Abstract
PGE2 is a natriuretic factor whose production is elevated after water deprivation (WD) but its role in dehydration natriuresis is not well-defined. The goal of the present study was to investigate the role of microsomal prostaglandin E synthase-1 (mPGES-1) in dehydration natriuresis. After 24-h WD, wild-type (WT) mice exhibited a significant increase in 24-h urinary Na+ excretion accompanied with normal plasma Na+ concentration and osmolality. In contrast, WD-induced elevation of urinary Na+ excretion was completely abolished in mPGES-1 knockout (KO) mice in parallel with increased plasma Na+ concentration and a trend increase in plasma osmolality. WD induced a 1.8-fold increase in urinary PGE2 output and a 1.6-fold increase in PGE2 content in the renal medulla of WT mice, both of which were completely abolished by mPGES-1 deletion. Similar patterns of changes were observed for urinary nitrate/nitrite and cGMP. The natriuresis in dehydrated WT mice was associated with a significant downregulation of renal medullary epithelial Na channel-α mRNA and protein, contrasting to unaltered expressions in dehydrated KO mice. By quantitative RT-PCR, WD increased the endothelial nitric oxide synthase (eNOS), inducible NOS, and neuronal NOS expressions in the renal medulla of WT mice by 3.9-, 1.48-, and 2.6-fold, respectively, all of which were significantly blocked in mPGES-1 KO mice. The regulation of eNOS expression was further confirmed by immunoblotting. Taken together, our results suggest that mPGES-1-derived PGE2 contributes to dehydration natriuresis likely via NO/cGMP.
Keywords: mPGES-1, PGE2, dehydration natriuresis, cGMP, nitric oxide
dehydration leads to the depletion of plasma volume and the increase of body fluid osmolality. Such changes trigger the homeostatic response to conserve the fluids through the thirst mechanism, vasopressin release, and potentiated renal reabsorption. During the early stage of dehydration, an important physiological response is an acute increase in urinary Na+ excretion, a phenomenon termed dehydration natriuresis. This response will help prevent the further rise of plasma Na+ concentration and extracellular tonicity. Dehydration natriuresis has been demonstrated in humans as well as in other mammalian species, including dog, sheep, rat, and mouse (1, 7, 18, 28, 31, 33, 36, 37). A number of factors such as neurohypophysial hormones and serum- and glucocorticoid-inducible kinase (Sgk1) are implicated in mediating dehydration natriuresis but the precise mechanism remains unclear.
PGE2 is a major prostanoid in the kidney and possesses natriuretic property owing to its ability to inhibit Na+ transport in the distal nephron (3, 6, 39, 43). The elevation of urinary PGE2 in response to dehydration accompanied with increased renal medullary cyclooxygenase (COX)-2 expression has also been well-demonstrated (29, 41, 47). However, the functional implication of dehydration-induced renal PGE2 synthesis is still incompletely understood.
To date, three PGE synthases (PGES) have been cloned, including microsomal PGES-1 (mPGES-1), microsomal PGES (mPGES-2), and cytosolic PGES (cPGES), of which mPGES-1 is the best characterized PGES (19, 22, 24, 25, 38). Within the kidney, mPGES-1 is most abundantly expressed in the collecting duct (12), an important nephron site for both production and action of PGE2. A series of studies from our group reveal an important role of mPGES-1-derived PGE2 in promoting urinary Na+ excretion under various conditions of extracellular volume expansion (21, 22, 24). The goal of the present study was to employ mPGES-1 knockout (KO) mice to investigate mPGES-1-derived PGE2 in dehydration natriuresis.
METHODS
Animals.
mPGES-1 wild-type (WT) and KO mice were originally generated by Trebino et al. (44). This mouse colony was propagated at the University of Utah and maintained on a mixed DBA/1lacJxC57/BL6x129/Sv background. In all studies, 3- to 4-mo-old male mice were used. All protocols employing mice were reviewed and approved by the University of Utah Institutional Animal Care and Use Committee.
Establishment of dehydration natriuresis mouse model.
Male mPGES-1 WT and KO mice were subjected to the 24-h water deprivation (WD) by removing the water bottles from the metabolic cages (Hatteras Instruments). The control groups of animals had free access to water and standard food, while the WD groups were provided only with standard food. Twenty-four-hour urine was collected. After the urine collection, mice were killed and the blood and kidneys were harvested. The urine electrolytes excretion, plasma osmolality, and Na+ concentration were measured. Kidney tissues were assayed for PGE2 content or subjected to quantitative (q)RT-PCR analysis of gene expression.
Enzyme immunoassay.
Urine samples were centrifuged for 5 min at 10,000 rpm and diluted 1:1 with enzyme immunoassay buffer. Concentrations of urinary and kidney tissue PGE2 were determined by enzyme immunoassay (Cayman Chemical, Ann Arbor, MI) according to the manufacturer's instructions. The urine cGMP (Cayman Chemical) and nitrate/nitrite (NOx; Cayman Chemical) levels were also determined following the manufacturer's instructions.
qRT-PCR.
Total RNA was isolated from renal tissues using TRIzol. One microgram of total RNA was denatured at 65°C for 5 min, and cDNA synthesis was then performed at 42°C for 1 h using Superscript reverse transcriptase (BRL, Gaithersburg, MD). Oligonucleotides were designed using Primer3 software (available at http://frodo.wi.mit.edu/). The sequences of the oligonucleotide primers in the public sequence are as shown in the Table 1. Quantitative (q) PCR amplification was performed using the SYBR Green Master Mix (Applied Biosystems) and the Prism 7500 Real-Time PCR Detection System (Applied Biosystems). Cycling conditions were 95°C for 10 min, followed by 40 repeats of 95°C for 15 s and 60°C for 1 min.
Table 1.
Sequences of primers for real-time PCR
| Gene | Primer Sequence | Accession Number |
|---|---|---|
| β-Actin | 5′-GCTCTGGCTCCTAGCACCAT-3′ | NM_007393 |
| 5′-GCCACCGATCCACACAGAGT-3′ | ||
| α-ENaC | 5′-GCTTCATCTTTACCTGTCGTTTC-3′ | NM_011324 |
| 5′-CCAGAGATTGGAGTTGTTCTTGT-3′ | ||
| β-ENaC | 5′-CAGTGGGGAGTCTTCATCC-3′ | NM_011325 |
| 5′-TCCTGGTGGTGTTGCTGT-3′ | ||
| γ-ENaC | 5′-CTGCTTCTTCGATGGGATG-3′ | NM_011236 |
| 5′-GACACCAGGAAGGGGTTGT-3′ | ||
| nNOS | 5′-CAGCCAAAGCAGAGATGAAA-3′ | D14552 |
| 5′-ATACGGGTTGTTGAGGACCA-3′ | ||
| iNOS | 5′-ACTGTGTGCCTGGAGGTTCT-3′ | NM_010927 |
| 5′-TCTCTGCCTATCCGTCTCGT-3′ | ||
| eNOS | 5′-GAGAGCGAGCTGGTGTTTG-3′ | NM_008713 |
| 5′-TGATGGCTGAACGAAGATTG-3′ |
ENaC, epithelial Na channel; nNOS, neuronal nitric oxide synthase; iNOS, inducible NOS; eNOS, endothelial NOS.
Immunoblotting.
Lysates of the kidney medullary tissue were stored at −80°C until assayed. Protein concentrations were determined using a Coomassie reagent. An equal amount of the whole tissue protein was denatured at 100°C for 10 min, separated by SDS-PAGE, and transferred to nitrocellulose membranes. The blots were blocked overnight with 5% nonfat dry milk in Tris-buffered saline (TBS), followed by incubation for 1 h with mouse polyclonal antibodies against endothelial nitric oxide synthase (eNOS; BD Transduction Laboratories, cat. no. 610297), epithelial Na channel-α (ENaC-α; StressMarq, cat. no. SPC-403D), and mouse monoclonal anti-β-actin (Sigma, cat. no. A1978). The blots were washed with TBS followed by incubation with goat anti-mouse horseradish peroxidase-conjugated secondary antibody for eNOS and β-actin. Immune complexes were detected using enhanced chemiluminescence methods. The immunoreactive bands were quantified using the Gel and Graph Digitizing System (Silk Scientific).
Data analysis.
Data are summarized as means ± SE. Statistical analysis was performed using one-way ANOVA or Student's t-test as appropriate. P < 0.05 was considered statistically significant.
RESULTS
Effect of mPGES-1 deletion on dehydration-induced natriuretic response.
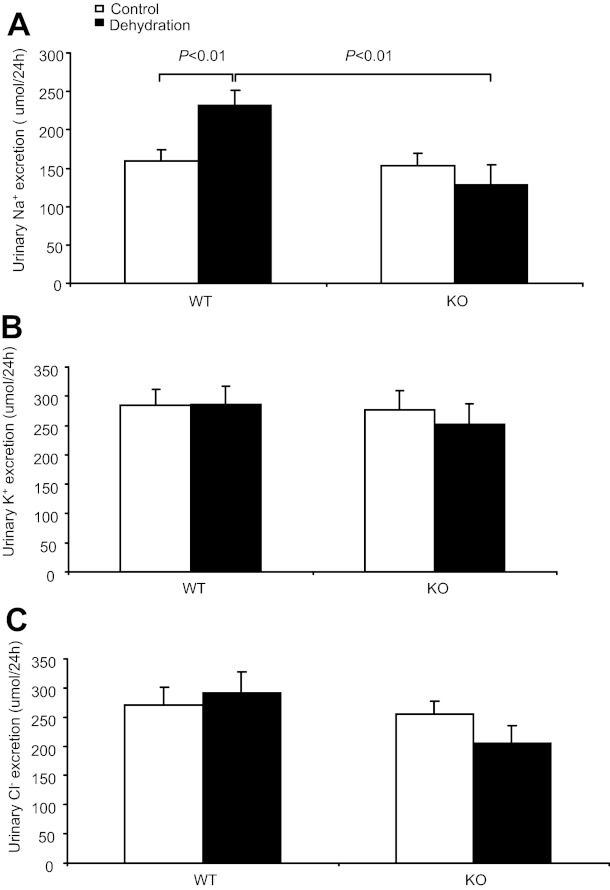
Dehydrated WT mice had increased urine Na+ (231.2 ± 19.8 vs. 159.2 ± 15.6 μmol/24 h, P < 0.01; Fig. 1A) and unaffected urine K+ (285.4 ± 31.7 vs. 284.1 ± 27.7 μmol/24 h, P > 0.05) and urine Cl− (290.98 ± 37.0 vs. 274.7 ± 30.3 μmol/24 h, P > 0.05) excretion (Fig. 1, B and C). However, in response to WD, the increase in urine Na+ output in the KO mice was completely blocked (128.6 ± 25.9 vs. 152.6 ± 17.3 μmol/24 h, P > 0.05; Fig. 1A), indicating the blunted dehydration natriuresis. WD reduced urine volume (0.72 ± 0.16 vs. 0.97 ± 0.1 ml, P < 0.05, n = 14–15) and elevated urine osmolality (2,658.1 ± 304.7 vs. 1,916.0 ± 157.1 mosmol/kgH2O, P < 0.01, n = 14–15) in WT mice. At baseline, neither urine volume nor urine osmoality was different between WT and KO strains. In contrast, in response to WD, the KO mice exhibited a smaller urine volume (0.4 ± 0.1 ml, P < 0.01 vs. WT/WD, n = 13) and higher urine osmolality (3,603.1 ± 180.7 mosmol/kgH2O, P < 0.01 vs. WT/WD, n = 13), suggesting enhanced urine concentrating ability.
Fig. 1.
Effect of microsomal PGE synthases (mPGES)-1 deletion on dehydration-induced natriuresis. A: 24-h urinary Na+ excretion. B: 24-h urinary K+ excretion. C: urinary Cl− excretion. Control group: n = 18–20. Dehydration group: n = 22–30. Data are means ± SE. WT, wild-type; KO, knockout.
Effect of mPGES-1 deletion on plasma sodium concentration and plasma osmolality after WD.
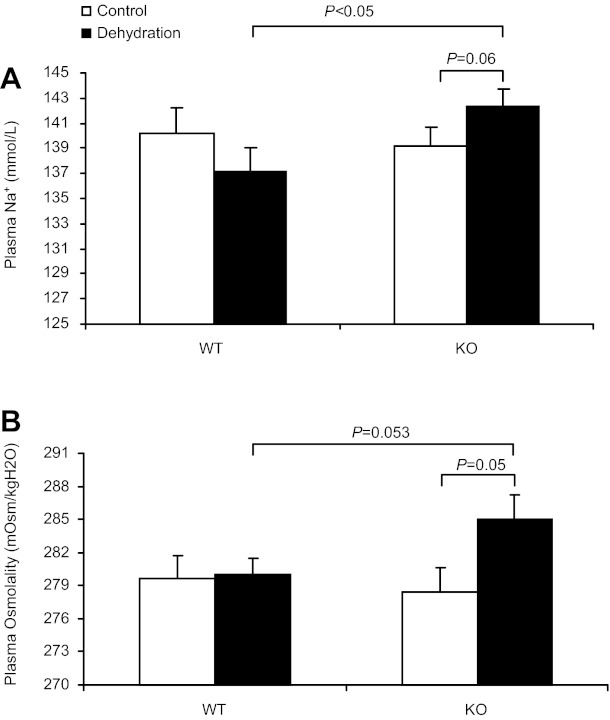
Impaired dehydration natriuresis may lead to hypernatremia and increased plasma osmolality. We therefore measured plasma Na+ concentration and osmolality in both WT and KO mice after 24-h WD. Indeed, dehydrated mPGES-1 KO mice displayed a significantly higher plasma Na+ concentration (KO/WD 142.3 ± 1.42 vs. WT/WD 137.1 ± 1.9 mmol/l, P < 0.05; Fig. 2A) and a trend increase of plasma osmolality (KO/WD 284.0 ± 2.34 vs. WT/WD 280.0 ± 1.5 mosmol/kgH2O, P = 0.053; Fig. 2B). It is likely that elevated plasma Na+ concentration and osmolality in dehydrated mPGES-1 KO mice are the direct consequence of impaired natriuretic response.
Fig. 2.
Effect of mPGES-1 deletion on plasma Na+ concentration and plasma osmolality after dehydration. A: plasma Na+ concentration. B: plasma osmolality. Control: n = 6–7. Dehydration: n = 7–9. Data are means ± SE.
Effects of mPGES-1 deletion on dehydration-induced renal PGE2 production.
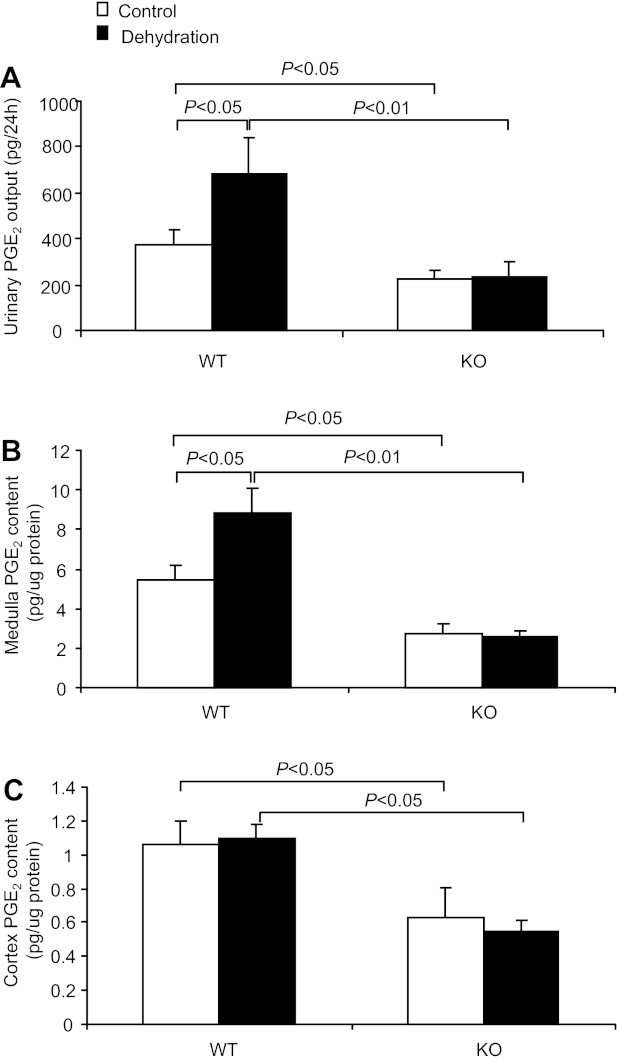
To evaluate mPGES-1 as a potential source of dehydration-induced renal PGE2 synthesis, we examined urinary PGE2 excretion and tissue PGE2 content in mPGES-1 WT and KO mice after 24-h WD. WD in WT mice significantly increased urinary PGE2 excretion (685.95 ± 158.8 vs. 376.0 ± 66.3 pg/24 h, P < 0.05; Fig. 3A) and this increase was absent in mPGES-1 KO mice (236.9 ± 58.1 vs. 221.8 ± 39.8 pg/24 h, P > 0.05; Fig. 3A). Interestingly, dehydration-induced increases in tissue PGE2 content in WT mice were observed in the renal medulla (8.87 ± 1.2 vs. 5.45 ± 0.7 pg/μg protein, P < 0.05; Fig. 3B) but not in the renal cortex (1.1 ± 0.08 vs. 1.06 ± 0.14, P > 0.05; Fig. 3C). In contrast, the medullary induction of PGE2 production was completely blocked in the KO mice (Fig. 3B). All these findings demonstrated that mPGES-1 is the predominant enzyme source of dehydration-induced renal PGE2 synthesis.
Fig. 3.
Effect of mPGES-1 deletion on dehydration-induced renal PGE2 synthesis. A: 24-h urinary PGE2 excretion. Control group: n = 7–8. Dehydration group: n = 11–13. B: PGE2 content in the renal medulla; n = 6–9 per group. C: PGE2 content in the renal cortex; n = 6–9 per group. Data are means ± SE.
Effect of mPGES-1 deletion on urinary NOx and cGMP excretion.
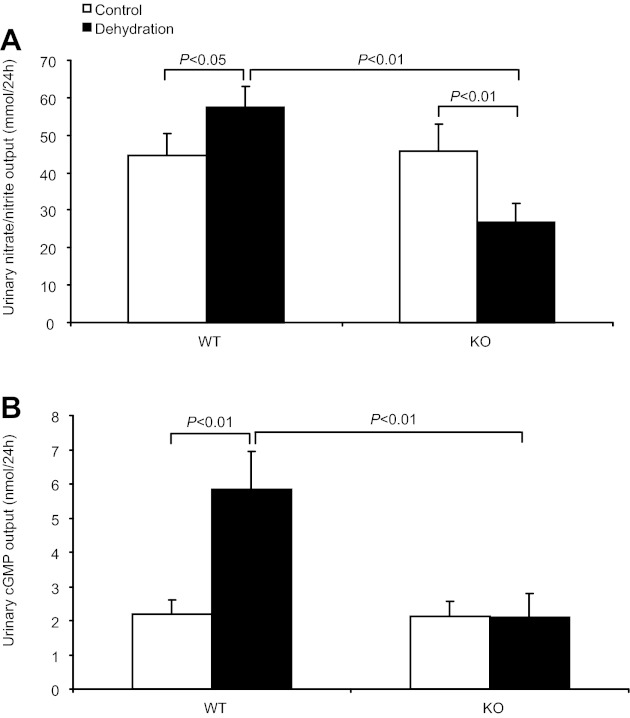
It is known that nitric oxide and cGMP play a very important role in mediating the natriuretic response under various physiological and pathological conditions. Moreover, we demonstrated the suppressed urinary nitric oxide and cGMP excretion in mPGES-1 KO mice when challenged with high-salt loading (24) or DOCA-salt (22). In the present study, 24-h WD slightly elevated urinary NOx excretion (57.5 ± 5.6 vs. 44.74 ± 5.5 mmol/24 h, P < 0.05; Fig. 4A) in WT mice. In contrast, mPGES-1 deletion significantly reduced urinary NOx excretion (26.8 ± 4.9 vs. 45.6 ± 7.5 mmol/24 h, P < 0.01; Fig. 4A) after 24-h WD. Similar results were observed for urinary cGMP excretion (Fig. 4B). These data suggested that mPGES-1-derived PGE2 mediated dehydration natriuresis possibly through nitric oxide and cGMP.
Fig. 4.
Effect of mPGES-1 deletion on urinary nitrate/nitrite (NOx) and cGMP excretion. A: 24-h urinary NOx excretion. Control: n = 21–25. Dehydration: n = 29–30. B: 24-h urinary cGMP excretion. Control: n = 16–17. Dehydration: n = 20–22. Data are means ± SE.
Effect of mPGES-1 deletion on renal ENaC expression after WD.
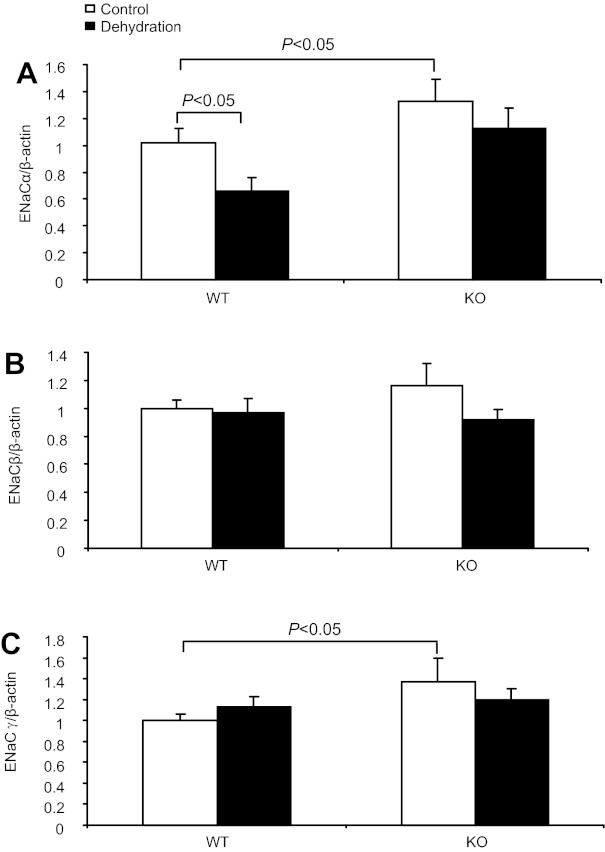
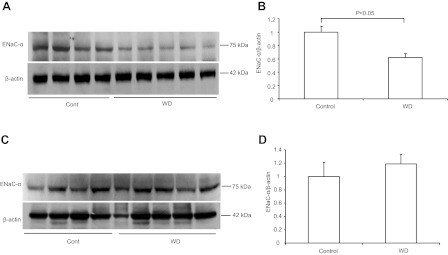
To test the possibility that PGE2 may promote Na+ excretion by inhibiting ENaC expression in the distal nephron, we examined the regulation of mRNA expression of the three ENaC subunits in the two genotypes after WD. By qRT-PCR, 24-h WD induced a 34% reduction of ENaC-α mRNA in WT mice, which was completely blocked in the KO mice (Fig. 5A). By Western blotting, WD resulted in a reduction of ENaC-α protein by 40% in WT mice (P < 0.05; Fig. 6, A and B), but without an effect in KO mice (Fig. 6, C and D). In contrast, renal mRNA expression of ENaC-β or -γ was unaffected by WD, irrespective of the genotype (Fig. 5, B and C).
Fig. 5.
Effect of mPGES-1 deletion on renal epithelial Na channel (ENaC) mRNA expression after dehydration. The mRNA expression of the 3 subunits of ENaC in the renal medulla of control and dehydrated WT mice was detected by quantitative (q)RT-PCR and normalized by β-actin. A: ENaC-α. B: ENaC-β. C: ENaC-γ; n = 6–9 per group. Data are means ± SE.
Fig. 6.
Regulation of renal medullary ENaC-α protein expression by dehydration. A: immunoblots of ENaC-α in the medulla of WT mice. B: densitometric analysis of ENaC-α protein expression in WT mice. C: immunoblots of ENaC-α in the medulla of KO mice. D: densitometric analysis of ENaC-α protein expression in KO mice; n = 4–5 per group. Data are means ± SE.
Regulation of renal medullary expression of eNOS, iNOS, and nNOS by WD.
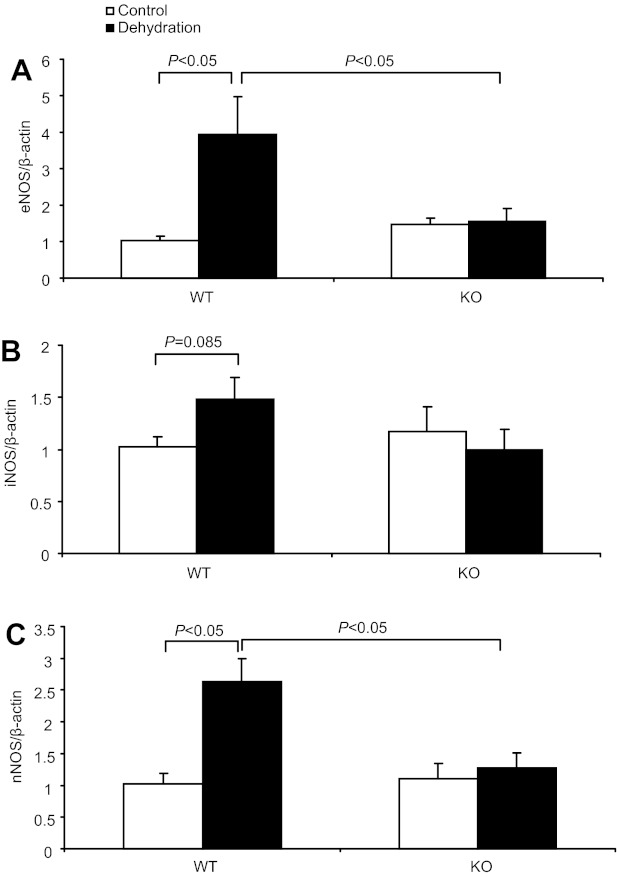
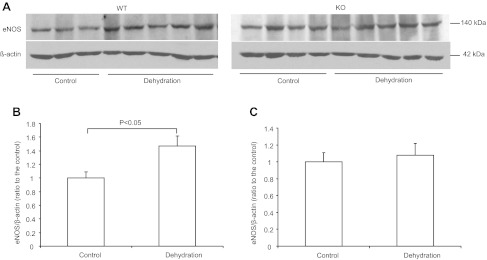
The altered urinary NOx and cGMP excretion in dehydrated mPGES-1 KO mice suggested a potential role of NO/cGMP system in mediating PGE2-elicited dehydration natriuersis. To determine the source of increased NO production in response to WD, we examined renal medullary expression of NOS isoforms in the two genotypes by qRT-PCR and immunoblotting. qRT-PCR demonstrated parallel increases in all three NOS isoforms, e.g., nNOS, iNOS, and eNOS, in the renal medulla of WT mice after WD; the increases were 2.6-fold for nNOS, 1.48-fold for iNOS, and 3.9-fold for eNOS (Fig. 7, A, B, C). In contrast, such alterations did not happen in KO mice (Fig. 7, A, B, C). We further confirmed the eNOS regulation by using immunoblotting analysis (Fig. 8, A, B, C).
Fig. 7.
Regulation of renal medullary endothelial nitric oxide synthase (eNOS), inducible (i)NOS, and neuronal (n)NOS mRNA expressions by dehydration. A: qRT-PCR detection of renal medullary eNOS mRNA expression. B: qRT-PCR detection of renal medullary iNOS mRNA expression. C: qRT-PCR detection of renal medullary nNOS mRNA expression. Control: n = 6–7. Dehydration: n = 7–9. Data are means ± SE.
Fig. 8.
Regulation of renal medullary eNOS protein expression by dehydration. A: immunoblots of eNOS in the medulla of WT and KO mice. B: densitometric analysis of eNOS protein expression in WT mice. C: densitometric analysis of eNOS protein expression in KO mice. Control: n = 3–5. Data are means ± SE.
DISCUSSION
Dehydration natriuresis is an established physiological phenomenon in many mammalian species (1, 7, 18, 28, 31, 33, 36, 37) but the underlying mechanisms, especially the renal mechanisms, are poorly characterized. The present study was undertaken to define the role of PGE2, a well-known natriuretic factor, in the occurrence of dehydration natriuresis, by examining the phenotype of mPGES-1 null mice after WD. We found that 24-h WD in WT mice increased urinary Na excretion, accompanied with increased urinary PGE2 output and renal medullary PGE2 concentration. In contrast, mPGES-1 null mice displayed a nearly complete blockade of the increases in urinary Na+ excretion and renal PGE2 synthesis. As a result, the null mice developed hypernatremeia and a trend increase in plasma osmolality. We further provide evidence that mPGES-1-derived PGE2 may elicit dehydration natriuresis through NO/cGMP. Overall, these results represent compelling evidence supporting an important role of mPGES-1-derived PGE2 in the occurence of dehydration natriuresis.
Along the nephron, the distal nephron is the major site for both production and action of PGE2. PGE2 synthesis in the collecting duct cells is modulated by osmolality (20). Along this line, it is consistently demonstrated that renal medullary COX-2 expression is induced by WD (13, 45); the opposite is true in that the COX-2 expression is reduced by water loading or chronic furosemide infusion that eliminates renal medullary osmotic gradient (4). Extensive in vitro studies have defined the signaling transduction pathway leading to osmotic regulation of COX-2 in collecting duct and renal medullary interstitial cells that primarily involve the activation of MAPK and NF-κB (13, 46). However, the functional implication of dehydration-induced renal medullary PGE2 synthesis is still poorly understood. Renal medullary PGE2 produced under WD is thought to antagonize the antidiuretic action of vasopressin, preserve the medullary blood flow, and improve cell survival but the precise role of PGE2 is unclear. mPGES-1 is a novel isomerase possessing PGE2 synthesizing activity in vivo and in vitro. The availability of mPGES-1 null mice offers a unique opportunity to examine the in vivo function of PGE2. The present study for the first time demonstrates that mPGES-1-derived PGE2 serves as a key regulator of urinary Na+ excretion during WD. In a separate study (23), we demonstrate that mPGES-1 deletion enhances urine concentrating capability after WD via stimulation of renal aquaporin-2 expression. The dual role of PGE2 facilitates the separate but coordinated regulation of salt and water handling at least during WD.
The NO/cGMP pathway represents the common mechanism triggering the natriuretic response under various physiopathological conditions. Indeed, dehydration natriuresis in WT mice is accompanied by elevated urinary NOx and cGMP excretion. Consistent with this finding, the mRNA levels of three NOS subtypes were upregulated in the medullary tissues of WT mice following WD, which are in a complete agreement with previous reports (30, 42). Strikingly, the elevation of urinary NO/cGMP along with the upregulation of NOS isoforms were completely abolished by mPGES-1 deletion. It is conceivable that PGE2 may signal through the NOS/NO/cGMP pathway to elicit the natriuretic response during WD. This finding is also in accordance with a series of our previous reports of defective NO and cGMP production in mPGES-1 null mice after high-salt loading or DOCA-salt treatment (22, 24). Other investigations also suggest the potential of PGE2 in stimulating NO and cGMP production in various renal or extrarenal systems (16, 17, 26, 40). Overall, these results support the existence of PGE2/NO/cGMP pathway that appears to be important in renal salt handling.
There is abundant evidence to suggest the involvement of central nervous system in response to the elevated sodium concentration and/or osmolality in cerebrospinal fluid after dehydration (2, 27, 32, 34, 35). Further efforts suggest that neurohypophysial hormones such as vasopressin (32) and oxytocin (9, 10, 18) may play a role. While vasopressin-deficient Brattleboro homozygous rats maintain the normal dehydration natriuresis response (11), arguing against the involvement of vasopressin in this process, consistent reports favor the involvement of oxtocin (5, 9–11, 18). There is definitive evidence that oxytocin processes natriuretic property independent of its antidiuretic activity and oxytocin receptor antagonism prevents dehydration natriuresis. Prostaglandins have been implicated to be a potential mediator of the natriuretic action of oxytocin. Further studies are needed to determine whether mPGES-1 serves as a molecular target of oxytocin in the kidney.
Microperfusion studies demonstrate that PGE2 via EP1 receptors inhibits Na+ transport in the distal nephron (14, 15). Considering ENaC as the major Na+ transport route in the distal nephron, it seems reasonable to speculate that ENaC may serve as a molecular target of PGE2 during WD. Indeed, we found that WD in WT mice induced significant downregulation of ENaC-α without affecting ENaC-β and ENaC-γ, which at least in part accounted for dehydration natriuresis. This downregulation was completely prevented in mPGES-1 null mice, suggesting that PGE2 may mediate dehydration natriuresis by targeting ENaC-α. Deletion of ENaC-α without disruption of ENaC-β and ENaC-γ in the collecting duct and connecting tubule caused the significant sodium imbalance (8), indicating that alteration of single ENaC subunit can affect the activity of the whole channel activity. Interestingly, mPGES-1 KO mice exhibited increased baseline level of of ENaC-α mRNA in the renal medulla, suggesting that mPGES-1-derived PGE2 may tonically suppress ENaC expression. Of note, the constitutive elevation of ENaC-α expression in mPGES-1 KO mice apparently did not cause obvious disturbance of Na+ balance at the basal condition. The reason for this phenomenon is unclear. The transcript change may not correlate with the channel activity or there might be some compensatory changes in other sodium channels or renal hemodynamic that may offset the influence of altered ENaC expression.
In summary, the present study examined the role of mPGES-1 in dehydration natriuresis by using mPGES-1 null mice. mPGES-1 deletion prevented dehydration natriuresis, leading to hypernatremia and a trend increase in plasma osmolality. This was associated with blockade of dehydration-induced increases in urinary and renal medullary PGE2 levels and in urinary NOx and cGMP excretion. Together, these results demonstrate that mPGES-1 mediates dehydration natriuresis likely through NO/cGMP pathway.
Perspectives
In light of the cardiovascular consequences associated with COX-2 inhibitors, there is rising interest in developing mPGES-1 inhibitors as the next generation of analgesics. It is of critical importance to understand the role of mPGES-1 in physiological processes. The present study supports the concept that mPGES-1 inhibition may lead to perturbation of sodium and water balance, particularly during WD.
GRANTS
This work was supported by VA Merit Review, National Basic Research Program of China 973 Program 2012CB517600 (No.2012CB517602), and National Institutes of Health Grant DK079162. T. Yang is an Established Investigator from American Heart Association and Research Career Scientist in Department of Veterans Affairs.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: Z.J., G.L., Y.S., and Y.K. performed experiments; Z.J. and T.Y. analyzed data; Z.J. prepared figures; Z.J., A.Z., and T.Y. drafted manuscript; G.G., A.Z., S.-F.Z., and T.Y. interpreted results of experiments; G.G., A.Z., S.-F.Z., and T.Y. edited and revised manuscript; A.Z. and T.Y. conception and design of research; A.Z. and T.Y. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Alexandra Panasiuk (University of Utah) and Maicy Downton (University of Utah) for administrative and technical assistance.
REFERENCES
- 1. Andersen LJ, Andersen JL, Pump B, Bie P. Natriuresis induced by mild hypernatremia in humans. Am J Physiol Regul Integr Comp Physiol 282: R1754–R1761, 2002 [DOI] [PubMed] [Google Scholar]
- 2. Bealer SL, Crofton JT, Share L. Hypothalamic knife cuts alter fluid regulation, vasopressin secretion, and natriuresis during water deprivation. Neuroendocrinology 36: 364–370, 1983 [DOI] [PubMed] [Google Scholar]
- 3. Breyer MD, Breyer RM. Prostaglandin E receptors and the kidney. Am J Physiol Renal Physiol 279: F12–F23, 2000 [DOI] [PubMed] [Google Scholar]
- 4. Castrop H, Vitzthum H, Schumacher K, Schweda F, Kurtz A. Low tonicity mediates a downregulation of cyclooxygenase-1 expression by furosemide in the rat renal papilla. J Am Soc Nephrol 13: 1136–1144, 2002 [DOI] [PubMed] [Google Scholar]
- 5. Chan WY. Renal prostaglandins and natriuretic action of oxytocin and vasopressin in rats. J Pharmacol Exp Ther 246: 603–609, 1988 [PubMed] [Google Scholar]
- 6. Chen J, Zhao M, He W, Milne GL, Howard JR, Morrow J, Hebert RL, Breyer RM, Chen J, Hao CM. Increased dietary NaCl induces renal medullary PGE2 production and natriuresis via the EP2 receptor. Am J Physiol Renal Physiol 295: F818–F825, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen S, Grigsby CL, Law CS, Ni X, Nekrep N, Olsen K, Humphreys MH, Gardner DG. Tonicity-dependent induction of Sgk1 expression has a potential role in dehydration-induced natriuresis in rodents. J Clin Invest 119: 1647–1658, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Christensen BM, Perrier R, Wang Q, Zuber AM, Maillard M, Mordasini D, Malsure S, Ronzaud C, Stehle JC, Rossier BC, Hummler E. Sodium and potassium balance depends on alphaENaC expression in connecting tubule. J Am Soc Nephrol 21: 1942–1951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Conrad KP, Gellai M, North WG, Valtin H. Influence of oxytocin on renal hemodynamics and electrolyte and water excretion. Am J Physiol Renal Fluid Electrolyte Physiol 251: F290–F296, 1986 [DOI] [PubMed] [Google Scholar]
- 10. Conrad KP, Gellai M, North WG, Valtin H. Influence of oxytocin on renal hemodynamics and sodium excretion. Ann NY Acad Sci 689: 346–362, 1993 [DOI] [PubMed] [Google Scholar]
- 11. Edwards BR, LaRochelle FT., Jr Antidiuretic effect of endogenous oxytocin in dehydrated Brattleboro homozygous rats. Am J Physiol Renal Fluid Electrolyte Physiol 247: F453–F465, 1984 [DOI] [PubMed] [Google Scholar]
- 12. Guan Y, Zhang Y, Schneider A, Riendeau D, Mancini JA, Davis L, Komhoff M, Breyer RM, Breyer MD. Urogenital distribution of a mouse membrane-associated prostaglandin E2 synthase. Am J Physiol Renal Physiol 281: F1173–F1177, 2001 [DOI] [PubMed] [Google Scholar]
- 13. Hao CM, Yull F, Blackwell T, Komhoff M, Davis LS, Breyer MD. Dehydration activates an NF-κB-driven, COX2-dependent survival mechanism in renal medullary interstitial cells. J Clin Invest 106: 973–982, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hebert RL, Jacobson HR, Breyer MD. Prostaglandin E2 inhibits sodium transport in rabbit cortical collecting duct by increasing intracellular calcium. J Clin Invest 87: 1992–1998, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hebert RL, Jacobson HR, Breyer MD. Triple signal transduction model for the mechanism of PGE2 actions in rabbit cortical collecting duct. Prostaglandins Leukot Essent Fatty Acids 42: 143–148, 1991 [DOI] [PubMed] [Google Scholar]
- 16. Hristovska AM, Rasmussen LE, Hansen PB, Nielsen SS, Nusing RM, Narumiya S, Vanhoutte P, Skott O, Jensen BL. Prostaglandin E2 induces vascular relaxation by E-prostanoid 4 receptor-mediated activation of endothelial nitric oxide synthase. Hypertension 50: 525–530, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Huang CN, Liu KL, Cheng CH, Lin YS, Lin MJ, Lin TH. PGE2 enhances cytokine-elicited nitric oxide production in mouse cortical collecting duct cells. Nitric Oxide 12: 150–158, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Huang W, Lee SL, Arnason SS, Sjoquist M. Dehydration natriuresis in male rats is mediated by oxytocin. Am J Physiol Regul Integr Comp Physiol 270: R427–R433, 1996 [DOI] [PubMed] [Google Scholar]
- 19. Ikeda-Matsuo Y, Ota A, Fukada T, Uematsu S, Akira S, Sasaki Y. Microsomal prostaglandin E synthase-1 is a critical factor of stroke-reperfusion injury. Proc Natl Acad Sci USA 103: 11790–11795, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jackson BA. Prostaglandin E2 synthesis in the inner medullary collecting duct of the rat: implications for vasopressin-dependent cyclic AMP formation. J Cell Physiol 129: 60–64, 1986 [DOI] [PubMed] [Google Scholar]
- 21. Jia Z, Aoyagi T, Kohan DE, Yang T. mPGES-1 deletion impairs aldosterone escape and enhances sodium appetite. Am J Physiol Renal Physiol 299: F155–F166, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jia Z, Aoyagi T, Yang T. mPGES-1 protects against DOCA-salt hypertension via inhibition of oxidative stress or stimulation of NO/cGMP. Hypertension 55: 539–546, 2010 [DOI] [PubMed] [Google Scholar]
- 23. Jia Z, Liu G, Dong Z, Zhang A, Yang T. mPGES-1 deletion potentiates urine concentrating capability after water deprivation. Am J Physiol Renal Physiol 302: F1005–F1012, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jia Z, Zhang A, Zhang H, Dong Z, Yang T. Deletion of microsomal prostaglandin E synthase-1 increases sensitivity to salt loading and angiotensin II infusion. Circ Res 99: 1243–1251, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Kamei D, Yamakawa K, Takegoshi Y, Mikami-Nakanishi M, Nakatani Y, Oh-Ishi S, Yasui H, Azuma Y, Hirasawa N, Ohuchi K, Kawaguchi H, Ishikawa Y, Ishii T, Uematsu S, Akira S, Murakami M, Kudo I. Reduced pain hypersensitivity and inflammation in mice lacking microsomal prostaglandin e synthase-1. J Biol Chem 279: 33684–33695, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Kunori S, Matsumura S, Okuda-Ashitaka E, Katano T, Audoly LP, Urade Y, Ito S. A novel role of prostaglandin E2 in neuropathic pain: blockade of microglial migration in the spinal cord. Glia 59: 208–218, 2011 [DOI] [PubMed] [Google Scholar]
- 27. Leksell LG, Denton DA, Fei DT, McKinley MJ, Muller AF, Weisinger RS, Young H. On the importance of CSF Na in the regulation of renal sodium excretion and renin release. Acta Physiol Scand 115: 141–146, 1982 [DOI] [PubMed] [Google Scholar]
- 28. Luke RG. Natriuresis and chloruresis during hydrogenia in the rat. Am J Physiol 224: 13–20, 1973 [DOI] [PubMed] [Google Scholar]
- 29. Machida K, Wakamatsu S, Izumi Y, Yosifovska T, Matsuzaki T, Nakayama Y, Kohda Y, Inoue T, Saito H, Tomita K, Nonoguchi H. Downregulation of the V2 vasopressin receptor in dehydration: mechanisms and role of renal prostaglandin synthesis. Am J Physiol Renal Physiol 292: F1274–F1282, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Martin PY, Bianchi M, Roger F, Niksic L, Feraille E. Arginine vasopressin modulates expression of neuronal NOS in rat renal medulla. Am J Physiol Renal Physiol 283: F559–F568, 2002 [DOI] [PubMed] [Google Scholar]
- 31. McKenna TM, Haines H. Sodium metabolism during acclimation to water restriction by wild mice, Mus musculus. Am J Physiol Regul Integr Comp Physiol 240: R319–R326, 1981 [DOI] [PubMed] [Google Scholar]
- 32. McKinley MJ, Denton DA, Coghlan JP, Harvey RB, McDougall JG, Rundgren M, Scoggins BA, Weisinger RS. Cerebral osmoregulation of renal sodium excretion–a response analogous to thirst and vasopressin release. Canadian J Physiol Pharmacol 65: 1724–1729, 1987 [DOI] [PubMed] [Google Scholar]
- 33. McKinley MJ, Denton DA, Nelson JF, Weisinger RS. Dehydration induces sodium depletion in rats, rabbits, and sheep. Am J Physiol Regul Integr Comp Physiol 245: R287–R292, 1983 [DOI] [PubMed] [Google Scholar]
- 34. McKinley MJ, Denton DA, Park RG, Weisinger RS. Cerebral involvement in dehydration-induced natriuresis. Brain Res 263: 340–343, 1983 [DOI] [PubMed] [Google Scholar]
- 35. McKinley MJ, Harvey RB, Vivas L. Reducing brain sodium concentration prevents post-prandial and dehydration-induced natriuresis in sheep. Acta Physiol Scand 151: 467–476, 1994 [DOI] [PubMed] [Google Scholar]
- 36. Merrill DC, Skelton MM, Cowley AW., Jr Humoral control of water and electrolyte excretion during water restriction. Kidney Int 29: 1152–1161, 1986 [DOI] [PubMed] [Google Scholar]
- 37. Metzler GH, Thrasher TN, Keil LC, Ramsay DJ. Endocrine mechanisms regulating sodium excretion during water deprivation in dogs. Am J Physiol Regul Integr Comp Physiol 251: R560–R568, 1986 [DOI] [PubMed] [Google Scholar]
- 38. Monrad SU, Kojima F, Kapoor M, Kuan EL, Sarkar S, Randolph GJ, Crofford LJ. Genetic deletion of mPGES-1 abolishes PGE2 production in murine dendritic cells and alters the cytokine profile, but does not affect maturation or migration. Prostaglandins Leukot Essent Fatty Acids 84: 113–121, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Parnova RG. Molecular mechanisms of action of prostaglandin E2 in the regulation of water osmotic permeability. Membr Cell Biol 13: 287–301, 2000 [PubMed] [Google Scholar]
- 40. Sakai M, Minami T, Hara N, Nishihara I, Kitade H, Kamiyama Y, Okuda K, Takahashi H, Mori H, Ito S. Stimulation of nitric oxide release from rat spinal cord by prostaglandin E2. Br J Pharmacol 123: 890–894, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schwertschlag U, Gerber JG, Barnes JS, Nies AS. Increased PGE2 excretion by dDAVP in humans depends on state of hydration. Am J Physiol Renal Fluid Electrolyte Physiol 250: F1008–F1012, 1986 [DOI] [PubMed] [Google Scholar]
- 42. Shin SJ, Lai FJ, Wen JD, Lin SR, Hsieh MC, Hsiao PJ, Tsai JH. Increased nitric oxide synthase mRNA expression in the renal medulla of water-deprived rats. Kidney Int 56: 2191–2202, 1999 [DOI] [PubMed] [Google Scholar]
- 43. Soodvilai S, Jia Z, Wang MH, Dong Z, Yang T. mPGES-1 deletion impairs diuretic response to acute water loading. Am J Physiol Renal Physiol 296: F1129–F1135, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Trebino CE, Stock JL, Gibbons CP, Naiman BM, Wachtmann TS, Umland JP, Pandher K, Lapointe JM, Saha S, Roach ML, Carter D, Thomas NA, Durtschi BA, McNeish JD, Hambor JE, Jakobsson PJ, Carty TJ, Perez JR, Audoly LP. Impaired inflammatory and pain responses in mice lacking an inducible prostaglandin E synthase. Proc Natl Acad Sci USA 100: 9044–9049, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yang T, Schnermann JB, Briggs JP. Regulation of cyclooxygenase-2 expression in renal medulla by tonicity in vivo and in vitro. Am J Physiol Renal Physiol 277: F1–F9, 1999 [DOI] [PubMed] [Google Scholar]
- 46. Yang T, Zhang A, Honeggar M, Kohan DE, Mizel D, Sanders K, Hoidal JR, Briggs JP, Schnermann JB. Hypertonic induction of COX-2 in collecting duct cells by reactive oxygen species of mitochondrial origin. J Biol Chem 280: 34966–34973, 2005 [DOI] [PubMed] [Google Scholar]
- 47. Zucker A, Nasjletti A, Schneider EG. Effect of water deprivation on urinary excretion of PGE2 in the dog. Am J Physiol Regul Integr Comp Physiol 245: R329–R333, 1983 [DOI] [PubMed] [Google Scholar]