Abstract
In a previous study, we demonstrated that human proximal tubular epithelial cells obtained from a commercial source metabolized extracellular 2′,3′-cAMP to 2′-AMP and 3′-AMP and extracellular 2′-AMP and 3′-AMP to adenosine (the extracellular 2′,3′-cAMP-adenosine pathway; extracellular 2′,3′-cAMP → 2′-AMP + 3′-AMP → adenosine). The purpose of this study was to investigate the metabolism of extracellular 2′,3′-cAMP in proximal tubular vs. thick ascending limb vs. collecting duct epithelial cells freshly isolated from their corresponding nephron segments obtained from rat kidneys. In epithelial cells from all three nephron segments, 1) extracellular 2′,3′-cAMP was metabolized to 2′-AMP and 3′-AMP, with 2′-AMP > 3′-AMP, 2) the metabolism of extracellular 2′,3′-cAMP to 2′-AMP and 3′-AMP was not inhibited by either 3-isobutyl-1-methylxanthine (phosphodiesterase inhibitor) or 1,3-dipropyl-8-p-sulfophenylxanthine (ecto-phosphodiesterase inhibitor), 3) extracellular 2′,3′-cAMP increased extracellular adenosine levels, 4) 3′-AMP and 2′-AMP were metabolized to adenosine with an efficiency similar to that of 5′-AMP, and 5) the metabolism of 5′-AMP, 3′-AMP, and 2′-AMP was not inhibited by α,β-methylene-adenosine-5′-diphosphate (CD73 inhibitor). These results support the conclusion that renal epithelial cells all along the nephron can metabolize extracellular 2′,3′-cAMP to 2′-AMP and 3′-AMP and can efficiently metabolize extracellular 2′-AMP and 3′-AMP to adenosine and that the metabolic enzymes involved are not the classical phosphodiesterases nor ecto-5′-nucleotidase (CD73). Because 2′,3′-cAMP is released by injury and because previous studies demonstrate that the extracellular 2′,3′-cAMP-adenosine pathway stimulates epithelial cell proliferation via adenosine A2B receptors, the present results suggest that the extracellular 2′,3′-cAMP-adenosine pathway may help restore epithelial cells along the nephron following kidney injury.
Keywords: 2′,3′-cAMP; 2′-AMP; 3′-AMP; proximal tubular epithelial cells; thick ascending limb epithelial cells; collecting duct epithelial cells
recent studies in isolated, perfused rat (14, 28) and mouse (11) kidneys demonstrate that kidneys can produce a positional isomer of 3′,5′-cAMP, namely 2′,3′-cAMP, and provide evidence for the concept that 2′,3′-cAMP derives from mRNA breakdown caused by energy depletion and renal injury (14). These studies also demonstrate that kidneys can release 2′,3′-cAMP into the extracellular space and metabolize extracellular 2′,3′-cAMP to 2′-AMP and 3′-AMP and can further metabolize extracellular 2′-AMP and 3′-AMP to adenosine (11, 14). Taken together, these investigations support the existence of a renal extracellular 2′,3′-cAMP-adenosine pathway (extracellular 2′,3′-cAMP → 2′-AMP + 3′-AMP → adenosine).
It is conceivable that adenosine generated by the 2′,3′-cAMP-adenosine pathway helps protect kidneys from acute injury by promoting the restoration of a healthy renal vascular architecture via preventing the overproliferation of renal vascular smooth muscle cells and glomerular mesangial cells, while stimulating the proliferation of renovascular endothelial cells. In this regard, recent studies demonstrate that preglomerular vascular smooth muscle cells, glomerular mesangial cells, and preglomerular vascular endothelial cells metabolize extracellular 2′,3′-cAMP to 2′-AMP and 3′-AMP and further metabolize these AMPs to adenosine (8, 13). Moreover, extracellular 2′,3′-cAMP inhibits the proliferation of preglomerular vascular smooth muscle cells and glomerular mesangial cells, while stimulating the proliferation of preglomerular vascular endothelial cells, and these effects are mediated via the production of adenosine that then activates adenosine A2B receptors (8, 13). Likewise, extracellular 2′-AMP and 3′-AMP inhibit proliferation of preglomerular vascular smooth muscle cells and glomerular mesangial cells and stimulate proliferation of preglomerular endothelial cells via adenosine acting on A2B receptors (8, 9).
It is also conceivable that adenosine generated by the 2′,3′-cAMP-adenosine pathway helps protect kidneys from injury by promoting the proliferation of tubular epithelial cells. Indeed, in human proximal tubular epithelial cells (commercial source), the extracellular 2′,3′-cAMP-adenosine pathway exists and strongly stimulates proliferation of these cells (8). However, it is unknown whether the 2′,3′-cAMP-adenosine pathway exists in primary cultures of proximal tubular epithelial cells and whether the pathway exists all along the nephron vs. only the proximal tubule. Therefore, in the present study, we cultured primary proximal, thick ascending limb and collecting duct renal epithelial cells from freshly isolated rat nephron segments and then studied the metabolism of extracellular 2′,3′-cAMP, 2′-AMP, and 3′-AMP in these cells.
METHODS
Cell culture.
Kidneys for isolation of tubular segments were obtained from adult, male Sprague-Dawley rats (Charles River, Wilmington, MA). The Institutional Animal Care and Use Committee approved all procedures. The investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996). Proximal tubules, thick ascending limbs, and collecting ducts were isolated using a Percoll solution centrifugation method previously described and validated in detail by us (10, 16). In this regard, the presence and absence of appropriate protein markers were used to validate the specificity of this isolation method (10, 16): bumetanide-sensitive cotransporter type 1 (marker specific for thick ascending limbs), thiazide-sensitive cotransporter (marker for distal tubules), aquaporin-2 (marker for collecting ducts), sodium-bicarbonate cotransporter type 1 (marker for proximal tubules), and sodium-hydrogen exchanger type 3 (also marker for proximal tubules). Cells were cultured from these isolated segments as previously described by us (10, 16). Briefly, freshly isolated specific tubular segments were washed in phosphate-buffered saline without calcium and magnesium and incubated for 15 min with collagenase type IV (1 mg/ml in 5 ml of DMEM F12) in a shaking water bath at 37°C. Ten milliliters of DMEM F12 with 10% fetal calf serum (FCS) were added, and the sample was centrifuged. Pellets were resuspended in DMEM F12 with 10% FCS, and 1 ml of the suspension was added to 75-cm2 flasks. Before cells were added, culture flasks were preconditioned by incubating with FCS for 30 min. The medium was changed after 2 days. After 4 days, the cells were detached with tyrpsin/EDTA, washed, and plated with DMEM F12 with 10% FCS. All experiments were performed in these cultures once the cells reached confluence (herein defined as “primary” cultures).
Metabolism experiments.
Cells were washed twice with HEPES-buffered Hanks balanced salt solution and then incubated for 1 h in 0.5 ml of Dulbecco's phosphate-buffered saline with HEPES (25 mmol/l) and NaHCO3 (13 mmol/l) in the presence and absence of 2′,3′-cAMP, 5′-AMP, 3′-AMP, or 2′-AMP with or without α,β-methylene-adenosine-5′-diphosphate (AMPCP; selective inhibitor of CD73) (32), 3-isobutyl-1-methylxanthine (IBMX; broad spectrum phosphodiesterase inhibitor) (2), 1,3-dipropyl-8-p-sulfophenylxanthine (DPSPX; ecto-phosphodiesterase inhibitor) (22, 29, 31), all from Sigma (St. Louis, MO). After 1-h incubation, the medium was collected, heated for 90 s at 100°C to denature enzymes, and then frozen at −80°C until assayed by liquid chromatography-tandem mass spectrometery (LC-MS/MS).
LC-MS/MS purine assay.
2′-AMP, 3′-AMP, 5′-AMP, and adenosine were obtained from Sigma. The internal standard (13C10-adenosine) was from Medical Isotopes (Pelham, NH). Purines were resolved by reversed-phase liquid chromatography (Agilent Zorbax eclipse XDB-C-18 column, 3.5-μm beads; 2.1 × 100 mm) and quantified using a triple quadrupole mass spectrometer (TSQ Quantum-Ultra, ThermoFisher Scientific, San Jose, CA) operating in the selected reaction monitoring mode with a heated electrospray ionization source as previously described in detail (14).
Statistics.
Results were analyzed statistically using the nonparametric Kruskal-Wallis one-way ANOVA on ranks test, with post hoc comparisons using the Kruskal-Wallis multiple-comparison Z-value test. The criterion of significance was P < 0.05. All values in text and figures are means ± SE.
RESULTS
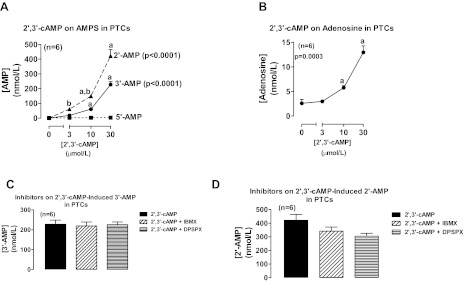
Incubation of rat proximal tubular epithelial cells with 2′,3′-cAMP significantly and concentration dependently increased medium levels of 2′-AMP and 3′-AMP, but not 5′-AMP, and the increase in 2′-AMP was significantly greater than the increase in 3′-AMP at 3 and 10 μmol/l of 2′,3′-cAMP (Fig. 1A). Also, 2′,3′-cAMP significantly and concentration dependently increased medium levels of adenosine (Fig. 1B). Neither IBMX (broad spectrum phosphodiesterase inhibitor) (2) nor DPSPX (ecto-phosphodiesterase inhibitor) (22, 29, 31) altered the metabolism of 2′,3′-cAMP to 3′-AMP (Fig. 1C) or 2′-AMP (Fig. 1D).
Fig. 1.
Line graphs illustrate the concentration-dependent effects of 2′,3′-cAMP on levels of 2′-AMP, 3′-AMP, and 5′-AMP (A) and adenosine (B) in the medium of cultures of rat proximal tubular cells (PTCs). Bar graphs illustrate the effects of 3-isobutyl-1-methylxanthine (IBMX; 1 mmol/l; broad spectrum phosphodiesterase inhibitor) and 1,3-dipropyl-8-p-sulfophenylxanthine (DPSPX; 1 mmol/l; ecto-phosphodiesterase inhibitor) on the metabolism of 2′,3′-cAMP to 3′-AMP (C) and 2′-AMP (D) in PTCs. P values in panels are from Kruskal-Wallis 1-way ANOVA on ranks. aP < 0.05 compared with basal (0) and bP < 0.05 comparing 2′-AMP levels with 3′-AMP levels at the indicated treatment concentration of 2′,3′-cAMP (Kruskal-Wallis multiple-comparison Z-value test).
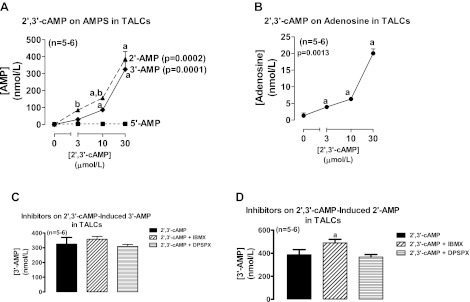
Incubation of rat thick ascending limb epithelial cells with 2′,3′-cAMP also significantly and concentration dependently increased medium levels of 2′-AMP and 3′-AMP, but not 5′-AMP, and again the increase in 2′-AMP was significantly greater than the increase in 3′-AMP at 3 and 10 μmol/l of 2′,3′-cAMP (Fig. 2A). In rat thick ascending limb epithelial cells, 2′,3′-cAMP significantly and concentration dependently increased medium levels of adenosine (Fig. 2B); but neither IBMX nor DPSPX inhibited the metabolism of 2′,3′-cAMP to 3′-AMP (Fig. 2C) or 2′-AMP (Fig. 2D).
Fig. 2.
Line graphs illustrate the concentration-dependent effects of 2′,3′-cAMP on levels of 2′-AMP, 3′-AMP, and 5′-AMP (A) and adenosine (B) in the medium of cultures of rat thick ascending limb cells (TALCs). Bar graphs illustrate the effects of IBMX (1 mmol/l; broad spectrum phosphodiesterase inhibitor) and DPSPX (1 mmol/l; ecto-phosphodiesterase inhibitor) on the metabolism of 2′,3′-cAMP to 3′-AMP (C) and 2′-AMP (D) in TALCs. P values in panels are from Kruskal-Wallis 1-way ANOVA on ranks. aP < 0.05 compared with basal (0) and bP < 0.05 comparing 2′-AMP levels with 3′-AMP levels at the indicated treatment concentration of 2′,3′-cAMP (Kruskal-Wallis multiple-comparison Z-value test).
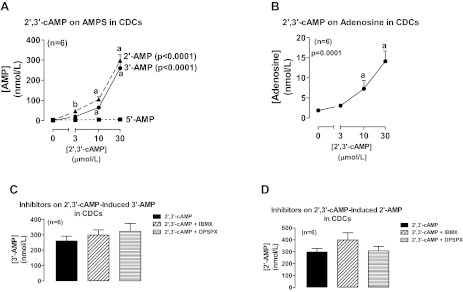
As with proximal tubular epithelial cells and thick ascending limb epithelial cells, incubation of rat collecting duct epithelial cells with 2′,3′-cAMP significantly and concentration dependently increased medium levels of 2′-AMP and 3′-AMP, but not 5′-AMP, and the increase in 2′-AMP was significantly greater than the increase in 3′-AMP at 3 μmol/l of 2′,3′-cAMP (Fig. 3A). In rat collecting duct epithelial cells, 2′,3′-cAMP significantly and concentration dependently increased medium levels of adenosine (Fig. 3B). Again, neither IBMX nor DPSPX altered the metabolism of 2′,3′-cAMP to 3′-AMP (Fig. 3C) or 2′-AMP (Fig. 3D).
Fig. 3.
Line graphs illustrate the concentration-dependent effects of 2′,3′-cAMP on levels of 2′-AMP, 3′-AMP, and 5′-AMP (A) and adenosine (B) in the medium of cultures of rat collecting duct cells (CDCs). Bar graphs illustrate the effects of IBMX (1 mmol/l; broad spectrum phosphodiesterase inhibitor) and DPSPX (1 mmol/l; ecto-phosphodiesterase inhibitor) on the metabolism of 2′,3′-cAMP to 3′-AMP (C) and 2′-AMP (D) in CDCs. P values in panels are from Kruskal-Wallis 1-way ANOVA on ranks. aP < 0.05 compared with basal (0) and bP < 0.05 comparing 2′-AMP levels with 3′-AMP levels at the indicated treatment concentration of 2′,3′-cAMP (Kruskal-Wallis multiple-comparison Z-value test).
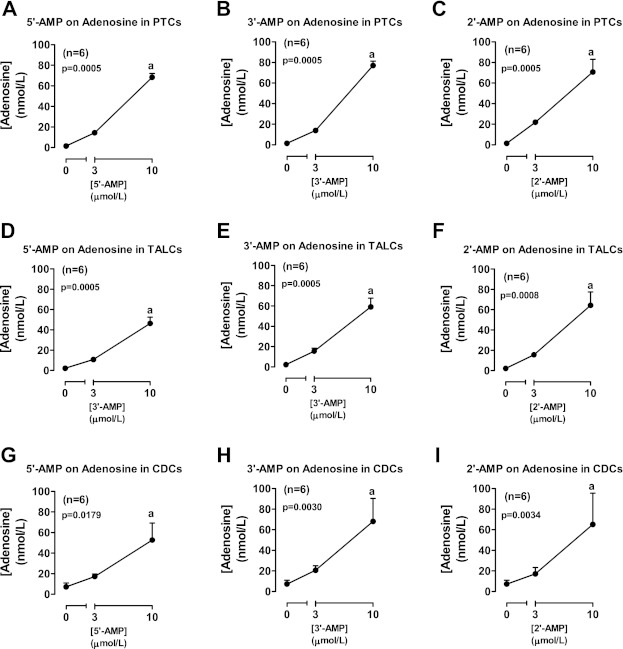
Incubation of rat proximal tubular epithelial cells with 5′-AMP (prototypical adenosine precursor; Fig. 4A), 3′-AMP (Fig. 4B), and 2′-AMP (Fig. 4C) significantly and concentration dependently increased levels of adenosine in the medium. Similar results were obtained when 5′-AMP, 3′-AMP, and 2′-AMP were incubated with rat thick ascending limb epithelial cells (Fig. 4, D, E, and F, respectively) or rat collecting duct epithelial cells (Fig. 4, G, H, and I, respectively). AMPCP (100 μmol/l; CD73 inhibitor) had no effect on the metabolism of 5′-AMP (Fig. 5A), 3′-AMP (Fig. 5B), or 2′-AMP (Fig. 5C) to adenosine.
Fig. 4.
Line graphs illustrate the concentration-dependent effects of 5′-AMP (A, D, G), 3′-AMP (B, E, H), and 2′-AMP (C, F, I) on levels of adenosine in the medium of cultures from rat PTCs (A, B, C), TALCs (D, E, F), and CDCs (G, H, I). P values in panels are from Kruskal-Wallis 1-way ANOVA on ranks. aP < 0.05 (Kruskal-Wallis multiple-comparison Z-value test) compared with basal (0).
Fig. 5.
Bar graphs illustrate the effects of α,β-methylene-adenosine-5′-diphosphate (AMPCP; 100 μmol/l; CD73 inhibitor) on adenosine levels in the medium of rat PTCs, TALCs, and CDCs incubated with 10 μmol/l of either 5′-AMP (A), 3′-AMP (B), or 2′-AMP (C).
DISCUSSION
An overwhelming body of solid evidence indicates that adenosine, an endogenous nucleoside, strongly protects against acute kidney injury (AKI) (1, 5, 7). In this regard, multiple mechanisms are likely involved with the participation of multiple adenosine receptors including A1 receptors (17–20, 27), A2A receptors (3, 23–26), and A2B receptors (6). Therefore, it is important to elucidate the mechanisms contributing to renal adenosine production and disposition. Interestingly, the increase in serum creatinine induced by renal ischemia-reperfusion injury is attenuated, rather than enhanced, in mice null for either CD39 or CD73 (21) and renal histopathology induced by ischemia-reperfusion injury is not changed in CD39 knockout mice and is actually improved in CD73 knockout mice (21). CD39 (converts ATP/ADP to 5′-AMP) and CD73 (converts 5′-AMP to adenosine) are thought to be the primary mediators of adenosine production. However, the findings that renal adenosine is protective yet knockdown of CD39 or CD73 does not worsen outcome suggest that alternative metabolic pathways are also important in forming adenosine during renal ischemia-reperfusion.
Our previous studies reveal that the 2′,3′-cAMP-adenosine pathway could exert biological effects via adenosine that should promote recovery of kidneys from AKI. In this regard, 2′,3′-cAMP, 2′-AMP, and 3′-AMP (via metabolism to adenosine and activation of A2B receptors) inhibit the proliferation of renal vascular smooth muscle cells and glomerular mesangial cells (9, 13), yet stimulate the proliferation of renovascular endothelial cells and renal epithelial cells (8). However, the concept that the 2′,3′-cAMP-adenosine pathway could be renoprotective would be even more compelling if it were the case that epithelial cells all along the nephron were capable of metabolizing 2′,3′-cAMP to 2′-AMP and 3′-AMP and capable of metabolizing 2′-AMP and 3′-AMP to adenosine. In this regard, the present study shows clearly that indeed epithelial cells in the proximal tubule, in the loop of Henle, and in the collecting duct can metabolize 2′,3′-cAMP to its corresponding AMPs and further metabolize the corresponding AMPs to adenosine. Therefore, the progrowth effects of the 2′,3′-cAMP-adenosine pathway could potentially promote epithelial regeneration all along the nephron.
The generation of extracellular AMPs from extracellular 2′,3′-cAMP potentially could involve two biochemical pathways: 1) generation of 2′-AMP via the action of ecto-2′,3′-cAMP-3′-phosphodiesterases hydrolyzing the 3′-phosphoester bond; 2) generation of 3′-AMP via the action of ecto-2′,3′-cAMP-2′-phosphodiesterases hydrolyzing the 2′-phosphoester bond. Interestingly, our previously published results indicate that in rat preglomerular vascular endothelial cells, production of 3′-AMP far exceeds production of 2′-AMP (8). In contrast, in human proximal tubular cells (commercial source), 2′-AMP production exceeds 3′-AMP biosynthesis (8). Thus, whether metabolism of 2′,3′-cAMP proceeds predominantly to 3′-AMP or 2′-AMP likely depends on the cell type and therefore the types of ecto-enzymes expressed. Consistent with our findings in human proximal tubular cells, in the present study 2′,3′-cAMP was metabolized more to 2′-AMP than 3′-AMP in all three types of renal epithelial cells. We conclude therefore that in renal epithelial cells, the dominant pathway for metabolism of extracellular 2′,3′-cAMP is conversion to 2′-AMP via ecto-2′,3′-cAMP-3′-phosphodiesterases.
In addition to the 2′,3′-cAMP-adenosine pathway, previous studies indicate that renal epithelial cells, from both proximal tubules and collecting ducts, express an extracellular 3′,5′-cAMP-adenosine pathway (extracellular 3′,5′-cAMP → 5′-AMP → adenosine) and that the ecto-3′,5′-cAMP-3′-phosphodiesterases that mediate this biochemical mechanism are inhibited by both IBMX and DPSPX (10, 16). The present studies demonstrate that unlike the extracellular 3′,5′-cAMP-adenosine pathway, the extracellular 2′,3′-cAMP-adenosine pathway is not inhibited by either IBMX or DPSPX, thus indicating a different set of ecto-enzymes mediating the two cAMP-adenosine pathways in renal epithelial cells.
Regarding the metabolism of 3′-AMP and 2′-AMP to adenosine, the present study demonstrates that conversion of these AMPs to adenosine in primary cultures of renal epithelial cells from rat proximal tubules, thick ascending limbs, and collecting ducts is as efficient as metabolism of 5′-AMP to adenosine, and the conversion of these AMPs to adenosine is not attenuated by the CD73 inhibitor AMPCP. This is consistent with our previous observations in human proximal tubular cells (8). Interestingly, in other cell types, including preglomerular vascular smooth muscle cells, glomerular mesangial cells, preglomerular vascular endothelial cells, aortic vascular smooth muscle cells, coronary artery vascular smooth muscle cells, microglia, and astrocytes, 5′-AMP generates more adenosine than do 3′-AMP or 2′-AMP (8, 9, 12, 13, 30). Also, in these other cell types, the conversion of 5′-AMP to adenosine is blocked by AMPCP, whereas the metabolism of 3′-AMP and 2′-AMP to adenosine is resistant to AMPCP (8, 9, 12, 13, 30). Thus, in renal epithelial cells, as opposed to all other examined cell types, the ecto-nucleotidase that metabolizes 5′-AMP is not CD73. Moreover, because 5′-AMP, 3′-AMP, and 2′-AMP are similarly efficacious with regard to generating adenosine in renal epithelial cells, the implication is that in renal epithelial cells 3′-AMP and 2′-AMP could be as important as 5′-AMP as precursors of adenosine.
An important issue is whether the results of our findings in epithelial cells isolated from rat kidneys can be extrapolated to human renal epithelial cells. Inasmuch as the results of the present study in rat proximal tubular epithelial cells are similar to our recent findings in human proximal tubular epithelial cells obtained from a commercial source (8), it seems likely that the results of the present study can be extended to human renal epithelial cells.
Hypoxia stimulates the adenosine system by increasing extracellular adenosine synthesis, by inhibiting the disposition of extracellular adenosine, and by increasing the expression of adenosine receptors (4). Since the 2′,3′-cAMP-adenosine pathway may protect against AKI and AKI is associated with renal hypoxia, it is conceivable that hypoxia may activate enzymes involved in the metabolism of 2′,3′-cAMP, and this hypothesis merits investigation.
In summary, the present results indicate that renal epithelial cells in the proximal tubule, thick ascending limb, and collecting duct can metabolize 2′,3′-cAMP to 2′-AMP and 3′-AMP and can convert 2′-AMP and 3′-AMP to adenosine. Combining this information with our previous findings that the extracellular 2′,3′-cAMP-adenosine pathway can stimulate the proliferation of renal epithelial cells and renovascular endothelial cells, while inhibiting the growth of renovascular smooth muscle cells and glomerular mesangial cells, suggests that the extracellular 2′,3′-cAMP-adenosine pathway (which is activated by renal injury) may be important in determining renal outcomes following AKI. Definitive testing of this hypothesis must await identification of the enzymes involved in the extracellular 2′,3′-cAMP-adenosine pathway so that renal outcomes following renal injury can be compared in animals with and without an intact extracellular 2′,3′-cAMP-adenosine pathway.
GRANTS
This study was supported by National Institutes of Health Grants DK091190, HL109002, HL069846, DK068575, and DK079307.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: E.K.J. conception and design of research; E.K.J. and D.G.G. analyzed data; E.K.J. interpreted results of experiments; E.K.J. prepared figures; E.K.J. drafted manuscript; E.K.J. and D.G.G. edited and revised manuscript; E.K.J. and D.G.G. approved final version of manuscript; D.G.G. performed experiments.
REFERENCES
- 1.Bauerle JD, Grenz A, Kim JH, Lee HT, Eltzschig HK. Adenosine generation and signaling during acute kidney injury. J Am Soc Nephrol 22: 14–20, 2011 [DOI] [PubMed] [Google Scholar]
- 2.Beavo JA, Reifsnyder DH. Primary sequence of cyclic nucleotide phosphodiesterase isozymes and the design of selective inhibitors. Trends Pharmacol Sci 11: 150–155, 1990 [DOI] [PubMed] [Google Scholar]
- 3.Day YJ, Huang L, Ye H, Linden J, Okusa MD. Renal ischemia-reperfusion injury and adenosine 2A receptor-mediated tissue protection: role of macrophages. Am J Physiol Renal Physiol 288: F722–F731, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Eltzschig HK, Carmeliet P. Hypoxia and inflammation. N Engl J Med 364: 656–665, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grenz A, Bauerle JD, Dalton JH, Ridyard D, Badulak A, Tak E, McNamee EN, Clambey E, Moldovan R, Reyes G, Klawitter J, Ambler K, Magee K, Christians U, Brodsky KS, Ravid K, Choi DS, Wen J, Lukashev D, Blackburn MR, Osswald H, Coe IR, Nurnberg B, Haase VH, Xia Y, Sitkovsky M, Eltzschig HK. Equilibrative nucleoside transporter 1 (ENT1) regulates postischemic blood flow during acute kidney injury in mice. J Clin Invest 122: 693–710, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Grenz A, Osswald H, Eckle T, Yang D, Zhang H, Tran ZV, Klingel K, Ravid K, Eltzschig HK. The reno-vascular A2B adenosine receptor protects the kidney from ischemia. PLoS Med 5: e137, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grenz A, Zhang H, Hermes M, Eckle T, Klingel K, Huang DY, Muller CE, Robson SC, Osswald H, Eltzschig HK. Contribution of E-NTPDase1 (CD39) to renal protection from ischemia-reperfusion injury. FASEB J 21: 2863–2873, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Jackson EK, Gillespie DG. Extracellular 2′,3′-cAMP and 3′,5′-cAMP stimulate proliferation of preglomerular vascular endothelial cells and renal epithelial cells. Am J Physiol Renal Physiol 303: F954–F962, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jackson EK, Gillespie DG, Dubey RK. 2′-AMP and 3′-AMP inhibit proliferation of preglomerular vascular smooth muscle cells and glomerular mesangial cells via A2B receptors. J Pharmacol Exp Ther 337: 444–450, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jackson EK, Mi Z, Zhu C, Dubey RK. Adenosine biosynthesis in the collecting duct. J Pharmacol Exp Ther 307: 888–896, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Jackson EK, Ren J, Cheng D, Mi Z. Extracellular cAMP-adenosine pathways in the mouse kidney. Am J Physiol Renal Physiol 301: F565–F573, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jackson EK, Ren J, Gillespie DG. 2′,3′-cAMP, 3′-AMP and 2′-AMP inhibit human aortic and coronary vascular smooth muscle cell proliferation via A2B receptors. Am J Physiol Heart Circ Physiol 301: H391–H401, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jackson EK, Ren J, Gillespie DG, Dubey RK. Extracellular 2′,3′-cyclic adenosine 5′-monophosphate is a potent inhibitor of preglomerular vascular smooth muscle cell and mesangial cell growth. Hypertension 56: 151–158, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jackson EK, Ren J, Mi Z. Extracellular 2′,3′-cAMP is a source of adenosine. J Biol Chem 284: 33097–33106, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jackson EK, Zacharia LC, Zhang M, Gillespie DG, Zhu C, Dubey RK. cAMP-adenosine pathway in the proximal tubule. J Pharmacol Exp Ther 317: 1219–1229, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Kim M, Chen SWC, Park SW, Kim M, D'Agati VD, Yang J, Lee HT. Kidney-specific reconstitution of the A1 adenosine receptor in A1 adenosine receptor knockout mice reduces renal ischemia-reperfusion injury. Kidney Int 75: 809–823, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee HT, Emala CW. Adenosine attenuates oxidant injury in human proximal tubular cells via A1 and A2a adenosine receptors. Am J Physiol Renal Physiol 282: F844–F852, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Lee HT, Emala CW. Protective effects of renal ischemic preconditioning and adenosine pretreatment: role of A1 and A3 receptors. Am J Physiol Renal Physiol 278: F380–F387, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Lee HT, Kim M, Jan M, Penn RB, Emala CW. Renal tubule necrosis and apoptosis modulation by A1 adenosine receptor expression. Kidney Int 71: 1249–1261, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Lu B, Rajakumar SV, Robson SC, Lee EKF, Crikis S, d′Apice AJF, Cowan PJ, Dwyer KM. The impact of purinergic signaling on renal ischemia-reperfusion injury. Transplantation 86: 1707–1712, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Mi Z, Jackson EK. Metabolism of exogenous cyclic AMP to adenosine in the rat kidney. J Pharmacol Exp Ther 273: 728–733, 1995 [PubMed] [Google Scholar]
- 23.Okusa MD. A2A adenosine receptor: a novel therapeutic target in renal disease. Am J Physiol Renal Physiol 282: F10–F18, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Okusa MD, Linden J, Huang L, Rieger JM, Macdonald TL, Huynh LP. A2A adenosine receptor-mediated inhibition of renal injury and neutrophil adhesion. Am J Physiol Renal Physiol 279: F809–F818, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Okusa MD, Linden J, Huang L, Rosin DL, Smith DF, Sullivan G. Enhanced protection from renal ischemia-reperfusion injury with A2A-adenosine receptor activation and PDE 4 inhibition. Kidney Int 59: 2114–2125, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Okusa MD, Linden J, Macdonald T, Huang L. Selective A2A adenosine receptor activation reduces ischemia-reperfusion injury in rat kidney. Am J Physiol Renal Physiol 277: F404–F412, 1999 [DOI] [PubMed] [Google Scholar]
- 27.Park SW, Kim JY, Ham A, Brown KM, Kim M, D'Agati VD, Lee HT. A1 adenosine receptor allosteric enhancer PD-81723 protects against renal ischemia-reperfusion injury. Am J Physiol Renal Physiol 303: F721–F732, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ren J, Mi Z, Stewart NA, Jackson EK. Identification and quantification of 2′,3′-cAMP release by the kidney. J Pharmacol Exp Ther 328: 855–865, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tofovic SP, Branch KR, Oliver RD, Magee WD, Jackson EK. Caffeine potentiates vasodilator-induced renin release. J Pharmacol Exp Ther 256: 850–860, 1991 [PubMed] [Google Scholar]
- 30.Verrier JD, Exo JL, Jackson TC, Ren J, Gillespie DG, Dubey RK, Kochanek PM, Jackson EK. Expression of the 2′,3′-cAMP-adenosine pathway in astrocytes and microglia. J Neurochem 118: 979–987, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zacher LA, Carey GB. cAMP metabolism by swine adipocyte microsomal and plasma membranes. Comp Biochem Physiol B Biochem Mol Biol 124: 61–71, 1999 [DOI] [PubMed] [Google Scholar]
- 32.Zimmermann H. 5′-Nucleotidase: molecular structure and functional aspects. Biochem J 285: 345–365, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]