Abstract
Background:
Micronucleus (MN) represents small, additional nuclei formed by the exclusion of chromosome fragments or whole chromosomes lagging at mitosis. MN rates, therefore, indirectly reflect chromosome breakage or impairment of the mitotic apparatus. During the last few decades, micronuclei (“MNi”) in oral exfoliated epithelial cells are widely used as biomarkers of chromosomal damage, genome instability and cancer risk in humans. However, until now only little attention has been given to the effect of different staining procedures on the results of these MN assays.
Aim:
To compare the MNi frequencies in oral exfoliated epithelial cells using three different stains, i.e.,Feulgen stain, Papanicolaou stain (Pap) and hemotoxylin and eosin stain (H and E).
Materials and Methods:
Oral exfoliated cells from 45 cases of potentially malignant disorders (15 oral submucous fibrosis, 15 lichen planus and 15 leukoplakia) and 15 controls with healthy mucosa, were taken and MNi frequencies (No. of MNi/1000 cells) were compared using three different stains.
Results:
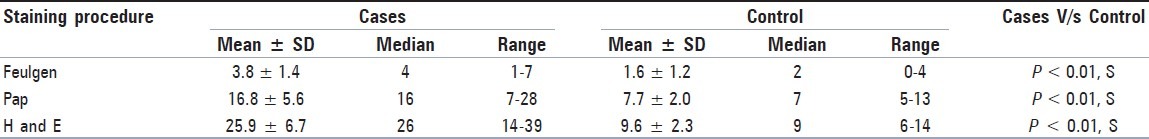
Mean MNi frequency in cases was found to be 3.8 with Feulgen stain, 16.8 with PAP and 25.9 with H and E. In controls, mean MNi frequency was 1.6 with Feulgen stain, 7.7 with PAP and 9.6 with H and E stain. Statistically significant value (P < 0.01) were observed when the three stains were compared together using Kruskal Walli's ANOVA test.
Conclusion:
Feulgen being a DNA-specific stain gave the least counts, although statistically significant results from the comparison of MNi frequency between cases and controls were obtained with all the three stains.
Keywords: Feulgen stain, hematoxylin and eosin stain, micronuclei, papanicolaou stain, potentially malignant disorders
Introduction
Micronucleus (MN) is the name given to the small nucleus that form whenever a chromosome or a fragment of a chromosome is not incorporated into one of the daughter nuclei during cell division. They are induced in cells by numerous genotoxic agents that damage the chromosomes. The damaged chromosomes, in the form of acentric chromatids or chromosome fragments, lag behind in anaphase when centric elements move towards the spindle poles. After telophase, the undamaged chromosomes, as well as the centric fragments, give rise to regular daughter nuclei. The lagging elements are included in the daughter cells, too, but a considerable proportion is transformed into one or several secondary nuclei, which are, as a rule, much smaller than the principal nucleus and are therefore called micronuclei.[1,2] Bigger micronuclei (MNi) result from exclusion of whole chromosome following damage to the spindle apparatus of the cell (aneugenic effect), whereas smaller MNi result from structural aberrations, thereby causing chromosomal fragments (clastogenic effect).[3,4]
An evaluation of the literature shows that a variety of different stains are used in MNi studies. Among the DNA-specific stains, the ones most widely used are Feulgen and acridine orange; in some experiments, 4’,6-diamidino-2-phenylindole (DAPI) and propidium iodide were also used. About 30% of the studies in epithelial cells were conducted with nonspecific stains (Giemsa, May-Grünwald-Giemsa, Papanicolaou and less frequently orcein and hematoxylin and eosin). However, only little attention has been, until now, given to the effect of different staining procedures on the results of MN assays.[5,6]
It has been established that in the head and neck region MNi are found at increased frequencies from normal mucosa to potentially malignant disorders (PMDs) to carcinoma, suggesting ever increasing chromosomal instability.[7] With this view in mind, the present study was carried out to investigate the effect of different stains on MNi frequencies in exfoliated oral epithelial cells. Oral exfoliated cells from patients with PMDs (i.e., cases) were taken and oral exfoliated cells were also taken from normal healthy subjects (i.e., controls). MNi frequencies were compared using three different stains, i.e., Feulgen stain, Papanicolaou (Pap) stain and hemotoxylin and eosin (H and E) stain.
Materials and Methods
Study sample consisted of 60 subjects and was divided into two groups as follows:
Group I (Controls): Control group comprised of 15 healthy subjects with clinically normal oral mucosa.
Group II (Cases): Comprised of 45 patients [15 oral submucous fibrosis (OSMF), 15 lichen planus and 15 leukoplakia] clinically diagnosed as having one of the PMDs of the oral cavity.
Relevant history of each patient, including their oral habits, was recorded thoroughly. Age-and sex-matched healthy subjects having no obvious oral lesions or habits of consumption of tobacco, other tobacco-related substances, or potentially toxic substances were selected as control group. Patients with provisional or confirmed diagnosis of any cancer were not included in the study. Written informed consents from these patients were taken for the procedures to be carried out on them.
Collection of exfoliated cells
Subjects were asked to rinse their mouth gently with water. Oral mucosal cells were scraped from lesional tissue in cases and from the buccal mucosa of control group using a slightly moistened cytobrush (Medscand Medical AB, Malmo, Sweden). The cells were immediately smeared on precleaned microscopic slides. Just prior to drying, the smears were fixed with commercially available spray fixative (available with the RAPIDPAP™ kit) for 15 minutes.
Cytological staining and evaluation
The cytosmears were separately stained with Feulgen, Pap and H and E stains.
The slides were mounted with cover glass using DPX mountant. All the slides were observed under a light microscope using low magnification (×400) for screening and high magnification (×1000) for counting of MNi.
Scoring criteria
The most commonly used method, i.e., the zigzag method, was followed for screening of slides. One thousand cells with intact nuclei and cell boundaries were counted on each slide. For the purpose of designating an extra nuclear body as a MN, the following criteria given by Tolbert et al.[8,9] were considered:
-
(a)
Rounded smooth perimeter suggestive of a membrane;
-
(b)
Less than a third the diameter of the associated nucleus, but large enough to discern shape and color;
-
(c)
Staining intensity similar to that of the nucleus;
-
(d)
Texture similar to that of nucleus;
-
(e)
Same focal plane as nucleus; and
-
(f)
Absence of overlap with, or bridge to, the nucleus.
Only those structures fulfilling the above-mentioned criteria were recorded as MNi [Figure 1]. The total number of MNi observed in 1000 intact epithelial cells was calculated as the MNi frequency. The data obtained was statistically analyzed. Kruskal-Walli's (KW) ANOVA test was used for multiple group comparisons and pairwise comparison was done by Wilcoxon's Signed Rank test. The Mann-Whitney test was applied for two group comparisons (Cases v/s. Controls). P-value of 0.05 or less was considered for statistical significance.
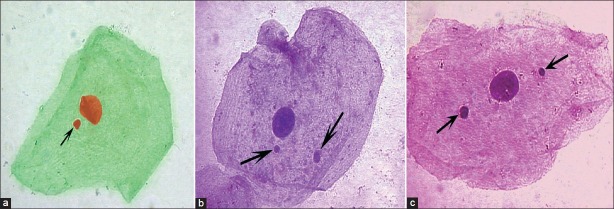
Figure 1.
Photomicrographs showing micronuclei (arrow) in oral exfoliated epithelial cells (a: Feulgen stain, b: Pap, c: H and E, ×1000)
Results
The mean age of the controls was found to be 35.1 ± 10.5 years, whereas for PMDs it was 39.1 ± 15.1 years. Any statistical significance of age between the cases and controls was ruled out by unpaired Student t-test (P = 0.26). The mean age of the subjects with OSMF was found to be 29.9 ± 10.0 years, for lichen planus it was 40.5 ± 15.6 years and for leukoplakia it was 46.8 ± 14.9 years. It was observed that subjects with leukoplakia had the highest mean age and the ones with OSMF had the lowest mean age as compared to the other two PMDs.
Among group I (control), out of 15 subjects, 9 (60%) were males and 6 (40%) were females. In group II (cases), out of 45 subjects, 35 (77.8%) were males and 10 (22.2%) were females. Any statistical significance of gender between the cases and controls was ruled out by the way of the Chi square test (P = 0.18). Among the different PMDs, except for lichen planus (F – 60%, M – 40%), the same pattern of males predominance was seen in OSMF (M – 100%, F – 0%) as well as in leukoplakia (M – 93.3%, F – 6.7%).
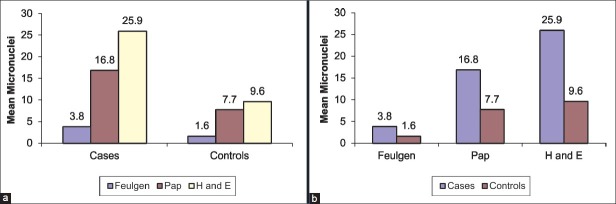
For the comparison of MNi frequency (No. of MNi/1000 cells) between the three staining procedures together, KW ANOVA test was used independently for cases [Table 1] and controls [Table 2]. A statistically significant (P < 0.01) result was obtained. The KW ANOVA was followed by Wilcoxon's Signed Rank Test for inter staining procedure comparison in cases and controls. Statistically significant (P < 0.01) results were obtained for each comparison being made independently [Figure 2a].
Table 1.
Comparison of micronuclei frequency between the three staining procedures together in cases (Kruskal Walli's ANOVA Test followed by Wilcoxon's Signed Rank Test)
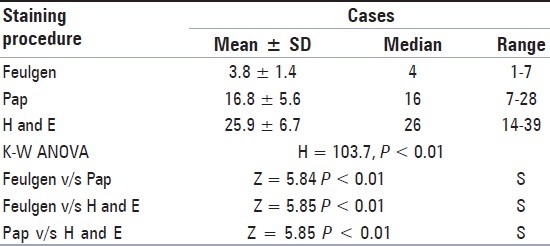
Table 2.
Comparison of micronuclei frequency between the three staining procedures together in control (Kruskal-Wallis ANOVA Test followed by Wilcoxon's Signed Rank Test)
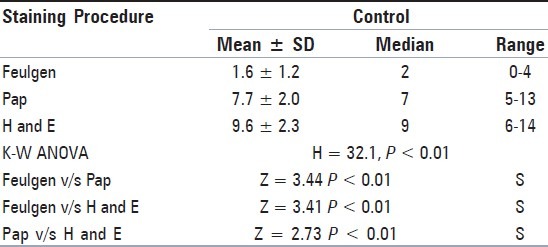
Figure 2.
(a) Comparison of MNi frequency (No. of MNi/1000 cells) between the three staining procedures together in cases and controls, (b) Comparison of the MNi frequency (No. of MNi/1000 cells) between the cases and controls for each of the three staining procedures
The Mann-Whitney test was used for comparison of the MNi frequency (No. of MNi/1000 cells) between the cases and controls for each of the three staining procedures [Table 3]. Statistically significant results (P < 0.01) were obtained in each of the staining procedures [Figure 2b].
Table 3.
Comparison of micronuclei frequency between cases and control for each of the three staining procedures (Mann-Whitney Test)
Discussion
Micronucleus (MN) represent small, additional nuclei formed by the exclusion of chromosome fragments or whole chromosomes lagging at mitosis. MN rates, therefore, indirectly reflect chromosome breakage or impairment of the mitotic apparatus. The quantitative detection of MNi is widely used for analysis of cytogenetic damage.[10,11]
About 30 years ago, Stich et al.[12] developed a protocol for MN assays with exfoliated buccal mucosa cells, which was widely used in occupational and lifestyle studies. It was repeatedly emphasized that this noninvasive method might be a suitable biomonitoring approach for the detection of increased cancer risk in humans because more than 90% of all human cancers are of epithelial origin.[13] The level of baseline chromosome damage in untreated cancer patients and also in various PMDs is much higher than in cancer-free controls. Therefore, MNi scoring can be used as a biomarker to identify different preneoplastic conditions much earlier than the manifestations of clinical features and might specifically be utilized in the screening of high-risk population for a specific cancer.[14]
Approximately more than 200 studies with epithelial cells have been published in the last 30 years and apart from cells of the oral mucosa, cells from other organs, such as nasal mucosa, cervix, bladder, esophagus and bronchi, were also used.[5]
However, until now only little attention has been given to the effect of different staining procedures on the results of MN assays. According to the results of the detailed survey of the current methodological and data acquisition statuses of laboratories, that have published papers on the buccal MN assay (discussed at eighth workshop of the Human MicroNucleus (HUMN) project held at Antalya, Turkey, 2007), more than 50% of the laboratories which participated in the survey used Feulgen stain and only one of the laboratory had used H and E stain.[6] According to the same survey the second most common stain used was May-Grünwald-Giemsa (MGG) stain, however Ayyad et al.[15] found better results for counting of MNi using Pap stain as compared to MGG stain.Thus in the present study an attempt was made to compare Feulgen stain (the most commonly stain used for MN assay), Pap stain (the most commonly used stain in routine exfoliative cytology) and H and E stain (the most easily available stain).
In the present study, among the cases taken together as a whole, Feulgen stain showed the least MNi frequency and H and E showed the maximum count, while the value obtained with Pap was intermediate between the former two. The same order was seen in the controls as well [Figure 2].
The possible explanation for the lowest count with the Feulgen stain could be its high DNA specificity and a clear transparent appearance of the cytoplasm which enables easy identification of MNi.[5] One shortcoming of this staining method apart from being highly technique sensitive (as observed during this study) is that it is relatively lengthy and may lead to the underscoring of MNi.[16] The increased MNi frequency observed with DNA nonspecific stains (Pap and H and E in this study) can be due to any of the following reasons:
Misinterpretation of nuclear anomalies like karyorrhexis, karyolysis, condensed chromatin, and binucleates as MNi, as significant correlation was observed between these anomalies and MNi count using DNA nonspecific stains in a study carried out by Nersesyan et al.[5]
Formation of keratin granules that are found in degenerated cells with nuclear anomalies. These round cytoplasmic bodies, which are formed as a consequence of cell injury, do not contain DNA and may be classified as MNi with nonspecific stains.[5]
Contamination by the bacteria that are commonly found in the mouth can interfere with MNi scoring. Bacteria can be differentiated from MNi by their characteristic shape, smaller size, color, staining intensity, and their presence upon and between buccal cells on the slide.[5,14]
Another common confounding issue is the small dye granules that may sometimes resemble MNi but usually have a slightly different refractility and color intensity.[5,14]
In the present study, these factors were conscientiously taken care of, to minimize the possibility of scoring these mimickers.
The explanation for a relatively higher count seen with H and E as compared to Pap stain warrants further evaluation. Since the scientific literature lacks sufficient relevant data, in relation to the use of H and E stain for MNi count in oral exfoliated cells, further detailed studies are required to ensure the validity of this stain.
The mean MNi frequency noted in cases was significantly higher (P < 0.01) than the one noted in controls, using each of the three stains [Figure 2b]. These results are in accordance with the ones observed in many studies carried out so far in PMDs.[7,10,17–21] Casartelli et al.[7] concluded that the gradual increase in MNi frequency from normal mucosal to precancerous lesion to carcinoma suggested a link of this biomarker with neoplastic progression. According to Samanta and Dey,[14] the various possible explanations for MNi formation in preneoplastic conditions include chromosomal aberrations, chromosome loss/breakage, mitotic apparatus dysfunctions, aneuploidy and genetic instability.
According to Belien et al.[22] multiples of 1000 epithelial cells should be observed for more standard results for evaluation of micronuclei, which necessitates automation.Since automation will hamper the cost effectiveness of this screening test, when applied to masses, so the authors of the present study restricted to 1000 cells and found statistically significant results.
The results of our study are in contrast to the ones observed in a similar study carried out by Nerseseyan et al.,[5] according to which, a statistically significant difference in MNi counts between smokers and nonsmokers was observed only with DNA nonspecific stains and not with the DNA-specific stains. The researchers (Nerseseyan et al.) validated their results by concluding that smoking does not induce a significant increase in MNi counts. Except for the Feulgen stain, none of the stains used in our study were common to the stains used in the above mentioned study, so any justification for the differences being observed cannot be optimally elaborated. A note on comparison between the two is made here only to emphasize the DNA specificity of Feulgen stain that was being observed in both the studies.
Conclusions
Being a DNA-specific stain, values with Feulgen stain were found to be lowest in measure. Because of the possibility of mis-interpretation of nuclear anomalies (e.g.,karyorrhexis, karyolysis, condensed chromatin, and binucleates) and keratin granules as MNi with nonspecific stains, a higher count was observed with Pap and H and E stain. Taking into consideration the drawbacks of Feulgen stain (i.e., technique sensitivity and time consuming procedure as compared to the other two staining procedures) on the one hand and at the same time statistically significant results being observed between the cases and controls in this study, using all the three stains, whether it be a DNA-specific stain (i.e., Feulgen stain) or DNA nonspecific stain (Pap and H and E stain), on the other hand, this study intends to lay a foundation for further detailed comparative studies to ensure the validation of DNA nonspecific stains in MN assays.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Schmid W. The micronucleus test. Mutat Res. 1975;31:9–15. doi: 10.1016/0165-1161(75)90058-8. [DOI] [PubMed] [Google Scholar]
- 2.Palve DH, Tupkari JV. Clinico-pathological correlation of micronuclei in oral squamous cell carcinoma by exfoliative cytology. J Oral Maxillofac Pathol. 2008;12:2–7. [Google Scholar]
- 3.Norppa H, Falck GC. What do micronuclei contain? Mutagenesis. 2003;18:221–33. doi: 10.1093/mutage/18.3.221. [DOI] [PubMed] [Google Scholar]
- 4.Sarto F, Finotto S, Giacomelli L, Mazotti D, Tomanin R, Levis AG. The micronucleus assay in exfoliated cells of the human buccal mucosa. Mutagenesis. 1987;2:11–7. doi: 10.1093/mutage/2.1.11. [DOI] [PubMed] [Google Scholar]
- 5.Nersesyan A, Kundi M, Atefie K, Hermann RS, Knasmuller S. Effect of staining procedures on the results of micronucleus assays with exfoliated oral mucosa cells. Cancer Epidemiol Biomarkers Prev. 2006;15:1835–40. doi: 10.1158/1055-9965.EPI-06-0248. [DOI] [PubMed] [Google Scholar]
- 6.Bonassi S, Biasotti B, Kirsch-Volders M, Knasmueller S, Zeiger E, Burgaz S, et al. State of the art survey of the buccal micronucleus assay: a first stage in the HUMN (XL) project initiative. Mutagenesis. 2009;24:295–302. doi: 10.1093/mutage/gep019. [DOI] [PubMed] [Google Scholar]
- 7.Casartelli G, Bonatti S, Ferrari MD, Scala M, Mereu P, Margarino G. Micronucleus frequencies in exfoliated buccal cells in normal mucosa, precancerous lesions and squamous cell carcinoma. Anal Quant Cytol Histol. 2000;22:486–92. [PubMed] [Google Scholar]
- 8.Tolbert PE, Shy CM, Allen JW. Micronuclei and other nuclear anomalies in Buccal Smears: A field test in snuff users. Am J of Epidemiol. 1991;134:840–50. doi: 10.1093/oxfordjournals.aje.a116159. [DOI] [PubMed] [Google Scholar]
- 9.Holland N, Bolognesi C, Kirsch-Volders M, Bonassi S, Zeiger E, Knasmuller S. The micronucleus assay in human buccal cells as a tool for biomonitoring DNA damage: The HUMN project perspective on current status and knowledge gap. Mutat Res. 2008;659:93–108. doi: 10.1016/j.mrrev.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 10.Bloching M, Hofmann , Lautenschlager C, Berghaus A, Grummt T. Exfoliative cytology of normal buccal mucosa to predict the relative risk of cancer in the upper aerodigestive tract using the MN-assay. Oral Oncol. 2000;36:550–5. doi: 10.1016/s1368-8375(00)00051-8. [DOI] [PubMed] [Google Scholar]
- 11.Jadhav K, Gupta N, Ahmed MB. Micronuclei: An essential biomarker in oral exfoliated cells for grading of oral squamous cell carcinoma. J Cytol. 2011;28:7–12. doi: 10.4103/0970-9371.76941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stich HF, San RH, Rosin MP. Adaptation of the DNA-repair and micronucleus tests to human cell suspensions and exfoliated cells. Ann N Y Acad Sci. 1983;407:93–105. doi: 10.1111/j.1749-6632.1983.tb47816.x. [DOI] [PubMed] [Google Scholar]
- 13.Singaraju M, Singaraju S, Parwani RN, Wanjari SP. Cytogenetic biomonitoring in petrol station attendants: A micronucleus study. J Cytol. 2012;29:1–5. doi: 10.4103/0970-9371.93208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Samanta S, Dey P. Micronucleus and its applications. Diagn Cytopathol. 2012;40:84–90. doi: 10.1002/dc.21592. [DOI] [PubMed] [Google Scholar]
- 15.Ayyad SB, Israel E, El-Setouhy M, Nasr GR, Mohamed MK, Loffredo CA. Evaluation of Papanicolaou stain for studying micronuclei in buccal cells under field conditions. Acta Cytol. 2006;50:398–402. doi: 10.1159/000325980. [DOI] [PubMed] [Google Scholar]
- 16.Casartelli G, Monteghirfo S, De Ferrari M, Bonatti S, Scala M, Toma S, et al. Staining of micronuclei in squamous epithelial cells of human oral mucosa. Anal Quant Cytol Histol. 1997;19:475–81. [PubMed] [Google Scholar]
- 17.Halder A, Chakraborty T, Mandal K, Gure PK, Das S, Raychowdhury R, et al. Comparative study of exfoliated oral mucosal cell micronuclei frequency in normal, precancerous and malignant epithelium. Int J Hum Genet. 2004;4:257–60. [Google Scholar]
- 18.Delfino V, Casartelli G, Garzogilo B, Scala M, Mereu P, Bonatti S, et al. Micronuclei and p53 accumulation in preneoplastic and malignant lesions of the head and neck. Mutagenesis. 2002;17:73–7. doi: 10.1093/mutage/17.1.73. [DOI] [PubMed] [Google Scholar]
- 19.Buajeeb W, Kraivaphan P, Amornchat C, Triratana T. Frequency of micronucleated exfoliated cells in oral lichen planus. Mutat Res. 2007;627:191–6. doi: 10.1016/j.mrgentox.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 20.Saran R, Tiwari RK, Reddy PP, Ahuja YR. Risk assessment of oral cancer in patients with pre-cancerous states of the oral cavity using micronucleus test and challenge assay. Oral Oncol. 2008;44:354–60. doi: 10.1016/j.oraloncology.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 21.Desai SS, Ghaisas SD, Jakhi SD, Bhide SV. Cytogenetic damage in exfoliated oral mucosal cells and circulating lymphocytes of patients suffering from precancerous oral lesions. Cancer Lett. 1996;109:9–14. doi: 10.1016/s0304-3835(96)04390-x. [DOI] [PubMed] [Google Scholar]
- 22.Belien JA, Copper MP, Braakhuis BJ, Snow GB, Baak JP. Standardization of counting micronuclei: definition of a protocol to measure genotoxic damage in human exfoliated cells. Carcinogenesis. 1995;16:2395–400. doi: 10.1093/carcin/16.10.2395. [DOI] [PubMed] [Google Scholar]