Abstract
Background:
Since the first introduction of Papanicolaou (Pap) stain in 1942 there have been many modifications. Of these, the Ultra-Fast Pap stain has become popular. This technique was further modified in India as many of the reagents were not available in our country. Our study was conducted by adapting this modified staining technique which involves the replacement of Gill's hematoxylin with Harris hematoxylin.
Aims:
The aim of our prospective study was to assess the use of the modified Ultra-Fast Pap stain (MUFP) for fine needle aspiration cytology (FNAC) of various organs in comparison with the standard rapid Pap stain.
Materials and Methods:
A total of 100 FNAC cases were studied by random sampling. Two smears were prepared for each case and stained by both, the MUFP and the rapid Pap stain. Scores were given and the quality index was calculated, followed by the statistical analysis. The number of cases was as follows: lymph node (43), thyroid (25), breast (23), salivary gland (02), and soft tissues (07). Scores were given on four parameters: Background of smears, overall staining pattern, cell morphology and nuclear staining. Quality index was calculated from the ratio of score achieved to the maximum score possible.
Statistical Analysis:
Results were analyzed using Mean, Median, Standard Deviation, ‘t’ paired test, ‘P’ value and M-diff for statistical significance.
Results:
Correct diagnosis was made in all cases. The quality index of MUFP smears was better compared to the rapid Pap stain in all the organs, and was statistically significant. MUFP smears showed a clear red blood cells background, transparent cytoplasm and crisp nuclear features.
Conclusion:
MUFP is a reliable and rapid technique for cytology diagnosis.
Keywords: Fine needle aspiration cytology, modified Pap stain, papanicolaou stain, rapid Pap stain, ultra-fast Pap stain
Introduction
The need for minimal turnaround time for assessing the fine needle aspiration smears has encouraged innovations in staining procedures that require lesser staining time with unequivocal cell morphology. Modifications have been developed in Papanicolaou stain to improve the staining quality and/or to minimize staining time.[1–4] Wet fixation is time consuming, shows drying artifacts and less retention of material compared to air dried smears. Air dried smears also have shortcomings such as magnification in the cell size, and often the morphology of the cells is not crisp. Ultra-Fast Papanicolaou (UFP) stain was introduced by Yang and Alvarez in 1995.[5] Kamal et al.[6] modified this technique because not all reagents used in UFP are readily available[5] and some of the thyroid aspirations showed nuclear ground glass appearance as an artifact.
The objective of this prospective study was to assess the feasibility and applicability of Modified Ultra-Fast Papanicolaou stain (MUFP) in fine needle aspiration smears of various organs in comparison to standard rapid Papanicolaou stain, and to assess the alternative use of Harris hematoxylin in place of Gill's hematoxylin.
Materials and Methods
This prospective study was carried out in the cytopathology laboratory of a large tertiary care teaching hospital. Fine needle aspiration was carried out from various organs as an outpatient procedure for patients referred from different clinical departments for diagnostic purpose. The number of specimens collected was as follows: lymph node (43), thyroid (25), breast (23), salivary gland (02), and soft tissue (07). Smears were kept for fixation with ether alcohol mixture for rapid Papanicolaou (Pap) stain and were air dried for MUFP staining. Air dried smears were then kept in normal saline for 30 seconds and in alcoholic formalin for 10 seconds. Stain preparation is listed at appendix.
Steps for staining
Tap water (6 slow dips)
Harris hematoxylin (30 seconds)
Tap water (6 slow dips)
Isopropyl alcohol 95% (6 dips)
EA-36 (15 seconds)
Isopropyl alcohol 95% (6 dips)
Isopropyl alcohol 100% (6dips)
Xylene (10 slow dips)
DPX
Mount with cover slip.
Total staining time was 130 seconds. In MUFP, Gill's hematoxylin, modified EA and isopropyl alcohol were used instead of Richard- Allan hematoxylin, Richard- Allan cytostain and 95% ethyl alcohol, respectively. In our study, Harris hematoxylin which is readily available, replaced Gill's hematoxylin. The quality of Ultra-Fast MUFP staining was assessed by considering the background, overall staining, cell morphology and nuclear characteristics of the cells in the smear [Table 1]. Maximum score possible for a single case, taking into account all the four parameters is 11. Maximum possible score is calculated by multiplying the number of cases by 11.
Table 1.
Assessment of the quality of Modified Ultra-Fast Papanicolaou staining

Quality index
Actual score obtained / Maximum score possible
The scores obtained for MUFP were compared with scores for the standard rapid Papanicolaou stain.
Results
Table 2 shows the scores and quality index for both the MUFP and rapid Pap stains. Compared to the rapid Pap stain, the quality index of MUFP is better. In MUFP, the minimum index obtained was 0.97 (for breast) and the maximum was 1.0 (for thyroid and soft tissues). In the rapid Pap staining, the minimum score was 0.9 (for breast) and the maximum, 0.95 (for soft tissue). In thyroid lesions, the quality index by rapid Pap was 0.91, whereas by MUFP was 1.0; clearly indicating that MUFP is best for the thyroid smears. Standard deviation and ‘t’ paired test was applied to check if the difference in the mean ratio of MUFP and rapid Pap is significant. Let μ1 -=mean ratio for MUFP; μ2-=mean ratio for rapid Pap.
Table 2.
Comparison of quality index scores of Modified Ultra-Fast Papanicolaou and rapid Papanicolaou stain
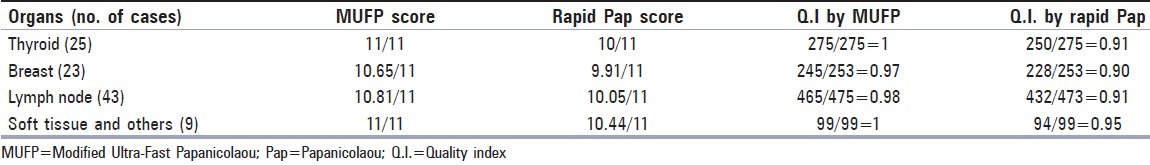
The t paired exam tests the following hypothesis: H0: μ1- μ2 = 0; Ha: μ1-μ2 ≠0. In all the organs, the obtained ‘t’ has a P value < a =.05 (α is the significance level we apply to check the null hypothesis), and thus reject H0. Thus the difference between the MUFP ratio and rapid Pap ratio (Mdiff) is significantly more than zero, as seen in the Table 2. In other words, in all the organs, the mean MUFP ratio is statistically significantly higher than the mean rapid Pap ratio.
Discussion
Fine needle aspiration cytology (FNAC) is one of the cheapest, fastest and easiest tools available for early detection and diagnosis of various lesions. Since its inception, Pap stain remains the traditional and preferred stain, not only for the gynecological cytology, but also the lesions of other organs. The different stains used for air dried smears, such, as May-Grünwald-Giemsa, Jenner- Giemsa and Diff-quick fail to offer the transparency for the study of subtle nuclear features as seen by the Pap stain. The traditional Pap stain involves wet fixation and subsequent staining, together requiring at least 30 minutes. To cut down the time, the rapid Pap stains were developed by Kline,[1] Tao[3] and Sato[7] with respective staining time of 4 minutes, 5 minutes and 90 seconds. However, the quality of rapid stains is usually not as satisfactory, as they show suboptimal cell morphology and still require wet fixation.[5] To overcome these problems, Yang and Alvarez[4] developed Ultra-Fast Pap (UFP) stain which is a hybrid of the technique by Romanowsky and conventional Pap stain, to reduce the staining time to 90 seconds.
This method involves 3 steps:
To make the cells appear larger due to air drying thus increasing resolution
To hemolyse the RBCs thus making the background clear;
To bring out vibrant colors in cells thus making the nucleoli distinct.
UFP is preferably used for thyroid FNAC[8–10] [Figure 1] and intra-operative cytology.[11] Kamal et al.[6] from India further modified the UFP stain (modified Ultra-Fast Pap stain) to overcome the problem of shortage of Richard- Allan hematoxylin, Richard- Allan cytostain and ethyl alcohol reagents in the Indian set-up. This method has a short staining time of 130 seconds, and also the cytomorphology can be well appreciated. We adapted Kamal's MUFP staining for evaluating the FNAC smears of various organs, by replacing Gill's hematoxylin with the easily available Harris hematoxylin, and compared the results with those of rapid Pap staining. A correct diagnosis was achieved in all the cases. In the present study, we obtained quality index taking into consideration four parameters: Smear background, staining pattern, cell morphology and nuclear characteristics. Index in majority of organs was very close to 1 [Table 2]. It was observed that quality index was lower in few lymph node smears diagnosed with metastatic squamous carcinoma. This is attributed to the omission of Orange-G (OG-6) component, which renders appreciation of cytoplasmic keratinization difficult [Figure 2]. OG-6 was not added because it gives a dirty orange background to the smears.
Figure 1.
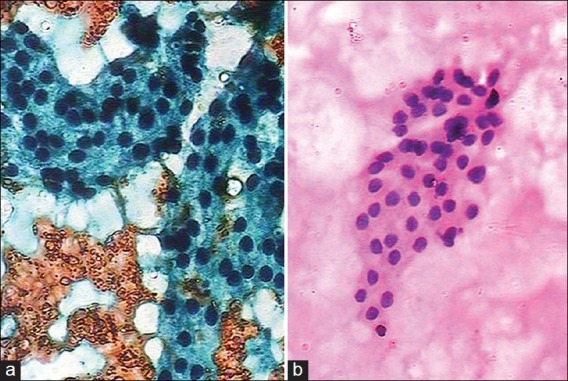
(a) Benign thyroid follicular cells obscured by blood (Rapid Pap, ×100); (b) Follicular cells in clean background (Modified Ultra-Fast Pap (MUFP) stain, ×100)
Figure 2.

(a) Metastatic squamous cells with orange cytoplasm (Rapid Pap, ×400); (b) Squamous cells without orange color (Modified Ultra-Fast Pap (MUFP) stain, ×400)
Few cases of breast FNAC smears showed suboptimal staining of single benign bare nuclei, probably due to their stromal nature. However, it was possible to achieve diagnosis in all cases as all myoepithelial cells in clusters, were well stained. Quality index score by MUFP stain was high for the thyroid and soft tissues, while for the rapid Pap stain, the score was high only for the soft tissues. It was further observed that the overall quality index scoring by MUFP stain was better than the rapid Pap stain, and this was statistically significant [Table 3].
Table 3.
Descriptive statistics for Modified Ultra-Fast Papanicolaou ratio and rapid Papanicolaou ratio

The advantages of modified Ultra-Fast pap stain (MUFP) stain compared to rapid Papanicolaou stain are as follows:
Staining solution can be prepared from locally available reagents.
Replacing Gill's hematoxylin with Harris hematoxylin does not alter the staining characteristics and gives equally good results.
As fixation is not required, the staining time is 130 seconds and therefore very useful for intraoperative cytology, rapid assessment of adequacy of samples and rapid diagnosis.
Background is clear, RBC free and thus helps in better interpretation. This is especially useful for smears of vascular organs like the thyroid and in identification of Reed Sternberg's cells of Hodgkin's lymphoma.
The technique provides good nuclear and cytoplasmic details as the cells appear large with crisp morphological features.
Air drying removes the artifactual changes seen in wet fixed smears due to poor fixation.
The technique causes no deleterious effect on immunophenotyping.
Cell loss with wet fixation is avoided, and therefore recommended for lipid rich tumors like lipoma.
The disadvantages of modified Ultra-Fast Pap stain (MUFP) stain compared to rapid Papanicolaou stain are as follows:
The method is technique sensitive as complete air drying should be strictly observed. Inadequate drying gives suboptimal results. Further, smears need to be properly prepared as thick smears don’t give satisfactory results.
Interpretation of cytoplasmic keratinization is not possible due to the omission of Orange-G.
Normal saline, Harris hematoxylin and EA-36 should be changed regularly.
Bipolar single nuclei are not stained properly.
Universal standardization of MUFP stain is recommended. Locally available solutions may influence the results adversely.
The solution is storage sensitive and the pH the alcoholic formalin should be maintained at 5.0; else can lead to poor staining.
Lesser staining time along with unequivocal morphological quality is undoubtedly the need of the hour for any cytopathology set-up. MUFP stain easily fulfills these criteria either equivalent to or better than the rapid Pap technique for cytological staining and study of various organs. MUFP stain is fast, reliable and can be done with locally available reagents, and therefore is especially useful in developing countries like India.
Appendix

Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Dighe SB, Ajit D, Pathuthara S, Chinoy R. Papinicolaou stain: Is it economical to switch to rapid, economical acetic acid papanicolaou stain? Acta Cytol. 2006;50:643–6. doi: 10.1159/000326034. [DOI] [PubMed] [Google Scholar]
- 2.Lemos LB, Baliga M, Cason Z. Ultrafast Papanicolaou stain: one year experience in a fine needle aspiration service. Acta Cytol. 1997;41:1630–1. doi: 10.1159/000332872. [DOI] [PubMed] [Google Scholar]
- 3.Pak HY, Yokota SB, Teplitz RL. Rapid staining techniques employed in fine needle aspirations. Acta Cytol. 1983;27:81–3. [PubMed] [Google Scholar]
- 4.Yang GC, Alvarez II. Ultrafast papanicolaou stain: an alternative preparation for fine needle aspiration cytology. Acta Cytol. 1995;39:55–60. [PubMed] [Google Scholar]
- 5.Shinde PB, Pandit AA. Application of modified ultrafast Papanicolaou stain in cytology of various organs. Diagn Cytopathol. 2006;34:135–9. doi: 10.1002/dc.20360. [DOI] [PubMed] [Google Scholar]
- 6.Kamal MM, Bodele A, Munshi MM, Bobhate SK, Kher AV. Efficacy of a modified ultrafast Papanicolaou stain for breast aspirates. Indian J Pathol Microbiol. 2000;43:417–21. [PubMed] [Google Scholar]
- 7.Sato M, Taniguchi E, Kagiya T, Nunobiki O, Yang Q, Nakamura M, et al. A modified rapid Papanicolaou stain for imprint smears. Acta Cytol. 2004;48:461–2. [PubMed] [Google Scholar]
- 8.Maruta J, Hashimoto H, Yamashita H, Noguchi S. Quick aspiration cytology of thyroid nodules by modified ultrafast Papanicolaou staining. Diagn Cytopathol. 2003;28:45–8. doi: 10.1002/dc.19999. [DOI] [PubMed] [Google Scholar]
- 9.Shidham VB, England JM, Maruta J, et al. Quick aspiration cytology for thyroid nodules by modified ultrafast Papanicolaou staining. Diagn Cytopathol. 2003;29:305. doi: 10.1002/dc.10341. [DOI] [PubMed] [Google Scholar]
- 10.Yang GC. Clear nuclei are specific to papillary carcinoma in thyroid FNA processed by ultrafast Papanicolaou stain for intra-operative cytology. Diagn Cytopathol. 2003;29:236–7. doi: 10.1002/dc.10350. [DOI] [PubMed] [Google Scholar]
- 11.Yang GC, Hoda SA. Combined use of the “scratch and smear” sampling technique and Ultrafast Papanicolaou stain for intraoperative cytology. Acta Cytol. 1997;41:1513–8. doi: 10.1159/000332868. [DOI] [PubMed] [Google Scholar]


