflammation.[5,6] Although it is not the primary function of eosinophils to phagocytose and kill bacteria, in vitro studies have shown that eosinophils can move towards the bacteria, ingest and kill them.[7,8] In one of these studies, Svensson and Wenneras[8] demonstrated that human eosinophils were capable of killing bacteria, specifically Staphylococcus aureus and Escherichia coli. While several studies have shown that eosinophils are less capable of killing bacteria than neutrophils,[9] it is reported that tissue-derived eosinophils were more efficient in phagocytosing E. coli than either neutrophils or macrophages.[5] Recently, Wong et al.[10] documented the expression of multiple toll-like receptors (TLRs) of human eosinophils by Western blot and flow cytometry, suggesting that eosinophils can recognize several bacteria through these TLRs. After recognizing bacteria, eosinophils become activated and secrete their cytoplasmic granules.[11] The antibacterial activity of eosinophils arises from both their cytotoxic proteins stored in these cytoplasmic granules and their ability to ascend a respiratory burst.[8] Furthermore, Persson et al.[6] indicated that the antibacterial effects of eosinophils due to their granules may be oxygen-independent.
Taken together these data, it is plausible to indicate that eosinophils may have a role in host defense against bacterial infection. In the present study, we have investigated whether there is an association between Actinomyces and eosinophil leucocytes using Pap smears. To the best of our knowledge, it is the first study regarding the relationship between these cells at light microscopic level.
Materials and Methods
Subjects
A total of 200 women aged 21-68 years with varied gynecological complaints were seen in the outpatient clinic. Before the pelvic examination, all women enrolled in this study completed a questionnaire that requested information on age, menstruation date, gravidity and clinical symptoms. Pregnant women were not included in this study. The study design was approved by the institutional ethics committee. Sampling was performed in accordance with the principles of The Declaration of Helsinki.
Pap smear microscopy
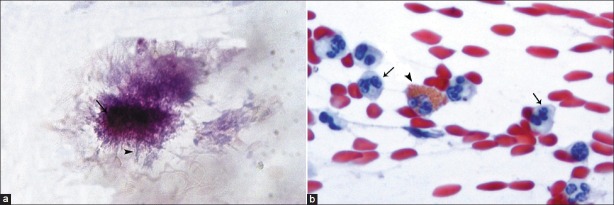
Cervicovaginal smears were taken from each patient using a cyto-brush and fixed with 96% ethanol without air-drying. They were stained using the routine Papanicolaou technique and examined promptly by an experienced cytologist. In this cytological examination, Actinomyces-like organisms (ALOs) were observed as dense, basophilic, central aggregations surrounded by radially oriented, filament-like structures [Figure 1a]. As is seen in [Figure 1b], pink staining granules and two-lobed nucleus are the most characteristic means of identifying eosinophil leucocytes.
Figure 1.
(a) ALOs showing dense, basophilic, central aggregations (arrow) surrounded by radially oriented, filamentlike structures (arrowhead) (Pap, ×400); (b) An eosinophil leukocyte (arrowhead) containing pink staining granules and two lobed nucleus and a few of PMNLs (arrow) (Pap, ×400)
Anaerobic culturing
Sterile swabs were collected from each patient for diagnosis by anaerobic culturing. Actinomyces were identified by both classical methods (culturing and Gram staining) and a BBL Crystal ANR ID kit (Becton Dickinson, Franklin Lakes, NJ), following the protocol recommended by the manufacturer.
Statistical analyses
Statistical analyses were conducted with χ2 test using the SPSS program, version 11.5. The limit for statistical significance was P=0.05.
Results
Six vaginal fluid samples (3%) revealed ALOs on Pap smear examination. Actinomyces were determined in 7 samples (3.5%) by anaerobic culturing. There was no agreement between results obtained by Pap smear microscopy and anaerobic culturing; only one sample was positive by both methods. Since the aim of this study was to determine whether there was a relationship between eosinophil leucocytes and ALOs, and since eosinophil leucocytes were detected only by Pap smear examination in this study, Pap smear microscopy was used as a reference method. Thus, six of the 200 patients (3%) diagnosed with ALOs on Pap smear examination became the study group. Patients without any infectious agents (n=134) were the control group.
When the study and control groups were compared statistically in view of the presence of eosinophil leucocytes [Figure 1b], it was found that there was a significant correlation between the presence of ALOs and eosinophil leucocytes (P<0.05) [Table 1]. Also, four of six patients with ALOs used an intrauterine contraceptive device (IUD). The type of IUDs was Cu T 380A.
Table 1.
Comparison of study and control groups in view of presence of eosinophils, PMNLs and macrophages
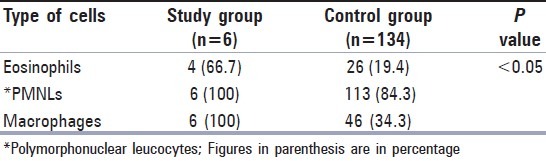
In this study, the presence of PMNLs and macrophages which are characteristic for Actinomyces infection was also examined. In all of six smears with ALOs, abundant PMNLs [Figure 1b] and macrophages were detected [Table 1]. As seen in Table 1, eosinophil leucocytes were observed less than PMNLs and macrophages in the study group.
Discussion
In the present study, Actinomyces were detected by both Pap smear microscopy and anaerobic culturing. However, the results obtained by Pap smear microscopy and anaerobic culturing were not concordant in diagnosis of Actinomyces. The identification and differentiation of these anaerobic bacteria from clinical samples are difficult because of their fastidious and slow growing nature.[12] Pap staining is the widely used, economical and practical method for early detection of genital infections.[13] Culturing is considered a gold standard for identifying the presence of these bacteria. However, it is very difficult to cultivate and identify these anaerobic organisms by using routine specimen handling and routine culturing techniques, and conventional culturing methods may yield ambiguous results.[14] Thus, the results obtained by both Pap smear microscopy and anaerobic culturing should be taken into consideration by clinicians to diagnose actinomycosis in time. In our study, since eosinophils were observed only on Pap smear examination, the results of this method were used as reference to evaluate the relationship between ALOs and eosinophils. Thus, patients with ALOs became the study group and those without any infectious agents were the control group.
The presence of eosinophil leucocytes was detected in both study and control groups. However, the percentage of eosinophil leucocytes was found to be higher in the study group (66.7%) than in the control group (19.4%). There was also a significant correlation between the presence of eosinophil leucocytes and ALOs (P<0.05). This finding suggests that eosinophil leucocytes might have a role in host defense against Actinomyces.
Although roles for eosinophils in host defenses against bacterial infections are still conflicting, recent data have documented the expression of TLRs on human eosinophils.[10] This finding has suggested that eosinophils can recognize multiple bacterial species via these TLRs. Then, they are activated and secrete their cytotoxic granule proteins located in their cytoplasm.[11] These cytotoxic proteins are eosinophil cationic protein (ECP), major basic protein (MBP) and eosinophil peroxidase (EPO).[8] In vitro studies showed that these proteins have an antibacterial activity.[5] Among these proteins, ECP was purified by Rosenberg et al.[15] and it was found that ECP is toxic to S. aureus which is a Gram-positive bacterium. The cytotoxic ability of ECP arises from making pores in the cell membranes.[8] Furthermore, Wong et al.[10] detected that peptidoglycan, a layer outside the plasma membrane of bacteria, could induce the release of ECP. MBP may aid in the recruitment of phagocytic cells such as neutrophils by stimulating the release of IL-8, whereas EPO catalyzes the generation of highly toxic reactive halogen species and nitric oxide derived oxidants.[6] While the release of ECP was only triggered at high concentrations of bacteria, MBP and EPO were released from eosinophils exposed to both low and high bacterial concentrations.[8] Recently, it has been reported that other proteins such as secretory phospholipase A2, bactericidal/permeability increasing protein (BPI), which is also stored in azurophil granules of neutrophils, and bacterial cell wall degrading enzyme N-acetylmuramyl-L-alanine-amidase present in eosinophil granules and they also possess bactericidal activity.[5,6,11] However, little is known about the role of eosinophils during bacterial infection.
In the early stage of Actinomyces infection, numerous neutrophils surround the mass of bacteria to engulf them. At the intermediate and late stages of infection, macrophages replace most of neutrophils. These foamy cells phagocytose the degenerated neutrophils including bacteria.[3] Although Actinomyces are easily phagocytosed and efficiently killed by these PMNLs and macrophages, the presence of Actinomyces in clusters can result from their escape from the destruction of host immune defense mechanisms.[16] In this study, the presence of PMNLs and macrophages which are characteristic for actinomycotic infections was also examined and abundant PMNLs and macrophages were observed in all of 6 smears with ALOs. We also detected that there was a statistically significant difference between the presence of ALOs and PMNLs as well as macrophages (P<0.05). This result was consistent with those of other authors.[3,16]
In literature, several studies have shown that eosinophils can kill bacteria less efficiently than neutrophils.[6] In this study, the percentage of PMNLs and macrophages was seen more than eosinophils in patients with ALOs. Also, eosinophil leucocytes were not observed in all patients with ALOs. According to these findings, it is plausible to suggest that PMNLs and macrophages are the main phagocytes in the host defense against ALOs and that, eosinophils may aid in the recruitment of these phagocytic cells.
In conclusion, there is a significant relationship between the presence of ALOs and eosinophil leucocytes. Thus, eosinophils might have a role in host defense against Actinomyces in order to aid PMNLs as well as macrophages by secreting cytoplasmic granules. However, further studies are needed to define the exact role of specific granule proteins of eosinophil leucocytes against bacterial infections.
Acknowledgment
The authors gratefully thank Prof. Dr. Gülşen Hasçelik for the use of the laboratory facilities at the Medical Microbiology Department, Faculty of Medicine, Hacettepe University.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.de Feiter PW, Soeters PB. Gastrointestinal actinomycosis: an unusual presentation with obstructive uropathy: report of a case and review of the literature. Dis Colon Rectum. 2001;44:1521–5. doi: 10.1007/BF02234610. [DOI] [PubMed] [Google Scholar]
- 2.Gorisek B, RebersekGorisek H, Kavalar R, Krajnc I, Zavrsnik S. Pelvic actinomycosis. Wien Klin Wochenschr. 1999;111:603–7. [PubMed] [Google Scholar]
- 3.Moral MA, Ohshima H, Maeda T, Hoshino E. Experimental chronic infection induced in mice by Actinomyces israelii entrapped in alginate gel. Arch Oral Biol. 1998;43:485–96. doi: 10.1016/s0003-9969(98)00015-6. [DOI] [PubMed] [Google Scholar]
- 4.Weller PF. Human eosinophils. J Allergy Clin Immunol. 1997;100:28–37. doi: 10.1016/s0091-6749(97)70237-9. [DOI] [PubMed] [Google Scholar]
- 5.Calafat J, Janssen H, Tool A, Dentener MA, Knol EF, Rosenberg HF, et al. The bactericidal/permeabilityincreasing protein (BPI) is present in specific granules of human eosinophils. Blood. 1998;91:4770–5. [PubMed] [Google Scholar]
- 6.Persson T, Andersson P, Bodelsson M, Laurell M, Malm J, Egesten A. Bactericidal activity of human eosinophilic granulocytes against Escherichia coli. Infect Immun. 2001;69:3591–6. doi: 10.1128/IAI.69.6.3591-3596.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yazdanbakhsh M, Eckmann CM, Bot AA, Roos D. Bactericidal action of eosinophils from normal human blood. Infect Immun. 1986;53:192–8. doi: 10.1128/iai.53.1.192-198.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Svensson L, Wenneras C. Human eosinophils selectively recognize and become activated by bacteria belonging to different taxonomic groups. Microbes Infect. 2005;7:720–8. doi: 10.1016/j.micinf.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 9.Cline MJ. Microbicidal activity of human eosinophils. J Reticuloendothel Soc. 1972;12:33–29. [PubMed] [Google Scholar]
- 10.Wong CK, Cheung PF, Ip WK, Lam CW. Intracellular signaling mechanisms regulating tolllike receptormediated activation of eosinophils. Am J Respir Cell Mol Biol. 2007;37:85–96. doi: 10.1165/rcmb.2006-0457OC. [DOI] [PubMed] [Google Scholar]
- 11.Linch SN, Kelly AM, Danielson ET, Pero R, Lee JJ, Gold JA. Mouse eosinophils possess potent antibacterial properties in vivo. Infect Immun. 2009;77:4976–82. doi: 10.1128/IAI.00306-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Westhoff C. IUDs and colonization or infection with Actinomyces. Contraception. 2007;75:S48–50. doi: 10.1016/j.contraception.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 13.Fitzhugh VA, Heller DS. Significance of a diagnosis of microorganisms on Pap smear. J Low Genit Tract Dis. 2008;12:40–51. doi: 10.1097/lgt.0b013e31813e07ff. [DOI] [PubMed] [Google Scholar]
- 14.Wang YH, Tsai HC, Lee SS, Mai MH, Wann SR, Chen YS, et al. Clinical manifestations of actinomycosis in Southern Taiwan. J Microbiol Immunol Infect. 2007;40:487–92. [PubMed] [Google Scholar]
- 15.Rosenberg HF. Recombinant human eosinophil cationic protein.Ribonuclease activity is not essential for cytotoxicity. J Biol Chem. 1995;270:7876–81. doi: 10.1074/jbc.270.14.7876. [DOI] [PubMed] [Google Scholar]
- 16.Figdor D, Davies J. Cell surfaces structures of Actinomyces israelii. Aust Dent J. 1997;42:125–8. doi: 10.1111/j.1834-7819.1997.tb00109.x. [DOI] [PubMed] [Google Scholar]