Abstract
The fossil record of land plants is an obvious source of information on the dynamics of mass extinctions in the geological past. In conjunction with the end-Permian ecological crisis, ≈250 million years ago, palynological data from East Greenland reveal some unanticipated patterns. We document the significant time lag between terrestrial ecosystem collapse and selective extinction among characteristic Late Permian plants. Furthermore, ecological crisis resulted in an initial increase in plant diversity, instead of a decrease. Paradoxically, these floral patterns correspond to a “dead zone” in the end-Permian faunal record, characterized by a paucity of marine invertebrate megafossils. The time-delayed, end-Permian plant extinctions resemble modeled “extinction debt” responses of multispecies metapopulations to progressive habitat destruction.
Extinction, the irreversible loss of species, is perhaps the most alarming symptom of the ongoing biodiversity crisis. In an attempt to understand long-term consequences of present-day biodiversity decline, past episodes of dramatic mass extinction therefore are investigated increasingly for consistent patterns of species responses to global environmental deterioration. At present, conceptual models linking patterns of mass extinction, survival, and recovery mainly are constrained by qualitative generalizations on temporal distribution of marine invertebrate lineages (1–4). Focus is commonly on the profound macroevolutionary consequences of mass extinctions.
A drawback of zoocentric models is their inadequacy to register population and community dynamics at the very climax of global ecological crisis. This is due mainly to a depleted record of invertebrate megafossils. Analogous to man-induced mass mortality in modern marine ecosystems, such depletions may appear as a “dead zone” between intervals with evidence of more diverse faunal communities (5, 6). This lowest level of observed biodiversity in the faunal extinction–recovery sequence is effected not only by definitive extinction of taxa, but also by temporary disappearances. Apart from successful emigration of motile animal species to refugia (2), this Lazarus effect also could reflect a strong reduction of population density to quantities that are below the detection level of the fossil record (7).
Land plants possess a number of features that yield both challenges and opportunities for the development of new insights into extinction dynamics at the time of “dead zone” formation. As a result of their immobility and restricted rate of dispersal, disappearance of plant taxa cannot be related to rapid emigration in the face of environmental disturbance. Refugia with suitable habitats for survival of plant taxa can develop only from progressive range fragmentation and range contraction of populations. Another feature of vegetation is its unique fossilization potential. Annually, land plants release huge amounts of pollen and spores. These can be transported readily by wind and water to a wide variety of terrestrial and marine depositional settings. Despite bias introduced by over- or underrepresentation, assemblages of dispersed pollen and spores broadly reflect the regional composition of terrestrial plant communities (8). In contrast to faunal records, therefore, successive pollen and spore assemblages from marine “dead zones” may provide the sample size needed to detect population and community responses to global ecological crisis. On the basis of palynological data from a “dead zone” in a Permian–Triassic (P-Tr) transition sequence from East Greenland, in this paper we document evidence of nonequilibrium vegetation dynamics resulting in selective but time-delayed extinctions among woody gymnosperms.
The End-Permian “Dead Zone”
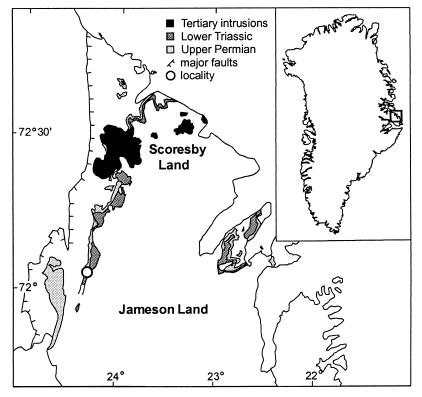
Latest Permian and earliest Triassic sediments in East Greenland (Fig. 1) are represented by the upper part of the Schuchert Dal Formation and the overlying Wordie Creek Formation. The predominantly fine-grained siliciclastic sediments of these formations were deposited in a narrow, elongate, shallow-marine basin. Rapid subsidence and high sedimentation rates produced one of the most expanded P-Tr sedimentary records in the world (10). At the margins of the basin, the P-Tr transition generally is marked by a hiatus, but in deepest parts of individual subbasins in southern Jameson Land, a complete sequence has been preserved (11).
Figure 1.
Locality map of Jameson Land and Scoresby Land, East Greenland, showing outcropping Late Permian and Early Triassic strata (modified after ref. 9).
In southern Jameson Land, distinctive Permian-type invertebrate macrofossils, including ammonoids (Paramexicoceras), solitary rugose corals, and brachiopods such as Martinia, disappear within the upper 5 m of the Schuchert Dal Formation (11). After a “dead zone,” macrofossils reappear 14 m above the base of the Wordie Creek Formation, when the bivalve Claraia appears and begins to dominate the low-diversity benthic macrofauna. Despite some possible Permian records (12), Claraia generally is regarded as an Early Triassic marker. After an 8-m sampling gap because of a lack of outcrop, a more diverse record starts at 23.5 m above the base of the Wordie Creek Formation. At this level, the fauna also contains the first unquestionable specimens of Hindeodus parvus, the conodont element that has been accepted recently to formally define the base of the Triassic at Meishan, China (ref. 12; International Union of Geological Sciences, http://www.iugs.org/iugs/news/excom01.htm). Despite the absence of macrofossils, the “dead zone” is by no means devoid of fauna. Some beds contain large amounts of very small (<1 mm) bivalves and gastropods. It is uncertain whether these are juveniles or dwarfed adults, but both possibilities may indicate stressed environmental conditions. In addition, there are monospecific blooms of the foraminifera Earlandia, similar to age-equivalent occurrences in other parts of the world (13). The carbon-isotope profile for carbonates displays relatively high δ13C values in the Schuchert Dal Formation that are followed by a significant decline in the basal part of the Wordie Creek Formation (11).
Palynological Analysis
A percentage diagram for selected spore and pollen categories from the P-Tr transition in Jameson Land is presented in Fig. 2. Information on the botanical affinity of the categories is summarized in Table 1. The pollen record of the upper part of the Schuchert Dal Formation reflects widespread, dense gymnosperm woodland. The abundance of Inaperturopollenites suggests a dominance of cordaites. The presence of a variety of pteridosperm taxa (particularly Peltaspermales) is evident from Vittatina, Weylandites, Striatoabieites, Protohaploxypinus, Lunatisporites, as well as the majority of alete bisaccoid pollen. The form-genera Scutasporites and Lueckisporites represent subordinate conifers. This vegetation type matches the general composition of the Late Permian Subangara flora, recognized on the basis of plant megafossil records from Eurasia (24). The low spore/pollen ratio confirms a limited role of herbaceous lycopsids and ferns. Although underrepresented in the percentage diagram, the consistent presence of fungal remains (Reduviasporonites) points to excessive decomposition of woody plant tissue (29, 30) and/or pathogenic fungal infection (14).
Figure 2.
Quantitative distribution pattern of selected spore and pollen types and carbon-isotope (δ13C) profile for carbonates (after ref. 11) from the P-Tr transition sequence in Jameson Land, East Greenland (for location, see Fig. 1). The marine macrofaunal “dead zone” embraces the interval between last appearances of the brachiopod Martinia and first appearances of the bivalve Claraia. Although the conodont element H. parvus is the first unquestionable indication of earliest Triassic age, the inception of Claraia may more accurately approximate the P-Tr boundary (11). Small, horizontal lines represent the position of palynological samples. Processed sample material is stored in the collection of the Laboratory of Palaeobotany and Palynology, Utrecht University. The recognized form-genera and other palynological categories typically reflect taxonomic diversity at generic and suprageneric levels; for botanical affinity of the types, see Table 1. Relative abundances are expressed as percentages of the total spore and pollen assemblages. Spore/pollen ratio represents counted number of spores of lycopsids, ferns, and bryophytes, divided by the total number of counted spores and pollen grains; cavate spore tetrads represent four spores. The diagram depicts the principal phases (a–e) of regional vegetation succession indicative of ecosystem collapse and initial recovery. a, decline of cordaite–pteridosperm woodland; b, proliferation of herbaceous lycopsids; c, establishment of diverse gymnosperm shrubland communities; d, renewed lycopsid proliferation; e, extinction of typical Late-Permian Subangaran gymnosperms.
Table 1.
Botanical affinity of principal Late Permian and Early Triassic spores and pollen from Greenland
| Spore/pollen category | Botanical affinity | Remarks |
|---|---|---|
| Reduviasporonites | Fungi | Hyphae and asexual fungal spores, in literature also known as Tympanicysta or Chordecystia. Morphologically similar to the extant anamorphic genus Rhizoctonia (14), which includes both ascomycetes and basidiomycetes (15). Although the wide morphological variation may include individual forms resembling vegetative Spirogyra cells (16), a suggested algal affinity is not in accordance with the highly resistant and nonfluorescent cell walls. |
| Maculatasporites | Bryophytes | The reticulate spores are alete or hilate, characters most frequently present in bryophyte spores (17). |
| Uvaesporites | Lycopsids (Selaginellales) | Lycopsid microspores. Similar forms have been found in situ in the Triassic/Jurassic fertile shoots of Selaginellites hallei (18, 19). |
| Densoisporites | Lycopsids (Isoetales, Selaginellales) | Although Densoisporites nejburgii is known to represent the microspores of Early Triassic Isoetales (Lycomeia rossica, Pleuromeia sternbergii; refs. 19–21), other forms (e.g., D. playfordii) are likely to represent Selaginellales (19). |
| Lundbladispora | Lycopsids (Isoetales, Selaginellales) | Although known from Early Triassic Isoetales, e.g., Isoetes beestoni (21), the form-genus may also include microspores with a selaginelloid affinity (19). |
| Cavate tetrads | Lycopsids (Isoetales, Selaginellales) | Lycopsid microspores belonging to Uvaesporites, Densoisporites, and Lundbladispora may sometimes retain a tetrad condition. In literature, such tetrads are also collectively known as Lapposisporites. |
| Rewanispora | Lycopsids (Selaginellales) | The cavate nature of the spores is consistent with a heterosporous lycopsid affinity, probably Selaginellales. |
| Other cavate spores | Lycopsids (Selaginellales) | A variety of cavate trilete spore types, occurring in subordinate amounts. The cavate nature of the spores is consistent with a heterosporous lycopsid affinity, probably Selaginellales. |
| Acavate trilete spores | Ferns | A wide variety of acavate trilete spore types, occurring in subordinate amounts. Forms are assignable to form-genera such as Punctatisporites, Scabratisporites, Cyclogranulatisporites, Anapiculatisporites, Apiculatisporites, Baculatisporites, and Verrucosisporites, characteristic of fossil fern taxa (19). |
| Inaperturopollenites | Cordaites | In literature, the form-genus has been related to the Araucariaceae. However, there are no Permian or Early Triassic records of this conifer family. Instead, in the Greenland material one may observe a strong similarity with the characteristic microverrucate exine structure of Cladaitina, pollen associated with the Ruffloria-type (Sub)Angaran cordaites (22, 23). |
| Alete bisaccoid pollen | Pteridosperms (Peltaspermales) | In general, Late Permian alete bisaccoid pollen is known from conifers and pteridosperms. In the Subangara flora, however, alete bisaccoid form-genera such as Alisporites and Falcisporites mainly represent peltasperms (23, 24). |
| Scutasporites | Conifers | Known from the Subangaran conifer cone Dvinostrobus sagittalis (23, 25). |
| Lueckisporites | Conifers (Majonicaceae) | Known from the Euramerican conifer Majonica alpina (26). |
| Striatoabieites Protohaploxypinus Lunatisporites | Pteridosperms (Peltaspermales) | Multitaeniate saccoid pollen assignable to form-genera such as Striatoabieites, Protohaploxypinus, and Lunatisporites is known from the Gondwana glossopterids, but in the Subangara flora these form-genera are associated exclusively with Late Permian peltasperms, e.g., Salpingocarpus bicornutus (23, 25). |
| Vittatina Weylandites | Pteridosperms (Peltaspermales) | In the Subangara flora, multitaeniate asaccate form-genera such as Vittatina and Weylandites are associated with Late Permian peltasperms, e.g., Permotheca (?) vittatinifera (23, 25). |
| Ephedripites | Gnetophytes | The form-genus corresponds morphologically and ultrastructurally to pollen of extant Welwitschia and Ephedra (27, 28). |
| Cycadopites | Cycads | Smooth monosulcate pollen is particularly known from Cycadales and Ginkgoales (19). Considering megafossil records, Late Permian forms from Greenland are likely to represent cycads. |
Parallel to the disappearance of marine invertebrate macrofauna, palynological data at the top of the Schuchert Dal Formation indicate a conspicuous dominance decline among woody plants (Fig. 2, phase a). Justified by the high spore/pollen ratio, data from the transition between the Schuchert Dal and Wordie Creek Formations reflect the rapid conversion to an open herbaceous vegetation (Fig. 2, phase b). Proliferation of a variety of cavate spore types demonstrates that plant communities are dominated by heterosporous lycopsids, both Selaginellales and Isoetales. It should be noted that a considerable part of the lycopsid microspores (Uvaesporites, Densoisporites, Lundbladispora) has retained a tetrad condition. Acavate spores and Maculatasporites indicate that ferns and bryophytes also play a prominent role. Subordinate pollen reflect a persistence of strongly reduced populations of most of the woody gymnosperm taxa.
In the next phase (Fig. 2, phase c), some of the residual woodland elements, notably pteridosperms, regain subdominance. In addition, the presence of Cycadopites and Ephedripites implies the appearance of other gymnosperm groups (cycads, gnetophytes) that were not present in the original communities. A subsequent rapid increase of spore/pollen ratios (Fig. 2, phase d) marks a renewed decline among woody elements. This decline is associated with the last, consistent occurrences of distinctive pollen types characteristic of late-Permian cordaites (Inaperturopollenites), pteridosperms (Vittatina, Weylandites), and conifers (Lueckisporites) (Fig. 2, phase e). The final disappearance of these Subangaran remnants approximates the end of the marine invertebrate “dead zone.”
End-Permian Floral Extinction Dynamics
The general trend in compositional changes between latest Permian and earliest Triassic palynological assemblages from East Greenland is consistent with (semi)quantitative information from many other parts of the world. In conjunction with the end-Permian ecological crisis, extinction among dominant gymnosperms appears to be a global event that dramatically affected terrestrial ecosystems. Justified by worldwide changes in vegetation structure and soil characteristics, as well as by strongly increased fungal activity, dieback of woody vegetation caused an unparalleled loss of standing biomass irrespective of floral provinciality and climatic zonation (29–34). However, because of the condensed or otherwise incomplete nature of most end-Permian palynological records, vegetation development at the climax of ecological crisis could be inferred only in very general terms. The successional changes in vegetation recorded in the expanded P-Tr section of East Greenland provide a more detailed picture of nonequilibrium dynamics that drove the degrading end-Permian terrestrial ecosystem over the resilience threshold, where ecosystem properties seek new equilibrium levels instead of returning to original conditions.
Already before the conversion from closed, competition-constrained woodland to open herbaceous vegetation, excessive fungal activity indicates destabilization of the terrestrial ecosystem. The succeeding ecosystem collapse (phases a and b) corresponds to a dramatic destruction and fragmentation of woodland habitat. Populations of competitive gymnosperms are decimated. The closed woodland becomes replaced by scattered trees and shrubs, whereas the vacated ecospace is colonized by opportunistic taxa that are capable of rapid population expansion into stressed environments (3) and thus profit from the ecological crisis. The herbaceous opportunists are dominated strongly by Selaginellales and Isoetales, already present as subordinate elements in the precrisis woodland environment, where they were largely suppressed by competition from woody gymnosperms. The role of heterosporous lycopsids is evidenced further by locally abundant megaspores as well as small vegetative remains of Selaginellales. In addition to the residual elements, however, stress-tolerant herbs also include some taxa not previously present.
Similar to other P-Tr palynological records in North America (35), Europe (36), Asia (37, 38), and Africa (39), dispersed microspores of heterosporous lycopsids are preserved regularly as unseparated tetrads that seem to be ineffective in reproduction. Also, corresponding megaspores can retain a tetrad condition. This striking phenomenon is likely to be a measure for chronic environmental stress that governs the nonequilibrium state of the ecosystem.
After ecosystem collapse and the initial opportunistic responses of a variety of herbs, open terrain becomes extensively recolonized by pteridosperm taxa that were common but nondominant in the precrisis woodland. On the other hand, the concomitant invasion of cycads and gnetophytes points to successful long-range migration. A southern source area for these groups is probable, because earlier records of Ephedra-like pollen are notably known from North America (40). Both residual and invader taxa may be regarded as ecological generalists, characterized by their ability to adjust to environmental change through phenotypic plasticity (3). Competitive Subangaran cordaites, pteridosperms, and conifers continue to exist as subordinate elements but qualify as “endangered species.” Herbaceous lycopsids, on the other hand, remain an important constituent of the resulting high-diversity shrubland communities.
Strong fluctuations in the relative dominance of both woody and herbaceous taxa imply that nonequilibrium dynamics characterize the newly established vegetation. Chronic environmental stress eventually results in renewed dieback of woody elements and a renewed proliferation of opportunistic lycopsids. This event approximates the end of the faunal “dead zone” at or close to the P-Tr boundary and marks the coup de grâce for the lingering Subangaran floral elements. It confirms the well documented, drastic loss of provinciality at the end of the Permian (41). The time-delayed extinction signals the onset of low-diversity, open shrubland vegetation, in which cosmopolitan lycopsid taxa continue to play a central role until full recovery of closed woodland ecosystems may occur at the transition between Early and Middle Triassic (42).
Time-Delayed Extinction
The P-Tr palynological record from East Greenland testifies that end-Permian floral extinction dynamics during formation of the marine “dead zone” is characterized by two unanticipated patterns: (i) there is a significant time lag between initiation of ecosystem collapse and selective extinction among gymnospermous taxa and (ii) there is an initial increase in regional plant diversity after ecosystem collapse, instead of a decrease.
Despite a more prolonged time scale, the observed pattern of time-delayed extinction of typical late-Permian plant taxa is remarkably compatible with model predictions, describing responses of multispecies metapopulations to progressive habitat destruction. These models indicate that habitat destruction can lead to an “extinction debt,” which is the delayed extinction of competitive species (43, 44). Extinction is preferential, in order of the best to the poorest competitors, as habitat destruction increases. As a consequence, species initially most abundant in undisturbed habitats are the most susceptible to eventual extinction. Similar to end-Permian vegetation development, surviving taxa display a strongly fluctuating pattern of relative abundances before a new equilibrium state is reached. As a direct result of a delayed extinction of competitive taxa, initial plant diversity is not affected significantly by environmental disturbance. Hence, any supplementary invasion of opportunistic herbs and/or generalistic shrubs would cause an increase in overall diversity of the mixed vegetation. In faunal extinction–recovery sequences, taxa that temporarily outlive the majority of their clade are known as “holdover taxa” (45), but their significance often is not emphasized.
The observed extinction delay falsifies current concepts that end-Permian extinctions are coincident with the negative shift in δ13C values for carbonates and sedimentary organic matter (46). This observation likely is a result of the condensed nature of many of the P-Tr transition sections in the Tethys realm, including the boundary stratotype at Meishan, South China. From the expanded record in East Greenland it is obvious that initiation of ecosystem collapse precedes the negative δ13C excursion, whereas plant extinctions take place long after the onset of the isotope event (Fig. 2; see also ref. 10). Through correlation with the Meishan section, the relative position of the δ13C decline and the P-Tr boundary constrain the actual duration of the end-Permian extinction delay. Radiometric dating at Meishan (47) suggests a time interval of ≈0.5 million years between the δ13C decline and the first appearance of H. parvus (46). Assuming a P-Tr boundary close to the inception of Claraia (10), and assuming uniform rates of siliciclastic sediment accumulation in the basal part of the Wordie Creek Formation, this figure would imply that the time lag between terrestrial ecosystem collapse and plant extinction had a duration of 0.5–0.6 million years.
Concluding Remarks
Because of its role in global biomass storage and its sensitivity to environmental change, land vegetation is one of the most obvious biota to be investigated for patterns of mass extinction, survival, and recovery. The present example suggests that current zoocentric concepts may be refined on the basis of quantitative palynological data sets from expanded P-Tr sections. End-Permian floral extinction dynamics indicates unexpected patterns that have not been recognized in faunal analyses. A search for “holdover taxa” and quantitative analysis of microfauna may reveal to what extent a prolonged “extinction-debt” effect and the related temporary diversity increase also are reflected in the marine faunal record.
Acknowledgments
We appreciate helpful discussion on the topic from participants in the International Geological Correlation Program Project (no. 335), “Biotic Recoveries from Mass Extinctions.” We thank Sydney Ash, Henk Brinkhuis, and Gene Mapes for constructive comments. This study was supported by the Council for Earth and Life Sciences of The Netherlands Organization for Scientific Research and the Natural Environment Research Council. This paper is Netherlands Research School of Sedimentary Geology publication no. 20010303 and University of Florida Contribution to Paleobiology publication no. 521.
Abbreviation
- P-Tr
Permian–Triassic
References
- 1.Kauffman E G, Erwin D H. Geotimes. 1995;14(3):14–17. [Google Scholar]
- 2.Kauffman E G, Harries P J. Geol Soc Sp Pap. 1996;102:15–39. [Google Scholar]
- 3.Harries P J, Kauffman E G, Hansen T A. Geol Soc Sp Pap. 1996;102:41–60. [Google Scholar]
- 4.Erwin D H. Trends Ecol Evol. 1998;13:344–349. doi: 10.1016/s0169-5347(98)01436-0. [DOI] [PubMed] [Google Scholar]
- 5.Harries P J, Kauffman E G. In: Extinction Events in Earth History. Kauffman E G, Walliser O H, editors. Berlin: Springer; 1990. pp. 277–298. [Google Scholar]
- 6.Håkansson E, Thomsen E. Palaeogeogr Palaeoclimatol Palaeoecol. 1999;154:67–85. [Google Scholar]
- 7.Wignall P B, Benton M J. J Geol Soc (London) 1999;156:453–456. [Google Scholar]
- 8.Traverse A. Paleopalynology. Boston: Unwin Hyman; 1988. [Google Scholar]
- 9.Piasecki S. Bull Geol Soc Denmark. 1984;32:139–144. [Google Scholar]
- 10.Stemmerik L, Christiansen F G, Piasecki S, Jordt B, Marcussen C, Nøhr-Hansen H. In: Arctic Geology and Petroleum Potential. Vorren T O, Bergsager E, Dahl-Stamnes Ø A, Holter E, Johansen B, Lie E, Lund T B, editors. Amsterdam: Elsevier; 1992. pp. 67–87. [Google Scholar]
- 11.Twitchett R J, Looy C V, Morante R, Visscher H, Wignall P B. Geology. 2001;29:351–354. [Google Scholar]
- 12.Yin H, Sweet W C, Glenister B F, Kotlyar G, Kozur H, Newell N D, Sheng J, Yang Z, Zakharov Y D. Newsl Stratigr. 1996;34:81–108. [Google Scholar]
- 13.Twitchett R J. Palaeogeogr Palaeoclimatol Palaeoecol. 1999;154:27–37. [Google Scholar]
- 14.Elsik W C. Palynology. 1999;23:35–41. [Google Scholar]
- 15.Hawskworth D L, Kirk P M, Sutton B C, Pegler D N. Dictionary of the Fungi. 8th Ed. Oxon, U.K.: CAB International; 1995. [Google Scholar]
- 16.Krassilov V A, Afonin S A, Barinova S S. Permophiles. 1999;35:16–17. [Google Scholar]
- 17.Playford G, Dettmann M E. In: Palynology: Principles and Applications. Jansonius J, McGregor D C, editors. Vol. 1. College Station, TX: Am. Assoc. of Stratigraphic Palynologists Foundation; 1996. pp. 227–260. [Google Scholar]
- 18.Lundblad B. Svensk Bot Tidskr. 1950;44:447–486. [Google Scholar]
- 19.Balme B E. Rev Palaeobot Palynol. 1995;87:81–323. [Google Scholar]
- 20.Yaroshenko O P. Paleontol Zh. 1985;19:110–117. [Google Scholar]
- 21.Retallack G J. J Paleontol. 1997;71:500–521. [Google Scholar]
- 22.Maheshwari H K, Meyen S V. Lethaia. 1975;8:103–123. [Google Scholar]
- 23.Gomankov A V, Meyen S V. Tr Geol Inst Akad Nauk SSSR. 1986;401:1–173. [Google Scholar]
- 24.Meyen S V. Biol Mem (India) 1982;7:1–109. [Google Scholar]
- 25.Meyen S V. Rev Palaeobot Palynol. 1997;96:351–447. [Google Scholar]
- 26.Clement-Westerhof J A. Rev Palaeobot Palynol. 1987;52:375–402. [Google Scholar]
- 27.Trevisan L. Pollen Spores. 1980;13:85–132. [Google Scholar]
- 28.Osborn J M, Taylor T N, De Lima M R. Rev Palaeobot Palynol. 1993;77:171–184. [Google Scholar]
- 29.Eshet Y, Rampino M R, Visscher H. Geology. 1995;23:967–970. [Google Scholar]
- 30.Visscher H, Brinkhuis H, Dilcher D L, Elsik W C, Eshet Y, Looy C V, Rampino M R, Traverse A. Proc Natl Acad Sci USA. 1996;93:2155–2158. doi: 10.1073/pnas.93.5.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Retallack G J. Science. 1995;267:77–80. doi: 10.1126/science.267.5194.77. [DOI] [PubMed] [Google Scholar]
- 32.Retallack G J. Geol Soc Am Bull. 1999;111:52–70. [Google Scholar]
- 33.Retallack G J, Veevers J J, Morante R. Geol Soc Am Bull. 1996;108:195–207. [Google Scholar]
- 34.Ward P D, Montgomery D R, Smith R. Science. 2000;289:1740–1743. doi: 10.1126/science.289.5485.1740. [DOI] [PubMed] [Google Scholar]
- 35.Utting J. Geol Surv Can Bull. 1994;478:1–107. [Google Scholar]
- 36.Haas J, Góczán F, Oravecz-Scheffer A, Barabás-Stuhl Á, Majoros G, Bérczi-Makk A. Mem Soc Geol It. 1986;34:221–241. [Google Scholar]
- 37.Tiwari R S, Meena K L. Palaeobotanist. 1988;37:210–214. [Google Scholar]
- 38.Shu O, Utting J. Rev Palaeobot Palynol. 1990;66:65–103. [Google Scholar]
- 39.Hankel O. Rev Palaeobot Palynol. 1992;72:129–147. [Google Scholar]
- 40.Wilson L R. Okla Geol Notes. 1962;22:91–96. [Google Scholar]
- 41.Yaroshenko O P. Paleontol Zh. 1997;31:168–177. [Google Scholar]
- 42.Looy C V, Brugman W A, Dilcher D L, Visscher H. Proc Natl Acad Sci USA. 1999;96:13857–13862. doi: 10.1073/pnas.96.24.13857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tillman D, May R M, Lehman C L, Nowak M A. Nature (London) 1994;371:65–66. [Google Scholar]
- 44.Loehle G, Li B L. Ecol Appl. 1996;6:784–789. [Google Scholar]
- 45.Hallam A, Wignall P B. Mass Extinctions and Their Aftermath. Oxford: Oxford Univ. Press; 1997. [Google Scholar]
- 46.Jin Y G, Wang Y, Wang W, Shang Q H, Cao C Q, Erwin D H. Science. 2000;289:432–436. doi: 10.1126/science.289.5478.432. [DOI] [PubMed] [Google Scholar]
- 47.Bowring S A, Erwin D H, Jin Y G, Martin M W, Davidek K, Wang W. Science. 1998;280:1039–1045. doi: 10.1126/science.280.5366.1039. [DOI] [PubMed] [Google Scholar]